Abstract
Background
Elderly patients with primary colorectal cancer (CRC) are less frequently treated with adjuvant chemotherapy than younger patients due to concerns regarding toxicity and efficiency. We investigated how age, performance status (PS) and comorbidity influence treatment outcomes.
Patients and methods
A retrospective single-centre study of 529 patients with stages II–III CRC treated with adjuvant chemotherapy (5-fluorouracil/capecitabine+/÷oxaliplatin) from 2001 to 2011 at Herlev Hospital, Denmark. Baseline characteristics, chemotherapy and outcome were analysed with respect to age after adjusting for PS and comorbidity.
Results
Elderly patients (>70 years) had significantly more comorbidity (p<0.001) and poorer PS (p=0.001) than younger patients. Elderly were more frequently treated with single-agent therapy (p=0.001) and at lower initial dose (p<0.001). There was no age-dependent difference in 3-year disease-free survival (DFS; HR 1.09, 95% CI 0.80 to 1.47, p=0.59), in grade 3–5 toxicity (29% vs 28%, p=0.86) or in 10-year CRC mortality (28%, HR 1.07, p=0.71). In elderly patients, a reduction in chemotherapy dose intensity compared with full dose had no impact on DFS or CRC mortality. Elderly patients receiving <50% of planned cycles had shorter DFS (HR=1.78, p=0.020) and higher CRC mortality (HR=2.17, p=0.027) than elderly receiving all cycles. Poor PS in younger and elderly patients was related to shorter DFS (HR=1.95, p=0.002; HR=1.6, p=0.035, respectively) and overall survival (OS; HR=2.28, p<0.001; HR=2.03, p=0.002). Comorbidity in younger patients was significantly related to shorter DFS (HR 2.72, p<0.001), OS (HR 3.16, p<0.001) and higher CRC mortality (HR 2.70, p=0.001).
Conclusions
Choice of regimen, primary dose reduction and given dose intensity in patients treated with adjuvant chemotherapy for CRC were highly dependent on age. However, age had no impact on DFS and CRC mortality. Comorbidity in younger patients and PS in all patients were associated with shorter DFS and higher CRC mortality.
Keywords: adjuvant chemotherapy, comorbidity, colorectal cancer, elderly, performance status
Key questions.
What is already known about this subject?
Elderly patients treated with adjuvant chemotherapy for colorectal cancer (CRC) are at greater risk of treatment related toxicity than younger patients. There are no precise guidelines for treating the elderly patients, but age is independently associated with reduced primary dose, to reduce risk of toxicity. A lower dose, however, might lead to an inefficient treatment with a greater risk of relapse.
What are the new findings?
In the ACCORE study choice of regimen and given dose intensity of adjuvant chemotherapy were highly dependent on age. However, age had no impact on disease-free survival (DFS) and CRC mortality. Comorbidity in younger patients and performance status in all patients were associated with shorter DFS and higher CRC mortality. No difference was found in DFS or CRC-related mortality in elderly patients who had undergone only 50% of all cycles compared to elderly patients who had received all cycles. Receiving reduced dose intensities compared to full dose intensity had no impact on cancer prognosis in elderly patients.
How might this impact on clinical practice?
The results suggest that lower dose intensity and fewer numbers of cycles could be sufficient adjuvant treatment for elderly patients with CRC. With a lower risk of toxicity, more frail elderly patients might receive treatment, with a reduced risk of recurrence and better prognosis for elderly patients as a result.
