Abstract
Prolonged survival in patients with chronic myeloid leukemia treated with BCR-ABL1-targeted tyrosine kinase inhibitors allows consideration of parenthood for patients on chronic therapy, but there are limited data about the effects of dasatinib on pregnancy. Pregnancy-related outcomes in dasatinib-treated patients or their partners reported to Bristol–Myers Squibb from clinical trials or healthcare providers through December 2013 were reviewed. Outcomes were available in 46/78 dasatinib-treated women (59%) and 33/69 partners of dasatinib-treated men (48%). Fifteen women (33%) delivered a normal infant; 18 (39%) and 8 (17%) had an elective or spontaneous abortion; and 5 (11%) had an abnormal pregnancy. There were 7 reports of fetal/infant abnormalities (encephalocele, renal tract abnormalities, and hydrops fetalis). Thirty of 33 (91%) infants fathered by dasatinib-treated men were reported normal at birth. Also, animal studies evaluated the impact of dasatinib on fertility, embryo-fetal toxicity, and development, suggesting that dasatinib may be a selective developmental toxicant. The outcomes of most pregnancies conceived by men treated with dasatinib were normal, but due to the small number of cases, further monitoring is required. Significant effects on pregnancy outcomes in women treated with dasatinib were found, supporting current recommendations that women avoid becoming pregnant during dasatinib treatment and be informed of fetal risks.
Introduction
The introduction of BCR-ABL1-targeted tyrosine kinase inhibitors (TKIs) has revolutionized the treatment of chronic myeloid leukemia (CML). As the likelihood of survival increases for CML patients, there is a heightened attention to quality of life issues and long-term treatment choices. In the dasatinib clinical trial program, approximately one-third of the women were younger than 46 years of age, including half of all women receiving first-line therapy.
Information on pregnancy outcomes in CML patients treated with TKIs is mostly from those treated with imatinib [1–3]. In more than 150 men who conceived while receiving imatinib, one infant was born with malrotation of the small intestine, and one stillbirth with malformations occurred [1,3]. In contrast, among 265 women who became pregnant while on imatinib, 60% of pregnancies resulted in a normal birth, 11% ended in spontaneous abortion, and at least 7% had fetuses with abnormalities [1–3].
In preclinical studies, second- or third-generation TKIs were found to have minor effects on fertility, although increased embryonic resorption, decreased implantation, and fewer viable embryos were reported in dasatinib-treated female animals. Various embryo-fetal toxicities were observed, including skeletal malformations [4–8].
There are a few clinical data regarding the effects of nilotinib and dasatinib on pregnancy. There were two normal pregnancies and one fetal abnormality in three pregnancies in female partners of nilotinib-treated men [3,4,9]. Nilotinib was stopped in the first trimester in three pregnancies in female patients: two were successful pregnancies [3,10] and one fetus had an omphalocele [3]. The timing of nilotinib treatment was unknown in another case of fetal abnormalities [4].
All nine reported pregnancies in partners of dasatinib-treated men resulted in the birth of normal infants [3,11–13]. In seven dasatinib-treated women who had pregnancies that went to term, six resulted in the delivery of a normal infant [3,11,14–17]. These six women stopped dasatinib in the first trimester. One pregnancy in a dasatinib-treated woman resulted in fetal abnormalities; however, dasatinib was continually administered through the second trimester [18]. Based on preclinical data and the lack of human experience, patients on TKIs are advised to use contraception as pregnancy is not recommended.
Here, we analyze all 147 pregnancies with parental exposure to dasatinib that were submitted to the Bristol–Myers Squibb (BMS) pharmacovigilance database. Pregnancy outcomes of women who either became pregnant while receiving dasatinib or initiated dasatinib to manage their disease after becoming pregnant, as well as the pregnancy outcomes of female partners of men treated with dasatinib at the time of conception, are reviewed. In addition, the results of pre-clinical studies to determine the reproductive toxicology of dasatinib are presented.
