Abstract
Ginger and its extracts have been used traditionally as anti-inflammatory remedies, with a particular focus on the medicinal properties of its phenolic secondary metabolites, the gingerols. Consistent with these uses, potent anti-arthritic effects of gingerol-containing extracts were previously demonstrated by our laboratory using an experimental model of rheumatoid arthritis, streptococcal cell wall (SCW)-induced arthritis. In this study, anti-inflammatory effects of ginger's other secondary metabolites, the essential oils (GEO), which contain terpenes with reported phytoestrogenic activity, were assessed in female Lewis rats with SCW-induced arthritis. GEO (28 mg/kg/d ip) prevented chronic joint inflammation, but altered neither the initial acute phase of joint swelling nor granuloma formation at sites of SCW deposition in liver. Pharmacologic doses of 17-β estradiol (200 or 600 μg/kg/d sc) elicited the same pattern of anti-inflammatory activity, suggesting that GEO could be acting as a phytoestrogen. However, contrary to this hypothesis, GEO had no in vivo effect on classic estrogen target organs, such as uterus or bone. En toto, these results suggest that ginger's anti-inflammatory properties are not limited to the frequently studied phenolics, but may be attributable to the combined effects of both secondary metabolites, the pungent-tasting gingerols and as well as its aromatic essential oils.
Keywords: ginger, essential oil, gingerol, arthritis, estradiol, mice, phytoestrogen, inflammation, rats, estrogen
Graphical Abstract
1. Introduction
Ginger (Zingiber officinale Roscoe, Zingiberaceae), a commonly used botanical in the United States [1], is primarily known for its anti-emetic properties [2]. However, it has also been used medicinally since antiquity as an anti-inflammatory [3-5]. In modern usage, particular attention has focused on the cyclooxygenase (COX)-inhibiting effects of the gingerols, phenolic compounds that are responsible for ginger's pungent taste, and their potential use in treating inflammatory disorders such as arthritis [4,5]. Previously, we demonstrated potent anti-arthritic effects of gingerol-containing extracts of ginger in an experimental model of rheumatoid arthritis (RA) [6]. However, crude extracts containing both of ginger's secondary metabolites, the gingerols and the essential oils, were even more potent in inhibiting joint swelling than gingerols alone.6 Having previously demonstrated anti-arthritic effects of both the phenolic and essential oil fractions of turmeric (Curcuma longa L, Zingiberaceae), a plant that is botanically and chemically related to ginger [7-10], we postulated that the essential oils of ginger could similarly be bioactive with respect to inhibition of joint inflammation and thus contribute to ginger's potential anti-arthritic effects.
To test this postulate, studies were undertaken to examine the joint protective effects of the isolated essential oils of ginger (GEO), secondary metabolites that are responsible for ginger's characteristic aroma [11,12]. For these studies, the streptococcal cell wall (SCW)-induced arthritis model of RA previously employed by our laboratory to test other ginger (and turmeric) extracts was employed to facilitate comparisons with chemically-related extracts [6-10]. In this model, the inflammatory reaction in response to streptococcal cell wall (SCW) deposition within joints recapitulates the histopathology of RA; female Lewis rats develop an initial, transient phase of joint swelling that is characterized by an influx of neutrophils and other inflammatory cells (acute phase, days 0-5), followed by a recrudescence of joint swelling that is associated with synovial hyperplasia and progressive destruction of periarticular bone by the invading synovium (chronic phase, days 10-28) [6-10,13]. Additionally, classic granulomas form within the liver at sites of hepatic SCW deposition [6,7,10,14], an inflammatory response that can be protective in certain settings, such as pulmonary tuberculosis where invading bacilli are walled off within granulomas, thus helping to quell the spread of infection [15,16].
In our previous studies, SCW-induced arthritis and granulomatous inflammation were each more effectively blocked by a crude ginger extract containing GEO and gingerols as compared to a gingerol-only fraction [6]. The crude extract almost completely prevented both phases of joint swelling (93% and 97% inhibition of acute and chronic arthritis, respectively), while the gingerol-only fraction was less effective (78% and 62% inhibition, respectively) [6]. The crude extract also blocked granulomatous inflammation by 76%, while the gingerol-only fraction was without effect [6]. Therefore, effects of isolated GEO on joint inflammation and the granulomatous hepatic response were tested here using the SCW model. In addition, because estrogenic effects have been reported in vitro for certain monoterpenes present in GEO [17,18], in vivo treatment effects of GEO in the SCW model were compared to those of 17-β estradiol (E2). While joint protective effects of estrogen have previously been reported in pre-clinical RA models and have been postulated for RA itself due to the clinical observation of improved disease activity during pregnancy [19], effects of estrogen in the SCW-model in female rats have, to our knowledge, not previously been reported.
2. Material and Methods
2.1. Preparation of GEO
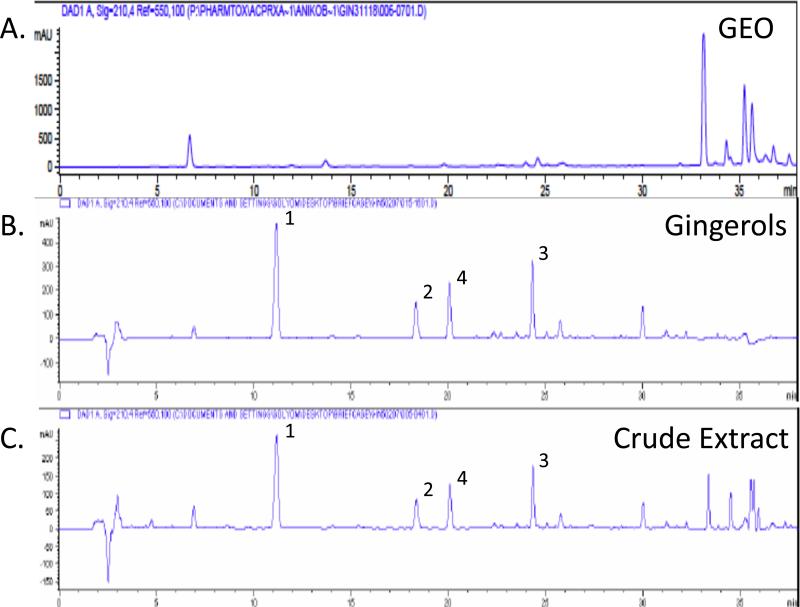
A crude ginger extract was prepared as previously described by extracting ground ginger rhizome (2500g) with CH2Cl2 (dichloromethane) at 25°C for 36 h (6.4% yield) [6,12]. After filtration, washing and work up, 40 g of the resultant extract (“crude ginger extract’) were applied to a silica gel column and sequentially eluted with solvents of increasing polarity to yield fractions 1 through 11, which were chemically characterized by HPLC (Figure 1C) and/or GC-MS and screened in vitro for their ability to inhibit PGE2 production from an LPS-stimulated human macrophage cell line, as previously described [6,12]. For the studies reported here, fraction 1, a lipophilic, sesquiterpene-containing gingerol-free fraction was used (23% yield, “GEO”; Figure 1A) [12]. In previous SCW experiments, essential oil-free fractions (fractions 4-9) containing gingerols and their derivatives, as identified by HPLC and GC-MS, were combined to constitute a single “gingerol fraction” (Figure 1B) (approximately 50% yield) that was used for in vivo testing [6,12].
