Abstract
Genetic variants and dysfunctional monocyte had been reported to be associated with infection susceptibility in advanced cirrhotic patients. This study aims to explore genetic predictive markers and relevant immune dysfunction that contributed to severe sepsis in febrile acute de-compensated cirrhotic patents. Polymorphism analysis of candidate genes was undergone in 108 febrile acute de-compensated cirrhotic patients and 121 healthy volunteers. Various plasma inflammatory/regulatory cytokines, proportion of classical (CD 16-, phagocytic) and non-classical (CD16+, inflammatory) monocytes, lipopolysaccharide (LPS)-stimulated toll-like receptor 4 (TLR4) and intracellular/extracellular cytokines on cultured non-classical monocytes, mCD14/HLA-DR expression and phagocytosis of classical monocytes were measured. For TLR4+896A/G variant allele carriers with severe sepsis, high plasma endotoxin/IL-10 inhibits HLA-DR expression and impaired phagocytosis were noted in their classical monocyte. In the same group, increased non-classical monocyte subset, enhanced LPS-stimulated TLR4 expression and TNFα/nitrite production, and systemic inflammation [high plasma soluble CD14 (sCD14) and total nitric oxide (NOx) levels] were noted. For CD14-159C/T variant allele carriers with severe sepsis, persist endotoxemia inhibited mCD14/HLA-DR expression and impaired phagocytosis of their classical monocyte. In the same group, increased non-classical monocyte subset up-regulated TLR4-NFκB-iNOS and p38MAPK pathway, stimulated TNFα/nitrite production and elicited systemic inflammation. In febrile acute de-compensated cirrhotic patients, TLR4+896A/G and CD14-159C/T polymorphisms-related non-classical and classical monocytes dysfunction resulted in increased severe sepsis risk. Malnutrition, high plasma endotoxin and sCD14 levels, single TLR4+896A/G or CD14-159C/T variant allele carriers and double variant allele carriers are significant predictive factors for the development of severe sepsis among them.
Introduction
Acute de-compensated cirrhotic patients are suffered from compromise hepatic immune surveillance, impaired phagocytosis and high severe sepsis-related mortality [1]. Poor response to bacterial challenge, increased susceptibility to bacterial infection, high severe sepsis-related mortality had been reported in advanced cirrhotic patients [2,3]. Fever is the nonspecific clinical manifestations for infection that frequently elicits suspicion of sepsis. So, it is important to search accurate predictors to early identify those febrile cirrhotic patients who are at high severe sepsis risk [2,3].
In response to bacterial invasion, Toll-like receptor-4(TLR4)/MD-2 complex and its co-receptor membrane-bound CD14 (mCD14) on leukocyte recognizes lipopolysaccharide (LPS) to activate NFκB-mediated pro-inflammatory signaling and phagocytosis [4–7]. An association between TLR4 genetic polymorphism and infection susceptibility has been reported in patients with and without cirrhosis [8–10]. Two forms of CD14 had been reported with soluble molecule (sCD14) and mCD14. It had been reported that CD14-159C/T polymorphism can affect mCD14 expression, plasma sCD14 levels, increased septic shock susceptibility [11–14].
The circulating non-classical (CD16+, inflammatory) monocytes produce pro-inflammatory cytokines TNFα and IL-6 and, in vitro, after LPS stimulation. Significantly increased CD16+ monocytes proportion can be found in sepsis and cirrhotic patients [15–17]. To initiate effective phagocytosis, HLA-DR expression on monocytes are essential for the presentation of peptides derived from ingested microbes to prevent severe sepsis [1,2]. So, low HLA-DR expression has been reported as poor prognostic markers for ICU mortaility in critically ill cirrhotic patients [18].
Variant allele of TNFα -308G/A affects TNFα transcription and increase septic shock susceptibility [19]. Elevated IL1β level enhances the severity of sepsis [20]. IL-6 stimulates TNFα and IL-1β release to amplify the inflammatory reaction. Both IL-6-597G/A and IL-6-174G/C polymorphism regulated IL-6 transcription rate and plasma IL-6 level, which is elevated in septic patients [21]. High plasma IL-6 helps in detecting severe sepsis in admitted patients with the suspicion of infection [22].
In febrile acute de-compensated cirrhotic patients, current study wants to explore the potential roles of candidate genetic variants and corresponding dys-regulation in the pathogenesis of increased severe sepsis risk. The interactions between genetic variants-related downstream in vivo and in vitro serologic and immunologic pathogenic changes among cases with and without sever sepsis were assessed.
Materials and Methods
The detail description was shown in the S1 File.
Patients and clinical data
Between May 1, 2013 and March 1, 2016, 108 febrile cirrhotic patients, aged between 38 and 80 years, admitted to our hospital for the treatment of an acute de-compensation [ascites, jaundice, hepatic encephalopathy (HE), spontaneous bacterial peritonitis (SBP) variceal bleeding, and hepatorenal syndrome (HRS)] were enrolled consecutively [23]. The diagnosis of cirrhosis was based on liver biopsy results or on clinical (presence of stigma of cirrhosis including spider angioma, plamar erythema, captus medusa, ascites, varcies, splenomegaly, etc), laboratory, and ultrasonographic (including elastrography) data. Fever was defined as body temperature above 39°C or above 38.5°C measured consecutively at two occasions at least 1 h apart. With distribution (30:70) of variant and wild-type alleles frequency of all tested gene polymorphisms, we estimated the number of febrile de-compensated cirrhotic patients needed to observe differences in susceptibility of severe sepsis at least 10% (D) and with a common variance of 20 (σ) by literatures [8,10,12] With the type 1 error risk of 5% (α), power of 80% (1-β) and, Type 2 error (β), 20%; it was estimated that 104 patients would be required in total. Notably, the power (80%) calculation used was sufficient to detect 40% (70–30%) difference in the allele frequency of all tested gene polymorphisms.
Exclusion criteria were: human immunodeficiency virus infection, previous transplantation or any other type of immunodeficiency, steroid treatment, pituitary or adrenal disease, hepatocellular carcinoma, severe chronic heart (New York Heart Association function class III or IV) or pulmonary disease (global initiative for chronic obstructive lung disease III or IV), chronic dialysis, acute respiratory distress syndrome, and refusal of patient to participate. Patients gave written informed consent to participate in the study which was approved by the Institutional Review Board Taipei Veterans General Hospital, Taiwan, R.O.C. (IRB number: 201303013AC, approved on 19/April/2013).
Demographic and the baseline clinical evaluation [the Child-Pugh, and model for end stage liver disease (MELD), APACH III scores] were completed within 48 hours of hospitalization. Subjective global nutritional assessment (SGNA) score of >1 (2 to 4) was defined as malnourish [24]. All clinical parameters especially newly developed systemic inflammatory response syndrome (SIRS) [24], sepsis and severe sepsis during admission and during 3-month follow-up; in-hospital and 3-month mortality and causes of death were carefully collected after enrolled. Sepsis was diagnosed as the presence of SIRS in combination with suspected or proven infection but without any evidence of organ dysfunction or the need for intravenous vasopressor drug support to maintain blood pressure. Severe sepsis was defined as sepsis that was temporally accompanied by the need for intravenous vasopressor drug support (excluding dopamine at ≦5μg/kg/min) to maintain blood pressure (despite adequate fluid resuscitation) along with the presence of perfusion abnormalities, or metabolic acidosis (pH≦7.3) or the development of respiratory, renal, hepatic, or hematological failure. After recruitment, admitted febrile de-compensated cirrhotic patients [25] were divided into severe sepsis group and non-severe sepsis group, which including uncomplicated, SIRS and sepsis cases. Among 108 enrolled febrile de-compensated cirrhotic patients, 9 uncomplicated cases, 18 SIRS cases and 34 sepsis cases were identified as non-severe sepsis group. By contrast, 47 severe sepsis cases were identified as severe sepsis group
Retrospectively, we determined the time of the first de-compensation of cirrhosis (ascites, variceal bleeding, encephalopathy or infection) and the period from this time until the first day of the present hospitalization/time of entering current study (pre-study period). Previous incidence and total episode of infections during the pre-study period were recorded.