Introduction
The incidence of colorectal cancer (CRC) increases with age.1 2 Since populations are getting older,3 more elderly patients will be diagnosed with CRC. Adjuvant chemotherapy with 5-fluorouracil (5-FU) or capecitabine after surgery for colon cancer (CC) stages II–III reduces recurrence rate and improves overall survival (OS).4 5 For elderly patients, adjuvant chemotherapy prolongs time to recurrence,6 and OS is higher in patients with CC >75 years receiving adjuvant chemotherapy.7 8 Although the benefit of single-agent treatment seems to be the same in elderly and younger patients, elderly patients are less frequently treated with adjuvant chemotherapy.9 In 5489 patients operated on for stage III CC, 63% of the patients aged 75–79 years received adjuvant chemotherapy, whereas only 14% of patients ≥85 years received treatment.7 Combination chemotherapy is considered standard therapy for patients with stage III CC, but in elderly patients the beneficial effect on disease-free survival (DFS) and OS of adding oxaliplatin to 5-FU/capecitabine is controversial.4 10–14
Many elderly patients with CRC are not treated with adjuvant chemotherapy because of high age, comorbidity, poor performance status (PS),8 or due to lack of social support and concerns regarding toxicity and efficiency. Elderly patients tend to receive no or less aggressive treatment, which could explain the higher rates of recurrence and the higher mortality seen in these patients.2 More information is needed in how comorbidity and PS influence treatment outcomes in elderly patients with CRC.
We investigated age-dependent differences and the role of PS and comorbidity in relation to type of regimen, dose intensity, number of cycles, toxicity, and time to recurrence and death in patients treated with adjuvant chemotherapy after radical resection of stage II–III CRC. We also searched for clinical biomarkers that could identify frail elderly patients with a high risk of toxicity and poor prognosis.
Methods
This is a retrospective single-centre study evaluating the efficacy and toxicity of adjuvant chemotherapy in patients who underwent radical resection and chemotherapy for primary CRC at the Department of Oncology, Herlev Hospital, Denmark, from January 2001 to January 2012. During this period, the adjuvant treatment given to patients with colon and rectum cancer was similar, that is, 6 months of 5-FU or capecitabine+/÷oxaliplatin (for details see online supplementary table S1).
esmoopen-2016-000087_S1.pdf (184.5KB, pdf)
Patients
Among patients planned to start adjuvant chemotherapy after surgery for CRC, 849 medical charts were reviewed and assessed for eligibility. Data were extracted and interpreted by one author (CML). Overall 320 patients were excluded, and 529 patients were found eligible for the present study (see online supplementary figure S2). Baseline characteristics included demographic data, comorbidities, body mass index (BMI), tobacco use, civil status, PS, tumour characteristics and blood samples (see online supplementary table S1). Cause of death was categorised into CRC mortality, other causes of death or unknown cause. Adverse events were interpreted according to the National Cancer Institute Common Toxicity Criteria (CTCAE) V.3.0. Grades 3–5 were defined as severe toxicity.
esmoopen-2016-000087_S2.pdf (78.4KB, pdf)
The statistical methods are described in detail in the online supplementary text S3.
esmoopen-2016-000087_S3.pdf (250.5KB, pdf)
Results
A total of 529 patients were included and divided into elderly patients (≥70 years, N=191) and younger patients (<70 years, N=338). Clinical and histopathological baseline characteristics are given in table 1. The mean follow-up for the two groups was 6.34 years (3 days to 13.80 years) for the younger group and 5.40 years (20 days to 13.75 years) for the elderly group. Elderly patients had significantly poorer PS and more comorbidity than the younger group. Data were therefore systematically analysed with and without adjustment for PS and comorbidity. The elderly patients were more likely to live alone and have right-side tumours than younger patients.
Table 1.
