Summary
The stem-loop binding protein (SLBP) binds to the 3′ end of histone mRNA and participates in 3′-processing of the newly synthesized transcripts, which protects them from degradation, and probably also promotes their translation. In proliferating cells, translation of SLBP mRNA begins at G1/S and the protein is degraded following DNA replication. These post-transcriptional mechanisms closely couple SLBP expression to S-phase of the cell cycle, and play a key role in restricting synthesis of replication-dependent histones to S-phase. In contrast to somatic cells, replication-dependent histone mRNAs accumulate and are translated independently of DNA replication in oocytes and early embryos. We report here that SLBP expression and activity also differ in mouse oocytes and early embryos compared with somatic cells. SLBP is present in oocytes that are arrested at prophase of G2/M, where it is concentrated in the nucleus. Upon entry into M-phase of meiotic maturation, SLBP begins to accumulate rapidly, reaching a very high level in mature oocytes arrested at metaphase II. Following fertilization, SLBP remains abundant in the nucleus and the cytoplasm throughout the first cell cycle, including both G1 and G2 phases. It declines during the second and third cell cycles, reaching a relatively low level by the late 4-cell stage. SLBP can bind the histone mRNA-stem-loop at all stages of the cell cycle in oocytes and early embryos, and it is the only stem-loop binding activity detectable in these cells. We also report that SLBP becomes phosphorylated rapidly following entry into M-phase of meiotic maturation through a mechanism that is sensitive to roscovitine, an inhibitor of cyclin-dependent kinases. SLBP is rapidly dephosphorylated following fertilization or parthenogenetic activation, and becomes newly phosphorylated at M-phase of mitosis. Phosphorylation does not affect its stem-loop binding activity. These results establish that, in contrast to Xenopus, mouse oocytes and embryos contain a single SLBP. Expression of SLBP is uncoupled from S-phase in oocytes and early embryos, which indicates that the mechanisms that impose cell-cycle-regulated expression of SLBP in somatic cells do not operate in oocytes or during the first embryonic cell cycle. This distinctive pattern of SLBP expression may be required for accumulation of histone proteins required for sperm chromatin remodelling and assembly of newly synthesized embryonic DNA into chromatin.
Keywords: SLBP, Histone mRNA, Mouse, Oocyte, Embryo, Translational control, Cell cycle
Introduction
The stem-loop binding protein (SLBP) is a 31-kDa RNA-binding protein that plays a central role in the stabilization and translation of mRNAs encoding the replication-dependent histones (Wang et al., 1996; Martin et al., 1997). The 3′-untranslated region of newly transcribed histone pre-mRNAs contains a stem-loop structure and a purine-rich element, termed the histone downstream element (HDE), about 10–15 nt downstream. SLBP binds via its central RNA-binding domain to the stem-loop sequence (Wang et al., 1996; Dominski et al., 2001). This binding stabilizes the association of the RNA component of the U7 small nuclear RNP to the HDE (Dominski et al., 1999; Muller et al., 2000). Following recruitment of other factors, the primary transcript is cleaved between the stem-loop and HDE, thus producing a mature mRNA (Stauber et al., 1986; Gick et al., 1987; Stauber and Schumperli, 1988; Harris et al., 1991; Dominski et al., 2002). Transcripts that do not undergo this processing reaction are rapidly degraded within the nucleus (Pandey et al., 1994). SLBP remains associated with processed histone mRNAs in the cytoplasm, and is thought to be necessary for their translation (Sun et al., 1992; Gallie et al., 1996; Hanson et al., 1996).
The expression of SLBP is tightly regulated during the somatic cell cycle (Whitfield et al., 2000). It is not detectable in G1, but begins to accumulate at the G1/S transition. It remains abundant during S-phase, and then declines rapidly during G2. SLBP remains undetectable until the subsequent G1/S transition. Regulation does not appear to occur at the level of transcription, as the amount of SLBP mRNA varies little during the cell cycle. Rather, changes in SLBP abundance are regulated through post-transcriptional mechanisms. Translation of the mRNA is up-regulated at the G1/S transition and remains elevated during S-phase. Following S-phase, SLBP is rapidly degraded through a proteasome-dependent pathway. Hence, as a result of its controlled synthesis and degradation, the expression of SLBP is restricted to G1/S and S-phase of the cell cycle (Whitfield et al., 2000). The cell cycle-regulated expression of SLBP is thus a major mechanism through which accumulation and translation of mRNAs encoding the replication-dependent histones are restricted to S-phase, although increased histone gene transcription also occurs at this time (Eliassen et al., 1998).
In contrast to somatic cells, accumulation and translation of replication-dependent histone mRNAs are not linked to S-phase in oocytes and embryos. Oogonia undergo a final S-phase during embryonic development of the female, then enter meiosis (and are termed oocytes) and a prolonged period of G2-arrest that may last for many years. Shortly before ovulation and fertilization, oocytes undergo meiotic maturation, during which they are released from G2-arrest, enter M-phase, and complete the first meiotic division before becoming arrested at metaphase II (reviewed by Wassarman, 1988). Following fertilization, embryos embark on a series of cleavage divisions consisting of alternating periods of DNA replication and mitosis with no increase in cell mass. In mammals, unlike frogs and flies, these early cycles are slow (15–20 hours) and include clearly defined G1 and G2 phases (Bolton et al., 1984; Howlett and Bolton, 1985). In the mouse, replication-dependent histone mRNAs and proteins are present in oocytes and embryos (Wassarman and Mrozak, 1981; Graves et al., 1985; Clarke et al., 1997; Wiekowski et al., 1997), and translation of the mRNAs is not coupled to DNA replication in early embryos (Wiekowski et al., 1997). These histones are required to replace the protamines of the sperm DNA and to assemble newly synthesized embryonic DNA into chromatin until the embryo becomes transcriptionally active.
To investigate the mechanism underlying this pattern of histone expression, we tested the hypothesis that SLBP expression and activity were not restricted to S-phase in oocytes and early embryos. We report that, in contrast to proliferating somatic cells, SLBP is expressed and possesses stem-loop binding activity both in oocytes and in embryos at all stages of the cell cycle. Thus, SLBP expression is uncoupled from progression through S-phase in the oocyte and early embryo. These results suggest that the factors regulating SLBP expression and activity may be distinct from those regulating other aspects of cell cycle progression. In addition, they provide a potential molecular explanation for the distinctive pattern of histone gene expression in oocytes and embryos, and they open the possibility that SLBP may perform additional roles in these cell types.
Materials and Methods
Collection and culture of oocytes and embryos
Fully-grown oocytes containing a visible germinal vesicle (GV) were collected by puncture of the ovarian antral follicles of CD-1 female mice aged 21–28 days, as previously described (Clarke et al., 1992). Immature oocytes free of adhering granulosa cells were cultured in bicarbonate-buffered minimal essential medium supplemented with sodium pyruvate, antibiotics, and 3 mg/ml bovine serum albumin at 37°C in a humidified atmosphere of 5% CO2 in air.
To obtain embryos at different stages of preimplantation development, CD-1 females (8 weeks or older, Charles River Canada) were injected with 7.5 IU of pregnant mares’ serum gonadotropin followed 44–48 hours later by 5 IU of human chorionic gonadotropin (hCG) and caged individually with males overnight. For early and late 1-cell embryos and early 2-cell embryos, plugged females were sacrificed at 16 hours post-hCG, and the 1-cell embryos were recovered and cultured until they reached the appropriate stage. For late 2-cell and older embryos, plugged females were sacrificed at 42 hours post-hCG, and the 2-cell embryos were flushed from the oviduct and cultured until they reached the appropriate stage. Embryo stages were obtained at the following times post-hCG, as previously described (Bolton et al., 1984; Howlett and Bolton, 1985; Smith and Johnson, 1986): early 1-cell (G1), 18 hours; mid 1-cell (S), 24 hours; late 1-cell (G2), 28 hours; early 2-cell (G1/S), 32 hours; late 2-cell (G2) 44 hours; early 4-cell (G1/S) 52 hours; late 4-cell (G2), 64 hours. Blastocysts were collected at 116 hours post-hCG. Embryos were cultured in 5 μl drops of KSOM (Lawitts and Biggers, 1993) under paraffin oil at 37°C in an atmosphere of 5% CO2 in air.