Methods
Pharmacovigilance database review. A cumulative review was performed using the BMS pharmacovigilance database, the repository for clinical trial and post-marketing safety data from worldwide sources, including scientific literature, to identify all reports of pregnancy and fetal outcomes in dasatinib-treated women or the female partners of dasatinib-treated men treated for any disease up to December 2013. Women were included if they were exposed to dasatinib at conception or at any time during pregnancy. Female partners of dasatinib-treated men were included if the man was on active treatment at the time of conception.
Of the 147 cases identified, approximately one-third were from BMS-led trials, with the remainder from spontaneous post-marketing reports. As per standard pharmacovigilance practice, follow-up data were sought in all cases; however, complete information was not always available.
Preclinical studies. Fertility and early embryonic development studies were performed in dasatinib-treated male and female rats (n = 100; 25 per sex per group), with evaluations on gestation day (GD) 16. Embryo-fetal toxicity was studied in both mated rat and rabbit dams treated with dasatinib on GD6–15 (rats) or GD7–19 (rabbits). Toxicokinetic evaluation was performed on GD15 (rats; n = 10) or GD19 (rabbits; n = 5), and toxicity endpoints were evaluated on GD20 (rats; n = 22) or GD29 (rabbits; n = 22). Peri- and postnatal development were evaluated in pregnant and lactating rats (n = 56; 8 per group) treated with dasatinib on GD16 to lactation day (LD) 3, GD21–LD8, or LD4–LD20. These studies were conducted once; however, each was powered according to international guidelines ICH S5(R2) to be sensitive for changes in the endpoints measured. All studies were approved by the BMS Institutional Animal Care and Use Committee. Further details can be found in the online Supporting Information.
Results
Clinical data from the pharmacovigilance database
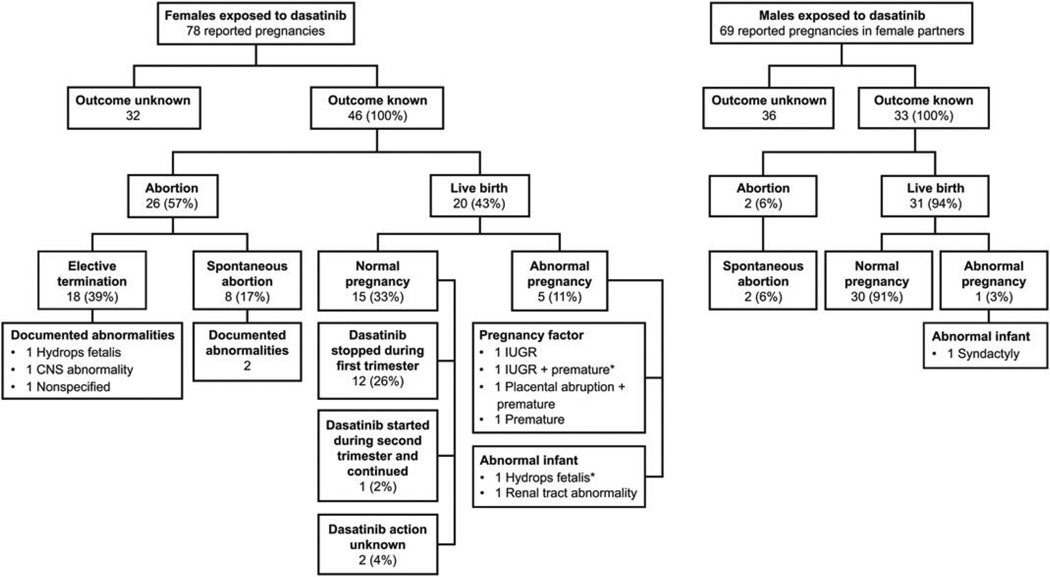
A total of 147 pregnancies were identified in the BMS pharmacovigilance database: 78 pregnancies in dasatinib-treated women and 69 pregnancies in female partners of dasatinib-treated men. One female partner of a male patient and one female patient were pregnant with twins. Of patients with known indications, 87 were treated for CML (17 first-line, 31 second-line, and 39 unknown) and 5 patients were treated for Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia. Twenty-three of 78 women treated with dasatinib were enrolled in a clinical trial. Pregnancy outcomes are summarized in Fig. 1.
