Abstract
For scientists working on gonochoric organisms, determining sex can be crucial for many biological questions and experimental studies, such as crossbreeding, but it can also be a challenging task, particularly when no sexual dimorphism is visible or cannot be directly observed. In metazoan parasites of the genus Schistosoma responsible for schistosomiasis, sex is genetically determined in the zygote with a female heterogametic ZW/ZZ system. Adult flukes have a pronounced sexual dimorphism, whereas the sexes of the larval stages are morphologically indistinguishable but can be distinguished uniquely by using molecular methods. Therefore, reliable methods are needed to identify the sex of larvae individuals. Here, we present an endpoint PCR-based assay using female-specific sequences identified using a genome-wide comparative analysis between males and females. This work allowed us to identify sex-markers for Schistosoma haematobium and Schistosoma bovis but also the hybrid between both species that has recently emerged in Corsica (France). Five molecular sex-markers were identified and are female-specific in S. haematobium and the hybrid parasite, whereas three of them are also female-specific in S. bovis. These molecular markers will be useful to conduct studies, such as experimental crosses on these disease-causing blood flukes, which are still largely neglected but no longer restricted to tropical areas.
Author Summary
Current global changes (environmental and anthropogenic) are expected to promote the spread and transmission of infectious diseases. One of the direct consequences of such changes is the modification of the geographical distribution of species, enabling natural hybridization. Such hybridization is already known to occur in schistosomes, and offspring have been shown to have superior virulence and invasive capacities. The recent outbreak of a hybrid between the human- and animal- infecting schistosomes, S. haematobium x S. bovis, in Europe (Corsica, France) clearly demonstrates this invasive capacity and raises the risk of zoonotic transmission. Therefore, it is important to study such hybrids, and experimental crosses are critical to address this issue. Here, we developed molecular sex markers for S. haematobium and S. bovis in order to distinguish gender and to be able to generate differentially introgressed hybrids, allowing us to investigate parasite fitness.
Introduction
Flatworm parasites of the genus Schistosoma are well known trematodes for their serious threat to human and animal health. These blood flukes cause schistosomiasis, a neglected disease which ranks second to malaria, in terms of morbidity and mortality [1]. More than 200 million people are infected worldwide, and the parasite is endemic to 78 countries in tropical and subtropical areas [2,3]. Schistosomes are endoparasites with a complex lifecycle involving two obligatory hosts. One is a freshwater mollusc, as an intermediate host, and humans or mammals represent definitive hosts. In the definitive host, monogamous couples of worms sexually reproduce, and females release hundreds of eggs daily. The elimination of eggs through host faeces or urine in a freshwater environment allows the development of free-swimming larvae called miracidia. These larvae actively search for their intermediate mollusc hosts, which they penetrate and develop via asexual multiplication to produce thousands of vertebrate infecting larvae (cercariae). A unique feature of the Schistosoma genus amongst other hermaphroditic trematodes is their gonochorism. Sexes are genetically determined during egg fertilization by a ZZ/ZW chromosomal system, where the female is the heterogametic sex [4]. Sexual dimorphism strongly characterises schistosome adult worms (i.e. a muscular adult male vs. a thin adult female). Furthermore, females do not fully mature in the absence of males. By contrast, male and female larval stages are morphologically indistinguishable [5,6].