Additionally, age and sex-matched unrelated 121 healthy controls and 51 afebrile compensated cirrhotic patients with available blood sample for genetic analysis were included as comparison group. Healthy controls were those whose visit our hospital for health check-up without clinical or biochemical evidence of liver, renal and cardiovascular disease. The afebrile compensated cirrhotic patients were identified from ongoing studies on cirrhosis in outpatient department of our hospital.
Genetic and serologic analysis
We selected candidate SNPs (S1 Fig) within bacterial recognition [(TLR4 +896A/G, Asp299Gly substitution mutation, rs4986790; TLR4 3′UTR, G/C, rs11536889) &(CD14, promoter region,-159C/T, rs2569190; CD14, 3′UTR, C/A, rs2563298)] and inflammatory response [(TNFα -308G/A, rs1800629; -238G/A, rs361525) & (IL-1β, -31, 5′-UTR, T/C, transition, rs1143627; IL-1β, +3954C/T, rs1143634) & (IL-6, -174G/C transversion, promoter region, rs1800795; IL-6, -597G/A, rs1800797)] genes that had available polymorphic information for a Han population by using Applied Biosystems SNP browser software version 3.0 [26].
Plasma soluble CD14 levels, IL-6, IL-1β, TNFα, IL-10 and total nitric oxide (NOx, nrtrite+nitrate), and endotoxin were measured by kits purchased from Biosource (USA), R&D Systems (Minneapolis, USA) and Lonza, Walkersville (MD).
Proportion of CD16- (classical) and CD16+ (non-classical) monocyte subsets
Individual′s peripheral blood mononuclear cells (PBMC) were isolated and CD14+CD16− and CD14+CD16+cells were separated by magnetic cell sorting, using MACS isolation kits by negative selection (Miltenyi Biotec, Bergisch Gladbach, Germany) for comparison between groups.
TLR4/HLA-DR expression on monocytes
The isolated CD16− and CD16+ monocytes were double-stained with anti-CD16-PE (BD Biosciences, USA) and anti-TLR4-APC (BD Biosciences, USA) antibodies. In a dose-finding preliminary experiment, the most potent stimulation of TLR4 expression on cells analyzed by flow cytometry (FACScan, BD Biosciences) was presented at 100ng/mL of Escherichia Coli LPS (Sigma-Aldrich, St, Louis, MO). Therefore, all the following acute in vitro experiments were divided into un-stimulated and stimulated [LPS, 100ng/mL] groups. The HLA-DR expression on CD16- (classical) and CD16+ (non-classical) monocytes were measured by staining with anti-HLA-DR-APC, anti-CD14-FITC (BD Biosciences, USA) and anti-CD16-PE antibodies.
Our preliminary experiments revealed that the TLR4 expression was not different between CD16- (classical) monocytes between groups. It is well-established that classical monocyte are phagocytic with no inflammatory attributes [15–17], so the LPS-stimulated extra- and intra-cellular inflammatory cytokines production and corresponding signals were only evaluated in cultured CD16+ (non-classical) monocytes.
Extracellular and intracellular cytokine assays of CD16+ monocytes
For both stimulated LPS (100ng/mL) and un-stimulated groups, the isolated CD16+ monocytes were incubated for 20 h, cell free supernatants were harvested and analyzed for IL-6, IL-1β, and TNFα production using a commercial ELISA kit (R&D, Minneapolis, MN). Additionally, supernatants nitrite concentration was measured by the Griess reaction.
The isolated CD16+ monocytes from individual subjects were stimulated with LPS (100ng/mL) for 5h. Manensin (1.7μg/mL; Sigma-Aldrich) was added to inhibit cytokine secretion. The CD16+ cells were stained with anti-TNFα-APC (BD Biosciences, USA) or anti-iNOS-APC (LifeSpan Biosciences, USA) with istotype control for 20 min at 4°C, fixed, permeabilized washed, and analyzed by flow cytometry.
Inflammatory mRNA and protein expressions in cultured CD16+monocytes
With and without LPS (100ng/mL) stimulation, NFkB-p65, iNOS, p38MAPKα:, p38MAPKβ, ERK1 mRNA/protein expressions in isolated CD16+ monocytes were measured with primers listed in S1 Table and antibodies purchased from R&D system, Minneapolis, MN; Abcam, Cambridge, MA, UK.
Membrane-bounded CD14 (mCD14) expression on classical CD16- (phagocytic) monocytes
Both for un-stimulated and stimulated (100ng/mL of LPS) groups of isolated CD16- monocytes, mCD14 expression was analyzed using the phycoerythrin (PE)-labeled anti-CD14 antibody (R&D Systems, Minneapolis, MN, USA) [27,28].
Phagocytic ability of CD16- (phagocytic) monocytes
Both for un-stimulated and stimulated (100ng/mL of LPS) groups, CD16- monocytes (2×105/well) were incubated in five replicates with Alexa Fluor 488 (AF488)-conjugated Escherichia coli BioParticles (Invitrogen). CD16- monocytes incubated with bioiparticles were used as positive controls, while monocytes without bioparticles were used as negative controls. The phagocytic indexes of the CD16- monocytes from different individual were calculated by the following formula, Phagocytic index = [(MFI of experiment-MFI of negative controls)/MFI of positive controls-MFI of negative controls]×100%, where MFI stands for mean fluorescence intensity [29].
Statistical analysis
The significance of differences in allelic frequencies between each group was determined by Fisher’s exact test. Differences in the distribution of alleles between the groups and deviation from Hardy-Weinberg equilibrium were assessed by Pearson χ2 test and likelihood-ratio χ2 tests of independence; 2×2 tables were used to compare allele distribution between any 2 groups. Continuous variables were expressed as mean and standard deviation (SD). Student′s t-test was used to compare continuous variables from two groups. For univariate and multivariate regression analysis, the third quartile of plasma sCD14 (3.7 μg/mL) and endotoxin (>2.3 EU/mL) levels at inclusion of all febrile acute de-compensated cirrhotic patients were used as cut-off values for high-risk group of severe sepsis.
Results
Basal Characteristics
Basically, the enrolled afebrile compensated cirrhotic patients were characterized by lower Child-Pugh class, lower MELD score, relative normal serum sodium concentration, higher serum albumin, lower serum bilirubin and less prothrombin time prolongation than febrile de-compensated cirrhotic patients (S2 Table). At inclusion, cases complicated with severe sepsis had significantly higher plasma sCD14, IL-10, NOx levels, higher proportion of moderate/massive ascites, lower serum albumin and more episodes of overall previous infection per patients than those non-severe sepsis cases (S2 Table and S1F Fig). Moreover, the APACH (acute physiologic and chronic health) III score (21.3±7.4 vs. 20.6±7.6), the SGNA (subjective global nutritional assessment) score (2.8±0.5 vs. 2.3±1.1), percentages of using beta-blockers/diuretics (60/64% vs. 65/50%), using oral antibiotics prophylaxis for SBP recurrence at inclusion (24% vs. 21%), percentage of previous ascite/variceal bleeding/SBP/encephalopathy (33/80/33/47% vs. 40/83/38/56%), causes of current admission [ascites/jaundice/encephalopathy/variceal bleeding/SBP/HRS (25/28/14/27/4/2% vs. 22/32/12/24/6/4%)], duration (months) from first de-compensation to entering study, serum bilirubin, prothrombin time prolongation,8plasma CRP level (3.6±0.8 vs. 2.9±1.7mg/dL) and plasma TNFα/IL-6/IL-1β levels were not different between febrile de-compensated cirrhotic patients with and without severe sepsis (S2 Table, S1G, S2A and S2C Figs).
