Abstract
A new metabolite, 2,4-dihydroxyquinoline (DHQ), was identified in cultures of the bacteria Pseudomonas aeruginosa and Burkholderia thailandensis. We found that the biosynthesis of DHQ correlates with the presence of a functional PqsA, which is a product of the pqsABCDE operon responsible for the synthesis of 4-hydroxy-2-alkylquinolines (HAQs) in P. aeruginosa. However, DHQ is not a degradation product or precursor of HAQs. This finding sheds some light on the poorly understood biosynthesis pathway of HAQs, which includes important communication signals regulating the expression of virulence factors.
Keywords: 2,4-dihydroxyquinoline; 4-hydroxy-2-alkylquinolines; mass spectrometry; natural product; Pseudomonas aeruginosa; PqsA
Introduction
Pseudomonas aeruginosa is a ubiquitous and versatile Gram-negative opportunistic bacterial pathogen primarily infecting immunocompromised individuals and those suffering from cystic fibrosis (Lyczak et al., 2000). P. aeruginosa produces a large number of extracellular products, many of which are virulence factors (Kipnis et al., 2006). The regulation of many of these virulence factors is controlled in a cell density-dependent manner through a process called quorum sensing (QS) (Rumbaugh et al., 2000; Juhas et al., 2005), an intercellular communication system used by many bacterial species to regulate the expression of a wide variety of survival and virulence mechanisms in response to cell density (Fuqua et al., 2001).
We have discovered a unique regulatory and signalling mechanism required for the full virulence of P. aeruginosa. This newly identified intercellular communication system is based on the production of a group of extracellular 4-hydroxy-2-alkylquinolines (HAQs) (Déziel et al., 2004; Lépine et al., 2004). These include the intercellular signalling molecules 4-hydroxy-2-heptylquinoline (HHQ) (Déziel et al., 2004) and its hydroxylated derivative 3,4-dihydroxy-2-heptylquinoline, also known as the Pseudomonas quinolone signal (PQS), involved in the QS regulatory system (Pesci et al., 1999; McKnight et al., 2000; Diggle et al., 2003). HAQs are primarily synthesised through the activity of enzymes encoded by the pqs-ABCDE and phnAB operons, both controlled by the MvfR transcriptional regulator (Cao et al., 2001; Gallagher et al., 2002; Déziel et al., 2004). HHQ and PQS have recently been revealed as inducers of MvfR (Wade et al., 2005; Xiao et al., 2006a). The production of a number of virulence factors and secondary metabolites known to be under the influence of the QS regulatory circuitry is reduced in an mvfR mutant (Cao et al., 2001; Gallagher et al., 2002; Diggle et al., 2003; Déziel et al., 2005).
Anthranilic acid (AA) is the precursor of all HAQs (Déziel et al., 2004) and PhnAB is primarily responsible for its synthesis (Calfee et al., 2001; Déziel et al., 2004). However, little is known about the exact role of the individual genes in the pqsABCDE operon. While, according to the P. aeruginosa genome annotation (www.pseudomonas.com), pqsBCD is related to 3-oxoacyl-[acyl-carrier-protein] synthases and would then be involved in synthesis of the 3-keto fatty acid moiety (Gallagher et al., 2002; Bredenbruch et al., 2005), PqsE is not required for the synthesis of HAQs (Gallagher et al., 2002; Déziel et al., 2004). PqsA displays similarity with co-enzyme A ligases, and more specifically with many enzymes that activate aromatic acids such as benzoate and 4-hydroxybenzoate, an indication that it might be involved in activation of the carbonyl of AA in the synthesis of HAQs. We report here that PqsA is also involved in the synthesis of 2,4-dihydroxyquinoline (DHQ), an extracellular metabolite that has not been described so far.
Results
Identification and quantification of DHQ by LC/MS
During LC/MS analysis of P. aeruginosa PA14 cultures supplemented with AA-d4, we noted, along with the expected labelling of HAQs, another ion presenting an additional 4-Da increase compared to the same culture with unlabelled AA. This ion exhibits a pseudomolecular ion [M+H]+ at m/z 162 and corresponds to a compound derived from AA that cannot be extracted from the culture medium, even after multiple extractions with ethyl acetate, an indication that it is very polar.