Figure 1.
HPLC-UV profiles (λ = 250 nm) of GEO (A), a gingerol-only fraction (B), and the crude DCM extract from which the GEO and gingerol fractions were derived (C). The 3 major gingerols (1, [6]-gingerol; 2, [8]-gingerol; 3, [10]-gingerol) and a primary gingerol degradation product (4, [6]-shogaol) are not present in the GEO fraction.
2.2. Animal Procedures
Animal studies were performed in accordance with institutional guidelines using approved protocols. Using the identical protocol previously described for assessment of other ginger extracts [6], female Lewis rats (Harlan, Indianapolis, IN) were administered a single intraperitoneal (i.p.) injection of vehicle (normal saline) or peptidoglycan-polysaccharide polymers (25 μg rhamnose/g body weight) isolated from the sonicated cell wall of Group A Streptococcus pyogenes (Lee Laboratories, Grayson, GA). At the indicated times, control and streptococcal cell wall (SCW)-treated animals received an intraperitoneal injection of botanical sample or vehicle (0.5-1 μL/g DMSO) [6]. Intraperitoneal treatments with the GEO extract or vehicle (1 ul/g DMSO) were administered as in our previous studies beginning 4 days prior to SCW administration and continuing daily until the beginning of the chronic phase (day 14), when treatment frequency was decreased to five days per week [6]. GEO was dosed at 28 mg/kg/d in order to: 1) facilitate comparison with the in vivo potency of isolated gingerols, which were dosed at 25 mg/kg/d [6], and to 2) approximate the GEO dose received by rats treated with the crude ginger extract in prior experiments (containing approximately 32 mg/kg/d GEO [and 25 mg/kg/d gingerols]) [6]. In separate experiments, in order to compare the anti-inflammatory effects of GEO to those of estrogen, rats were treated beginning 4 days prior to SCW injection with 17-β estradiol (E2, Sigma, St. Louis, MO) using pharmacologic doses with demonstrated anti-arthritic efficacy in other rat arthritis models [20-22] that also normalize bone parameters and prevent uterine atrophy in ovariectomized rats [23]25] (200 μg/kg or 600 μg/kg subcutaneously five times a week, as indicated, vs. vehicle alone [1 μl/g sesame oil]). Joint inflammation was determined in a blinded fashion by daily assessment of arthritic index (AI) in each distal limb using standard criteria (0 = normal; 1 = slight erythema and edema; 2 = increased edema with loss of landmarks; 3 = marked edema; 4 = marked edema with ankylosis on flexion) [19,20,23,24]. Bone mineral density (BMD) of the total femur was determined using a Piximus densitometer (GE Lunar, Madison, WI) at end of experiment (days 28-30) as previously described [8]. To monitor for possible toxic effects of treatments in normal or SCW-treated animals, daily weights were recorded, and serum creatinine and alanine aminotransferase (ALT) levels in blood samples obtained 28 days after injection of SCW (or vehicle) were determined using a Hemagen Diagnostics Endocheck Plus Chemistry Analyzer to monitor for possible renal- or hepatotoxicity, respectively [6,8]. Circulating white blood cell counts and hematocrit in whole blood were assayed using a Hemavet 880 analyzer (CDC Technologies, Oxford, CT), with manual determination of differential WBC counts [6,8,25].
2.3. Histology
Liver specimens obtained 28 days after SCW injection were fixed in 10% formalin and embedded in paraffin [6,7,10,14]. Hepatic granuloma formation was assessed in hematoxylin and eosin (H&E) stained sections of liver in a blinded fashion using standard criteria [6,7,10,14].
2.4. Statistical Analyses
All values are presented as mean ± the SEM, unless otherwise stated, with statistical significance determined by ANOVA or non-parametric Kruskal-Wallis analysis with post hoc testing, non-parametric Mann Whitney analysis, or Fisher's Exact Test, as appropriate, using Instat 3.0b software (Graphpad, San Diego, CA).
3. Results
3.1. Isolation and characterization of GEO
Fraction 1, a lipophilic sesquiterpenoid-containing essential oil fraction isolated as described in the methods from a crude ginger extract (23% yield), was gingerol-free, as determined by HPLC (“GEO”, Figure 1A). The HPLC profile of the GEO can be compared to that of a previously tested: 1) GEO-free fraction containing gingerols and their derivatives (“gingerol fraction” [50% yield], 38% by weight of the 3 most common gingerols, Figure 1B) [6], or 2) the crude ginger extract itself (“crude extract”, 18% by weight of the 3 most common gingerols, Figure 1C) [6]. The IC50 for inhibition of PGE2 production in vitro by the GEO fraction (IC50 = 0.411 μg extract/ml) was 20-fold lower than those previously reported for the gingerol fraction or gingerol-containing crude ginger extract (IC50 = 0.06-0.07 μg extract/ml or 0.01-0.02 μg gingerols/ml) [6].
3.2. In vivo effect of ginger essential oils (GEO) on joint inflammation
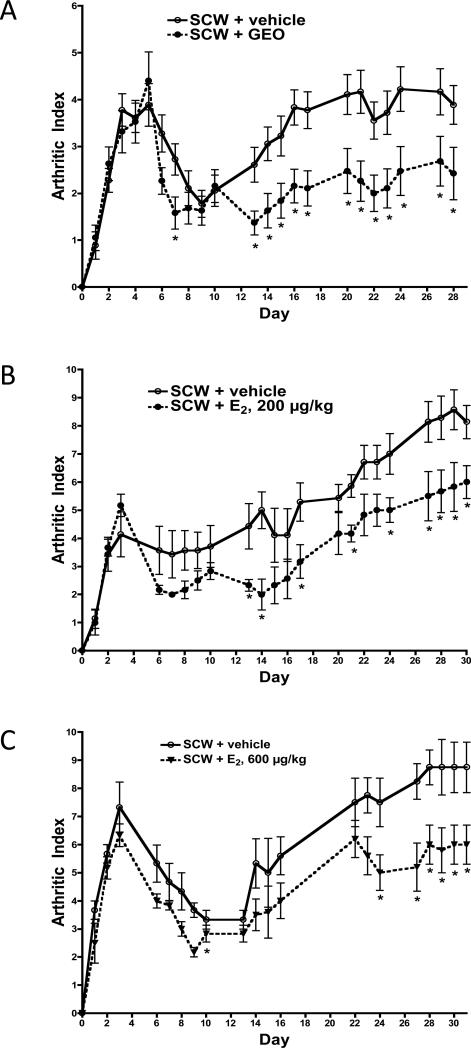
A GEO dosing scheme (28 mg/kg/d beginning 4 days prior to SCW injection) similar to that previously used for the crude extract (25 mg gingerols/kg with GEO ≈ 32 mg/kg/d) or gingerol fraction (25 mg gingerols/kg/d) was employed to explore whether the excess joint protection previously documented with the crude (vs. gingerol only) extract in SCW-induced arthritis could be attributable to bioactivity of its GEO content [6]. Acute joint swelling in SCW-injected rats was unaltered by GEO treatment (Figure 2A, day 3). In contrast, GEO treatment significantly inhibited the chronic phase of arthritis (days 13-28), decreasing joint swelling by 38% on day 28 (Figure 2A).