Distribution of variant allele of candidate SNPs between groups
In comparison with healthy controls, higher frequencies of TLR4+896A/G and CD14-159C/T variant alleles were noted among severe sepsis and non-severe sepsis febrile de-compensated cirrhotic patients as well as afebrile compensated cirrhotic patients (Table 1 and S3 Table). Higher frequencies of TLR4+896A/G and CD14-159C/T variant alleles were noted in febrile de-compensated cases than afebrile compensated cases (Table 1). In comparison with non-severe sepsis cases, higher frequencies of TLR4+896A/G and CD14-159C/T variant alleles were noted in severe sepsis cases. However, the percentage of carrying variant alleles of the following SNPs including TLR4 3′UTR, G/C; CD14 ′UTR, C/A; TNFα -238G/A; IL-1β -31T/C; IL-1β +3954C/T; IL-6 -174G/C; IL-6 -597G/A were not different among healthy controls, severe sepsis febrile de-compensated cirrhotic patients, non-severe sepsis febrile de-compensated cirrhotic patients and afebrile compensated cirrhotic patients (Table 1 and S3 Table).
Table 1. Comparison of distribution (%) of variant allele carriers of candidate SNPs among groups.
| Severe sepsis cases (n = 47) | Non-severe sepsis cases (n = 61) | Significance (severe vs. non-severe sepsis cases) | Febrile de-compensated cirrhotic patients (n = 108) | Afebrile compensated cirrhotic patients (n = 51) | Significance (febrile vs. afebrile cases) | Healthy controls (n = 121) | Significance (healthy controls vs. afebrile cases) | |
|---|---|---|---|---|---|---|---|---|
| TLR4 +896A/G | 16 (34%) ##,** | 15(25%) # | <0.001 | 31(29%) ‡ | 10(20%) | 0.018 | 17(14%) ‡ | 0.04 |
| G–allele carriers | 3.7 [0.23–4.25] | 2.13 [1.302–4.908] | 2.05 [1.34–5.17] | |||||
| TLR4 3′UTR, G/C | 10(21%) | 12(20%) | 0.28 | 22(20%) | 8(16%) | 0.15 | 13(11%) | 0.08 |
| C–allele carriers | 1.89 [0.65–5.96] | 1.28 [0.49–3.82] | 1.23 [0.57–2.69] | |||||
| CD14 -159C/T | 18 (38%) ##,** | 15(25%) # | <0.001 | 33(31%) ‡ | 12(24%) | 0.029 | 21(17%) ‡ | 0.032 |
| T-allele carriers | 2.7 [1.1–3.9] | 1.98 [1.234–8.214] | 1.75 [1.07–4.23] | |||||
| CD14 3′UTR,C/A | 8(17%) | 9(15%) | 0.15 | 17(16%) | 7(14%) | 0.36 | 13(11%) | 0.19 |
| A-allele carriers | 1.47 [0.94–2.3] | 0.89 [0.22–3.36] | 1.06 [0.78–1.43] | |||||
| TNFα -308G/A | 14 (30%) # | 16 (26%) | 0.305 | 30(28%) | 14(27%) | 0.31 | 28(23%) | 0.166 |
| A-allele carriers | 1.01 [099–1.028] | 1.16 [1.25–5.13] | 1.27 [0.94–1.73] |
Cases: de-compensated cirrhotic patients; categorical variables were expressed as case number and percentage [%, case No. of variant allele carrier/variant+wild-type allele carriers] of variant alleles carriers; the unlisted case No. (%) of corresponding wild-type allele carriers were case number in different groups minus variant allele carriers {100-[% of variant allele carriers]}; severe sepsis/non-severe sepsis cases: febrile de-compensated cirrhotic patients with severe sepsis/without severe sepsis
#P <0.01 &
##P <0.001 vs. healthy controls
*P <0.01 &
**P <0.001 vs. non-severe sepsis cases.
‡P<0.05 vs. afebrile compensated cirrhotic patients; Descriptive significance between groups were showed as P-value [odd ratio, OR (95% confidence interval, CI)].
Characteristics of cases with different genotypes
Significantly, more overall previous episodes of infection (S1F Fig), higher proportion of previous SBP episodes, and severe sepsis cases were observed in TLR4+896A/G and CD14-159C/T variant allele carriers than wild-type carriers (Table 2). Among TLR4+896A/G and CD14-159C/T variant allele carriers, significantly higher plasma sCD14 and NOx levels were noted than their comparative groups. Plasma IL-10 level was significantly higher in TLR4+896A/G variant allele carrier than their controls but similar between variant and wild-type CD14-159C/T allele carriers (Table 2).
Table 2. Characteristics of febrile acute de-compensated cirrhotic patients with variant/wild-type allele carriers (variant/wild-type).
| Different carrier of TLR4 +896A/G polymorphism | variant allele (n = 22) | wild-type allele (n = 86) | P values |
| Gender (male/female) ratio | 15/7 (68/32%) | 62/24 (72/28%) | 0.35 |
| Child-Pugh class (A/B+C) (%) | 5/17(13/77%) | 19/67(22/78%) | 0.48 |
| Severity of ascites (no+mild/moderate+massive)(%) | 9/13(41/59%) | 40/46(47/53%) | 0.65 |
| Model of end stage liver disease (MELD) score | 17.9± 6.4 | 19.3± 4.1 | 0.33 |
| Acute physiologic and chronic health (APACH) III score | 22.1 ±5.9 | 19.8± 6.5 | 0.24 |
| [C-reactive protein, (CRP)] mg/dL | 4.1±1.1 | 3.9 ±1.7 | 0.41 |
| [sCD14] μg/mL | 6.9±0.5* | 3.7±0.4 | 0.002 |
| [IL-10] pg/mL | 17.5 ±2.2* | 9.7±1.4 | 0.01 |
| [total nitric oxide (NOx)] μM | 38.5± 3.5* | 19.3 ±8.7 | 0.005 |
| Previous spontaneous bacterial peritonitis (SBP) | 10(45%)** | 29(34%) | <0.001 |
| Percentage of cases complicated with severe sepsis (%) | 13(59%)* | 27(31%) | 0.005 |
| Different carrier of CD14 -159C/T polymorphism | variant allele (n = 33) | wild-type allele (n = 75) | |
| Gender (male/female) ratio | 22/11(67/33%) | 55/20(73/27%) | 0.95 |
| Child-Pugh class (A/B+C) (%) | 5/28(15/85%) | 19/56(25/75%) | 0.69 |
| Ascites (no+mild/moderate+massive)(%) | 13/20(39/61%) | 36/39(48/52%) | 0.38 |
| MELD score | 18.3± 2.5 | 18.4± 5.6 | 0.52 |
| APACH III score | 22.3 ±5.4 | 24.1±6.3 | 0.29 |
| [CRP] mg/dL | 5.7±1.9 | 4.6 ±2.1 | 0.26 |
| [sCD14] μg/mL | 4.7±0.8* | 1.5±0.4 | 0.003 |
| [IL-10] pg/mL | 16.8 ±1.3 | 14.3±5.7 | 0.15 |
| [NOx] μM | 37.5 ±2.9* | 21.4 ±6.8 | 0.012 |
| Previous SBP | 13(39%)** | 21(28%) | <0.001 |
| Percentage of cases complicated with severe sepsis (%) | 14(42%)* | 23(31%) | 0.026 |
Data are mean±SD; categorical variables were expressed as case number [percentage (%) of frequency]
*P <0.01
**P <0.001 vs. TLR4 +896 A/G&CD14 -159C/T, wild-type allele carriers.
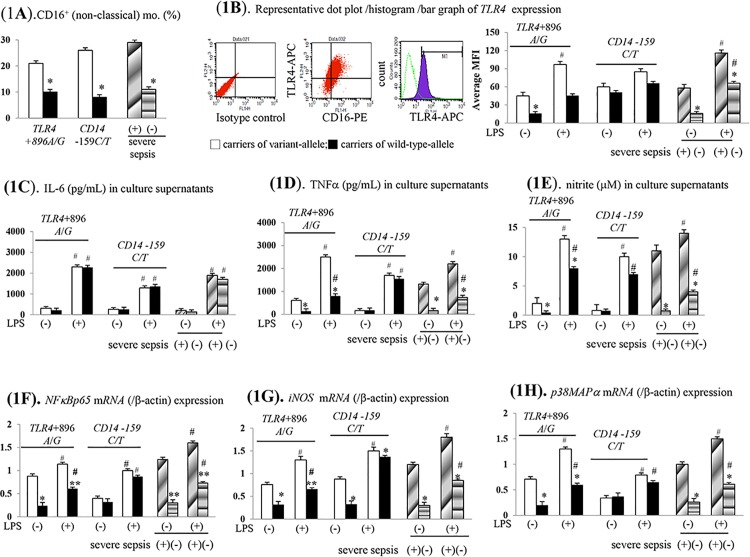
Increased CD16+ (non-classical) monocyte subset was associated with high TLR4/TNFα/iNOS expression and extracellular TNFα/nitrite production
The baseline and LPS-stimulated TLR4 expression on the CD16- (classical) monocytes were not different between variant and wild-type allele carriers of TLR4+896A/G or CD14-159C/T as well as between non-severe sepsis and severe sepsis cases (data not shown).