Figure 1.
Pregnancy outcomes in dasatinib-treated women and the female partners of dasatinib-treated men. CNS, central nervous system; IUGR, intrauterine growth restriction. *One woman had both maternal issues during her pregnancy (IUGR 1 premature) and delivered an abnormal infant with hydrops fetalis.
Pregnancy outcomes in dasatinib-treated women
Pregnancy outcomes in 46 of 78 patients (59%; Fig. 1) for whom outcomes were known were reported. The median age of these 46 women was 30.5 years (range, 17–40). Twenty of 46 pregnancies (43%) resulted in live births, with delivery of a normal infant at term in 15 (33%) and abnormal pregnancies in 5 (11%). Pregnancies were terminated in 26 of 46 cases (57%): 18 elective and 8 spontaneous abortions.
Maternal issues occurred in 4 women (Fig. 1), leading to one intrauterine growth retardation (IUGR) observed at term (100 mg/day dasatinib stopped during first trimester and replaced with interferon-α), one IUGR with premature delivery at week 28 (dasatinib dose unknown, administered weeks 17–24), one placental abruption at week 34 with premature delivery of a normal infant (180 mg/day dasatinib stopped at week 5), and one premature birth (month 7) of an infant small for dates (140 mg/day dasatinib stopped during first trimester).
Congenital abnormalities were identified in seven infants: two after birth and five after abortion (two spontaneous, three elective; Fig. 1). An infant with renal tract abnormalities (bilateral pyelocaliceal dilatation and kidney enlargement) was delivered live at week 36 to a woman who was treated with 60 mg/day dasatinib at conception. Dasatinib was discontinued at weeks 4–6 and interferon-α was initiated. In the second case, a woman diagnosed with CML while pregnant was started on dasatinib (unknown dose) in the second trimester (weeks 17–24). At week 28, hydrops fetalis was diagnosed and an emergency Cesarean section was performed. The infant was alive at birth but died within 24 hr with hydrops fetalis and lung hypoplasia. This infant was the child of a woman who also had maternal issues during her pregnancy (IUGR with premature delivery at week 28).
The two fetuses in which abnormalities were identified after elective termination had CNS abnormalities and hydrops fetalis (Fig. 1). In the first case, dasatinib (dose unknown) was stopped at week 4. Parietooccipital encephalocele and premature closure of the cranial vault sutures anterior and median fossa were identified. There is an unconfirmed suggestion that this fetus was exposed to hydroxyurea. The gestational date of the surgical abortion was not provided. The second case has been reported previously [18]. A woman receiving imatinib 400 mg/day from the time of conception was switched to dasatinib 100 mg/day at week 6. An ultrasound at week 16 showed that the fetus had hydrops fetalis. Fetal blood sampling found leukopenia and thrombocytopenia and a fetal serum dasatinib level of 3 ng/mL, compared with a maternal level of 4 ng/mL. Fetal autopsy after termination at week 17 confirmed subcutaneous edema, ascites, pleural effusion, microretrognathia, and hypertelorism. The fetal genotype was normal. Details on the remaining three fetal abnormalities are not known.
Forty-two of 46 women (91%) were taking dasatinib at the time of conception. In most cases (32/42; 76%), dasatinib was stopped on confirmation of pregnancy in the first trimester. This includes 12 women who had normal pregnancies, three with maternal issues (IUGR, premature, and premature with placental abruption), and two with fetal abnormalities (renal tract and CNS abnormalities). One of the four women not on dasatinib at conception was unaware that she was pregnant when dasatinib was started in the first trimester and chose to have an elective abortion. The remaining three pregnant women were treated with dasatinib later in pregnancy to manage underlying CML. Of these, one patient went on to deliver a normal infant at term after starting dasatinib 100 mg/day at week 17. The other two women had fetuses that developed hydrops fetalis. One woman on dasatinib 100 mg/day from week 6 terminated her pregnancy at week 17. The other took dasatinib (dose unknown) during weeks 17–24 and delivered prematurely at week 28, and the infant survived 1 day.
