Abstract
Background
Recently, many studies have shown that the serum lactate dehydrogenase (LDH) level is related to the prognosis of renal cell carcinoma (RCC). We launched this meta-analysis to assess the prognostic value of serum LDH in patients with RCC.
Methods
We searched PubMed, Embase and Web of Science for information on serum LDH and the outcome of RCC through June 14, 2016. The hazard ratio (HR) and its 95% confidence interval (CI) for overall survival (OS) and progression-free survival (PFS) were extracted and integrated from the matching studies.
Results
A total of 29 studies including 6629 patients with RCC were incorporated in this meta-analysis. Patients whose serum LDH levels were elevated had a lower OS (HR = 2.13, 95% CI = 1.69–2.69, P < 0.001). Meanwhile, the pooled data showed that a higher serum LDH level was a negative prognostic factor for PFS (HR = 1.74, 95% CI = 1.48–2.04, P < 0.001). Furthermore, subgroup analyses indicated elevated serum LDH was associated with poor survival in different tumor types. Elevated serum LDH was significantly associated with worse prognosis for patients with all stages of RCC (OS, HR = 2.41, 95% CI = 1.09–5.33), metastatic RCC (OS, HR = 2.62, 95% CI 1.57–2.59; CSS, HR = 1.79, 95% CI 1.49–2.15), and non-metastatic RCC (OS, HR = 3.67, CI = 1.33–10.13). Besides, elevated serum LDH also indicated a worse prognosis in subgroups of cut-off values, analysis types and ethnicity.
Conclusions
Our results show that serum LDH levels are associated with the outcomes of RCC and can be used as a valuable biomarker for monitoring prognoses.
Introduction
Renal cell carcinoma (RCC) is a common malignant tumor. The estimated number of new cases of RCC in the United States in 2015 was 61560, representing approximately 3.7% of all new cases, and the estimated deaths were 14080, accounting for nearly 2.4% of the total cancer mortality[1]. Early-stage RCC patients treated with nephrectomy may have a satisfying five-year survival expectancy[2, 3]. Once metastasis occurs, molecular-targeted therapy and immunotherapy are the only ways to treat RCC due to its poor response to radiotherapy and chemotherapy[4–9]. TNM staging is typically used to predict the survival of RCC patients in clinical medicine. Relying on this index, we can propose individualized treatments. However, patients with RCC in the same stage may suffer different outcomes, which urges us to find new and more precise biomarkers to assess the prognosis of RCC.
Aerobic glycolysis is the most prominent characteristic of a cancer cell[10]. A large amount of lactate is produced during this process. Lactate dehydrogenase (LDH) is a glycolysis enzyme that catalyzes the conversion of pyruvate into lactate and that could potentially play an important role in tumor metabolism. In recent years, a large number of studies have demonstrated that serum LDH levels are linked to the prognosis of RCC[11–15]. LDH could be a cheap and simple prognostic index that could be applied in everyday oncologic clinical practice rather than to stratify patients in clinical trials. However, due to different conditions, parameters and other factors included in the studies, the prognostic value of serum LDH in patients with RCC is still inconsistent. Therefore, we conducted this systematic literature review and meta-analysis to research the relationship between serum LDH levels and the prognosis of RCC.
Materials and Methods
Search strategy
PubMed, Embase and Web of Science were searched systematically through June 14, 2016. The terms included in the search strategy were as follows: “lactate dehydrogenase or LDH” (all fields), “kidney cancer or renal cancer or renal carcinoma or renal cell carcinoma” (all fields) and “prognosis or prognostic or survival or outcome” (all fields). To guarantee the validity of the retrieved reference lists, each article was screened by two different researchers (Jie Shen and Zhen Chen).
Selection criteria
Studies were included in our meta-analysis if the following criteria were met: (1) the patients suffered from RCC only, without other malignant tumors; (2) the serum LDH levels were calculated, and the relationship between serum LDH and RCC was mentioned in the article; (3) the values for the hazard ratio (HR) and its 95% confidence interval (CI), overall survival (OS) or disease-free survival (DFS) were mentioned or could be extrapolated; and (4) the studies were written in English. Studies were excluded based on the following criteria: (1) reviews, letters, comments, case reports and conference abstracts; (2) studies with duplicate data; and (3) missing data for further analysis.
Data extraction
The data were extracted from the articles by two independent authors (Shen and Chen). Details included author, year of publication, country, patient numbers, age of patients, duration of follow-up care, ethnicity, cut-off value, T stage, Furman grade, treatment method, HR and relative 95% CI. In case survival data could only be found in Kaplan–Meier curves, we had to use software designed by Jayne F Tierney and Matthew R Syde[16] to digitize and extract HR values and 95% CIs. Moreover, disputed data were only included after discussion between both authors.
Statistical analysis
High and low levels of serum LDH were determined according to the cut-off values provided in the articles. In addition, the pooled HRs and their 95% CIs were estimated to evaluate the effective impact of the serum LDH level on survival. We found an observed HR above 1 to be indicative for a poorer prognosis for RCC patients with high serum LDH level. Heterogeneity of combined HRs was tested by applying a Cochran’s Q test and Higgins I-squared statistics. If the P value was less than 0.05 and/or I2 was larger than 50%, indicative for a significant heterogeneous distribution, a random-effects model (DerSimonian-Laird method) was used. Otherwise, a fixed-effects model (the Mante-Haenszel method) was implemented. The factors that may have led to the existence of heterogeneity were further analyzed. Publication bias was assessed by using Begg’s and Egger’s tests. All data analyses were performed with STATA 12.0 (Stata Corporation, College Station, TX, USA). Applying a two-sided test, a P value lower than 0.05 was defined as statistically significant.