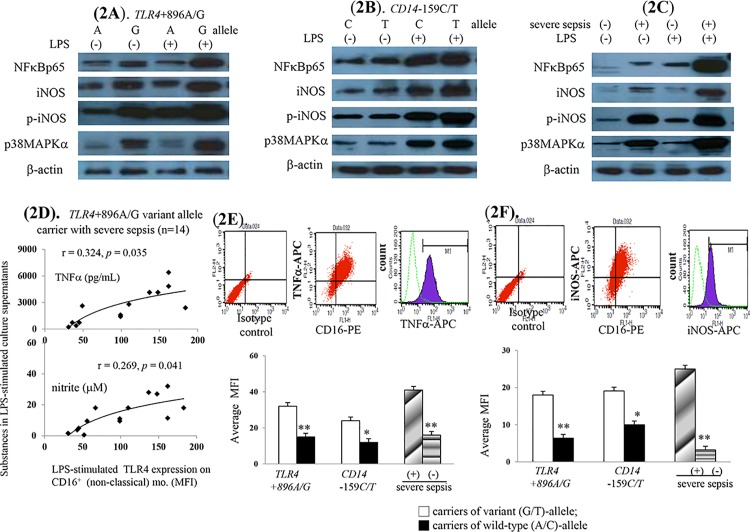
Significantly, increased proportion of CD16+ monocyte subsets were observed in TLR4+896A/G or CD14-159C/T variant allele carriers and severe sepsis cases (Fig 1A). In supernatant on cultured CD16+ monocytes of variant TLR4+896A/G allele carriers and severe sepsis cases, significantly higher baseline and LPS-stimulated TLR4 over-expression was accompanied by TNFα and nitrite over-production (Fig 1B & 1D & 1E). Corresponding to the enhanced LPS-stimulated TNFα/nitrite production, the significantly up-regulated NFκBp65, p-iNOS and p38MAPα mRNA and protein expressions were found on cultured monocyte of TLR4+896A/G variant allele carriers and severe sepsis cases (Fig 1F–1H, Fig 2A–2C).
Fig 1. Various LPS-stimulated pro-inflammatory profiles on CD16+ (non-classical, inflammatory) monocytes of different cases.
(A). percentage (%) of CD16+ monocyte subset; (B). representative flow cytometric dot plots/histograms of TLR4 expression (10000 counts); (C-E). LPS-stimulated extracellular production of various substances. (F-H). various mRNA expressions; *or** P < 0.05 or 0.01 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases; # P <0.05 vs. un-stimulated groups; MFI: mean fluorescence intensity.
Fig 2. Contribution of increased CD16+ (non-classical, inflammatory) monocytes subsets on the increased severe sepsis risk among TLR4+896A/G or CD14-159C/T variant alleles carriers.
(A-C). LPS-stimulated various protein expressions on CD16+ monocytes of all cases; (D). positive correlation between LPS-stimulated TLR4 expression and TNFα/nitrite production; (E,F). increased LPS-stimulated intracellular TNFα/iNOS levels. *or** P < 0.05 or P < 0.001 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases.
In variant TLR4+896 allele carriers with severe sepsis, a significantly positive correlation was noted between LPS-stimulated TLR4 expression and corresponding TNFα/nitrite production (Fig 2D). However, above correlation was loss among variant CD14-159C/T allele carriers with severe sepsis (S3B Fig). In line with increased extracellular LPS-stimulated TNFα/nitrite levels, significantly higher intracellular TNFα/iNOS level confirm the cellular sources of TNFα/nitrite from circulating CD16+ monocytes of these cases (Fig 2E & 2F).
Compared to un-stimulated states, higher LPS-stimulated IL-6/IL-1β levels in CD16+ monocytes supernatant was observed among all cases. Nonetheless, the magnitudes of these LPS-stimulated changes were not different between variant and wild-type TLR4+896A/G or CD14-159C/T allele carriers as well as between non-severe sepsis and severe sepsis cases (Fig 1C, S2D Fig).
Significantly, acute LPS incubation stimulated the TNFα, iNOS, TNFα/nitrite, NFκBp65, iNOS and p38MAPα in CD16+ monocytes of both variant and wild-type CD14-159C/T allele carriers (Fig 1B & 1D–1H). Nonetheless, the baseline and LPS-stimulated above mention signals were not different between variant and wild-type CD14-159C/T allele carriers (Fig 1B & 1D–1H).
Down-regulated HLA-DR expression on monocytes increase severe sepsis risk
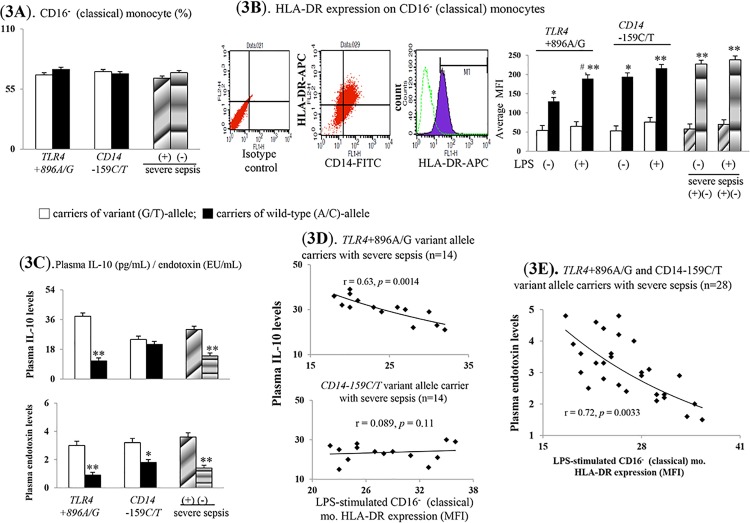
Fig 3A observed that the CD16- monocyte subset proportions were not different between cases carrying TLR4+896A/G or CD14-159C/T variant allele and severe sepsis. Both on the CD16- and CD16+ monocyte, the significantly lower baseline and LPS-stimulated HLA-DR expression were observed in cases carrying variant TLR4+896A/G or CD14-159C/T allele carriers and severe sepsis (Fig 3B, S3A Fig).
Fig 3. Profound pro-inflammatory responses are accompanied by CD16- classical (phagocytic) monocyte-related abnormalities.
(A). percentage (%) of CD16- monocyte subset; (B). surface HLA-DR expression on CD16- monocyte; (C). plasma endotoxin and IL-10 levels; correlation between LPS-stimulated surface HLA-DR expression on CD16- monocyte and plasma IL-10 (D)/endotoxin (E) levels. *or** P < 0.05 or P < 0.001 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases. # P <0.05 vs. un-stimulated groups.
Significantly, higher plasma endotoxin and IL-10 levels were observed in cases carrying variant TLR4+896A/G allele and severe sepsis (Fig 3C). Among variant CD14-159C/T allele carriers, higher plasma endotoxin levels rather than plasma IL-10 levels were noted than wild-type carriers.
