Abstract
Background
The chromosomal region 6q23 has been found to be associated with multiple sclerosis (MS) predisposition through genome wide association studies (GWAS). There are four independent single nucleotide polymorphisms (SNPs) associated with MS in this region, which spans around 2.5 Mb. Most GWAS variants associated with complex traits, including these four MS associated SNPs, are non-coding and their function is currently unknown. However, GWAS variants have been found to be enriched in enhancers and there is evidence that they may be involved in transcriptional regulation of their distant target genes through long range chromatin looping.
Aim
The aim of this work is to identify causal disease genes in the 6q23 locus by studying long range chromatin interactions, using the recently developed Capture Hi-C method in human T and B-cell lines. Interactions involving four independent associations unique to MS, tagged by rs11154801, rs17066096, rs7769192 and rs67297943 were analysed using Capture Hi-C Analysis of Genomic Organisation (CHiCAGO).
Results
We found that the pattern of chromatin looping interactions in the MS 6q23 associated region is complex. Interactions cluster in two regions, the first involving the rs11154801 region and a second containing the rs17066096, rs7769192 and rs67297943 SNPs. Firstly, SNPs located within the AHI1 gene, tagged by rs11154801, are correlated with expression of AHI1 and interact with its promoter. These SNPs also interact with other potential candidate genes such as SGK1 and BCLAF1. Secondly, the rs17066096, rs7769192 and rs67297943 SNPs interact with each other and with immune-related genes such as IL20RA, IL22RA2, IFNGR1 and TNFAIP3. Finally, the above-mentioned regions interact with each other and therefore, may co-regulate these target genes.
Conclusion
These results suggest that the four 6q23 variants, independently associated with MS, are involved in the regulation of several genes, including immune genes. These findings could help understand mechanisms of disease and suggest potential novel therapeutic targets.
Introduction
Genome wide association studies (GWAS) have been pivotal in identifying genetic associations with single nucleotide polymorphisms (SNPs) in many complex diseases, including multiple sclerosis (MS) [1–4]. MS is an inflammatory demyelinating disease of the central nervous system (CNS) and is a common cause of chronic neurological disability, showing moderate heritability (λs ~6.3) [5]. Similar to many autoimmune diseases, the major histocompatibility complex (MHC) represents the largest single genetic risk factor for MS, with multiple non-HLA loci, discovered in large international GWAS, contributing smaller individual effects to disease susceptibility. Due to the extensive overlap of genetic loci between multiple autoimmune diseases, the International Multiple Sclerosis Genetics Consortium (IMSGC) conducted a large study using the Illumina Immunochip genotyping array, identifying 48 new and validating 49 previously discovered non-MHC susceptibility variants for MS [6]. Among these variants, four mapped to the 6q23 region, which is also associated with other autoimmune diseases including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), celiac disease (CeD), type 1 diabetes (T1D), inflammatory bowel disease (IBD), psoriasis (Ps) and psoriatic arthritis (PsA), and containing several candidate genes, such as TNFAIP3, AHI1 and IL22RA2 [7–13].
The 6q23 locus, like many other GWAS loci, shows extensive overlap with many other autoimmune diseases and demonstrates a complex pattern of different associations attributable to different diseases. This sharing of associated loci led to the Immunochip array which contains three regions densely mapped and capturing four independent associations with MS (Table 1 and Fig 1) in the 6q23 region. The first, tagged by rs11154801, is located within an intron of the AHI1 gene required for both cerebellar and cortical development. The second region, tagged by rs17066096, is an intergenic region 87kb 5’ of IL20RA and 12kb 3’ of IL22RA2. The third region covers 430kb, encompassing a PTPN11 pseudogene (RP11-95M15.2), TNFAIP3 and several lncRNAs and contains two independent associations (rs7769192 & rs67297943). Interestingly, while other SNP associations are shared between autoimmune diseases, the MS associated SNPs are unique to MS alone (S1 Data). As such, these MS associated SNPs could offer an insight into the mechanisms affecting MS at this locus.
Table 1. MS 6q23 Immunochip associated regions.
| Region Co-ordinates (GRCh37) | Index SNP | Reported Gene | MAF | P | OR | ||
|---|---|---|---|---|---|---|---|
| Chr. | Start | End | |||||
| 6 | 134946991 | 135498875 | rs11154801 | AHI1 | 0.37 | 1.80 x 10−20 | 1.12 |
| 6 | 136685539 | 136875216 | rs17066096 | IL22RA2 | 0.23 | 1.60 x 10−23 | 1.14 |
| 6 | 137231416 | 137660037 | rs7769192a | - | 0.45 | 3.30 x 10−09 | 1.08 |
| rs67297943 | TNFAIP3 | 0.22 | 5.50 x 10−13 | 1.11 | |||
All P values are from the joint analysis by Beecham et al.[6]. Chr., chromosome; MAF, minor allele frequency; OR, odds ratio.
a, P values and ORs shown after conditioning on rs67297943.
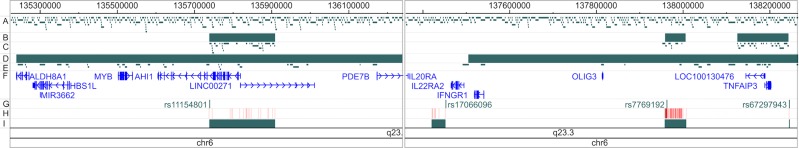
Fig 1. Overview of MS 6q23 Immunochip associated regions.
Tracks are labelled as follows: A–HindIII restriction fragments; B–LD regions targeted in ‘region’ Capture Hi-C; C–HindIII restriction fragments targeted in ‘region’ Capture Hi-C; D–Gene regions targeted in ‘promoter’ Capture Hi-C; E–HindIII restriction fragments targeted in ‘promoter’ Capture Hi-C; F–RefSeq genes (packed for clarity); G–MS index SNPs; H–Density of MS LD SNPs (r2 ≥ 0.8) and I–MS LD regions. The genomic region chr6:136,238,000–137,360,000 has been omitted for clarity. All co-ordinates are based on GRCh37. Generated using the WashU EpiGenome Browser (http://epigenomegateway.wustl.edu/browser/).
