Abstract
Objective
To evaluate the benefit–risk profile (BRP) of oxycodone/naloxone (OXN) and tapentadol (TAP) in patients with chronic low back pain (cLBP) with a neuropathic component (NC) in routine clinical practice.
Methods
This was a blinded end point analysis of randomly selected 12-week routine/open-label data of the German Pain Registry on adult patients with cLBP-NC who initiated an index treatment in compliance with the current German prescribing information between 1st January and 31st October 2015 (OXN/TAP, n=128/133). Primary end point was defined as a composite of three efficacy components (≥30% improvement of pain, pain-related disability, and quality of life each at the end of observation vs baseline) and three tolerability components (normal bowel function, absence of either central nervous system side effects, and treatment-emergent adverse event [TEAE]-related treatment discontinuation during the observation period) adopted to reflect BRP assessments under real-life conditions.
Results
Demographic as well as baseline and pretreatment characteristics were comparable for the randomly selected data sets of both index groups without any indicators for critical selection biases. Treatment with OXN resulted formally in a BRP noninferior to that of TAP and showed a significantly higher primary end point response vs TAP (39.8% vs 25.6%, odds ratio: 1.93; P=0.014), due to superior analgesic effects. Between-group differences increased with stricter response definitions for all three efficacy components in favor of OXN: ≥30%/≥50%/≥70% response rates for OXN vs TAP were seen for pain intensity in 85.2%/67.2%/39.1% vs 83.5%/54.1%/15.8% (P= ns/0.031/<0.001), for pain-related disability in 78.1%/64.8%/43.8% vs 66.9%/50.4%/24.8% (P=0.043/0.018/0.001), and for quality of life in 76.6%/68.0%/50.0% vs 63.9%/54.1%/34.6% (P=0.026/0.022/0.017). Overall, OXN vs TAP treatments were well tolerated, and proportions of patients who either maintained a normal bowel function (68.0% vs 72.2%), reported no central nervous system side effects (91.4% vs 89.5%), or completed the 12-week evaluation period without any TEAE-related treatment discontinuations (93.0% vs 92.5%) were similar for both index medications (P= ns for each comparison).
Conclusion
In daily practice, the BRP of OXN proved to be noninferior to that of TAP in patients with cLBP-NC, but showed a superior efficacy if stricter analgesic response definitions were evaluated.
Keywords: oxycodone/naloxone, tapentadol, chronic low back pain, neuropathic component, noninterventional study, German Pain Registry, random data selection, blinded end point analysis, benefit–risk profile
Background
Chronic low back pain (cLBP) is a highly prevalent cause for medical consultation and a major reason for disability and quality of life (QoL) restrictions in industrialized countries.1 Its manifestation with clinical signs indicating the pathophysiological involvement of neuropathic processes represents the most common form of a neuropathic pain syndrome and affects between 20% and 35% of cLBP patients.2 Among the spectrum of cLBP patients, those with a neuropathic component (NC) usually report higher pain intensity levels as well as more and more severe disabilities with respect to daily-life activities and QoL as compared to those suffering from nociceptive pain.3,4
Several treatment guidelines have been developed worldwide to improve cLBP management, and although different nonpharmacologic strategies are recommended, management primarily relies on pharmacologic treatments. Recommended approaches for cLBP usually include acetaminophen, non-steroidal anti-inflammatory drugs, and low-potency opioids, according to the stepwise analgesic pain ladder approach of the World Health Organization (WHO), supplemented by muscle relaxants in case of a proven increase in muscle tone, and adjuvant agents (eg, tricyclic antidepressants, selective serotonin–norepinephrine reuptake inhibitors, or Ca2+-channel modulating antiepileptic agents) if cLBP patients present with clinical signs suggestive for NC.5–11
Irrespective of the underlying pathomechanisms or the clinical pain phenomenology, potent WHO Step III opioid analgesics are currently recommended as second- or third-line alternatives only, for patients where either first-line medications did not achieve an adequate response, are associated with intolerable side effects, or are even contraindicated.11–14 Main reasons for these limitations are frequent and bothersome adverse events (AEs) experienced by a substantial proportion of opioid-treated patients (eg, fatigue, headaches, dizziness, nausea, vomiting, and opioid-induced constipation [OIC]), which significantly impair daily functioning as well as QoL and interfere with the analgesic effects.15–18 Although most of these AEs are transient and can be prevented or at least diminished by appropriate countermeasures, OIC often persists over time and evolves – with a prevalence of 40% in opioid-treated patients – into the most common and most burdensome complication of long-term treatment with opioid analgesics, resulting in an increased use of health care resources and a significant loss of productivity.19–26 Recommended countermeasures to treat or even prevent this opioid-related AEs (eg, fluid and fiber intake, laxatives, and stool softeners) suffer from a lack of high-quality evidence regarding efficacy and safety27–30 and have proven ineffective for the majority of patients, as they are not able to address the underlying OIC mechanisms adequately.31–36
On the basis of the published evidence, WHO Step III analgesics are more or less comparably effective for patients suffering from chronic nonmalignant pain such as cLBP,13,37,38 but differ with respect to their pharmacokinetics and side effect profiles, especially with respect to OIC.39 Subsequently, both aspects gained in importance, and the latter has evolved into a primary criterion for differential treatment approaches, promoting the use of oxycodone/naloxone (OXN) and tapentadol (TAP), the two recently approved WHO Step III analgesics that differ significantly in comparison to the conventional μ-opioid receptor agonists of this class.
Among the currently available traditional WHO Step III opioids, the fixed-dose combination of prolonged-release (PR) oxycodone (a semisynthetic opioid agonist with a mixed activity on μ- and κ-receptors, twice as potent as oral controlled release morphine) with a low dose of PR naloxone (a highly potent peripherally acting μ-opioid receptor antagonist with a systemic bioavailability of less than 2%, narrowing its antagonistic activity to the prehepatic structures of the gastrointestinal [GI] tract), was the first WHO Step III analgesic with a dual mechanism on μ-opioid receptors providing not only relief of OIC via the antagonistic effects of naloxone, but also analgesia via its agonistic combination partner oxycodone.40–42 Initially approved in Germany in 2006 for severe cancer- and noncancer-related pain that can only sufficiently be treated with opioid analgesics, OXN evolved rapidly into the most prevalently prescribed oral WHO Step III analgesic for chronic nonmalignant pain irrespective of its clinical phenomenology or underlying mechanisms in several European countries. In 2014, OXN was approved by the US Food and Drug Agency for the treatment of pain severe enough to require daily, round-the-clock, long-term opioid treatment and for which alternative treatment options are inadequate.43
In 2010, PR tapentadol (TAP) was approved in Europe, and in 2011, in the USA, raising expectations toward a superior efficacy vs conventional opioid analgesics especially for neuropathic pain due its preclinically proven dual mode of action as a mild μ-opioid receptor agonist (with a relative potency of 2% vs morphine) and as a selective norepinephrine reuptake inhibitor.44 Although clinical evidence exists to support both, its equivalent effectiveness for nociceptive as well as neuropathic pain and its superior safety profile in comparison to conventional WHO Step III opioids, tapentadol has so far not unequivocally been shown to be really superior effective to any other opioid at alleviating neuropathic pain – a target indication due to its special mode of action. Recently, Baron et al45 published results of a prospective randomized open-label noninferiority study comparing OXN vs TAP in opioid-naïve cLBP patients with an NC and reported some data suggesting a superior efficacy of TAP vs OXN. However, high and unbalanced attrition rates (51.6%/23.1% of OXN/TAP patients dropped out during the 3-week titration phase and 62.5%/33.8% during the whole 12-week study) and extensive imputation for data missing due to informative reasons raised several concerns about the generalizability of the results reported, leaving more questions open than answered.46,47
As both WHO Step III analgesics enjoy an increasing popularity among physicians, the German Pain Association and the German Pain League initiated the present noninterventional evaluation of routine data provided by the German Pain Registry focusing on patients with moderate-to-severe cLBP-NC refractory to other analgesics to gain further insight into their differential effects and their benefit–risk profiles (BRP) under real-life conditions. Objectives of this analysis were to assess the BRP for both treatments using a composite definition that incorporates patient-reported information on pain intensity, pain-related disabilities in daily-life activities and QoL, occurrence of OIC, side effects affecting the central nervous system (CNS), and the ability to remain on treatment for a period long enough to assess its efficacy.
Patients and methods
Study design
This is a noninterventional cohort study of randomly selected patients with cLBP-NC who received either TAP or OXN as part of routine care. Real-world data of the German Pain Registry – a national pain treatment register developed by the Institute of Neurological Sciences on behalf of the German Pain Association – that have originally been prospectively sampled for routine care purposes was analyzed. Data were entered using electronic case report forms provided by the German Pain Registry and the related online documentation service iDocLive®. Patient questionnaires provided by this system were those recommended by the German Pain Association, the German Pain Society, and the German Pain League and cover a broad spectrum of validated instruments addressing among other parameters pain intensity, pain-related disabilities in daily life, QoL, bowel function, use of analgesics, and adjuvant therapies as well as treatment-related AEs, etc.48,49 Data were documented by patients themselves during routine use of the electronic documentation tools provided by the German Pain Registry and supplemented by related physician information, where appropriate.
Data sets with an a priori defined treatment evaluation period of at least 12 weeks at baseline were selected for patients who fulfilled the inclusion and exclusion criteria mentioned below and for whom a treatment with one of both index medications has been newly initiated between 1st January and 31st October 2015. Treatment initiation was defined as no index medication coverage in the prior 90 days, and the date of treatment initiation was set as the index date for the definition of the 12-week data selection period. Based on that sample, data for this analysis were randomly selected based on a hidden random select list to address possible treatment biases and substance preferences of participating physicians, and a blinded end point analysis has been performed focusing on a combination of several patient-reported efficacy and tolerability end points that are known to be important for the benefit–risk assessments of patients. Main advantage of such a study design is the assessment of clinical outcomes under conditions that allow both the enrollment of data from a broad patient population (in our case patients who require one of both index medications owing to elsewhere-refractory moderate-to-severe cLBP-NC) as well as open-label non-interventional dose adjustments and the discretionary use of concomitant medications on an as-needed basis (which reflect clinical practice) but with the advantage of some kind of randomization and rigorous evaluation of study end points by blinded data analyses.50,51 Analgesic treatments followed medical requirements according to the decision of the participating physicians and based exclusively on individual patient needs without any external specifications.
After baseline evaluation (prior onset of treatment with index medication), patients completed standardized pain diaries on a weekly basis and provided information on their current health status and their response to the medication via the German Pain Diary. Formally, no predefined study visits were scheduled. However, interim visits were possible at all times according to individual patient needs and established procedures (eg, if patients had to be closely monitored due to commencement of treatment, inadequate pain control, tolerability issues, and/or AEs).
This noninterventional treatment evaluation was conducted in accordance with the principles of the Declaration of Helsinki, conformed to relevant national and regulatory requirements, and was approved by the independent ethics committe of the State Authorisation Association for Medical Issues Baden-Württemberg. All patients provided written informed consent prior participation in the German Pain Registry, and this study was registered in the electronic database of the European Medicine Agency for noninterventional studies (ENCEPP/SDPP/11234). All analyses were performed with anonymized data derived from the German Pain Registry to comply with national guidelines on protection of data privacy. Data selection based on a temporary selection key list as defined by the inclusion and exclusion criteria mentioned below.
Patient data eligibility criteria
Data selected for this study were those of male and nonpregnant, nonlactating female patients who were at least 18 years of age with a documented history of moderate-to-severe nonmalignant cLBP-NC, previously treated with WHO Step I or II analgesics and/or adjuvant treatments, who experienced either insufficient pain relief and/or unacceptable side effects, and who got an round-the-clock therapy with one of both index medications. The NC of these cLBP patients was evaluated using the modified seven-item version of the painDetect questionnaire (PDQ7),52 and patients were required to have a PDQ7 score of ≥11 at baseline (with scores of 11–18 classified as “mixed” and scores of 19–35 classified as “neuropathic”) to qualify their data set for this analysis.