Immunoblotting
Oocytes or embryos were collected in 5 μl of loading buffer (Harlow and Lane, 1988) and frozen at –80°C until use. Samples were electrophoresed through 10% or 12% polyacrylamide gels in Tris glycine buffer (25 mM Tris, 250 mM glycine, 0.1% SDS, pH 8.3). Proteins were transferred onto a PVDF membrane (Amersham) for 120–150 minutes at 70 V in a transfer buffer containing 25 mM Tris, 192 mM glycine, 20% v/v methanol, pH 8.3. Membranes were soaked for 30 minutes in blocking solution (5% non-fat milk in TBS), and then incubated with gentle agitation in anti-SLBP antiserum (Wang et al., 1996; Whitfield et al., 2000) diluted 1:4000 in blocking buffer at 4°C overnight. Following three washes in TBS containing 0.1% Tween-20 (TBST), membranes were incubated for 1 hour in biotinylated anti-rabbit IgG (Jackson Immunoresearch) diluted 1:5000 in TBST, washed as above, incubated for 30 minutes in streptavidin-horse radish peroxidase (Amersham) diluted 1:1000 in TBST, and washed as above. Immunoreactive proteins were revealed using ECL Plus (Amersham) according to the manufacturer’s instructions.
Immunofluorescence
Oocytes and embryos were freed of the zona pellucida using acidified (pH 2.5) Tyrode’s solution, washed in PBS, and fixed for 15 minutes at room temperature in a freshly prepared solution of 4% paraformaldehyde in PBS. The fixed cells were stored in a blocking solution of PBS, 3% BSA, 0.5% Triton X-100 at 4°C for up to one week. To detect SLBP, oocytes or embryos were transferred to affinity-purified anti-SLBP diluted 1:1000 in blocking solution and incubated with agitation overnight at 4°C. They were then rinsed twice for 15 minutes in blocking solution, incubated for 1 hour at room temperature in FITC-conjugated donkey anti-rabbit IgG (Jackson Laboratories) diluted 1:100 in blocking solution, washed twice as previously described and mounted on a microscope slide in a drop of Moviol (Hoechst) containing 1 μg/ml of DAPI to stain the DNA and 2.5% (w/v) 1,4-diazabicyclo[2.2.2]octane (Sigma) to retard extinction of the fluorescent signal. The cells were examined using an Olympus BX60 microscope equipped for epifluorescence with fluorescein and UV filter sets and linked to an Applied Imaging analysis system.
Reverse transcription (RT) and polymerase chain reaction (PCR)
RNA was purified, reverse-transcribed, and the cDNA amplified as previously described (Mohamed et al., 2001). For each experiment, RNA was prepared from 25–50 oocytes or embryos and cDNA from 8 oocyte/embryo-equivalents was used in the PCR reaction. Each PCR cycle (40 cycles) consisted of 1 minute at 94°C, 30 seconds at 57°C and 45 seconds at 72°C. Primers and positions on the published mRNA sequence (GenBank locus NM_009193) were 81-GGTTATGGGAGTCGCCGCGA- 100 and 880-TAACTCATGGCAGAGAAGTC- 861. These primers should generate an 800-nt fragment from cDNA, but are separated by 1.14 kb in genomic DNA (GenBank locus AC079504). In all experiments, samples prepared without reverse-transcriptase were included to ensure that the PCR product was cDNA-dependent. Ten microliters (one-fifth) of amplified product was electrophoresed through an 8% (w/v) polyacrylamide gel prepared in 45 mM Tris-borate, 1 mM EDTA, pH 8.0, stained using ethidium bromide, and photographed using a UV transilluminator.
Phosphatase treatment
Oocytes or embryos were transferred to a microfuge tube in a minimal volume of medium and stored at –80°C. After thawing, each tube received 5 μl of phosphatase buffer (Boehringer) containing protease inhibitors (Boehringer) and 1 μl of phosphatase (Boehringer). The tubes were incubated for 15 minutes at 37°C, after which 5 μl of 2× Laemmli buffer was added. The samples were processed for immunoblotting as described above.
Mobility-shift assays
Fifty oocytes or embryos collected at MII, G1, S or G2 of the first cell cycle, or at the blastocyst stage were lysed in 0.1% NP-40, 50 mM Tris-HCl [pH 7.5], 150 mM NaCl in a final volume of 25 μl and stored at –80°C until use. Five hundred oocytes collected from 15- day-old mice were collected and stored in 25 μl of the same buffer. An appropriate volume of each extract was mixed on ice with 0.5 pmol of end-labeled, 30 nt stem-loop RNA either in the presence or absence of 1 μg of protein A-purified SLBP antibody in a total volume of 20 μl. Each reaction also contained 10 μg tRNA as a competitor for non-specific RNA binding activities, 20 mM EDTA, 10 μg BSA and 5 μl Buffer D (20 mM HEPES-KOH, [pH 7.9], 100 mM KCl, 0.2 mM EDTA [pH 8.0], 20% glycerol). Following a 10 minute incubation, complexes were resolved on a 7% native polyacrylamide gel (37.5 parts acrylamide to 1 part bisacrylamide) containing Tris-borate- EDTA buffer. The gel was dried and subject to autoradiography. The preparation and labeling of the stem-loop RNA and protein A-purification of the SLBP antibody have been described previously (Dominski et al., 1999; Wang et al., 1996).
Drugs
Puromycin (Sigma) was dissolved in water at 10 mg/ml and used at 10 μg/ml. Nocodazole (Sigma) was dissolved in water at 150 μg/ml and used at 0.15 μg/ml. For each experiment, the effectiveness of the drugs was confirmed by their ability to prevent cleavage. Phosphatase (Boehringer) was stored according to the manufacturer’s instructions. Roscovitine (Sigma) was dissolved in DMSO at 10 mM and used at 100 μM. For each experiment, its effectiveness was confirmed by its ability to prevent germinal vesicle breakdown (GVBD) in a sample of oocytes. U0126 (Calbiochem) was dissolved in DMSO at 10 mM and used at 0.5 μM. For each experiment, its effectiveness was confirmed by immunoblotting using an anti-phospho-ERK antibody (Santa Cruz).
Results
SLBP accumulates during meiotic maturation and remains abundant in early embryos
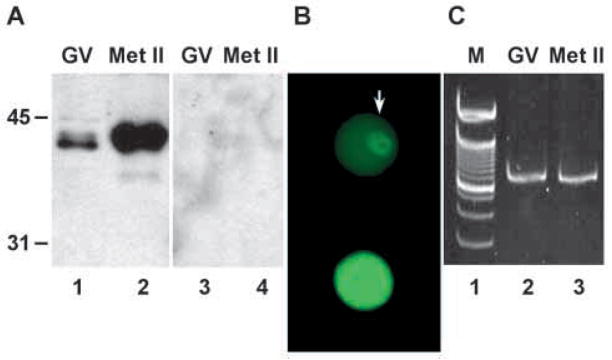
To determine whether mouse oocytes contain SLBP, oocytes containing a germinal vesicle (GV; these possess an intact nuclear membrane and are at late G2 of the cell cycle) were immunoblotted using an antiserum raised against the thirteen C-terminal amino acids of mouse SLBP. Blots prepared from fifty oocytes displayed two immunoreactive bands of relative molecular mass (Mr) slightly less than 45 kDa (Fig. 1A, lane 1), the major species migrating more rapidly than the minor species, as previously described in somatic cells (Wang et al., 1996; Whitfield et al., 2000). Neither species was detected when the antiserum had been preabsorbed against the peptide immunogen (Fig. 1A, lane 3). Thus, the anti-SLBP antiserum recognizes a protein in oocytes that exhibits the same Mr and doublet pattern during SDS-PAGE as the SLBP of somatic cells. Analysis of oocytes using affinity-purified antibody revealed that immunoreactivity was concentrated in the germinal vesicle, although the cytoplasm was also stained above background levels (Fig. 1B, upper panel). These results indicate that SLBP is present in fully grown G2-arrested oocytes and is present at a higher concentration in the nucleus than the cytoplasm.