Baseline characteristics
| Age groups |
Start dose |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Missing | <70 years N (%) N=338 |
≥70 years N (%) N=191 |
p Value | 100% N (%) N=459 |
≤75% N (%) N=67 |
p Value | |
| Age | 0 | Median (range) | 61 (31–69) | 74 (70–85) | 64 (31–82) | 76 (44–85) | <0.001 | |
| Sex | 0 | F | 161 (48) | 95 (50) | 0.71 | 212 (46) | 41 (61) | 0.03 |
| M | 177 (52) | 96 (50) | 247 (54) | 26 (39) | ||||
| PS | 60 | 0 | 250 (85) | 125 (71) | 0.001 | 337 (84) | 35 (56) | <0.001 |
| 1 | 40 (14) | 44 (25) | 60 (15) | 24 (38) | ||||
| 2+ | 4 (1) | 6 (3) | 6 (1.5) | 4 (6.4) | ||||
| Civil status | 31 | Alone | 72 (23) | 62 (34) | 0.007 | 113 (26) | 19 (29) | 0.73 |
| Married | 245 (77) | 119 (66) | 317 (74) | 46 (71) | ||||
| BMI | 136 | Median (range) | 24.3 (16.5–45.3) | 23.9 (17–49.8) | 0.11 | 24.2 (17–49.8) | 23.9 (16.5–32.6) | 0.52 |
| Number of comorbidities | 0 | 0 | 166 (49) | 45 (24) | <0.001 | 197 (43) | 12 (18) | <0.001 |
| 1 | 89 (26) | 67 (35) | 134 (29) | 22 (33) | ||||
| 2 | 42 (12) | 44 (23) | 70 (15) | 16 (24) | ||||
| 3 | 22 (7) | 23 (12) | 31 (6.8) | 13 (19) | ||||
| 4+ | 19 (6) | 12 (6) | 27 (5.9) | 4 (6.0) | ||||
| Smoking | 44 | Non-smoker | 154 (50) | 90 (51) | 0.86 | 205 (49) | 37 (60) | 0.14 |
| Smoker | 155 (50) | 86 (49) | 215 (51) | 25 (40) | ||||
| Surgery | 19 | Elective | 256 (79) | 140 (76) | 0.60 | 344 (78) | 49 (77) | |
| Acute | 70 (21) | 44 (23) | 99 (22) | 15 (23) | ||||
| Tumour location* | 0 | Right | 128 (38) | 98 (51) | 0.009 | 210 (46) | 26 (39) | 0.12 |
| Left | 163 (48) | 75 (39) | 60 (13) | 5 (7.5) | ||||
| Rectum | 47 (14) | 18 (9) | 189 (41) | 36 (54) | ||||
| Tumour stage† | 24 | II | 44 (14) | 26 (14) | 0.97 | 62 (14) | 8 (12) | 0.83 |
| III | 278 (86) | 157 (86) | 375 (86) | 57 (88) | ||||
| MSI | 265 | Instability | 20 (12) | 16 (17) | 0.34 | 31 (14) | 5 (13) | 1.00 |
| White cell counts | 50 | Median (range) | 6.8 (2.8–22.8) | 7.2 (3.4–19.8) | 0.04 | 6.8 (2.8–23) | 7.4 (4.1–18) | 0.02 |
| Neutrophils | 55 | Median (range) | 4.38 (1.68–82) | 4.88 (1.61–84) | 0.05 | 4.44 (1.6–84) | 5.2 (2.4–81) | 0.21 |
| Lymphocytes | 54 | Median (range) | 1.91 (0.56–45) | 1.75 (0.46–37) | 0.02 | 1.9 (0.6–45) | 1.6 (0.46–15) | 0.003 |
| Haemoglobin | 48 | Median (range) | 7.9 (5.5–10.8) | 7.6 (5.8–10,5) | 0.046 | 7.9 (5.6–11) | 7.5 (5.5–11) | 0.01 |
| Platelets | 53 | Median (range) | 333 (127–1080) | 323 (131–1310) | 0.11 | 328 (127–1310) | 331 (139–833) | 0.75 |
| LDH | 57 | Median (range) | 174 (84–894) | 179 (106–640) | 0.09 | 177 (84–894) | 172 (105–389) | 0.45 |
| CEA | 133 | Median (range) | 1.5 (0.3–84.4) | 1.6 (0.3–90.2) | 0.17 | 1.5 (0.3–90) | 1.7 (0.5–26) | 0.95 |
| Creatinine | 50 | Median (range) | 76 (46–292) | 79 (50–209) | 0.06 | 77 (46–292) | 78 (51–167) | 0.04 |
*Right=proximal colon, left=distal colon.
†Stage II: T3–T4 N=0, stage III: T1–T4 and N1–N2.
BMI, body mass index; CEA, carcino embryonic antigen; F, female; LDH, lactate dehydrogenase; M, male; MSI, microsatelite instability; PS, performance status.