Among TLR4+896A/G variant allele carriers with severe sepsis, a significant negative correlation was noted between plasma IL-10 levels and LPS-stimulated HLA-DR expression on CD16- monocyte (Fig 3D). Among both TLR4+896A/G and CD14-159C/T variant allele carriers with severe sepsis, a significant negative correlation was found between plasma endotoxin level and LPS-stimulated HLA-DR expression on CD16- monocyte (Fig 3E).
Decreased mCD14 density on CD16- (classical) monocyte
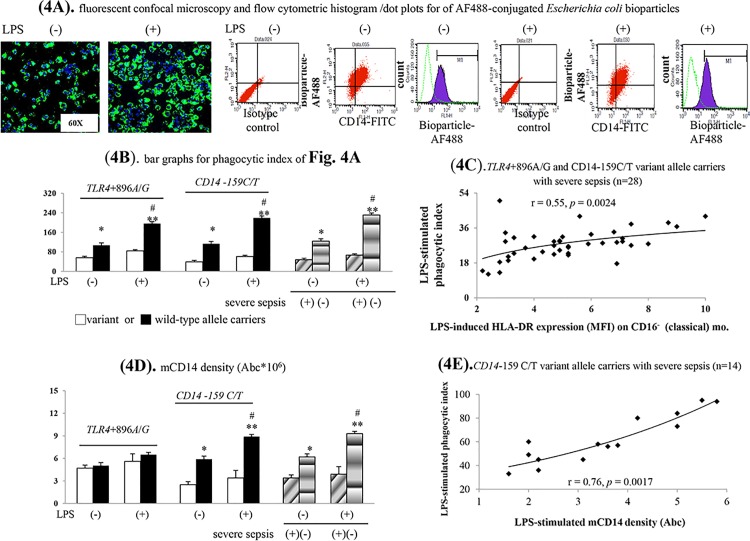
Among cultured CD16- monocytes collected from cases carrying wild-type TLR4+896A/G or CD14-159C/T allele and non-severe sepsis, acute LPS incubation significantly stimulated HLA-DR/mCD14 expressions and phagocytosis (Figs 3B, 4B & 4D). Nonetheless, above mention phenomena was disappeared among CD16- monocytes collected from cases carrying variant TLR4+896A/G or CD14-159C/T allele and severe sepsis.
Fig 4. Various phagocytic profiles of CD16- (classical, phagocytic) monocyte of different cases.
(A).representative photomicrographs/flow cytometric histogram/dot plots and (B) bar graph for phagocytic index; correlation between LPS-stimulated surface HLA-DR expression on CD16- monocyte (C)/mCD14 density (E) and phagocytic index; (D). LPS-stimulated membrane bound-CD14 densities. Abc: antigen binding capacity; * P < 0.05 or ** P < 0.001 vs. TLR4/CD14 genes variant alleles carriers/severe sepsis cases; # P <0.05 vs. un-stimulated groups.
The phagocytic abilities of CD16- monocyte were significantly decreased among cases carrying variant TLR4+896A/G or CD14-159C/T allele and severe sepsis (Fig 4A & 4B). Notably, the magnitude of LPS-stimulated HLA-DR expression determined the phagocytosis of CD16- monocyte in TLR4+896A/G and CD14-159C/T variant allele carriers with severe sepsis (Fig 4C).
Additionally, the mCD14 density was remarkably decreased on CD16- monocyte of CD14-159C/T variant allele carriers and severe sepsis cases (Fig 4D). Further analysis indicated that there was a positive correlation between LPS-stimulated mCD14 densities and phagocytic index on CD16- monocyte in CD14-159C/T variant allele carriers with severe sepsis (Fig 4E). By contrast, there were no correlation between LPS-stimulated mCD14 densities and phagocytic index on CD16- monocyte in TLR4+896A/G variant allele carriers with severe sepsis (S3C Fig).
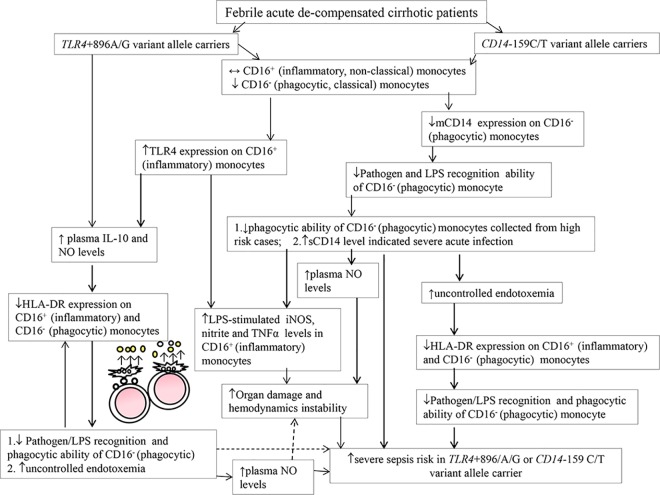
Summative mechanisms for severe sepsis risk in cases (Fig 5)
Fig 5. Schematic representation of key different findings compared to corresponding wild-type allele carriers for the mechanisms of increase severe sepsis risk in febrile acute de-compensated cirrhotic patients carrying TLR4+896/A/G or CD14-159C/T variant alleles in current study.
TLR4: gene of toll-like receptor 4; LPS: lipopolysaccharide.
Both TLR4+896A/G and CD14-159C/T variant allele carriers shared common increased CD16+ (non-classical, inflammatory) monocyte subset–related mechanisms for increased severe sepsis risk (Figs 1B, 2D–2F). The up-regulated TLR4 expression-increased TNFα/iNOS/nitrite levels and subsequently systemic acute inflammatory status (high plasma sCD14 and total NO levels) resulted in severe sepsis among them (Figs 1 & 2, S2 Table, Table 2). High plasma endotoxin/IL-10-related HLA-DR down-regulation and impaired phagocytosis of CD16- (classical, phagocytic) monocytes also resulted in increased severe sepsis risk among TLR4+896A/G and CD14-159C/T variant allele carriers (Figs 3, 4A & 4B). Further, low mCD14 density on CD16- (phagocytic) monocytes contributed to increased severe sepsis risk in CD14-159C/T variant allele carriers (Figs 3 & 4).
Predictors for severe sepsis
Among all cases, 59% of TLR4+896A/G variant allele carriers complicated with severe sepsis whereas 43% of CD14-159C/T variant allele carriers complicated with severe sepsis (Table 2).
In univariate analysis, the odd ratio for predicting severe sepsis among TLR4+896A/G variant allele carriers was 2.41 whereas 1.82 for CD14-159C/T variant allele carriers (Table 3). Additionally, 13% of them were double TLR4+896A/G and CD14-159C/T variant allele carriers. Accordingly, higher percentage (69%) of double TLR+896A/G and CD14-159C/T variant allele carriers were complicated with severe sepsis compared to either single TLR+896A/G or CD14-159C/T variant alleles carriers (59% or43%). In multivariate analysis, malnutrition (SGNA score>1), high plasma sCD14 level (>3.7 μg/mL), high endotoxin level (>2.3 EU/mL), single or double TLR4 +896A/G or CD14 -159C/T variant alleles carriers were associated with increased severe sepsis risk in febrile de-compensated cirrhotic patients (Table 3).