Figure 2.
Effects of GEO or E2 on joint inflammation. Female Lewis rats were injected on day 0 with SCW (or vehicle) to induce arthritis with daily GEO, E2, or vehicle treatments starting 4 days prior to SCW injection as described in Methods and Materials. Joint swelling in limbs of SCW-injected rats was assessed at times indicated and expressed as mean arthritic index (AI, mean ± SEM)) (scale 0-4/limb for total possible score of 16) with statistical significance assessed by ANOVA with Mann Whitney analysis. *p < 0.05, treated vs. vehicle control. A. GEO (28 mg/kg, n = 19) or vehicle alone (DMSO, 1 μl/g, n = 18) were dosed ip daily 5-7 times a week. B. E2 (200 μg/kg, n = 9) or vehicle alone (sesame seed oil, μl/g, n = 9) were dosed sc daily 5 times a week. C. E2 (600 μg/kg, n = 9) or vehicle alone (sesame seed oil, μl/g, n = 9) were dosed sc daily 5 times a week.
3.3. In vivo effect of GEO on hepatic granuloma formation
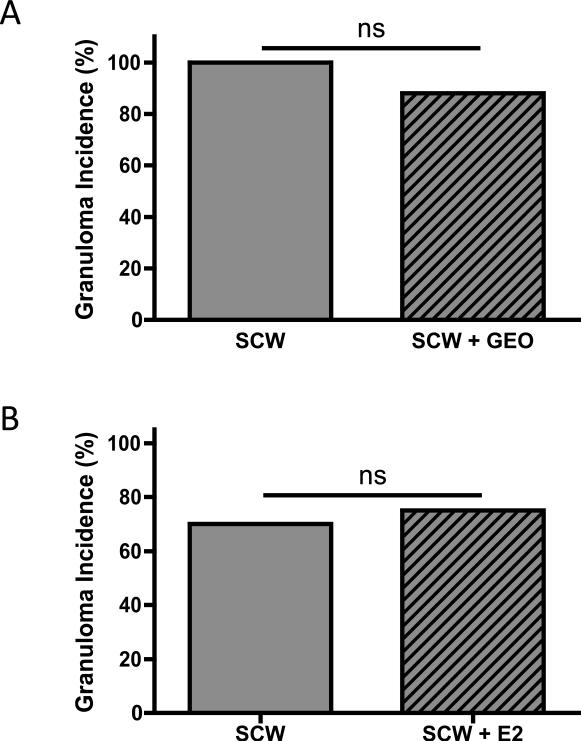
In previous experiments, the crude ginger extract decreased the incidence of granuloma formation at site of SCW deposition in the liver by 76% while gingerol-only extracts were without effect [6], suggesting that blockade of granuloma formation by the crude extract might be attributable to its the GEO content. However, treatment of SCW-injected animals with GEO in isolation did not alter the granulomatous response; the incidence of granuloma formation in the livers of SCW-injected rats was unchanged in GEO vs. vehicle-treated animals (Figure 3A).
Figure 3.
Effects of GEO or E2 on incidence of hepatic granuloma formation. Female Lewis rats were injected on day 0 with SCW (or vehicle) to induce arthritis with daily GEO, E2, or vehicle treatments starting 4 days prior to SCW injection as described in Methods and Materials. The incidence of granuloma formation on day 28-30 was assessed histologically as described in SCW-injected animals. Statistical significance was determined by Fisher's exact test. NS = non-significant. A. GEO (28 mg/kg, n = 8) or vehicle alone (DMSO, 1 μl/g, n = 8) were dosed ip daily 5-7 times a week. B. E2 (200 μg/kg or 600 μg/kg, n = 12) or vehicle alone (sesame seed oil, μl/g, n = 10) were dosed sc daily 5 times a week. The E2 doses tested did not alter granuloma incidence when analyzed separately (data not shown) or in combination, as demonstrated here.
3.4. Tolerability of GEO
Mortality, which only occurred in SCW-injected animals, was not altered by GEO (vs. vehicle) treatment in control or SCW-injected rats (Table1). GEO treatment did not alter body weight, which tends to decrease in SCW-treated rats, in control or SCW-injected rats (Table 1). Circulating leukocyte counts, which are elevated in SCW-injected rats, were unchanged by GEO treatment in control or SCW-injected rats (Table 1). Neutrophil, monocyte and lymphocyte counts were similarly unaltered by GEO treatment in control or SCW-injected animals (Table 1). Blood hematocrit values, which decrease in SCW-injected animals, were unchanged by GEO treatment in SCW-injected or control animals (Table 1). Hepatic function, which remained normal in SCW-injected animals as assessed by ALT, was unaltered by GEO treatment in control or SCW-injected animals (Table 1). Creatinine levels, which were unchanged by GEO treatment in control animals, were slightly but statistically increased in SCW-injected animals treated with GEO, a change that is of questionable physiologic significance given its small magnitude (Table 1).
Table 1.
Tolerability of GEO in Normal and Arthritic Rats
| Toxicity Monitoring | Control (vehicle) | GEO | SCW | SCW + GEO |
|---|---|---|---|---|
| Mortality (number died/total) | 0% (0/9) | 0% (0/5) | 4% (1/23) | 7% (2/29) |
| Body Weight (g) | 159.7 ± 4.6 | 158.6 ± 6.8 | 146.7 ± 3.5 | 143.3 ± 2.5a |
| ALT (U/L) | 17.6 ± 3.0 | 13.9 ± 3.3 | 13.4 ± 1.6 | 13.1 ± 2.5 |
| Creatinine (mg/dL) | 0.24 ± 0.02 | 0.26 ± 0.05 | 0.28 ± 0.02 | 0.34 ± 0.02a |
| COMPLETE BLOOD COUNT | ||||
| WBC (K/μl) | 9.1 ± 1.3 | 10.2 ± 1.9 | 18.04 ± 1.5b | 18.2 ± 2.0b |
| Neutrophils (K/μl) | 1.6 ± 0.5 | 2.9 ± 1.1 | 8.0 ± 1.2b | 7.2 ± 1.0b |
| Lymphocytes (K/μl) | 6.5 ± 1.0 | 6.9 ± 0.9 | 9.1 ± 0.7 | 10.1 ± 1.2 |
| Monocytes (K/μl) | 0.24 ± 0.03 | 0.35 ± 0.07 | 0.90 ± 0.09a | 1.21 ± 0.21c |
| Hematocrit (%) | 44.9 ± 1.3 | 39.9 ± 4.0 | 34.7 ± l.2b | 34.3 ± 1.2b |
Values are expressed as mean ± SEM with statistical significance of differences between all groups determined by ANOVA with post-hoc testing or Fisher's exact test, as appropriate.
p < 0.05 vs. vehicle
p<0.01 vs. vehicle
p<0.001. vs. vehicle.