Fig. 1.
Expression of SLBP in oocytes and eggs. (A) Fifty germinal vesicle (GV)-stage oocytes and metaphase II (Met II) eggs were immunoblotted using anti-SLBP antiserum (lanes 1,2) or using anti- SLBP pre-incubated with the immunogenic peptide (lanes 3,4). Molecular weight markers are indicated to the left of the blots. (B) Oocytes were stained with purified anti-SLBP and analyzed by immunofluoresence. (Upper panel) GV-stage oocyte. The arrow indicates the nucleus. (Lower panel) Metaphase II egg. The photographs were taken using identical conditions. (C) Twenty-five GV-stage (lane 2) or metaphase II (lane 3) oocytes were subjected to RT-PCR using primers derived from the mouse SLBP cDNA sequence that were expected to amplify a fragment of 800 nt. Lane 1 shows 100-nt ladder; intense band near middle of gel is 700 nt. No signal was obtained when reverse transcriptase was omitted from the reaction (not shown).
Upon hormonal stimulation of the somatic follicular cells, fully grown oocytes undergo meiotic maturation. During maturation, the oocyte undergoes germinal vesicle breakdown (GVBD), enters M-phase of the cell cycle, completes the first meiotic division, and becomes arrested at metaphase of meiosis II. Oocytes at metaphase II contained substantially more SLBP than oocytes at prophase I, as determined both by immunoblotting (Fig. 1A, lane 2) and immunofluorescence (Fig. 1B, lower panel). SLBP in M-phase oocytes was uniformly distributed throughout the egg. In contrast to the increase in SLBP protein during maturation, the quantity of SLBP mRNA did not detectably increase as evaluated by RT-PCR (Fig. 1C), consistent with the general transcriptional arrest during oocyte maturation (Masui and Clarke, 1979; Wassarman, 1988). SLBP protein quantity did not increase when prophase I oocytes were incubated for up to 12 hours in the presence of the proteasomal inhibitor, MG132, implying that the increase during maturation is not due to reduced proteasome-dependent degradation (data not shown).
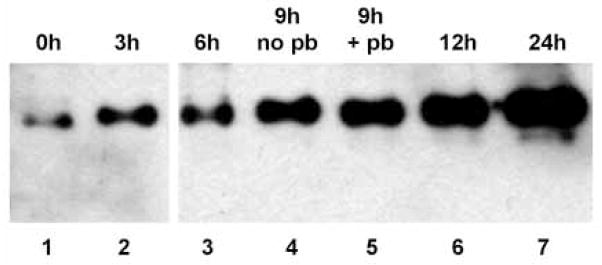
To determine the timing of SLBP accumulation during meiotic maturation, we took advantage of the fact that when GV-stage oocytes are removed from ovarian follicles and placed in culture, they undergo maturation to metaphase II. During maturation in vitro, GVBD has occurred by 2 hours of incubation and the first polar body has been expelled by 9–12 hours. Immature oocytes were collected, placed in culture, and samples were collected at 3-hour intervals. Only oocytes that had undergone GVBD were collected at each time point (except the 0-hour sample which contained GV-stage oocytes). By 3 hours of incubation, a significant increase in the amount of SLBP was detectable (Fig. 2, lanes 1,2), and the SLBP showed a slower mobility suggestive of phosphorylation (see below). As maturation continued, the amount of SLBP progressively increased. When oocytes that had reached metaphase II, as indicated by the presence of the first polar body (Fig. 2, lane 5), were incubated for an additional 3 or 15 hours, the quantity of SLBP continued to increase (Fig. 2, lanes 6,7). Thus, by shortly after GVBD, SLBP has begun to accumulate in maturing oocytes and it continues to accumulate even after the oocytes have reached metaphase II.
Fig. 2.
Accumulation of SLBP during oocyte maturation. GV-stage oocytes were collected and incubated for the period of time indicated at the top of each lane, then immunoblotted using the anti-SLBP antiserum. All oocytes in the 0 hour group contained a GV; all oocytes in the other groups had undergone germinal vesicle breakdown. Oocytes in the 9 hour group were separated into those that had or had not emitted the first polar body (pb). All oocytes in the 12 and 24 hour groups had produced a polar body. Lanes 1 and 2 contain 50 oocytes; all other lanes contain 25 oocytes.
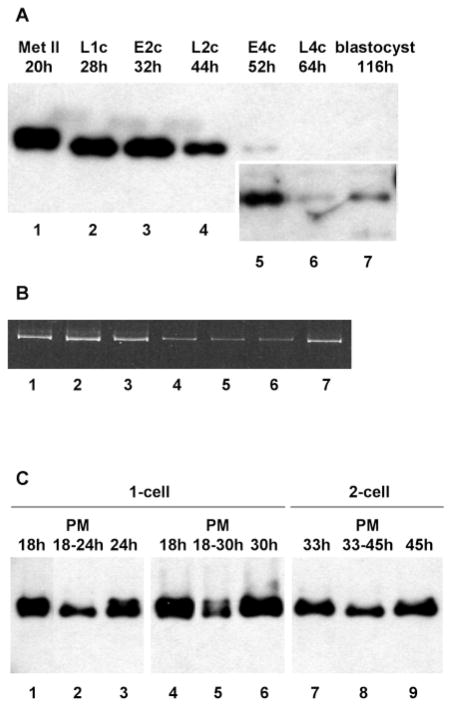
To determine whether the high level of SLBP in mature oocytes was maintained during early embryonic development, embryos at different stages of preimplantation development were collected and analyzed by immunoblotting. SLBP remained abundant throughout the first cell cycle and the early portion of the second cell cycle (Fig. 3A, lanes 2,3). Notably, there was no reduction in SLBP level at the end of the first S-phase or during mitosis to the 2-cell stage, in contrast to its degradation at the end of S-phase in somatic cells (Whitfield et al., 2000). However, SLBP levels had decreased significantly by the late 2-cell stage (Fig. 3A, lane 4). A further decrease was evident between the late 2-cell stage and the early 4-cell stage, and SLBP declined to a very low level by the late 4-cell stage (Fig. 3A, lanes 5,6). SLBP remained present at a low level throughout subsequent preimplantation development, as exemplified by the signal in the lane containing 100 embryos (about 3200 cells) at the blastocyst stage (Fig. 3A, lane 7). SLBP mRNA was present throughout preimplantation development, but the amount of RT-PCR product declined after the 2-cell stage (Fig. 3B) which is consistent with the widespread loss of maternal mRNA that occurs at this time (Paynton et al., 1988), and increased again by the blastocyst stage presumably as a result of the increased cell number. Thus, the quantity of SLBP protein remains high during the first two cell cycles, then declines to a much lower level once the embryo reaches the 4-cell stage and remains low during subsequent development.
Fig. 3.
(A) Abundance of SLBP during early embryogenesis. Unfertilized eggs and embryos were collected at the stages and times (hours post-hCG injection) indicated, and immunoblotted using the anti-SLBP antiserum. The inset within lanes 5–7 shows a longer exposure of the same blot. Met II, metaphase II eggs; L1c, late 1-cell; E2c, early 2-cell; L2c, late 2-cell; E4c, early 4-cell; L4c, late 4-cell. All lanes contain 25 embryos, except lane 7 which contains 100 embryos. (B) Twenty-five metaphase II oocytes (lane 1) or 1-cell (lane 2), 2-cell (lane 3), 4-cell (lane 4), 8-cell (lane 5), morula (lane 6), or blastocyst (lane 7) embryos were subjected to RT-PCR using primers derived from the mouse SLBP cDNA sequence that were expected to amplify a fragment of 800 nt. No signal was obtained when reverse transcriptase was omitted from the reaction (not shown). (C) Effect of the protein synthesis inhibitor, puromycin (PM), on SLBP quantity in early embryos. Embryos at the 1- or 2- cell stage were collected at the indicated times (hours post-hCG; lanes 1,4,7). Embryos were incubated in the presence (lanes 2,5,8) or absence (lanes 3,6,9) of puromycin for the period indicated after which embryos were analyzed by immunoblotting using the anti- SLBP antiserum. 25 embryos per lane.