Patient data exclusion criteria
Exclusion criteria for this study applied data sets containing contraindications as listed in the German prescribing information of both analgesics, and additionally addressed situations as well as conditions that would confound the analysis and/or interpretation of the observational results. Therefore, data were excluded from this analysis if patients reported a hypersensitivity to any of the product constituents, or if they documented severe respiratory depression, chronic obstructive airway disease, pulmonary hypertension, severe bronchial asthma, paralytic ileus, moderate-to-severe hepatic impairment and/or renal impairment, or any other condition in which a therapy with a strong WHO Step III analgesic is usually contraindicated. In addition, data of patients who were reported to suffer from an irritable bowel syndrome, GI diseases, and significant structural abnormalities of the GI tract, who participated in a clinical research study involving a new chemical entity or an experimental drug within 30 days prior onset of treatment, or in whom a scheduled surgery was performed during the observational period were excluded as well.
Index medication
Patients received their index medication in the course of routine clinical practice and based on their individual needs. Initial starting dose, dose titration, and further dose adjustments followed the recommendations given in the marketing authorization in existence at the time of this study and were documented in the German prescribing information.53,54 For opioid-naïve patients, the recommended starting dose of OXN vs TAP was 10/5 vs 50 mg (each corresponding to 20 mg morphine equivalent [MEQ]) of a PR preparation twice daily (bid). Data sets describing initial dosages exceeding twice the recommended starting dose were excluded from this analysis. Any dose adjustments, prescriptions of analgesic comedication, rescue medication, or laxatives were done at the discretion of the physician and due to the individual needs of the patients.
Study assessments
Efficacy evaluation
Effectiveness was evaluated from the patient’s perspective only. Efficacy assessments were performed on the basis of patient-reported information documented for pain intensity, pain-related disabilities in daily-life activities/functionality, and QoL. Pain intensity measures based on the low back pain (LBP) intensity index (LBPIX) were calculated as arithmetic mean of the lowest, average, and highest 24-hour LBP intensities on a 100 mm visual analog scale (VAS100; 0= “no pain” and 100= “worst pain conceivable”). LBP-related disabilities in daily life were assessed with a modified version of the pain disability index (mPDI), which recorded the degree of LBP-related functional restrictions in daily-life activities on the basis of an eleven-point numerical rating scale (NRS11; 0= “none” and 10= “worst conceivable”) with respect to seven distinct domains (related to “home and family activities”, “recreation”, “social activities”, “occupation”, “self-care/personal maintenance”, “sleep”, and “overall QoL”).55,56 QoL was measured using the three-level, five dimension EuroQol questionnaire (EQ-5D-3L), which includes the five dimensions “mobility”, “self-care”, “usual activities”, “pain/discomfort”, and “anxiety/depression” rated as “no problems”, “some problems”, or “extreme problems”.57 The ratings were then converted to a weighted EQ-5D index score ranging from 0 (“worst possible state of health”) to 1 (“best possible state of health”). All parameters were recorded by patients retrospectively for the last 7 days, starting at baseline (to assess the situation prior onset of the index medication) and covering the whole observation period with regular assessments at the end of each treatment week. Other end points were the global impression of change assessed by both the patient and clinician, using the Patient Global Impression of Change and Clinician Global Impression of Change, respectively.58
Safety and tolerability measures
Safety assessments consisted of monitoring all treatment-emergent AEs (TEAEs), collected via respective patient questionnaires provided by the German Pain Registry. For this evaluation, TEAEs were defined as any untoward medical occurrence reported by a patient receiving an index medication and did not necessarily have a causal relationship with the trial treatment. OIC was assessed using data from the validated bowel function index (BFI), which was calculated as the mean of three items recorded retrospectively by patients for the last days on the basis of a 100 mm horizontal visual analog scale (VAS100) at the end of each treatment week.59,60 Single BFI items were as follows: ease of defecation (0= “easy/no difficulty” and 100= “severe difficulty”), feeling of incomplete bowel evacuation (0= “not at all” and 100= “very strong”), and personal judgment of constipation (0= “not at all” and 100= “very strong”). In addition, the number of complete spontaneous bowel movements (CSBMs; defined as a stool not induced by rescue medication or other external measures and associated with a sensation of complete evacuation) in the last days and the use of laxatives were used to evaluate the degree of constipation.
Random selection
Data selection followed a simple random sampling procedure based on an a priori defined randomization list for both index treatments (OXN or TAP; 1:1 ratio, block size 10) to prevent selection and accidental bias. Opioid treatments of patients whose records were basically classified as appropriate for data selection were sequentially (in order of their treatment initiation dates) compared with those given in this random list and – in case of accordance – finally selected for this analysis.
Statistical analysis
The aim of this noninterventional treatment evaluation study was to demonstrate the noninferiority of the BRP of OXN vs TAP for the treatment of “neuropathic” cLBP under real-life conditions. Primary criteria for this comparison were the treatment contrasts for the frequency of patients reporting a combination of six efficacy and tolerability end points important for a successful treatment under real-life conditions: a clinically relevant (ie, ≥30% vs baseline) relief with respect to 1) pain intensity, 2) functionality, and 3) QoL, each at the end of the observation period compared to baseline, without 4) any AE-related treatment discontinuations, 5) CNS side effects, or 6) a BFI deterioration beyond the normal reference range. The primary end point of this study was the percentage of responders, ie, patients who fulfilled all the mentioned criteria (Table 1). Secondary end points evaluated the percentages of patients for the individual components 1) to 5) of the primary composite end point mentioned earlier. In addition, the proportions of patients who achieved improvements of 50% and 70% with respect to pain, pain-related disabilities in daily life, and QoL were determined as recommended by the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials to gain further information on the analgesic power of the index medications evaluated.61 Safety analyses addressed the percentages of patients with 1) TEAEs, 2) TEAE-related treatment discontinuations, as well as 3) spectrum and 4) characteristics of TEAEs reported.
Table 1.
Primary end point
| Primary (combined) study endpoint | OXN (n=128) |
TAP (n=133) |
OR (95% CI) | Significance |
|---|---|---|---|---|
| n | 51 | 34 | 1.929 (1.139–3.264) | P=0.014 |
| % (95% CI) | 39.8 (31.8–48.5) | 25.6 (18.9–35.6) | ||
| Average dose; mg MEQ; mean (SD) | 113.9 (31.3) | 119.4 (28.5) | P=0.419 |
Notes: Data show the absolute (n) and relative (%, 95% CI) proportion of responding patients treated with OXN (n=128) and TAP (n=133). For the primary end point, responders were defined as patients who presented at the end of observation with a combination of 1) lack of a premature treatment discontinuation, 2) a normal BFI, 3) no CNS side effects, and 4) an at least 30% improvement (vs baseline) with respect to any of the following three efficacy parameters: low back pain intensity index, pain-related disabilities in daily life (assessed with the mPDI), and QOL (assessed with the EQ5D-3L).
Abbreviations: OXN, oxycodone/naloxone; TAP, tapentadol; OR, odds ratio; 95% CI, 95% confidence interval; mg, milligram; MEQ, morphine equivalent; SD, standard deviation; CNS, central nervous system; BFI, bowel function index; EQ5D-3L, five-dimensional (three-level) European quality-of-life questionnaire; mPDI, modified pain intensity index; QOL, quality of life.
Data analyses were performed for a modified intent-to-treat (ITT) population, which consisted of the data sets of all randomly selected patients who 1) took at least one dose of the index medication and 2) had at least one postbaseline/postdose measure. Sample size estimations, performed prior to this study resulted in a required number of 120 data sets for each treatment group, providing a 90% power to conclude the noninferiority of OXN vs TAP with respect to the combined BRP end point, based on an anticipated responder rate of 30% for TAP, a noninferiority margin of 10%, and a two-sided Type I error of 2.5%. Assuming an attrition/dropout rate of ~20% and a ~40% selection rate (based on the hidden random list), a total of 300 patient data sets per treatment group were to be collected to ensur ~120 evaluable patient data sets per ITT group.
Linear interpolation was used to impute intermittent missing scores, and the last observation carried forward method was to impute missing scores after early discontinuation. The corresponding completed data set built the basis for all primary and secondary end point analyses. Additional “completer case analyses” and “as observed data” analyses were scheduled in case of a critical attrition asymmetry between treatment groups (ie, between group dropout difference ≥10%).
Descriptive and inferential statistical analyses were performed. For continuous variables, descriptive statistics were summarized by the number of patients (n), the mean, standard deviation (SD), and 95% confidence intervals (95% CIs) of the mean, median, and range (minimum – maximum) values. For categorical and ordinal variables, data were summarized by frequency number (n) and percentage (%) of participants in each category; where appropriate, 95% CIs were added. For between-group comparisons of continuous/categorical variables, Student’s t-test/Pearson’s χ2 test was used. For within group, (eg, pre–post) comparisons, paired samples t-tests were performed. All statistical tests were carried out using a two-sided significance level of 0.05. Since all comparisons, except those for the primary end point, were considered secondary, the respective analyses were exploratory and not adjusted for multiplicity.
Results
Patient disposition
Between 1st March and 31st October 2015, 13,314 pain patients actively participated in the German Pain Registry and used 2,457,228 validated documentation tools (on average 185 per patient) to report on their pain problems and their response to treatments. Roughly two-thirds of these patients (9,085; 68.2%) recorded a back pain problem, and of those, 637 (6.9%) formally fulfilled the inclusion and exclusion criteria of this study. After screening of their baseline data for completeness and conformity with the evaluation plan, the data of 579 patients were finally classified appropriate, and of those, the data of 261 patients (45.1%) were randomly selected for this analysis (128 for OXN, 133 for TAP; see patient disposition in Figure 1), resulting in an ITT population which met the predefined requirements of the sample size estimation considering a statistical power of 90%. With 30/128 patients treated with OXN (23.4%) and 30/133 patients treated with TAP (22.6%), attrition rates were well balanced and comparable for both index treatments. The main reason for a treatment discontinuation within Weeks 1–12 after initiation of the index treatment was insufficient tolerability of treatments (38/261, 14.6%), followed by TEAEs (19/261, 7.3%) and inadequate analgesic efficacy (16/261, 6.1%).
Figure 1.