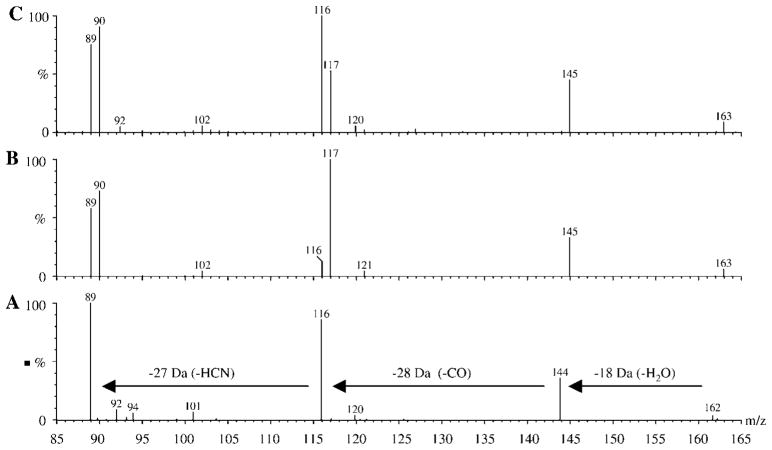
Its MS/MS spectrum shows the loss of 18 Da (loss of water) from the [M+H]+ ion (Figure 1A), to produce an ion at m/z 144. This ion further fragments with the loss of 28 Da (CO) to yield an ion at m/z 116, which then produces an ion at m/z 89 after the loss of 27 Da (HCN). The precursor molecule incorporates the aromatic ring of AA and likely contains the nitrogen of AA as well, considering the odd molecular weight of the molecule. Exact mass measurement of the m/z 162 ion using a Fourier-transform mass spectrometer (FT-MS) gave a value of 162.0548, which corresponds to an elemental composition of C9H8NO2. These observations are compatible with the structure of 2,4-dihydroxyquinoline (DHQ) (Figure 2). This was confirmed using authentic DHQ, which presents the same retention time and the same MS/MS spectrum (data not shown). The fragmentation sequence presented above was confirmed by FT-MS using sustained off-resonance irradiation (SORI). Using exact mass measurement, we confirmed that the ion at m/z 144.0444 m/z (C9H6NO) fragments to produce only one ion at 116.0494 (C8H6N), thus proving that the 28-Da loss observed in MS/MS corresponds to the loss of CO.
Figure 1. Mass spectra of DHQ.
(A) MS/MS spectrum of the m/z 162 ion of DHQ observed in a PA14 culture. (B) MS/MS spectrum of the m/z 163 ion corresponding to DHQ in a PA14 culture fed α13C-labelled AA. (C) MS/MS spectrum of the m/z 163 ion of DHQ in a culture fed 1000 mg/l acetic acid-2-13C.
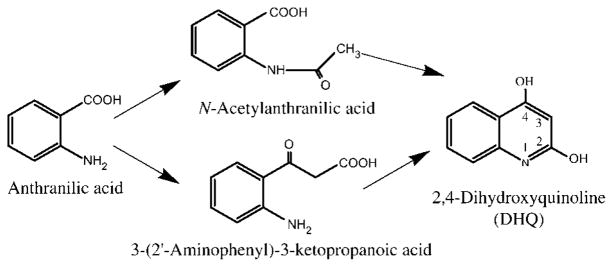
Figure 2.
Putative intermediates in the formation of DHQ corresponding to the addition of an acetate moiety onto AA.
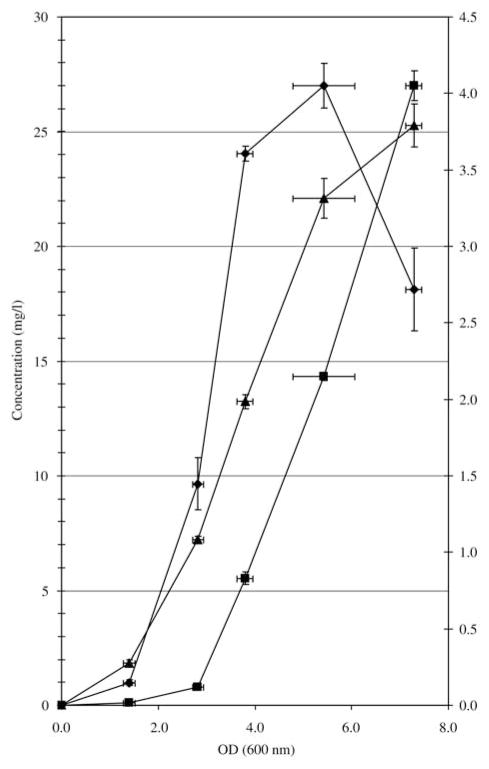
Using a calibration curve of commercial DHQ, the rate of DHQ production in a PA14 culture was measured (Figure 3), which clearly indicates that DHQ production is initiated at the end of the logarithmic growth phase, like HHQ, the precursor of PQS. Under the specified growth conditions, DHQ production peaks at 26.9±0.96 mg/l at an OD600 of 5.8. Production by P. aeruginosa strain PAO1 is similar, while strain PAK does not produce any detectable DHQ. We have previously shown that the latter strain does not produce HAQs (Lépine et al., 2003). DHQ was also found in cultures of Bulkholderia thailandansis strain E264, but at a much lower concentration (0.26±0.26 mg/l at an OD600 of 7.5).