3.5. In vivo effect of 17ß estradiol on joint inflammation and hepatic granuloma formation
Using the same dosing strategies as for GEO (treatment beginning 4 days prior to SCW injection), treatment with 200 ug/kg E2, a dose that normalizes BMD and prevents uterine atropy in OVX rats [23], had no effect on acute joint swelling (Figure 2B, day 3) but decreased chronic joint inflammation by 31% (Figure 2B, day 28) in ovary-intact SCW-injected female rats. A three-fold higher E2 dose (600 ug/kg) had exactly the same effect (Figure 2C); the initial phase of joint swelling was unchanged (Figure 2C, day 3) while chronic inflammation was significantly decreased (Figure 2C, day 28). The magnitude of E2's inhibitory effect on chronic joint inflammation was the same for both doses, blocking joint swelling by one third (Figures 2B and 2C), suggesting that this was the maximal achievable anti-arthritic effect. Hepatic granuloma formation was unaltered by treatment with either dose of E2 (Figure 3B).
3.6. Comparative effects of GEO and 17-ß estradiol on classic estrogen-responsive tissues
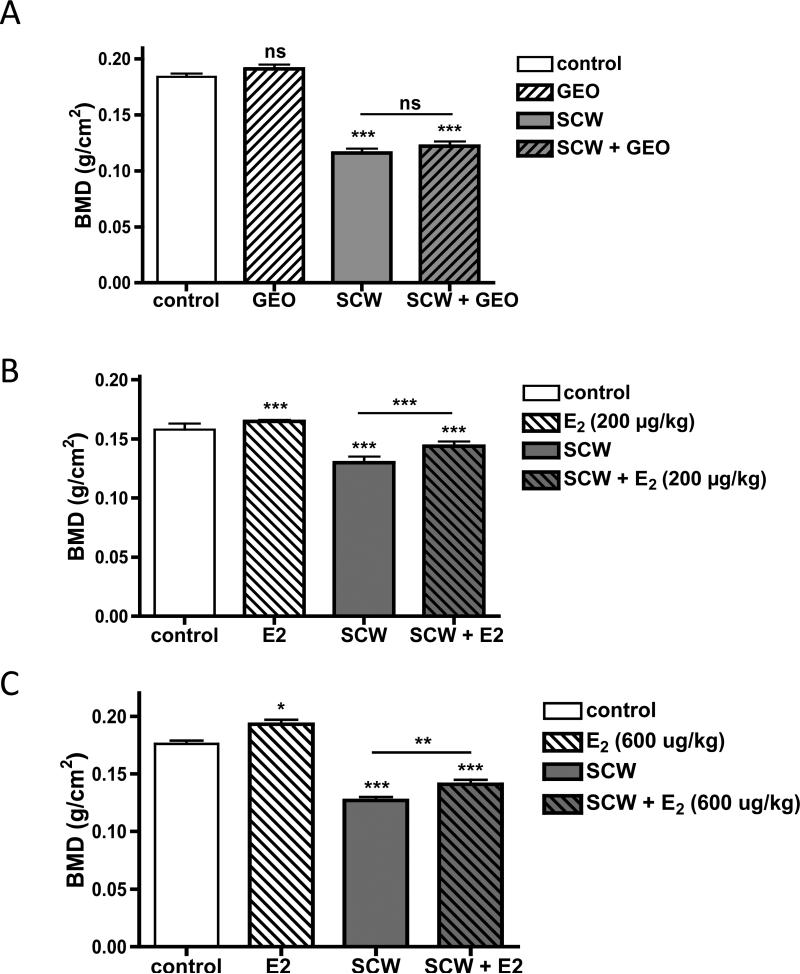
Because GEO effects on joint and granulomatous inflammation in the SCW model mirrored those of E2, the effects of both treatments on classic estrogen-responsive tissues were compared to explore the possibility that in vivo effects of GEO could be attributed to ER agonist activity. In non-arthritic control animals, femoral BMD was unchanged after one month of GEO treatment (Figure 4A, GEO vs. control). In contrast, E2 treatment of the same duration at low (200 ug/kg/d) or high doses (600 mg/kg/d E2) increased BMD by 4% or 10%, respectively (Figure 4B&C, control vs. E2). Femoral BMD in arthritic SCW-injected animals, which decreased by 28-37% as compared to non-arthritic control animals in these experiments, was unaltered by GEO (Figure 4A, SCW vs. SCW + GEO) but was significantly increased (11%) by treatment with either dose of E2 (Figure 4B&C, SCW vs. SCW + E2).
Figure 4.
Effects of GEO or E2 on femoral BMD. Female Lewis rats were injected on day 0 with vehicle (control) or SCW to induce arthritis with daily GEO, E2, or vehicle treatments starting 4 days prior to SCW injection as described in Methods and Materials. Femoral BMD (mean ± SEM, n = 8-36/group) was assessed at time of sacrifice on day 28-30 using a Piximus densitometer as described in Materials and Methods. Statistical significance was determined by ANOVA with post-hoc testing. * p < 0.05 vs. control. ** p < 0.01 vs. SCW. *** p < 0.001 vs. control or SCW, as indicated. NS = non significant. A. GEO (28 mg/kg) or vehicle alone (DMSO, 1 μl/g) were dosed ip daily 5-7 times a week. B. E2 (200 μg/kg) or vehicle alone (sesame seed oil, μl/g) were dosed sc daily 5 times a week. C. E2 (600 μg/kg) or vehicle alone (sesame seed oil, μl/g) were dosed sc daily 5 times a week.
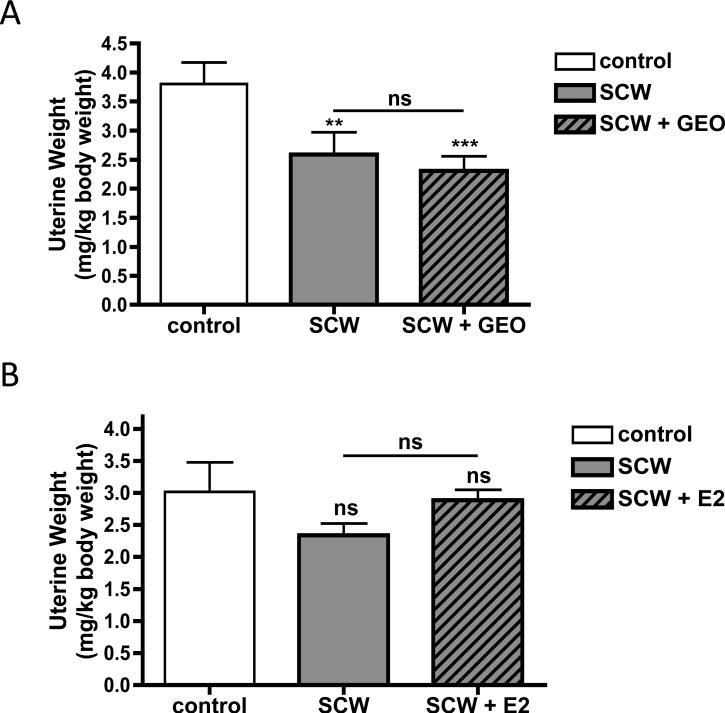
In ovary intact female rats injected with SCW, uterine weights tended to decrease slightly, a trend of small magnitude, compared to OVX rats [23,26], that achieved statistical significance in certain experiments (Figure 5A and B, SCW vs. control). Uterine weights, while lower in GEO treated animals, were statistically unchanged by GEO treatment in SCW-injected rats (Figure 5A). In ovary–intact SCW-injected animals treated with a 17-β estradiol dose that prevents uterine atrophy in OVX animals (200 ug/kg/d) [23], an opposing trend towards increased uterine weight did not achieve statistical significance. To more clearly assess possible in vivo estrogenic effects of GEO on the uterus, effects of GEO on uterine weights were determined in ovariectomized (OVX) Lewis rats [26]. Uterine weights in OVX Lewis rats, which decreased to 11% of sham controls after one month (sham, 3.84 ± 0.37 mg uterus/kg body weight vs. OVX, 0.43 ± 0.03 mg/kg), were unaltered by one month of GEO treatment using the same dosing scheme as in the arthritis experiments (OVX + GEO, 0.55 ± 0.06 mg/kg body weight, p > 0.05 vs. OVX).