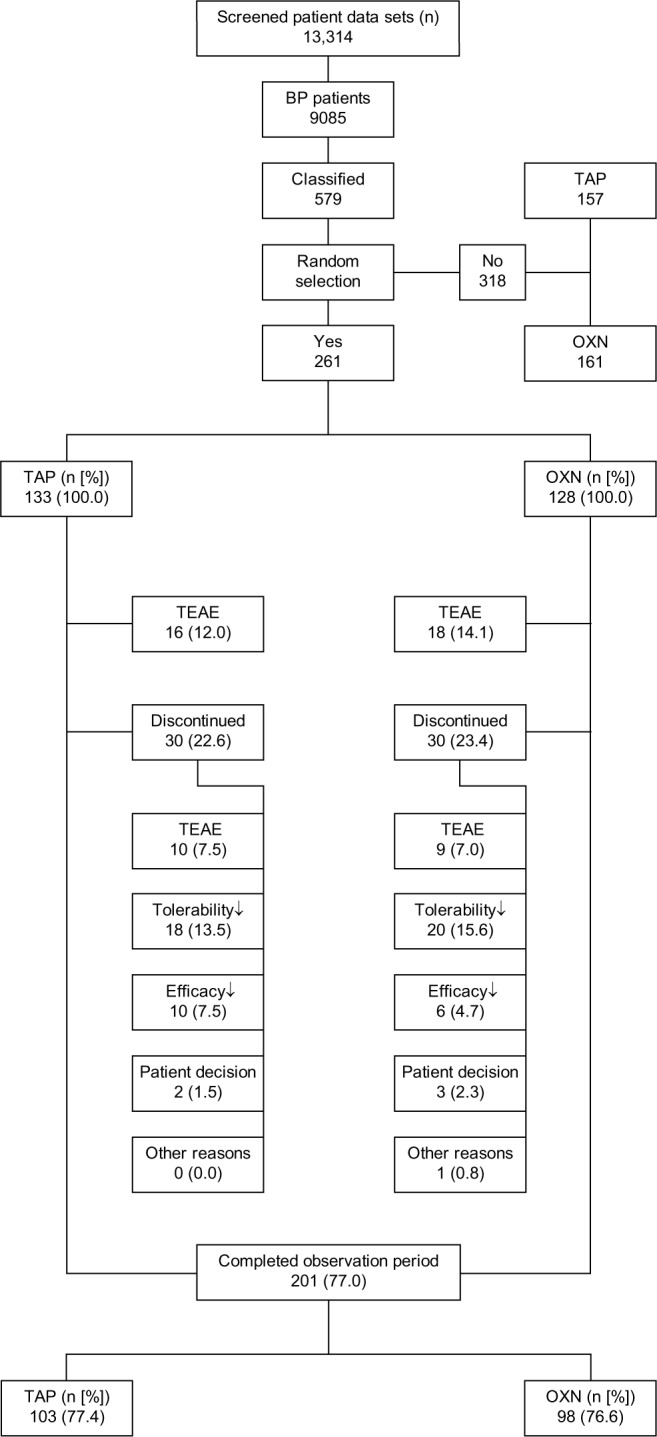
Patient data set disposition.
Abbreviations: BP, back pain; TAP, tapentadol; OXN, oxycodone/naloxone; n, number of patients; %, percentage of patients; TEAE, treatment emergent adverse event
Baseline characteristics
Baseline demographics were essentially comparable between both treatment groups and are presented in Table 2. Mean age (±SD) was 46.9±9.6 (median: 47, range: 20–71) years, and 59.4% (155/261) of the participants were female. About 60.5% (158/261) or six out of ten back pain (BP) patients suffered for longer than 6 months prior to baseline; 82.4% (215/261) or eight out of ten reported a treatment by at least five different physicians (average 5.8±1.4, median: 6, range: 3–10), and 83.9% (219/261), ie, the same proportion of patients, documented a pretreatment with at least five analgesic medications (on average 6.3±1.9, median: 6, range: 3–13). Nonopioid analgesics were the most frequently used treatments reported by 98.9% (n=258/261) of patients as the prior index medication, followed by nonsteroidal anti-inflammatory drugs (96.2%, 251/261), antidepressants (70.1%, 183/261), WHO Step II opioids (69.3%, n=181/261), and antiepileptic agents (37.5%, 98/261). Around 37.2% (97/261), a third of patients, presented with advanced pain chronification (Stage III) according to the Mainz Pain Staging System,35 and 59.0% (n=154/261) suffered from a high disability with either moderate (Grade III; n=100/261, 38.3%) or severe (Grade IV; n=54/261, 20.7%) limitations according to the von Korff pain grading scale.36 For both assessments, OXN patients showed significantly stronger impairments compared to those treated with TAP.
Table 2.
Patient demographic and baseline characteristics
| Parameter | OXN (n=128) |
TAP (n=133) |
Significance OXN vs TAP |
|---|---|---|---|
| Age (years); mean (SD) | 46.3 (10.2) | 47.6 (9.0) | P=0.287 |
| Proportion >65 years; | 1 (0.8) | 2 (1.5) | P=0.584 |
| n (%) | |||
| Gender: female; n (%) | 75 (58.6) | 80 (60.2) | P=0.798 |
| Height (cm); mean (SD) | 171.2 (9.5) | 171.8 (8.8) | P=0.602 |
| Weight (kg); mean (SD) | 81.0 (16.1) | 80.6 (17.6) | P=0.878 |
| BMI; kg/m2; mean (SD) | 27.5 (4.9) | 27.2 (4.8) | P=0.552 |
| Obesity (BMI:>30.0); n(%) | 31 (23.3) | 30 (23.4) | P=0.751 |
| Back pain duration | 77 (60.2) | 81 (60.9) | P=0.902 |
| >6 months; n (%) | |||
| Number of physicians involved; median (range) | 5 (3–10) | 6 (3–10) | P=0.369 |
| Number of analgesics received prior enrollment; median (range) | 6 (3–11) | 6 (3–13) | P=0.488 |
| Nonopiod analgesics; n (%) | 126 (98.4) | 132 (99.2) | P=0.375 |
| NSAIDS; n (%) | 123 (96.1) | 128 (96.2) | P=0.652 |
| Low-potent (WHO Step II) opioid analgesics; n (%) | 85 (66.4) | 96 (72.2) | P=0.133 |
| Antidepressants; n (%) | 93 (72.7) | 90 (67.7) | P=0.165 |
| Anticonvulsants; n (%) | 47 (36.7) | 51 (38.3) | P=0.553 |
| MPSS, Stage 1; n (%) | 14 (10.9) | 23 (17.3) | P=0.038 |
| II; n (96) | 55 (43.0) | 72 (54.1) | |
| III; n (%) | 59 (46.1) | 38 (28.6) | |
| von Korff Grade 1; n (%) | 11 (8.6) | 16 (12.0) | P=0.001 |
| 2; n (%) | 31 (24.2) | 49 (36.8) | |
| 3; n (%) | 48 (37.5) | 52 (39.1) | |
| 4; n (%) | 38 (29.7) | 16 (12.0) | |
| Pain detect score | 68 (53.1) | 84 (63.2) | P=0.259 |
| 11–17; n (%) | |||
| 18–35; n (%) | 60 (46.9) | 49 (36.8) | |
| Lowest 24-hour pain intensity (VAS100); mean (SD) | 18.1 (17.2) | 18.8 (18.7) | P=0.756 |
| Average 24-hour pain intensity (VAS100); mean (SD) | 66.1 (11.2) | 67.4 (12.4) | P=0.357 |
| Highest 24-hour pain intensity (VAS100); mean (SD) | 80.9 (21.5) | 81.1 (15.6) | P=0.913 |
| TTT; VAS100; mean (SD) | 25.6 (12.1) | 26.1 (9.8) | P=0.675 |
| LBPIX; VAS100; mean (SD) | 55.0 (9.3) | 55.6 (9.9) | P=0.622 |
| Pain-related disability (mPDI; NRS70); mean (SD) | 43.9 (12.0) | 42.1 (12.2) | P=0.245 |
| Quality-of-life (EQ5D-3L); mean (SD) | 0.386 (0.322) | 0.388 (0.318) | P=0.961 |
| BFI; VAS100; mean (SD) | 16.7 (17.1) | 14.9 (15.5) | P=0.387 |
| Proportion with normal BFI (≤28.8 mm VAS); n (%) | 101 (78.9) | 114 (85.7) | P=0.044 |
Abbreviations: OXN, oxycodone/naloxone; TAP, tapentadol; BMI, body mass index; NSAIDS, nonsteroidal anti-inflammatory drugs; MPSS, Mainz pain staging system; TTT, tailored treatment target; LBPIX, low back pain intensity index; EQ5D-3L; EQ5D-3L, five-dimensional (three-level) European quality-of-life questionnaire; BFI, Bowel function index; VAS, visual analog scale.
Baseline pain intensity as well as all other pain-related patient measures were comparable among treatment groups. Scores for lowest, average, and highest 24-hour pain intensities for OXN/TAP were 18.1±17.2/18.8±18.7, 66.1±11.2/67.4±12.4, and 80.9±21.5/81.1±15.6 mm VAS100. Corresponding LBPIX, mPDI, and EQ5D-3L scores for OXN/TAP were 55.0±9.3/55.6±9.9 mm VAS100, 43.9±12.0/42.1±12.2 NRS70, and 0.386±0.322/0.388±0.318, respectively. Average PDQ7 scores at baseline were 18.0±3.6 and ranged from 14 to 35. About 41.8% (109/261), four out of ten patients, presented with PDQ7 scores ≥18 and had, therefore, per definition, a positive rating for an NC at baseline, whereas the remaining scored 11–17, and so their pain was therefore formally classified as of “mixed” or “unclear” pathophysiology.
Despite numerically equal BFI scores for both groups, the proportion of patients with normal scores at baseline was significantly lower for OXN (101/128, 78.9%) vs TAP (114/133, 85.7%; P=0.044).
Randomization effects
To exclude selection effects as a possible source of bias, we compared the demographic and baseline data of those 261 patients whose data were randomly selected for this analysis with the data of the 318 patients not selected. Selection rates were comparable for both index treatments (TAP: 133 of 290, 45.9%; OXN: 128 of 289, 44.3%; P=0.704), and between-group analyses revealed comparable demographic characteristics with only insignificant baseline differences, thus minimizing the risk that the treatment-related effects were significantly influenced by hidden selection effects.
WHO Step III treatments
Average initial starting doses were 35.5±10.9 (median: 40, range: 20–60) mg MEQ for TAP and 28.0±10.7 (median: 30, range: 10–50) mg MEQ for OXN, which were comparable among treatment groups, as were dose titration and the maintenance dose. At the end of the study, patients treated with TAP received on average 127.6±25.6 (median: 120, range: 60–180) mg MEQ per day and those with OXN 117.2±31.7 (median: 120, range: 30–180) mg MEQ per day, corresponding to a daily dose of 318.9±63.9 (median: 300, range: 150–450) mg tapentadol and 57.6±15.9 (median: 60, range: 15–90) mg oxycodone.
Primary end point
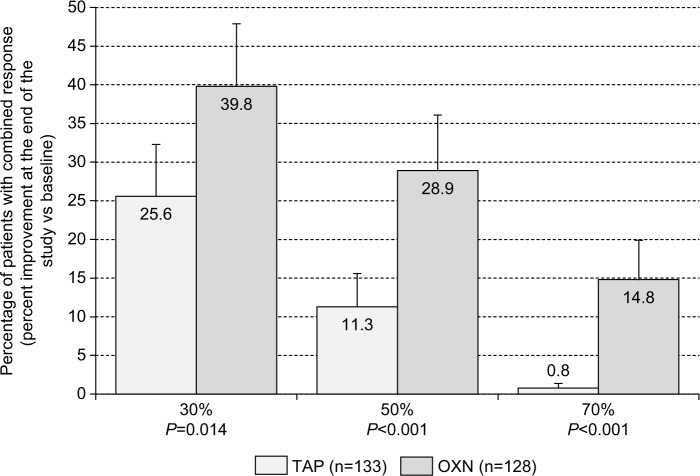
Overall, 34 of 133 patients treated with TAP (25.6%, 95% CI: 18.9%–35.6%) reached the primary composite end point, and 51 of those 128 patients treated with OXN (39.8%, 95% CI: 31.8%–48.5%), thus formally confirming the noninferiority of OXN vs TAP according to the criteria prespecified in the evaluation plan (Table 1). As the between-group difference of 14.2% exceeded the noninferiority margin of 10% in favor of the comparative agent OXN and the 95% CIs of the corresponding odds ratio (OR: 1.929, 1.139–3.264) lay entirely above “1”, a supplemental superiority analysis has been conducted that finally confirmed a significantly superior effect of OXN vs TAP (P=0.014).