Treatment, doses and compliance of chemotherapy
Choice of single-agent or combination treatment was associated with age (see online supplementary figure S2). The odds of receiving single-agent therapy increased by 10.6% per year from the age of 65 years (see online supplementary figure S4). The likelihood of receiving reduced primary dose was significantly higher for the elderly patients before and after adjustment for PS and comorbidity (OR 6.92, 95% CI 3.54 to 13.52, p<0.001). Patients given primary dose reduction had a significantly higher median age (76 vs 64, p<0.001), poorer PS (p<0.001) and more comorbidity (p<0.001) than patients receiving full dose chemotherapy (table 1).
esmoopen-2016-000087_S4.pdf (37.3KB, pdf)
The proportion of patients receiving a full cumulative dose of capecitabine was similar in elderly and younger patients, but elderly patients were less likely to receive full dose of 5-FU (OR 0.20, p=0.02) and oxaliplatin in the Folfox regimen (OR 0.43, p=0.03) after adjustment for PS and comorbidity (see online supplementary table S5).
esmoopen-2016-000087_S5.pdf (254KB, pdf)
The percentage of patients receiving all cycles of capecitabine and 5-FU was similar in the two groups. Elderly patients were less likely to receive all cycles of oxaliplatin in the Capeox regimen (OR 0.47, p=0.04), but after adjusting for PS and comorbidity, the difference was not significant (see online supplementary table S6).
esmoopen-2016-000087_S6.pdf (253.7KB, pdf)
The distributions of dose intensity and compliance are given in online supplementary figure S7.
esmoopen-2016-000087_S7.pdf (93.7KB, pdf)
Adverse events
The prevalence of toxicity is shown in online supplementary table S8. Toxicity (grades 3–5) was similar in the two groups (29% vs 28%, p=0.86). Death due to toxicity was observed in one elderly patient (0.52%) and in three younger patients (0.89%). There was no age-dependent difference in hospitalisations (rate ratio (RR) 1.09, p=0.79) or length of hospital stay (RR 0.87, p=0.13) between the two age groups. Diagnoses causing hospitalisation were similar in younger and elderly patients (see online supplementary table S9).
esmoopen-2016-000087_S8.pdf (297KB, pdf)
esmoopen-2016-000087_S9.pdf (327.5KB, pdf)
Outcomes
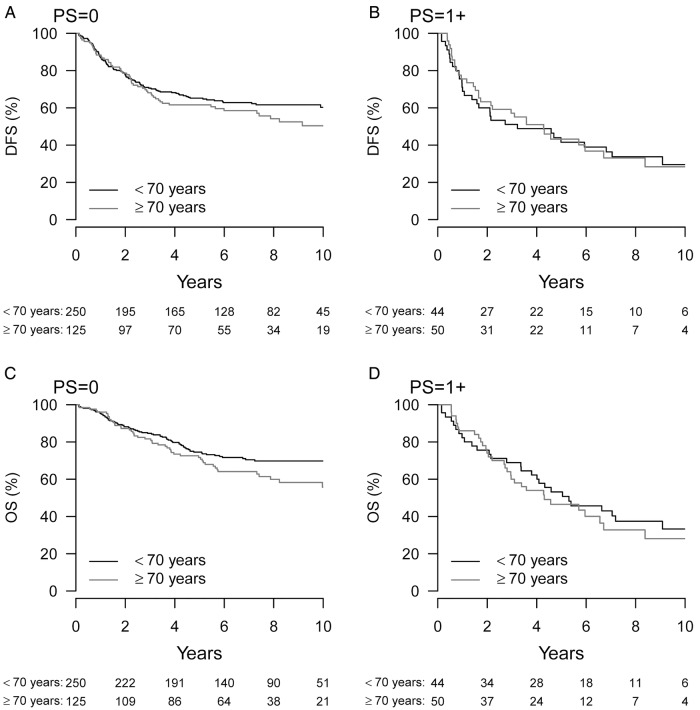
There was no significant difference in 3-year DFS in the two age groups (HR 1.09, p=0.59). Seventy per cent of the elderly patients with PS=0 and 55% of the elderly with PS+1 were disease free and alive at 3 years (see online supplementary figure S10). The corresponding numbers for younger patients were 70% (PS=0) and 60% (PS+1), respectively. Figure 1A, B illustrates that 10-year DFS was also similar in both age groups.