Recently, an outbreak of urogenital schistosomiasis occurred in Corsica [7,8]. This French Mediterranean Island is particularly attractive for tourists, and its population increases from 300,000 to 3 million people in summer season. With more than 100 people infected in Corsica, this outbreak demonstrates how easily a tropical disease, possibly under the influence of global changes (i.e. climate change and human migration), could emerge in temperate continental areas [9]. Urogenital schistosomiasis is usually caused by S. haematobium. This species is widely present in Africa and in the Arabic Peninsula, and is the only schistosome species in the vessels of the urogenital tract (mainly bladder) of humans. Surprisingly, molecular investigations (sequencing of the mitochondrial cox1 gene and nuclear ribosomal internal transcribed spacer DNA = ITS) have revealed that the parasite incriminated in the Corsican outbreak was not a pure S. haematobium parasite, but a hybrid between S. haematobium and S. bovis [10,11]. S. bovis is a parasite of the same monophyletic group referred to as the S. haematobium group [12]. However it is not a human, but rather a livestock parasite, infecting cows, sheep or goats, and lives in the mesentery instead of the bladder vessels. This hybrid parasite makes the epidemiological situation complex and raises the risk of zoonotic transmission. One relevant question is the importance of the hybrid vigour of a parasite in such situation. Parasite hybridization has been shown to impact parasite infectivity, virulence, transmission and/or host specificity in fungal, viral, bacterial and parasitic helminths (see [13] for recent synthesis). Such hybrid vigour has already been demonstrated in the laboratory, with S. haematobium x S. intercalatum (a parasite of human belonging to the same S. haematobium group) crosses. These last hybrids have a better capacity to invade and develop into their respective molluscan or experimental rodent hosts compared with both parental species [14]. Hybrid vigour of the parasite is therefore an important aspect to study, and experimental crosses are critical to explore the hybridisation process. Such experimental crosses necessitate controlling the sex of cercariae proposed to experimental rodent host infection. Because larvae sex cannot be distinguished morphologically, molecular markers based on W-specific regions are crucial to identify the larval form. Such sex markers have already been developed in S. mansoni [15–18]. It has been shown previously that massive sequencing methods allow rapid comparisons of genomes and identification of sex-specific sequences in S. mansoni [18]. Therefore, we developed a robust and reliable endpoint PCR method to distinguish the sexes of S. bovis and S. haematobium at any developmental stage. The markers can also be applied efficiently to the hybrid strain recovered from Corsica.
Materials and Methods
Parasitological methods
Schistosome species, hosts and origin of the parasite strains used in this study are summarized in Table 1. For each strain, molluscs were individually exposed to a single miracidium of unknown sex. Twenty-eight (for S. bovis) to 45 days (for S. haematobium or the Corsican hybrid strain) after miracidium exposure, molluscs released either male or female clonal populations of cercariae. Cercariae from single molluscs were used to individually infect hamster definitive hosts. Three months after exposing hamsters to cercariae, adult worms were recovered by portal perfusion as described previously [19]. Only at this step, are male and female morphologically distinct. Individual male and female worms were stored for subsequent molecular analyses. Male and female of the hybrid strain were used for whole genome sequencing and the subsequent identification of sex markers (see below). Thereafter, these markers were tested on male and female of the three strains (Table 1). Detailed methods employed for molluscan and rodent infections were described previously [20,21].
Table 1. Schistosome species, hosts and origins of the parasite strains.
| Schistosome species | Intermediate host | Definitive host | Origin | Year of isolation | References |
|---|---|---|---|---|---|
| S. haematobium x S. bovis | Bulinus truncatus | Mesocricetus auratus | Cavu River, Corsica, (France) | 2014 | [10,11] |
| S. haematobium | Bulinus truncatus | Mesocricetus auratus | Barombi Kotto Lake, (Cameroon) | 2015 | This study |
| S. bovis | Planorbarius metidjensis | Mesocricetus auratus | Villar de la Yegua-Salamanca, (Spain) | 1970 | [22] |
Identification of sex markers
Genome sequencing
Genomic DNA was separately sequenced from males and females (one pool of 10 males and one pool of 40 females) of S. haematobium x S. bovis hybrid adult worms (produced in rodents after recovering eggs from urine of an infected human patient) using Illumina HiSeq 2000 PE100 technology. DNA was extracted using Qiamp DNA microkit tissue protocol from Qiagen. After the lysis step, we used 4 μl (100 mg/ml) of RNase A (Qiagen) for 2 min at room temperature to digest RNA. The final elution step was performed twice with 35 μl of Tris HCl 5 mM pH 8.5. DNA quantification was performed using a QubitTM dsDNA HS Assay Kit (Invitrogen), and concentrations were estimated at 12.7 ng/μl for males and 12.8 ng/μl for females. DNA was then sent to Genome Quebec for library construction from 700 ng of genomic DNA (for each sex). A total of 192,363,250 and 186,790,310 reads were produced from males and females, respectively.
In silico analysis
The sequencing reads were filtered for quality (PHRED score < 30 for 90% of read length; Q30/90%). Then, we removed primers and adapters with the algorithm cutadapt [23]. Finally, ~144 million (75%) reads of high quality for males and females remained for subsequent analysis.