Secondary end points
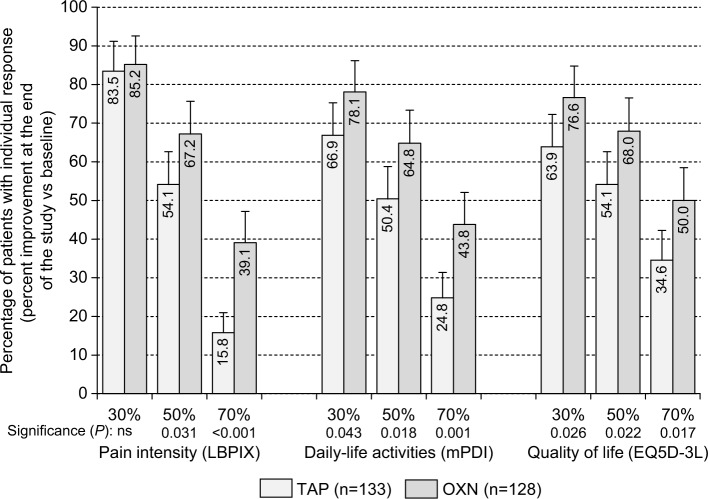
Complementary between-group analyses with respect to each single component of the efficacy and safety parameters underlying the primary composite end point are shown in Table 3. For OXN, a ≥30% BP relief was found in 85.2% of patients and as such was comparable to those seen with TAP (83.5%, OR: 1.14, P=0.706). A greater proportion of patients who reported a ≥30% improvement in functionality/QoL at the end of observation were on OXN (78.1%/76.6%) than on TAP (66.9%/63.9%; OR: 1.77/1.85; P=0.043/0.026), resulting in a combined efficacy responder rate of 57.8% for OXN vs 41.4% for TAP (OR: 1.95, P=0.008). TEAE-related premature treatment discontinuations were comparable for OXN vs TAP (7.0% vs 7.5%) as well as the proportion of patients without any CNS side effects (91.4% vs 89.5%) and those with a normal BFI at the end of observation (68.0% vs 72.2%), resulting in a combined safety/tolerability responder rate of 60.9% for OXN vs 64.7% for TAP (OR: 0.85, P=0.534).
Table 3.
Single-component analysis of all six parameters constituting the primary study end point
| Primary endpoint components | OXN (n=128) |
TAP (n=133) |
OR (95% CI) | Significance |
|---|---|---|---|---|
| Efficacy endpoints | ||||
| 1) LBPIX improvement ≥30% at W12 vs BL n | 109 | 111 | 1.137 (0.583–2.218) | P=0.706 |
| Percent (95% CI) | 85.2 (78.0–90.3) | 83.5 (76.2–88.8) | ||
| Average dose; mg MEO; mean (SD) | 112.7 (31.3) | 119.4 (28.5) | P=0.419 | |
| 2) mPDI improvement ≥30% at W12 vs BL n | 100 | 89 | 1.766 (1.015–3.070) | P=0.043 |
| Percent (95% CI) | 78.1 (70.2–84.4) | 66.9 (58.5–74.3) | ||
| Average dose; mg MECl; mean (SD) | 110.4 (35.6) | 118.2 (35.2) | P=0.126 | |
| 3) EQ5D-3L improvement>30% at W12 vs BL n | 98 | 85 | 1.845 (1.074–3.168) | P=0.026 |
| Percent (95% CI) | 76.6 (68.5–83.1) | 63.9 (55.5–71.6) | ||
| Average dose; mg MECl; mean (SD) | 113.5 (33.4) | 124.7 (29.5) | P=0.019 | |
| 1–3) Combined efficacy endpoints n | 74 | 55 | 1.945 (1.188–3.179) | P=0.008 |
| Percent (95% CI) | 57.8 (49.2–66.0) | 41.4 (33.3–49.9) | ||
| Average dose; mg MECl; mean (SD) | 117.6 (31.7) | 124.9 (28.3) | P=0.191 | |
| Safety/tolerability endpoints | ||||
| 4) No TEAE-related treatment discontinuation n | 119 | 123 | 1.075 (0.422–2.739) | P=0.880 |
| Percent (95% CI) | 93.0 (87.2–96.3) | 92.5 (86.7–95.9) | ||
| Average dose; mg MEO; mean (SD) | 109.6 (36.4) | 121.1 (30.6) | P=0.008 | |
| 5) No CNS-side effects n | 117 | 119 | 1.251 (0.546–2.869) | P=0.596 |
| Percent (95% CI) | 91.4 (85.3–95.1) | 89.5 (83.1–93.6) | ||
| Average dose; mg MEQ mean (SD) | 108.4 (37.4) | 120.7 (31.2) | P=0.007 | |
| 6) Normal bowel function (BFI< 28.8) at W12n | 87 | 96 | 0.818 (0.481–1.391) | P=0.457 |
| Percent (95% CI) | 68.0 (59.5–75.4) | 72.2 (64.0–79.1) | ||
| Average dose; mg MEQ; mean (SD) | 104.0 (37.1) | 116.3 (33.6) | P=0.020 | |
| 4–6) Combined safety/tolerability endpoints n | 78 | 86 | 0.853 (0.516–1.409) | P=0.534 |
| Percent (95% CI) | 60.9 (52.3–69.0) | 64.7 (56.2–72.3) | ||
| Average dose; mg MEQ; mean (SD) | 105.3 (36.5) | 118.8 (32.0) | P=0.012 | |
Notes: Data of the upper panel show the absolute (relative) proportion of patients, who presented with a ≥30% improvement (vs baseline) with respect to the LBPIX, pain-related disabilities in daily life (assessed with the mPDI), QOL (assessed with the EQ5D-3L), and the aggregate of all three efficacy parameters. Data of the lower panel show the absolute (relative) proportion of patients, who completed the 12-week treatment observation without any adverse event-related premature discontinuation, who experienced no CNS-related adverse events and, who reported a normal bowel function index at the end of the study), and a combination of all three safety parameters.
Abbreviations: OXN, oxycodone/naloxone; TAP, tapentadol; OR, odds ratio; 95% CI, 95% confidence interval; TEAE, treatment-emergent adverse event; CNS, central nervous system; W12; LBPIX, low back pain intensity index; W12, treatment Week 12 (end of observation); BL, baseline; mPDI, modified pain intensity index; QOL, quality of life; EQ5D-3L, five-dimensional (three-level) European quality-of-life questionnaire; mg, milligram; MEQ, morphine equivalent; SD, standard deviation.
Further efficacy analyses
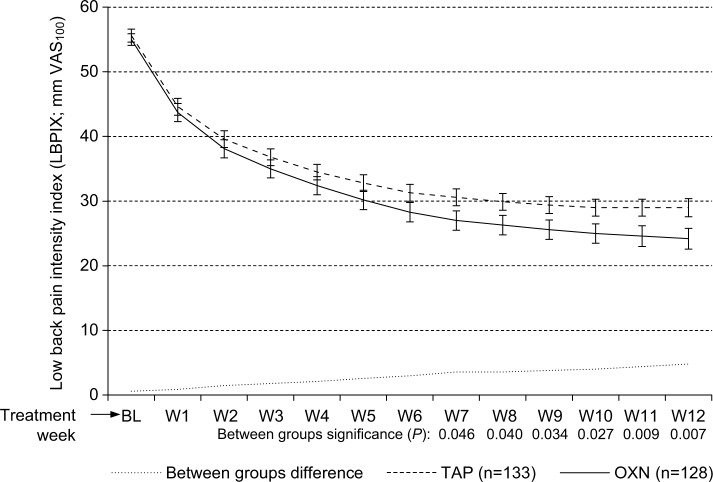
Treatment was followed by a significant pain relief in both treatment groups: absolute (VAS100)/relative (% vs baseline) change for patients treated with OXN vs TAP was −14.4 (−51.1%) vs −13.9 (−51.2%; P= ns) for the lowest 24-hour pain intensity, was −35.0 (−53.6%) vs −31.2 (−46.5%; P=0.032) for the average 24-hour pain intensity, and was −48.9 (−60.8%) vs −41.0 (−49.0%; P<0.001) for the highest 24-hour pain intensity. LBPIX – the pain intensity measure used for the assessment of the primary end point – dropped for OXN/TAP from a baseline score of 55.0±9.3/55.6±9.9 mm VAS100 to 23.6±15.8/28.5±13.9 mm VAS100 (P<0.001 for both treatments) at the end of Week 12 (Figure 2 and Table 4). Corresponding absolute (VAS100)/relative (% vs baseline) LBPIX improvements were 31.4±15.4/57.5±26.0 for OXN vs 27.1±14.4/48.3±24.4 for TAP (P=0.021/0.003). Proportion of patients with LBPIX scores ≤30 mm VAS100 increased significantly from baseline to the end of study with both treatments (P<0.001 for each treatment); however, this was significantly more with OXN (71.9%) vs TAP (60.2%; P=0.046). In parallel, the percentage of patients who were able to reduce their LBPIX scores to or even below their tailored treatment target – an a priori (at baseline) set LBPIX VAS100 level that defines an individual pain intensity threshold that allows patients to manage their daily-life activities – were significantly higher for OXN than for TAP (58.6% [75/128] vs 42.9% [57/133]; P=0.011).
Figure 2.
Change of the LBPIX (mean ±95% CIs) during the course of the 12-week observation with TAP (n=133, dashed line) and OXN (n=128, solid line).
Notes: LBPIX improved significantly for both treatment groups vs baseline (P<0.001 for each), but was significantly superior for OXN vs TAP (P<0.05 from the end of Week 7 until the end of Week 12). Dotted line in the lower part of the graph marks the between group difference.
Abbreviations: VAS100, 100 mm visual analog scale (0: no pain, 100: worst pain conceivable); BL, baseline; W1–W12, treatment Weeks 1–12; LBPIX, low back pain intensity index; 95% CIs, 95% confidence intervals; OXN, oxycodone/naloxone; TAP, tapentadol.
Table 4.
LBPIX data at baseline vs end of observation at Week 12
| LBPIX | OXN (n=128) |
TAP (n=133) |
Significance OXN vs TAP |
|---|---|---|---|
| LBPIX at BL (mm VAS); mean (SD) | 55.0 (9.3) | 55.6 (9.9) | P=0.622 |
| LBPIX at Week 12 (mm VAS); mean (SD) | 23.6 (15.8) | 28.5 (13.9) | P=0.009 |
| LBPIX difference absolute (mm VAS) vs BL; mean (SD) | 31.4 (15.4) | 27.1 (14.4) | P=0.021 |
| LBPIX difference relative (%)vs BL; mean (SD) | 57.5 (26.0) | 48.3 (24.4) | P=0.003 |
| Significance Week 12 vs BL | P<0.001 | P<0.001 | |
| Patients with LBPIX ≤30 mm VAS at BL; n (%) | 1 (0.8) | 1 (0.8) | P=1.000 |
| Patients with LBPIX ≤30 mm VAS at Week 12; n (%) | 93 (72.7) | 81 (60.9) | P=0.044 |
| Difference; absolute, n (relative, %) | 92 (71.9) | 80 (60.2) | P=0.046 |
| Significance Week 12 vs BL | P<0.001 | P<0.001 | |
| Patients with LBPIX relief ≤TTT at Week 12; n (%) | 75 (58.6) | 57 (42.9) | P=0.011 |
Abbreviations: LBPIX, low back pain intensity index; OXN, oxycodone/naloxone; TAP, tapentadol; VAS, visual analog scale; SD, standard deviation; TTT, tailored treatment target; BL, baseline
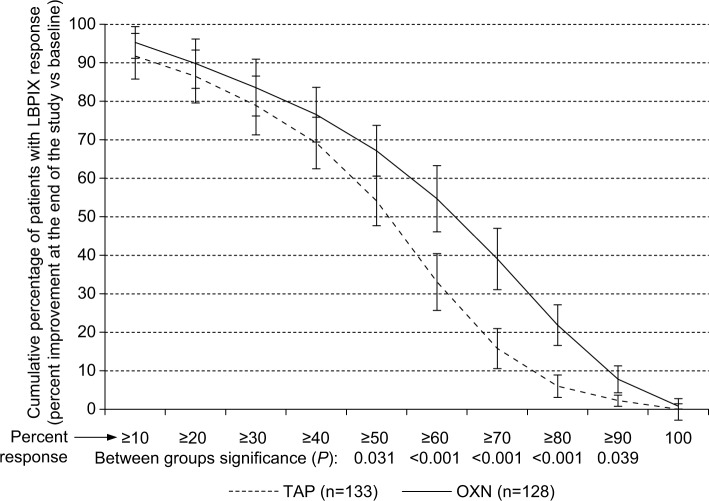
Analysis of the percentages of patients who reported a distinct LBPIX improvement at the study end vs baseline revealed a superior analgesic efficacy of OXN vs TAP with more challenging responder definitions beyond the already mentioned ≥30% criteria (Figure 3). Comparable treatment effects were also seen for pain-related disabilities in daily life and QoL, as shown in Figure 4. Although ≥30% response rates were either comparable (for LBPIX) or slightly different (for mPDI and EQ5D-3L), between-group differences increased for all three efficacy parameters with escalating responder definitions in favor of OXN. A modified primary end point analysis based – beyond the already reported ≥30% response rate – also on ≥50% and ≥70% responses for LBPIX, mPDI, and EQ5D-3L is shown in Figure 5. Although proportions of patients who reached those different (≥30%/≥50%/≥70%) responder end points decreased within both treatment groups, the between-group differences did not and confirmed the superior efficacy of OXN vs TAP, especially if stricter efficacy response criteria were applied for pain intensity, pain-related disabilities, and QoL.