Figure 1.
Kaplan-Maier survival curves of 10-year DFS (A and B) and OS (C and D) according to PS 0 and PS≥1 in patients treated with adjuvant chemotherapy after surgery for stages II–III CRC. The patients are divided into elderly (≥70 years, grey) and younger (<70 years, black). CRC, colorectal cancer; DFS, disease-free survival; OS, overall survival; PS, performance status.
esmoopen-2016-000087_S10.pdf (72KB, pdf)
In addition, 2-year OS was similar (HR=1.16, p=0.53). At 2 years, 90% of the elderly patients with PS=0 and 80% of the elderly with PS+1 were alive. The corresponding numbers for younger patients were 90% (PS=0) and 75% (PS+1; see online supplementary figure S10). Figure 1C, D suggests a small difference in the 10-year OS between the two age groups, PS=0. After adjustment for PS and comorbidity, no significant difference existed in 10-year survival between the age groups (HR 1.35, p=0.051). The 10-year CRC mortality was identical in the two groups (HR=1.03, p=0.88), but elderly patients had a higher mortality due to other causes of death, also after adjustment for PS and comorbidity (HR=1.83, p=0.02; figure 2).
Figure 2.
The 10-year cumulative incidence of CRC death or death due to other causes in patients treated with adjuvant chemotherapy after surgery for stages II–III CRC. The patients are divided into elderly (≥70 years, grey) and younger (<70 years, black). CRC, colorectal cancer; DFS, disease-free survival; OS, overall survival; PS, performance status.
Baseline correlation analyses
For both elderly and younger patients, poor PS was significantly correlated with shorter DFS (see online supplementary table S11) and shorter OS (see online supplementary table S12). In younger patients, poor PS was also related to both cancer-related mortality (see online supplementary table S13) and death from other causes (see online supplementary table S14). The effect of poor PS on hospitalisation was significantly larger in elderly than in younger patients (p=0.041; online supplementary table S15). High comorbidity in younger patients but not in elderly patients was significantly associated with shorter DFS (see online supplementary table S11), shorter OS (see online supplementary table S12), cancer-related mortality (see online supplementary table S13) and other causes of death (see online supplementary table S14). The effect of comorbidity on OS was significantly greater in younger than in elderly patients (p=0.022).
esmoopen-2016-000087_S11.pdf (280.6KB, pdf)
esmoopen-2016-000087_S12.pdf (280.7KB, pdf)
esmoopen-2016-000087_S13.pdf (280.9KB, pdf)
esmoopen-2016-000087_S14.pdf (354KB, pdf)
esmoopen-2016-000087_S15.pdf (281KB, pdf)
Overweight (BMI 25–30) was after adjustment for PS and comorbidity significantly correlated with shorter DFS in elderly patients (see online supplementary table S11).
High serum carcino embryonic antigen in elderly and younger patients was related to shorter DFS, shorter OS and higher cancer-related mortality (see online supplementary tables S11–S13). Elevated serum lactate dehydrogenase and high white cell count (WCC) were associated with shorter DFS and OS (see online supplementary tables S11 and S12). High WCC in the younger patients were also associated with severe toxicity (see online supplementary table S16).
esmoopen-2016-000087_S16.pdf (354.1KB, pdf)
Treatment-related analyses
For elderly patients, combination therapy compared with single-agent therapy was related to longer DFS (HR 0.58, p=0.016; see online supplementary table S17) and longer OS (HR 0.49, p=0.003; see online supplementary table S16) and this difference was significantly different from younger patients (page=0.04).
esmoopen-2016-000087_S17.pdf (264KB, pdf)
Elderly patients receiving <50% of planned cycles of capecitabine/5-FU had shorter DFS (HR=1.78, p=0.020; see online supplementary table S17), shorter OS (HR=2.12, p=0.003; see online supplementary table S18) and higher CRC-related death (HR=2.17, p=0.028; see online supplementary table S19) than elderly patients given all cycles.
esmoopen-2016-000087_S18.pdf (267.8KB, pdf)
esmoopen-2016-000087_S19.pdf (267.7KB, pdf)
Younger patients receiving <75% of planned cycles of capecitabine/5-FU had shorter DFS (HR=2.24, p=0.017; see online supplementary table S17), shorter OS (HR=2.80, p=0.003; see online supplementary table S18) and higher CRC-related death (HR=3.26, p=0.001; see online supplementary table S19) than younger patients given all cycles. Furthermore, younger patients receiving dose intensity below 50% compared with dose intensity >90% had higher CRC mortality (HR=3.15, p=0.02; see online supplementary table S19).