To discriminate sex-specific sequences from unbalanced genomic introgression, high quality sequencing reads were aligned to either S. haematobium, S. bovis or to a chimeric concatenate of both genomes (allowing each read, depending on its origin, to map against the more similar location in one or the other species’ genome). We used the SchistoDB S. haematobium genome [24] and the S. bovis draft assembly genome available at the Sanger institute website. The short alignment tool Bowtie2 [25] was used; mapping was performed in single-end with pre-set parameters (—sensitive -D 15 -R 2 -L 22 -i S, 1,1.15) and by restricting to uniquely mapped reads. Because the assembly of the S. bovis genome (362 Mb) is highly fragmented (>100,000 scaffolds; N50 = 7.0 kb), most effort concentrated on mapping reads to the genome of S. haematobium (385 Mb genome; 3 833 scaffolds; N50 = 307 kb).
Two independent and complementary methods were used to detect female specific sequences, with 1) a visual inspection of reads aligned to the genomes of parental species (example in S1 Fig), and 2) a copy number variation detection using CNV-seq between male and female reads [26]. The visual inspection of genomic read alignments was performed using the Integrative Genomics Viewer (IGV) [27] to find regions showing exclusive coverage in females. Scaffolds and contigs were randomly inspected in the genomes of S. bovis and S. haematobium for potential female specific sequences. The CNV-seq method was used to compare coverage between males and females along the genome. The minimum window size was set to 500 bp, log2 ratio to 0.6 and p-value stringency to 10−6. S. bovis and S. haematobium reference genomes were used, alternatively, and regions showing specific coverage for females were selected.
Consensus candidate sequences were extracted using IGV, and primers were designed using the program Primer3Plus [28]. In order to confirm specificity, primers were compared (by BLAST) against the genome that they were originally identified in.
Validation of sex markers
DNAs of single adult worms of each sex were extracted, as recommended from the QIAGEN QIAamp DNA Micro Kit protocol for isolation of genomic DNA from tissues. The final elution was in 40 μl of buffer AE (10 mM Tris·Cl, 0.5 mM EDTA, pH 9.0). Sex markers were also tested on cercariae of S. haematobium. DNA extraction from cercariae was conducted as published by our colleagues [29].
We used a multiplex approach with the GAPDH gene as an internal PCR control (Table 2) The reactions were carried out in a total volume of 12.5 μl containing GoTaq Flexi Reaction Buffer (Promega), 1.5 mM of MgCl2, 0.4 μM of each primers of both markers (at 10 μM each), 0.2 μM of dNTP solution (at 10 μM each), 0.5 U of GoTaq G2 Hot Start Polymerase (Promega, USA) and 10 ng of DNA template (except no-DNA controls). The PCR protocol was: an initial denaturation phase at 95°C for 5 min, followed by 35 cycles at 95°C for 30s, 57°C for 30s, 72°C for 40s, and a final extension at 72°C for 10 min. PCR products were examined on 1.5% agarose gels using a 100 bp-DNA Ladder (Promega) for size estimation. PCR amplifications were performed using biological duplicates of adult male and female worms of S. haematobium, S. bovis and the hybrid, and using seven replicates of a pool of 5 cercariae recovered from molluscs infected by only one miracidium (i.e. one sex). The sex of the clonal populations of cercariae was confirmed by infecting hamsters, and morphological examination of adults that developed. In total, 35 individuals were used to validate sex markers (Fig 1 and S2 Fig). Female-specific amplicons were sent for sequencing (Genoscreen, Lille, France) for each target species.
Table 2. Summary information of the sex-specific markers identified.