Figure 3.
Percent response vs baseline (±95% CI) with respect to the LBPIX at the end of the 12-week observation with tapentadol (n=133, dashed line) and oxycodone/naloxone (n=128, solid line).
Notes: Proportion of patients who showed a definite LBPIX relief was significantly superior for OXN vs TAP (P<0.05) for ≥50%–≥90% response criteria.
Abbreviations: 95% CI, 95% confidence interval; LBPIX, low back pain intensity index; TAP, tapentadol; OXN, oxycodone/naloxone.
Figure 4.
Proportion of patients (percent ±95% CIs) who reported an individual ≥30%/≥50%/≥70% improvement (vs baseline) with respect to pain intensity (LBPIX, left), pain-related disabilities in daily life (mPDI, middle), and QOL (EQ5D-3L, right) at the end of the 12-week observation with TAP (n=133, light gray), and OXN (n=128, dark gray).
Abbreviations: 95% CIs, 95% confidence intervals; LBPIX, low back pain intensity index; mPDI, modified pain intensity index; QOL, quality of life; EQ5D-3L, five-dimensional (three-level) European quality-of-life questionnaire; TAP, tapentadol; OXN, oxycodone/naloxone; ns, not significant.
Figure 5.
Proportion of patients (percent ±95% CIs) who recorded a ≥30%/≥50%/≥70% improvement (vs baseline) with respect to all the three efficacy parameters (low back pain intensity index, pain-related disabilities in daily life, and QOL) at the end of the 12-week observation with TAP (n=133, light gray), and OXN (n=128, dark gray) in combination with all the three safety/tolerability parameters as used for the primary end point analysis (eg, no TEAE-related study discontinuation, no CNS side effects, normal BFI at the end of observation).
Abbreviations: 95% CIs, 95% confidence intervals; TAP, tapentadol; OXN, oxycodone/naloxone; QOL, quality of life; CNS, central nervous system; BFI, bowel function index; TEAE, treatment-emergent adverse event.
Response is dependent on clinical phenomenology at baseline
A primary end point analysis for subgroups of patients suffering from “mixed” or “neuropathic” types of LBP revealed differential effects of both treatments. Although OXN showed a superior effect vs TAP for BP patients who presented clinically with a “mixed” phenomenology (response rate: 42.7% vs 19.1%; OR: 3.160; P=0.002), both treatments proved comparably effective for patients with a more “neuropathic” type of pain (response rate: 36.7% for both).
Change in clinical phenomenology
Neuropathic symptoms improved comparably with both treatments. Average PDQ7 scores dropped with OXN/TAP from 18.2±3.7 17.7±3.4 at baseline to 12.4±6.3/12.7±5.2 at the study end (P<0.001 for both treatments). In parallel, the proportion of patients with PDQ7 scores ≥18 changed with OXN/TAP from 46.9% (60/128)/36.8% (49/133) at baseline to 20.3% (26/128)/21.8 (29/133) at the end of Week 12. Vice versa the proportion of patients with PDQ7 scores ≤10 (indicating a primarily “nociceptive” pain phenomenology) increased up to 44.5% (57/128) for OXN and 36.1% (48/133) for TAP.
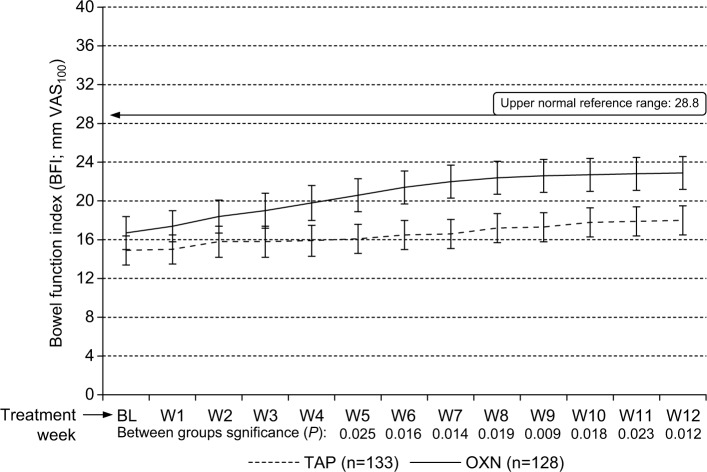
Tolerability analyses
Bowel function index
As expected with the introduction of opioid agonistic WHO Step III analgesics, bowel function worsened from baseline to the end of study (Figure 6). BFI scores increased from baseline to the study end from 14.9±15.5 to 18.0±15.5 (P<0.001) with TAP and from 16.7±17.1 to 23.2±17.6 (P<0.001) with OXN. Between-group analyses showed different BFI changes with significantly lower absolute/relative BFI increments at the end of Week 12 vs baseline for TAP (3.1±4.6 mm VAS100/3.6%±5.1%) in comparison to OXN (6.5±10.2 mm VAS100/7.7%±14.4%; P=0.012). However, percentages of patients with BFI scores within the normal range (ie, ≤28.8)60 at the end of observation were 72.2% (96/133) for TAP, and 68.0 (87/128) for OXN comparable (P=0.457).
Figure 6.
Change of BFI (mean ±95% CIs) during the course of the 12-week observation for TAP (n=133, dashed line) vs OXN (n=128, solid line).
Notes: BFI increased slightly for both treatment groups vs baseline, but significantly less for tapentadol vs oxycodone/naloxone (P<0.05 from treatment Weeks 5–12). However, average BFI scores as well as corresponding 95% CIs for both treatment groups remained within the normal BFI reference range. Horizontal line marked at “28.8” indicates the upper normal reference range of the BFI.
Abbreviations: VAS100, 100 mm visual analog scale (0: no bowel dysfunction, 100: worst bowel dysfunction conceivable); BL, baseline; W1–W12, treatment Weeks 1–12; TAP, tapentadol; OXN, oxycodone/naloxone; BFI, bowel function index; 95% CIs, 95% confidence intervals.
Complete spontaneous bowel movements
Average number of CSBMs per week changed with OXN from 4.3±1.5 at baseline to 4.2±1.8 at the end of observation and with TAP from 4.1±1.6 to 4.2±1.6. Percentages of patients with four or more CSBMs per week at the study end were 68.8% for OXN (88/128) vs 69.2% (92/133) for TAP (P=0.941).
Use of laxatives
The percentage of patients who used prescription laxatives prior initiation of index treatment was 28.6% for TAP (38/133) and 23.4% (30/128) for OXN. The proportion of patients without using laxatives changed insignificantly from baseline to the study end for both treatments (TAP: 71.4 → 75.2% [95 → 100/133], OXN: 76.6 → 68.8% [98 → 88/128]), as did the proportion of patients who used nonprescription laxatives as well as dietary measures and/or exercise changes (TAP: 23.3 → 17.3% [31 → 23/133], OXN: 18.0 → 21.1% [23 → 27/128]). Overall, five TAP patients (3.8%) who took any of these measures at baseline, stopped treatment until the end of observation, whereas ten OXN patients (7.8%), who took initially none, started to do so during the 12-week observation.
Analyses of the available patient information on the use of laxatives revealed a mixed, however, comparable, utilization pattern for both treatment groups evaluated. At baseline, 15 patients (5.7%) – 7 (5.5%) of the OXN and eight (6.0%) of the TAP group (P=0.850) – reported the daily use of laxatives and 53 (20.3%) – 23 (18.0%) of the OXN and 30 (22.6%) of the TAP group (P=0.357) – an intake several times per week. At the end of the evaluation period, 24 patients documented the use of laxatives on a daily basis – 13 (10.3%) vs 11 (8.3%) for OXN vs TAP (P=0.598) – and further 49 several times per week – 27 (21.1%) vs 22 (16.5%) for OXN vs TAP (0.346).
Safety evaluation
All study medications were well tolerated, without significant differences in the overall nature or severity of TEAEs, or related treatment discontinuations. As shown in Figure 1, 14.1% of OXN patients (18/128) reported at least one TEAE in comparison to 12.0% (16/133) for TAP (OR: 1.20, 95% CI: 0.58–2.46; P= ns). Two or more TEAEs were reported with OXN by 7.0% (9/128) and with TAP by 6.8% (9/133). TEAE-related treatment discontinuations were seen in 7.0% with OXN (9/128) vs 7.5% with TAP (10/133; OR: 0.93, 95% CI: 0.37–2.37; P= ns). Overall, 69 TEAEs were reported, 35 in relation with OXN and 34 with TAP. A detailed TEAE analysis (Table 5) revealed that with 43 events (62.3%), the majority of those TEAEs affected the CNS, followed by 21 events (30.4%) affecting the GI tract, four (5.8%) affecting the skin, and one (1.5%) affecting the metabolic system. “Somnolence” as a reportable TEAE was the most frequently documented drug-related AE, noted with OXN/TAP in 7.0%/7.5%, followed by abdominal pain (5.5%/1.5%), dizziness (3.1%/3.0%), and constipation (4.7%/0.8%). Of the 69 events, most of them were mild or moderate, with 17 (24.6%) classified as mild and 36 (52.2%) as moderate, and only 16 (23.2%) as severe. In all cases, TEAEs recovered completely, either after treatment discontinuation (60.9%, 42/69) or with supportive drug treatment (39.1%, 27/69).
Table 5.
