Abstract
We hypothesized that blood levels of GABA and γ-hydroxybutyric acid (GHB), biomarkers of succinic semialdehyde dehydrogenase deficiency (SSADHD), would correlate with age. GABA and GHB were quantified in plasma and red blood cells (RBCs) from eighteen patients (age range 5–41 years; median 8). Both metabolites negatively correlated with age (P<0.05). Plasma and RBC GHB declined with age, reaching a nadir and approximate steady-state by 10 years. Declining plasma GABA achieved this approximate steady state at 30–40 years of age. These biomarker relationships may reflect further GABA- and GHB-ergic neurotransmission imbalances that correlate with the onset of adolescent/adulthood neuropsychiatric morbidity and epilepsy in SSADHD.
Introduction
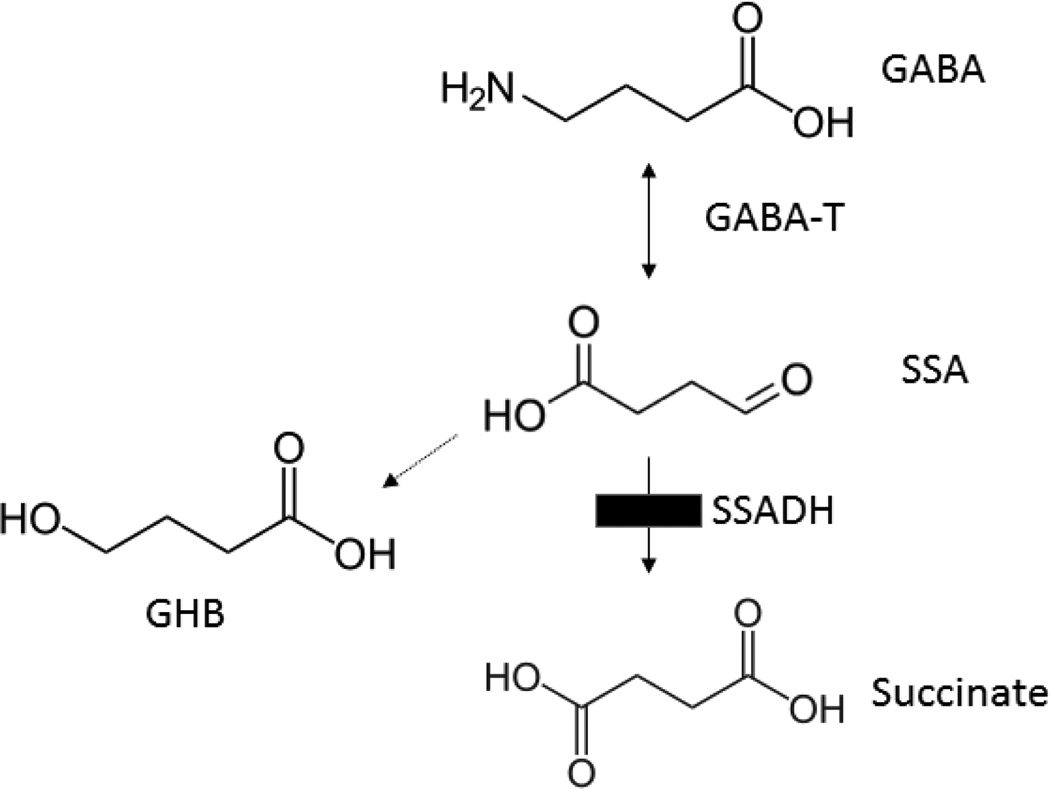
A rare disorder of GABA catabolism, SSADHD has been reported in approximately 200 patients worldwide (Malaspina et al 2016). The phenotype is non-specific, with developmental delay, hypotonia, absence of formulated speech, and the onset of neuropsychiatric morbidity (obsession-compulsion, attention-deficit, oppositional defiant disorders) in adolescence to adulthood. Autism spectrum disorder is frequently considered in the differential diagnosis (Malaspina et al 2016; Parviz et al 2014). Detection of SSADHD requires measurement of γ-hydroxybutyrate (GHB; the biochemical hallmark) in peripheral biofluids, although GABA is also elevated in CSF (Gibson et al 1995; 2003). Both compounds have either neurotransmitter, or neuromodulatory, roles in CNS (Maitre et al 2016; Fig. 1).