To determine whether on-going protein synthesis was required to maintain the high level of SLBP in early embryos, 1- and 2-cell embryos were treated with the protein synthesis inhibitor, puromycin. Embryos treated for either 6 or 12 hours contained less SLBP than control embryos processed at the same time, although a substantial fraction of SLBP remained (Fig. 3C, compare lanes 2 vs 3, 5 vs 6, and 8 vs 9). This implies that SLBP, which has accumulated during meiotic maturation, continues to be synthesized during the first two cell cycles.
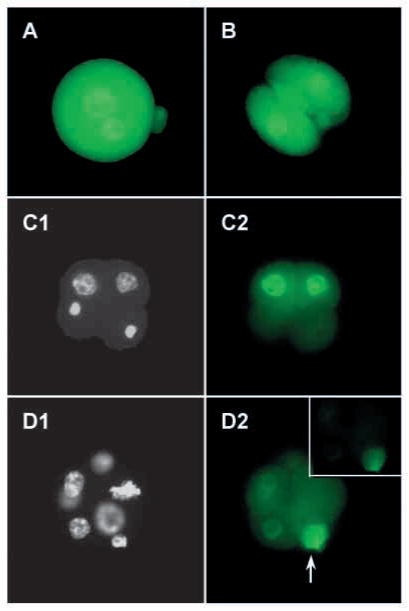
As revealed by immunofluorescence, SLBP was present in the nucleus and cytoplasm during the early embryonic cell cycles, including both the male and the female pronucleus at the 1-cell stage (Fig. 4). Some SLBP was also present in the second polar body (Fig. 4A), and remained there as the embryo developed (Fig. 4D2). The intensity of staining was consistently greater in the nucleus suggesting that, as in oocytes, SLBP preferentially accumulates in this compartment. Embryos fixed at G1, S, and G2 of the first three cell cycles showed no obvious differences in the nucleo-cytoplasmic distribution of SLBP (data not shown). However, embryos fixed early in a cell cycle occasionally contained very small nuclei, which presumably were at late telophase following a recent mitosis. SLBP was not detectable in these nuclei, although it was present in the surrounding cytoplasm (Fig. 4C). This suggests that SLBP accumulates in the nuclei following each mitosis. In embryos beyond the 4-cell stage, SLBP staining was only weakly detected by immunofluorescence (Fig. 4D), consistent with the relatively low quantity detected by immunoblotting.
Fig. 4.
Intracellular distribution of SLBP during early embryogenesis. (A) 1-cell. (B) 2-cell. (C1) Early 4-cell; DAPI stain to reveal DNA. The two lower nuclei appear to be at late telophase/early interphase. (C2) Early 4-cell; SLBP staining. (D1) 5- to 8-cell; DAPI stain. Five nuclei, one set of metaphase chromosomes and the DNA of the second polar body (arrow) are visible. (D2) 5- to 8-cell; SLBP staining. Long exposure reveals weakly stained nuclei and cytoplasm of embryo and strongly stained polar body (arrow). (Inset) Same embryo, exposure for the same duration as A–C.
SLBP is the sole stem-loop binding activity in oocytes and embryos
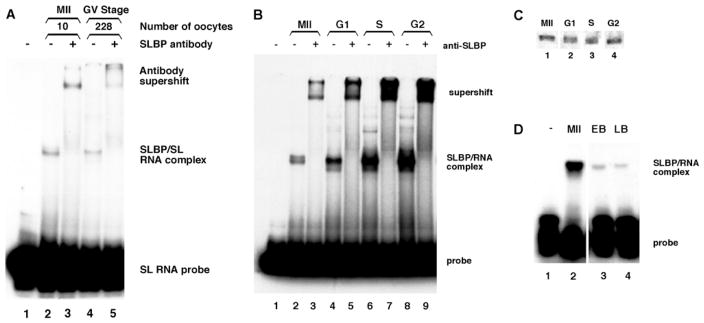
As SLBP exerts its biological function through binding to the stem-loop region of mRNAs encoding histones, we tested whether the SLBP in oocytes and embryos was active in RNA binding and whether there were other factors that could bind the 3′-end of the histone mRNA. To this end, we employed a mobility-shift assay in which cellular lysates are mixed with a radiolabelled histone mRNA stem-loop and then fractionated by electrophoresis to determine whether migration of the labelled stem-loop is retarded (Williams and Marzluff, 1995; Wang et al., 1996). Both GV-stage and metaphase II oocytes contained an activity able to bind the stem-loop sequence. Ten metaphase oocytes contained more binding activity than over 200 GV-stage oocytes (Fig. 5A, lanes 2,4), which is consistent with the accumulation of SLBP during oocyte maturation as analyzed by Western blotting (Fig. 1A; Fig. 2A). Furthermore, all of the binding activity in both GV and metaphase oocytes was supershifted by addition of the anti-SLBP antibody (Fig. 5A, lanes 3,5). This strongly suggests that the single SLBP species recognized by the antibody accounts for all of the detectable stem-loop binding activity in oocytes.
Fig. 5.
(A) Stem-loop binding activity of lysates of oocytes. Lysates from 10 metaphase II oocytes (lanes 2,3) or 228 GV-stage oocytes (lanes 4,5) were incubated with a radioactive stem-loop RNA and the complexes resolved by native gel electrophoresis. In lanes 3 and 5, the antibody to SLBP was added to the extract prior to electrophoresis. (B) Stem-loop binding activity of lysates of oocytes and 1-cell embryos. Lysates from 20 oocytes or embryos were incubated with a radiolabelled stem-loop RNA and resolved on native polyacrylamide gels. MII, metaphase II oocytes; G1, 1-cell embryos, 18 hours post-hCG; S, 1-cell embryos 24 hours post-hCG; G2, 1-cell embryos 28 hours post-hCG. Presence of the SLBP antibody in the binding reaction is indicated (+). (C) Immunoblot of samples used for stem-loop binding assay. Five oocyte- or embryo-equivalents were immunoblotted using the anti-SLBP antiserum. Stages correspond to those in Fig. 5B. (D) Stem-loop binding activity of blastocysts compared with metaphase II oocytes. Lysates from equal numbers of oocytes or blastocysts were incubated with a radiolabelled stem-loop RNA and resolved on native polyacrylamide gels. MII, metaphase II oocytes; EB, early blastocysts; LB, late blastocysts.
The same assay was used to evaluate the stem-loop binding activity in embryo lysates. Stem-loop binding activity was present in 1-cell embryos at G1, S, and G2 of the cell cycle (Fig. 5B, lanes 4,6,8). There was no substantial difference in the binding activity of metaphase II oocytes and the G1-phase embryos (Fig. 5B, compare lanes 2 and 4). This suggests that the phosphorylation of SLBP at M-phase does not affect its ability to bind the histone mRNA stem-loop in vitro. The doublet observed in the complexes from metaphase II samples (lane 2) is a result of the phosphorylation of SLBP (J.A.E. and W.F.M., unpublished; see below). The amount of SLBP binding activity increased, however, as embryos progressed through the first cell cycle (compare lanes 4, 6 and 8), even though the amount of SLBP remained essentially unchanged as analyzed by Western blotting (Fig. 5C). Extracts prepared from early and late blastocyst embryos also contained stem-loop binding activity (Fig. 5D, lanes 3,4). The amount of activity was considerably lower than in metaphase II oocytes (compare lane 2 vs 3 and 4), in agreement with the reduced amount of SLBP detected by Western blotting at these stages (Fig. 3). As in the oocytes, all detectable stem-loop binding activity in embryos was supershifted by the addition of the anti- SLBP antibody (Fig. 5B, lanes 3,5,7,9), demonstrating that both oocytes and embryos contain a single stem-loop binding activity.