Overall TEAE experience
| TEAEs | OXN (n=128), n (%) | TAP (n=133), n (%) | Significance |
|---|---|---|---|
| Number of TEAEs | 35 | 34 | |
| Number of serious TEAEs | − | − | |
| Subjects with TEAEs | 18 (14.1) | 16 (12.0) | ns |
| Subjects with ≤2 TEAEs | 9 (7.0) | 9 (6.8) | ns |
| Most common TEAEs | |||
| Somnolence | 9 (7) | 10 (7.5) | ns |
| Constipation | 6 (4.7) | 1 (0.8) | ns |
| Headache | − (−) | 6 (4.5) | 0.017 |
| Nausea | 2 (1.6) | 3 (2.3) | ns |
| Dizziness | 4 (3.1) | 4 (3) | ns |
| Abdominal pain | 7 (5.5) | 2 (1.5) | ns |
| Sleep problems | 3 (2.3) | − (−) | ns |
| Vomiting | − (−) | 1 (0.8) | ns |
| Sweating | 1 (0.8) | 1 (0.8) | ns |
| Others | 3 (2.3) | 6 (4.5) | ns |
| Affected organ classes | |||
| Nervous system | 18 (14.1) | 25 (18.8) | ns |
| Gastrointestinal system | 15 (11.7) | 6 (4.5) | 0.032 |
| Skin | 2 (1.6) | 2 (1.5) | ns |
| Metabolic system | − (−) | 1 (0.8) | ns |
| Intensity | |||
| Mild | 10 (7.8) | 7 (5.3) | ns |
| Moderate | 18 (14.1) | 18 (14.1) | ns |
| Severe | 7 (5.5) | 9 (6.8) | ns |
| Countermeasures | |||
| Pharmacotherapy | 15 (11.7) | 12 (9) | ns |
| Treatment discontinuation (TEAEs) | 20 (15.6) | 22 (16.5) | ns |
| Treatment discontinuation (patients) | 9 (7) | 10 (7.5) | ns |
| Treatment discontinuation for any reasons (patients) | 35 (27.3) | 30 (22.6) | ns |
Abbreviations: OXN, oxycodone/naloxone; TAP, tapentadol; n, number of patients; %, percentage of patients; TEAEs, treatment-emergent adverse events; ns, not significant.
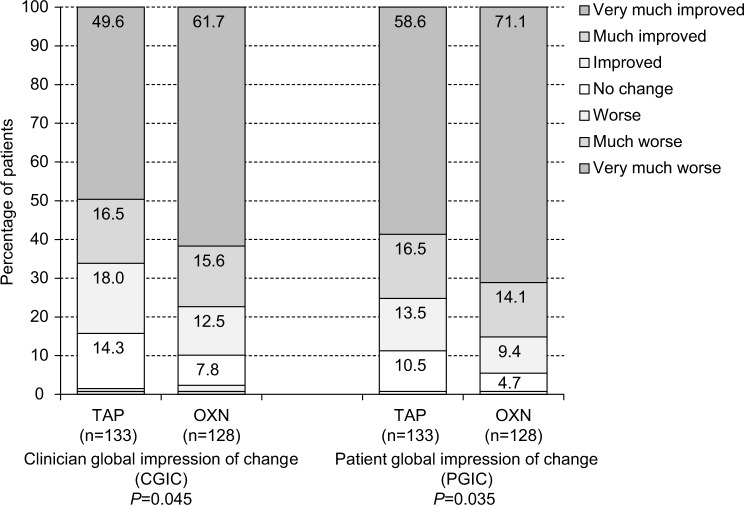
Global impression of change
Clinician/patient rated global impression of treatment-related changes at the end of the 12-week observation period vs baseline were good, with only minor differences between both index treatments (Figure 7). Physicians scored treatment-related changes with OXN/TAP in 77.3% (99/133)/66.2% (88/128; P=0.045) and patients in 85.2% (109/128)/75.2% (100/133; P=0.035) as “very much” or “much improved”. Overall, physicians and patients reported treatment-related improvements in 87.0% (227/261) and 91.6% (239/261).
Figure 7.
CGIC (left panel) and (right panel) ratings, both at the end of the 12-week observation.
Notes: Summary percentages for the “very much improved” and “much improved” categories for both scales were significantly higher (CGIC: P=0.045; PGIC: P=0.035) for the OXN group (n=128) vs the TAP group (n=133).
Abbreviations: CGIC, Clinician global impression of change; PGIC, patient global impression of change; TAP, tapentadol; OXN, oxycodone/naloxone.
Discussion
When considering appropriate treatment strategies for individuals with cLBP-NC, physicians have to balance the efficacy alongside safety and tolerability of treatments under consideration. This noninterventional treatment evaluation of routine data comparing flexible dose regimens of TAP and OXN, designed to reflect usual analgesic use in clinical practice, provides a comprehensive real-life assessment of the BRP of both WHO Step III analgesics in cLBP patients with an NC. Based on a composite response definition that incorporates a combination of six different items – chosen to reflect day-to-day challenges in balancing efficacy with tolerability in daily practice – both index medications proved efficacious and well tolerated. However, differences were observed with the degree of pain relief, which proved to be stronger with OXN in comparison to TAP if stricter response criteria were used.
This outcome is in contrast to the data of the randomized open-label study on both index treatments in patients with cLBP-NC published by Baron et al45 in 2015. However, in contrast to our evaluation that was based on routine data gathered under conditions of daily life, dosing in the Baron study followed a prespecified schedule (formally based on information given in the SmPCs of both index medications) and patients had to reach a definite dose level combined with a definite pain response at the end of the 3-week titration period, to qualify for the following 9-week evaluation period. This peculiarity of the Baron study did not really reflect daily-life titration approaches, which usually focus on a stepwise uptitration of the medication based on individual response and tolerability issues, without the need to reach a definite dose and/or a definite response at a distinct time point after treatment initiation. In addition, uptitration of a WHO Step III analgesic under daily-life conditions lasts normally longer than 3 weeks, and patients and physicians generally do not terminate a drug treatment initiated in response to a chronic pain condition such as cLBP-NC in patients elsewhere refractory to other approaches after 3 weeks, if either response or tolerability are not as expected, but try to improve patient’s response by further dose adjustments to the individual needs. Due to the fact that the analgesic compound in OXN is a μ-opioid receptor agonist about twice as potent as morphine,62 while the μ-opioid receptor activity of TAP is approximately only 2% vs morphine,63 the combination of a forced titration process with definite treatment termination criteria focusing on tolerability issues as used in the Baron study resulted in a one-sided privilege of TAP vs OXN. Not surprisingly, the attrition rates for OXN (51.6%) at the end of the initial titration phase reported in the Baron study were significantly higher compared to those reported for TAP (23.1%) – as well as those observed by us for the first three treatment weeks with OXN (n=5/128, 3.9%) and TAP (n=5/133; 3.8%) – and due to the predefined techniques used to impute missing data for the final end point analyses unavoidably followed by a statistically superior analgesic response in favor of TAP.
As reported, overall attrition rates found in our analysis of 12-week data gathered under real-life conditions without any formal third party or other external influences were comparable for both treatments (OXN: 23.4%, TAP: 22.6%), highlighting the importance of an individualized titration process over time. Based on that, OXN did not only prove formally noninferior to TAP with respect to the primary end point, but even superior – due to its significantly stronger analgesic effects seen for treatment-related improvements of pain intensity, pain-related disabilities in daily life, and QoL. The differentiation between patients who presented with an improvement of pain and pain-related restrictions of ≥30%, ≥50%, or even ≥70% at the end of the 12-week observation period vs baseline as consequence of a treatment with OXN vs TAP showed clearly that the treatment of patients suffering from some kind of neuropathic pain with a high-potent opioid receptor agonist must not necessarily be inferior to the treatment with a low-potent opioid analgesic for whom preclinical studies also report a combined activity via norepinephrine-reuptake inhibition.44,64 In fact, data from individual randomized, placebo-controlled trials with TAP in patients suffering from diabetic neuropathy,65 from structured literature reviews,66 and from recent meta-analyses on the efficacy of different treatments used for neuropathic pain11 have so far been unable to indisputably prove a superior analgesic effect of TAP vs placebo and/or potent WHO Step III opioids. This issue does not necessarily mean that the combined approach described for TAP is not effective for patients with neuropathic pain but shows that new approaches proven in preclinical studies do not necessarily translate into relevant improvements vs established treatments if given under daily-life conditions of routine care.
Overall, both index treatments proved equally tolerated and showed only marginal differences with respect to occurrence, spectrum, and severity of side effects reported. In general, TEAE prevalence was significantly lower for both index medications evaluated compared to those reported by randomized controlled trials. However, in contrast to these previous studies, the assessment of side effect data in the current analysis based solely on spontaneous self-reports of patients, which are known to either yield a significantly lower percentage of AE instances and also cover predominantly events more debilitating in nature relative to regularly scheduled assessments with standardized AE questionnaires as they are usually performed in traditional trials – noninterventional in nature or not.67
The majority of TEAEs reported were mild or moderate in intensity, resolved spontaneously without specific counter measures, and occurred – to our surprise – with two exceptions (headaches and GI problems) equally frequently with both index medications. Despite the fact that the worsening in BFI was biometrically significant vs baseline in patients treated with OXN but not in those treated with TAP, the proportion of patients who presented with BFI scores above the upper reference range at the end of the observation period was comparable for both index treatments (OXN: 32.0% vs TAP: 27.8%, P=0.457). Constipation (4.7% vs 0.8%) and abdominal pain (5.5% vs 1.5%) were the two GI-related TEAEs that differentiated numerically best between OXN vs TAP, but resulted only in combination in a significantly superior tolerability in favor of TAP (11.7% vs 4.5%, P=0.032), reflecting not only the significantly improved OIC profile of OXN vs oxycodone and other potent μ-opioid receptor agonists, but also the favorable GI profile of the WHO Step III analgesic TAP, which exhibits only minor μ-opioid receptor activity. This overall favorable tolerability profile of both index medications is in contrast to the data of the randomized treatment comparison of TAP vs OXN published by Baron et al45 as already mentioned, where OXN treatment was associated with a significantly increased risk of constipation and other side effects vs TAP leading to premature treatment discontinuations of 51.6% vs 23.1% during the titration phase and of 62.5% vs 33.8% for the whole study. Major differences to our evaluation that – at least from our point of view – count responsible for these extraordinary attrition rates were a predefined dosing schedule as well as the requirement to achieve a distinct treatment response at the end of the 3-week titration phase to gain access to the real treatment evaluation phase in the Baron study, that obviously forced physicians to increase dosages according to the given titration schedule and not according to the individual needs of their patients. As a consequence, average (±SD) 3-week dosages for TAP vs OXN were substantially higher in the Baron study (259.0±80.1 vs 45.0±18.3 mg) compared to those observed in this treatment evaluation (164.5±50.8 vs 27.8±11.4 mg). On the other hand, these data show that TAP has an overall beneficial tolerability profile that allows a rapid titration without provoking significant side effects even by nonpain specialists, which might help with respect to the differentiation of both index medications for different user populations.
To the knowledge of the authors, this is the first time that such a multifactorial composite definition of response reflecting the complexity of daily-life considerations has been used for the scientific evaluation of treatment effects observed with routine data gathered under real-life conditions in patients suffering from some kind of neuropathic pain, and hence, the rationale for the choice of the response criteria underlying our primary BRP end point deserves some discussion. The combined weighting of different factors covering efficacy as well as tolerability and safety aspects of a treatment – something natural in daily routine care for pain patients – has not gained importance in clinical trials as it has in daily life due to few methodological issues reluctantly seen by authorities and scientists. Most of these concerns focus on a potential bias, as there may be competing risks between different end points, and therefore it is important to particularly look whether a positive composite end point camouflages a negative individual outcome or dilutes the effect of the treatments under evaluation on other end points. As a consequence, regulatory authorities usually require all components of a composite end point to be analyzed separately to reassure if a treatment affects all components or just a single outcome68–70 – a requirement fulfilled by the current analysis.
In general, the use of a composite end point for the evaluation of treatment effects is justified if the individual components of the composite are clinically meaningful and of similar importance to the patient and if the expected effects on each component are similar, based on biological plausibility (which is, in the end, the rationale for using a composite end point).70–72 As pain is not only a symptom but also a cause for functional disability and restrictions in physical and mental QoL, the combined use of all three aspects for the definition of response sounds rational for daily-life evaluations. And the same is true for the tolerability appraisements chosen in our study, as the overall ability to stay on a treatment without conflicting side effects combined with least problems in those two organ systems known to be usually affected in most patients treated with a potent WHO Step III opioid – the CNS and the GI tract – is of upmost importance.