| Name | Primer sequences (5’-3’) | Scaffold | Position | Amplicon size | Female specificity | ||
|---|---|---|---|---|---|---|---|
| S. h | S. b | S.h x S.b | |||||
| GAPDH | Forward | KL251991 (S. haematobium) SBOI.contig.33617.2504 (S. bovis) | 15,936–16,493 | 558 bp | No | No | No |
| CGACCATTGATGCAGCTAAA | |||||||
| Reverse | 1,000–1,557 | ||||||
| TTCCAAAATCCCCTTCATTG | |||||||
| WSh1 | Forward | KL253191 | 39–225 | 187 bp | Yes | NA | Yes |
| GCGTTCCGTTTAAAACATCG | |||||||
| Reverse | |||||||
| GTCCATGTGAGGGAATTTCG | |||||||
| WSh2 | Forward | KL252440 | 84–322 | 239 bp | Yes | NA | Yes |
| GAATCGATGACACTGGCGTA | |||||||
| Reverse | |||||||
| CCACTGTCCTTCGGAATTGT | |||||||
| WShSb1 | Forward | KL252782 | 3,138–3,334 | 197 bp | Yes | Yes | Yes |
| CCACTAGAGTCGTCGTCGTG | |||||||
| Reverse | |||||||
| GCTGCCGAATCCATAACAAA | |||||||
| WShSb2 | Forward | KL252782 | 8,258–8,487 | 230 bp | Yes | Yes | Yes |
| GTTGAAATTCGCTGCTGGAT | |||||||
| Reverse | |||||||
| AATGGTTTTGGACGGAATTG | |||||||
| WShSb3 | Forward | KL252440 | 18,445–18,637 | 193 bp | Yes | Yes | Yes |
| GGTGGTCAGGCATTGATTCT | |||||||
| Reverse | |||||||
| CATGTTTAGGCGCTTCAGGT | |||||||
NA: no amplification. S. h: Schistosoma haematobium. S. b: Schistosoma bovis
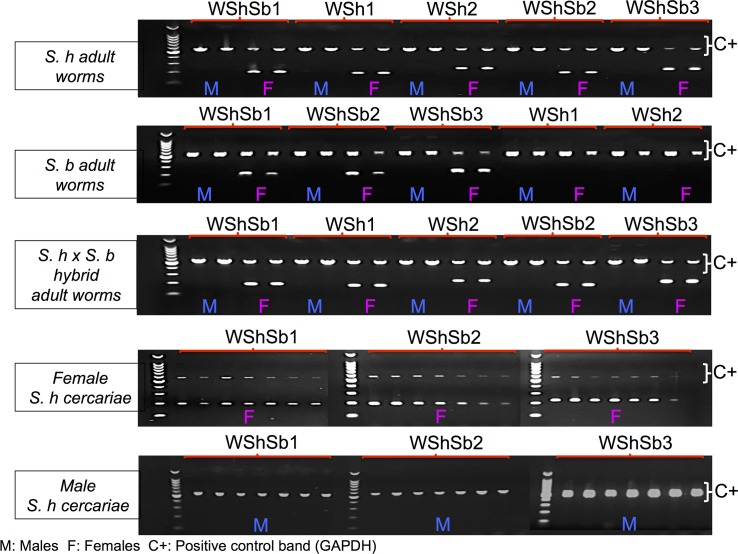
Fig 1. Diagnostic PCR assay of female-specific markers in males and females Schistosoma haematobium (S. h), Schistosoma bovis (S. b) and their hybrids.
The first three gels correspond to PCR amplifications on adult worms (biological duplicates for males and females) of the 5 female specific markers identified with our in silico approach. Note that upper-bands correspond to the GAPDH gene control (558 bp), whereas lower bands correspond to female-specific amplification (see Table 2 for amplicon sizes). Note also that amplification is not effective for amplification from the genome of S. bovis using the two markers WSh1 and Wsh2. The last two gels show sex-specific amplification for the three markers, efficient in both species on a batch of cercariae from molluscs mono-miracidially infected with S. haematobium.
Real-time quantitative PCR to test the efficiency of primers
Reactions were performed on pools of 3 females of Schistosoma haematobium or Schistosoma bovis using technical triplicates. The reaction mixture of a final volume of 10 μl consisted of standard reaction mix (Takyon SYBR assays; 1x), 1 μM of each primer and DNA template. The cycling protocol was: an initial denaturation step at 95°C for 5 min, followed by 40 cycles of denaturation (95°C for 10 s), annealing (56°C for 20 s) and extension (72° C for 25 s). Primer efficiencies was calculated using the slope of the standard curve on serial dilutions from 100 ng to 0.4 ng with efficiency = -1 + 10(-1/slope).
Ethics Statement
All experiments on hamsters were carried out according to national ethical standards established in the writ of February 1st, 2013 (NOR: AGRG1238753A), setting the conditions for approval, planning and operation of establishments, breeders and suppliers of animals used for scientific purposes and controls. The French Ministry of Agriculture and Fishery (Ministère de l’Agriculture et de la Pêche), and the French Ministry for Higher Education, Research and Technology (Ministère de l’Education Nationale de la Recherche et de la Technologie) approved the experiments carried out for this study and provided permit A66040 for animal experimentation. The investigator possesses the official certificate for animal experimentation delivered by both ministries (Décret n° 87–848 du 19 octobre 1987; number of the authorization 007083).