SLBP is phosphorylated at meiotic and mitotic M-phase
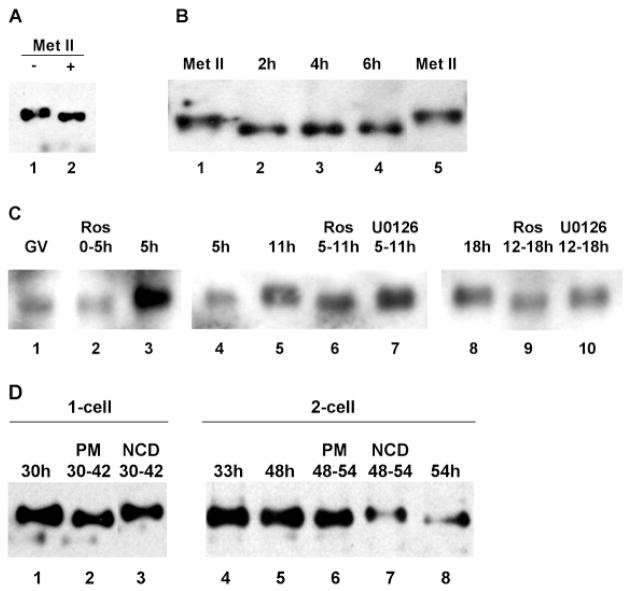
As shown in Fig. 2, the electrophoretic mobility of SLBP decreased during meiotic maturation. This mobility shift was evident shortly after GVBD and remained throughout maturation to metaphase II. To test whether phosphorylation contributed to the decrease in electrophoretic mobility, metaphase oocyte lysates were treated with phosphatase prior to immunoblotting. Phosphatase treatment converted SLBP to the fast-migrating form observed in GV-stage oocytes (Fig. 6A), indicating that SLBP becomes phosphorylated during meiotic maturation.
Fig. 6.
Control of SLBP phosphorylation. (A) Extracts from 20 metaphase II oocytes were incubated without (lane 1) or with phosphatase treatment (lane 2) and immunoblotted using the anti-SLBP antiserum. (B) Dephosphorylation of SLBP following oocyte activation. Unfertilized eggs were parthenogenetically activated and samples were collected at the indicated times after activation and analyzed by immunoblotting using the anti-SLBP antiserum (lanes 2–4). Lanes 1 and 5 are oocytes arrested at metaphase II; 25 eggs per lane. (C) Control by cyclin-dependent kinase of SLBP phosphorylation. (Lanes 1–3) GV-stage oocytes were collected; one sample was harvested immediately (lane 1, 80 oocytes) and the others were incubated for 5 hours in the presence (lane 2, 75 oocytes) or absence (lane 3, 67 oocytes) of roscovitine. (Lanes 4–7) GV-stage oocytes were collected and incubated for 5 hours. Those that underwent GVBD were either harvested immediately (lane 4) or at 11 hours (lane 5) or treated for the indicated period with roscovitine (lane 6) or U0126 (lane 7); 25 oocytes per lane. (Lanes 8–10) GV-stage oocytes were collected and incubated for 12 hours. Those that produced a polar body were incubated for an additional 6 hours in control medium (lane 8) or in the presence of roscovitine (lane 9) or U0126 (lane 10); 25 oocytes per lane. All samples were immunoblotted using the anti-SLBP antiserum. The films showing lanes 1–3, 4-7, and 8–10 were not exposed for the same lengths of time. (D) Phosphorylation of SLBP at mitotic M-phase. Embryos at the 1- or 2-cell stage were collected at the indicated times (hours post-hCG). Puromycin (PM, lanes 2 and 6) or nocodazole (NCD, lanes 3 and 7) treatment was performed for the period indicated, after which embryos were collected for immunoblotting using the anti-SLBP antiserum. All control embryos had cleaved by the end of the treatment period, whereas none of the drug-treated embryos had done so; 25 eggs per lane.
To test whether phosphorylation of SLBP was reversed when activated oocytes exited M-phase, metaphase II oocytes were exposed to SrCl2 to induce parthenogenetic activation and samples were collected at different times after exposure to SrCl2. By 2 hour post-activation, SLBP had been converted to the fast-migrating dephosphorylated form (Fig. 6B). This form remained predominant at 4 hour and 6 hour post-activation. Conversion of SLBP to the fast-migrating form following activation is also evident in Fig. 3A (compare lanes 1 and 2). Thus, the phosphorylation of SLBP upon entry into M-phase of meiosis is reversed upon activation and exit from M-phase.
The rapid phosphorylation of SLBP following GVBD and its rapid dephosphorylation following activation suggested that phosphorylation might depend on cyclin-dependent kinase 1 (cdk-1) activity, which increases rapidly at GVBD and remains high throughout maturation and in oocytes arrested in metaphase II, and falls rapidly after completion of meiosis (Choi et al., 1991). To test this, oocytes at different stages of maturation were treated with roscovitine, an inhibitor of cdk activities (Meijer et al., 1997; Deng and Shen, 2001). When oocytes were treated at prophase I, they failed to undergo germinal vesicle breakdown and SLBP remained in its fast-migrating, hypophosphorylated form (Fig. 6C, lanes 1–3). Moreover, SLBP did not accumulate (Fig. 6C, lane 3), suggesting that accumulation of SLBP also depends on cdk1 kinase activity. When oocytes that had undergone GVBD and contained slow-migrating SLBP were treated with roscovitine for 6 hours, SLBP was converted to the fast-migrating form by the end of the treatment period (lanes 4–6). Following transfer of oocytes to roscovitine-free medium, SLBP was converted back to the slow-migrating form (data not shown), indicating that the effect of the drug was reversible. In contrast, exposure of oocytes to an inhibitor of the ERK MAP kinases, U0126, did not affect the electrophoretic mobility of SLBP (lane 7). Similarly, when oocytes that had reached metaphase II were treated with roscovitine, the fast-migrating form of SLBP was predominant (Fig. 6C, lane 9), whereas the slow-migrating form was predominant following treatment with U0126 (Fig. 6C, lane 10). These results indicate that cdk1 activity is continuously necessary during maturation to maintain the bulk of SLBP in its slow-migrating phosphorylated form, suggesting that it can be rapidly dephosphorylated during this time.
Since SLBP becomes phosphorylated by a cdk-dependent pathway in maturing oocytes, we then examined whether SLBP also becomes phosphorylated at mitotic M-phase of early embryos, when there also is high cdk1 activity. One- and 2-cell embryos were transferred at times corresponding to late G2 of each cell cycle to medium either containing nocodazole to arrest them at M-phase, or containing puromycin to prevent the transition from G2 to M-phase, or were left untreated. Six or 12 hours later, when the untreated controls had undergone cleavage, all groups were collected for immunoblotting. As shown in Fig. 6D, SLBP in both 1- and 2-cell embryos treated with nocodazole displayed the retarded electrophoretic mobility indicating it was phosphorylated (lanes 3,7). This change in mobility was not observed in the puromycin-treated embryos (lanes 2,6), however, indicating that the presence of the slow-migrating SLBP in the nocodazole-treated embryos was not a non-specific consequence of arresting cell-cycle progression. In addition, puromycin treatment at late G2 of the second cell cycle prevented the rapid decrease in SLBP (Fig. 6D, lane 6), while treatment with nocodazole did not (Fig. 6D, lane 7), consistent with a degradation of SLBP at late G2 or entry into mitosis of the second cell cycle. Thus, SLBP also becomes phosphorylated at mitotic M-phase of the early embryonic cell cycles, probably by a cdk1- dependent pathway, even though SLBP remains stable during this time.
Discussion
Expression and activity of SLBP in mouse oocytes and embryos
We have shown that SLBP is present at a low level in the nucleus of fully grown GV-stage oocytes, which are arrested at late G2 of the cell cycle. Upon entry of oocytes into M-phase during meiotic maturation, the amount of SLBP increases substantially and it becomes phosphorylated by a roscovitine-sensitive mechanism. Following egg activation and exit from M-phase, SLBP becomes dephosphorylated. It remains abundant throughout the first embryonic cell cycle and early stages of the second cell cycle, then decreases progressively at the late 2-cell and 4-cell stages, and it is found both in the nucleus and at a lower concentration in the cytoplasm. It is present at a relatively low level in blastocysts. Oocytes and embryos all contain an activity able to bind stem loop-containing RNA, and this is supershifted by anti-SLBP antibody. Thus, SLBP is expressed and is able to bind stem-loop RNA independently of progression to S-phase in oocytes and during the first two cell cycles in mouse embryos.