Fig. 1. GABA metabolism.
Abbreviations: GABA, γ-aminobutyric acid; GABA-T, GABA-transaminase; SSADH, succinic semialdehyde dehydrogenase; SSA, succinic semialdehyde; GHB, γ-hydroxybutyric acid. The site of the block in patients with SSADHD is indicated by the filled black box.
The natural history of SSADHD remains unknown, and a longitudinal study that may define natural history is lacking. Nonetheless, anecdotal evaluation of reported cases has highlighted the onset of neuropsychiatric morbidity in adolescence and adulthood (Parviz et al 2014; Pearl et al 2014). Moreover, Knerr and coworkers have identified a more severe disease course in a subset of patients, in which the epileptic disorder appears more severe (Knerr et al 2007). Importantly, some patients have expired with SUDEP (sudden unexplained death in epilepsy) in adulthood (Lapalme-Remis et al 2015; Knerr et al 2010; Gibson and Pearl, unpublished). To explore the natural history of SSADHD, we have sought to identify biomarkers in physiological fluids which may provide pathophysiological insight into disease evolution. Here, we examine the levels of both GABA and GHB in urine, plasma and RBCs to address potential age-dependent correlations. Our working hypothesis centered on the prediction that blood levels of both biomarkers would reflect biomarker disturbances in CNS, providing insight into disease progress and evolution.
Methods
Whole blood and urine were obtained from patients with SSADHD, obligate heterozygotes (parents) and control individuals with informed consent (IRB 12678). For the physiological fluids evaluated, the number of control individuals were: eight (plasma total GABA and RBC GHB), eleven (plasma GHB), and thirteen (urine GHB). Control individuals were not age-matched to patients, although we speculate that the age-range of both cohorts overlapped. The majority of controls were adults, although some were siblings of patients (children and adolescents) whose genetic status was unknown. For control individuals, gender status was identified only for seven, and age identified only for four, making correlative analyses between metabolic findings and age/gender challenging. Patient age range was 5–41 years (median, 8 years), and ages were available for all. Clinical details were not available on patients at the time of sample shipment, and to our knowledge none of the patients was concurrently receiving vigabatrin or other antiepileptic which may have artefactually elevated plasma total GABA, as is the case for GABA in cerebrospinal fluid for patients receiving vigabatrin (Gibson et al 1995; Casado et al 2014).
Blood samples, upon receipt in the laboratory, were fractionated into plasma, mixed polymorphonuclear cells, and packed red blood cells. Although GABA levels have been effectively quantified in platelets isolated from whole blood (Kowa et al 1992), we opted for isolation of plasma since an objective was to determine if total GABA levels might be elevated in this compartment and potentially inform GABA concentrations in whole bloodspots for eventual newborn screening of SSADHD. Moreover, since we have recently demonstrated that both GHB and GABA are significantly elevated in brain and peripheral organs (kidney, liver) of SSADH-deficient mice (Vogel et al, in revision), we assume that peripheral GABA metabolism mimics the pathway of metabolism shown in brain. The latter underscores the utility of measuring these biomarkers in peripheral tissues (plasma, red blood cells) as a surrogate for brain, or even cerebrospinal fluid, measurements. Total GABA in plasma, and GHB in urine, plasma and RBC extracts were quantified as described (Kok et al 1993; Gibson et al 1990). Statistical analysis was performed using GraphPad Prizm 6.0 (San Diego, CA).