In our evaluation, separate analyses for each of the three efficacy and tolerability end points as well as each individual component of these aggregates offer a detailed in-depth view on the effects of both index medications. All individual components of both of our composite end points were not only clinically meaningful, but also based on daily-life procedures and allowed the combined evaluation for individual patients mirroring daily-life procedures in contrast to the groupwise comparisons usually used in controlled trials such as the Baron study, which frequently hide individual response rates behind average scores. Main drivers for the treatment effect differences seen for OXN vs TAP in our evaluation were the combined efficacy end point (reached by 57.8% vs 41.4%; P=0.008), and among its components, the percentage of patients who reported at least a 30% improvement in QoL at the end of the 12-week observation period vs baseline (76.6% vs 63.9%; P=0.026). In contrast to that, tolerability analyses showed comparable effects of both index treatments both for the combined tolerability aggregate, as well as its individual components.
An important aspect of our evaluation was the fact that all analyses of the BRP were based on the labeled dosages of both index treatments, chosen, initiated, uptitrated, and maintained without any external influences, solely based on the individual needs of the patients seeking help. Despite the fact that we found no indicators for specific selection effects, it seems at least probable that there are some. Physicians usually try to use the most effective and tolerable treatment option for their patients, which involves a trade-off between the expected benefit and the potential risk based on individual patient circumstances that must not necessarily be identifiable by standardized patient records. From that point of view, it must be taken into consideration that patient selection for both treatments suffered some risk for some kind of positive selection for both index medications, and therefore it is not the least surprising that the treatment effects and tolerability data reported here showed for both agents an overall improved BRP vs those reported by randomized controlled trials. However, these issues are part of daily-life considerations and mirror current treatment strategies for patients with cLBP-NC much better than those randomized treatment selections performed by controlled trials.
Strengths and limitations
Several limitations should be noted, including that this treatment effect evaluation was based on observational and open-label real-world data gathered via an electronic treatment registry as part of daily routine. Since selection of patients and choice of treatment was based solely on the discretion of the physicians and their clinical judgments, medications were allocated in a nonrandomized fashion, a factor that might interfere – irrespective of the random select approach we used to overcome this limitation – with the treatment effects reported. This design may also be considered as setting an imbalance in clinical equipoise, since the repeatedly published effects of TAP for different pain types with an NC and the advantages of OXN for OIC suffer some risk of a biased patient selection, irrespective of the fact that we found none in our analyses.
Further obvious limitations of this analysis are the lack of a placebo group, the restricted ability to differentiate between outcomes that are due to adoption of the index treatments or due to other unrecognized changes in the population under study, and the fact that entering data into the German Pain Registry requires the active participation of physician and pain treatment centers and the implementation of the online documentation service iDocLive as part of routine care. Although both the lack of a placebo control and the restricted ability to correlate treatment and effect have to be accepted as inevitable issues in daily-life evaluations of routine medical care and the accompanying aspects of internal vs external validity are a well-known issue intensively discussed between scientists and practitioners, data analysis based on nonrepresentative or artificially collected patient samples might significantly interfere with the generalizability of the results obtained. To exclude selection effects as a possible source of bias, we compared demographic and baseline data of randomly selected vs nonselected cLBP-NC patients and found comparable demographic characteristics and only insignificant baseline differences. Physicians and centers building the network of the German Pain Registry covered the whole spectrum of medical disciplines involved in pain management and were homogenously distributed among Germany, hence minimizing the risk of geographical or other systemic patient selection biases. However, all participants were board-certified pain specialists accredited by the German Pain Association, well experienced with WHO Step III analgesics and their differential use in patients with chronic nonmalignant pain. This special qualification might be the reason for the overall low and comparable attrition rates of both index medications and should be kept in mind when treatment strategies based on WHO Step III analgesics are adopted by less experienced physicians. In addition, patients treated by these specialists may differ from patients who consult primary care physicians, which should also be taken into consideration before translating the results of this evaluation to the general population of cLBP-NC patients.
Due to the use of routine data, there was neither a systematic monitoring of treatment compliance nor a formal recording of possible opioid addiction or treatment abuse. However, both index medications are known to have a lower risk of abuse compared with traditional WHO Step III analgesics.
A formal issue interfering with common Good Clinical Practice standards for conducting clinical trials and non-interventional studies is that none of the patient-reported data derived from the German Pain Registry for evaluation purposes like this one can be confirmed via medical charts, laboratory results, or treatment schedules, and this is due to the fact that the direct electronic data entry performed under the conditions of routine care does not provide evaluable materials for independent source data verification processes. Moreover, German data protection guidelines forced us to perform any analyses of data obtained from the German Pain Registry only with anonymized data sets, which excludes any possibilities for backward tracing or the identification of individual patients, pain management centers, or pain specialists.
On the other hand, this special design can also be considered as a unique strength of our analysis since it allows physicians not only to optimize a drug’s efficacy and safety in clinical practice, which does not itself endorse balanced treatment strategies, but focuses almost exclusively on patient-relevant and, especially, patient-reported outcomes, sampled as part of a routine data registry established to improve patient care under real-life conditions.
Restrictions of the range of variables collected by users was – in contrast to most other routine data systems – not really a problem, as most of the information generated by administrative and clinical processes via the German Pain Registry was based on standardized documentation tools (eg, German Pain Questionnaire and German Pain Diary) mutually developed, agreed, and recommended for routine use by respective medical associations in Germany (the German Pain Association and the German Pain Society) and the German Pain League (Germanys largest umbrella group for self-regulating communities of pain and palliative care patients) in 2006.73 Both standard tools contain a broad spectrum of validated self-assessment instruments (covering physical, mental, psychological, and social aspects of pain, previous, and current pain treatments, comorbidities as well as concomitant medication, etc.) sensitive for baseline as well as ongoing evaluations during the course of a pain treatment and fulfill the official requirements of the German National Association of Statutory Health Insurance Physicians for a quality-assured standard documentation tool for pain medicine.74
Another important factor in favor of this registry-based treatment evaluation is that in contrast to usual studies (interventional or not), neither physicians nor patients got any reimbursements for their study-related data collection activities, a factor eliminating the risk of treatments initiated not for the medical sake of an individual patient but for economic reasons. German practice registry participation and the use of the online documentation tool iDocLive are complimentary for physicians who are members of the German Pain Association and free of charge for all patients – irrespective of their health insurance coverage.
Conclusion
Caring for patients with moderate to severe and elsewhere-refractory cLBP-NC requires clinicians to carefully weigh benefits and disadvantages of a longer term opioid therapy and to select the most appropriate treatment with respect to the individual patient needs and the BRP provided under daily-life conditions The data presented here consist of a broad range of efficacy and tolerability measures matching daily-life assessments and clearly indicate that, under the conditions of routine care, both index treatments – OXN as well as TAP – proved to be effective, safe, and well-tolerated options when using flexible dose regimens as usual in clinical practice. Under these conditions, both treatments caused not only a biometrically significant but also clinically relevant improvement of pain, pain-related disabilities, and QoL and showed a favorable safety profile with only minor and clinically irrelevant differences with respect to prevalence, spectrum, and intensity of TEAEs reported. However, formally confirmed to be noninferior to TAP, OXN – a potent μ-opioid receptor agonist – showed a superior efficacy profile, particularly if stricter response criteria were applied in comparison to those usually used in clinical trials.
Acknowledgments
Data of this analysis has been presented at the Annual Congress of the German Pain Association, March 3–5, 2016, Frankfurt, Germany, and at the 8th World Congress of the World Institute of Pain (WIP), May 20–23, 2016, New York, USA. The concept for this evaluation of routine data provided by the German Pain Registry has been developed by MAU at the Institute of Neurological Sciences (IFNAP) on behalf of the German Pain Association (Deutsche Gesellschaft für Schmerzmedizin, DGS) and the German Pain League (Deutsche Schmerzliga, DSL), and its realization has been funded in part (~30%) by an unrestricted scientific grant from Mundipharma, Germany. Neither Mundipharma nor any of its employees exerted any influence on the data acquisition, the conduct of this analysis, or on the interpretation and publication of the results.
Footnotes
Disclosure
MAU and GHHMS are physicians and independent of any significant/relevant financial or other relationship to the sponsor, except for minor reimbursements for occasional lecture or consulting fees. Both are honorary members of the management boards of the German Pain Association and the German Pain League. The German Pain Registry is hosted by an independent contract research organization by order of the German Pain Association and under control of the Institute of Neurological Sciences and collects standardized data from daily routine medical care since January 2000. MAU received financial support and/or expenses in form of research money, consultancy fees, and/or renumerations for lecture activities from Almirall, Archimedes, Bene-Arzneimittel, Grünenthal, Janssen-Cilag, Menarini, MSD, Mucos, Mundipharma, Pfizer, PharmAllergan, Pro-Strakan, and TEVA. GHHMS received financial support and/or expenses in form of research money, consultancy fees, and/or renumerations for lecture activities from Allergan Ltd, ALMIRALL S.A., Grünenthal, Mundipharma, Pfizer, PharmAllergan, ProStrakan, and TEVA. The authors report no other conflicts of interest in this work.
References
- 1.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freynhagen R, Baron R. The evaluation of neuropathic components in low back pain. Curr Pain Headache Rep. 2009;13:185–190. doi: 10.1007/s11916-009-0032-y. [DOI] [PubMed] [Google Scholar]
- 3.Beith ID, Kemp A, Kenyon J, Prout M, Chestnut TJ. Identifying neuropathic back and leg pain: a cross-sectional study. Pain. 2011;152:1511–1516. doi: 10.1016/j.pain.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Smart KM, Blake C, Staines A, Doody C. Self-reported pain severity, quality of life, disability, anxiety and depression in patients classified with ‘nociceptive’, ‘peripheral neuropathic’ and ‘central sensitisation’ pain. The discriminant validity of mechanisms-based classifications of low back (±leg) pain. Man Ther. 2012;17:119–125. doi: 10.1016/j.math.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin RH, O’Connor AB, Backonja M, et al. Pharmacologic management of neuropathic pain: evidence based recommendations. Pain. 2007;132(3):237–251. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Moulin DE, Clark AJ, Gilron I, et al. Canadian Pain Society Pharmacological management of chronic neuropathic pain – consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12(1):13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22(5):467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 8.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Attal N, Cruccu G, Baron R, et al. European Federation of Neurological Societies EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17(9):1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, O’Connor AB, Audette J, et al. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc. 2010;85(3 Suppl):S3–S14. doi: 10.4065/mcp.2009.0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: systematic review, meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015;14(2):162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahan M, Mailis-Gagnon A, Wilson L, Srivastava A, National Opioid Use Guideline Group Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can Fam Physician. 2011;57(11):1257–1266. [PMC free article] [PubMed] [Google Scholar]
- 13.Häuser W, Bock F, Engeser P, Tölle T, Willweber-Strumpf A, Petzke F. Long-term opioid use in non-cancer pain. Dtsch Arztebl Int. 2014;111(43):732–740. doi: 10.3238/arztebl.2014.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med. 2015;162:276–286. doi: 10.7326/M14-2559. [DOI] [PubMed] [Google Scholar]
- 15.Grimby A, Rosenhall U. Health-related quality of life and dizziness in old age. Gerontology. 1995;41(5):286–298. doi: 10.1159/000213696. [DOI] [PubMed] [Google Scholar]
- 16.Terwindt G, Ferrari MD, Tijhuis M, Groenen SM, Picavet HS, Launer LJ. The impact of headache on quality of life in the general population: the GEM study. Neurology. 2000;55(5):624–629. doi: 10.1212/wnl.55.5.624. [DOI] [PubMed] [Google Scholar]
- 17.Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8(Suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 18.Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007;61(7):1181–1187. doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh TD. Prevention of opioid side effects. J Pain Symptom Manage. 1990;5(6):362–367. doi: 10.1016/0885-3924(90)90031-e. [DOI] [PubMed] [Google Scholar]
- 20.McNicol E, Horowicz-Mehler N, Fisk RA, et al. American Pain Society Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4(5):231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 21.Oderda GM, Evans RS, Lloyd J, et al. Cost of opioid related adverse drug events in surgical patients. J Pain Symptom Manage. 2003;25(3):276–283. doi: 10.1016/s0885-3924(02)00691-7. [DOI] [PubMed] [Google Scholar]
- 22.Leslie K. Current depth of anaesthesia monitors: jacks of all trades and masters of none? Anaesth Intensive Care. 2007;35(3):333–334. doi: 10.1177/0310057X0703500302. [DOI] [PubMed] [Google Scholar]
- 23.Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–406. doi: 10.1345/aph.1H386. [DOI] [PubMed] [Google Scholar]
- 24.Cook SF, Lanza L, Zhou X, et al. Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther. 2008;27(12):1224–1232. doi: 10.1111/j.1365-2036.2008.03689.x. [DOI] [PubMed] [Google Scholar]
- 25.Holzer P, Ahmedzai SH, Niederle N, et al. Opioid-induced bowel dysfunction in cancer related pain: causes, consequences, and a novel approach for its management. J Opioid Manag. 2009;5(3):145–151. doi: 10.5055/jom.2009.0015. [DOI] [PubMed] [Google Scholar]
- 26.Wan Y, Corman S, Gao X, Liu S, Patel H, Mody R. Economic burden of opioid-induced constipation among long-term opioid users with noncancer pain. Am Health Drug Benefits. 2015;8(2):93–102. [PMC free article] [PubMed] [Google Scholar]
- 27.Miles CL, Fellowes D, Goodman ML, Wilkinson S. Laxatives for the management of constipation in palliative care patients. Cochrane Database Syst Rev. 2006;(4):CD003448. doi: 10.1002/14651858.CD003448.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Chou R, Fanciullo GJ, Fine PG, et al. American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tack J. Current and future therapies for chronic constipation. Best Pract Res Clin Gastroenterol. 2011;25(1):151–158. doi: 10.1016/j.bpg.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Adult Cancer Pain. [Accessed May 7, 2016]. V 2.2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/pain.pdf.