Eight of 32 women who stopped dasatinib in the first trimester were then treated with interferon-α during the remainder of pregnancy, and one was maintained on hydroxyurea. All but two pregnancies with known outcome resulted in a normal birth (interferon-α: one delivery of a live infant with renal tract abnormalities and one IUGR).
Pregnancy outcomes in female partners of dasatinib-treated men
Pregnancy outcomes have been provided for 33 of 69 (48%) pregnancies in female partners of dasatinib-treated men (Fig. 1). Of these, 30 (91%) resulted in full-term deliveries of normal infants, two (6%) resulted in spontaneous abortions (one at week 12 and one unknown), and one (3%) resulted in the birth of an infant at term with syndactyly.
Preclinical studies
Female rats were mated to determine if dasatinib affected fertility or early embryonic development. There were no effects of dasatinib on fertility across dasatinib doses tested (Table I). Increased embryolethality was observed with clinically relevant doses of dasatinib (Table I), indicating that dasatinib is a selective reproductive toxicant.
TABLE I.
Preclinical Study to Determine the Effects of Dasatinib on Fertility and Early Embryonic Development
| Male rats | Female rats | |||||
|---|---|---|---|---|---|---|
| Dasatinib (mg/kg/day) | ||||||
| Drug-related changesa | 2.5 | 5 | 10 | 2.5 | 5 | 10 |
| Mating rate | NC | NC | NC | NC | NC | NC |
| Fertility rate | NC | NC | NC | NC | NC | NC |
| Embryolethality | NC | NC | NC | NC | I | I |
| Postimplantation loss of embryos | NC | NC | NC | NC | I | I |
| Seminal vesicle weights with fluid | NC | NC | D | N/A | N/A | N/A |
| Viability | NC | NC | NC | NC | NC | NC |
| Mild/moderate dehydration | NC | I | I | NC | NC | I |
| Body weight (males) | NC | D | D | N/A | N/A | N/A |
| Body weight gain (females) | N/A | N/A | N/A | NC | D | D |
| Food intake | NC | D | D | NC | NC | D |
Compared with untreated controls.
I. increased; D, decreased; NC, no change; N/A, not applicable.
The embryo-fetal toxicity of dasatinib was investigated in female rats and rabbits. When dasatinib was administered to rats on GD6–15 (organogenesis), embryolethality was observed at all doses (Table II). Viable fetuses were observed when dasatinib 2.5 or 5 mg/kg/day was administered, but skeletal abnormalities, such as bending of the scapula or humerus and reduced ossification of the sternebrae and thoracic vertebral centra, were observed. Maternal toxicity was not observed. In rabbits, dasatinib was administered on GD7–19 (organogenesis). At clinically relevant dasatinib doses, there was no change in embryolethality, but dasatinib treatment resulted in fetal abnormalities, including delayed ossification of fetal lumbar vertebrae and pelvis, irregular ossification of the hyoid, and the presence of cervical ribs (Table II). Maternal toxicity was not observed. These results indicate that dasatinib is a selective developmental toxicant.
TABLE II.
Preclinical Studies to Determine the Embryo-Fetal Toxicity of Dasatinib
| Rats | Rabbits | ||||||
|---|---|---|---|---|---|---|---|
| Dasatinib (mg/kg/day) | |||||||
| Drug-related effectsa | 2.5 | 5 | 10 | 20 | 0.5 | 2 | 6 |
| Maternal effects | |||||||
| Body weight gain during dosing | D | D | D | D | NC | NC | NC |
| Body weight gain postdosing | D | D | D | N/A | NC | NC | NC |
| Body weight postdosing | D | D | D | D | NC | NC | NC |
| Embryo-fetal effects | |||||||
| Fetal skeletal abnormalities | I | I | N/A | N/A | I | I | I |
| Embryolethality | I | I | I | I | NC | NC | |
| NC | No surviving fetuses | NC | NC | I | I NC | ||
| NC | NC | ||||||
Compared with untreated controls.
I, increased; D, decreased; NC, no change; N/A, not applicable.