Results
Sixty primer couples were designed and tested in this study. None of the primers designed on genome of S. bovis gave significant sex-specific results. Concerning primers designed on S. haematobium genome, we identified 5 female specific sequences for pure S. haematobium as well as for the hybrid form of the parasite, 3 of which can also discriminate female from male individuals in S. bovis (Table 2, Fig 1, and S2 Fig). Three different scaffolds of S. haematobium genome were identified as being at least in part restricted to females and thus W-chromosome specific (KL252782, KL253191, KL252440). The GAPDH gene (control) was retrieved from both S. haematobium and S. bovis genomes, whereas none of the sex-specific markers were detected in the S. bovis draft assembly. The results of PCR amplification of these markers are presented in Fig 1. The correspondence between the sexes of S. haematobium cercariae determined by PCR, and the observation of these parasites as adult worms following hamster perfusion confirmed the reliability of our approach. Furthermore, we tested the efficiencies of our primers using real-time quantitative PCR, and obtained efficiencies around 100% for all primer couples with a single peak in melting curves. DNA amplification was still detectable below 30 Ct for 1.5 ng of DNA template for all markers. The sequenced amplicons (S1 File) were always identical between S. haematobium and the hybrid from Corsica. The sequences were highly conserved with S. bovis (100% identical for WShSb3, 97% and 97,4% for WShSb1 and WShSb2, respectively (S2 File).
Discussion
In the present work, we used massive sequencing data from a field S. haematobium x S. bovis hybrid schistosome strain, allowing us to successfully identify 5 female-specific markers on 3 different scaffolds from the draft genome of S. haematobium, thus corresponding to previously unplaced W-specific regions. No sex marker candidates were confirmed by PCR when they were selected based on reads aligned against the genome of S. bovis. Furthermore, we could not locate the female-specific markers originating from the S. haematobium draft genome in the S. bovis genome. This can be explained by the relatively poor quality of the present genome for S. bovis, which emphasises the need to improve the assembly. Despite this, among 5 female-specific sex markers in S. haematobium, 3 can also be used for S. bovis. The conservation of three of the markers for S. bovis females was expected due to the close relatedness of both species, which are in the same monophyletic group [12]. The two markers, Wsh1 and Wsh2, did not amplify either female or male in S. bovis. All of the markers are functional for the S. haematobium x S. bovis hybrid and identical to the S. haematobium sequence, which is consistent with current work in our laboratory that suggests higher levels of introgression of S. haematobium than S. bovis in the hybrid schistosome from Corsica.
We first tested existing molecular sex markers designed for S. mansoni [18] on S. bovis and S. haematobium parasites, but no amplification was achieved. The sex-specific markers identified in the present study were also tested on 2 males and 2 females S. mansoni, and could not amplify any sequence. S. mansoni does not belong to the S. haematobium group but to another monophyletic group of Schistosoma (referred to as S. mansoni group) [12]. The fact that sex chromosome structure, inferred by C-banding method, is known to be different among schistosome species may explain the absence of cross-group amplification [4]. Further investigations are also needed to test whether these sex markers are applicable to other species of the S. haematobium group. Indeed, the Schistosoma genus harbours a high diversity of species, and the S. haematobium group is the most diverse and includes 3 species that infect humans (i.e. S. haematobium, S. intercalatum, and S. guineensis) and 6 species infecting animals (S. bovis, S. curassoni, S. kisumuensis, S. leiperi, S. margrebowiei and S. mattheei), mainly ruminants and/or rodents. Interestingly, species of the S. haematobium group have frequently been incriminated in hybridization phenomena. Natural hybrids have been observed between schistosome species infecting humans (i.e. S. haematobium x S. intercalatum [30,31], S. haematobium x S. mansoni [32], S. haematobium x S. guineensis [33,34], between schistosome species infecting animals (S. bovis x S. curassoni [35,36] and between both, humans and animal infecting schistosomes (S. mansoni x S. rodhaini [37,38], S. haematobium x S. bovis [11,35,39], S. haematobium x S. mattheei [40], S. haematobium x S. curassoni [35]. These last crosses are particularly important because they raise a risk of animal reservoir hosts and zoonotic transmission. A possible way of distinguishing these hybrid forms from pure parasites would be to use both mitochondrial (cox1) and nuclear (e.g., ITS) markers. In addition, being able to rely on sex-specific markers for such species will enable the identification of sex of the clonal cercariae pool proposed for hamster infection, and therefore will offer the opportunity to better characterise the ecological and medical impacts of schistosome hybrids. Another interesting aspect to be developed using such sex-markers would be to create a linkage map for S. haematobium and S. bovis, as has been achieved for S. mansoni [41].