However, due to the design of GWAS, these lead genetic associations do not necessarily represent the causal variant but instead a number of variants in strong linkage disequilibrium with them. In addition, associated SNPs have generally been annotated to the closest, most biologically plausible gene. Evidence suggests that GWAS discovered SNPs in general, including these associations within 6q23, are enriched in cell-type specific enhancer regions [14,15] which can regulate gene expression. Additionally, an individual’s genotype can influence this expression (expression quantitative trait loci (eQTL)), potentially leading to disease. It has been shown that enhancers can regulate genes located some distance away through long-range chromatin interactions [16]. Therefore, confidently assigning causal SNPs, genes and cell types to these and other GWAS signals remains a major challenge. Potential long-range interactions have previously been prohibitive to investigate as methods, such as 3C and Hi-C, required interacting regions to be considered a priori or, lacked throughput and resolution. Capture Hi-C was developed to overcome these limitations by enriching a Hi-C library using RNA baits designed to specific restriction fragments. This approach reduces library complexity, increases power and subsequently allows the identification of statistically significant chromatin interactions at a restriction fragment resolution (~4kb). As part of a large study investigating the interactions with associated regions in four autoimmune diseases [17], several sites within the 6q23 region were targeted, including associated regions and promoters of nearby genes (Table 1 and Fig 1). Our Capture Hi-C data represents a unique opportunity to explore this region for MS and offer an insight into the mechanisms specifically affecting MS at this locus and how they compare with other autoimmune diseases. The aim of this study was to use this chromatin interaction experiment to explore the unique genetic associations with MS in the 6q23 region to identify possible target causal genes whose expression could be perturbed in at risk individuals. The ultimate goal is to help translate GWAS findings into clinical benefit, as the identification of causal genes can pinpoint biological mechanisms altered in disease and suggest potential therapeutic targets or drug repositioning.
We demonstrate that the MS associated region 6q23 presents numerous, complex chromatin looping interactions clustered in two regions. The first contains SNPs located within the AHI1 gene, tagged by rs11154801, and correlated with expression, which interact with the AHI1 promoter thereby supporting the gene candidature of AHI1. Interestingly, these SNPs also interact with other potential candidate genes such as SGK1 and BCLAF1, suggesting they may regulate multiple loci. The second region encompasses the rs17066096, rs7769192 and rs67297943 associated regions and interact with each other and with immune-related genes, such as IL20RA, IL22RA2, IFNGR1 and TNFAIP3. Additionally, these regions interact with each other and therefore, may co-regulate these target genes.
Materials and Methods
MS SNP Associations & Regions
All MS SNP associations in the 6q23 region were taken from the IMSGC Immunochip study [6]. All SNPs in linkage disequilibrium (LD) (r2≥0.8) with each lead Immunochip MS SNP were identified using European samples from the 1000 Genomes Phase 3 release. Associated regions for each lead association were defined by the two terminal SNPs in LD.
Cell culture
B-lymphoblastoid cell lines (LCL) were obtained directly from Coriell Institute for Medical Research (catalogue number GM12878). Cells were grown in vented 25cm2 cell culture flasks containing 10-20mls of Roswell Park Memorial Institute (RPMI)-1640 + 2mM L-glutamine culture medium, supplemented with 15% foetal bovine serum (FBS). Flasks were incubated upright at 37°C/5% CO2. Cultures were regularly monitored to maintain a cell density between 2×105–5×105 viable cells/ml. Cells were split every 2 days into fresh medium until they reached a maximum density of 1×106 cells/ml.
Jurkat E6.1 human leukaemic T-lymphoblast cells were obtained directly from LGC Standards (catalogue number ATCC® TIB-152™). Cells were grown in vented 25cm2 cell culture flasks containing 10-20mls of RPMI-1640 + 2mM L-glutamine, supplemented with 10% FBS. Flasks were incubated upright at 37°C/5% CO2 and the cultures regularly monitored to maintain a cell density between 3×105–9×105 viable cells/ml.
These cell lines are not listed in the in the database of commonly misidentified cell lines maintained by ICLAC, were authenticated using STR analysis and were tested for mycoplasma contamination (MycoSEQ® Mycoplasma Detection System, 4460625, Life Technologies).
Capture Hi-C
Capture Hi-C data was produced as part of a larger study targeting all regions associated with four autoimmune diseases (RA, JIA, PsA and T1D) and separately, all promoters within these regions [17]. Briefly, all promoters within 1Mb of associated SNPs were selected and RNA baits were designed to the ends of all fragments within 500bp of the transcription start sites. Separately, associated regions were defined by SNPs in LD (r2≥0.8) and all restriction fragments not selected for the promoter capture experiment were targeted. Experiments were performed using human T-cell (Jurkat) and B-cell (GM12878) lines. Capture Hi-C libraries were sequenced using 75bp paired-end reads on an Illumina HiSeq 2500. Resulting reads were mapped to restriction fragments and filtered using the Hi-C User Pipeline (HICUP http://www.bioinformatics.babraham.ac.uk/projects/hicup). Chromatin interactions were analysed using CHiCAGO (Capture Hi-C Analysis Of Genomic Organisation [18], http://regulatorygenomicsgroup.org/chicago), a publicly available, open-source, bespoke statistical model for detecting significant interactions in Capture Hi-C data at a single restriction fragment resolution. Further filtering was carried out using the BEDTools v2.21.0 pairtobed command to identify significant interactions involving the MS associated regions.
Chromatin interactions identified in the Capture Hi-C data were further validated against dense Hi-C data generated by Rao et al. [19] in GM12878 cells. No data was available for the Jurkat T-cell line. Raw contact matrices and normalisation matrices for GM12878 cells at 5kb resolution were obtained from GEO accession GSE63525. Observed and expected contact matrices were normalised using the Knight and Ruiz normalisation matrices as described in the accompanying documentation. Observed/expected (O/E) values were calculated and further filtered by O/E ≥5 and normalised read count ≥ 5. BEDTOOLS was used to obtain the overlap of interactions observed in our data and the Rao et al. [19] data.