In an exploratory peri- and postnatal development study in rats, females were administered dasatinib from GD16, the approximate onset of parturition (GD21), or during early lactation (LD4). A large proportion of pups died when dasatinib 5 or 10 mg/kg/day was initiated on GD16 (25% and 89%, respectively) or on GD21 (57% and 60%, respectively; Table III). There were no reported deaths of pups treated with 5 mg/kg/day when dasatinib was started at LD4, but 34% of pups died when exposed to dasatinib 10 mg/kg/day. Pleural effusion was observed in pups, most of which had died (Table III). Therefore, indirect exposure of pups to dasatinib from the end of organogenesis through LD4 is usually incompatible with survival and can result in abnormalities.
TABLE III.
Exploratory Peri- and Postnatal Development Preclinical Study
| Rat pups (n) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dasatinib (mg/kg/day) | ||||||||||||
| 0 | 5 | 10 | ||||||||||
| Start of dasatinib treatment | Died | M | F | NE | Died | M | F | NE | Died | M | F | NE |
| Gestation day 16 | 2 (2%) | N/A | N/A | N/A | 23 (25%) | 25 | 22 | 45 | 57 (89%) | 4 | 1 | 59 |
| Pleural effusion | N/A | N/A | N/A | N/A | 18 | 13 | 7 | N/A | 0 | 0 | 0 | N/A |
| Gestation day 21 | 1 (2%) | N/A | N/A | N/A | 58 (57%) | 22 | 20 | 60 | 46 (60%) | 20 | 10 | 47 |
| Pleural effusion | N/A | N/A | N/A | N/A | 16 | 9 | 7 | N/A | 30 | 20 | 10 | N/A |
| Lactation day 4 | 0 | 40 | 28 | 33 | 0 | 29 | 42 | 32 | 33 (34%) | 31 | 26 | 40 |
| Pleural effusion | 0 | 0 | 0 | N/A | 0 | 0 | 0 | N/A | 25 | 10 | 15 | N/A |
F, viable female; M, viable male; N/A, not applicable; NE, not examined.
In male rats, no changes were observed in mating, fertility, or embryonic development at any administered dasatinib dose, despite the observed decrease in absolute and relative seminal vesicle weights with fluid observed with dasatinib 10 mg/kg/day (Table I).
Discussion
A review of the BMS pharmacovigilance database was performed to determine pregnancy and fetal outcomes in dasatinib-treated women and the female partners of dasatinib-treated men. Overall, 147 pregnancies were identified: 78 in dasatinib-treated women and 69 in female partners of dasatinib-treated men. These numbers are higher than expected based on prescribing information that recommends avoiding pregnancy while on dasatinib and represent the largest reported pregnancy series of patients treated with any second- or third-generation TKI. Despite the limitations of evaluating pharmacovigilance data, this analysis provides useful insight for physicians and patients when considering management of CML during pregnancy, and lifestyle choices during long-term treatment.
Pregnancy outcomes in female partners of dasatinib-treated men were good, with 91% reporting a normal outcome. Only 6% resulted in spontaneous abortion. The reported frequency of spontaneous abortion in the general population may be as high as 31% [19], although rates from 11% to 22% are typically reported [20,21]. Thus, the reported incidence is within that expected in the general population. The lack of direct effects on testis, male reproductive function, implantation, and embryogenesis in the off-spring of dasatinib-treated male animals further supports this. Currently, the prescribing information for dasatinib (and other TKIs) has no restrictions regarding fathering children for men receiving these medications.
An infant with syndactyly was born of a female partner of a dasatinib-treated man. Syndactyly is the most common congenital malformation of the limbs and is usually the result of the digits failing to separate between weeks 6 and 8. With no preclinical data suggesting an effect on sperm, and the low probability of significant fetal exposure at weeks 6–8, it is possible that dasatinib exposure had no impact. Still, these limited data cannot exclude a detrimental effect. Men should be counseled on the potential risks of dasatinib, and the use of contraception should be recommended.