Results
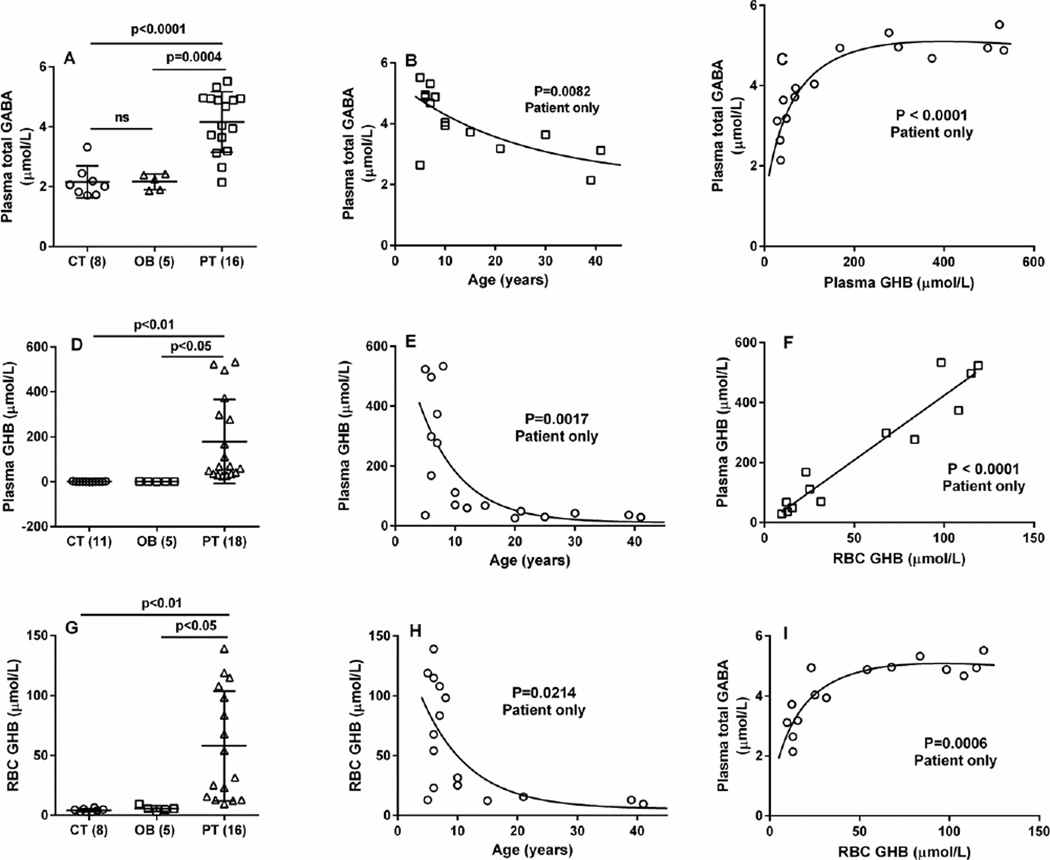
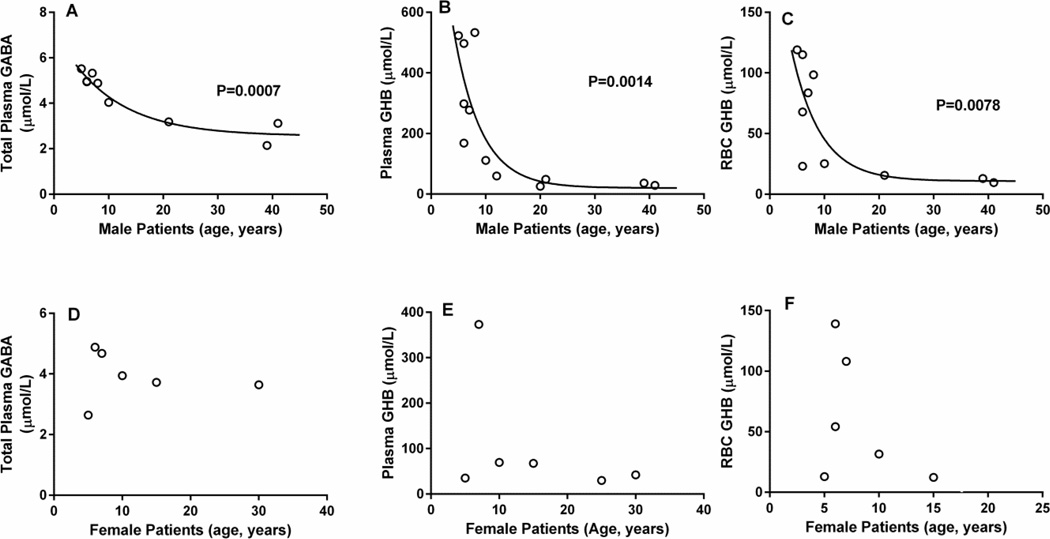
Plasma total GABA values were: control, 2.16 ± 0.19 µmol/L (mean ± SEM, n=8; median, 2.06, range 1.7–3.32); obligate heterozygotes (parents), 2.16 ± 0.12 (n=5; median 2.24, range 1.86–2.42); SSADHD, 4.16 ± 0.25 (n=16, median 4.04; range 2.14–5.52; p < 0.001 compared to control and obligate heterozygotes, two tailed t test) (Fig. 2A). When stratified for patient age, plasma total GABA showed a significant negative correlation (Fig. 2B; P = 0.0082, Spearman coefficient, 10 male, 6 female). A negative correlation was also observed when evaluating only male patients (P = 0.0007), but not with female patients(Fig. 3A, D).