Figure 3.
Production of DHQ (◆), PQS (■) (left axis) and HHQ (▲) (right axis) in a growing culture of P. aeruginosa strain PA14. Results represent the average of triplicates.
Genes involved in DHQ production
Feeding HHQ-d4 or PQS-d4 to a PA14 culture did not lead to labelling of DHQ, proving that DHQ is not a degradation product of these HAQs. The fact that AA is a precursor of both HAQs and DHQ, along with their structural similarities, prompted us to investigate whether enzymes required for the synthesis of HAQs could also be involved in the synthesis of DHQ. DHQ production by various PA14 mutants for the pqsABCDE genes was therefore investigated. While pqsA, pqsB, pqsC and pqsD are all essential for HAQ production (Gallagher et al., 2002; Déziel et al., 2004), we found that only the pqsA−mutant does not produce DHQ. As expected, complementation of the pqsA− mutant with pLG14, a plasmid carrying pqsABC, restored the production of DHQ (0.20±0.022 mg/l). The pqsB− mutant, in which only pqsA is transcribed, produced 0.50±0.036 mg/l of DHQ, supporting the model in which PqsA is involved in the conversion of AA into DHQ. Importantly, the DHQ concentration increased to 1.2±0.066 mg/l when PQS was added to a pqsB− culture, showing that the enzyme system responsible for DHQ production is controlled by MvfR, for which PQS acts as an activator.
To support this genetic evidence pointing towards PqsA as an enzyme responsible for DHQ synthesis, 6-fluoroanthranilic acid (6-FABA), an inhibitor of PqsA activity, was fed to the bacteria. 6-FABA inhibits HAQ synthesis without affecting bacterial growth (Lesic et al., submitted for publication). Because of its structural analogy with AA, we believe that 6-FABA competitively hinders PqsA function by occupying its active site. Addition of 1.5 mM 6-FABA completely prevented DHQ production in strain PA14. In agreement with the presumed mode of action of this inhibitor, supplementation with 3 mM AA to a medium containing 1.5 mM 6-FABA partially restored DHQ production (1.97±0.14 mg/l), another indication that HAQs and DHQ share a common enzymatic pathway through PqsA.
Biosynthesis of DHQ
The DHQ biosynthetic pathway was then investigated. Knowing that the aromatic ring of AA is incorporated into DHQ, as shown above with AA-d4, we investigated whether the carbon of the carbonyl group is also conserved. A PA14 culture was fed 100 mg/l α13C-labelled AA. The observed m/z 163 peak displayed 101.6% of the intensity of the peak at m/z 162, while the natural 13C abundance in DHQ yields a normal intensity of 10.5%. Therefore the ratio of labelled to unlabelled DHQ was 83.1%. When 100 mg/l AA-d4 was fed to the bacteria, the ratio of labelled to unlabelled DHQ was 80.6%. This shows that the α-carbon of AA is incorporated to the same extent as the aromatic moiety of AA and that all the carbons of AA are incorporated into DHQ.
This leaves two carbon atoms unaccounted for in the biosynthesis of DHQ. To verify if these two carbons originate from the direct addition of acetate onto AA, PA14 cultures were supplemented with 1000 mg/l sodium acetate-2-13C. The resulting m/z 163 peak had 35% of the intensity of that at m/z 162 (data not shown), and thus a labelled to unlabelled DHQ ratio of 24.5%. This indicates that at least one carbon from exogenously supplied acetate is incorporated into DHQ.
Two plausible DHQ precursors that include AA and an acetate moiety can therefore readily be envisioned: N-acetyl-AA and 3-(2′-aminophenyl)-3-ketopropanoic acid (Figure 2). The first would be the N-acetylation product of AA, which could undergo cyclisation of the methyl group with an activated carbonyl of AA. The second could be the result of coupling of the methyl group of acetate onto the activated carbonyl of AA, followed by cyclisation of the acetyl carbonyl and the amine of AA. In both cases, direct incorporation of 2-13C acetic acid would result in labelling at the 3-position of DHQ.
