Abstract
Purpose
The effects of impaired spatiotemporal vision in amblyopia on visuomotor skills have rarely been explored in detail. The goal of this study was to examine the influences of amblyopia on visually guided reaching.
Methods
Fourteen patients with anisometropic amblyopia and 14 control subjects were recruited. Participants executed reach-to-touch movements toward targets presented randomly 5° or 10° to the left or right of central fixation in three viewing conditions: binocular, monocular amblyopic eye, and monocular fellow eye viewing (left and right monocular viewing for control subjects). Visual feedback of the target was removed on 50% of the trials at the initiation of reaching.
Results
Reaching accuracy was comparable between patients and control subjects during all three viewing conditions. Patients’ reaching responses were slightly less precise during amblyopic eye viewing, but their precision was normal during binocular or fellow eye viewing. Reaching reaction time was not affected by amblyopia. The duration of the acceleration phase was longer in patients than in control subjects under all viewing conditions, whereas the duration of the deceleration phase was unaffected. Peak acceleration and peak velocity were also reduced in patients.
Conclusions
Amblyopia affects both the programming and the execution of visually guided reaching. The increased duration of the acceleration phase, as well as the reduced peak acceleration and peak velocity, might reflect a strategy or adaptation of feedforward/feedback control of the visuomotor system to compensate for degraded spatiotemporal vision in amblyopia, allowing patients to optimize their reaching performance.
A primary function of the senses (e.g., vision) is to collect information to guide motor behaviors, and one of the brain’s main tasks is to perform sensorimotor transformation—a process that involves integrating sight, sound, and other sensory information for the preparation and execution of purposeful action. Visuomotor skills that seem ordinary, such as reaching and picking up a coffee cup, typing on a keyboard, or catching a ball, actually require complex and accurate sensorimotor processing. Sensorimotor processing can be conceptualized in three stages: localization, motor planning, and movement execution.1,2 For instance, to pick up a cup successfully, sensory information about the target, including its location (both direction and distance) and properties (size, shape, and orientation), as well as the initial location of the arm, head, and trunk, have to be detected and encoded by the central nervous system.
Sensory input must be then transformed into a frame of reference appropriate for the effector movement. For example, eye movements are planned and executed in a gaze-centered coordinate frame, whereas arm movements are programmed in a shoulder-centered coordinate frame.1,3 During the movement execution stage, visual and proprioceptive feedback arising from the receptors is monitored, updated, and compared with the corollary discharge4,5 so that the motor system can detect errors and correct them quickly to achieve optimal performance.6 In most everyday situations, input from all sensory modalities is used during goal-directed movements, but vision provides a major input for all three stages. This is exemplified by the observation that reaching movements are most accurate when the target and the hand are visible throughout the movement.7
Amblyopia is an impairment of spatiotemporal vision8 –13 that is caused by inadequate stimulation of the eyes during early childhood.14 It is most commonly associated with strabismus, anisometropia, and, more rarely, image degradation caused by congenital cataract.15 Vision provides a major sensory input in guiding hand movements. It is surprising that very few studies have examined how degraded vision affects visuomotor skills in people with amblyopia. Previous studies reported that adults16 –18 and children19 with amblyopia exhibit more perceptual localization errors when viewing with their amblyopic eye. Another study20 reported significant deficits on a standardized clinical test of fine motor skills in children with amblyopia. These studies,16 –20 however, did not provide quantitative kinematic measures of the eye movement or the reaching response. A recent study21 examined prehension skills of adults with amblyopia using objective recordings of their reaching and grasping movements. It was found that patients’ movements were slower and that they exhibited spatiotemporal deficits in the final approach phase of reaching and grasping.
To date, the effects of impaired spatiotemporal vision on feedforward (planning stage) and feedback (execution stage) control during visuomotor tasks in people with amblyopia have not been investigated systematically. In the present study, we investigated how degraded visual input in patients with anisometropic amblyopia affects the control of basic visuomotor skills—the accuracy, precision, and speed of reaching to visual targets. We manipulated the visual feedback of the target to examine how well patients with amblyopia program their reaching movement based on the initial (peripheral) view of the target (feedforward control) and how effectively they use visual feedback of the target during movement to improve accuracy and precision. We found that the motor control system in people with amblyopia compensates for their degraded vision by extending the duration of the acceleration phase and by reducing the peak acceleration and peak velocity to optimize reaching performance. Our group has recently reported on the effects of anisometropic amblyopia on saccades during reaching movements.22
Materials and Methods
Patients
Fourteen patients with anisometropic amblyopia were recruited (3 males, 11 females; age 27 ± 9 years; Table 1). All participants underwent complete orthoptic assessment, including visual acuity testing using the Snellen chart, measurement of eye alignment using the prism cover test, measurement of refractive errors, and stereoacuity testing using the Titmus test. Anisometropic amblyopia was defined as amblyopia in the presence of a difference in refractive error between the two eyes of ≥1 diopter (D) of spherical or cylindrical power.20,21,23–26 All patients had visual acuity between 20/30 and 5/400 in the amblyopic eye, 20/20 or better in the fellow eye, and an interocular acuity difference ≥2 lines. Eleven patients had mild amblyopia (visual acuity of 20/30 to 20/60) and fine to gross stereopsis, whereas three had severe amblyopia (visual acuity of 20/400 to 5/400) and no stereopsis. Four patients were orthophoric and 10 had monofixation syndrome27 (microtropia ≤ 8PD [prism diopters] as a result of a foveal scotoma arising from the anisometropia; it is not the cause of the amblyopia), inability to bifixate, and presence of fusional vergence. Fourteen visually normal participants (7 males, 7 females; age 32 ± 11 years) served as control subjects. They had normal or corrected-to-normal visual acuity of 20/20 or better in each eye and stereoacuity ≤40 arc sec. Exclusion criteria were any ocular cause for reduced visual acuity, previous intraocular surgery, or any neurologic disease. All participants were right-handed. The study was approved by the Research Ethics Board at The Hospital for Sick Children, and all protocols adhered to the guidelines of the Declaration of Helsinki. Informed consent was obtained from each participant.
Table 1.
