Abstract
Aims
In the current study, we aimed to examine primary parotid squamous cell carcinoma (ParSCC) for the presence of HR-HPV and associated molecular alterations.
Methods and results
Eight cases of ParSCC were retrieved after a detailed clinicopathologic review to exclude a possibility of metastasis and/or extension from another primary site. HR-HPV status was determined based on immunohistochemistry (IHC) for p16 protein expression and by chromogenic in situ hybridization (CISH) for HR-HPV. All cases were genotyped by multiplexed mass spectrometry assay interrogating 91 hotspot mutations in 8 cancer-related genes (EGFR, KRAS, NRAS, BRAF, PIK3CA, AKT1, MEK1 and ERBB2), and studied by fluorescence in situ hybridization (FISH) for PTEN copy number alteration. Three of 8 cases (37.5%) were positive for presence of HR-HPV by CISH and p16 IHC. One of three (33%) HR-HPV-positive cases harbored PTEN hemizygous deletion, and one (33%) HR-HPV-positive case harbored PIK3CA E545K somatic mutation. No alteration of PTEN-PI3K pathway was detected in HR-HPV-negative tumors. Over a median follow-up period of 66.2 months, only the patient with HR-HPV-positive PIK3CA-mutated tumor died of his disease, while the remaining 7 patients were disease free.
Conclusions
Given the established etiologic role of HR-HPV in other head and neck squamous cell carcinoma, it is likely that HR-HPV represents an oncogenic driver in the pathogenesis of more than one third of ParSCC. Presence of HR-HPV in ParSCC may be coupled with alterations in PTEN-PI3K pathway. HR-HPV and molecular characterization of a larger number of ParSCC is needed to determine the clinical significance of these findings.
Keywords: human papillomavirus, squamous cell carcinoma, parotid gland, p16, in situ hybridization
Introduction
High risk human papillomavirus (HR-HPV) is a well-established oncogenic agent causing about 5% of human cancers worldwide, in particular the vast majority of cervical and oropharyngeal carcinomas, and a subset of anogenital squamous cell carcinoma 1–3. Over the past two decades, the prevalence of HR-HPV in oropharyngeal squamous cell carcinoma (SCC) has increased dramatically, from 21% prior to 1990 to 75% at present 3, 4. More importantly, recent research has shown that HR-HPV is an important prognostic and predictive biomarker in oropharyngeal squamous carcinoma. HPV-positive oropharyngeal SCC tends to affect relatively younger individuals that those usually affected by conventional HPV-negative SCC, and is associated with a favorable overall prognosis, excellent loco-regional control, prolonged disease specific survival, and improved response to radiation therapy 5, 6.
The clinical significance of HR-HPV in head and neck SCC outside of the oropharynx, in particular salivary glands, remains unclear. A handful of published studies have investigated the effects of HPV, including HR-HPVs, in epithelial neoplasms, benign or malignant, of salivary glands 7–15. The results were somewhat inconsistent with a documented incidence of HPV ranging from nil to 100%. In addition, the majority of prior published studies have employed polymerase chain reaction (PCR)-based assays. Despite its well-documented high sensitivity, a positive PCR result does not necessarily imply the integration of the viral oncogenes into the host DNA, which is the first initiating step leading to malignant transformation. Primary squamous cell carcinoma of the parotid gland (parSCC) is a rare and aggressive malignant epithelial tumor with a 5-year disease specific survival rate of 33% to 50% 16, 17. To date, only one published study has reported HPV positivity in a single case of squamous cell carcinoma of salivary gland, using a nested two-step-PCR assay and customized primers targeting both high risk and low risk HPVs 8.
In the present study, our aim was to examine ParSCC for the presence of HR-HPV and any associated molecular alterations. Additionally, we performed molecular and cytogenetic analyses to explore the molecular alterations of HPV-positive and negative parSCCs.
Material and Methods
Case selection and characteristics and Confirmation of HPV status using p16 immunohistochemistry and CISH
The study was approved by the institutional review board. Eight cases fulfilling the following criteria were retrieved from the pathology database: 1) patients who underwent surgical resection at Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, US) between 2000 to 2014; 2) a final pathological diagnosis of squamous cell carcinoma of the parotid gland (parSCC); 3) no documented prior history of squamous cell carcinoma in the head and neck region; 4) no squamous cell carcinoma outside of the parotid gland detected on extensive radiological, clinical, and endoscopic work-up; 5) no evidence of direct connection of the tumor to the skin on radiological studies, macroscopic and microscopic examination; and 6) an epicenter of the tumor within the parotid gland by imaging and/or macroscopic examination. All histologic and immunohistochemistry slides were reviewed by four head and neck pathologists. The pathologic and clinical stages were assigned using the American Joint Committee on Cancer (AJCC) staging manual 18.