Treatment was not disrupted in men who fathered children, but almost all female patients stopped or interrupted dasatinib upon confirmation of pregnancy, with potential negative effects on disease control (data not collected). In imatinib-treated women who became pregnant, interruption of therapy resulted in a loss of complete hematologic remission in 50% and an increase in Ph+ metaphases in 60% [1]. In patients with deep molecular responses at conception, those who stopped imatinib during pregnancy and resumed after delivery reached deep molecular responses again (if they had lost them) quickly. This suggests the possibility of safe therapy manipulation during pregnancy, particularly for patients with good disease control at the time of interruption [22,23]. Based on this evidence, contraception should be suggested for women, and pregnancy should be planned only after stable response is reached.
Of the 46 pregnancies in dasatinib-treated women, there were 20 live births of which 15 (33%) resulted in full-term deliveries of normal infants. In 12 of 15, dasatinib was discontinued in the first trimester. Three women received treatment later in pregnancy, and only one resulted in a normal pregnancy (dasatinib initiated at week 17). The fetuses of the other two women both developed hydrops fetalis after initiating dasatinib at weeks 6 or 17. One of these women was exposed to dasatinib in the second trimester (weeks 6–17) and electively aborted at week 17. Fetal exposure to dasatinib transplacentally was documented, with drug serum levels approximately 75% of the maternal concentrations. This is consistent with the placental transfer of dasatinib observed in animals [24]. The hydrops fetalis could have been associated with unwanted pharmacology and a different susceptibility to the effects of dasatinib on marrow suppression, although fluid retention effects could have also contributed. The effects observed in fetuses exposed later in gestation are consistent with the effects observed in the peri- and postnatal preclinical studies. Although the data are scant, this should discourage the use of dasatinib at any time during pregnancy.
Twenty-six women had a spontaneous (17%) or elective (39%) abortion. Although the exact number of fetuses with abnormalities is not known, five cases were reported and the details were known in two. One fetus with encephalocele and premature closure of the cranial vaults was exposed to dasatinib at the time of neural tube formation (up to week 4) [25]. Therefore, it is possible that this was a result of dasatinib treatment. The other fetus with structural abnormalities (microretrognathia and hypertelorism) had been exposed to imatinib in the first 6 weeks of gestation, prior to switching to dasatinib, and was aborted with hydrops fetalis in the second trimester. Skeletal abnormalities were reported in the off-spring of dasatinib-treated animals, and were also reported in the fetuses of imatinib-treated women [2]. These findings may suggest a class effect due to the spectrum of tyrosine kinases inhibited by these agents [26,27].
Lastly, regarding the infant born with renal tract abnormalities, the renal calyceal system develops during the third month of pregnancy [28], which was after the cessation of dasatinib. However, the possible effect of dasatinib on the development of the Wolffian duct system and mesonephros, which occurs earlier in pregnancy and lays the foundation for the more mature renal tract elements, cannot be excluded [29]. This patient was also treated with interferon-α for most of the pregnancy. The effect of exposure to interferon-α on the emergence of this abnormality is not known. Renal tract abnormalities were also observed in 2 of 12 infants with abnormalities reported following exposure to imatinib [2].
The underlying mechanism behind these fetal defects is unknown; however, the disruption of platelet-derived growth factor receptor-α (PDGFR-α) signaling by dasatinib may be involved [27]. Similar fetal abnormalities to those reported here were observed in mice homozygous null for mutations in Pdgfra following exposure to dasatinib at doses roughly equivalent to standard doses in humans [30,31].
Pregnancy-related issues reported in dasatinib-treated women included IUGR, premature delivery, and placental abruption. While all these could potentially be the result of natural causes, this cannot be known. Placental abruption, which occurs in approximately 1% of pregnancies in the United States [32], could reflect alterations in placental function and bleeding diathesis related to therapy.
In summary, review of the BMS pharmacovigilance database showed no apparent risk to the offspring of dasatinib-treated male patients, although the numbers are too small to be definitive. In contrast, in dasatinib-treated women, there are definite risks to the fetus, in the form of skeletal malformations and detrimental pharmacological effects. These data are consistent with reports of dasatinib-treated animals, and humans treated with imatinib. These results support the current recommendations that women taking dasatinib should avoid becoming pregnant, and should not receive dasatinib at any time while pregnant. The optimal management of CML during pregnancy requires further study.