Results
Search results
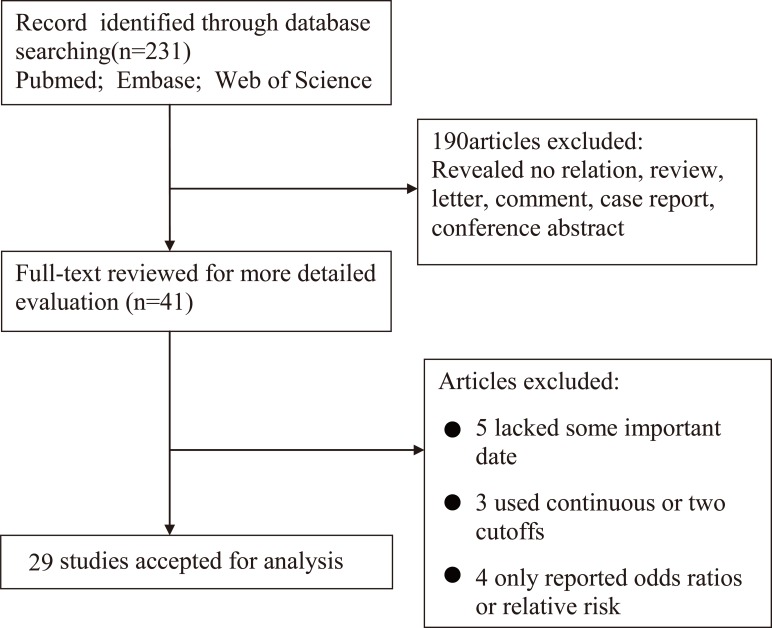
The process of searching and filtering of articles is shown in Fig 1. Initially, a total of 231 articles were listed. After scanning the titles, abstracts, publication categories and full text of each article, 41 articles were shortlisted. Among these, 12 articles were excluded (five lacked some important data, three used continuous or two cut-offs, and four only reported odds ratios or relative risk). Finally, 29 articles, representing 6629 patients with RCC, were included in this meta-analysis[11–15, 17–40].
Fig 1. Flow diagram of the study selection process.
Study characteristics
The characteristics of the 29 eligible studies are presented in Table 1. The studies were originally published between 2002 and 2016. The 6629 patients were from Canada, China, Czech, Denmark, Germany, Italy, Japan, Netherlands, Poland, Turkey and the USA. Furthermore, Asian nationals account for 19.4% of the patients (1289 patients) and Caucasian patients account for the remaining 80.6% (5340 patients). In this study, 2287 patients in 12 articles were treated with multiple therapies, while 3465 patients in another 12 articles were treated with targeted therapy only. The remaining 1123 patients received surgery only. Meanwhile, OS was mentioned in 25 articles, and PFS was found in 9 articles.
Table 1. Main characteristics of all studies included in the meta-analysis.
| Author | Year | Country | Number | Age | Follow-up | Ethnicity | Cut-off value | T stage | Furman | Tumor type | Survival analysis | Source of HR | Multivariate analysis | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chrom | 2016 | Poland | 266 | 61(22–85) | 46.1(41.2–51) | Caucasian | ULN | 45/68/109/8 | 13/127/71/28 | mRCC | OS | Report | yes | targeted therapy |
| Song | 2016 | China | 74 | 51.4(20.1–86.8) | 38.3(2.3–53.9) | Asian | 1.5ULN | 27/35/11/1 | NR | mRCC | OS | Report | yes | MT |
| Song | 2016 | China | 155 | 55.3(17.9–86.8) | 36.3(4.3–110.5) | Asian | 1.5ULN | 48/36/11 | NR | mRCC | OS/PFS | Report | yes | targeted therapy |
| Matrana | 2015 | USA | 88 | 65(34–90) | 29.3(25.4–33.2) | Caucasian | 1.5ULN | 19/42 | NR | mRCC | PFS | SC | yes | surgery |
| Fukushima | 2015 | Japan | 92 | 65(37–91) | 19(1–142) | Asian | 1.5ULN | NR | NR | mRCC | OS | Report | yes | MT |
| Sasaki | 2015 | Japan | 126 | 67 | 30.8 | Asian | 250U/L | NR | NR | All stage | OS | Report | yes | MT |
| Bodnar | 2015 | Poland | 58 | 60(41–78) | NR | Caucasian | ULN | NR | NR | mRCC | OS | Report | yes | targeted therapy |
| Kubackova | 2014 | Czech | 836 | 59(21–83) | NR | Caucasian | 1.5ULN | NR | NR | All stage | OS/PFS | Report | no | targeted therapy |
| Girgis | 2014 | Canada | 385 | NR | NR | Caucasian | NR | 190/35/49/33/78(x) | 31/180/103/22/49(x) | Non-mRCC | OS | SC | yes | surgery |
| Malik | 2014 | USA | 70 | 56.5(44–76) | NR | Caucasian | 1.5ULN | NR | NR | All stage | OS | Report | yes | MT |
| Poprach | 2014 | Czech | 319 | 62(45–77) | 15 | Caucasian | 1.5ULN | NR | NR | mRCC | OS/PFS | Report | no | MT |
| Amato | 2014 | USA | 57 | NR | NR | Caucasian | 1.5ULN | NR | 13/39(1,2–3,4) | All stage | OS | Report | yes | MT |
| Cetin | 2014 | Turkey | 59 | 60 (34–80) | 15(2–59) | Caucasian | ULN | NR | NR | mRCC | OS | Report | no | MT |