Finally, the sex markers we have identified might be used to study sex-specific population structures of schistosome larvae in the field. Such sex specific structures with males being more randomly distributed than females has been observed for S. mansoni [42]. Our sex markers would allow the testing of bias dispersal in larval populations of S. haematobium or of S. bovis.
To conclude we demonstrate throughout this work that comparing male and female genomic data is relatively straightforward and could offer a reliable way of identifying sex-specific sequences. These markers are essential for experimental crosses and to design appropriate protocols for studying schistosome species within the S. haematobium group which are currently of great concern in a context of disease emergence both in Africa and Europe.
Supporting Information
- Female (a) and male (b) reads aligned against S. haematobium x S. bovis concatenated genome using pre-set parameters.
- Female (c) and male (d) reads aligned against S. haematobium x S. bovis concatenated genome using unique read alignment.
Female specific sequences are easily recognisable and selected regions (red) were confirmed with our end-point PCR approach.
(TIF)
The top panel corresponds to PCR amplifications of female-specific markers from 6 females and 5 males of Schistosoma haematobium. All five sex markers are efficient in distinguishing male from female individuals. The bottom panel corresponds to PCR amplification of the 3 sex markers also efficient in Schistosoma bovis. Five males and 5 females were readily distinguished using primers WShSb1, WShSb2 and WShSb3. Note that the upper bands correspond to the GAPDH gene control (558 bp), whereas lower bands correspond to female-specific amplifications (see Table 2 for amplicon sizes).
(TIF)
(FASTA)
(DOC)
Acknowledgments
We would like to thank Cécile Saint-Béat, and Nathalie Arancibia for rodent and snail maintenance. We also acknowledge the contribution of the McGill University and Genome Quebec Innovation Centre, Montréal, Canada.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.King CH. Parasites and poverty: The case of schistosomiasis. Acta Tropica. 2010. pp. 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grossman a I, Short RB, Cain GD. Karyotype evolution and sex chromosome differentiation in Schistosomes (Trematoda, Schistosomatidae). Chromosoma. 1981;84: 413–30. [DOI] [PubMed] [Google Scholar]
- 5.Basch PF. Why do schistosomes have separate sexes? Parasitol Today. 1990;6: 160–163. [DOI] [PubMed] [Google Scholar]
- 6.Loker ES, Brant S V. Diversification, dioecy and dimorphism in schistosomes. Trends Parasitol. 2006;22: 521–528. 10.1016/j.pt.2006.09.001 [DOI] [PubMed] [Google Scholar]
- 7.Holtfreter MC, Moné H, Müller-Stöver I, Mouahid G, Richter J. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Eurosurveillance. 2014;19. [DOI] [PubMed] [Google Scholar]
- 8.Berry A, Fillaux J, Martin-Blondel G, Boissier J, Iriart X, Marchou B, et al. Evidence for a permanent presence of schistosomiasis in Corsica, France, 2015. Eurosurveillance. 2016;21. [DOI] [PubMed] [Google Scholar]
- 9.Boissier J, Moné H, Mitta G, Bargues MD, Molyneux D, Mas-Coma S. Schistosomiasis reaches Europe. Lancet Infect Dis. Elsevier; 2015;15: 757–8. 10.1016/S1473-3099(15)00084-5 [DOI] [PubMed] [Google Scholar]
- 10.Boissier J, Grech-Angelini S, Webster BL, Allienne J-F, Huyse T, Mas-Coma S, et al. Outbreak of urogenital schistosomiasis in Corsica (France): an epidemiological case study. Lancet Infect Dis. 2016;3099: 1–9. [DOI] [PubMed] [Google Scholar]
- 11.Moné H, Holtfreter MC, Allienne JF, Mintsa-Nguéma R, Ibikounlé M, Boissier J, et al. Introgressive hybridizations of Schistosoma haematobium by Schistosoma bovis at the origin of the first case report of schistosomiasis in Corsica (France, Europe). Parasitol Res. 2015;114: 4127–4133. 10.1007/s00436-015-4643-4 [DOI] [PubMed] [Google Scholar]