Acknowledgments
Conflict of interest: J.E.C. has received research support from and acted as a consultant for Ariad, BMS, Novartis, and Pfizer and received research support from Teva. E.A. has acted as a consultant for BMS. E.C. has received honoraria and acted as a consultant for BMS and Novartis. M.G. was an employee of BMS at the time of manuscript development. N.W. is an employee of BMS. J.F.A. has received honoraria from and has acted as a consultant for Ariad, BMS, Novartis, and Pfizer.
The authors would like to thank all participating study sites and the patients and physicians who reported the spontaneous cases used in this BMS-sponsored analysis. J.F.A. is grateful for the support of the NIHR Biomedical Research Centre. Thank you to Kary Thompson for assistance with the preclinical studies. Professional medical writing and editorial assistance was provided by Samantha L. Dwyer, PhD, and Rebecca A. Rozich, PhD, of StemScientific, an Ashfield Company, part of UDG Healthcare plc, funded by BMS. The authors did not receive financial compensation from BMS for authoring this manuscript.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Author Contributions
All authors provided feedback and guidance on the analysis and interpretation of the results, critically reviewed and provided revisions to the manuscript, and approved the final draft for submission. N.W. critically reviewed all pregnancy cases in the BMS pharmacovigilance database and contributed to writing the initial draft of the manuscript. M.G. designed, performed, and interpreted the preclinical experiments.
References
- 1.Ault P, Kantarjian H, O’Brien S, et al. Pregnancy among patients with chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2006;24:1204–1208. doi: 10.1200/JCO.2005.04.6557. [DOI] [PubMed] [Google Scholar]
- 2.Pye SM, Cortes J, Ault P, et al. The effects of imatinib on pregnancy outcome. Blood. 2008;111:5505–5508. doi: 10.1182/blood-2007-10-114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abruzzese E, Trawinska MM, Perrotti AP, De Fabritiis P. Tyrosine kinase inhibitors and pregnancy. Mediterr J Hematol Infect Dis. 2014;6:e2014028. doi: 10.4084/MJHID.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bristol-Myers Squibb Company. Sprycel (dasatinib) [prescribing information] Princeton, NJ: 2015. [Google Scholar]
- 5.Novartis Pharmaceuticals Corporation. Gleevec (imatinib) [prescribing information] East Hanover, NJ: 2015. [Google Scholar]
- 6.Novartis Pharmaceuticals Corporation. Tasigna (nilotinib) [prescribing information] East Hanover, NJ: 2015. [Google Scholar]
- 7.Pfizer, Inc. Bosulif (bosutinib) [prescribing information] New York, NY: 2014. [Google Scholar]
- 8.Ariad Pharmaceuticals, Inc. Iclusig (ponatinib) [prescribing information] Cambridge, MA: 2014. [Google Scholar]
- 9.Zhou L, You JH, Wu W, et al. Pregnancies in patients with chronic myeloid leukemia treated with tyrosine kinase inhibitor. Leuk Res. 2013;37:1216–1221. doi: 10.1016/j.leukres.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 10.Conchon M, Sanabani SS, Bendit I, et al. Two successful pregnancies in a woman with chronic myeloid leukemia exposed to nilotinib during the first trimester of her second pregnancy: Case study. J Hematol Oncol. 2009;2:42. doi: 10.1186/1756-8722-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes J, O’Brien S, Ault P, et al. Pregnancy outcomes among patients with chronic myeloid leukemia treated with dasatinib. Blood. 2008;112 abstract 3230. [Google Scholar]
- 12.Gentile M, Guido M, Lucia E, et al. Favorable conception and pregnancy involving a male patient affected by chronic myeloid leukemia while taking dasatinib. Leuk Lymphoma. 2014;55:709–710. doi: 10.3109/10428194.2013.811240. [DOI] [PubMed] [Google Scholar]