| Atkinson | 2014 | USA | 185 | Over 18 | NR | Caucasian | 927 | NR | NR | mRCC | OS/PFS | Report | yes | targeted therapy |
| Nakano | 2013 | Japan | 36 | 65.7 | 13(2–48) | Caucasian | 200U/L | NR | NR | mRCC | PFS | Report | yes | targeted therapy |
| Kamba | 2013 | Japan | 144 | 62.9(19–86) | 2–218 | Caucasian | 1.5ULN | NR | NR | mRCC | PFS | Report | yes | MT |
| Motzer | 2013 | USA | 1059 | 60(24–87) | NR | Caucasian | 1.5ULN | NR | NR | mRCC | OS/PFS | Report | yes | targeted therapy |
| Armstrong | 2012 | USA | 404 | 59.4(23–86) | NR | Caucasian | ULN | NR | NR | mRCC | OS | Report | yes | targeted therapy |
| Du | 2012 | China | 286 | 55.72(28–77) | NR | Asian | 1.5ULN | 165/55/52/4 | 17/134/112/23 | mRCC | OS | Report | yes | surgery |
| Shinohara | 2011 | Japan | 473 | 64(32–87) | 18 | Asian | 1.5ULN | NR | NR | mRCC | OS | Report | yes | MT |
| Abel | 2011 | USA | 75 | 60(23–80) | 15 | Caucasian | ULN | 14/16/34/11 | 12/20/4/32/7(Ⅱ/Ⅲ/Ⅳ/unknown/high grade) | mRCC | OS | Report | yes | targeted therapy |
| Zhang | 2011 | China | 83 | 51(27–75) | 27(12–46) | Asian | 315IU/L | NR | NR | mRCC | PFS | Report | yes | targeted therapy |
| Aben | 2011 | Netherlands | 328 | 67.6±11 | NR | Caucasian | 1.5ULN | NR | 14/32/43/13/226 (uknown) | mRCC | OS | Report | yes | MT |
| Richey | 2011 | USA | 188 | 60.8(18.2–83.9) | 13.1(1.0–64.4) | Caucasian | ULN | 45/28/89/26 | NR | mRCC | OS | Report | yes | targeted therapy |
| Jeppesen | 2010 | Denmark | 120 | 58.3(29–73) | 48–72 | Caucasian | 1.5ULN | NR | NR | mRCC | OS | Report | yes | MT |
| Donskov | 2006 | Denmark | 120 | 57(19–74) | 57(32–73) | Caucasian | 1.5ULN | NR | NR | mRCC | OS | Report | yes | targeted therapy |
| Peccatori | 2005 | Italy | 70 | NR | 10 | Caucasian | 300U/L | NR | NR | All stage | OS | Report | yes | surgery |
| Lehmann | 2004 | Germany | 48 | 63(35–82) | 125.3(33.4–156) | Caucasian | 183U/L | 44761 | NR | Non-mRCC | OS | Report | no | surgery |
| Atzpodien | 2002 | Germany | 425 | NR | 20(0–157) | Caucasian | 220U/L | NR | NR | mRCC | OS | Report | yes | MT |
Abbreviation: RCC renal cell carcinoma, mRCC metastatic renal cell carcinoma, Non-mRCC non- metastatic renal cell carcinoma, OS overall survival, PFS progression-free survival, HR hazard ratio, NR not report, MT multiple therapy, SC survival curve, ULN upper limits of normal, x status unknown.
Quality assessment
We assessed the quality of the 29 eligible studies included in our meta-analysis following the guideline of the Newcastle-Ottawa Scale (NOS)[41]. The quality of the studies varied from a score of 5 to 9, with a mean of 6. A higher score suggested the use of better methodologies. Therefore, all 29 studies were included in the subsequent analysis.
Overall survival
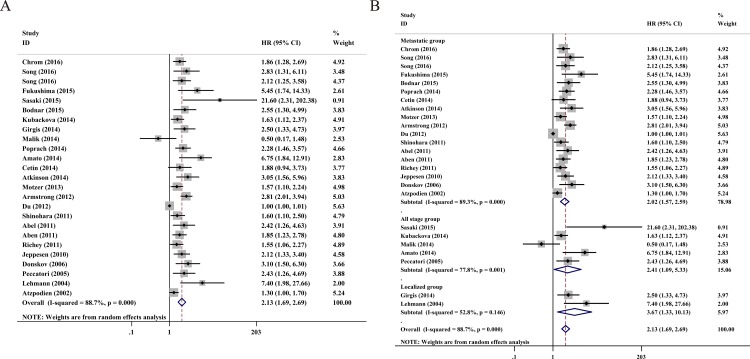
In total, 25 studies, including 6278 RCC patients, provided valid data for OS and are presented in Fig 2A. We used a random model to pool the HRs because of statistical heterogeneity (I2 = 88.7%, P < 0.001). The pooled data demonstrated that high serum LDH predicts worse OS (HR = 2.13, 95% CI = 1.69–2.69, P < 0.001).
Fig 2.
A. Forest plots of studies evaluating hazard ratios of elevated serum LDH level in all renal cell carcinoma (RCC) for overall survival. B. Forest plot of the relationship between elevated serum LDH level and overall survival in patients with different tumor types.