Expression quantitative trait loci (eQTLs) analysis
Publicly available datasets from Westra, et al. [20], the GEUVADIS analysis (http://www.ebi.ac.uk/arrayexpress/files/E-GEUV-1/analysis_results/) and Raj et al. [21] were queried directly. Additional datasets were also queried through HaploReg [22]. Two whole-genome gene expression datasets were also available in-house: CD4+ and CD8+ T-cells from 21 healthy individuals of the National Repository of Healthy Volunteers (NRHV), The University of Manchester. Written informed consent was obtained from all subjects. Ethical approval was obtained from North West Centre for Research Ethics Committee (REC: 99/8/84). Samples were all of Caucasian ancestry with a median age of 50.5 years (26–82 years) and comprised of 8 males and 13 females. mRNA was isolated from sorted cell subsets, quality and concentration assessed using the Agilent Bioanalyzer and Nanodrop, before cDNA/cRNA conversion using Illumina TotalPrep RNA Amplification Kits. 750ng of cRNA was hybridised to HumanHT-12 v4 Expression BeadChip arrays according to the manufacturer’s protocol before being scanned on the Illumina iScan system. Raw expression data were exported from Illumina GenomeStudio and analysed using the R Bioconductor package ‘limma’ 81. Briefly, the neqc function was used for log2 transformation of the data, background correction and quantile normalisation using control probes. Principal Component Analysis was used to detect batch effects. The cDNA/cRNA conversion produced the largest batch effect in both cohorts and was corrected using ComBat (in R Bioconductor package sva) (http://bioconductor.org/packages/release/bioc/html/sva.html). Genome-wide genotype data was generated using the Illumina HumanCoreExome BeadChip kit. Genotype data was aligned to the 1000 genomes reference strand, pre-phased using SHAPEIT2 (v2.r727), before imputation using IMPUTE2 (v2.3.0) with the 1000 genome reference panel Phase 1. Imputed data was hard-called to genotypes using an INFO score cut-off of 0.8 and posterior probability of 0.9. The effect of the SNPs on gene expression was analysed using MatrixEQTL (v 2.1.0) (http://www.bios.unc.edu/research/genomic_software/Matrix_eQTL/) with an additive linear model. Only SNPs within 4Mb of a gene expression probe were considered to be cis-eQTL.
Bioinformatics Refinement of SNPs
SNPs were annotated using data from HaploReg v4.1 [22] and RegulomeDB v1.1 [23] for each LD SNP and combined with our Capture Hi-C data. SNPs attaining a RegulomeDB score of ≥5 and showing evidence of chromatin interactions in either cell type were selected as potentially causal and merit further investigation.
Results and Discussion
Capture Hi-C
Chromatin interactions at the 6q23 locus were analysed as part of a larger study that included all known risk loci for RA, juvenile idiopathic arthritis (JIA), PsA and T1D. We performed two different Capture Hi-C experiments: firstly, the Region Capture targeted the LD regions (r2>0.8) for all SNPs associated with each disease (Fig 1B); secondly, the Promoter Capture targeted all known gene promoters overlapping a region 500kb upstream and downstream of the lead disease associated SNP (Fig 1D). Capture Hi-C libraries were generated for two cell lines: GM12878, a B-lymphoblastoid cell line, and Jurkat, a CD4+ T-lymphoblastoid cell line.
Our Capture Hi-C experiments revealed that the 6q23 region presents a complex pattern of chromosomal interactions, highlighting both new and previously implicated genes for disease risk. Overall, 827 unique interactions involving MS 6q23 associated regions were observed across both cell lines and both capture experiments (promoter & region) (Fig 2 and S1 Fig). Each cell line demonstrated similar interaction patterns in both capture experiments. Encouragingly, there was a high degree of support from previously published Hi-C data obtained in GM12878 cells [19], with between 81% and 90% of interactions identified through our Capture Hi-C also being seen at an observed/expected ratio of ≥5 in previously published data.
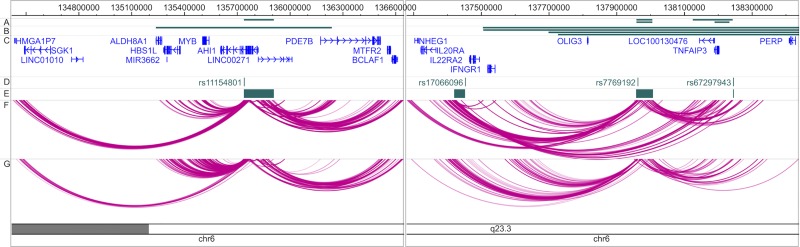
Fig 2. Overview of MS 6q23 interactions.
Tracks are labelled as follows: A–LD regions targeted in ‘region’ Capture Hi-C; B–Gene regions targeted in ‘promoter’ Capture Hi-C; C–RefSeq genes (packed for clarity); D–MS index SNPs; E–MS LD regions; F–Interactions observed in the GM12878 B-cell line and G–Interactions observed in the Jurkat T-cell line. Promoter and region Capture Hi-C experiments have been merged for clarity. The genomic region chr6:136,650,000–137,280,000 has been omitted for clarity. All co-ordinates are based on GRCh37. Generated using the WashU EpiGenome Browser (http://epigenomegateway.wustl.edu/browser/).
The numerous chromosomal interactions detected appeared to cluster in two genomic locations, involving the rs11154801 region and a region containing the other three independent MS associations: rs17066096, rs7769192 and rs67297943 (Fig 2).