Table 3. Regression analysis of predictive factors for complicated with severe sepsis in febrile de-compensated cirrhotic patients.
| Parameter at inclusion | Odd Ratio(95% CI) | P values | |
|---|---|---|---|
| (A). Univariate analysis | |||
| Child-Pugh class B+C | 1.23(0.41–2.16) | 0.81 | |
| Moderate+massive ascites | 1.18 (0.82–1.75) | 0.53 | |
| Model of end stage liver disease (MELD) score | 0.943(0.36–1.89) | 0.25 | |
| Plasma acute C-reactive protein level (CRP, mg/dL) | 0.67(0.94–4.67) | 0.79 | |
| Acute physiologic and chronic health (APACH) III score | 1.58(1.04–2.24) | 0.23 | |
| High [sCD14] (>3.7μg/mL) | 1.73 (0.42–2.13) | 0.007 | |
| High [endotoxin] (>2.3 EU/mL) | 1.36 (1.08–2.3) | 0.014 | |
| Subjective global nutritional assessment (SGNA) score>1 | 2.688(0.244–2.87) | 0.029 | |
| Previous spontaneous bacterial peritonitis (SBP) | 0.842(1.18–2.34) | 0.45 | |
| TLR4 +896 A/G, variant(G)–allele carriers | 2.41 (0.275–2.71) | 0.02 | |
| CD14–159 C/T, variant ((T)-allele carriers | 1.82(0.783–2.51) | 0.01 | |
| Double TLR4 +896A/G and CD14 -159C/T variant–alleles carriers | 4.37 (0.25–1.27) | 0.0039 | |
| (B). Multivariate analysis | |||
| Subjective global nutritional assessment (SGNA) score>1 | 3.714(0.737–4.123) | 0.0014 | |
| High [sCD14] (>3.7μg/mL) | 2.198(0.045–2.65) | 0.006 | |
| High [endotoxin] (>2.3 EU/mL) | 3.105(0.89–3.142) | 0.0032 | |
| TLR4 +896 A/G, variant ((G)–allele carriers | 2.967(0.593–5.123) | 0.004 | |
| CD14–159 C/T, variant ((T)-allele carriers | 3.11(0.71–5.2) | 0.001 | |
| Double TLR4 +896A/G and CD14 -159C/T variant–alleles carriers | 5.44(0.065–3.1) | 0.002 | |
SGNA score of >1 (2 to 4) indicated malnutrition; cut-off values for high plasma IL-10 and endotoxin levels were defined as greater than third quartile of data of severe sepsis and non-severe sepsis case.
Discussion
Early innate immune responses to LPS are critical for determining resistance to bacterial infection; the same responses are important driving forces behind the pathophysiology of sepsis in infected individuals. Unregulated responses to bacterial agonists can cause immune-suppression, which may lead to secondary infections, or to over-stimulated inflammatory responses that can cause severe sepsis, shock, and death in cirrhosis and non-cirrhosis [5–7,30–32].
Guarner-Argente et al. reported a trend towards a higher incidence of bacterial infections in de-compensated cirrhotic patients whose carrying TLR4 D299G variant genotype compared to wild-type carriers [10]. Appenrodt et al. has demonstrated an association between NOD2 (nucleotide-binding oligomerization domain containing 2) variants and the SBP-related mortality in de-compensated cirrhotic patients [33]. However, the roles and immunologic mechanisms of genetic polymorphism on the increased severe sepsis risk had never been explored in febrile acute de-compensated cirrhotic patients.
Thoughtfully, the correlation among the plasma sCD14/endotoxin/NOx levels and various immune regulatory effectors (LPS-stimulated TLR4, mCD14, NFκBp65 and iNOS expression and corresponding intracellular and extracellular IL-6, TNFα, nitrite levels on cultured classical and non-classical monocytes) activities were surveyed in our current study. Notably, current study revealed that variant alleles of TLR4+896A/G and CD14-159C/T modulate immunologic protein abundance (increase plasma sCD14 levels/up-regulated TLR4 expression on non-classical inflammatory monocyte and down-regulated HLA-DR and mCD14 expression on classical monocyte) and function (impaired phagocytosis of classical monocytes) in our febrile acute de-compensated cirrhotic patients with severe sepsis.
It was suggested that chronic endotoxemia is associated with increased plasma IL-10 levels in cirrhotic patients [30,34]. In de-compensated cirrhotic patients, high IL-10 levels had been reported to be associated with decreased monocyte phagocytic ability [1,9,30]. In line with previous studies, carriers of variant allele of TLR4 +896A/G in our febrile acute de-compensated cirrhotic patients did show increased LPS-stimulated IL-10 production and complicated with higher frequency (59%) of severe sepsis compared to wild-type allele carriers (32%).
For host protection from pathogen, the LPS-stimulated NFκB/MAP kinases and TNF-α release depend on the up-regulation of TLR4 expression on activated monocytes [4,5,9]. Sequentially, the up-regulated NFκB-MAP kinases, over-produced TNF-α and iNOS-derived NO (nitrite) involved in the development of severe sepsis and septic shock [35–38]. Not surprisingly, in our TLR4+896A/G and CD14-159C/T variant alleles carriers with severe sepsis, increased TLR4 expression was accompanied by the up-regulation of in vitro LPS-stimulated NFκB, TNF-α, and iNOS signals on their cultured non-classical (CD16+, inflammatory) monocytes.
Membrane bound CD14 (mCD14) are pattern recognition receptors generate early innate immune response against bacterial pathogens. Decreased mCD14 expression on activated classical (phagocytic) monocyte may decrease the pathogen elimination ability of individuals. Previous study had revealed elevated levels of sCD14 in patients with sepsis [39]. Additionally, low monocyte mCD14 and high plasma sCD14 level can predict 28-day mortality in patients with community acquired infections [27,28]. Notably, in our febrile acute de-compensated cirrhotic patients with severe sepsis, low mD14 expression is characterized by the decreased LPS-stimulated phagocytic ability of classical monocyte, whereas high plasma sCD14 level indicated uncontrolled sepsis among CD14-159C/T variant allele carriers.
Reduced monocyte HLA-DR expression has been reported in patients with septic de-compensation of acute-on-chronic liver failure [30]. In advanced cirrhotic patients, high plasma IL-10 levels can negatively regulated their HLA-DR expression on monocytes [30]. In our TLR4+896A/G variant allele carriers with severe sepsis, a negative correlation was noted between plasma IL-10 and LPS-stimulated CD16- (classical) monocyte HLA-DR expression. Due to persistent endotoxemia, acute de-compensated cirrhotic patients were suffered from the down-regulation of monocyte HLA-DR expression and immune paralysis [1,30].
Accordingly, it was reasonable to observe a significant negative correlation between plasma endotoxin levels and LPS-stimulated CD16- (classical) monocyte HLA-DR expression in our TLR4+896A/G or CD14-159C/T variant allele carriers with severe sepsis. It had been reported that iNOS-derived NO can down-regulate HLA-DR expression and suppress systemic monocyte activation [40]. In our TLR4+896A/G or CD14-159C/T variant allele carriers with severe sepsis, the significantly higher LPS-stimulated iNOS expression and nitrite production on their cultured non-classical (inflammatory) monocytes and significantly higher plasma NOx levels compared to their control groups were observed.
Our study revealed that LPS-stimulated IL-1β/IL-6 production in cultured supernatant of non-classical monocyte and plasma IL-1β/IL-6 levels were not significantly different between our TLR4+896A/G or CD14-159C/T variant and wild-type allele carriers as well as between severe sepsis and non-severe sepsis cases. Actually, recent meta-analysis reported a lack of association between IL-6 -174G/C polymorphism and sepsis risk [41]. In our study, the lack of association between the TNFα-308G/A, TNFα-238G/A, IL-6-174G/C, IL-6-597G/A, IL-1β-31T/C and +3954C/T polymorphisms and severe sepsis risk suggested that these inflammatory cytokines might influence sepsis progression via mechanisms other than regulations by these polymorphisms. Probably, the phenotypic heterogeneity of the sepsis syndrome including the infection location, and the amount of time passed since the onset of infection, as well as other individual parameters including background and environmental factors, contributed to some dis-concordance with previous reports.