Fig. 2. Metabolic correlations as a function of age, and between metabolites, in patients with SSADH deficiency.
(A, D, G) Graphic representation of metabolites level as a function of genotype. Abbreviations employed: CT, control (number of individuals in parentheses); OB, obligate heterozygotes (parents); PT, patients with SSADH deficiency. Data shown as mean with SEM. Statistical analysis employed a two-way t test. (B, E, F) Correlation of metabolites with age (patients only). Statistical analysis employed the Spearman coefficient. (C–I) Metabolite interrelationships, in which the Spearman coefficient was employed for 2C and 2I, and the Pearson coefficient for Fig. 2F.
Fig. 3. Metabolic correlations for patients with SSADH deficiency as a function of gender.
(A–C) male patients; (D–F) female patients. For male patients, statistical analysis employed the Spearman coefficient. There was no statistically significant correlation for female patients, but the low number of participants may have impacted this analysis.
Plasma GHB values were: control, 1.14 ± 0.23 µmol/L (mean ± SEM, n=11; median 0.8, range 0.5–2.9); obligate heterozygotes, 1.14 ± 0.23 (n=5; median, 1.1, range 0.5–1.9); SSADHD, 179.1 ± 44.1 (n=18, median 67.6, range 25.5–533; p < 0.05 compared to both groups, two-tailed t test) (Fig. 2D). Plasma GHB showed a significant negative correlation with age (Fig. 2E; P = 0.0017, Spearman coefficient, 12 male, 6 female patients). A negative correlation was also observed when evaluating only male patients (P = 0.0014), but not with female patients (Fig. 3B, E). RBC GHB levels were: control, 4.19 ± 0.45 µmol/L (mean ± SEM, n=8; median 3.9, range 2.8–6.4); obligate heterozygotes, 5.56 ± 1.05 (n=5; median 5.4, range 2.9–9.3); SSADHD, 58.0 ± 11.5 (n=16, median 54.2, range 9.6–139; p < 0.05 compared to both groups, two-tailed t test) (Fig. 2G). RBC GHB showed a significant negative correlation (Fig. 2H; P = 0.0214, Spearman coefficient, 12 male and 6 female patients). A negative correlation was also observed when evaluating only male patients (P = 0.0078), but not with female patients (Fig. 3C, F). Urine samples were also available from 18 patients, although age characteristics were known only for 8 patients. Urine GHB levels were: control, 1.68 ± 0.77 mmol/mol creatinine (mean ± SEM, n=13; median, 0.4, range 0.12–10.1); obligate heterozygotes, 1.84 ± 1.37 (n=14; median 0.4, range 0.10–19.6); SSADHD, 158.5 ± 27.0 (n=18, median 153, range 26.8–514; p < 0.0001 compared to both groups, two-tailed t test). There was no correlation between urine GHB concentration and age (data not shown).