Clinical Characteristics of Study Patients with Anisometropic Amblyopia
| Patient | Visual Acuity (Snellen chart)
|
Refractive Error
|
LE | Stereoacuity (arc sec) | Alignment | ||
|---|---|---|---|---|---|---|---|
| Age (y) | RE | LE | RE | ||||
| 1 | 33 | 20/15 | 20/30 | −0.75 | +2.00 | 140 | Orthophoria |
| 2 | 29 | 20/50 | 20/15 | +2.50+0.75×50 | +0.25 | 3000 | Monofixation RE |
| 3 | 47 | 20/40 | 20/15 | +2.75+2.25×60 | Plano | 400 | Monofixation RE |
| 4 | 14 | 20/50 | 20/15 | +3.25+1.25×90 | +2.00 | 50 | Orthophoria |
| 5 | 18 | 20/15 | 20/40 | Plano | +2.00+0.25×130 | 60 | Orthophoria |
| 6 | 20 | 20/15 | 20/50 | Plano | +1.50 | 120 | Orthophoria |
| 7 | 35 | 20/15 | 20/60 | −4.25 | −0.75 | 3000 | Monofixation LE |
| 8 | 36 | 20/15 | 20/400 | −5.25 | −12.00 | Negative | Monofixation LE |
| 9 | 25 | 20/40 | 20/15 | +1.00+0.25×22 | Plano | 400 | Monofixation RE |
| 10 | 28 | 20/20 | 20/400 | +4.00 | +6.00+1.75×90 | Negative | Monofixation LE |
| 11 | 21 | 20/30 | 20/15 | +1.50 | Plano | 3000 | Monofixation RE |
| 12 | 20 | 5/400 | 20/20 | −2.00 | −3.00+0.75×15 | Negative | Monofixation RE |
| 13 | 19 | 20/20 | 20/40 | −3.50+1.50×90 | −3.50+2.50×102 | 200 | Monofixation LE |
| 14 | 36 | 20/15 | 20/40 | −1.50 | +1.50+1.00×15 | 200 | Monofixation LE |
LE = left eye; RE = right eye.
Apparatus
Reaching movements of the upper limb were recorded using an infrared illumination-based motion capture system (Optotrak Certus; Northern Digital Inc., Waterloo, Canada). This system is noninvasive and allows for precise tracking of 3D motion of the limb (spatial accuracy, 0.1 mm; resolution, 0.01 mm; sampling frequency, 200 Hz). The coordinate system was defined relative to the computer screen used to present the visual stimulus (see next section) as follows: x-axis, horizontal plane (azimuth); y-axis, vertical plane (elevation); and z-axis, median plane (depth). The system was calibrated before the experiment by using a four-marker digitizing probe to define the coordinate frame for the reaching movement. Two infrared markers (4-mm diameter) were affixed to the tip of the index finger and the wrist joint of the participant’s right hand. A force sensitive resistor (FSR; Tekscan, Boston, MA), 15 mm in diameter, was placed on the table at the participant’s midline 28 cm from the computer screen and 17 cm from the participant. The FSR was used to trigger the initiation of each trial and to control when the visual target was switched off during a trial.
Eye movements were recorded binocularly at 200 Hz using a video-based pupil/iris tracking system (Chronos Vision, Berlin, Germany). This system has a maximum resolution of 6 min arc over a range of ±20° and a linearity of <0.5% for both horizontal and vertical eye movements. Before each experiment, horizontal and vertical calibrations were performed for each eye using fixation targets at five locations: 0° and ±10° horizontally and vertically.
Experimental Conditions and Procedure
The visual stimulus was a white circle (visual angle, 0.25°) presented on a black background generated by a custom-written technical computing program (MatLab; MathWorks, Natick, MA) and presented on a 20-inch CRT computer screen (Diamond Pro 2070SB [NEC/Mitsubishi, Itasca, IL]; resolution 1600 × 1200 at 85 Hz) located 42 cm from the subject using a visual stimulus generator (ViSaGe; Cambridge Research Systems, Cambridge, UK). In three-dimensional space, the distance from the starting position of the index finger to the computer screen was 43 cm. Testing was conducted in a dimly lit room. Participants were seated at a table with their heads stabilized on a chin rest. At the start of each trial, the right hand was placed on the table and the index finger was placed on the FSR at midline 28 cm from the screen. Participants fixated a white cross on the screen that was centered vertically at their eye level and horizontally along their midsagittal plane. After a variable delay of 1.5 to 3 seconds, the fixation cross was extinguished and the target appeared (i.e., there was no temporal gap between fixation and target) randomly at four eccentricities ±5° or ±10° from central fixation along the azimuth. The participants were instructed to look at and reach to the target as fast and as accurately as possible. On 50% of the trials, the target was switched off at the onset of hand movement (i.e., as soon as the finger was lifted from the FSR [target OFF condition]). On these trials, participants were instructed to reach to the remembered location of the target. On the remaining 50% of the trials, the target remained visible throughout the trial (target ON condition). The target OFF and ON conditions were randomly interleaved.
The experiments were performed under three viewing conditions: binocular, monocular amblyopic eye, and monocular fellow eye. For control subjects, viewing was binocular, monocular left eye, and monocular right eye. Data were collected in blocks for each viewing condition, and the order of viewing conditions was randomized across subjects. Participants completed 10 trials in each combination of the experimental conditions for a total of 240 trials. The inter-trial interval varied among trials and was at least 5 seconds. Practice trials were completed before the start of the experiment to familiarize subjects with the experimental procedure.
Statistical Analysis
Hand position data were filtered using a second-order, dual-pass (bidirectional) Butterworth filter with a cutoff frequency of 7.5 Hz. Hand velocity was obtained using a two-point differentiation method. Position data were differentiated twice to obtain acceleration. A custom-written technical computing program (MatLab; MathWorks) was used to identify the initiation of the hand movement, defined here as when the velocity of the finger in the y-axis (i.e., elevation) exceeded 30 mm/s. The end of the reaching movement was identified as when the finger reached the computer screen and the velocity of the finger in the z-axis (depth) fell to and stayed below 30 mm/s. All trials were inspected visually to ensure that the reaching movement was identified correctly by the program.
Reaching performance was quantified by calculating the end point constant error and variable error along the azimuth (horizontal) and elevation (vertical) direction. Constant error was defined as the mean distance between the fingertip and the target location along the azimuth and elevation at the end of the movement. Variable error was the dispersion (i.e., within-subject SD) of the movement end points along the azimuth and elevation and their resultant combined vector (i.e., azimuth and elevation combined).
The kinematics of the reaching movement were assessed by calculating the following parameters: reaction time (defined as the interval between onset of the visual stimulus and the initiation of reaching), total movement time (the interval between reaching initiation and the end of movement), peak acceleration, peak velocity, the duration of the acceleration phase (the interval from movement onset to peak velocity, i.e., the zero-crossing on the acceleration trajectory), and duration of the deceleration phase (the interval from peak velocity to the end of hand movement). Peak acceleration, peak velocity, and duration of the acceleration phase reflect the programming of the movement (i.e., feedforward control), whereas peak deceleration and duration of deceleration phase reflect online (i.e., feedback) control.28 All kinematic parameters were calculated based on the z-axis of the finger, which represents the primary direction of movement in depth.
Reaching accuracy and precision, as well as kinematic parameters, were submitted to a repeated-measures mixed ANOVA with group as a between-subjects factor (control subjects and patients) and three within-subjects factors: viewing condition (binocular, monocular amblyopic eye, and monocular fellow eye viewing; for control subjects, binocular, left eye monocular viewing, and right eye monocular viewing), visual feedback of target (target ON, and OFF), and target location (±5°, ±10°). To investigate the effects of severity of amblyopia on reaching performance, repeated-measures ANOVA was performed on each outcome measure. The ANOVA had severity of amblyopia as a between-subjects factor (mild [20/30 to 20/60] and severe [20/400 to 5/400]) and viewing condition as a within-subjects factor. All statistical analyses were performed using a statistics software package (SAS 9.2; SAS Institute, Cary, NC). The significance level was set at P < 0.05. Any significant main effects and interactions were analyzed further using Tukey’s HSD test.