Serum CRP level, MELD score and APACH score, were well known as a predictor of severity of inflammation/disease. However, the parameters revealed non-significance to predict severe sepsis in current study. In fact, the study about the predictor for severe sepsis in febrile de-compensated cirrhotic patients is limited. It had been suggested that the concentration of lipopolysaccharide binding protein (LBP) is associated inversely with disease severity scores and outcomes in critically ill cirrhotic patients with severe sepsis [42]. Another study reported that serum LPB predicts severe bacterial infection in cirrhotic patients with ascites [43]. In non-cirrhotic patients, serum C-reactive protein (CRP) level had been reported as the risk factor for severe sepsis in children with cancer and febrile neutropenia [44]. Nonetheless, CRP is primarily produced in the liver and its specificity as diagnostic and prognostic tool for infection is limited in cirrhotic patients with compromised liver function, which will underestimate the severity of acute infection. MELD score is a representative maker for cirrhosis severity as well as criteria in donor liver allocation systems. In liver transplant recipients, MELD score >23 is reported to predict ICU stay >10 days without modify survival [45]. The APACHE is ICU-specific prognostic scores. In cirrhotic patients admitted to ICU, MELD score predict short mortality better than APACH scores [46]. In our admitted febrile de-compensated cirrhotic patients, the lack of roles of serum CRP level, MELD score and APACH score in prediction svere sepsis might be due to different studied population contrast to other non-cirrhotic critically ill patients.
Using multi-modalities comprehensive approaches, our data showed the contribution on the TLR4+896A/G and CD14-159C/T polymorphism-related immune dysfunction including increased non-classical (inflammatory) monocyte proportion-related LPS hyper-inflammatory response and decreased classical (phagocytic) monocyte proportion-related impaired phagocytosis in febrile acute de-compensated cirrhotic patients complicated with severe sepsis. In addition to regular predictive factors such as malnutrition, high plasma endotoxin and sCD14 levels for development of severe sepsis in high risk groups, the novel predictive factors were either single or double TLR4+896A/G and CD14-159C/T variant alleles carriers observed in current study. Accordingly, treatment strategies should change from uniform management to rapid stratifications and sub-categorization, with subsequent aggressive targeted therapeutic intervention in those most at risk.
Supporting Information
(A-E) Schematic representative SNPs that explored within various cytokines genes in current study; the comparison of (F) previous episode of infection during the pre-study period and (G) duration of first de-compensation to entering study (pre-study period) between cases with and without severe sepsis. * P < 0.05 or ** P < 0.001 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases. Pre-study period: first de-compensation of cirrhosis and the period from this time until the first day of the present hospitalization/time of entering current study.
(TIF)
(A-C). Plasma TNFα, IL-6 and IL-1β levels of all cases; (D). LPS-stimulated IL-1β production; (E,F). LPS-stimulated p38MAPβ and ERK1 mRNA expression on CD16+ (non-classical) monocytes of all cases; #p<0.05 vs. un-stimulated group.
(TIF)
(A). surface HLA-DR expression on CD16+ monocyte; (B).correlation between LPS-stimulated surface HLA-DR expression on CD16- monocyte and levels of TNFα/nitrite in the LPS-stimulated on the culture supernatant in CD14-159C/T variant allele carrier with severe sepsis. (C). correlation between LPS-stimulated mCD14+ density (Abc) on CD16- (classical) monocyte and LPS-stimulated phagocytic index TLR4+896A/G variant allele carriers with severe sepsis. * P < 0.05 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases; #p<0.05 vs. un-stimulated group.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We especially thank Yun-Ru Wang, Che-Rui Chang, Fan-Yi Jhan and Yan-Ling Lin for their excellent technical supports.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Science Council (grant: 105-2314-B-010-024-MY3) and the Taipei Veterans General Hospital (grants: V104-023, V105C-010, VGHUST105-G1-2-2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Albillos A, Lario M, Álvarez-Mon M. (2014) Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J Hepatol 61,1385–1396. 10.1016/j.jhep.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 2.Gustor T, Durand F, Lebrec D, Vincent JL, Moreau R. (2009) Severe sepsis in cirrhosis. Hepatology 50:2022–2033. 10.1002/hep.23264 [DOI] [PubMed] [Google Scholar]
- 3.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, et al. (2010) Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology 139:1246–1256. 10.1053/j.gastro.2010.06.019 [DOI] [PubMed] [Google Scholar]
- 4.Triantfilou M, Triantafilou K. (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation clusters. Trends Immunol 23:301–304. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KV (2000). Toll-signalling pathways in the innate immune response. Curr Opin Immunol 12: 13–19. [DOI] [PubMed] [Google Scholar]
- 6.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. (2006) Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue. Shock 26: 430–437. 10.1097/01.shk.0000228797.41044.08 [DOI] [PubMed] [Google Scholar]
- 7.Beutler B. (2002) TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol 270:109–120. [DOI] [PubMed] [Google Scholar]
- 8.Lorenz E, Mira JP, Frees KL, Schwartz DA. (2002) Relevance of mutations in the TLR4 receptor in patients with gram-negative septic shock. Arch Intern Med 162(9): 1028–1032. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Wu W, Yang Y, Yang Q, Song G, Wu Y, et al. (2015) Decreased Tim-3 expression is associated with functional abnormalities of monocytes in de-compensated cirrhosis without overt bacterial infection. J Hepatol 63: 60–67. 10.1016/j.jhep.2015.02.020 [DOI] [PubMed] [Google Scholar]
- 10.Guarner-Argente C, Sanchex E, Román E, Concepción M, Poca M, et al. (2010) Toll-like receptor 4 D299G polymorphism and the incidence of infection in cirrhotic patients. Aliment Pharmacol Ther 31: 1192–1199. 10.1111/j.1365-2036.2010.04291.x [DOI] [PubMed] [Google Scholar]
- 11.Rosas-Tarac AG, Revol A, Salinas-Carmona MC, Rendon A, Caballero-Olin G, Arce-Mendoza AY. (2007) CD14 C(-159)T polymorphism is a risk factor for development of pulmonary tuberculosis. J Infect Dis 196(11): 1698–1706. 10.1086/522147 [DOI] [PubMed] [Google Scholar]
- 12.Gibot S, Cariou A, Drouet L, Rossignol M, Ripoll L. (2002) Association between a genomic polymorphism within the CD14 locus and septic shock susceptibility and mortality rate. Crit Care Med .30(5): 969–973. [DOI] [PubMed] [Google Scholar]
- 13.Levan TD, Michel O, Dentener M, Thorn J, Vertongen F, Beijer L, et al. (2008) Association between CD14 polymorphisms and serum soluble CD14 levels: effect of atopy and endotoxin inhalation. J Allergy Clin Immunol 121(2): 434–440. 10.1016/j.jaci.2007.08.050 [DOI] [PubMed] [Google Scholar]
- 14.Masson S, Caironi P, Spanuth E, Thomae R, Panigada M, Sangiorgi G, et al. (2014) Presepsin (soluble CD14 subtype) and procalcitonin levels for mortality prediction in sepsis: data from the Albumin Italian Outcome Sepsis trial. Crit Care 18(1): R6 10.1186/cc13183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ziegler-Heitbrock L. (2007) The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol 81(3): 584–592. 10.1189/jlb.0806510 [DOI] [PubMed] [Google Scholar]
- 16.Liaskou E, Zimmermann HW, Li KK, Oo YH, Suresh S, Stamataki Z, et al. (2013) Monocyte subsets in human liver disease show distinct phenotypic and functional characteristics. Hepatology 57(1): 385–398. 10.1002/hep.26016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, et al. (2010) Functional contribution of elevated circulating and hepatic non-classical CD14+CD16+ monocytes to inflammation and human liver fibrosis. PLoS One 5(6): e11049 10.1371/journal.pone.0011049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, et al. (2009) Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int 29(4): 536–543. 10.1111/j.1478-3231.2008.01870.x [DOI] [PubMed] [Google Scholar]