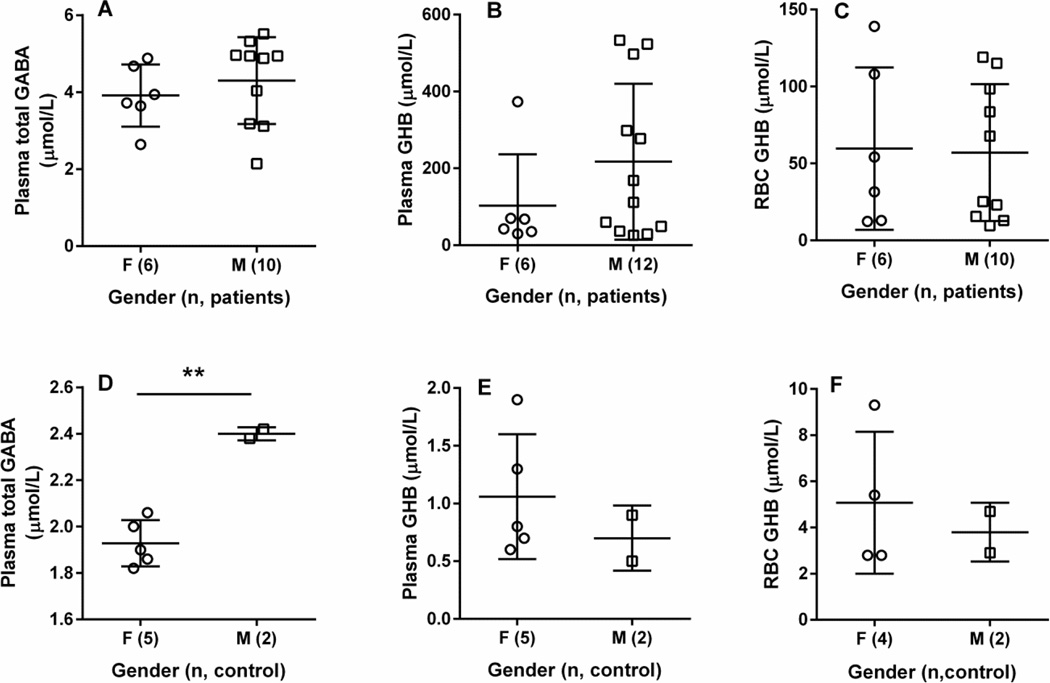
Correlations between plasma total GABA and GHB revealed a profile which attained a plateau at total GABA levels of ~5 µmol/L and GHB concentrations ~300 µmol/L (Fig. 2C; P < 0.0001). Similar results were seen for plasma total GABA (~ 5 µmol/L) and RBC GHB of ~ 65 µmol/L (P = 0.0006; Fig. 2I). The relationship between plasma and RBC GHB was linear (Fig. 2F; P < 0.0001, Pearson coefficient). For patients, there was no significant difference with respect to gender for any of the metabolites evaluated (Figs. 4A–C). Information on gender and age characteristics for the control cohort were, unfortunately, limited (Fig. 4). For the few control individuals for whom gender was known, plasma and RBC GHB levels were not significantly different (Figs. 4E, F), but only 2 individuals were identified as male. For plasma total GABA, the mean value in males was significantly higher in males as compared to females (Fig. 4A), but for males the control cohort contained only 2 individuals.
Fig. 4. Metabolic comparisons within SSADH-deficient patient and control cohorts as a function of gender.
(A–C) gender comparisons for patients with SSADH deficiency (f, female; m, male, with parenthetical values representing the number of subjects studied). Statistical analysis employed a two-way t test, and no significant differences were observed for any metabolite. (D–F) gender comparisons within the control cohort. Gender characteristics were available only on a limited number of individuals. For plasma total GABA, a significantly higher mean value was observed in males vs. females (Fig. 4D; two way t test), but only 2 males populated this group.