The rs11154801 LD block spans 170.4kb and contains several enhancers overlapping SNPs in strong LD with the lead association. It demonstrates a complex array of several long-range (>100kb) as well as shorter (<100kb) chromatin interactions (Fig 2 and S2 Fig) in both cell lines. Shorter internal chromatin interactions include ones with restriction fragments flanking the AHI1 gene (previously assigned as the candidate gene to this variant) promoter, and SNPs in LD with rs11154801. We show how these SNPs, within the introns of AHI1, interact with the promoter region thereby supporting the AHI1 hypothesis in both cell lines. Mutations in AHI1 have been shown to cause Joubert syndrome [24], an autosomal recessive neurological condition causing symptoms including neonatal breathing abnormalities and mental retardation. Furthermore, it has been suggested that AHI1 is required for both cerebellar and cortical development in humans and is expressed in the brain [25].
However, this locus may be more complex than previously thought, as long-range chromatin interactions, although more numerous in B-cells, were observed between the enhancer region and other compelling candidates such as SGK1 and BCLAF1 in both cell lines. The interaction with SGK1 represents a >1.2Mb interaction and Sgk1 knockout mice have been shown to have a reduced incidence of disease severity in experimental autoimmune encephalomyelitis (EAE), a mouse model of MS [26]. Other long-range interactions include those to the MYC and PDE7B gene regions.
Finally, rs11154801 also showed an interaction in both B and T-cells with a region encompassing the promoters of BCLAF1, MTFR2, an antisense gene (RP13-143G15.4) overlapping PDE7B and a lincRNA (RP3-406A7.7). The BCLAF1 gene encodes a transcriptional repressor which interacts with BCL2-family proteins, is expressed in the brain and overexpression can lead to cell apoptosis [27]. In lymphocytes, dysregulation of BCL2-family proteins has been shown to lead to a reduction of pro-apoptotic BCL-2 members and survival of T-cells in MS [28]. Additionally, BCLAF1 has also been shown to be crucial in the homeostasis of T- and B-cell lineages and proliferation of T-cells [29]. These interactions suggest that the associations at this locus may have different or additional effects on disease than just the previously assigned AHI1 gene.
The second cluster of chromatin interactions involve the remaining MS associated SNPs in the 6q23 region, rs17066096, rs7769192 and rs67297943. Our Capture Hi-C results showed that these three SNPs interact with each other and also with several genes with immune function such as IL20RA, IL22RA2, IFNGR1 and TNFAIP3, suggesting that these variants may be involved in the regulation of common immune pathways (Fig 2).
The first two interacting regions in this cluster, identified by the promoter Capture Hi-C experiment and tagged by rs17066096, were only observed in B-cells and involved the IL22RA2 and IFNGR1 gene promoters, almost 71kb and 113kb away, respectively. The restriction fragment overlapping the rs17066096 LD block shows evidence of enhancer activity, as predicted by ChromHMM, and the furthest 5’ SNP in LD with the index SNP is located within this enhancer.
The region tagged by rs7769192 spans 50kb and contains 72 SNPs in LD, 21 of which are perfectly correlated with the lead SNP. It shows evidence of multiple enhancers and in addition to the previously mentioned interaction with the rs17066096 region, interacts with five other regions, in both cell lines. The first of these is located >500kb 5’ of rs7769192 and contains the IL20RA gene. The next two regions, located over 400kb away, are shared with the rs17066096 region and contain the IL22RA2 and IFNGR1 genes, providing further evidence of the interplay between the two associated regions. The product of the IFNGR1 gene is a subunit of the interferon gamma (IFN-γ) receptor whose ligand, IFN-γ, is important in adaptive immunity and has been linked to many different autoimmune diseases [30]. The IL20RA and IL22RA2 genes both encode receptors for members of the IL-20 sub-family of cytokines and both exhibit a pro-inflammatory effect [31]. Additionally, anti-IL20 therapy has recently been shown to be effective in the treatment of RA and psoriasis [32,33]. Although anti-IL20 therapy was not developed as a result of Capture Hi-C, the discovery of interactions between these genes and autoimmune disease associations, demonstrates the power of this technique to inform drug discovery or repositioning. Further evidence for anti-IL20 therapy comes from the interaction with IL22RA2. IL22RA2 encodes a soluble receptor which binds to and inhibits IL-22, a cytokine which can stimulate pro-inflammatory epithelial defence mechanisms [34], preventing the interaction with its cell surface receptor. This evidence suggests that blocking the IL-20 pathway may be effective in the treatment of MS and other autoimmune diseases.
The fourth region is located 178kb 3’ of the associated region and contains multiple promoters of the TNFAIP3 gene as well as a non-coding processed transcript (RP11-356I2.4) of unknown function. The role of TNFAIP3 in autoimmunity is well established and the gene product A20 is a protein that is induced by tumour necrosis factor (TNF) and inhibits NFκB activation and TNF-mediated apoptosis [35]. This locus within the 6q23 region is one of the most important autoimmunity risk loci, as it contains multiple SNPs strongly associated with many autoimmune diseases, including MS, RA, SLE, CeD, IBD, psoriasis and PsA, among others. Variants associated with most autoimmune diseases map to the TNFAIP3 gene or its vicinity, including the MS SNPs rs17066096, rs7769192 and rs67297943.
The rs67297943 SNP is located within a predicted enhancer element in B-cells and also in a 48.8kb region showing multiple enhancer marks in both B and T-cells. Furthermore, the adjacent restriction fragment to rs67297943 interacts with the IL20RA promoter region only in B-cells in this experiment.
Our Capture Hi-C results suggest that rs17066096, rs7769192 and rs67297943 physically interact with several immune genes with pro-inflammatory roles, such as IL20RA, IL22RA2, IFNGR1 and TNFAIP3, indicating that they may be involved in the inflammatory processes that typically occurs in autoimmunity. Conversely, rs11154801, which is exclusively associated to MS and not other autoimmune diseases, interacts with genes with neurological function, like AHI1, SGK1 and BCLAF1. Intriguingly, these two separate regions, over 2.3Mb apart, interact with each other in T-cells but not B-cells (S1 Fig), suggesting that these pathways may converge to give rise to disease-specific MS mechanisms in a stimulus and cell type specific manner. In this regard, it has been previously shown that there is a correlation between chromatin interactions and gene co-expression [36–39] and it has been hypothesised that multiple co-regulated genes can interact and share regulatory elements at specialised ‘transcription factories‘ [16]. Our data possibly supports this idea and suggests a possible co-regulation of genes in this region in MS.