- 12.Lawton SP, Hirai H, Ironside JE, Johnston DA, Rollinson D. Genomes and geography: genomic insights into the evolution and phylogeography of the genus Schistosoma. Parasit Vectors. 2011;4: 131 10.1186/1756-3305-4-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King KC, Stelkens RB, Webster JP, Smith DF, Brockhurst MA. Hybridization in Parasites: Consequences for Adaptive Evolution, Pathogenesis, and Public Health in a Changing World. PLoS Pathog. 2015;11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webster BL, Southgate VR. Compatibility of Schistosoma haematobium, S. intercalatum and their hybrids with Bulinus truncatus and B. forskalii. Parasitology. 2003;127: 231–242. [DOI] [PubMed] [Google Scholar]
- 15.Boissier J, Durand P, Moné H. PCR effectiveness for sexing Schistosoma mansoni cercariae: Application for sexing clonal cercarial populations. Mol Biochem Parasitol. 2001;112: 139–141. [DOI] [PubMed] [Google Scholar]
- 16.Chevalier FD, Le W, Carolina A, Mattos A De, Loverde PT. Real-time PCR for sexing Schistosoma mansoni cercariae. Mol Biochem Parasitol. Elsevier B.V.; 2016;205: 35–38. 10.1016/j.molbiopara.2016.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasser RB, Morahan G, Mitchell GF. Sexing single larval stages of Schistosoma mansoni by polymerase chain reaction. Mol Biochem Parasitol. 1991;47: 255–258. [DOI] [PubMed] [Google Scholar]
- 18.Portela J, Grunau C, Cosseau C, Beltran S, Dantec C, Parrinello H, et al. Whole-genome in-silico subtractive hybridization (WISH) using massive sequencing for the identification of unique and repetitive sex-specific sequences: the example of Schistosoma mansoni. BMC Genomics. 2010;11: 387 10.1186/1471-2164-11-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Théron a, Pages JR, Rognon a. Schistosoma mansoni: distribution patterns of miracidia among Biomphalaria glabrata snail as related to host susceptibility and sporocyst regulatory processes. Exp Parasitol. 1997;85: 1–9. 10.1006/expr.1996.4106 [DOI] [PubMed] [Google Scholar]
- 20.Boissier J, Chlichlia K, Digon Y, Ruppel A, Moné H. Preliminary study on sex-related inflammatory reactions in mice infected with Schistosoma mansoni. Parasitol Res. 2003;91: 144–150. 10.1007/s00436-003-0943-1 [DOI] [PubMed] [Google Scholar]
- 21.Boissier J, Rivera ER, Moné H. Altered behavior of the snail Biomphalaria glabrata as a result of infection with Schistosoma mansoni. J Parasitol. 2003;89: 429–433. 10.1645/0022-3395(2003)089[0429:ABOTSB]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 22.Silva ML, Vicente FS, Avelino IC, Martin VR. Susceptibility of Planorbarius metidjensis from Portugal and Spain to Schistosoma bovis from Salamanca, Spain. Malacologia. 1977;16: 251–254. [PubMed] [Google Scholar]
- 23.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal. 2011;17: 10. [Google Scholar]
- 24.Young ND, Jex AR, Li B, Liu S, Yang L, Xiong Z, et al. Whole-genome sequence of Schistosoma haematobium. Nat Genet. 2012;44: 221–225. 10.1038/ng.1065 [DOI] [PubMed] [Google Scholar]
- 25.Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10: R25 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie C, Tammi MT. CNV-seq, a new method to detect copy number variation using high-throughput sequencing. BMC Bioinformatics. 2009;10: 80 10.1186/1471-2105-10-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thorvaldsdóttir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief Bioinform. 2013;14: 178–192. 10.1093/bib/bbs017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132: 365–386. [DOI] [PubMed] [Google Scholar]
- 29.Beltran S, Galinier R, Allienne JF, Boissier J. Cheap, rapid and efficient DNA extraction method to perform multilocus microsatellite genotyping on all Schistosoma mansoni stages. Mem Inst Oswaldo Cruz. 2008;103: 501–503. [DOI] [PubMed] [Google Scholar]
- 30.Southgate VR, Wijk HB, Wright CA. Schistosomiasis at Loum, Cameroun; Schistosoma haematobium, S. intercalatum and their natural hybrid. Zeitschrift für Parasitenkd. 1976;49: 145–159. [DOI] [PubMed] [Google Scholar]