To investigate the spatiotemporal dynamics of the reaching movement, we examined whether the duration of the acceleration and duration of the deceleration phases correlated with overall reaching precision in both groups in each viewing condition. Multiple regression analysis was performed to assess the relative weighting of the duration of these two phases on reaching precision using the standardized regression coefficient (β) and the associated P value. The value of the β weight reflects the proportion of the variance explained in the dependent variable (reaching precision) by the changes in the independent variables: duration of the acceleration phase (i.e., the interval from movement onset to peak velocity) and duration of the deceleration phase (i.e., the interval from peak velocity to the end of hand movement). Separate multiple regressions were performed on the group mean data from patients and control subjects during the three viewing conditions using the statistics software package (SAS 9.2; SAS Institute).
Results
Reaching Performance
Accuracy
There was no significant difference between patients and control subjects for reaching accuracy (i.e., constant error) along either the azimuth (horizontal) or the elevation (vertical) direction. Mean (± SD) accuracy was 0.26 ± 4.1 mm in patients and 0.03 ± 3.6 mm in control subjects along the azimuth direction (F(1,26) = 0.23, P = 0.604), and it was −1.53 ± 4.9 mm in patients and 0.22 ± 3.6 mm in control subjects along the elevation direction (F(1,26) = 1.62, P = 0.173). Of particular note, there was no significant interaction between group (patients and control subjects) and visual feedback of the target (ON and OFF), or between group and viewing condition (amblyopic eye, fellow eye, and binocular viewing) for reaching accuracy. There was no significant difference in reaching accuracy between patients with mild amblyopia (0.48 ± 3.96 mm) and those with severe amblyopia (−0.55 ± 4.25 mm; F(1,12) = 3.01, P = 0.108).
Precision
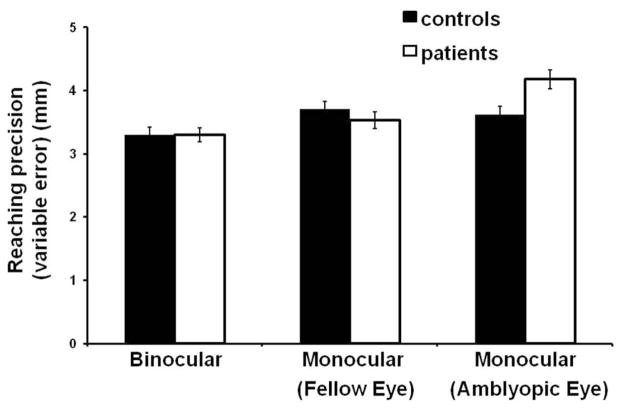
There was a significant interaction between group (patients and control subjects) and viewing condition (amblyopic eye, fellow eye, and binocular viewing) for reaching precision (i.e., variable error) along the azimuth (F(2,52) = 4.63, P = 0.014). Post hoc tests revealed that patients were less precise when viewing with the amblyopic eye (4.25 ± 1.26 mm) in comparison with viewing binocularly (3.32 ± 1.19 mm) and with the fellow eye (3.61 ± 1.3 mm) and in comparison with control subjects during all three viewing conditions (binocular 3.21 ± 1.26 mm, left eye 3.65 ± 1.3 mm, right eye 3.55 ± 1.4 mm) (Fig. 1). However, the effect was very small; the difference in mean precision between amblyopic eye viewing in patients and monocular viewing in control subjects was <0.7 mm, indicating that precision in patients behaviorally was relatively normal. In addition, there was no difference in precision between patients with mild amblyopia (4.09 ± 1.67 mm) and those with severe amblyopia (4.51 ± 1.24 mm) during amblyopic eye viewing.
Figure 1.
Mean precision (variable error) of the reaching movement along the azimuth direction for control subjects and patients across the three viewing conditions. Patient performance was significantly less precise when viewing with the amblyopic eye (P = 0.014). Error bars, ±1 SE.
Both patients and control subjects made less precise movements along the azimuth when the target was switched off at the initiation of reach than when the target was present throughout the trial (F(1,26) = 5.94, P = 0.022). For patients, mean precision (variable error) was 3.53 ± 1.3 mm when the target was ON and 3.81 ± 1.5 mm when the target was OFF. For control subjects, mean precision was 3.43 ± 1.3 mm when the target was ON and 3.66 ± 1.3 mm when the target was OFF.
Mean precision was not significantly different between patients and control subjects in any viewing conditions along the elevation direction (F(2,52) = 2.16, P = 0.139). No other significant main effect or interaction was observed for reaching precision.
Reaching Kinematics
Reaction Time
There were no significant main effects or interactions for mean reaction time. Reaching reaction time was 353 ± 66 ms for patients and 334 ± 86 ms for control subjects (F(1,26) = 0.44, P = 0.584).
Total Movement Time
Patients had significantly longer total mean movement time (647 ± 116 ms) than control subjects (547 ± 126 ms; F(1,26) = 5.29, P = 0.003). Total movement time was significantly shorter for both groups when visual feedback of the target was absent compared with when visual feedback was present (F(1,26) = 19.87, P < 0.001). In patients, total movement time decreased to 638 ± 115 ms when visual feedback was absent compared with 656 ± 116 ms when visual feedback was present. Similarly, total movement time in control subjects decreased to 539 ± 119 ms in the absence of visual feedback compared with 566 ± 132 ms in the presence of visual feedback. There was no significant difference in total movement time between patients with mild amblyopia (640 ± 115 ms) and those with severe amblyopia (672 ± 114 ms; F(1,12) = 0.21, P = 0.656). No other significant main effect or interaction was observed for total movement time.