In order to further test for heterogeneity among the studies, we performed this meta-analysis on discrete subgroups, including ethnicity, cut-off value, analysis type and tumor type. High serum LDH was linked to a worse OS for all stages of RCC (HR = 2.41, 95% CI = 1.09–5.33, P = 0.03), metastatic RCC (HR = 2.02, 95% CI = 1.57–2.59, P <0.001) and non-metastatic RCC (HR = 3.67, 95% CI = 1.33–10.13, P = 0.012), as shown in Table 2 and Fig 2B. Serum LDH also showed a statistically significant connection with poor OS in the remaining subgroups: Asian RCC patients (HR = 2.22, 95% CI = 1.27–3.87, P = 0.005), Caucasian RCC patients (HR = 2.06, 95% CI = 1.73–2.44, P <0.001), multivariate analysis (HR = 2.11, 95% CI = 1.63–2.71, P <0.001), univariate analysis (HR = 1.98, 95% CI = 1.52–2.56, P <0.001), 1.5 ULN (HR = 1.96, 95% CI = 1.43–2.68, P <0.001), and others (HR = 2.21, 95%CI = 1.73–2.83, P <0.001).
Table 2. Pooled hazard ratios for OS according to subgroup analyses.
| Outcome subgroup | No. of patients | No. of studies | HR (95% CI) | P value | Model | heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| Overall survival | 6278 | 25 | 2.13(1.69–2.69) | <0.001 | random | 88.7% | <0.001 |
| Ethnicity | |||||||
| Asian | 1206 | 6 | 2.22(1.27–3.87) | 0.005 | random | 86.4% | <0.001 |
| Caucasian | 5072 | 19 | 2.06(1.73–2.44) | <0.001 | random | 54.3% | 0.003 |
| Cut-off value | |||||||
| 1.5ULN | 4414 | 13 | 1.96(1.43–2.68) | <0.001 | random | 87.9% | <0.001 |
| Others | 1864 | 12 | 2.21(1.73–2.83) | <0.001 | random | 59.4% | 0.004 |
| Analysis type | |||||||
| Multivariate | 5016 | 21 | 2.11(1.63–2.71) | <0.001 | random | 89% | <0.001 |
| Univariate | 1262 | 4 | 1.98(1.52–2.56) | <0.001 | fixed | 43.3% | 0.152 |
| Tumor type | |||||||
| All stage | 1159 | 5 | 2.41(1.09–5.33) | 0.03 | random | 77.8% | 0.001 |
| Metastasis | 4686 | 18 | 2.02(1.57–2.59) | <0.001 | random | 89.3% | <0.001 |
| Non-metastasis | 433 | 2 | 3.67(1.33–10.13) | 0.012 | random | 52.8% | 0.146 |
Abbreviation: OS = overall survival; HR = hazard ratio; CI = confidence interval.
Sensitivity analysis
Each study was tested for the impact of a single study on the pooled HRs. One study deviated from the rest and may have influenced the stability of the result. After excluding this study, the pooled HRs and 95% CI for OS were 2.11 and 1.8–2.48, which is a further indication of the strength of our meta-analysis.
Progression-free survival
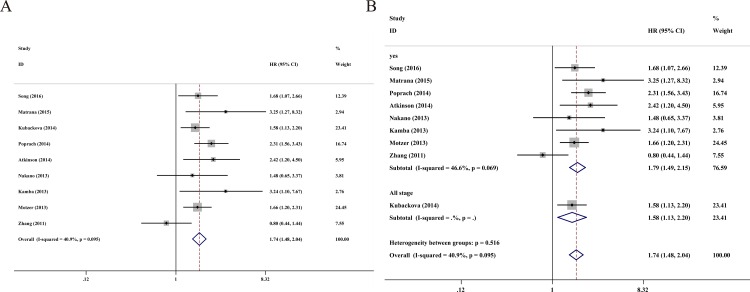
Next, 9 studies contained a total 2905 RCC patients with valid data for PFS, as showed in Fig 3A. A fixed-effect model was used in the analysis of PFS due to the absence of statistical heterogeneity (I2 = 40.9%, P = 0.095). Additionally, the pooled data demonstrated that high serum LDH predicts worse PFS (HR = 1.74, 95% CI = 1.48–2.04, P < 0.001). A subgroup analysis was conducted simultaneously in order to evaluate the heterogeneity of these studies (Table 3 and Fig 3B). A separate meta-analysis, including 5 studies and 2487 Caucasian patients, had a pooled HR for PFS of 1.87 (95% CI = 1.55–2.25; P < 0.001) with moderate heterogeneity among studies (I2 = 11%, P = 0.343). Other subgroups, such as the analysis type and tumor type for PFS, were shown to be statistically linked to serum LDH.
Fig 3.
A. Forest plots of studies evaluating hazard ratios of elevated serum LDH level in all renal cell carcinoma (RCC) for progression-free survival. B. Forest plot of the relationship between elevated serum LDH level and progression-free survival in patients with different tumor types.