Figure 5.
Effects of GEO or E2 on uterine weights. Female Lewis rats were injected on day 0 with vehicle (control) or SCW to induce arthritis with daily GEO, E2, or vehicle treatments starting 4 days prior to SCW injection as described in Methods and Materials. Uterine weights, expressed as a ratio of body weight (mg uterus/kg body weight, average ± SEM with n = 8-13/group), were assessed at time of sacrifice on day 28-30. Statistical significance was determined by ANOVA with post-hoc testing. ** p < 0.01 vs control. *** p < 0.001 vs control. NS = non significant. A. GEO (28 mg/kg) or vehicle alone (DMSO, 1 μl/g) were dosed ip daily 5-7 times a week. B. E2 (200 μg/kg) or vehicle alone (sesame seed oil, μl/g) were dosed sc daily 5 times a week.
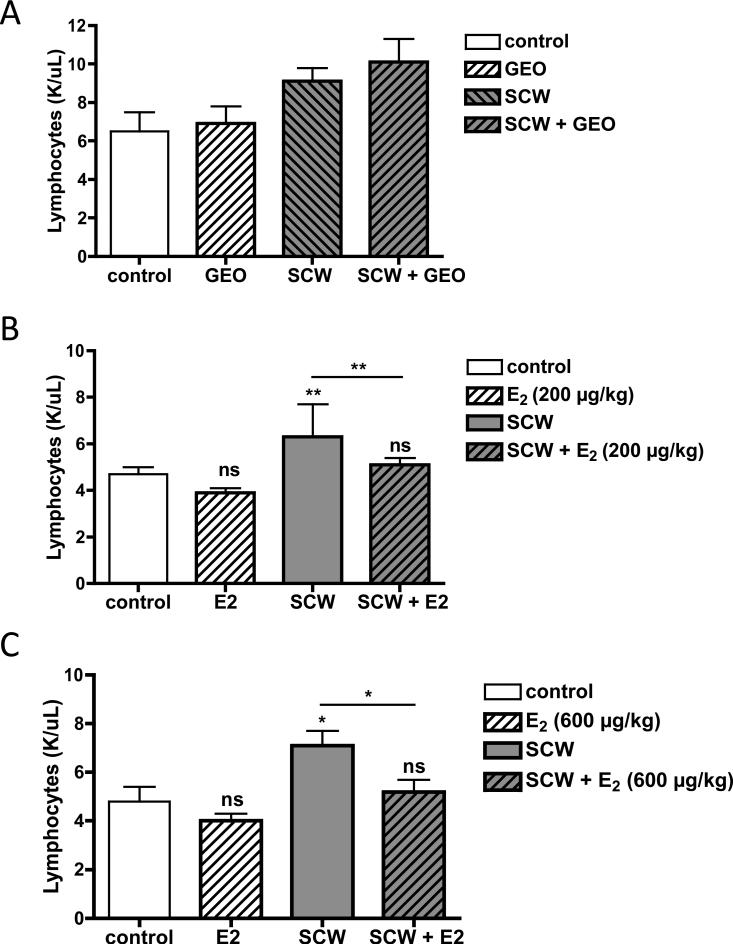
Consistent with previous reports, lymphocyte counts were decreased in estrogen-treated animals [27], an effect that achieved statistical significance in SCW-injected animals treated with either E2 dose (Figure 6B and C). This effect was lymphocyte-specific as total white counts, which are primarily elevated in SCW-injected animals due to increased neutrophils (Table 1), were unchanged by E2-treatment (data not shown). In contrast, lymphocyte counts in SCW-injected animals treated with GEO tended to be higher, but were statistically unchanged (Figure 6A).
Figure 6.
Effects of GEO or E2 on circulating lymphocyte counts. Female Lewis rats were injected on day 0 with vehicle (control) or SCW to induce arthritis with daily GEO, E2, or vehicle treatments starting 4 days prior to SCW injection as described in Methods and Materials. Lymphocyte counts (mean ± SEM, n = 4-16/group) were assessed at time of sacrifice on day 28-31 as described in Materials and Methods. Statistical significance was determined by ANOVA with post-hoc testing. * p < 0.05 vs. control or SCW, as indicated. ** p < 0.01 vs. control or SCW, as indicated. NS = non significant. A. GEO (28 mg/kg) or vehicle alone (DMSO, 1 μl/g) were dosed ip daily 5-7 times a week. B. E2 (200 μg/kg) or vehicle alone (sesame seed oil, μl/g) were dosed sc daily 5 times a week. C. E2 (600 μg/kg) or vehicle alone (sesame seed oil, μl/g) were dosed sc daily 5 times a week.
4. Discussion
Previous arthritis studies by our laboratory demonstrating a greater joint protective effect of a crude ginger extract containing gingerols and GEO as compared to gingerols alone (when normalized to gingerol content) suggested that the crude extract's GEO content could account for its enhanced anti-inflammatory effect in an experimental model of rheumatoid arthritis (RA) [6]. Consistent with this hypothesis, isolated administration of GEO in the experiments described here did significantly reduce joint swelling in experiments using the same SCW-induced model of RA. However, unlike the protective effects of gingerol-containing extracts, which prevented both early and late manifestations of joint inflammation [6], GEO treatment only offered protection during the later chronic, joint-destructive phase of arthritis. With respect to protection during the later stages of arthritis in this model, the totality of experimental findings suggests that a possible additive effect of GEO (38% inhibition) when combined with gingerols (62% inhibition) [6] could explain the increased effectiveness of the GEO- + gingerol-containing crude extract, which blocked chronic joint inflammation by 97% when administered at a dose delivering similar amounts of each of the secondary metabolites [6]. The complete lack of effect of GEO in limiting acute joint inflammation suggests a possible divergence in the mechanism of GEO's joint protective effects as compared to the gingerols, whose anti-arthritic efficacy was, in fact, greater during acute (vs. chronic) arthritis [6].
Previously, to our knowledge, only two interventions have been reported to prevent chronic (but not acute) joint inflammation in the SCW-model: 1) depletion/inactivation of T cells [28,29], or 2) treatment with human chorionic gonadotropin (hCG), which protects via an unknown mechanism [30]. In the SCW model, T cells are required for generating the chronic (but not acute) recrudescence of joint inflammation in the SCW model and are also key mediators of the granulomatous response at sites of SCW deposition in the liver; both of these inflammatory responses are inhibited by T cell depletion/inactivation [14,28,29]. In contrast, GEO inhibited chronic joint inflammation but had no effect on granulomatous inflammation in the SCW model; thus, it is unlikely that the joint protective effects of GEO can be attributed to in vivo modulation of T cell function.