Acceleration Phase
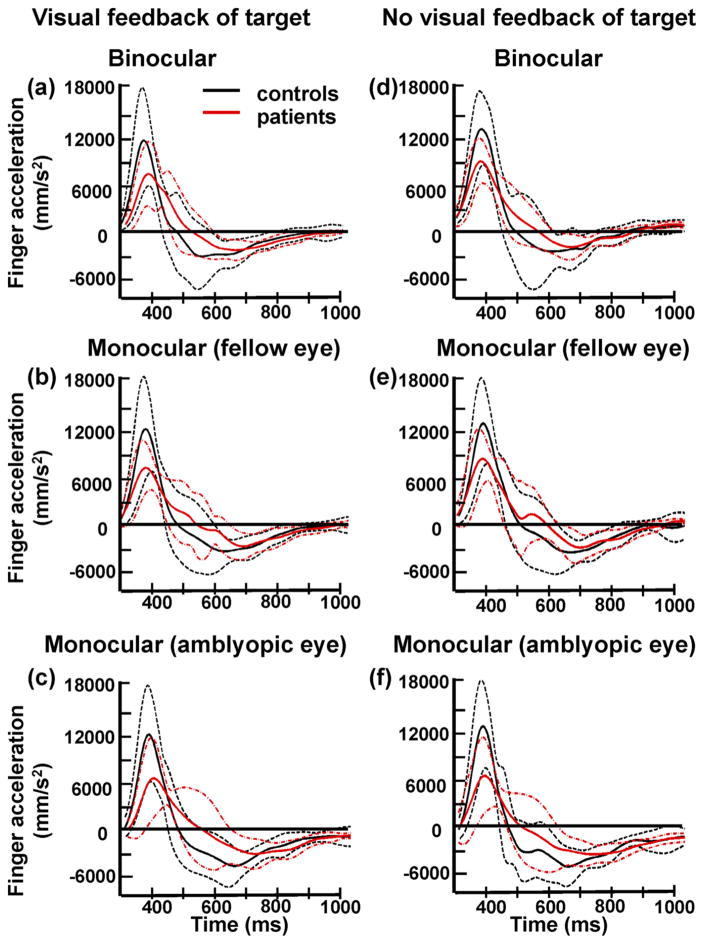
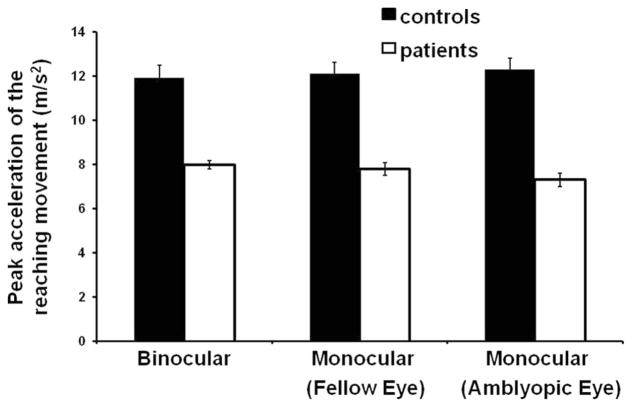
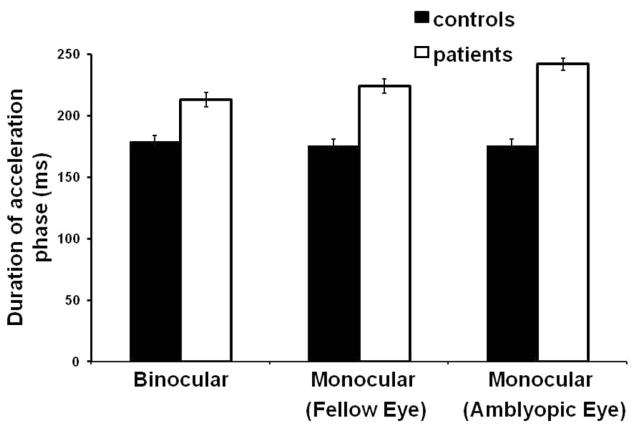
Mean acceleration profiles for all control subjects and patients in all three viewing conditions are shown in Figure 2. Patients exhibited lower mean peak acceleration (7.7 ± 2.6 m/s2) compared with control subjects (12.1 ± 5.4 m/s2; F(1,26) = 8.02, P = 0.009), regardless of viewing conditions (Figs. 2, 3). Figure 2 also shows that patients reached peak velocity later than control subjects (i.e., delayed zero-crossing on the acceleration trace), indicating that the duration of the acceleration phase was increased in patients. Indeed, the mean duration of the acceleration phase was significantly longer in patients (226 ± 60 ms) than in control subjects (177 ± 53 ms; F(1,26) = 7.31, P = 0.012), irrespective of the viewing condition (Figs. 2, 4). Peak velocity was also significantly reduced in patients (0.83 ± 0.18 m/s) compared to control subjects (1.06 ± 0.31 m/s; F(1,26) = 5.72, P = 0.024). There were no differences between patients with mild amblyopia and those with severe amblyopia in peak acceleration ([mild] 7.7 ± 2.5 m/s2 vs. [severe] 7.8 ± 2.9 m/s2; F(1,12) = 0.01, P = 0.947), duration of the acceleration phase ([mild] 231 ± 60 ms vs. [severe] 211 ± 56 ms; F(1,12) = 0.32, P = 0.585) and peak velocity ([mild] 0.83 ± 0.18 m/s vs. [severe] 0.82 ± 0.17 m/s; F(1,12) = 0.01, P = 0.964). No other significant main effect or interaction was observed during the acceleration phase.
Figure 2.
Mean acceleration trajectory (solid line) and the corresponding SD (dashed lines) of all control subjects (black lines) and patients (red lines) when they reached to the 10° target with (a–c) or without (d–f) visual feedback of the target. Viewing was binocular (a, d), monocular with the fellow eye (b, e), or monocular with the amblyopic eye (c, f). In all three viewing conditions, patients had lower mean peak acceleration and prolonged duration of the acceleration phase (indicated by the delayed zero-crossing) than did control subjects.
Figure 3.
Mean peak acceleration of the reaching movement for control subjects and patients across all three viewing conditions. Patients had significantly lower peak acceleration in all viewing conditions (P = 0.009). Error bars, ±1 SE.
Figure 4.
Mean duration of the acceleration phase of reaching movement for control subjects and patients across all three viewing conditions. Patients had significantly longer duration of the acceleration phase in all viewing conditions (P = 0.012). Error bars, ±1 SE.
Deceleration Phase
There was no significant difference in mean peak deceleration between patients (4.2 ± 1.5 m/s2) and control subjects (5.6 ± 2.5 m/s2; F(1,26) = 3.33, P = 0.080). There was also no significant difference in the duration of the deceleration phase between patients (420 ± 88 ms) and control subjects (371 ± 99 ms; F(1,26) = 1.95, P = 0.175). The duration of the deceleration phase, however, was affected by visual feedback for both groups (F(1,26) = 20.77, P < 0.001). Post hoc tests revealed that the duration of the deceleration phase was shorter for both groups when visual feedback of the target was absent compared with the condition when visual feedback was present. In patients, the duration of the deceleration phase decreased to 411 ± 89 ms when visual feedback was absent compared with 430 ± 88 ms when visual feedback was present. Similarly, duration of the deceleration phase in control subjects decreased to 362 ± 91 ms in the absence of visual feedback compared with 380 ± 106 ms in the presence of visual feedback. There were no differences between patients with mild amblyopia and those with severe amblyopia in peak deceleration ([mild] 4.1 ± 1.3 m/s2 vs. [severe] 4.7 ± 2.2 m/s2; F(1,12) = 0.51, P = 0.488) and duration of the deceleration phase ([mild] 410 ± 89 ms vs. [severe] 460 ± 75 ms; F(1,12) = 1.17, P = 0.302). No other significant main effect or interaction was observed during the deceleration phase.