To determine the position of the label in DHQ when the bacteria were fed sodium acetate-2-13C, it was necessary to determine whether the loss of CO originates from the 2- or the 4-position of DHQ. Thus, the m/z 163 ion arising from the α13C enriched AA feeding experiment was analysed by MS/MS (Figure 1B). The m/z 163 ion showed the same initial loss of water to produce the m/z 145 ion, which then mainly lost 28 Da to produce the m/z 117 ion. In this experiment, the 13C label can only be in the 4-position, and because a loss of 29 Da was not observed, it can be deduced that the carbon in the 4-position cannot be the one involved in the loss of CO. Thus, the loss of CO originates from the other carbon bearing an oxygen at position 2. This also shows that the oxygen involved in the initial loss of water must originate from the 4-position.
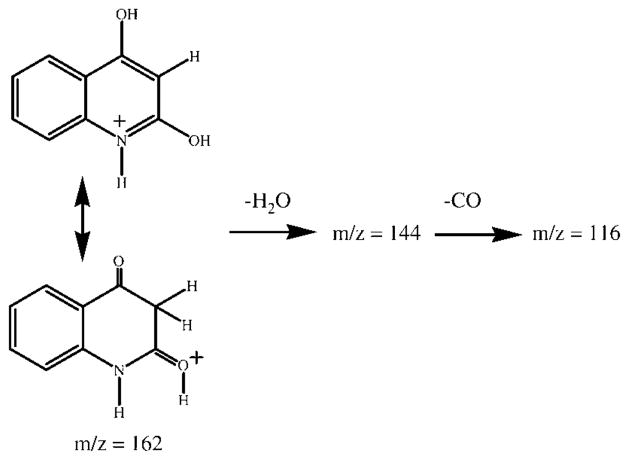
We were also interested in identifying the second proton involved in the initial loss of water (Figure 4). This could be either one of the easily exchangeable protons on the nitrogen or hydroxyl, or the aromatic proton adjacent to the hydroxyl groups. Deuterium exchange was carried out to label the three easily exchangeable protons located on the nitrogen or the hydroxyl groups, and MS/MS analysis of the [M+H]+ ion was performed to determine whether the molecule loses 19 or 20 Da. After 1 h at room temperature in D2O containing 1% acetic acid-d, we obtained an increase of 4 Da instead of exchanging only three protons. This fourth proton is likely the aromatic proton adjacent to the two hydroxyl groups, as it is a relatively acidic proton adjacent to two carbonyls in the tautomeric form represented in Figure 4. Nevertheless, the MS/MS spectrum of the transient m/z 165 ion, which corresponds to the exchange of only three protons, shows predominantly the loss of 19 Da. This could only be due to loss of the slower exchangeable proton at position 3, adjacent to the two carbonyls (Figure 4). The exchange of four hydrogen atoms in D2O implies that the molecule tautomerises into the diketo form to a considerable extent, as depicted in Figure 4, and that the 2-carbon exists, at least transiently, as a carbonyl, which is reflected in the loss of CO from the m/z 145 ion.
Figure 4.
Representation of the tautomeric forms of the pseudo-molecular ion of DHQ.
When sodium acetate-2-13C was added to the cultures, the intensity observed for the m/z 163 peak was 35% of that of m/z 162, instead of the 10.5% normally expected for naturally occurring 13C. Thus, 30% of the intensity of this m/z 163 ion is due to unlabelled DHQ. The MS/MS spectrum of m/z 163 shows predominantly an m/z 116 ion corresponding to the loss of 29 Da from m/z 145 (Figure 1C). Although the m/z 117 ion observed in this spectrum is relatively abundant compared to m/z 116, it arises mostly from the 28-Da loss from naturally 13C enriched DHQ, which represents 30% of the mother m/z 163 ion and should represent 30% of m/z 116. As m/z 116 has twice the intensity of the m/z 117 ion, this implies that most of the label is at the 2-position. This is not in agreement with the scheme proposed in Figure 2, or at least it indicates that if N-acetyl-AA is indeed the precursor, it is not the direct product of addition of the exogenously added acetic acid onto AA. Adding N-acetyl-AA to a PA14 culture did not increase the amount of DHQ produced. This leaves open the possibility that 3-(2′-aminophenyl)-3-ketopropanoic acid could be a precursor of DHQ, although it cannot be formed by the direct addition of exogenously applied acetic acid onto AA.