Table 3. Pooled hazard ratios for PFS according to subgroup analyses.
| Outcome subgroup | No. of patients | No. of studies | HR (95% CI) | P value | Model | heterogeneity | |
|---|---|---|---|---|---|---|---|
| I2 (%) | P | ||||||
| progression-free survival | 2905 | 9 | 1.74(1.48–2.04) | <0.001 | fixed | 40.9% | 0.095 |
| Ethnicity | |||||||
| Asian | 418 | 4 | 1.48(0.89–2.48) | 0.133 | random | 57.2% | 0.072 |
| Caucasian | 2487 | 5 | 1.87(1.55–2.25) | <0.001 | fixed | 11% | 0.343 |
| Cut-off value | |||||||
| 1.5ULN | 2601 | 6 | 1.84(1.54–2.19) | <0.001 | fixed | 5.7% | 0.38 |
| Others | 304 | 3 | 1.4(0.7–2.79) | 0.344 | random | 67.3% | 0.047 |
| Analysis type | |||||||
| Multivariate | 1750 | 7 | 1.67(1.36–2.06) | <0.001 | fixed | 45.8% | 0.086 |
| Univariate | 1155 | 2 | 1.88(1.30–2.73) | 0.001 | random | 52% | 0.149 |
| Tumor type | |||||||
| All stage | 836 | 1 | 1.58(1.13–2.20) | 0.007 | fixed | - | - |
| Metastasis | 2069 | 8 | 2.02(1.57–2.59) | <0.001 | fixed | 46.6% | 0.069 |
Abbreviation: DFS = disease-free survival; HR = hazard ratio; CI = confidence interval.
Publication bias
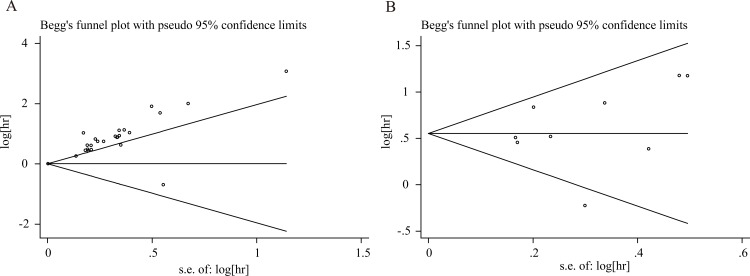
We assessed the publication bias of OS and PFS. The publication bias of all studies was evaluated using funnel plots and Egger’s and Begg’s tests. Funnel plots of OS and PFS are presented in Fig 4A and 4B, respectively. The figures show an existing publication bias for OS (P <0.01 using Egger’s test), while a publication bias for PFS does not exist (P = 0.531 using Egger’s test).
Fig 4. Funnel plots for the evaluation of potential publication bias.
(A) Overall survival for all renal cell carcinoma; (B) Progression-free survival for all renal cell carcinoma.
Discussion
Aerobic oxidation and glycolysis are two different pathways of glucose metabolism responding to distinct demands of oxygen. In most mammalian cells, glycolysis is inhibited under aerobic conditions and mitochondria oxidize pyruvate producing mainly CO2 and H2O. This phenomenon is known as the Pasteur effect[42]. However, in tumor cells, glycolysis is extremely elevated regardless of oxygen availability, which is called the Warburg effect[10]. As a result of high glycolysis, the production of lactate is enhanced, creating an acidic microenvironment in the tissues. Normal cells become necrotic due to the change of the transmembrane H+ gradient and the degradation of the extracellular matrix in an acidic microenvironment[43]. However, tumor cells can resist acidosis and survive in low pH conditions. Meanwhile, acidosis can inhibit the immune response to tumor antigens, which promotes the proliferation and invasion of tumor cells[44].
LDH is an essential substance in the Warburg effect and is available in almost all tissues. It has six different iso-enzymes[45]. These iso-enzymes are assembled in a homo- or hetero-tetramer structure by two protein subunits: LDHA and LDHB. Different combinations of LDHA and LDHB characterize five subtypes of LDH (LDH-1 to LDH-5)[45]. A third protein subunit, which is named LDHC, forms a testis-specific subtype, known as LDH-6[46]. The predominant function of LDH is the catalyzation of the reversible reaction of pyruvate to lactate. NAD+ is generated concomitantly alongside this process and is essential for the continuous generation of ATP to keep glycolysis running. Among LDH subtypes, the catalytic efficiency of LDH-5 is the highest[47]. When RCC tissue necrosis occurred, high level of intracellular LDH was released into blood, improving the serum LDH concentration[10]. Moreover, when distant metastasis occurred, tumor cells can disrupt neighboring organs such as liver, lung, and bones. The injury of those organs can also elevate serum LDH level[47–50]. Recently, several studies have proved that reduced LDH expression can inhibit the invasion and metastasis of cancer cells by decreasing their ability to proliferate and their resistance to chemotherapy[51]. In conclusion, drugs targeting LDH may represent new approaches for the treatment of RCC.
To our knowledge, this meta-analysis is the most comprehensive research to synthetically analyze the prognostic value of serum LDH in patients with RCC. We conclude that an elevated serum LDH is an objective biomarker for worse OS and PFS in RCC patients. Meanwhile, subgroup analyses demonstrated that elevated serum LDH remained a good biomarker regardless of ethnic background, analysis type and HR diagnostic method.
However, this meta-analysis has some limitations. First, we only considered articles mentioning the HR and the 95% CI, while other articles were excluded because they only reported odds ratios and relative risk for survival. Second, due to different standards for the use of cut-off values in the articles, the validity of serum LDH as an important role to predict the prognosis of RCC patients may have been affected. Moreover, serum LDH could have been influenced by other non-neoplastic conditions, such as muscular dystrophy, anemia, heart failure, hepatitis and lung disease. Most articles contained in this meta-analysis did not investigate those factors. Regardless, serum LDH is an important and meaningful biomarker to assess the prognosis of patients with RCC.