These results demonstrate that the cell-cycle dynamics of SLBP synthesis and degradation differ markedly in oocytes and early embryos as compared to somatic cells. First, in somatic cells, SLBP becomes phosphorylated at the end of S-phase (Whitfield et al., 2000), probably by cdk2, and this targets it for proteasome-dependent degradation (L.X. Zheng and W.F.M., unpublished). Thus, no SLBP is detected in G2 cells (Whitfield et al., 2000). In contrast, the presence of SLBP in G2-arrested oocytes, together with the inability of a proteasome inhibitor to increase SLBP quantity at this stage, implies that the mechanism that degrades SLBP following S-phase in somatic cells is not active in oocytes.
Second, the substantial accumulation of SLBP during M-phase of meiotic maturation indicates that it is abundantly synthesized at this stage of the cell cycle, whereas this is not the case in somatic cells. In the case of other proteins that accumulate during oocyte maturation, including tissue-type plasminogen activator [tPA (Huarte et al., 1987)], HPRT (Paynton et al., 1988), mos (O’Keefe et al., 1989; Paules et al., 1989; Gebauer et al., 1994), FGF receptor (Culp and Musci, 1999) cyclin B (Polanski et al., 1998; Barkoff et al., 2000; Tay et al., 2000) and spindlin (Oh et al., 2000), this is due to increased translation of existing mRNAs. Translation of these mRNAs is regulated by U-rich sequences, termed adenylation control elements (ACE) or cytoplasmic polyadenylation elements (CPE), that are located in the 3′-untranslated region (utr) of the mRNA within about 100 nt of the polyadenylation signal (Richter, 1995; Gray and Wickens, 1998; Oh et al., 2000). The ACE likely also represses translation of these mRNAs in immature oocytes (Stutz et al., 1997; Stutz et al., 1998). The increase in SLBP synthesis in M-phase oocytes may depend on interaction of oocyte factors with analogous elements in the 3′-utr of SLBP mRNA.
Third, our results indicate that, in early embryonic cells, the quantity of SLBP depends primarily on progression through development or on time elapsed since fertilization, rather than on cell cycle stage. The lifespan of most of the oocyte proteins inherited by the embryo is not known. However, most maternal mRNA is degraded by the 2-cell stage (Paynton et al., 1988) and we observed a decrease in the amount of RT-PCR product detected at this stage. Hence, a loss of maternal SLBP mRNA may be one factor contributing to the drop in SLBP levels near the end of the second cell cycle. Indeed, as indicated by the reduced quantity present in 1- and 2-cell embryos exposed to puromycin, SLBP is both synthesized and degraded following fertilization. These considerations suggest that the large amount of SLBP present during the early embryonic cell cycles represents maternal inheritance, both of the protein and of the mRNA that is translated after fertilization. This maternal supply becomes depleted near the end of the second cell cycle and during the third cell cycle, and the much smaller quantity of SLBP detectable in late 4-cell embryos and blastocysts is the product of embryonic gene activity. Thus, SLBP may become appropriately cell cycle-regulated, with protein expression restricted to S-phase, beginning at the fourth cell cycle.
The persistence of SLBP throughout the cell cycle allowed us to establish that it underwent an M-phase-specific phsophorylation that was reversed upon exit from M-phase. These results do not exclude that SLBP may also be phosphorylated, perhaps at different sites, at other phases of the cell cycle. M-phase phosphorylation is sensitive to roscovitine, implying that it requires the activity of cyclin-dependent kinases. The timing of SLBP phosphorylation during M-phase, as well as its rapid dephosphorylation following egg activation, closely matches the activity of cdk1 [cdc2 (Choi et al., 1991)], which suggests that this is likely the regulatory kinase. As roscovitine treatment of oocytes at mid-or late maturation led to dephosphorylation of much of the SLBP, it appears that on-going cdk1 activity during maturation is required to maintain the bulk of SLBP in its M-phase phosphorylated form.
SLBP in oocytes and at all stages of the first cell cycle was able to bind RNA containing the histone stem-loop in vitro. These results indicate that the M-phase phosphorylation of SLBP did not prevent its stem-loop binding activity. They also reveal that the stem-loop binding activity increased as cells progressed through the first cell cycle, even though the quantity of SLBP protein did not. This could mean that SLBP stem-loop binding is enhanced by co-factors whose activity increases during progression through the cell cycle, or that some of the maternal histone mRNA becomes degraded during this period of time, thus releasing SLBP that can bind to the labeled probe in vitro. It will be important to establish whether SLBP at G1, G2 and M-phase of the cell cycle is able to promote processing and translation of stem-loop mRNAs.
SLBP and histone gene expression in oocytes and embryos
Histone gene expression in mammalian oocytes and early embryos differs in several respects from proliferating somatic cells. For example, replication-dependent histone mRNAs are stable and accumulate in oocytes arrested at G2 (Wassarman and Mrozak, 1981; Graves et al., 1985; Clarke et al., 1997), whereas they are degraded at G2 in somatic cells. The distinctive expression of SLBP in oocytes and embryos suggests a potential mechanistic basis for these differences. As SLBP stabilizes histone mRNA by stimulating 3′-processing of the primary transcripts and remains associated with the histone mRNP, it is likely that the expression of SLBP in oocytes enables these cells to store replication-dependent histone mRNAs during G2 arrest.
In addition, the increase in SLBP during maturation immediately precedes the increased efficiency of histone synthesis in fertilized mouse eggs (Schultz, 1986; Wiekowski et al., 1997). SLBP is required for the translation of stem-loop histone mRNAs (Sun et al., 1992; Gallie et al., 1996), and we have recently shown that SLBP stimulates translation of histone mRNA in vivo and in vitro (Sanchez and Marzluff, 2002). The SLBP that accumulates during maturation may play a role in activating translation of histone mRNA during oocyte maturation and after fertilization, thus providing the histone proteins necessary both for repackaging the sperm chromatin and for synthesis of histone proteins for the first S-phase. The fact that SLBP in oocytes and embryos at all stages of the cell cycle can bind the histone mRNA stem-loop is consistent with such a function.
SLBP in oocytes and embryos of other species
SLBP has also been identified in oocytes and early embryos of Xenopus (Wang et al., 1999), Drosophila (Sullivan et al., 2001) and C. elegans (Kodama et al., 2002; Pettitt et al., 2002). However, cell cycle-dependent changes in expression and activity have been examined only in Xenopus oocytes and not in embryos of any of these species. Xenopus oocytes express two SLBP species (Wang et al., 1999). xSLBP1 is similar in amino acid sequence to the SLBP identified in mouse and human. It is present at high levels in G2-arrested growing oocytes but, unlike mouse SLBP, does not accumulate substantially upon entry into M-phase during meiotic maturation. xSLBP1 persists at a high level during early embryogenesis, but its expression during these cell cycles, which differ from mammalian cell cycles in that they lack gap phases, has not been examined. xSLBP2 is encoded by a separate gene and is similar to xSLBP1 only in the RNA binding domain. It also is present in G2-arrested growing oocytes, but is degraded at oocyte maturation. Histone mRNAs are mainly bound to xSLBP2 during oocyte growth, but exchange this for xSLBP1 during meiotic maturation (Wang et al., 1999). xSLBP2, although able to bind to the histone mRNA stem-loop, does not support pre-mRNA processing (Ingledue et al., 2000). It is thought be necessary for storage of the large quantity of translationally silent histone mRNAs that accumulate during oocyte growth (Wang et al., 1999).
Our results demonstrate that, in contrast to Xenopus, mouse oocytes and embryos contain only a single SLBP species. First, the amount of stem-loop binding activity, as measured by the band-shift assay, changed in parallel with the quantity of SLBP, as measured by immunoblotting, at different developmental stages. Second, at all developmental stages, all of the in vitro- binding activity was supershifted by addition of the anti-SLBP. These results imply that the cells do not contain an SLBP2-like protein. Similarly, only one SLBP is present in oocytes of Drosophila (Sullivan et al., 2001) and in the genome of Drosophila (Sullivan et al., 2001) and C. elegans (Martin et al., 1997), and a search of the human genome did not reveal any other genes with similarity to human SLBP in the RNA-binding domain. This difference between mouse and Xenopus may reflect the much greater accumulation of histone mRNAs during amphibian oogenesis and the unique mechanism for storing them in translationally inactive form.