Correlation of Duration of Acceleration and Deceleration Phases with Reaching Precision
Standardized regression coefficients (β) of the duration of the acceleration phase and the duration of the deceleration phase are shown in Table 2. In control subjects, only the duration of the deceleration phase, but not the duration of the acceleration phase, correlated significantly with overall reaching precision in all viewing conditions (β ≥ −0.79; P < 0.001). In contrast, in addition to a significant correlation in patients between reaching precision and duration of the deceleration phase (β ≥ −0.57; P < 0.010), the duration of acceleration phase correlated significantly with reaching precision in all three viewing conditions: amblyopic eye (β = −0.54; P = 0.003), fellow eye (β = −0.34; P = 0.028), and binocular viewing (β = −0.41; P = 0.027).
Table 2.
Results of Multiple Regression Analysis
| Viewing Condition | Control Subjects
|
Patients
|
Acceleration Phase
|
Deceleration Phase
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mode I Fit (R2) | Acceleration Phase
|
Deceleration Phase
|
Model Fit (R2) | |||||||
| β | P | β | P | β | P | β | P | |||
| Binocular | 0.79 | − 0.16 | 0.406 | −0.79 | 0.001 | 0.78 | −0.41 | 0.027 | −0.61 | 0.003 |
| Monocular (fellow eye) | 0.78 | −0.05 | 0.778 | −0.91 | <0.001 | 0.81 | −0.34 | 0.029 | −0.75 | <0.001 |
| Monocular (amblyopic eye) | 0.84 | −0.25 | 0.114 | −0.82 | <0.001 | 0.78 | −0.54 | 0.003 | −0.57 | 0.002 |
Results show the relative contribution (standardized regression coefficient β) of the duration of the acceleration phase and the duration of the deceleration phase to overall reaching precision for control subjects and patients in different viewing conditions.
Discussion
The present study examined the effects of impaired spatiotemporal vision on the dynamics of visually guided reaching movements in patients with anisometropic amblyopia. The major findings in our patients are that reaching performance (accuracy and precision) was relatively normal during a simple reaching task, reaching reaction time was normal, duration of the acceleration phase was increased and peak acceleration and peak velocity were reduced under all viewing conditions, whereas duration of the deceleration phase and mean peak deceleration were not significantly different. Additionally, in both patients and control subjects, the absence of visual feedback of the target led to a small decrease in precision and to shorter total movement time and duration of the deceleration phase.
Comparison with Previous Studies
In a previous study of strabismic amblyopia,29 patients were asked to press a button as soon as they saw a centrally presented target. The reaction time of their manual responses was found to be longer during amblyopic eye viewing. Information on reaching accuracy and precision, however, was unavailable because they were not relevant in this task. A more recent study by Grant et al.21 reported longer reaction time and more errors during a reaching and grasping task in patients with different types of amblyopia. The discrepancy between our normal reaction time results and those of previous studies might have been due to several factors. First, we included patients with anisometropic amblyopia only, whereas previous studies included patients with strabismic amblyopia only29 or those with different types of amblyopia.21 It has been demonstrated that different types of amblyopia exhibit distinctive patterns of visual deficits,8 which may in turn affect visually guided manual behaviors differentially. Second, it is known that in strabismic amblyopia, detection of a visual stimulus is more impaired when it is presented in the central visual field than when it appears in the peripheral visual field.30 The prolonged reaction time observed in a previous study29 of patients with strabismic amblyopia might be associated with the use of a central target. Third, we investigated a simple reaching response by using a two-dimensional stimulus presented on a computer screen. In contrast, a previous study21 examined reaching and grasping using three-dimensional stimuli (cylinders). Their experiments involved grip scaling and grip orientation constraints, a substantially more complicated motor response than reaching alone. The accuracy and precision requirements associated with reaching and grasping in the study by Grant et al.21 were also higher in terms of the potential cost. For example, if the target was not localized accurately, the approaching hand might collide with the target. In addition, if the grip was not scaled appropriately, there was a potential for the target to slip and fall. In contrast, in our study, the potential cost of missing the small target during reaching was relatively low. Although our subjects were instructed to reach as fast and as accurately as possible, they were not penalized for making any error. Overall, it is perhaps not surprising that people with amblyopia have prolonged reaction time but still have increased errors during reaching and grasping of a 3D target because reaching and grasping require programming of a more complex motor response that involves different neural networks.31
Our findings of a prolonged duration of acceleration phase in patients but a normal duration of the deceleration phase are different from those reported by Grant et al.,21 who found a normal duration of the initial reaching and grasping phase followed by a prolonged low-velocity “late” phase in patients. The discrepant findings between the present and previous studies21 might be related to a difference in the study populations and in the complexity of the tasks, as discussed. The discrepancy could also have arisen from how the data were analyzed. In the present study, we defined the duration of the acceleration phase as the interval from movement onset to peak velocity. This variable was not reported in the previous study21; instead, those authors reported and defined the duration of the initial phase of movement as the interval from movement onset to peak deceleration. Similarly, the duration of the deceleration phase in the present study was defined as the interval after peak velocity to the end of movement, whereas the previous study defined the duration of the low-velocity phase as the interval from peak deceleration to object contact.
We reported recently the effects of anisometropic amblyopia on saccadic eye movements during reaching movements in the same group of subjects.22 Similarly to those reaching kinematics and performance, we found that patients with mild amblyopia had saccadic deficits comparable to those in patients with severe amblyopia (i.e., longer saccade latencies, increased variability in reaction time and saccade amplitude compared with control subjects). The lack of statistically significant effects of severity of amblyopia on saccade and reaching performance may well be attributed to the small number of patients in the two subgroups and the unequal sample size between the two subgroups. In addition, because we used a simple high-contrast target, it remains to be seen whether the severity of amblyopia would have a different effect during tasks that require foveal vision and extraction of detailed features.
Reaching Kinematics and Performance
Despite degraded visual inputs, our patients exhibited relatively normal reaching performance (accuracy and precision) in all viewing conditions compared with control subjects. This came as a surprise to us at first; however, it is well known that the accuracy and precision of a movement cannot be judged independently of its timing.32,33 This is because during the execution of any movement, there is typically a tradeoff between the speed of the movement and its accuracy, in accordance with Fitts’ law.34 –36 It is thus possible that normal performance can be maintained in patients with amblyopia by altering the timing of the movement, such as reaching reaction time, and the duration of the acceleration and deceleration phases.
We found that our patients had normal reaching reaction time, suggesting that reaction time did not play a major role in maintaining their normal performance of reaching movement. Could the normal performance be related to a prolonged acceleration phase? We investigated this possibility by performing a regression analysis to assess the correlation between duration of the acceleration phase and reaching precision. Previous studies have reported that normal participants use visual feedback during the deceleration phase to improve the accuracy and precision of their hand movement.37 In agreement with previous findings,37 a longer duration of the deceleration phase was correlated with better precision in our control subjects, whereas the duration of the acceleration phase had no significant effect on reaching precision. In contrast, in patients, we found that the duration of both the deceleration and the acceleration phase contributed significantly to reaching precision in all viewing conditions.