A higher rate of severe toxicity was observed in elderly and younger patient receiving combination therapy compared with single-agent therapy (HR=3.69, p=0.001 and HR=2.28, p=0.006, respectively; see online supplementary table S19).
Discussion
In the present study, we investigated the age-dependent (<70 vs ≥70 years) difference in adjuvant chemotherapy and outcomes in patients who underwent radical surgery for stages II–III CRC). The cut-off at 70 years is also used in international treatment guidelines15 and is frequently used in the literature and made it possible to compare our results to other relevant studies.10 13 15 These two age groups were, as expected, not comparable, a higher degree of comorbidity and poor PS being seen in the elderly group. There was also a difference in tumour localisation: elderly right-sided, younger group left-sided dominance. The elderly patients were also more likely to live alone, which is known to be a prognostic factor for poorer OS.16 17
In the elderly group receiving combination therapy compared with single-agent therapy was related to higher DFS and OS, but not to death due to CRC. A longer DFS in both age groups treated with combination chemotherapy suggests that oxaliplatin was also effective in the elderly. This is in contrast to other findings,10 only a small beneficial effect of oxaliplatin being seen in patients >75 years.7 This speaks in favour of giving single-agent chemotherapy to frail elderly and combination therapy to fit elderly patients.
We found that the primary dose of adjuvant chemotherapy was significantly lower in elderly patients, who also had more comorbidity and poorer PS. Interestingly, primary dose reduction had no significant impact on DFS or CRC-related death. Age-based primary dose reduction is not routinely recommended, but older age is independently associated with primary dose reduction in patients with solid tumours.18 However, a reduction in toxicity, hospitalisation or discontinuation of therapy was not found in this study in relation to age.
In our study, elderly patients received a lower dose intensity of 5-FU and oxaliplatin, but with no impact on DSF, OS and CRC mortality. These results support other findings; adjustments in dose intensity and regimens still have a survival benefit in elderly patients.8
We found that the number of received cycles significantly correlated with survival. Younger patients who received <75% of planned cycles had a shorter DFS and OS and higher CRC-related death. However, no significant difference was seen between 75% of planned cycles and full treatment. Elderly patients who received <50% of planned cycles had higher CRC mortality. Our results are consistent with Kim et al,19 who found that survival was better in patients receiving at least 75% of expected cycles, but dose intensity below 75% did not affect OS. These results suggest that primary dose reduction in all elderly patients may improve the chance of completing the treatment regimen, but one should be cautious with making this conclusion based on this retrospective data which is a limitation of this study. Therefore, we need randomised control trials including frail elderly patients to illuminate this clinical issue.
In the literature, only 50% of patients with stages II–III CRC complete 6 months’ adjuvant treatment.20 21 In the large ongoing SCOT study, more than 6000 patients included,22 the efficiency of 3 months’ oxaliplatin-based adjuvant chemotherapy is compared with 6 months’ standard treatment. The survival data are expected in 2017.
Our retrospective single-centre study has several strengths. One researcher (CML) collected and interpreted all data to minimise interpersonal bias. We assessed both cancer-related mortality and other causes of death. This is crucial in evaluating the effect of chemotherapy in elderly patients, who have a higher overall mortality. Study limitations include small sample size in subanalyses of adverse effects. Data were collected from 2001 to 2012, and analysis of some clinical biomarkers was not undertaken during the first period (eg, tumour characteristics, RAS and BRAF mutation status, and presence of microsatellite instability). The follow-up programme only every sixth month might have delayed discover of recurrence.