In conclusion, this meta-analysis demonstrated that an elevated serum LDH level was connected to a poor prognosis for patients with RCC. The serum LDH was a convenient and cost-effective prognostic indicator, which could be utilized to divide risk stratification and formulate individualized treatments for patients with RCC. Considering the limitation of the present analysis, the more detailed mechanism why or how LDH activity in RCC tumors could elevate serum LDH level is still unclear. We will need to conduct further multicenter studies to confirm our findings and explore the mechanism deeply.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by the National Science Foundation of Jiangsu Province (Grant no.BK20150251).
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. Epub 2015/01/07. 10.3322/caac.21254 . [DOI] [PubMed] [Google Scholar]
- 2.Tan HJ, Norton EC, Ye Z, Hafez KS, Gore JL, Miller DC. Long-term survival following partial vs radical nephrectomy among older patients with early-stage kidney cancer. JAMA. 2012;307(15):1629–35. Epub 2012/04/19. 10.1001/jama.2012.475 307/15/1629 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan HJ. Survival benefit of partial nephrectomy: Reconciling experimental and observational data. Urol Oncol. 2015;33(12):505 e21-4. Epub 2015/09/04. 10.1016/j.urolonc.2015.08.002 S1078-1439(15)00399-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4.Macleod LC, Tykodi SS, Holt SK, Wright JL, Lin DW, Tretiakova MS, et al. Trends in Metastatic Kidney Cancer Survival From the Cytokine to the Targeted Therapy Era. Urology. 2015;86(2):262–8. Epub 2015/07/23. 10.1016/j.urology.2015.05.008 S0090-4295(15)00476-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 5.Clyne M. Kidney cancer: predicting survival after targeted therapy for mRCC. Nat Rev Urol. 2013;10(10):554 Epub 2013/09/04. 10.1038/nrurol.2013.205 nrurol.2013.205 [pii]. . [DOI] [PubMed] [Google Scholar]
- 6.Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989–2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008;113(9):2457–63. Epub 2008/10/01. 10.1002/cncr.23851 . [DOI] [PubMed] [Google Scholar]
- 7.Vallet S, Pahernik S, Hofner T, Tosev G, Hadaschik B, Duensing S, et al. Efficacy of targeted treatment beyond third-line therapy in metastatic kidney cancer: retrospective analysis from a large-volume cancer center. Clin Genitourin Cancer. 2015;13(3):e145–52. Epub 2015/01/19. 10.1016/j.clgc.2014.12.012 S1558-7673(14)00286-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 8.Grivas PD, Redman BG. Immunotherapy of kidney cancer. Curr Clin Pharmacol. 2011;6(3):151–63. Epub 2011/08/11. BSP/CCP/E-Pub/0051 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9.Pizza G, De Vinci C, Lo Conte G, Maver P, Dragoni E, Aiello E, et al. Immunotherapy of metastatic kidney cancer. Int J Cancer. 2001;94(1):109–20. Epub 2001/10/23. 10.1002/ijc.1426 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4(11):891–9. Epub 2004/11/02. nrc1478 [pii] 10.1038/nrc1478 . [DOI] [PubMed] [Google Scholar]
- 11.Sasaki T, Onishi T. Pretherapeutic Plasma Fibrinogen Level is an Independent Survival Predictor in Renal Cell Carcinoma. Oncol Res Treat. 2015;38(7–8):374–8. Epub 2015/08/19. 10.1159/000435896 000435896 [pii]. . [DOI] [PubMed] [Google Scholar]
- 12.Fukushima H, Nakanishi Y, Kataoka M, Tobisu K, Koga F. Prognostic Significance of Sarcopenia in Patients with Metastatic Renal Cell Carcinoma. J Urol. 2016;195(1):26–32. Epub 2015/08/21. 10.1016/j.juro.2015.08.071 S0022-5347(15)04604-2 [pii]. . [DOI] [PubMed] [Google Scholar]
- 13.Song Y, Du C, Zhang W, Sun Y, Yang L, Cui C, et al. Body mass index and age are additional prognostic factors in patients with metastatic renal cell carcinoma treated with tyrosine kinase inhibitors. Urol Oncol. 2016;34(6):258 e15-22. Epub 2016/01/25. 10.1016/j.urolonc.2015.12.008 S1078-1439(15)00586-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 14.Song Y, Du CX, Zhang W, Sun YK, Yang L, Cui CX, et al. Impact of Cytoreductive Nephrectomy on Survival in Patients with Metastatic Renal Cell Carcinoma Treated by Targeted Therapy. Chin Med J (Engl). 2016;129(5):530–5. Epub 2016/02/26. 10.4103/0366-6999.177001 ChinMedJ_2016_129_5_530_177001 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matrana MR, Bathala T, Campbell MT, Duran C, Shetty A, Teegavarapu P, et al. Outcomes of Unselected Patients with Metastatic Clear-Cell Renal Cell Carcinoma Treated with Front-Line Pazopanib Therapy Followed by Vascular Endothelial Growth Factor Receptor Tyrosine Kinase Inhibitors (VEGFR-TKI) or Mammalian Target of Rapamycin Inhibitors (mTORi): A Single Institution Experience. BJU Int. 2015. Epub 2015/11/18. 10.1111/bju.13374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 Epub 2007/06/09. 1745-6215-8-16 [pii] 10.1186/1745-6215-8-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chrom P, Stec R, Semeniuk-Wojtas A, Bodnar L, Spencer NJ, Szczylik C. Fuhrman Grade and Neutrophil-To-Lymphocyte Ratio Influence on Survival in Patients With Metastatic Renal Cell Carcinoma Treated With First-Line Tyrosine Kinase Inhibitors. Clin Genitourin Cancer. 2016. Epub 2016/03/17. S1558-7673(16)30033-7 [pii] 10.1016/j.clgc.2016.02.005 . [DOI] [PubMed] [Google Scholar]