- 19.Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, et al. (1999) Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA 282: 561–568. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt JH, Copeland EM 3rd, Moldawer LL. (1995) Interleukin-1 and interleukin-1 antagonism in sepsis, systemic inflammatory response syndrome, and septic shock. Shock 3(4): 235–251. [DOI] [PubMed] [Google Scholar]
- 21.Terry CF, Loukaci V, Green FR. (2000) Cooperative influence of genetic polymorphisms on interleukin-6 transcriptional regulation. J Biol Chem 275: 18138–1844. 10.1074/jbc.M000379200 [DOI] [PubMed] [Google Scholar]
- 22.Uusitalo-Seppala R, Koskinen P, Leino A, Peuravuori H, Vahlberg T, Rintala EM. (2011) Early detection of severe sepsis in the emergency room: diagnostic value of plasma C-reactive protein, procalcitonin, and interleukin-6. Scand J Infect Dis 43: 883–890. 10.3109/00365548.2011.600325 [DOI] [PubMed] [Google Scholar]
- 23.Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. (2013) Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute de-compensation of cirrhosis. Gastroenterology 144: 1426–1437. 10.1053/j.gastro.2013.02.042 [DOI] [PubMed] [Google Scholar]
- 24.Gatta A, Amodio P, Merkel C, Merkel C, Di Pascoli L, Boffo G, et al. (2001) Nutrition and survival in patients with liver cirrhosis. Nutrition 17(6): 445–450. [DOI] [PubMed] [Google Scholar]
- 25.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference. (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864–874. [PubMed] [Google Scholar]
- 26.International HapMap Consortium. (2003) The international Hap-Mao project. Nature 426: 789–796. 10.1038/nature02168 [DOI] [PubMed] [Google Scholar]
- 27.Repo H. (2005) Peripheral blood phagocyte CD14 and CD11b expression on admission to hospital in relation to mortality among patients with community-acquired infection. Inflamm Res 54(10): 428–434. 10.1007/s00011-005-1374-5 [DOI] [PubMed] [Google Scholar]
- 28.Aalto H, Takala A, Kautiainen H, Siitonen S, Repo H. (2007) Monocyte CD14 and soluble CD14 in predicting mortality of patients with severe community acquired infection. Scand J Infect Dis 39(6–7): 596–603. 10.1080/00365540701199808 [DOI] [PubMed] [Google Scholar]
- 29.Yang YY, Hsieh SL, Lee PC, Yeh YC, Lee KC, Hsieh YC, et al. (2014) Long-term cannabinoid type 2 receptor agonist therapy decreases bacterial translocation in rats with cirrhosis and ascites. J Hepatol 61(5): 1004–1013. 10.1016/j.jhep.2014.05.049 [DOI] [PubMed] [Google Scholar]
- 30.Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, et al. (2007) Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol 46: 816–826. 10.1016/j.jhep.2006.12.018 [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. (2002) The immunopathogenesis of sepsis. Nature. 420, 885–891. 10.1038/nature01326 [DOI] [PubMed] [Google Scholar]
- 32.van der Poll T, Opal SM. (2008) Host-pathogen interactions in sepsis. Lancet Infect Dis 8(1): 32–43. 10.1016/S1473-3099(07)70265-7 [DOI] [PubMed] [Google Scholar]
- 33.Appenrodt B, Grunhage F, Gentemann MG, Thyssen L, Sauerbruch T, Lammert F, et al. (2010) Nucletodie-binding oligomerization domain containing 2 (NOD2) variants are genetic risk factors for death and spontaneous bacterial peritonitis. Hepatology 51(4): 1327–1333. 10.1002/hep.23440 [DOI] [PubMed] [Google Scholar]
- 34.Von Baehr V, Docke WD, Plauth M, Liebenthal C, Küpferling S, Lochs H, et al. (2000) Mechanisms of endotoxin tolerance in patients with alcoholic liver cirrhosis: role of interleukin 10, interleukin 1 receptor antagonist, and soluble tumor necrosis factor receptors as well as effector cell desensitization. Gut 47: 281–287. 10.1136/gut.47.2.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Means TK, Pavlovich RP, Roca D, Vermeulen MW, Fenton MJ. (2000) Activation of TNF-alpha transcription utilizes distinct MAP kinase pathways in different macrophages population. J Leukoc Biol 67: 885–928. [DOI] [PubMed] [Google Scholar]
- 36.Abraham E. (2003) Nuclear factor-kappaB and its role in sepsis-associated organ failure. J Infect Dis 187: S364–S369. 10.1086/374750 [DOI] [PubMed] [Google Scholar]
- 37.Kothari N, Bogra J, Abbas H, Kohli M, Malik A, Kothari D, et al. (2013) Tumor necrosis factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine 61(2): 676–681. 10.1016/j.cyto.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 38.Fortin CF, McDonald PP, Fülöp T, Lesur O. (2010) Sepsis, leukocytes, and nitric oxide (NO): an intricate affair. Shock 33(4): 344–352. 10.1097/SHK.0b013e3181c0f068 [DOI] [PubMed] [Google Scholar]
- 39.Landmann R, Reber AM, Sansano S, Zimmerli W. (1996) Function of soluble CD14 in serum from patients with septic shock. J Infect Dis 173(3): 661–668. [DOI] [PubMed] [Google Scholar]
- 40.Dijkstra G, Zandvoort AJ, Kobold AC, de Jager-Krikken A, Heeringa P, van Goor H, et al. (2002) Increased expression of inducible nitric oxide synthase in circulating monocytes from patients with active inflammatory bowel disease. Scand J Gastroenterol 37(5): 546–554. [DOI] [PubMed] [Google Scholar]
- 41.Gao- JW, Zhang AQ, Pan W, Yue CL, Zeng L, Gu W, et al. (2015) Association between IL6-174G/C polymorphism and the risk of sepsis and mortality: a systemic review and meta-analysis. PloS One 10(3): e0118843 10.1371/journal.pone.0118843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YY, Lien JM, Peng YS, Chen YC, Tian YC, Fang JT, et al. (2014) Lipopolysaccharide binding protein in cirrhotic patients with severe sepsis. J Chin Med Assoc 77(2):68–74. 10.1016/j.jcma.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 43.Albillos A, de-la-Hera A, Alvarez-Mon M. (2004) Serum lipopolysaccharide-binding protein prediction of severe bacterial infection in cirrhotic patients with ascites. Lancet 63:1608–1610. [DOI] [PubMed] [Google Scholar]
- 44.Santolaya ME, Alvarez AM, Aviles CL, Becker A, King A, Mosso C, et al. (2008) Predictors of Severe Sepsis Not Clinically Apparent During the First Twenty-Four Hours of Hospitalization in Children With Cancer, Neutropenia, and Fever: A Prospective, Multicenter Trial. Pediatr Infect Dis J 27:538–543. 10.1097/INF.0b013e3181673c3c [DOI] [PubMed] [Google Scholar]
- 45.Oberkofler CE, Dutkowski P, Stocker R, Schuepbach RA, Stover JF, Clavien PA, et al. (2010) Model of end stage liver disease (MELD) score greater than 23 predicts length of stay in the ICU but not mortality in liver transplant recipients. Crit Care 14(3):R117 10.1186/cc9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cholongitas E, Senzolo M, Patch D, Kwong K, Nikolopoulou V, Leandro G, et al. (2006) Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther 23(7):883–893. 10.1111/j.1365-2036.2006.02842.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A-E) Schematic representative SNPs that explored within various cytokines genes in current study; the comparison of (F) previous episode of infection during the pre-study period and (G) duration of first de-compensation to entering study (pre-study period) between cases with and without severe sepsis. * P < 0.05 or ** P < 0.001 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases. Pre-study period: first de-compensation of cirrhosis and the period from this time until the first day of the present hospitalization/time of entering current study.
(TIF)
(A-C). Plasma TNFα, IL-6 and IL-1β levels of all cases; (D). LPS-stimulated IL-1β production; (E,F). LPS-stimulated p38MAPβ and ERK1 mRNA expression on CD16+ (non-classical) monocytes of all cases; #p<0.05 vs. un-stimulated group.
(TIF)
(A). surface HLA-DR expression on CD16+ monocyte; (B).correlation between LPS-stimulated surface HLA-DR expression on CD16- monocyte and levels of TNFα/nitrite in the LPS-stimulated on the culture supernatant in CD14-159C/T variant allele carrier with severe sepsis. (C). correlation between LPS-stimulated mCD14+ density (Abc) on CD16- (classical) monocyte and LPS-stimulated phagocytic index TLR4+896A/G variant allele carriers with severe sepsis. * P < 0.05 vs. TLR4/CD14 variant alleles carriers/severe sepsis cases; #p<0.05 vs. un-stimulated group.
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.