Finally, it should be noted that the amount of SLBP in the oocyte and 1-cell embryo is much higher than the amount of SLBP in the blastocyst embryo, even though the levels of histone mRNA are similar at these two stages (Giebelhaus et al., 1983; Graves et al., 1985). There thus appears to be a large excess of SLBP in the mature oocyte and early embryo, compared with the need for SLBP for synthesis and translation of histone mRNA. This taken together with the expression of SLBP throughout the cell cycle open the possibility that SLBP may perform other functions not directly linked to histone mRNA metabolism (Abbott et al., 1999).
Acknowledgments
This work was supported by grants from the Medical Research Council and Canadian Institutes for Health Research to H.J.C., by NIH grant GM58921 to W.F.M., by the NICHD and National Institutes of Health, and through Cooperative Agreement U54HD35041 as part of the Specialized Cooperative Centers Program in Reproductive Research. P.A. and M.J.C. were supported by fellowships from the Royal Victoria Hospital Research Institute. S.S. was supported by the McGill Work-Study program.
References
- Abbott JA, Marzluff WF, Gall JG. The stem-loop binding protein (SLBP1) is present in coiled bodies of the Xenopus germinal vesicle. Mol Biol Cell. 1999;10:487–499. doi: 10.1091/mbc.10.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkoff AF, Dickson KS, Gray NK, Wickens M. Translational control of cyclin B1 mRNA during meiotic maturation: coordinated repression and cytoplasmic polyadenylation. Dev Biol. 2000;220:97–109. doi: 10.1006/dbio.2000.9613. [DOI] [PubMed] [Google Scholar]
- Bolton VN, Oades PJ, Johnson MH. The relationship between cleavage, DNA replication, and gene expression in the mouse 2- cell embryo. J Embryol Exp Morphol. 1984;79:139–163. [PubMed] [Google Scholar]
- Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34 cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–795. doi: 10.1242/dev.113.3.789. [DOI] [PubMed] [Google Scholar]
- Clarke HJ, Oblin C, Bustin M. Developmental regulation of chromatin composition during mouse embryogenesis: somatic histone H1 is first detectable at the 4-cell stage. Development. 1992;115:791–799. doi: 10.1242/dev.115.3.791. [DOI] [PubMed] [Google Scholar]
- Clarke HJ, Bustin M, Oblin C. Chromatin modifications during oogenesis in the mouse: Removal of somatic subtypes of histone H1 from oocyte chromatin occurs post-natally through a post-transcriptional mechanism. J Cell Sci. 1997;110:477–487. doi: 10.1242/jcs.110.4.477. [DOI] [PubMed] [Google Scholar]
- Culp PA, Musci TJ. c-mos and cdc2 cooperate in the translational activation of fibroblast growth factor receptor-1 during Xenopus oocyte maturation. Mol Biol Cell. 1999;10:3567–3581. doi: 10.1091/mbc.10.11.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng MQ, Shen SS. A specific inhibitor of p34 cdc2/cyclin B suppresses fertilization-induced calcium oscillations In mouse eggs. Biol Reprod. 2001;62:873–878. doi: 10.1095/biolreprod62.4.873. [DOI] [PubMed] [Google Scholar]
- Dominski Z, Zheng LX, Sanchez R, Marzluff WF. Stem-loop binding protein facilitates 3′-end formation by stabilizing U7 snRNP binding to histone pre-mRNA. Mol Cell Biol. 1999;19:3561–3570. doi: 10.1128/mcb.19.5.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominski Z, Erkmann JA, Greenland JA, Marzluff WF. Mutations in the RNA binding domain of stem-loop binding protein define separable requirements for RNA binding and for histone pre-mRNA processing. Mol Cell Biol. 2001;21:2008–2017. doi: 10.1128/MCB.21.6.2008-2017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominski Z, Erkmann JA, Yang X, Sànchez R, Marzluff WF. A novel zinc finger protein is associated with U7 snRNP amd interacts with the stem-loop binding protein in the histone pre-mRNP to stimulate 3′-end processing. Genes Dev. 2002;16:58–71. doi: 10.1101/gad.932302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen KA, Baldwin A, Sikorski EM, Hurt MM. Role for a YY1-binding element in replication-dependent mouse histone gene expression. Mol Cell Biol. 1998;18:7106–7118. doi: 10.1128/mcb.18.12.7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie DR, Lewis NJ, Marzluff WF. The histone 3′-terminal stem-loop is necessary for translation in Chinese hamster ovary cells. Nucleic Acids Res. 1996;24:1954–1962. doi: 10.1093/nar/24.10.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F, Xu W, Cooper GM, Richter JD. Translational control by cytoplasmic polyadenylation of c-mos mRNA is necessary for oocyte maturation in the mouse. EMBO J. 1994;13:5712–5719. doi: 10.1002/j.1460-2075.1994.tb06909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gick O, Krämer A, Vasserot A, Birnstiel ML. Heat-labile regulatory factor is required for 3′ processing of histone precursor mRNAs. Proc Natl Acad Sci USA. 1987;84:8937–8940. doi: 10.1073/pnas.84.24.8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebelhaus DH, Heikkila JJ, Schultz GA. Changes in the quantity of histone and actin messenger RNA during the development of preimplantation mouse embryos. Dev Biol. 1983;98:148–154. doi: 10.1016/0012-1606(83)90343-3. [DOI] [PubMed] [Google Scholar]
- Graves RA, Marzluff WF, Giebelhaus DH, Schultz GA. Quantitative and qualitative changes in histone gene expression during early mouse embryo development. Proc Natl Acad Sci USA. 1985;82:5685–5689. doi: 10.1073/pnas.82.17.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NK, Wickens M. Control of translation initiation in animals. Annu Rev Cell Dev Biol. 1998;14:399–458. doi: 10.1146/annurev.cellbio.14.1.399. [DOI] [PubMed] [Google Scholar]
- Hanson RJ, Sun J, Willis DG, Marzluff WF. Efficient extraction and partial purification of the polyribosome-associated stem-loop binding protein bound to the 3′ end of histone mRNA. Biochemistry. 1996;35:2146–2156. doi: 10.1021/bi9521856. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies: a Laboratory Manual. New York: Cold Spring Harbour Press; 1988. [Google Scholar]
- Harris ME, Bohni R, Schneiderman MH, Ramamurthy L, Schumperli D, Marzluff WF. Regulation of histone mRNA in the unperturbed cell cycle: evidence suggesting control at two posttranscriptional steps. Mol Cell Biol. 1991;11:2416–2424. doi: 10.1128/mcb.11.5.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett SK, Bolton VN. Sequence and regulation of morphological and molecular events during the first cell cycle of mouse embryogenesis. J Embryol Exp Morphol. 1985;87:175–206. [PubMed] [Google Scholar]
- Huarte J, Belin D, Vassalli A, Strickland S, Vassalli JD. Meiotic maturation of mouse oocytes triggers the translation and polyadenylation of dormant tissue-type plasminogen activator mRNA. Genes Dev. 1987;1:1201–1211. doi: 10.1101/gad.1.10.1201. [DOI] [PubMed] [Google Scholar]
- Ingledue TC, III, Dominski Z, Sanchez R, Erkmann JA, Marzluff WF. Dual role for the RNA-binding domain of Xenopus laevis SLBP1 in histone pre-mRNA processing. RNA. 2000;6:1635–1648. doi: 10.1017/s1355838200000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y, Rothman JH, Sugimoto A, Yamamoto M. The stem-loop binding protein CDL-1 is required for chromosome condensation, progression of cell death and morphogenesis in Caenorhabditis elegans. Development. 2002;129:187–196. doi: 10.1242/dev.129.1.187. [DOI] [PubMed] [Google Scholar]
- Lawitts JA, Biggers JD. Culture of preimplantation embryos. Methods Enzymol. 1993;225:153–164. doi: 10.1016/0076-6879(93)25012-q. [DOI] [PubMed] [Google Scholar]