- 31.Burchard GD, Kern P. Probable hybridization between S. intercalatum and S. haematobium in western Gabun. Trop Geogr Med. 1985;37: 119–123. Available: http://www.ncbi.nlm.nih.gov/pubmed/4035773 [PubMed] [Google Scholar]
- 32.Huyse T, Van den Broeck F, Hellemans B, Volckaert FAM, Polman K. Hybridisation between the two major African schistosome species of humans. Int J Parasitol. Australian Society for Parasitology Inc.; 2013;43: 687–689. 10.1016/j.ijpara.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Webster BL, Tchuem Tchuenté LA, Jourdane J, Southgate VR. The interaction of Schistosoma haematobium and S. guineensis in Cameroon. J Helminthol. 2005;79: 193–197. [DOI] [PubMed] [Google Scholar]
- 34.Moné H, Minguez S, Ibikounlé M, Allienne J- F, Massougbodji A, Mouahid G. Natural Interactions between S. haematobium and S. guineensis in the Republic of Benin. ScientificWorldJournal. 2012;2012: 793420 10.1100/2012/793420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webster BL, Diaw OT, Seye MM, Webster JP, Rollinson D. Introgressive Hybridization of Schistosoma haematobium Group Species in Senegal: Species Barrier Break Down between Ruminant and Human Schistosomes. PLoS Negl Trop Dis. 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rollinson D, Southgate VR, Vercruysse J, Moore PJ. Observations on natural and experimental interactions between Schistosoma bovis and S. curassoni from West Africa. Acta Trop. 1990;47: 101–114. [DOI] [PubMed] [Google Scholar]
- 37.Morgan J a T, DeJong RJ, Lwambo NJS, Mungai BN, Mkoji GM, Loker ES. First report of a natural hybrid between Schistosoma mansoni and S. rodhaini. J Parasitol. 2003;89: 416–418. 10.1645/0022-3395(2003)089[0416:FROANH]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 38.Steinauer ML, Hanelt B, Mwangi IN, Maina GM, Agola LE, Kinuthia JM, et al. Introgressive hybridization of human and rodent schistosome parasites in western Kenya. Mol Ecol. 2008;17: 5062–5074. 10.1111/j.1365-294X.2008.03957.x [DOI] [PubMed] [Google Scholar]
- 39.Huyse T, Webster BL, Geldof S, Stothard JR, Diaw OT, Polman K, et al. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitchford RJ. Observations on a possible hybrid between the two schistosomes S. haematobium and S. mattheei. Trans R Soc Trop Med Hyg. 1961;55: 44–51. [DOI] [PubMed] [Google Scholar]
- 41.P Criscione CD, Valentim CLL, Hirai H, LoVerde PT, Anderson TJC. Genomic linkage map of the human blood fluke Schistosoma mansoni. Genome Biol. 2009;10: R71 10.1186/gb-2009-10-6-r71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prugnolle F, De Meeûs T, Durand P, Sire C, Théron A. Sex-specific genetic structure in Schistosoma mansoni: Evolutionary and epidemiological implications. Mol Ecol. 2002;11: 1231–1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
- Female (a) and male (b) reads aligned against S. haematobium x S. bovis concatenated genome using pre-set parameters.
- Female (c) and male (d) reads aligned against S. haematobium x S. bovis concatenated genome using unique read alignment.
Female specific sequences are easily recognisable and selected regions (red) were confirmed with our end-point PCR approach.
(TIF)
The top panel corresponds to PCR amplifications of female-specific markers from 6 females and 5 males of Schistosoma haematobium. All five sex markers are efficient in distinguishing male from female individuals. The bottom panel corresponds to PCR amplification of the 3 sex markers also efficient in Schistosoma bovis. Five males and 5 females were readily distinguished using primers WShSb1, WShSb2 and WShSb3. Note that the upper bands correspond to the GAPDH gene control (558 bp), whereas lower bands correspond to female-specific amplifications (see Table 2 for amplicon sizes).
(TIF)
(FASTA)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.