A series of compounds containing two carbons were tested to determine if they could be precursors of DHQ. Oxalic and glycolic acid had no effect on DHQ production, but glyoxylic acid totally inhibited DHQ. It was thought that this effect could be rationalised by inhibition of the Krebs cycle glyoxylate shunt and that the precursor could be a product of the Krebs cycle. Indeed, itaconate also inhibits DHQ production. However, fumarate and oxaloacetate have no effect.
It is still possible that exogenously supplied AA is metabolised by the bacteria and transformed into another compound that is the precursor of DHQ. As AA is a precursor of tryptophan, this amino acid was added to the culture along with other known tryptophan metabolites to determine the effect on DHQ production. The metabolites tested were 2-aminoacetophenone, N-for-mylacetophenone, indole, skatole, kynurenic acid and indoleacetic acid. However, no increase in DHQ production was observed. Finally, 4-hydroxyquinoline was also tested to verify whether it could be the precursor of DHQ through simple hydroxylation at the 2 position, but the amount of DHQ did not increase either.
Two anthranilate synthases are present in P. aeruginosa: PhnAB, controlled by MvfR and the primary source of AA for HAQ synthesis, and TrpEG, which especially contributes to tryptophan synthesis (Essar et al., 1990). A phnAB mutant is still capable of producing HAQs and DHQ, but at lower concentration (18.9±0.69 mg/l at OD 4.5) than the wild-type strain. A double trpE−phnAB− mutant grown in M63 minimal medium supplemented with 1% dextrose and tryptophan or AA still produces DHQ and HAQs, confirming that a third source of AA exists through degradation of tryptophan, apparently via the kynurenine pathway (Kurnasov et al., 2003).
When 3 mM AA-d4 or α13C-AA was added to cultures, DHQ and all the HAQs were almost quantitatively labelled with an additional increase of 4 and 1 Da, respectively. Quantitative labelling of 4 Da was also found for DHQ and HAQs when tryptophan-d5 was added to the same minimal medium. Therefore, endogenous production of AA by PhnAB and TrpEG caused the incomplete labelling of DHQ and HAQs observed when the wild-type strain or phnAB mutant was cultivated with AA-d4.
Biological activity of DHQ
Since PQS and HHQ act as inducers of the activity of MvfR (Wade et al., 2005; Xiao et al., 2006a), we investigated whether DHQ could perform a similar function by activating transcription of the pqsABCDE operon. However, DHQ had no effect on the activity of the pqsA-lacZ reporter and DHQ supplementation did not alter the production of HAQs. In addition, DHQ does not increase the production of blue phenazine pyocyanin, in contrast to PQS (Diggle et al., 2003). This behaviour is similar to the effect of the other principal HAQ produced by P. aeruginosa, HQNO (Déziel et al., 2004; Xiao et al., 2006a). Instead, the latter molecule inhibits the growth of Gram-positive bacteria (Machan et al., 1992; Déziel et al., 2004; Hoffman et al., 2006). Still, DHQ did not inhibit growth of Pseudomonas fluorescens, Escherichia coli, Vibrio harveyi, Chromobacterium violaceum, Staphylococcus aureus, Bacillus subtilis or Cryptococcus neoformans (data not shown).
Discussion
It is interesting to find that such a simple molecule as DHQ, which is produced at up to 26.9 mg/l (Figure 3) by the widely studied bacterium P. aeruginosa, has not been reported previously, perhaps because this compound is insoluble in most organic solvents, and therefore is not easily isolated by simple extraction. The fact that DHQ is produced by the two HAQ-positive P. aeruginosa strains PAO1 and PA14, but not by the HAQ-negative strain PAK, first shows that there is a relationship between DHQ and HAQ production. This is further highlighted by the observation that B. thailandensis strain E264, which produces some HAQ congeners as described in the B. thailandensis strain E30 (Diggle et al., 2006) and possesses a pqsABCDE homologue, also produces significant amounts of DHQ.