- 18.Bodnar L, Stec R, Cierniak S, Synowiec A, Wcislo G, Jesiotr M, et al. Clinical usefulness of PI3K/Akt/mTOR genotyping in companion with other clinical variables in metastatic renal cell carcinoma patients treated with everolimus in the second and subsequent lines. Ann Oncol. 2015;26(7):1385–9. Epub 2015/05/13. 10.1093/annonc/mdv166 mdv166 [pii]. . [DOI] [PubMed] [Google Scholar]
- 19.Kubackova K, Bortlicek Z, Pavlik T, Melichar B, Linke Z, Pokorna P, et al. Prognostic factors in renal cell carcinoma patients treated with sorafenib: results from the Czech registry. Target Oncol. 2015;10(3):385–92. Epub 2014/10/12. 10.1007/s11523-014-0343-8 . [DOI] [PubMed] [Google Scholar]
- 20.Girgis H, Masui O, White NM, Scorilas A, Rotondo F, Seivwright A, et al. Lactate dehydrogenase A is a potential prognostic marker in clear cell renal cell carcinoma. Mol Cancer. 2014;13:101 Epub 2014/06/03. 10.1186/1476-4598-13-101 1476-4598-13-101 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malik L, Parsons H, Mahalingam D, Ehler B, Goros M, Mejia A, et al. Clinical outcomes and survival of advanced renal cancer patients in phase I clinical trials. Clin Genitourin Cancer. 2014;12(5):359–65. Epub 2014/03/04. 10.1016/j.clgc.2014.01.011 S1558-7673(14)00019-6 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poprach A, Pavlik T, Melichar B, Kubackova K, Bortlicek Z, Svoboda M, et al. Clinical and laboratory prognostic factors in patients with metastatic renal cell carcinoma treated with sunitinib and sorafenib after progression on cytokines. Urol Oncol. 2014;32(4):488–95. Epub 2013/12/11. 10.1016/j.urolonc.2013.09.011 S1078-1439(13)00384-0 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Amato RJ, Flaherty A, Zhang Y, Ouyang F, Mohlere V. Clinical prognostic factors associated with outcome in patients with renal cell cancer with prior tyrosine kinase inhibitors or immunotherapy treated with everolimus. Urol Oncol. 2014;32(3):345–54. Epub 2013/12/11. 10.1016/j.urolonc.2013.09.008 S1078-1439(13)00351-7 [pii]. . [DOI] [PubMed] [Google Scholar]
- 24.Cetin B, Afsar B, Deger SM, Gonul II, Gumusay O, Ozet A, et al. Association between hemoglobin, calcium, and lactate dehydrogenase variability and mortality among metastatic renal cell carcinoma. Int Urol Nephrol. 2014;46(6):1081–7. Epub 2013/12/07. 10.1007/s11255-013-0613-x . [DOI] [PubMed] [Google Scholar]
- 25.Atkinson BJ, Kalra S, Wang X, Bathala T, Corn P, Tannir NM, et al. Clinical outcomes for patients with metastatic renal cell carcinoma treated with alternative sunitinib schedules. J Urol. 2014;191(3):611–8. Epub 2013/09/11. 10.1016/j.juro.2013.08.090 S0022-5347(13)05338-X [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, et al. Hand-foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn J Clin Oncol. 2013;43(10):1023–9. Epub 2013/08/21. 10.1093/jjco/hyt110 hyt110 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Kamba T, Yamasaki T, Teramukai S, Shibasaki N, Arakaki R, Sakamoto H, et al. Improvement of prognosis in patients with metastatic renal cell carcinoma and Memorial Sloan-Kettering Cancer Center intermediate risk features by modern strategy including molecular-targeted therapy in clinical practice. Int J Clin Oncol. 2014;19(3):505–15. Epub 2013/07/03. 10.1007/s10147-013-0581-2 . [DOI] [PubMed] [Google Scholar]
- 28.Motzer RJ, Escudier B, Bukowski R, Rini BI, Hutson TE, Barrios CH, et al. Prognostic factors for survival in 1059 patients treated with sunitinib for metastatic renal cell carcinoma. Br J Cancer. 2013;108(12):2470–7. Epub 2013/05/23. 10.1038/bjc.2013.236 bjc2013236 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armstrong AJ, George DJ, Halabi S. Serum lactate dehydrogenase predicts for overall survival benefit in patients with metastatic renal cell carcinoma treated with inhibition of mammalian target of rapamycin. J Clin Oncol. 2012;30(27):3402–7. Epub 2012/08/15. 10.1200/JCO.2011.40.9631 JCO.2011.40.9631 [pii]. . [DOI] [PubMed] [Google Scholar]
- 30.Du J, Zheng JH, Chen XS, Yang Q, Zhang YH, Zhou L, et al. High preoperative plasma fibrinogen is an independent predictor of distant metastasis and poor prognosis in renal cell carcinoma. Int J Clin Oncol. 2013;18(3):517–23. Epub 2012/05/23. 10.1007/s10147-012-0412-x . [DOI] [PubMed] [Google Scholar]