Discussion
Total GABA, as well as GHB in plasma and RBC extracts, revealed a significant negative correlation with age (Fig. 2B, E, H). Low plasma GABA has previously been associated with neuropsychiatric morbidity. Plasma GABA was negatively correlated with age and aggressiveness in subjects having a first-degree relative with primary unipolar depressive disorder (Bjork et al 2001), and positively correlated with depression and apathy and negatively correlated with age in severe Alzheimer disease (Lanctot et al 2007). The phenotype of SSADHD often includes neuropsychiatric morbidity in adolescence to adulthood, including attention deficit-hyperactivity, oppositional defiant and obsessive compulsive disorders (Malaspina et al 2016). We found tapering of levels for GHB at about 10 years of age, whereas this same tapering for plasma total GABA was not evident until at least 30 years of age. The lowered levels of GHB may track with the onset of neuropsychiatric morbidity in patients (Parviz et al 2014), but further studies are needed to verify this hypothesis. Moreover, we know that a subset of patients with SSADHD has a more severe phenotype, featuring adult-onset epilepsy (Parviz et al 2014), and there have been instances of SUDEP (sudden unexplained death in epilepsy) in adulthood (Lapalme-Remis et al 2015; Knerr et al 2010; Gibson and Pearl, unpublished observation). Previously, Casado and coworkers (2014) documented that GABA levels in cerebrospinal fluid from 55 pediatric controls showed a progressive increase with age. Unfortunately, we could not assess this age-related parameter in our control cohort since information on age characteristics was limited.
Several publications have highlighted alterations in GABAergic neurotransmission in SSADHD (Pearl et al 2009; Reis et al 2012; Buzzi et al 2006), both in the orthologue mouse model and patients, and it may be reasonable to assume that high levels of GABA and GHB during ontogeny induces an altered balance between GABAergic/GHBergic neurotransmission with that of excitatory glutamatergic neurotransmission (Jansen et al 2008; Vogel et al 2016; Talaei et al 2016). Our prediction is that as levels of GABA and GHB fall with age, this balance is likely disrupted and correlates with neuropsychiatric morbidity in patients. A comprehensive longitudinal evaluation of SSADHD, correlating brain and blood levels of GABA and GHB with age and phenotype, could address this issue.
Our study represents the first identification of elevated total GABA in the plasma of patients with SSADHD. This is relevant for several reasons. First, appropriate testing for GHB in order to achieve accurate diagnosis may be overlooked due to the non-specific neurological phenotype (Lapalme-Remis et al 2015). Additionally, clinical trials for SSADHD are either underway or completed (Pearl et al 2014; www.clinicaltrials.gov), and a therapeutic pipeline is under development using the corresponding murine model (Malaspina et al 2016). Accordingly, early detection of the disease, preferably through newborn screening, would be desirable. This may be even more compelling since many patients with SSADHD are not diagnosed until at least 2 years of age, such that the early course of the disease is largely unknown (Malaspina et al 2016). Thus, early detection via newborn screening could begin to outline the early natural history of SSADHD, while informing symptomatic management and providing families with important predictive information. Forni and colleagues (20130 presented methodology for quantitation of GHB in newborn bloodspots. The method, however, is not readily interfaced to current newborn screening platforms, which quantifies nitrogenous species amino acids, L-carnitine analogs; (Therrell et al 2015). Accordingly, plasma total GABA may provide a more rapid path to newborn screening of SSADHD, although it is likely that other neurological conditions could result in elevated GABA levels in newborn bloodspots (e.g., neuropsychiatric conditions, GABA-transaminase deficiency, etc).
Since the discovery of SSADHD in the early 1980s (Jakobs et al 1981), GHB has been employed as the biochemical hallmark of the disease, and its identification in physiological fluids has enabled accurate diagnosis. Later studies on SSADHD also revealed elevations of GABA in the CNS, using both cerebrospinal fluid analyses as well as magnetic resonance spectroscopy (Gibson et al 1995; 2003; Novotny et al 2003). We now demonstrate, for the first time, that measurement of GABA and GHB in peripheral biofluids may inform about the natural history of the disorder, and thereby provide a mechanism by which to predict the sequence and timing of the onset of symptoms and complications with development.
Acknowledgments
The authors gratefully acknowledge the families who contributed samples for this study, as well as the ongoing support of the SSADH association (www.ssadh.net) and Speragen, Inc.