- Martin F, Schaller A, Eglite S, Schumperli D, Muller B. The gene for histone RNA hairpin binding protein is located on human chromosome 4 and encodes a novel type of RNA binding protein. EMBO J. 1997;16:769–778. doi: 10.1093/emboj/16.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masui Y, Clarke HJ. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Meijer L, Borgne A, Mulner O, Chong JPJ, Blow JJ, Inagaki N, In-agaki M, Delcros JG, Moulinoux JP. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem. 1997;243:527–536. doi: 10.1111/j.1432-1033.1997.t01-2-00527.x. [DOI] [PubMed] [Google Scholar]
- Mohamed OA, Bustin M, Clarke HJ. High-mobility group proteins 14 and 17 maintain the timing of early embryonic development in the mouse. Dev Biol. 2001;229:237–249. doi: 10.1006/dbio.2000.9942. [DOI] [PubMed] [Google Scholar]
- Muller B, Link J, Smythe C. Assembly of U7 small nuclear ribonucleoprotein particle and histone RNA 3′ processing in Xenopus egg extracts. J Biol Chem. 2000;275:24284–24293. doi: 10.1074/jbc.M003253200. [DOI] [PubMed] [Google Scholar]
- Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. doi: 10.1242/dev.127.17.3795. [DOI] [PubMed] [Google Scholar]
- O’Keefe SJ, Wolfes H, Kiessling AA, Cooper GM. Microinjection of antisense c-mos oligonucleotides prevents meiosis II in the maturing mouse egg. Proc Natl Acad Sci USA. 1989;86:7038–7042. doi: 10.1073/pnas.86.18.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey NB, Williams AS, Sun JH, Brown VD, Bond U, Marzluff WF. Point mutations in the stem-loop at the 3′ end of mouse histone mRNA reduce expression by reducing the efficiency of 3′ end formation. Mol Cell Biol. 1994;14:1709–1720. doi: 10.1128/mcb.14.3.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules RS, Buccione R, Moschel RC, Vande Woude GF, Eppig JJ. Mouse Mos protooncogene product is present and functions during oogenesis. Proc Natl Acad Sci USA. 1989;86:5395–5399. doi: 10.1073/pnas.86.14.5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettitt J, Crombie C, Schümperli D, Müller B. The Caenorhabditis elegans histone hairpin-binding protein is required for core histone expression and is essential for embryonic and postembryonic cell division. J Cell Sci. 2002;115:857–866. doi: 10.1242/jcs.115.4.857. [DOI] [PubMed] [Google Scholar]
- Paynton BV, Rempel R, Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs durng oocyte maturation and early embryonic development in the mouse. Dev Biol. 1988;129:304–314. doi: 10.1016/0012-1606(88)90377-6. [DOI] [PubMed] [Google Scholar]
- Polanski Z, Ledan E, Brunet S, Louvet S, Verlhac MH, Kubiak J, Maro B. Cyclin synthesis controls the progression of meiotic maturation in mouse oocytes. Development. 1998;125:4989–4997. doi: 10.1242/dev.125.24.4989. [DOI] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus cdk2-cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Richter JD. Dynamics of poly(A) addition and removal during development. In: Hershey JWB, Mathews MB, Sonenberg N, editors. Translational Control. Cold Spring Harbour, New York: Cold Spring Harbor Laboratory Press; 1995. pp. 481–503. [Google Scholar]
- Sanchez R, Marzluff WF. The stem-loop binding protein is required for efficient translation of histone mRNA in vivo and in vitro. Mol Cell Biol. 2002;22:7093–7104. doi: 10.1128/MCB.22.20.7093-7104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz GA. Utilization of genetic information in the preimplantation embryo. In: Rossant J, Pederson RA, editors. Experimental Approaches to Mammalian Development. Cambridge University Press; 1986. pp. 239–265. [Google Scholar]
- Smith RK, Johnson MH. Analysis of the third and fourth cell cycles of mouse early development. J Reprod Fertil. 1986;76:393–399. doi: 10.1530/jrf.0.0760393. [DOI] [PubMed] [Google Scholar]
- Stauber C, Schümperli D. 3′ processing of pre-mRNA plays a major role in proliferation-dependent regulation of histone gene expression. Nucleic Acids Res. 1988;16:9399–9413. doi: 10.1093/nar/16.20.9399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauber C, Lüscher B, Eckner R, Lotscher E, Schümperli D. A signal regulating mouse histone H4 mRNA levels in a mammalian cell cycle mutant and sequences controlling RNA 3′ processing are both contained within the same 80-bp fragment. EMBO J. 1986;5:3297–3303. doi: 10.1002/j.1460-2075.1986.tb04643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz A, Huarte J, Gubler P, Conne B, Belin D, Vassalli JD. In vivo antisense oligodeoxynucleotide mapping reveals masked regulatory elements in an mRNA dormant in mouse oocytes. Mol Cell Biol. 1997;17:1759–1767. doi: 10.1128/mcb.17.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz A, Conne B, Huarte J, Gubler P, Volkel V, Flandin P, Vassalli JD. Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev. 1998;12:2535–2548. doi: 10.1101/gad.12.16.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan E, Santiago C, Parker ED, Dominski Z, Yang X, Lanzotti DJ, Ingledue TC, Marzluff WF, Duronio RJ. Drosophila stem loop binding protein coordinates accumulation of mature histone mRNA with cell cycle progression. Genes Dev. 2001;15:173–187. doi: 10.1101/gad.862801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Pilch DR, Marzluff WF. The histone mRNA 3′ end is required for localization of histone mRNA to polyribosomes. Nucleic Acids Res. 1992;20:6057–6066. doi: 10.1093/nar/20.22.6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay J, Hodgman R, Richter JD. The control of cyclin B1 mRNA translation during mouse oocyte maturation. Dev Biol. 2000;221:1–9. doi: 10.1006/dbio.2000.9669. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Whitfield ML, Ingledue TC, III, Dominski Z, Marzluff WF. The protein that binds the 3′ end of histone mRNA: a novel RNA-binding protein required for histone pre-mRNA processing. Genes Dev. 1996;10:3028–3040. doi: 10.1101/gad.10.23.3028. [DOI] [PubMed] [Google Scholar]
- Wang ZF, Ingledue TC, Dominski Z, Sanchez R, Marzluff WF. Two Xenopus proteins that bind the 3′ end of histone mRNA: implications for translational control of histone synthesis during oogenesis. Mol Cell Biol. 1999;19:835–845. doi: 10.1128/mcb.19.1.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman PM. The mammalian ovum. In: Knobil E, et al., editors. The Physiology of Reproduction. New York: Raven Press; 1988. pp. 69–102. [Google Scholar]
- Wassarman PM, Mrozak SC. Program of early development in the mammal: synthesis and intracellular migration of histone H4 during oogenesis n the mouse. Dev Biol. 1981;84:364–371. doi: 10.1016/0012-1606(81)90405-x. [DOI] [PubMed] [Google Scholar]
- Whitfield MW, Zheng LX, Baldwin A, Ohta T, Hurt MM, Marzluff WF. SLBP, the protein that binds the 3′ end of histone mRNA, is cell cycle regulated by both translational and post-translational mechanisms. Mol Cell Biol. 2000;20:4188–4198. doi: 10.1128/mcb.20.12.4188-4198.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiekowski M, Miranda M, Nothias JY, DePamphilis ML. Changes in histone synthesis and modification at the beginning of mouse development correlate with the establishment of chromatin mediated repression of transcription. J Cell Sci. 1997;110:1147–1158. doi: 10.1242/jcs.110.10.1147. [DOI] [PubMed] [Google Scholar]
- Williams AS, Marzluff WF. The sequence of the stem and flanking sequences at the 3′ end of histone mRNA are critical determinants for the binding of the stem-loop binding protein. Nucleic Acid Res. 1995;23:654–662. doi: 10.1093/nar/23.4.654. [DOI] [PMC free article] [PubMed] [Google Scholar]