- 31.Shinohara N, Abe T, Mochizuki T, Kashiwagi A, Kanagawa K, Maruyama S, et al. Is Memorial Sloan-Kettering Cancer Center risk classification appropriate for Japanese patients with metastatic renal cell carcinoma in the cytokine era? Urol Oncol. 2013;31(7):1276–82. Epub 2011/10/01. 10.1016/j.urolonc.2011.08.009 S1078-1439(11)00278-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 32.Abel EJ, Culp SH, Tannir NM, Tamboli P, Matin SF, Wood CG. Early primary tumor size reduction is an independent predictor of improved overall survival in metastatic renal cell carcinoma patients treated with sunitinib. Eur Urol. 2011;60(6):1273–9. Epub 2011/07/26. 10.1016/j.eururo.2011.07.008 S0302-2838(11)00745-7 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang HL, Zhu Y, Wang CF, Yao XD, Zhang SL, Dai B, et al. Erythrocyte sedimentation rate kinetics as a marker of treatment response and predictor of prognosis in Chinese metastatic renal cell carcinoma patients treated with sorafenib. Int J Urol. 2011;18(6):422–30. Epub 2011/04/13. 10.1111/j.1442-2042.2011.02761.x . [DOI] [PubMed] [Google Scholar]
- 34.Aben KK, Heskamp S, Janssen-Heijnen ML, Koldewijn EL, van Herpen CM, Kiemeney LA, et al. Better survival in patients with metastasised kidney cancer after nephrectomy: a population-based study in the Netherlands. Eur J Cancer. 2011;47(13):2023–32. Epub 2011/04/05. 10.1016/j.ejca.2011.03.002 S0959-8049(11)00146-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 35.Richey SL, Culp SH, Jonasch E, Corn PG, Pagliaro LC, Tamboli P, et al. Outcome of patients with metastatic renal cell carcinoma treated with targeted therapy without cytoreductive nephrectomy. Ann Oncol. 2011;22(5):1048–53. Epub 2010/12/01. 10.1093/annonc/mdq563 mdq563 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeppesen AN, Jensen HK, Donskov F, Marcussen N, von der Maase H. Hyponatremia as a prognostic and predictive factor in metastatic renal cell carcinoma. Br J Cancer. 2010;102(5):867–72. Epub 2010/02/11. 10.1038/sj.bjc.6605563 6605563 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donskov F, von der Maase H. Impact of immune parameters on long-term survival in metastatic renal cell carcinoma. J Clin Oncol. 2006;24(13):1997–2005. Epub 2006/05/02. 24/13/1997 [pii] 10.1200/JCO.2005.03.9594 . [DOI] [PubMed] [Google Scholar]
- 38.Peccatori J, Barkholt L, Demirer T, Sormani MP, Bruzzi P, Ciceri F, et al. Prognostic factors for survival in patients with advanced renal cell carcinoma undergoing nonmyeloablative allogeneic stem cell transplantation. Cancer. 2005;104(10):2099–103. Epub 2005/10/13. 10.1002/cncr.21477 . [DOI] [PubMed] [Google Scholar]
- 39.Lehmann J, Retz M, Nurnberg N, Schnockel U, Raffenberg U, Krams M, et al. The superior prognostic value of humoral factors compared with molecular proliferation markers in renal cell carcinoma. Cancer. 2004;101(7):1552–62. Epub 2004/09/21. 10.1002/cncr.20549 . [DOI] [PubMed] [Google Scholar]
- 40.Atzpodien J, Royston P, Wandert T, Reitz M. Metastatic renal carcinoma comprehensive prognostic system. Br J Cancer. 2003;88(3):348–53. Epub 2003/02/06. 10.1038/sj.bjc.6600768 6600768 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. Epub 2010/07/24. 10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 42.Racker E. History of the Pasteur effect and its pathobiology. Mol Cell Biochem. 1974;5(1–2):17–23. Epub 1974/11/15. . [DOI] [PubMed] [Google Scholar]
- 43.Rozhin J, Sameni M, Ziegler G, Sloane BF. Pericellular pH affects distribution and secretion of cathepsin B in malignant cells. Cancer Res. 1994;54(24):6517–25. Epub 1994/12/15. . [PubMed] [Google Scholar]
- 44.Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69(4):522–30. Epub 2001/04/20. . [PubMed] [Google Scholar]
- 45.Krieg AF, Rosenblum LJ, Henry JB. Lactate dehydrogenase isoenzymes a comparison of pyruvate-to-lactate and lactate-to-pyruvate assays. Clin Chem. 1967;13(3):196–203. Epub 1967/03/01. . [PubMed] [Google Scholar]
- 46.Goldberg E. Immunochemical specificity of lactate dehydrogenase-X. Proc Natl Acad Sci U S A. 1971;68(2):349–52. Epub 1971/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer Lett. 2015;358(1):1–7. Epub 2014/12/22. 10.1016/j.canlet.2014.12.035 S0304-3835(14)00790-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 48.Hermes A, Gatzemeier U, Waschki B, Reck M. Lactate dehydrogenase as prognostic factor in limited and extensive disease stage small cell lung cancer—a retrospective single institution analysis. Respir Med. 2010;104(12):1937–42. Epub 2010/08/20. 10.1016/j.rmed.2010.07.013 S0954-6111(10)00334-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 49.Gatenby RA. The potential role of transformation-induced metabolic changes in tumor-host interaction. Cancer Res. 1995;55(18):4151–6. Epub 1995/09/15. . [PubMed] [Google Scholar]
- 50.Gatenby RA, Gawlinski ET. A reaction-diffusion model of cancer invasion. Cancer Res. 1996;56(24):5745–53. Epub 1996/12/15. . [PubMed] [Google Scholar]
- 51.Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279(20):3898–910. Epub 2012/08/18. 10.1111/j.1742-4658.2012.08748.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.