Only patients receiving at least one dose of chemotherapy were assessed for eligibility in our study. Thus, many frail and elderly people were excluded from the start, perhaps accounting for the low occurrence of grades 3–5 toxicity (<30%) found in the ACCORE study compared with other studies.8 19 At the same time, frail and elderly patients more frequently received single-agent therapy and primary reduced doses, which probably have decreased the occurrence of toxicity, but without affecting the outcome. Despite the fact that the elderly patients more frequently received single-agent therapy (selection bias), their DFS and CRC mortality were similar to the younger patients. In accordance with Sanoff et al,23 we found no increased hospitalisation among older patients. Hamza et al24 found a similar low occurrence of severe toxicity among patients below and above 70 years but presented no data on DFS or cancer-specific mortality. No excess toxicity was found in patients with CC ≥75 years with good PS and no comorbidity.21
We suggest that treatment decisions, including dose intensity and choice of regimen, should not be made according to chronological age alone. Patients should be assessed with a combination of different measures like PS, comorbidities, functional and psychocognitive status, and risk of recurrence.15
In conclusion, we found an age-dependent difference in choice of chemotherapy regimen, primary dose reduction and given dose intensity. Interestingly, no age-dependent differences in DFS or CRC-related mortality were observed, but comorbidity and PS were related to shorter DFS and higher CRC mortality. To maintain (optimise) treatment efficacy and minimise toxicity, we need randomised control trials that include frail elderly patients and investigate optimal dose and number of treatments.
Our results generate the hypothesis ‘in adjuvant chemotherapy for primary CRC in elderly patients primary dose reduction to 75% is as effective as full dose treatment but with an expected decrease in toxicity as a result’. We suggest a randomised controlled trial comparing full dose to primary dose reduction (75%) in elderly patients with comorbidity and functional decline.
Acknowledgments
The authors thank Professor Stig E Bojesen, Department of Biochemistry, Herlev and Gentofte Hospital, University of Copenhagen, Denmark, for the biochemical results.
Footnotes
Contributors: CML, DN, JSJ, KKV, ABC and FR designed the study; CML extracted and interpreted data. CD did the statistical calculations; CML wrote the first paper draft and JSJ, DN, KKV, CD, ABC and FN reviewed it critically. All authors contributed to and approved the final version of the manuscript.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: To get access to data in the clinical ACCORE database, please contact; CML mobile: 0045-60141841, email: cecilia.margareta.lund.01@regionh.dk.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 2.Braendegaard Winther S, Baatrup G, Pfeiffer P et al. , Academy of Geriatric Cancer Research (AgeCare). Trends in colorectal cancer in the elderly in Denmark, 1980–2012. Acta Oncol 2016;55(Suppl 1):29–39. 10.3109/0284186X.2015.1114674 [DOI] [PubMed] [Google Scholar]
- 3.Christensen K, Doblhammer G, Rau R et al. Ageing populations: the challenges ahead. Lancet 2009;374:1196–208. 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gill S, Loprinzi CL, Sargent DJ et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806. 10.1200/JCO.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 5.Gray R, Barnwell J, McConkey C et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9. 10.1016/S0140-6736(07)61866-2 [DOI] [PubMed] [Google Scholar]
- 6.Sargent DJ, Goldberg RM, Jacobson SD et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7. 10.1056/NEJMoa010957 [DOI] [PubMed] [Google Scholar]
- 7.Sanoff HK, Carpenter WR, Stürmer T et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34. 10.1200/JCO.2011.41.1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoeben KW, van Steenbergen LN, van de Wouw AJ et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol 2013;24:974–9. 10.1093/annonc/mds576 [DOI] [PubMed] [Google Scholar]