It could be argued that the differences observed between cell types for the rs1154801 region and the rs17066096 region could also be attributable to genotype differences in the cell lines (Table 2). While the overall pattern of chromatin interactions is similar for the rs11154801, rs17066096 and MYB regions, the intensity of interactions observed does vary between cell lines and could be due to carriage of risk alleles in the B-cell line, absent in the T-cell line. It is therefore important to validate the chromatin interactions in a genotype specific manner.
Table 2. Associated SNP genotypes for GM12878 (B) and Jurkat (T) cell lines.
| SNP | Risk Allele | GM12878 | Jurkat | ||
|---|---|---|---|---|---|
| Genotype | Number of risk alleles | Genotype | Number of risk alleles | ||
| rs11154801 | A | CA | 1 | CC | 0 |
| rs17066096 | G | AA | 0 | AG | 1 |
| rs7769192 | A | AA | 2 | unknown* | |
| rs67297943 | C | TC | 1 | unknown* | |
*Neither directly genotyped nor suitable proxies available on array
Expression Quantitative Trait Loci (eQTLs)
Public databases were interrogated for evidence of eQTLs for MS associated SNPs in the 6q23 region (rs11154801, rs17066096, rs7769192 and rs67297943) and all SNPs in LD (r2>0.8) with them. The SNPs within the intergenic region 5’ of the AHI1 gene, tagged by rs11154801 and interacting with the AHI1 gene promoter, are correlated with AHI1 mRNA expression in multiple tissues, including brain, nerve and whole blood. This further supports that AHI1 is one of the causal genes within the 6q23 region.
Although many of the interactions detected in the Capture Hi-C experiment are between regions which show enhancer activity and regions which show active transcription, no eQTL evidence from public databases was observed for any of the other MS associated SNPs investigated, other than rs11154801. This could be due to the distance cut-offs used to define cis effects or the selection of the correct cell type and stimulatory conditions, as eQTLs are known to be highly cell type and stimulus specific.
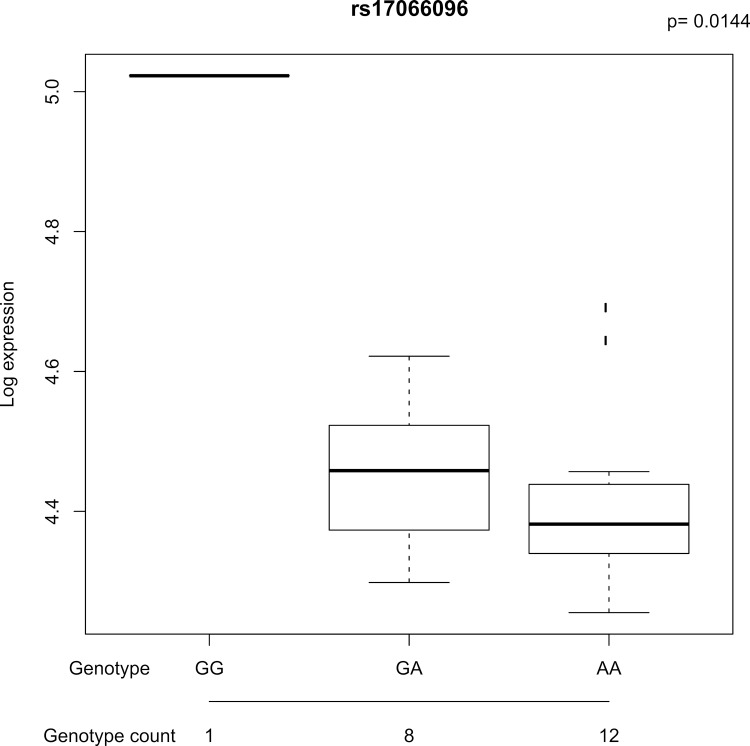
However, using in-house eQTL data on CD4+ and CD8+ primary T-cells from healthy donors, rs17066096 was shown to be correlated with expression of IL20RA in CD8+ T-cells (P = 0.01, Fig 3). Although the design of the Capture Hi-C experiment did not allow for the testing of chromatin interactions between the rs17066096 region and IL20RA, this eQTL suggests IL20RA could be important in the pathogenesis of MS in CD8+ T-cells, which have previously been shown to contribute to disease [40]. This data also confirmed the eQTL between rs11154801 and AHI1 in both CD4+ and CD8+ T-cells (P = 2.0x10-4 and 0.02 respectively). Full eQTL results for all MS SNPs are presented in S2 Data. No other eQTLs were identified between MS SNPs and genes showing chromatin interactions, this may again be due to the selection of the correct cell type and stimulatory conditions.
Fig 3. Increased expression of IL20RA in primary CD8+ T-cells from 21 healthy individuals carrying the G risk allele of rs17066096, P = 0.01.
The three different genotypes for the SNPs are displayed on the X axis and gene expression levels on the Y axis. Error bars indicate standard deviation.
Bioinformatics Refinement of SNPs
By utilising publically available data on regulatory elements obtained through HaploReg and RegulomeDB and augmenting with our Capture Hi-C data, we were able to refine large numbers of potential causal SNPs for three out of the four MS associated regions, by strong evidence of being in both a relevant cell-type enhancer region and interacting with a gene promoter (Table 3 and S3 Data). For the rs11154801 region, 6 SNPs were identified out of 19 potential candidates; for the rs17066096, 3 SNPs were selected from a total of 7 in LD with the index association; and finally, for the rs7769192 region, we refined the potential candidates to 4 SNPs out of 72 in LD with rs7769192. No SNPs were found in LD with rs67297943 and although this SNP shows enhancer marks in 6 tissues, no interactions were identified with this region and as such further refinement was not possible. It will be imperative to follow-up putative SNPs and genes with functional assays and demonstrate their contribution to disease in relevant cell types in a biological context using genome editing techniques.