While neither GEO- nor gingerols-only [6] altered granulomatous inflammation in the SCW model, the crude ginger extract profoundly inhibited this response [6]. There are two possible explanations for this constellation of findings: 1) either a combination of GEO + gingerols is required for inhibition of granulomatous inflammation, or 2) additional chemical moieties, such as the polar polysaccharides that are also uniquely present in the crude extract (e.g. column chromatography fraction 11) [12], mediate this anti-inflammatory effect. Support for the later explanation can be found in our previous studies examining the effects of turmeric extracts in this same model of RA; only turmeric extracts containing polar compounds were capable of inhibiting granulomatous inflammation [7,10]. Immunomodulatory effects of polysaccharide moieties have previously been reported in other settings [31-33]. In the context of RA, where reactivation of tuberculosis has been reported in response to certain anti-inflammatory treatments [16], inhibition of the protective granulomatous response by ginger- (or turmeric-) derived polysaccharides could similarly result in untoward clinical effects, making polysaccharide-containing ginger extracts less desirable.
The joint protective effects of essential oils derived from ginger, which modestly (38%) inhibited chronic joint inflammation, are very different from those previously reported by our laboratories for the essential oils of turmeric, which profoundly blocked joint inflammation throughout the entire course of disease (90-100%) [10]. Sesquiterpenes are major constituents of the essential oils of turmeric (ar-turmerone, α-turmerone and ß-turmerone)10 and ginger (zingiberene and ar-curcumene) [34-43], while the monoterpene isomers, neral and geranial, collectively referred to as citral, are only present in GEO and comprise a variable but significant amount by weight (16-75%) [35,37,39-43]. Because estrogenic effects have been reported previously for citral in vitro [17,18], including low affinity estrogen receptor (ER) binding and estrogenic activity, the in vivo effects of GEO were compared to those of 17ß-estradiol in the SCW model. The anti-inflammatory effects of GEO vs. estradiol were identical; both provided a similar magnitude of protection against chronic (but not acute) joint inflammation while leaving hepatic granuloma formation untouched. This similarity in anti-inflammatory activity suggests that joint protection by GEO could be attributable to ER-mediated effects of its monoterpene moieties. Running contrary to this postulate, however, GEO treatment in SCW or OVX rats did not recapitulate estrogen's effects on classic estrogen target tissues; GEO did not enhance uterine weight in intact or OVX rats, increase bone density or suppress lymphocyte number. This lack of GEO effect in vivo on classic estrogen target organs mirrors that reported in vivo for purified citral [18], an outcome that could be explained by the ≥10,000-fold lower ERα and ERß binding affinity of these monoterpenes as compared to E2 itself [18]. At the same time, the possibility that GEO could be acting as a selective estrogen receptor modulator (SERM), having tissue specific effects that allowed for estrogenic effects in inflamed joints, but not uterus or bone, cannot be discounted by these data [44].
In conclusion, the totality of our experimental studies exploring the anti-inflammatory effects of ginger extracts containing one or both of ginger's secondary metabolites, suggest that the enhanced anti-arthritic efficacy of chemically complex ginger extracts (vs. gingerol-only or essential oil-only fractions) [6] is likely due to additive effects of both classes of secondary metabolites present in the crude extract, the gingerols and essential oils, while its ability to block granulomatous inflammation is likely attributable to the polar compounds that are also present in the crude extract. Because reactivation of tuberculosis due to inhibition of granulomatous inflammatory processes can be an unwanted side effect of anti-inflammatory arthritis treatments [16], these findings suggest that a ginger-derived extract that contains joint-protective gingerols and essential oils but lacks polysaccharides and other polar compounds, may have the best efficacy and safety profile for use in arthritis. While we cannot rule out the possibility that ginger's essential oils may be acting as phytoestrogens in blocking joint inflammation in this model due to the similarly of their anti-arthritic effects with those of estrogen, the complete lack of evidence of classic in vivo effects of GEO in other estrogen-responsive tissues makes this less likely, unless GEO have SERM-like properties. In this event, a careful evaluation of potential adverse (estrogenic) effects of GEO on mammary tissue would also be of great importance prior to clinical use given the possible breast cancer promoting effects of estrogens [44].
One major limitation of the studies presented here is the use of an ip (not oral) dosing strategy to facilitate a comparison of the bioactivity of different ginger (and turmeric) constituents independent of differences in their oral bioavailability. While turmeric, one of the top selling herbs in the United States [45], has been frequently proposed and studied as an anti-arthritic agent [46,47] and patients will RA are frequent users of alternative treatments [48], much less is known about the pharmacokinetics and potential anti-arthritic benefits of ginger. Human studies have demonstrated good tolerability following oral administration of up to 2 g/d of a hydroalcoholic ginger root extract containing 5% gingerols (rat equivalent dose of 12 mg gingerols/kg/d) and an unknown quantity of essential oils [49], suggesting that the gingerol doses evaluated in our previous experimental arthritis studies utilizing crude and essential oil depleted extracts are clinically relevant [6]. Oral doses of GEO up to 500 mg/kg/d in rats have been demonstrated to be well tolerated with anti-inflammatory effects (human equivalent dose of 4 g/d) [50,51]. While oral doses of GEO were not tested here, oral doses of turmeric essential oils in a similar dosing range (560 mg/kg/d) were well tolerated with anti-arthritic efficacy in the SCW model, although their protective effect was much more attenuated as compared to ip dosing [10]. Clinical studies evaluating the anti-arthritic efficacy of ginger preparations in RA are completely lacking, while a small number of studies in osteoarthritic patients evaluating poorly characterized extracts containing ginger, alone or in combination with other herbs, report biological responses [52-55]. The studies presented here suggest that clinical investigation of the utility and safety of ginger dietary supplements to quell joint disease in inflammatory arthritis may be warranted, but should clearly include careful consideration and characterization of the chemical content of the products to be tested.
Highlights.
While anti-inflammatory effects of ginger are often attributed to its gingerols, each of its secondary metabolites, the essential oils and the gingerols, are joint protective in an experimental arthritis model.
Anti-arthritic effects of ginger essential oils (GEO) mirrored those of 17-ß estradiol.
However, GEO had no effect on classic estrogen-responsive organs.
GEO were well tolerated.
GEO and gingerols may have additive joint protective effects in arthritis.
Acknowledgments
Funding Source: This work was supported by the Office of Dietary Supplements (ODS) and the National Center for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH) (5R21AT004182) and the Arthritis Foundation (to JLF). The contents are solely the responsibility of the authors and do not necessarily represent the official views of NCCIH, ODS, NIH or the Arthritis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CHEMICAL COMPOUNDS STUDIED IN THIS ARTICLE
17 beta estradiol (PubChem CID: 5757), 6-gingerol (PubChem CID: CID: 442793), geranial (PubChem CID: 638011), neral (PubChem CID: 643779).