- 13.Oweini H, Otrock ZK, Mahfouz RA, Bazarbachi A. Successful pregnancy involving a man with chronic myeloid leukemia on dasatinib. Arch Gynecol Obstet. 2011;283:133–134. doi: 10.1007/s00404-010-1501-6. [DOI] [PubMed] [Google Scholar]
- 14.Bayraktar S, Morency B, Escalon MP. Successful pregnancy in a patient with chronic myeloid leukaemia exposed to dasatinib during the first trimester. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.05.2010.2975. bcr0520102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conchon M, Sanabani SS, Serpa M, et al. Successful pregnancy and delivery in a patient with chronic myeloid leukemia while on dasatinib therapy. Adv Hematol. 2010;2010:136252. doi: 10.1155/2010/136252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroll T, Ames MB, Pruett JA, Fenske TS. Successful management of pregnancy occurring in a patient with chronic myeloid leukemia on dasatinib. Leuk Lymphoma. 2010;51:1751–1753. doi: 10.3109/10428194.2010.497982. [DOI] [PubMed] [Google Scholar]
- 17.Dine G, Levert M, Rehn Y, et al. Two successful successive pregnancies in a woman with CML treated with dasatinib and temporary peg-interferon. J US China Med Sci. 2013;10:128–133. [Google Scholar]
- 18.Berveiller P, Andreoli A, Mir O, et al. A dramatic fetal outcome following transplacental transfer of dasatinib. Anticancer Drugs. 2012;23:754–757. doi: 10.1097/CAD.0b013e328352a8fe. [DOI] [PubMed] [Google Scholar]
- 19.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 20.Nybo Andersen AM, Wohlfahrt J, Christens P, et al. Maternal age and fetal loss: Population based register linkage study. BMJ. 2000;320:1708–1712. doi: 10.1136/bmj.320.7251.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ammon AL, Galindo C, Li DK. A systematic review to calculate background miscarriage rates using life table analysis. Birth Defects Res A Clin Mol Teratol. 2012;94:417–423. doi: 10.1002/bdra.23014. [DOI] [PubMed] [Google Scholar]
- 22.Sor'a F, De Matteis S, Bajer J, et al. Persistence of molecular remission throughout pregnancy in CML after imatinib. Leuk Res. 2009;33:e6–e7. doi: 10.1016/j.leukres.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Garderet L, Santacruz R, Barbu V, et al. Two successful pregnancies in a chronic myeloid leukemia patient treated with imatinib. Haematologica. 2007;92:e9–e10. doi: 10.3324/haematol.10935. [DOI] [PubMed] [Google Scholar]
- 24.He K, Lago MW, Iyer RA, et al. Lacteal secretion, fetal and maternal tissue distribution of dasatinib in rats. Drug Metab Dispos. 2008;36:2564–2570. doi: 10.1124/dmd.108.022764. [DOI] [PubMed] [Google Scholar]
- 25.O’Rahilly R, Muller F. Significant features in the early prenatal development of the human brain. Ann Anat. 2008;190:105–118. doi: 10.1016/j.aanat.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 27.Vandyke K, Fitter S, Zannettino AC. The tyrosine kinase inhibitor dasatinib (SPRYCEL) inhibits chondrocyte activity and proliferation. Blood Cancer J. 2011;1:e2. doi: 10.1038/bcj.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itatani H, Koide T, Okuyama A, et al. Development of the calyceal system in the human fetus. Invest Urol. 1979;16:388–394. [PubMed] [Google Scholar]
- 29.Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol. 2009;325:6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soriano P. The PDGF alpha receptor is required for neural crest cell development and for normal patterning of the somites. Development. 1997;124:2691–2700. doi: 10.1242/dev.124.14.2691. [DOI] [PubMed] [Google Scholar]
- 31.Ding H, Wu X, Bostrom H, et al. A specific requirement for PDGF-C in palate formation and PDGFR-alpha signaling. Nat Genet. 2004;36:1111–1116. doi: 10.1038/ng1415. [DOI] [PubMed] [Google Scholar]
- 32.Ananth CV, Oyelese Y, Yeo L, et al. Placental abruption in the United States, 1979 through 2001: Temporal trends and potential determinants. Am J Obstet Gynecol. 2005;192:191–198. doi: 10.1016/j.ajog.2004.05.087. [DOI] [PubMed] [Google Scholar]