Table 3. Refined MS SNPs based on bioinformatics and Capture Hi-C data.
| LD SNP | Index SNP | r2 | RegulomeDB Score | Promoter histone marks | Enhancer histone marks | DNAse | Proteins bound | Motifs changed | GRASP QTL hits | Selected eQTL hits | GENCODE genes | Gene Interactions* |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs11154801 | rs11154801 | 1 | 1d | BLD | BLD | BLD | Nkx2 | 20 hits | 46 hits | AHI1 | AHI1, ALDH8A1,HBS1L, MYB | |
| rs7759971 | rs11154801 | 0.99 | 1f | BLD | BLD, STRM, SKIN | 1 hit | 46 hits | AHI1 | ALDH8A1, HBS1L, MYB, SGK1 | |||
| rs13197384 | rs11154801 | 0.92 | 1a | 24 tissues | 53 tissues | 42 bound proteins | 13 altered motifs | 1 hit | 44 hits | AHI1 | BCLAF1, HBS1L, HMGB1P17, MTFR2, PDE7B | |
| rs7750586† | rs11154801 | 0.98 | 1f | GI, BLD | 10 tissues | 22 tissues | CTCF, RAD21, SMC3 | 2 hits | 53 hits | LINC00271 | ||
| rs9399148 | rs11154801 | 0.95 | 1f | 8 tissues | LNG, GI, BLD | KAP1 | GR | 2 hits | 52 hits | LINC00271 | MTFR2, PDE7B | |
| rs5880258 | rs11154801 | 0.95 | 4 tissues | MUS, MUS | Pbx-1 | 21 hits | LINC00271 | MTFR2, PDE7B | ||||
| rs1322553 | rs17066096 | 0.96 | 5 | BLD | IPSC, BLD | BLD | 4 altered motifs | 44kb 3' of IL22RA2 | IFNGR1, IL22RA2, TNFAIP3 | |||
| rs17066063 | rs17066096 | 0.96 | 3a | BLD | BRST, BLD, SKIN | 7 tissues | 6 bound proteins | Pou2f2, TCF4 | 1 hit | 41kb 3' of IL22RA2 | IFNGR1, IL22RA2, TNFAIP3 | |
| rs12214014 | rs17066096 | 0.97 | 5 | BLD | GR | 36kb 3' of IL22RA2 | IL22RA2, TNFAIP3 | |||||
| rs9321623 | rs7769192 | 0.97 | 4 | FAT, SKIN, MUS | 15 tissues | 7 tissues | Rad21 | 36kb 5' of RP11-95M15.1 | IL20RA, IL22RA2 | |||
| rs7769192 | rs7769192 | 1 | 4 | 6 tissues | 18 tissues | CTCF | HNF1, Irf, Pax-6 | 32kb 5' of RP11-95M15.1 | IFNGR1, IL20RA, IL22RA2 | |||
| rs11758213‡ | rs7769192 | 1 | 2a | GI, BLD, LIV | 39 tissues | CTCF, RAD21, SMC3 | 4 altered motifs | 12kb 5' of RP11-95M15.1 | ||||
| rs10607561 | rs7769192 | 0.93 | 3a | BLD | NFKB, SETDB1 | Foxp1, RREB-1 | 4.2kb 3' of RP11-95M15.1 | TNFAIP3 |
r2 has been rounded to 2 decimal places.
*—Only protein coding genes are shown for clarity.
†rs7750586 showed strong evidence of regulatory activity but was not involved in any interactions.
‡rs11758123 only interacts with lincRNAs. BLD, blood; BRST, breast; FAT, adipose tissue; GI, smooth muscle; IPSC, induced pluripotent stem cells; LIV, liver; LNG, lung; MUS, muscle; SKIN, skin; STRM, stromal connective.
Conclusion
In conclusion, our work has strengthened the case for the AHI1 gene candidate but also identified other potential MS gene targets, such as SGK1, BCLAF1 IL20RA, IL22RA2, IFNGR1 and TNFAIP3. Additionally, we have shown a possible co-regulation of MS GWAS associations in the 6q23 region, which could help elucidate the pathogenesis of MS as well as other autoimmune diseases. These targets require further functional investigation which has been informed by the bioinformatics analysis. While the MS associations show evidence of interacting with other genes with no obvious role in MS pathogenesis, it is likely that they share regulatory elements within this region. It is however important to investigate these interactions, in addition to ones highlighted in this analysis to fully explore disease pathogenesis.
Whilst the interactions identified require further independent validation, the unique experimental design using complementary capture baits (region and promoter captures) provides robust evidence of chromatin interactions. Additionally, validation with chromatin interactions identified by Rao et al. [19] further add to the confidence of the observed interactions. While Capture Hi-C offers much greater resolution for chromatin interactions than Hi-C, observed interactions are still limited by the restriction enzyme used and do not pinpoint the interactions to specific enhancers. As such further work will be necessary to confirm causal enhancers and how they affect gene regulation. The use of cell lines is a limitation of the study but the experimental requirement of high cell numbers for Capture Hi-C makes the use of primary cells challenging. However, it is essential that further experiments are performed in primary cells to fully elucidate how chromatin interactions can effect gene regulation in MS. Despite these limitations of Capture Hi-C, it is clear that this technique is a powerful approach to link genes to their regulatory elements and this work has identified several candidate causal genes for MS. Additionally it has been proposed that by using genetic evidence to select drug targets, it could double the success rate in clinical development [41]. Since Capture Hi-C has the potential to identify causal genes for genetic associations, it provides a way to enhance this further. This is exemplified by the identification of chromatin interactions between MS associations and the IL20RA and IL22RA2 genes, showing a potential use of anti-IL20 therapy in MS, and highlights the potential of Capture Hi-C to provide novel therapeutic targets or drug repositioning to improve patient outcome.