- 9.Jessup JM, Stewart A, Greene FL et al. Adjuvant chemotherapy for stage III colon cancer: implications of race/ethnicity, age, and differentiation. JAMA 2005;294:2703–11. 10.1001/jama.294.21.2703 [DOI] [PubMed] [Google Scholar]
- 10.McCleary NJ, Meyerhardt JA, Green E et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6. 10.1200/JCO.2013.49.6638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Accordino MK, Neugut AI, Hershman DL. Cardiac effects of anticancer therapy in the elderly. J Clin Oncol 2014;32:2654–61. 10.1200/JCO.2013.55.0459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.André T, Boni C, Navarro M et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 13.Yothers G, O'Connell MJ, Allegra CJ et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74. 10.1200/JCO.2011.36.4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller DG, Tabernero J, Maroun J et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71. 10.1200/JCO.2010.33.6297 [DOI] [PubMed] [Google Scholar]
- 15.Papamichael D, Audisio RA, Glimelius B et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463–76. 10.1093/annonc/mdu253 [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Gan L, Liang L et al. The influence of marital status on stage at diagnosis and survival of patients with colorectal cancer. Oncotarget 2015;6:7339–47. 10.18632/oncotarget.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aizer AA, Chen MH, McCarthy EP et al. Marital status and survival in patients with cancer. J Clin Oncol 2013;31:3869–76. 10.1200/JCO.2013.49.6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gajra A, Klepin HD, Feng T et al. Predictors of chemotherapy dose reduction at first cycle in patients age 65 years and older with solid tumors. J Geriatr Oncol 2015;6:133–40. 10.1016/j.jgo.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CA, Spratlin JL, Armstrong DE et al. Efficacy and safety of single agent or combination adjuvant chemotherapy in elderly patients with colon cancer: a Canadian cancer institute experience. Clin Colorectal Cancer 2014;13:199–206. 10.1016/j.clcc.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 20.Hermosillo-Rodriguez J, Anaya DA, Sada Y et al. The effect of age and comorbidity on patient-centered health outcomes in patients receiving adjuvant chemotherapy for colon cancer. J Geriatr Oncol 2013;4:99–106. 10.1016/j.jgo.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 21.Jensen SA, Vilmar A, Sørensen JB. Adjuvant chemotherapy in elderly patients (>or=75 yr) completely resected for colon cancer stage III compared to younger patients: toxicity and prognosis. Med Oncol 2006;23:521–31. 10.1385/MO:23:4:521 [DOI] [PubMed] [Google Scholar]
- 22.Iveson T, Kerr R, Saunders MP et al. MLTJWCERESHA: toxicity and quality of life data from SCOT: an international phase III randomized (1:1) noninferiority trial comparing 3 vs 6 months of oxaliplatin-based adjuvant chemotherapy. J Clin Oncol 2015;33:Abstract 3514. [Google Scholar]
- 23.Sanoff HK, Carpenter WR, Freburger J et al. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: a population-based analysis. Cancer 2012;118:4309–20. 10.1002/cncr.27422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamza S, Bouvier AM, Rollot F et al. Toxicity of oxaliplatin plus fluorouracil/leucovorin adjuvant chemotherapy in elderly patients with stage III colon cancer: a population-based study. Ann Surg Oncol 2014;21:2636–41. 10.1245/s10434-013-3438-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2016-000087_S1.pdf (184.5KB, pdf)
esmoopen-2016-000087_S2.pdf (78.4KB, pdf)
esmoopen-2016-000087_S3.pdf (250.5KB, pdf)
esmoopen-2016-000087_S4.pdf (37.3KB, pdf)
esmoopen-2016-000087_S5.pdf (254KB, pdf)
esmoopen-2016-000087_S6.pdf (253.7KB, pdf)
esmoopen-2016-000087_S7.pdf (93.7KB, pdf)
esmoopen-2016-000087_S8.pdf (297KB, pdf)
esmoopen-2016-000087_S9.pdf (327.5KB, pdf)
esmoopen-2016-000087_S10.pdf (72KB, pdf)
esmoopen-2016-000087_S11.pdf (280.6KB, pdf)
esmoopen-2016-000087_S12.pdf (280.7KB, pdf)
esmoopen-2016-000087_S13.pdf (280.9KB, pdf)
esmoopen-2016-000087_S14.pdf (354KB, pdf)
esmoopen-2016-000087_S15.pdf (281KB, pdf)
esmoopen-2016-000087_S16.pdf (354.1KB, pdf)
esmoopen-2016-000087_S17.pdf (264KB, pdf)
esmoopen-2016-000087_S18.pdf (267.8KB, pdf)
esmoopen-2016-000087_S19.pdf (267.7KB, pdf)