Abbreviations
- GABA
4-aminobutyrate
- GABA-T
GABA-transaminase
- SSADH
succinic semialdehyde dehydrogenase
- SSADHD
succinic semialdehyde dehydrogenase deficiency
- GHB
γ-hydroxybutyric acid
- NBS
newborn screening
Footnotes
- EEJ: acquisition and analysis of data
- KRV: acquisition and analysis of data; figure development
- GSS: acquisition and analysis of data
- PLP: conception and design of study; drafting of manuscript
- JBR: conception and design of study; drafting of manuscript
- KMG: conception and design of study; data analysis; manuscript drafting
Conflicts of Interest: The authors cumulatively declare they have no, and no perceived, conflicts of interest.
Ethics approval: Approved by WSU IRB (Institutional Review Board for Human Studies)
References
- Bjork JM1, Moeller FG, Kramer GL, et al. Plasma GABA levels correlate with aggressiveness in relatives of patients with unipolar depressive disorder. Psychiatry Res. 2001 Mar 25;101(2):131–136. doi: 10.1016/s0165-1781(01)00220-7. [DOI] [PubMed] [Google Scholar]
- Buzzi A, Wu Y, Frantseva MV, et al. Succinic semialdehyde dehydrogenase deficiency: GABAB receptor-mediated function. Brain Res. 2006 May 23;1090(1):15–22. doi: 10.1016/j.brainres.2006.02.131. [DOI] [PubMed] [Google Scholar]
- Casado M, Molero M, Sierra C, Garcia-Cazorla A, Ormazabal A, Artuch R. Analysis of cerebrospinal fluid γ-aminobutyric acid by capillary electrophoresis with laser-induced fluorescence detection. Electrophoresis. 2014;35:1181–1187. doi: 10.1002/elps.201300261. [DOI] [PubMed] [Google Scholar]
- Forni S, Pearl PL, Gibson KM, et al. Quantitation of gamma-hydroxybutyric acid in dried blood spots: feasibility assessment for newborn screening of succinic semialdehyde dehydrogenase (SSADH) deficiency. Mol Genet Metab. 2013 Jul;109(3):255–259. doi: 10.1016/j.ymgme.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson KM, Aramaki S, Sweetman L, et al. Stable isotope dilution analysis of 4-hydroxybutyric acid: an accurate method for quantification in physiological fluids and the prenatal diagnosis of 4-hydroxybutyric aciduria. Biomed Environ Mass Spectrom. 1990 Feb;19(2):89–93. doi: 10.1002/bms.1200190207. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Jakobs C, Ogier H, et al. Vigabatrin therapy in six patients with succinic semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 1995;18(2):143–146. doi: 10.1007/BF00711750. [DOI] [PubMed] [Google Scholar]
- Gibson KM, Gupta M, Pearl PL, et al. Significant behavioral disturbances in succinic semialdehyde dehydrogenase (SSADH) deficiency (gamma-hydroxybutyric aciduria) Biol Psychiatry. 2003 Oct 1;54(7):763–768. doi: 10.1016/s0006-3223(03)00113-6. [DOI] [PubMed] [Google Scholar]
- Jakobs C, Bojasch M, Mönch E, et al. Urinary excretion of gamma-hydroxybutyric acid in a patient with neurological abnormalities. The probability of a new inborn error of metabolism. Clin Chim Acta. 1981 Apr 9;111(2–3):169–178. doi: 10.1016/0009-8981(81)90184-4. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Struys E, Jakobs C, et al. Neurotransmitter alterations in embryonic succinate semialdehyde dehydrogenase (SSADH) deficiency suggest a heightened excitatory state during development. BMC Dev Biol. 2008 Nov 28;8:112. doi: 10.1186/1471-213X-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knerr I, Pearl PL, Bottiglieri T, et al. Therapeutic concepts in succinate semialdehyde dehydrogenase (SSADH; ALDH5a1) deficiency (gamma-hydroxybutyric aciduria). Hypotheses evolved from 25 years of patient evaluation, studies in Aldh5a1−/− mice and characterization of gamma-hydroxybutyric acid pharmacology. J Inherit Metab Dis. 2007 Jun;30(3):279–294. doi: 10.1007/s10545-007-0574-2. [DOI] [PubMed] [Google Scholar]