- 31.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182(5A Suppl):11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 32.Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010 pii: 2407. [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar L, Barker C, Emmanuel A. Opioid-induced constipation: pathophysiology, clinical consequences, and management. Gastroenterol Res Pract. 2014;2014:141737. doi: 10.1155/2014/141737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5):835–842. doi: 10.1038/ajg.2011.30. [DOI] [PubMed] [Google Scholar]
- 35.Choi YS, Billings JA. Opioid antagonists: a review of their role in palliative care, focusing on use in opioid-related constipation. J Pain Symptom Manage. 2002;24(1):71–90. doi: 10.1016/s0885-3924(02)00424-4. [DOI] [PubMed] [Google Scholar]
- 36.Poulsen JL, Brock C, Olesen AE, Nilsson M, Drewes AM. Evolving paradigms in the treatment of opioid-induced bowel dysfunction. Therap Adv Gastroenterol. 2015;8(6):360–372. doi: 10.1177/1756283X15589526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noble M, Treadwell JR, Tregear SJ, et al. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst Rev. 2010;(1):CD006605. doi: 10.1002/14651858.CD006605.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lauche R, Klose P, Radbruch L, Welsch P, Häuser W. Opioids in chronic noncancer pain – are opioids different? A systematic review and meta-analysis of efficacy, tolerability and safety in randomized head-to-head comparisons of opioids of at least four week’s duration. Schmerz. 2015;29(1):73–84. doi: 10.1007/s00482-014-1432-4. [DOI] [PubMed] [Google Scholar]
- 39.Smith HS. Opioids and neuropathic pain. Pain Physician. 2012;15(3 Suppl):ES93–ES110. [PubMed] [Google Scholar]
- 40.Smith K, Hopp M, Mundin G, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20(4):427–439. doi: 10.1517/13543784.2011.563236. [DOI] [PubMed] [Google Scholar]
- 41.DePriest AZ, Miller K. Oxycodone/naloxone: role in chronic pain management, opioid-induced constipation, and abuse deterrence. Pain Ther. 2014;3(1):1–15. doi: 10.1007/s40122-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis MP, Goforth HW. Oxycodone with an opioid receptor antagonist: a review. J Opioid Manag. 2016;12(1):67–85. doi: 10.5055/jom.2016.0313. [DOI] [PubMed] [Google Scholar]
- 43.US Food and Drug Administration Targiniq Medication Guide (issue: July 2014) [Accessed May 7, 2016]. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/205777lbl.pdf.
- 44.Schröder W, Tzschentke TM, Terlinden R, et al. Synergistic interaction between the two mechanisms of action of tapentadol in analgesia. J Pharmacol Exp Ther. 2011;337(1):312–320. doi: 10.1124/jpet.110.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baron R, Likar R, Martin-Mola E, et al. Effectiveness of tapentadol prolonged release (PR) compared with oxycodon/naloxone PR for the management of severe chronic low back pain with a neuropathic component: a randomized, controlled, open-label, phase 3b/4 study. Pain Pract. 2016;16(5):580–599. doi: 10.1111/papr.12308. 10.1111/papr.12308. [DOI] [PubMed] [Google Scholar]
- 46.Überall MA, Müller-Schwefe GHH, Emrich O, et al. Threats and risks of standardized imputation of drop-out-related missing values using LOCF in clinical studies with analgesics. MMW Fortschr Med. 2015;157(Suppl 7):19–26. [Google Scholar]
- 47.Bacallao J, Casado AJ. Methodological issues on a clinical trial to test tapentadol prolonged release vs. oxycodone/naloxone prolonged release. Pain Pract. 2016;16(3):E56–589. doi: 10.1111/papr.12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deutsche Schmerzgesellschaft Deutscher Schmerzfragebogen [German pain questionnaire] [Accessed May 7, 2016]. Available at http://www.dgss.org/deutscher-schmerzfragebogen.
- 49.Deutsche Schmerzgesellschaft Handbuch zum Deutschen Schmer-zfragebogen [Manual for the German pain questionnaire] [Accessed May 7, 2016]. Available at http://www.dgss.org/fileadmin/pdf/12_DSF_Manual_2012.2.pdf.
- 50.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Blood Press. 1992;1(2):113–119. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 51.Cryer B, Li C, Simon LS, Singh G, Stillman MJ, Berger MF. GI-REASONS: a novel 6-month, prospective, randomized, open-label, blinded endpoint (PROBE) trial. Am J Gastroenterol. 2013;108(3):392–400. doi: 10.1038/ajg.2012.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cappelleri JC, Bienen EJ, Koduru V, Sadosky A. Measurement properties of painDETECT by average pain severity. Clinicoecon Outcomes Res. 2014;6:497–504. doi: 10.2147/CEOR.S68997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palexia retard Retardtableten Product Information. Aug, 2010. Aug, 2015. [Accessed May 7, 2016]. Available from: https://www.gelbe-liste.de/produkte/palexia-retard-100-mg-retardtabletten_528663/fachinformation.
- 54.Targin Product Information. Aug, 2011. Jul, 2015. [Accessed May 7, 2016]. Available from: https://www.gelbe-liste.de/produkte/targin-10-mg-5-mg-retard-tabletten_486051/fachinformation.
- 55.Tait RC, Chibnall JT, Krause S. The pain disability index: psychometric properties. Pain. 1990;40(2):171–182. doi: 10.1016/0304-3959(90)90068-O. [DOI] [PubMed] [Google Scholar]
- 56.Casser HR, Hüppe M, Kohlmann T, et al. German pain questionnaire and standardised documentation with the KEDOQ-Schmerz. Schmerz. 2012;26(2):168–175. doi: 10.1007/s00482-011-1142-0. [DOI] [PubMed] [Google Scholar]
- 57.The EuroQol Group EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 58.Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM) Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 59.Rentz AM, Yu R, Mueller-Lissner S, Leyendecker P. Validation of the bowel function index to detect clinically meaningful changes in opioid-induced constipation. J Med Econ. 2009;12(4):371–383. doi: 10.3111/13696990903430481. [DOI] [PubMed] [Google Scholar]
- 60.Ueberall MA, Mueller-Lissner S, Buschmann-Kramm C, Bosse B. The bowel function index for evaluating constipation in pain patients: definition of a reference range for a non-constipated population of pain patients. J Int Med Res. 2011;39:41–50. doi: 10.1177/147323001103900106. [DOI] [PubMed] [Google Scholar]
- 61.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 62.Riley J, Eisenberg E, Müller-Schwefe G, Drewes AM, Arendt-Nielsen L. Oxycodone: a review of its use in the management of pain. Curr Med Res Opin. 2008;24(1):175–192. doi: 10.1185/030079908x253708. [DOI] [PubMed] [Google Scholar]
- 63.Tzschentke TM, Jahnel U, Kogel B, et al. Tapentadol hydrochloride: a next-generation, centrally acting analgesic with two mechanisms of action in a single molecule. Drugs Today. 2009;45(7):483–496. doi: 10.1358/dot.2009.45.7.1395291. [DOI] [PubMed] [Google Scholar]
- 64.Gonçalves L, Friend LV, Dickenson AH. The influence of μ-opioid and noradrenaline reuptake inhibition in the modulation of pain responsive neurones in the central amygdala by tapentadol in rats with neuropathy. Eur J Pharmacol. 2015;749:151–160. doi: 10.1016/j.ejphar.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302–2309. doi: 10.2337/dc13-2291. [DOI] [PubMed] [Google Scholar]
- 66.Coluzzi F, Ruggeri M. Clinical and economic evaluation of tapentadol extended release and oxycodone/naloxone extended release in comparison with controlled release oxycodone in musculoskeletal pain. Curr Med Res Opin. 2014;30(6):1139–1151. doi: 10.1185/03007995.2014.894501. [DOI] [PubMed] [Google Scholar]
- 67.Robinson CB, Fritch M, Hullett L, et al. Development of a protocol to prevent opioid-induced constipation in patients with cancer: a research utilization project. Clin J Oncol Nurs. 2000;4(2):79–84. [PubMed] [Google Scholar]
- 68.Lauer MS, Topol EJ. Clinical trials – multiple treatments, multiple end points, and multiple lessons. JAMA. 2003;289(19):2575–2577. doi: 10.1001/jama.289.19.2575. [DOI] [PubMed] [Google Scholar]
- 69.International Conference on Harmonization Guideline E9, Statistical Principles for Clinical Trials. [Accessed May 7, 2016]. Available at http://www.ich.org/filead-min/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf.
- 70.Committee for Proprietary Medicinal Products (CPMP) Points to Consider on Multiplicity Issues in Clinical Trials. [Accessed May 7, 2016]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003640.pdf.
- 71.Freemantle N, Calvert M, Wood J, Eastaugh J, Griffin C. Composite outcomes in randomized trials. Greater precision but with greater uncertainty? JAMA. 2003;289(19):2554–2559. doi: 10.1001/jama.289.19.2554. [DOI] [PubMed] [Google Scholar]
- 72.Montori VM, Permanyer-Miralda G, Ferreira-González I, et al. Validity of composite endpoints in clinical trials. BMJ. 2005;330:594–596. doi: 10.1136/bmj.330.7491.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nagel B, Pfingsten M, Lindena G, Nilges P. DGSS, editor. Handbuch Deutscher Schmerzfragebogen. [Accessed May 7, 2016]. Herausgegeben von. Available at http://www.dgss.org/fileadmin/pdf/12_DSF_Manual_2012.2.pdf.
- 74.Kassenärztliche Bundesvereinigung Qualitätssicherungsvereinbarung zur schmerztherapeutischen Versorgung chronisch schmerzkranker Patienten gem. § 135 Abs. 2 SGB V; 2005 (Qualitätssicherungsvereinbarung Schmerztherapie) [Accessed May 7, 2016]. Available at http://daris.kbv.de/daris.asp.