Feedforward versus Feedback Control
More than a century ago, Woodworth38 suggested that reaching movements are composed of two stages: an initial motor program stage followed by a corrective stage. The terms used today to describe these stages are feedforward and feedback control.6 During feedforward control, a sophisticated approximation of the required motor plan is specified based on internal models of eye, head, and limb configuration,39 – 43 but this plan is subject to both variable and systematic errors, such as gaze-dependent errors.44,45 To compensate for these errors and for unpredictable motion of the reaching target, fine adjustments to the limb trajectory are made during the movement based on visual and proprioceptive feedback about the locations of the target and the limb.6,37,46 – 49 Kinematic markers, such as peak acceleration, peak velocity, and duration of the acceleration phase are highly dependent on target location and reflect the initial motor program (i.e., feedforward stage), whereas peak deceleration and duration of the deceleration phase can be modified based on visual and proprioceptive feedback acquired earlier in the movement.48
In general, higher peak acceleration during a reaching movement is associated with higher force variability and greater end point error.50 We postulate that the lower peak acceleration and peak velocity and the prolonged acceleration phase may represent a strategy or adaptation of feedforward control to optimize reaching performance in face of the degraded visual input in amblyopia. The additional processing time during the acceleration phase could be related to stimulus acquisition (i.e., detection and localization) or programming of the required motor plan by using internal models of eye, head, and limb orientation. Our data suggest that the prolonged acceleration phase was unlikely to be related to stimulus acquisition. If it were, we would have expected a longer duration of the acceleration phase during amblyopic eye viewing. Duration of the acceleration phase, however, did not vary significantly among different viewing conditions in the patients, suggesting that encoding of visual stimulus was not a major contributing factor to the prolonged processing time. Instead, the extra processing time might be related to sensorimotor transformation in the face of noisy signals in the amblyopic visual system. It has been shown that patients with amblyopia exhibit a marked loss of efficiency (threshold elevation at all noise levels) and increased random internal noise.51–53 According to the minimum-variance theory proposed by Harris and Wolpert,54,55 perceptual and motor performance are limited by the noise present at various processing stages (e.g., because of uncertainty of the sensory signals, inherent task ambiguity, and noise in the motor neural commands). The neural control signals are corrupted by noise whose variance increases with the size of the control signal (i.e., signal-dependent noise).55 For example, the variability of motor errors is proportional to movement amplitude so that a movement of larger amplitude requires a larger control signal, which, in turn, results in greater end point variability. The minimum-variance theory suggests that the central nervous system optimizes the movement parameters to minimize the variance of the final limb position. In amblyopia, it is possible that because the visual sensory signals are degraded by increased noise, additional processing is required by patients to adjust their motor planning during the acceleration phase. This allows them to compensate for the greater noise and to minimize the variability of motor errors to achieve good reaching performance.
The prolonged acceleration phase might also reflect a strategy or adaptation of feedback control to optimize reaching performance. When reaching is initiated, information related to the motor command (i.e., the reafferent movement-related signals) and visual information are updated and integrated continually by the central nervous system to optimize performance.56 –58 Specifically, in visually normal people during the earlier part of movement (i.e., before peak velocity), visual and proprioceptive signals about hand position are updated continuously to compute and update the movement vector.59 – 64 In our patients with amblyopia, the duration of the acceleration phase might have been extended to provide more time to process feedback information to improve performance. It is also possible that the temporal integration of visual and proprioceptive signals about limb position/velocity or the relative weighting given to these two signals may differ from those of visually normal people because of increased visual noise in amblyopia. We are conducting experiments to explore these possibilities.
The Role of Visual Feedback
Visual feedback plays a critical role in motor control.65 When visual feedback of the target is removed at the onset of reaching, visually normal people exhibit a significant decrease in total movement time, primarily because of a shorter deceleration phase, whereas the duration of the acceleration phase is unchanged.37,66 The results from our study are in agreement with previous findings, showing that when the target was extinguished at reaching onset, both control subjects and patients had a shorter total movement time because of the shorter duration of the deceleration phase. We postulate that when the target was visible during the entire movement, both control subjects and patients had better precision, which suggests that they were able to use visual feedback during the deceleration phase to fine-tune the reaching trajectory. When the target was extinguished after movement onset, however, visual feedback of the target was no longer available. Therefore, both control subjects and patients might have tried to reach the target as quickly as possible while the memory of target location was still robust, which resulted in a shorter duration of the deceleration phase. It is also possible that reaching was faster because there was less sensory information to process when the target disappeared.
Temporal modification of movement (by changing the total movement time and the duration of the deceleration phase) as the movement is unfolding reflects the ability of the motor system to adapt the kinematics of hand movement quickly to changing and unpredictable contexts. Our results showed that as in control subjects, patients were able to adjust the temporal dynamics of their reaching movement quickly. Interestingly, despite shorter total movement time when the target was switched off at the initiation of reaching compared with when it remained visible, patients’ accuracy and precision were comparable to those of control subjects. These findings suggest that patients were able to alter the initial motor program based on information acquired early in the movement. In other words, when the target disappeared unpredictably at the initiation of reaching, the movement plan could still be updated based on the initial view of the target and on the current location of the hand derived from proprioceptive and visual input.
In conclusion, the present study demonstrates that the programming and execution of visually guided reaching is altered in anisometropic amblyopia. Patients with anisometropic amblyopia adopted a different but effective kinematic strategy by modifying the timing of their reaching movement (lower peak acceleration and peak velocity and extended acceleration phase) to achieve good reaching performance. Our results lead to several interesting questions. For example, how does the degraded visual input in patients with anisometropic amblyopia impact spatiotemporal coordination between the eye movement and manual motor systems during reaching? How do these changes differ among different subtypes of amblyopia? Further studies are under way to investigate these issues.
Acknowledgments
Supported by Canadian Institutes of Health Research Grants MOP 89763 and MOP 57853; Leaders Opportunity Fund from the Canadian Foundation for Innovation; and the Department of Ophthalmology and Vision Sciences and Research Training Centre, The Hospital for Sick Children.