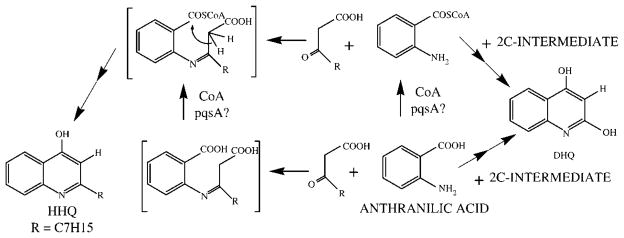
The fact that DHQ and HAQs share structural similarities and that they both have AA as a common precursor implies some common steps in their biosynthetic pathways. However, HAQ production requires all the genes of the pqsABCD cluster, while DHQ production only needs pqsA, as it is still produced in a polar pqsB− mutant, but is completely absent in a polar pqsA mutant. Complementation of a pqsA− mutant by a plasmid containing pqsA also restored DHQ production. The closing of the second aromatic ring of HAQs and DHQ with retention of the original carbonyl carbon of AA requires activation of this function. PqsA shows homologies with CoA ligases, and more specifically with CoA ligases involved in the activation of aromatic carbonyls, such as those of benzoate and 4-hydroxybenzoate. Bredenbruch et al. (2005) hypothesised that PqsA might be involved in the activation of the carbonyl of the 3-ketofatty acid precursor of HAQs. Our results rather show that PqsA is more likely involved in the activation of the carbonyl group of AA, as there is no fatty acid-derived alkyl chain in DHQ. This activation could directly occur on AA to produce an intermediate anthranilyl-CoA or occur after an eventual coupling between the AA nitrogen and the active ketone of the 3-ketofatty acid precursor (Figure 5). The involvement of an anthranilyl-CoA ester in the biosynthesis of HHQ has already been suggested (Ritter and Luckner, 1971; Gallagher et al., 2002). For DHQ, we initially hypothesised that acetate could be the source of the additional two carbons and that PqsA could be involved either in the final ring-closing reaction or in synthesis of the two putative precursors depicted in Figure 2. Feeding the bacteria with acetate-2-13C confirmed that at least one carbon from acetate is incorporated into DHQ, but detailed analysis of the various MS/MS spectra showed that exogenous acetate is not directly incorporated into AA, as the location of the label is not where it would be expected if exogenous acetate were directly incorporated into these two putative precursors. This also explains the relatively small ratio of labelled versus non-labelled compounds (24.5%) observed when acetate-2-13C was added to the culture medium, even if a very high concentration (1000 g/l) of acetate was used.
Figure 5.
Proposed pathway for the formation of DHQ and HHQ.
The ratio of labelled versus non-labelled compounds when AA-d4 was added, although more considerable than with acetate, did not exceed 1:1, despite the amount of AA-d4 added (100 mg/l), even if we observed that all the carbon atoms of AA are incorporated into DHQ. One possibility is that the intracellular concentration of endogenous AA is much higher than the concentration added to the culture medium. This was later confirmed, as labelling was almost quantitative when the double mutant trpE−phnAB− was used. We were also able to confirm that tryptophan degradation contributes to the endogenous pool of AA using the double mutant fed labelled tryptophan, even though addition of a variety of tryptophan degradation intermediates did not lead to an increase in DHQ production. The fact that the ratio of labelled versus unlabeled compound in DHQ is the same as that observed for the HAQs in the same experiment rather indicates that it is more likely that DHQ and HAQs directly incorporate exogenous AA once diluted in the intracellular pool, rather than incorporating AA through one of its metabolites.
All these results indicate that AA is directly used by the bacteria for coupling with an as yet unidentified metabolite of acetic acid. The inhibitory effects of glyoxylate and itaconate support the hypothesis of the involvement of the Krebs cycle and/or the glyoxylate shunt in the generation of this metabolite. One of these coupling reactions involves PqsA, which acts by activating the carboxylate of AA, probably through the formation of a CoA thioester bond, as suggested by the amino acid sequence homologies of PqsA with aromatic CoA ligases. This could be observed through the inhibition of DHQ production by 6-FABA, an inhibition rescued by addition of more AA.
The precise biological role of DHQ remains to be determined. It is not involved in the regulation of MvfR activity or in the biosynthesis of HAQs and has no antimicrobial effects against a large range of microbial species.
Overall, the results of these experiments indicate that DHQ biosynthesis proceeds through the action of PqsA, an enzyme required for the biosynthesis of HAQs. The structure of DHQ strongly suggests that PqsA is involved in the activation of the carbonyl function of AA, a chemical step that is required for closing of the second aromatic ring of DHQ and HAQs. As HHQ and PQS are modulators of the activity of MvfR, which controls the expression of a large number of virulence factors, it is important to understand the initial steps of HAQ synthesis. The identification of DHQ as a metabolite produced through PqsA will help in defining the function of this enzyme.