- Knerr I, Gibson KM, Murdoch G, et al. Neuropathology in succinic semialdehyde dehydrogenase deficiency. Pediatr Neurol. 2010 Apr;42(4):255–258. doi: 10.1016/j.pediatrneurol.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok RM, Howells DW, van den Heuvel CC, et al. Stable isotope dilution analysis of GABA in CSF using simple solvent extraction and electron-capture negative-ion mass fragmentography. J Inherit Metab Dis. 1993;16(3):508–512. doi: 10.1007/BF00711667. [DOI] [PubMed] [Google Scholar]
- Kowa H, Shimomura T, Takahashi K. Platelet gamma-aminobutyric acid levels in migraine and tension-type headache. Headache. 1992;32:229–232. doi: 10.1111/j.1526-4610.1992.hed3205229.x. [DOI] [PubMed] [Google Scholar]
- Lanctot KL, Herrmann N, Rothenburg L, Eryavec G. Behavioral correlates of GABAergic disruption in Alzheimer’s disease. Int Psychogeriatr. 2007;19(1):151–158. doi: 10.1017/S1041610206003899. [DOI] [PubMed] [Google Scholar]
- Lapalme-Remis S, Lewis EC, De Meulemeester C, et al. Natural history of succinic semialdehyde dehydrogenase deficiency through adulthood. Neurology. 2015 Sep 8;85(10):861–865. doi: 10.1212/WNL.0000000000001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitre M, Klein C, Mensah-Nyagan AG. Mechanisms for the Specific Properties of γ-Hydroxybutyrate in Brain. Med Res Rev. 2016 Apr;36(3):363–388. doi: 10.1002/med.21382. [DOI] [PubMed] [Google Scholar]
- Malaspina P, Roullet J-B, Pearl PL, et al. Succinic semialdehyde dehydrogenase deficiency (SSADHD): Pathophysiological complexity and multifactorial trait associations in a rare monogenic disorder of GABA metabolism. Neurochem. Intl. doi: 10.1016/j.neuint.2016.06.009. available on-line June 14, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny EJ, Jr, Fulbright RK, Pearl PL, et al. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54(Suppl 6):S25–S31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- Parviz M, Vogel K, Gibson KM, Pearl PL. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy. 2014 Nov 25;3(4):217–227. doi: 10.3233/PEP-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Gibson KM, Quezado Z, et al. Decreased GABA-A binding on FMZ-PET in succinic semialdehyde dehydrogenase deficiency. Neurology. 2009 Aug 11;73(6):423–429. doi: 10.1212/WNL.0b013e3181b163a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl PL, Schreiber J, Theodore WH, et al. Taurine trial in succinic semialdehyde dehydrogenase deficiency and elevated CNS GABA. Neurology. 2014 Mar 18;82(11):940–944. doi: 10.1212/WNL.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Cohen LG, Pearl PL, et al. GABAB-ergic motor cortex dysfunction in SSADH deficiency. Neurology. 2012 Jul 3;79(1):47–54. doi: 10.1212/WNL.0b013e31825dcf71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talaei SA, Azami A, Salami M. Postnatal development and sensory experience synergistically underlie the excitatory/inhibitory features of hippocampal neural circuits: Glutamatergic and GABAergic neurotransmission. Neuroscience. 2016 Mar 24;318:230–243. doi: 10.1016/j.neuroscience.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Therrell BL, Padilla CD, Loeber JG, et al. Current status of newborn screening worldwide: 2015. Semin Perinatol. 2015 Apr;39(3):171–187. doi: 10.1053/j.semperi.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Gibson KM. mTOR inhibitors rescue premature lethality and attenuate dysregulation of GABAergic/glutamatergic transcription in murine succinate semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis. 2016 Aug 12; doi: 10.1007/s10545-016-9959-4. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KR, Ainslie GR, Jansen EEW, Salomons GS, Gibson KM. Therapeutic relevance of mTOR inhibition in murine succinate semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. Biochim Biophys Acta. doi: 10.1016/j.bbadis.2016.10.009. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]