Footnotes
Disclosure: E. Niechwiej-Szwedo, None; H.C. Goltz, None; M. Chandrakumar, None; Z. Hirji, None; J.D. Crawford, None; A.M.F. Wong, None
References
- 1.Desmurget M, Pelisson D, Rossetti Y, Prablanc C. From eye to hand: planning goal-directed movements. Neurosci Biobehav Rev. 1998;22:761–788. doi: 10.1016/s0149-7634(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 2.Crawford JD, Medendorp WP, Marotta JJ. Spatial transformations for eye-hand coordination. J Neurophysiol. 2004;92:10–19. doi: 10.1152/jn.00117.2004. [DOI] [PubMed] [Google Scholar]
- 3.Snyder LH. Coordinate transformations for eye and arm movements in the brain. Curr Opin Neurobiol. 2000;10:747–754. doi: 10.1016/s0959-4388(00)00152-5. [DOI] [PubMed] [Google Scholar]
- 4.Sommer MA, Wurtz RH. Brain circuits for the internal monitoring of movements. Annu Rev Neurosci. 2008;31:317–338. doi: 10.1146/annurev.neuro.31.060407.125627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donaldson IM. The functions of the proprioceptors of the eye muscles. Philos Trans R Soc Lond B Biol Sci. 2000;355:1685–1754. doi: 10.1098/rstb.2000.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- 7.Prablanc C, Pelisson D, Goodale MA. Visual control of reaching movements without vision of the limb, I: role of retinal feedback of target position in guiding the hand. Exp Brain Res. 1986;62:293–302. doi: 10.1007/BF00238848. [DOI] [PubMed] [Google Scholar]
- 8.McKee SP, Levi DM, Movshon JA. The pattern of visual deficits in amblyopia. J Vis. 2003;3:380–405. doi: 10.1167/3.5.5. [DOI] [PubMed] [Google Scholar]
- 9.Levi DM. Visual processing in amblyopia: human studies. Strabismus. 2006;14:11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- 10.Hess RF, Wang YZ, Demanins R, Wilkinson F, Wilson HR. A deficit in strabismic amblyopia for global shape detection. Vision Res. 1999;39:901–914. doi: 10.1016/s0042-6989(98)00157-6. [DOI] [PubMed] [Google Scholar]
- 11.Sireteanu R, Baumer CC, Sarbu C, Iftime A. Spatial and temporal misperceptions in amblyopic vision. Strabismus. 2007;15:45–54. doi: 10.1080/09273970601180263. [DOI] [PubMed] [Google Scholar]
- 12.Bonneh YS, Sagi D, Polat U. Spatial and temporal crowding in amblyopia. Vision Res. 2007;47:1950–1962. doi: 10.1016/j.visres.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 13.Barrett BT, Pacey IE, Bradley A, Thibos LN, Morrill P. Nonveridical visual perception in human amblyopia. Invest Ophthalmol Vis Sci. 2003;44:1555–1567. doi: 10.1167/iovs.02-0515. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Ophthalmology. [Accessed September 6, 2010];Amblyopia Preferred Practice Pattern. 2007 http://one.aao.org/CE/PracticeGuidelines/PPP.
- 15.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 16.Levi DM, Klein SA. Spatial localization in normal and amblyopic vision. Vision Res. 1983;23:1005–1017. doi: 10.1016/0042-6989(83)90011-1. [DOI] [PubMed] [Google Scholar]
- 17.Sireteanu R, Fronius M. Human amblyopia: structure of the visual field. Exp Brain Res. 1990;79:603–614. doi: 10.1007/BF00229328. [DOI] [PubMed] [Google Scholar]
- 18.Mansouri B, Hansen BC, Hess RF. Disrupted retinotopic maps in amblyopia. Invest Ophthalmol Vis Sci. 2009;50:3218–3225. doi: 10.1167/iovs.08-2914. [DOI] [PubMed] [Google Scholar]
- 19.Fronius M, Sireteanu R, Zubcov A. Deficits of spatial localization in children with strabismic amblyopia. Graefes Arch Clin Exp Ophthalmol. 2004;242:827–839. doi: 10.1007/s00417-004-0936-5. [DOI] [PubMed] [Google Scholar]
- 20.Webber AL, Wood JM, Gole GA, Brown B. The effect of amblyopia on fine motor skills in children. Invest Ophthalmol Vis Sci. 2008;49:594–603. doi: 10.1167/iovs.07-0869. [DOI] [PubMed] [Google Scholar]
- 21.Grant S, Melmoth DR, Morgan MJ, Finlay AL. Prehension deficits in amblyopia. Invest Ophthalmol Vis Sci. 2007;48:1139–1148. doi: 10.1167/iovs.06-0976. [DOI] [PubMed] [Google Scholar]
- 22.Niechwiej-Szwedo E, Goltz HC, Chandrakumar M, Hirji ZA, Wong AMF. Effects of anisometropic amblyopia on visuomotor behavior, I: saccadic eye movements. Invest Ophthalmol Vis Sci. 2010;51:6348–6354. doi: 10.1167/iovs.10-5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weakley DR., Jr The association between nonstrabismic anisometropia, amblyopia, and subnormal binocularity. Ophthalmology. 2001;108:163–171. doi: 10.1016/s0161-6420(00)00425-5. [DOI] [PubMed] [Google Scholar]
- 24.Huang CB, Zhou J, Lu ZL, Feng L, Zhou Y. Binocular combination in anisometropic amblyopia. J Vis. 2009;9:11–16. doi: 10.1167/9.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caputo R, Frosini R, De Libero C, Campa L, Magro EF, Secci J. Factors influencing severity of and recovery from anisometropic amblyopia. Strabismus. 2007;15:209–214. doi: 10.1080/09273970701669983. [DOI] [PubMed] [Google Scholar]
- 26.Agrawal R, Conner IP, Odom JV, Schwartz TL, Mendola JD. Relating binocular and monocular vision in strabismic and anisometropic amblyopia. Arch Ophthalmol. 2006;124:844–850. doi: 10.1001/archopht.124.6.844. [DOI] [PubMed] [Google Scholar]
- 27.Parks MM. Th monofixation syndrome. Trans Am Ophthalmol Soc. 1969;67:609–657. [PMC free article] [PubMed] [Google Scholar]
- 28.Jeannerod M. The interaction of visual and proprioceptive cues in controlling reaching movements. In: Humphrey DR, Freund HJ, editors. Motor Control: Concepts and Issues. Chichester, UK: John Wiley & Sons; 1991. pp. 277–291. [Google Scholar]
- 29.Hamasaki DI, Flynn JT. Amblyopic eyes have longer reaction times. Invest Ophthalmol Vis Sci. 1981;21:846–853. [PubMed] [Google Scholar]
- 30.Hess RF, Pointer JS. Differences in the neural basis of human amblyopia: the distribution of the anomaly across the visual field. Vision Res. 1985;25:1577–1594. doi: 10.1016/0042-6989(85)90128-2. [DOI] [PubMed] [Google Scholar]
- 31.Culham JC, Valyear KF. Human parietal cortex in action. Curr Opin Neurobiol. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Smyrnis N, Evdokimidis I, Constantinidis TS, Kastrinakis G. Speed-accuracy trade-off in the performance of pointing movements in different directions in two-dimensional space. Exp Brain Res. 2000;134:21–31. doi: 10.1007/s002210000416. [DOI] [PubMed] [Google Scholar]
- 33.Dean M, Wu SW, Maloney LT. Trading off speed and accuracy in rapid, goal-directed movements. J Vis. 2007;7:11–12. doi: 10.1167/7.5.10. [DOI] [PubMed] [Google Scholar]
- 34.Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- 35.MacKenzie SI, Buxton WAS. Extending Fitts’ law to two-dimensional tasks. Proceedings of ACM CHI: 1992 Conference on Human Factors in Computing Systems. 1992:219–226. [Google Scholar]