References
- 1.Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data 2004. 343:1–19. [PubMed] [Google Scholar]
- 2.Palatty PL, Haniadka R, Valder B, Arora R, Baliga MS. Ginger in the prevention of nausea and vomiting: a review. Crit Rev Food Sci Nutr. 2013;53:659–69. doi: 10.1080/10408398.2011.553751. [DOI] [PubMed] [Google Scholar]
- 3.Ali BH, Blunden G, Tanira MO, Nemmar A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem Toxicol. 2008;46:409–20. doi: 10.1016/j.fct.2007.09.085. [DOI] [PubMed] [Google Scholar]
- 4.Grzanna R, Lindmark L, Frondoza CG. Ginger--an herbal medicinal product with broad anti-inflammatory actions. J Med Food. 2005;8:125–32. doi: 10.1089/jmf.2005.8.125. [DOI] [PubMed] [Google Scholar]
- 5.Chrubasik S, Pittler MH, Roufogalis BD. Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine. 2005;12:684–701. doi: 10.1016/j.phymed.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Funk JL, Frye JB, Oyarzo JN, Timmermann BN. Comparative effects of two gingerol-containing Zingiber officinale extracts on experimental rheumatoid arthritis. J Nat Prod. 2009;72:403–7. doi: 10.1021/np8006183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funk JL, Oyarzo JN, Frye JB, Chen G, Lantz RC, Jolad SD, Solyom AM, Timmermann BN. Turmeric extracts containing curcuminoids prevent experimental rheumatoid arthritis. J Nat Prod. 2006;69:351–5. doi: 10.1021/np050327j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Funk JL, Frye JB, Oyarzo JN, Kuscuoglu N, Wilson J, McCaffrey G, Stafford G, Chen G, Lantz RC, Jolad SD, Solyom AM, Kiela PR, Timmermann BN. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452–64. doi: 10.1002/art.22180. [DOI] [PubMed] [Google Scholar]
- 9.Funk JL, Timmermann BN. Translational investigation of turmeric for arthritis treatment: a review of lessons learned. Natural Product Communication. 2006;1:1061–1066. [Google Scholar]
- 10.Funk JL, Frye JB, Oyarzo JN, Zhang H, Timmermann BN. Anti-arthritic effects and toxicity of the essential oils of turmeric (Curcuma longa L.). J Agric Food Chem. 2010;58:842–9. doi: 10.1021/jf9027206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Timmerman BN, Funk JL. Multidisciplinary Studies of Anti-Inflammatory Botanicals: Ginger and Turmeric. In: Carkeet C, Grann K, Randolph RK, Venzon DS, Izzy S, editors. Phytochemicals: Health Promotion and Therapeutic Potential. CRC Press; Boca Raton, FL: 2012. pp. 47–72. [Google Scholar]
- 12.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–35. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Cromartie WJ, Craddock JG, Schwab JH, Anderle SK, Yang CH. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahl SM, Allen JB, Dougherty S, Evequoz V, Pluznik DH, Wilder RL, Hand AR, Wahl LM. T lymphocyte-dependent evolution of bacterial cell wall-induced hepatic granulomas. J Immunol. 1986;137:2199–209. [PubMed] [Google Scholar]
- 15.Ramakrishnan L. Revisiting the role of the granuloma in tuberculosis. Nat Rev Immunol. 2012;12:352–66. doi: 10.1038/nri3211. [DOI] [PubMed] [Google Scholar]
- 16.Salgado E, Gómez-Reino JJ. The risk of tuberculosis in patients treated with TNF antagonists. Expert Rev Clin Immunol. 2011;7:329–40. doi: 10.1586/eci.11.6. [DOI] [PubMed] [Google Scholar]
- 17.Geldof AA, Engel C, Rao BR. Estrogenic action of commonly used fragrant agent citral induces prostatic hyperplasia. Urol Res. 1992;20:139–44. doi: 10.1007/BF00296526. [DOI] [PubMed] [Google Scholar]
- 18.Howes MJ, Houghton PJ, Barlow DJ, Pocock VJ, Milligan SR. Assessment of estrogenic activity in some common essential oil constituents. J PharmPharmacol. 2002;54:1521–8. doi: 10.1211/002235702216. [DOI] [PubMed] [Google Scholar]
- 19.Islander U, Jochems C, Lagerquist MK, Forsblad-d'Elia H, Carlsten H. Estrogens in rheumatoid arthritis; the immune system and bone. Mol Cell Endocrinol. 2011;335:14–29. doi: 10.1016/j.mce.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Yoshioka T, Hagino H, Yamasaki D, Okano T, Teshima R. Effect of estrogen replacement therapy on arthritis and bone mineral density in estrogen-replete rats with collagen-induced arthritis. Mod Rheumatol. 2008;18:23–8. doi: 10.1007/s10165-007-0011-2. [DOI] [PubMed] [Google Scholar]
- 21.Yamasaki D, Enokida M, Okano T, Hagino H, Teshima R. Effects of ovariectomy and estrogen replacement therapy on arthritis and bone mineral density in rats with collagen-induced arthritis. Bone. 2001;28:634–40. doi: 10.1016/s8756-3282(01)00426-4. [DOI] [PubMed] [Google Scholar]
- 22.Waksman Y, Hod I, Friedman A. Therapeutic effects of estradiol benzoate on development of collagen-induced arthritis (CIA) in the Lewis rat are mediated via suppression of the humoral response against denatured collagen type II (CII). Clin Exp Immunol. 1996;103:376–83. doi: 10.1111/j.1365-2249.1996.tb08290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow JW, Lean JM, Chambers TJ. 17 beta-estradiol stimulates cancellous bone formation in female rats. Endocrinology. 1992;130:3025–32. doi: 10.1210/endo.130.5.1572310. [DOI] [PubMed] [Google Scholar]
- 24.Wronski TJ, Cintrón M, Doherty AL, Dann LM. Estrogen treatment prevents osteopenia and depresses bone turnover in ovariectomized rats. Endocrinology. 1988;123:681–6. doi: 10.1210/endo-123-2-681. [DOI] [PubMed] [Google Scholar]
- 25.Funk JL, Frye JB, Davis-Gorman G, Spera AL, Bernas MJ, Witte MH, Weinand ME, Timmermann BN, McDonagh PF, Ritter L. Curcuminoids limit neutrophil-mediated reperfusion injury in experimental stroke by targeting the endothelium. Microcirculation. 2013;20:544–54. doi: 10.1111/micc.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright LE, Frye JB, Timmermann BN, Funk JL. Protection of trabecular bone in ovariectomized rats by turmeric (Curcuma longa L.) is dependent on extract composition. J Agric Food Chem. 2010;58:9498–504. doi: 10.1021/jf101873f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn G, Hardegg W. Quantitative studies of haematological values in long-term ovariectomized, ovariohysterectomized and hysterectomized rats. Lab Anim. 1991;25:40–5. doi: 10.1258/002367791780808248. [DOI] [PubMed] [Google Scholar]
- 28.Yocum DE, Allen JB, Wahl SM, Calandra GB, Wilder RL. Inhibition by cyclosporin A of streptococcal cell wall-induced arthritis and hepatic granulomas in rats. Arthritis Rheum. 1986;29:262–73. doi: 10.1002/art.1780290215. [DOI] [PubMed] [Google Scholar]
