Fig 1D is missing from Fig 1. Please see the corrected Fig 1 here.
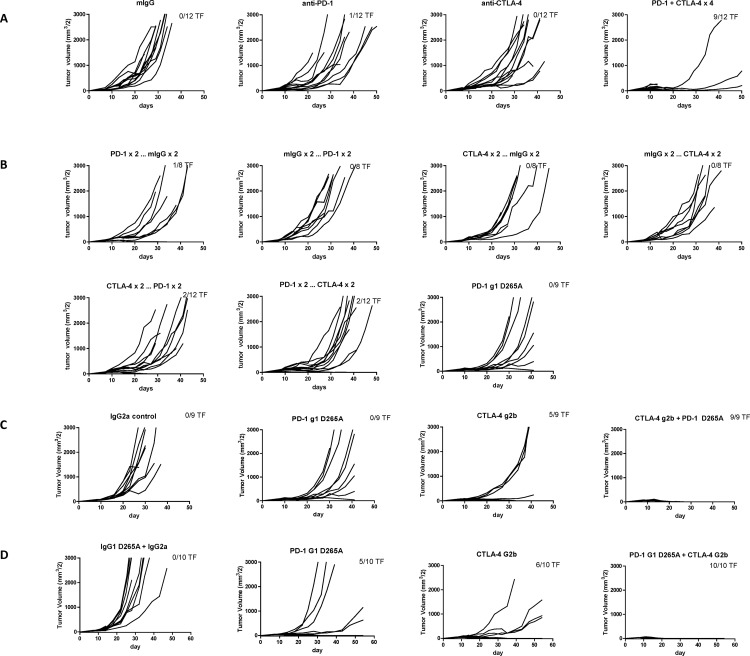
Fig 1. Antitumor Responses of Anti-CTLA-4 and Anti-PD-1 Antibodies in Staged MC38 and CT26 Tumor Models.
A-B. Groups of 8–12 C57/BL6 mice were sourced from Taconic and subcutaneously injected with 2×106 MC38 cells. After tumors were measured on day 7, mice were randomized (58 mm3 mean tumor volume per group) and then treated with the designated mAb (200 μg/dose IP) followed by additional doses on days 10, 14, and 17. A. Groups were treated with 4 doses of single or combined agents. Anti-PD-1 vs control p = 0.0176; anti-PD-1 and anti-CTLA-4 vs control p< 0.0001. B. Sequential dosing, where 4 doses were given as 2 doses of one mAb followed by 2 doses of the other mAb and the converse. Anti-CTLA-4 followed by anti-PD-1 vs control p = 0.0250; anti-PD-1 followed by anti-CTLA-4 vs control p = 0.0015. Tumor volumes were measured twice weekly. The number of tumor-free (TF) mice per group is indicated. C-D. Groups of 10 BALB/c mice sourced from CRL (C) or HAR (D) Laboratories were subcutaneously injected with 1×106 CT26 cells. After tumors were measured on day 7, mice were randomized (C: 56 mm3 and D: 35 mm3 mean tumor volume) and then treated with the designated mAb (200 μg/dose IP) followed by additional doses on days 10, 14 (HAR mice), or 10, 14, 17 (CRL mice). Anti-CTLA-4 vs control p = 0.0035; anti-CTLA-4 and anti-PD-1 vs control p<0.0001. Tumor volumes were measured twice weekly. The number of TF mice per group is indicated.
Reference
- 1.Selby MJ, Engelhardt JJ, Johnston RJ, Lu L- S, Han M, Thudium K, et al. (2016) Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS ONE 11(9): e0161779 doi:10.1371/journal.pone.0161779 [DOI] [PMC free article] [PubMed] [Google Scholar]