- 36.Murata A. Extending effective target width in Fitts’ law to a two-dimensional pointing task. Int J Hum-Comput Interaction. 1999;11:137–152. [Google Scholar]
- 37.Elliott D. Discrete vs. continuous visual control of manual aiming. Hum Mov Sci. 1991;10:393–418. [Google Scholar]
- 38.Woodworth RS. The accuracy of voluntary movement. Psychol Rev. 1899;3(monograph suppl):1–119. [Google Scholar]
- 39.Henriques DY, Medendorp WP, Gielen CC, Crawford JD. Geometric computations underlying eye-hand coordination: orientations of the two eyes and the head. Exp Brain Res. 2003;152:70–78. doi: 10.1007/s00221-003-1523-4. [DOI] [PubMed] [Google Scholar]
- 40.Blohm G, Crawford JD. Computations for geometrically accurate visually guided reaching in 3-D space. J Vis. 2007;7:1–22. doi: 10.1167/7.5.4. [DOI] [PubMed] [Google Scholar]
- 41.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010:18. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 42.Diedrichsen J, Shadmehr R, Ivry RB. The coordination of movement: optimal feedback control and beyond. Trends Cogn Sci. 2009;14:31–39. doi: 10.1016/j.tics.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabes PN. The planning and control of reaching movements. Curr Opin Neurobiol. 2000;10:740–746. doi: 10.1016/s0959-4388(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 44.Henriques DY, Klier EM, Smith MA, Lowy D, Crawford JD. Gaze-centered remapping of remembered visual space in an open-loop pointing task. J Neurosci. 1998;18:1583–1594. doi: 10.1523/JNEUROSCI.18-04-01583.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Henriques DY, Crawford JD. Direction-dependent distortions of retinocentric space in the visuomotor transformation for pointing. Exp Brain Res. 2000;132:179–194. doi: 10.1007/s002210000340. [DOI] [PubMed] [Google Scholar]
- 46.Sarlegna F, Blouin J, Bresciani JP, Bourdin C, Vercher JL, Gauthier GM. Target and hand position information in the online control of goal-directed arm movements. Exp Brain Res. 2003;151:524–535. doi: 10.1007/s00221-003-1504-7. [DOI] [PubMed] [Google Scholar]
- 47.Paulignan Y, Jeannerod M, MacKenzie C, et al. Selective perturbation of visual input during prehension movements, 2: the effects of changing object size. Exp Brain Res. 1991;87:407–420. doi: 10.1007/BF00231858. [DOI] [PubMed] [Google Scholar]
- 48.Paulignan Y, MacKenzie C, Marteniuk R, Jeannerod M. Selective perturbation of visual input during prehension movements, 1: the effects of changing object position. Exp Brain Res. 1991;83:502–512. doi: 10.1007/BF00229827. [DOI] [PubMed] [Google Scholar]
- 49.Elliott D, Binsted G, Heath M. The control of goal-directed limb movements: correcting errors in the trajectory. Hum Mov Sci. 1999;18:121–136. [Google Scholar]
- 50.Schmidt RA, Zelaznik H, Hawkins B, Frank JS, Quinn JT., Jr Motor-output variability: a theory for the accuracy of rapid motor acts. Psychol Rev. 1979;47:415–451. [PubMed] [Google Scholar]
- 51.Xu P, Lu ZL, Qiu Z, Zhou Y. Identify mechanisms of amblyopia in Gabor orientation identification with external noise. Vision Res. 2006;46:3748–3760. doi: 10.1016/j.visres.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Levi DM, Klein SA, Chen I, Levi DM, Klein SA, Chen I. What limits performance in the amblyopic visual system: seeing signals in noise with an amblyopic brain: the response of the amblyopic visual system to noise. J Vis. 2008;8:1–23. doi: 10.1167/8.4.1. [DOI] [PubMed] [Google Scholar]
- 53.Levi DM, Klein SA, Chen I. The response of the amblyopic visual system to noise. Vision Res. 2007;47:2531–2542. doi: 10.1016/j.visres.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- 55.Wolpert DM. Probabilistic models in human sensorimotor control. Hum Mov Sci. 2007;26:511–524. doi: 10.1016/j.humov.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Proteau L, Isabelle G. On the role of visual afferent information for the control of aiming movements toward targets of different sizes. J Mot Behav. 2002;34:367–384. doi: 10.1080/00222890209601954. [DOI] [PubMed] [Google Scholar]
- 57.Bedard P, Proteau L. On-line vs. off-line utilization of peripheral visual afferent information to ensure spatial accuracy of goal-directed movements. Exp Brain Res. 2004;158:75–85. doi: 10.1007/s00221-004-1874-5. [DOI] [PubMed] [Google Scholar]
- 58.Proteau L, Roujoula A, Messier J. Evidence for continuous processing of visual information in a manual video-aiming task. J Mot Behav. 2009;41:219–231. doi: 10.3200/JMBR.41.3.219-231. [DOI] [PubMed] [Google Scholar]
- 59.Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST. Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci. 1999;2:563–567. doi: 10.1038/9219. [DOI] [PubMed] [Google Scholar]
- 60.Vindras P, Desmurget M, Viviani P. Error parsing in visuomotor pointing reveals independent processing of amplitude and direction. J Neurophysiol. 2005;94:1212–1224. doi: 10.1152/jn.01295.2004. [DOI] [PubMed] [Google Scholar]
- 61.Vesia M, Yan X, Henriques DY, Sergio LE, Crawford JD. Transcranial magnetic stimulation over human dorsal-lateral posterior parietal cortex disrupts integration of hand position signals into the reach plan. J Neurophysiol. 2008;100:2005–2014. doi: 10.1152/jn.90519.2008. [DOI] [PubMed] [Google Scholar]
- 62.Proteau L, Masson G. Visual perception modifies goal-directed movement control: supporting evidence from a visual perturbation paradigm. Q J Exp Psychol A. 1997;50:726–741. doi: 10.1080/713755729. [DOI] [PubMed] [Google Scholar]
- 63.Hansen S, Elliott D, Tremblay L. Online control of discrete action following visual perturbation. Perception. 2007;36:268–287. doi: 10.1068/p5629. [DOI] [PubMed] [Google Scholar]
- 64.Grierson LE, Elliott D. Kinematic analysis of goal-directed aims made against early and late perturbations: an investigation of the relative influence of two online control processes. Hum Mov Sci. 2008;27:839–856. doi: 10.1016/j.humov.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Elliott D, Helsen WF, Chua R. A century later: Woodworth’s (1899) two-component model of goal-directed aiming. Psychol Bull. 2001;127:342–357. doi: 10.1037/0033-2909.127.3.342. [DOI] [PubMed] [Google Scholar]
- 66.Prablanc C, Echallier JE, Jeannerod M, Komilis E. Optimal response of eye and hand motor systems in pointing at a visual target, II: static and dynamic visual cues in the control of hand movement. Biol Cybern. 1979;35:183–187. doi: 10.1007/BF00337063. [DOI] [PubMed] [Google Scholar]