- 29.Allen JB, Malone DG, Wahl SM, Calandra GB, Wilde RL. Role of the thymus in streptococcal cell wall-induced arthritis and hepatic granuloma formation. Comparative studies of pathology and cell wall distribution in athymic and euthymic rats. J Clin Invest. 1985;76:1042–56. doi: 10.1172/JCI112057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song XY, Zeng L, Jin W, Pilo CM, Frank ME, Wahl SM. Suppression of streptococcal cell wall-induced arthritis by human chorionic gonadotropin. Arthritis Rheum. 2000;43:2064–72. doi: 10.1002/1529-0131(200009)43:9<2064::AID-ANR18>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 31.Chandrasekaran CV, Sundarajan K, Edwin JR, Gururaja GM, Mundkinajeddu D, Agarwal A. Immune-stimulatory and anti-inflammatory activities of Curcuma longa extract and its polysaccharide fraction. Pharmacognosy Res. 2013;5:71–9. doi: 10.4103/0974-8490.110527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue GG, Chan BC, Hon PM, Kennelly EJ, Yeung SK, Cassileth BR, Fung KP, Leung PC, Lau CB. Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 2010;47:342–7. doi: 10.1016/j.ijbiomac.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonda R, Tomoda M, Shimizu N, Kanari M. Characterization of polysaccharides having activity on the reticuloendothelial system from the rhizome of Curcuma longa. Chem Pharm Bull (Tokyo) 1990;38:482–6. doi: 10.1248/cpb.38.482. [DOI] [PubMed] [Google Scholar]
- 34.Antonious GF, Kochhar TS. Zingiberene and curcumene in wild tomato. J Environ Sci Health B. 2003;38:489–500. doi: 10.1081/PFC-120021668. [DOI] [PubMed] [Google Scholar]
- 35.Buddhakala N, Talubmook C, Sriyotha P, Wray S, Kupittayanant S. Inhibitory effects of ginger oil on spontaneous and PGF2alpha-induced contraction of rat myometrium. Planta Med. 2008;74:385–91. doi: 10.1055/s-2008-1034323. [DOI] [PubMed] [Google Scholar]
- 36.El-Ghorab AH, Nauman M, Anjum FM, Hussain S, Nadeem MA. comparative study on chemical composition and antioxidant activity of ginger (Zingiber officinale) and cumin (Cuminum cyminum). J Agric Food Chem. 2010;28:8231–7. doi: 10.1021/jf101202x. [DOI] [PubMed] [Google Scholar]
- 37.Gupta S, Pandotra P, Ram G, Anand R, Gupta AP, Husain K, Bedi YS, Mallavarapu GR. Composition of a monoterpenoid-rich essential oil from the rhizome of Zingiber officinale from north western Himalayas. Nat Prod Commun. 2011;6:93–6. [PubMed] [Google Scholar]
- 38.Jeena K, Liju VB, Kuttan R. A preliminary 13-week oral toxicity study of ginger oil in male and female Wistar rats. Int J Toxicol. 2011;30:662–70. doi: 10.1177/1091581811419023. [DOI] [PubMed] [Google Scholar]
- 39.Kiran CR, Chakka AK, Amma KP, Menon AN, Kumar MM, Venugopalan VV. Influence of cultivar and maturity at harvest on the essential oil composition, oleoresin and [6]-gingerol contents in fresh ginger from northeast India. J Agric Food Chem. 2013;61:4145–54. doi: 10.1021/jf400095y. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira de Melo GA, Grespan R, Fonseca JP, Farinha TO, da Silva EL, Romero AL, Bersani-Amado CA, Cuman RK. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J Nat Med. 2011;65:241–6. doi: 10.1007/s11418-010-0479-5. [DOI] [PubMed] [Google Scholar]
- 41.Sasidharan I, Venugopal VV, Menon AN. Essential oil composition of two unique ginger (Zingiber officinale Roscoe) cultivars from Sikkim. Nat Prod Res. 2012;26:1759–64. doi: 10.1080/14786419.2011.571215. [DOI] [PubMed] [Google Scholar]
- 42.Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Chemistry, antioxidant and antimicrobial investigations on essential oil andoleoresins of Zingiber officinale. Food Chem Toxicol. 2008;46:3295–302. doi: 10.1016/j.fct.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Wohlmuth H, Smith MK, Brooks LO, Myers SP, Leach DN. Essential oil composition of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe) grown in Australia. J Agric Food Chem. 2006;54:1414–9. doi: 10.1021/jf0521799. [DOI] [PubMed] [Google Scholar]
- 44.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–65. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith T, Lynch ME, Johnson J, Kawa K, Bauman H, Blumenthal M. Herbal Dietary Supplement Sales in US Increase 6.8% in 2014. HerbalGram. 2015;107:52–59. [Google Scholar]
- 46.Sunagawa Y, Katanasaka Y, Hasegawa K, Morimoto T. Clinical applications of curcumin. PharmaNutrition. 2015;3:131–135. [Google Scholar]
- 47.Marino A, Paterniti I, Cordaro M, Morabito R, Campolo M, Navarra M, Esposito E, Cuzzocrea S. Role of natural antioxidants and potential use of bergamot in treating rheumatoid arthritis. PharmaNutrition. 2015;3:53–59. [Google Scholar]
- 48.Efthimiou P, Kukar M, Mackenzie CR. Complementary and alternative medicine in rheumatoid arthritis: no longer the last resort. HSS J. 2010;6:108–11. doi: 10.1007/s11420-009-9133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zick SM, Djuric Z, Ruffin MT, Litzinger AJ, Normolle DP, Alrawi S, Feng MR, Brenner DE. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeena K, Liju VB, Kuttan R. A preliminary 13-week oral toxicity study of ginger oil in male and female Wistar rats. Int J Toxicol. 2011;30:662–70. doi: 10.1177/1091581811419023. [DOI] [PubMed] [Google Scholar]
- 51.Nogueira de Melo GA, Grespan R, Fonseca JP, Farinha TO, da Silva EL, Romero AL, Bersani-Amado CA, Cuman RK. Inhibitory effects of ginger (Zingiber officinale Roscoe) essential oil on leukocyte migration in vivo and in vitro. J Nat Med. 2011;65:241–246. doi: 10.1007/s11418-010-0479-5. [DOI] [PubMed] [Google Scholar]
- 52.Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Andersen LA, Ibfelt HH, Christensen K, Jensen ON, Barslev J. A randomized, placebo-controlled, cross-over study of ginger extracts and ibuprofen in osteoarthritis. Osteoarthritis Cartilage. 2000;8:9–12. doi: 10.1053/joca.1999.0264. [DOI] [PubMed] [Google Scholar]
- 53.Altman RD, Marcussen KC. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44:2531–8. doi: 10.1002/1529-0131(200111)44:11<2531::aid-art433>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 54.Chopra A, Saluja M, Tillu G, Sarmukkaddam S, Venugopalan A, Narsimulu G, Handa R, Sumantran V, Raut A, Bichile L, Joshi K, Patwardhan B. Ayurvedic medicine offers a good alternative to glucosamine and celecoxib in the treatment of symptomatic knee osteoarthritis: a randomized, double-blind, controlled equivalence drug trial. Rheumatology (Oxford) 2013;52:1408–17. doi: 10.1093/rheumatology/kes414. [DOI] [PubMed] [Google Scholar]
- 55.Niempoog S, Pawa KK, Amatyakul C. The efficacy of powdered ginger in osteoarthritis of the knee. J Med Assoc Thai. 2012;95(Suppl 1):S59–64. [PubMed] [Google Scholar]