Supporting Information
Disease abbreviations are as follows: CEL–Coeliac disease; CRO–Crohn’s disease; MS–Multiple sclerosis; PBC–Primary biliary cirrhosis; PSO–Psoriasis; RA–Rheumatoid arthritis; SLE–Systemic lupus erythematosus; T1D –Type 1 diabetes; UC–Ulcerative colitis; SJO—Sjogren Syndrome.
(XLSX)
(XLSX)
Refined SNPs are highlighted in green.
(XLSX)
Tracks are labelled as follows: A–LD regions targeted in ‘region’ Capture Hi-C; B–Gene regions targeted in ‘promoter’ Capture Hi-C; C–GENCODE Genes V17; D–MS index SNPs; E–MS LD regions; F–Interactions observed in the GM12878 B-cell line and G–Interactions observed in the Jurkat T-cell line. All co-ordinates are based on GRCh37.
(TIF)
Tracks are labelled as follows: A–LD regions targeted in ‘region’ Capture Hi-C; B–Gene regions targeted in ‘promoter’ Capture Hi-C; C–RefSeq genes (packed for clarity); D–MS index SNPs; E–MS LD regions; F–Interactions observed in the GM12878 B-cell line and G–Interactions observed in the Jurkat T-cell line. All co-ordinates are based on GRCh37.
(TIF)
Acknowledgments
The authors would like to acknowledge the Faculty of Life Sciences Genomics Facility and the assistance given by IT Services and the use of the Computational Shared Facility at The University of Manchester. We thank Dr Kathryn Steel for carrying out cell separation and gene expression profiling of healthy volunteers.
Data Availability
Raw data and HindIII restriction fragment interaction counts are available in the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE69600.
Funding Statement
Wellcome Trust Research Career Development Fellowship (095684); Arthritis Research UK (grant numbers 20385, 20571); Wellcome Trust (097820/Z/11/B); European Union’s FP7 Health Programme (FP7-HEALTH-F2-2012-305549, Euro-TEAM, FP7/2007-2013); Innovative Medicines Initiative (BeTheCure project 115142); Medical Research Council (MR/K015346/1); National Institute for Health Research Manchester Musculoskeletal Biomedical Research Unit; Biotechnology and Biological Sciences Research Council UK (BBS/E/B/000C0405).
References
- 1.De Jager PL, Jia X, Wang J, de Bakker PIW, Ottoboni L, Aggarwal NT, et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41: 776–82. 10.1038/ng.401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, et al. Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med. 2007;357: 851–62. 10.1056/NEJMoa073493 [DOI] [PubMed] [Google Scholar]
- 3.Patsopoulos NA, Esposito F, Reischl J, Lehr S, Bauer D, Heubach J, et al. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol. 2011;70: 897–912. 10.1002/ana.22609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476: 214–9. 10.1038/nature10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemminki K, Li X, Sundquist J, Hillert J, Sundquist K. Risk for multiple sclerosis in relatives and spouses of patients diagnosed with autoimmune and related conditions. Neurogenetics. 2009;10: 5–11. 10.1007/s10048-008-0156-y [DOI] [PubMed] [Google Scholar]
- 6.Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45: 1353–60. 10.1038/ng.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PIW, Maller J, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39: 1477–82. 10.1038/ng.2007.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fung EYMG, Smyth DJ, Howson JMM, Cooper JD, Walker NM, Stevens H, et al. Analysis of 17 autoimmune disease-associated variants in type 1 diabetes identifies 6q23/TNFAIP3 as a susceptibility locus. Genes Immun. 2009;10: 188–91. 10.1038/gene.2008.99 [DOI] [PubMed] [Google Scholar]
- 9.Graham RR, Cotsapas C, Davies L, Hackett R, Lessard CJ, Leon JM, et al. Genetic variants near TNFAIP3 on 6q23 are associated with systemic lupus erythematosus. Nat Genet. 2008;40: 1059–61. 10.1038/ng.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-kappaB pathways. Nat Genet. 2009;41: 199–204. 10.1038/ng.311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois PCA, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, et al. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42: 295–302. 10.1038/ng.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowes J, Orozco G, Flynn E, Ho P, Brier R, Marzo-Ortega H, et al. Confirmation of TNIP1 and IL23A as susceptibility loci for psoriatic arthritis. Ann Rheum Dis. 2011;70: 1641–4. 10.1136/ard.2011.150102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491: 119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fairfax BP, Humburg P, Makino S, Naranbhai V, Wong D, Lau E, et al. Innate immune activity conditions the effect of regulatory variants upon monocyte gene expression. Science. 2014;343: 1246949 10.1126/science.1246949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farh KK-H, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518: 337–43. 10.1038/nature13835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoenfelder S, Clay I, Fraser P. The transcriptional interactome: gene expression in 3D. Curr Opin Genet Dev. 2010;20: 127–33. 10.1016/j.gde.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 17.Martin P, McGovern A, Orozco G, Duffus K, Yarwood A, Schoenfelder S, et al. Capture Hi-C reveals novel candidate genes and complex long-range interactions with related autoimmune risk loci. Nat Commun. 2015;6: 10069 10.1038/ncomms10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cairns J, Freire-Pritchett P, Wingett SW, Várnai C, Dimond A, Plagnol V, et al. CHiCAGO: robust detection of DNA looping interactions in Capture Hi-C data. Genome Biol. Cold Spring Harbor Labs Journals; 2016;17: 127 10.1186/s13059-016-0992-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao SSP, Huntley MH, Durand NC, Stamenova EK, Bochkov ID, Robinson JT, et al. A 3D Map of the Human Genome at Kilobase Resolution Reveals Principles of Chromatin Looping. Cell. 2014;159: 1665–80. 10.1016/j.cell.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westra H-J, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45: 1238–43. 10.1038/ng.2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj T, Rothamel K, Mostafavi S, Ye C, Lee MN, Replogle JM, et al. Polarization of the effects of autoimmune and neurodegenerative risk alleles in leukocytes. Science. 2014;344: 519–23. 10.1126/science.1249547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40: D930–4. 10.1093/nar/gkr917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22: 1790–7. 10.1101/gr.137323.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valente EM, Brancati F, Silhavy JL, Castori M, Marsh SE, Barrano G, et al. AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders. Ann Neurol. 2006;59: 527–34. 10.1002/ana.20749 [DOI] [PubMed] [Google Scholar]