Materials and methods
Chemicals
3,4,5,6-Tetradeutero-AA (AA-d4) and anthranilic-α 13C acid were from CDN Isotopes (Pointe-Claire, Canada). Sodium acetate-2-13C, deuteroacetic acid, DHQ, and all other chemicals were from Sigma-Aldrich (Oakville, ON, Canada). N-Formylacetophenone was synthesised according to Hoenicke et al. (2002).
Bacteria, plasmids, and culture conditions
P. aeruginosa strains PA14, PAO1, and PAK and B. thailandansis strain E264 (ATCC) were used. PA14 pqsA, pqsB and pqsD mutants were TnphoA insertional mutants (Déziel et al., 2004). pLG14, which is pUCP18 carrying pqsABC, was from Gallagher et al. (2002), and pGX5 is a pqsA-lacZ transcriptional reporter (Xiao et al., 2006b). A trpE (No. 40990) mutant was obtained from the non-redundant PA14 transposon library (Liberati et al., 2006) and used for construction of the double mutant trpE−phnAB− mutant, which was generated by allelic exchange using the suicide vector pEX18Ap and sucrose counterselection as previously described (Déziel et al., 2004). Mutagenesis results in the deletion of most of phnAB and leaves 300 bp of the 5′ end of phnA and 300 bp of the 3′ end of phnB. The deletion was confirmed by PCR and sequencing. As expected, the double mutant trpE−phnAB− was not viable in minimal medium, as it is unable to produce AA, the precursor of tryptophan. Unless otherwise stated, all bacteria were cultivated in tryptic soy broth (Difco, Sparks, MD, USA) at 37°C in a rotary shaker and samples were taken when cultures had reached an OD600 between 5.0 and 5.5. Sample preparation was performed with methanol as previously described, using 5,6,7,8-tetradeutero-PQS as the internal standard (Déziel et al., 2005). All experiments were performed in triplicate and the standard deviation is indicated.
Mass spectrometry
MS analyses were performed on a Quattro II (Waters, Mississauga, ON, Canada) triple-quadrupole mass spectrometer in positive electrospray ionisation mode. Collision-induced dissociation was performed with argon as collision gas at 2×10−3 mTorr. The mass spectrometer was coupled to a HP 1100 HPLC system (Agilent Technologies, Saint-Laurent, QC, Canada) equipped with a 3×150 mm C8 Luna reverse-phase column (Phenomenex, Torrance, CA, USA). A water/acetonitrile gradient with 1% acetic acid was used as the mobile phase at a flow rate of 0.4 ml/min split to 10% through a Valco T-piece. Quantification of DHQ and other HAQs was performed in full-scan mode using PQS-d4 as the internal standard. The elemental composition of fragment ions was determined using a 7-T FTMS instrument using SORI (Varian, Mississauga, ON, Canada).
Acknowledgments
Work in F.L.’s laboratory was funded by NSERC. Work in E.D.’s laboratory was supported by funding from the Canadian Institutes of Health Research (CIHR). Work in L.G.R.’s laboratory was funded by NIH (R01AI063433) and SCH (grant #8850).
References
- Bredenbruch F, Nimtz M, Wray V, Morr M, Müller R, Häussler S. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J Bacteriol. 2005;187:3630–3635. doi: 10.1128/JB.187.11.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee MW, Coleman JP, Pesci EC. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2001;98:11633–11637. doi: 10.1073/pnas.201328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Krishnan G, Goumnerov B, Tsongalis J, Tompkins R, Rahme LG. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc Natl Acad Sci USA. 2001;98:14613–14618. doi: 10.1073/pnas.251465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déziel E, Gopalan S, Tampakaki AP, Lépine F, Padfield KE, Saucier M, Xiao G, Rahme LG. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- Déziel E, Lépine F, Milot S, He J, Mindrinos MN, Tompkins RG, Rahme LG. Analysis of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines (HAQs) reveals a role for 4-hydroxy-2-heptylquinoline in cell-to-cell communication. Proc Natl Acad Sci USA. 2004;101:1339–1344. doi: 10.1073/pnas.0307694100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Lumjiaktase P, Dipilato F, Winzer K, Kunakorn M, Barrett DA, Chhabra SR, Camara M, Williams P. Functional genetic analysis reveals a 2-Alkyl-4-quinolone signaling system in the human pathogen Burkholderia pseudomallei and related bacteria. Chem Biol. 2006;13:701–710. doi: 10.1016/j.chembiol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Diggle SP, Winzer K, Chhabra SR, Worrall KE, Camara M, Williams P. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol. 2003;50:29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- Essar DW, Eberly L, Hadero A, Crawford IP. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990;172:884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acylhomoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- Gallagher LA, McKnight SL, Kuznetsova MS, Pesci EC, Manoil C. Functions required for extracellular quinolone signaling by Pseudomonas aeruginosa. J Bacteriol. 2002;184:6472–6480. doi: 10.1128/JB.184.23.6472-6480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenicke K, Borchert O, Gruning K, Simat TJ. ‘Untypical aging off-flavor’ in wine: synthesis of potential degradation compounds of indole-3-acetic acid and kynurenine and their evaluation as precursors of 2-aminoacetophenone. J Agric Food Chem. 2002;50:4303–4309. doi: 10.1021/jf011672r. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Deziel E, D’Argenio DA, Lepine F, Emerson J, McNamara S, Gibson RL, Ramsey BW, Miller SI. Selection for Staphylococcus aureus small-colony variants due to growth in the presence of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2006;103:19890–19895. doi: 10.1073/pnas.0606756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhas M, Eberl L, Tummler B. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ Microbiol. 2005;7:459–471. doi: 10.1111/j.1462-2920.2005.00769.x. [DOI] [PubMed] [Google Scholar]
- Kipnis E, Sawa T, Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med Mal Infect. 2006;36:78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kurnasov O, Jablonski L, Polanuyer B, Dorrestein P, Begley T, Osterman A. Aerobic tryptophan degradation pathway in bacteria: novel kynurenine formamidase. FEMS Microbiol Lett. 2003;227:219–227. doi: 10.1016/S0378-1097(03)00684-0. [DOI] [PubMed] [Google Scholar]
- Lépine F, Déziel E, Milot S, Rahme LG. A stable isotope dilution assay for the quantification of the Pseudomonas quinolone signal in Pseudomonas aeruginosa cultures. Biochim Biophys Acta. 2003;1622:36–41. doi: 10.1016/s0304-4165(03)00103-x. [DOI] [PubMed] [Google Scholar]
- Lépine F, Milot S, Déziel E, He J, Rahme LG. Electrospray/mass spectrometric identification and analysis of 4-hydroxy-2-alkylquinolines (HAQs) produced by Pseudomonas aeruginosa. J Am Soc Mass Spectrom. 2004;15:862–869. doi: 10.1016/j.jasms.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci USA. 2006;103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyczak JB, Cannon CL, Pier GB. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 2000;2:1051–1060. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Machan ZA, Taylor GW, Pitt TL, Cole PJ, Wilson R. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J Antimicrob Chemother. 1992;30:615–623. doi: 10.1093/jac/30.5.615. [DOI] [PubMed] [Google Scholar]
- McKnight SL, Iglewski BH, Pesci EC. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J Bacteriol. 2000;182:2702–2708. doi: 10.1128/jb.182.10.2702-2708.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesci EC, Milbank JBJ, Pearson JP, McKnight S, Kende AS, Greenberg EP, Iglewski BH. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96:11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter C, Luckner M. Biosynthesis of 2-n-alkyl-4-hydroxyquinoline derivatives (pseudane) in Pseudomonas aeruginosa. Eur J Biochem. 1971;18:391–400. doi: 10.1111/j.1432-1033.1971.tb01255.x. [DOI] [PubMed] [Google Scholar]
- Rumbaugh KP, Griswold JA, Hamood AN. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect. 2000;2:1721–1731. doi: 10.1016/s1286-4579(00)01327-7. [DOI] [PubMed] [Google Scholar]
- Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, Pesci EC. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol. 2005;187:4372–4380. doi: 10.1128/JB.187.13.4372-4380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G, Deziel E, He J, Lepine F, Lesic B, Castonguay MH, Milot S, Tampakaki AP, Stachel SE, Rahme LG. MvfR, a key Pseudomonas aeruginosa pathogenicity LTTR-class regulatory protein, has dual ligands. Mol Microbiol. 2006a;62:1689–1699. doi: 10.1111/j.1365-2958.2006.05462.x. [DOI] [PubMed] [Google Scholar]
- Xiao G, He J, Rahme LG. Mutation analysis of the Pseudomonas aeruginosa mvfR and pqsABCDE gene promoters demonstrates complex quorum-sensing circuitry. Microbiology. 2006b;152:1679–1686. doi: 10.1099/mic.0.28605-0. [DOI] [PubMed] [Google Scholar]