- 25.Dixon-Salazar T, Silhavy JL, Marsh SE, Louie CM, Scott LC, Gururaj A, et al. Mutations in the AHI1 gene, encoding jouberin, cause Joubert syndrome with cortical polymicrogyria. Am J Hum Genet. 2004;75: 979–87. 10.1086/425985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496: 513–7. 10.1038/nature11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasof GM, Goyal L, White E. Btf, a novel death-promoting transcriptional repressor that interacts with Bcl-2-related proteins. Mol Cell Biol. 1999;19: 4390–404. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=104398&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharief MK, Matthews H, Noori MA. Expression ratios of the Bcl-2 family proteins and disease activity in multiple sclerosis. J Neuroimmunol. 2003;134: 158–65. Available: http://www.ncbi.nlm.nih.gov/pubmed/12507784 [DOI] [PubMed] [Google Scholar]
- 29.McPherson JP, Sarras H, Lemmers B, Tamblyn L, Migon E, Matysiak-Zablocki E, et al. Essential role for Bclaf1 in lung development and immune system function. Cell Death Differ. 2009;16: 331–9. 10.1038/cdd.2008.167 [DOI] [PubMed] [Google Scholar]
- 30.Pollard KM, Cauvi DM, Toomey CB, Morris K V, Kono DH. Interferon-γ and systemic autoimmunity. Discov Med. 2013;16: 123–31. Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3934799&tool=pmcentrez&rendertype=abstract [PMC free article] [PubMed] [Google Scholar]
- 31.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29: 71–109. 10.1146/annurev-immunol-031210-101312 [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb AB, Krueger JG, Sandberg Lundblad M, Göthberg M, Skolnick BE. First-In-Human, Phase 1, Randomized, Dose-Escalation Trial with Recombinant Anti-IL-20 Monoclonal Antibody in Patients with Psoriasis. PLoS One. 2015;10: e0134703 10.1371/journal.pone.0134703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Šenolt L, Leszczynski P, Dokoupilová E, Göthberg M, Valencia X, Hansen BB, et al. Efficacy and Safety of Anti-Interleukin-20 Monoclonal Antibody in Patients With Rheumatoid Arthritis: A Randomized Phase IIa Trial. Arthritis Rheumatol (Hoboken, NJ). 2015;67: 1438–48. 10.1002/art.39083 [DOI] [PubMed] [Google Scholar]
- 34.Rutz S, Eidenschenk C, Ouyang W. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252: 116–32. 10.1111/imr.12027 [DOI] [PubMed] [Google Scholar]
- 35.Catrysse L, Vereecke L, Beyaert R, van Loo G. A20 in inflammation and autoimmunity. Trends Immunol. 2014;35: 22–31. 10.1016/j.it.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 36.Dong X, Li C, Chen Y, Ding G, Li Y. Human transcriptional interactome of chromatin contribute to gene co-expression. BMC Genomics. BioMed Central; 2010;11: 704 10.1186/1471-2164-11-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Homouz D, Kudlicki AS. The 3D Organization of the Yeast Genome Correlates with Co-Expression and Reflects Functional Relations between Genes. Khodursky AB, editor. PLoS One. Public Library of Science; 2013;8: e54699 10.1371/journal.pone.0054699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lan X, Witt H, Katsumura K, Ye Z, Wang Q, Bresnick EH, et al. Integration of Hi-C and ChIP-seq data reveals distinct types of chromatin linkages. Nucleic Acids Res. 2012;40: 7690–7704. 10.1093/nar/gks501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schoenfelder S, Sexton T, Chakalova L, Cope NF, Horton A, Andrews S, et al. Preferential associations between co-regulated genes reveal a transcriptional interactome in erythroid cells. Nat Genet. 2010;42: 53–61. 10.1038/ng.496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huseby ES, Huseby PG, Shah S, Smith R, Stadinski BD. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol. 2012;3: 64 10.3389/fimmu.2012.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tipney H, Painter JL, Shen J, Nicoletti P, Shen Y, Floratos A, et al. The support of human genetic evidence for approved drug indications. Nat Genet. Nature Research; 2015;47: 1–7. 10.1038/ng.3314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disease abbreviations are as follows: CEL–Coeliac disease; CRO–Crohn’s disease; MS–Multiple sclerosis; PBC–Primary biliary cirrhosis; PSO–Psoriasis; RA–Rheumatoid arthritis; SLE–Systemic lupus erythematosus; T1D –Type 1 diabetes; UC–Ulcerative colitis; SJO—Sjogren Syndrome.
(XLSX)
(XLSX)
Refined SNPs are highlighted in green.
(XLSX)
Tracks are labelled as follows: A–LD regions targeted in ‘region’ Capture Hi-C; B–Gene regions targeted in ‘promoter’ Capture Hi-C; C–GENCODE Genes V17; D–MS index SNPs; E–MS LD regions; F–Interactions observed in the GM12878 B-cell line and G–Interactions observed in the Jurkat T-cell line. All co-ordinates are based on GRCh37.
(TIF)
Tracks are labelled as follows: A–LD regions targeted in ‘region’ Capture Hi-C; B–Gene regions targeted in ‘promoter’ Capture Hi-C; C–RefSeq genes (packed for clarity); D–MS index SNPs; E–MS LD regions; F–Interactions observed in the GM12878 B-cell line and G–Interactions observed in the Jurkat T-cell line. All co-ordinates are based on GRCh37.
(TIF)
Data Availability Statement
Raw data and HindIII restriction fragment interaction counts are available in the NCBI Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE69600.