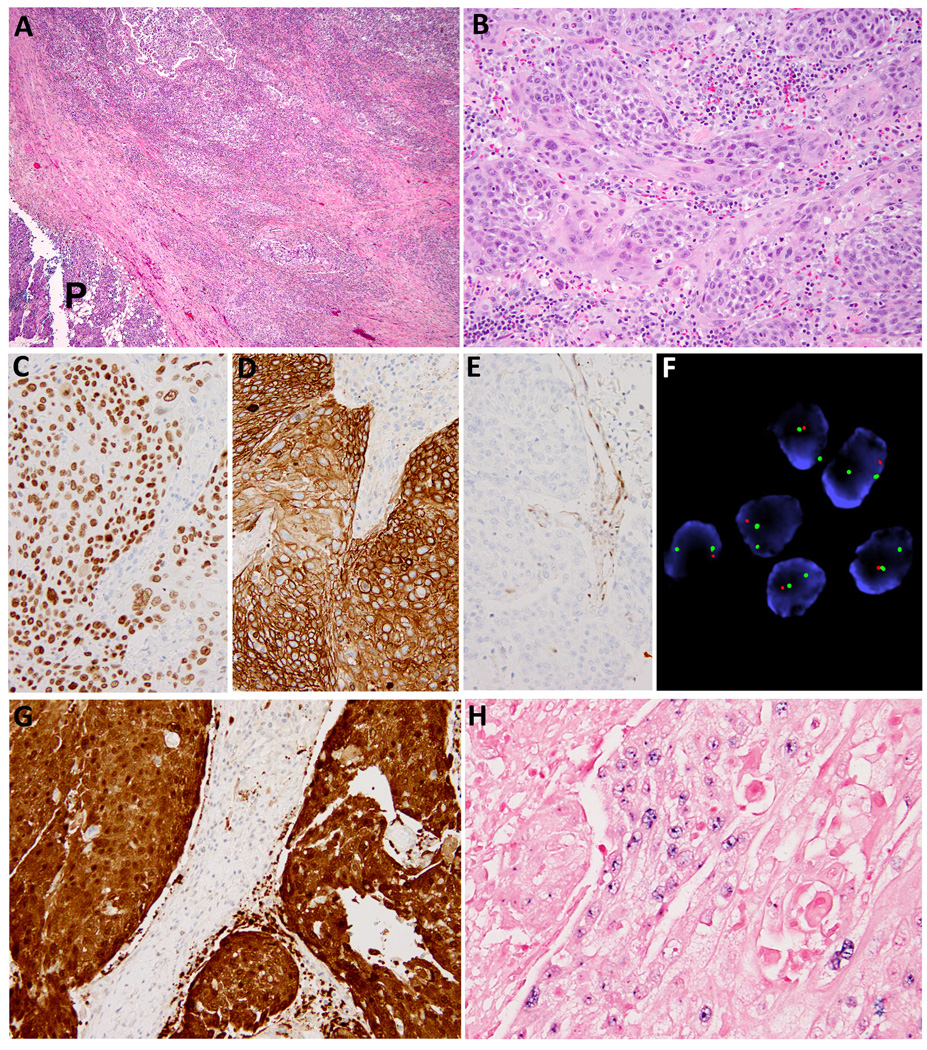
Immunohistochemistry studies were performed to confirm the presence of squamous differentiation and to exclude diagnostic mimics. Antibodies and ISH probes utilized are listed in Table 1. HPV status was confirmed using p16 immunostain and CISH against HPV types 16, 18, 31, 33, and 51. Immunopositivity for p16 was defined as a diffuse and strong cytoplasmic and nuclear stain of p16 in at least 70% of tumor cells (Figure 1G). A carcinoma was considered as positive for HR-HPV only when the integrated pattern was observed (Figure 1H). Additionally, FISH for PLAG1, MAML2 or EWSR-1 loci was performed in three individual cases to exclude diagnostic mimickers.
Table 1.
Antibodies and in situ hybridization probes utilized in the current study.
| Antibody specificity | Clone | Dilution | Source |
|---|---|---|---|
| p16 | E6H4 | RTU | Ventana Medical Systems Inc |
| p63 | 4A4 | RTU | Ventana Medical Systems Inc |
| p40 | PC373 | 1: 3000 | EMD Millipore |
| Cytokeratin 5/6 | D5/16B4 | RTU | Ventana Medical Systems Inc |
| Cytokeratin 34βE12 | 34BE12 | RTU | Ventana Medical Systems Inc |
| Calponin | EP789Y | RTU | Ventana Medical Systems Inc |
| Smooth muscle actin | asm-1 | 1:50 | Vector laboratory |
| S100 | Z0311 | 1:8000 | DAKO |
| PTEN | 6H2.1 | 1:100 | DAKO |
| Androgen receptor | AR441 | 1:200 | DAKO |
| CISH probe for high risk HPV type 16, 18, 3133, and 51 |
PATHO-GENE® | NA | ENZO Life Sciences Inc. |
| FISH probe for PTEN | Vysis® LSI PTEN/CEP 10 FISH probe |
NA | Abbott Laboratories |
RTU: ready to use
Figure 1.
Clinico-pathological features captured included: age, gender, smoking history, duration of clinical follow-up, disease status at the last follow-up, clinical staging, size of the tumor, tumor necrosis, mitotic index,, perineural invasion, lymphovascular invasion, AJCC pT and pN stages 18.
Molecular and cytogenetic analyses
All cases were genotyped using a multiplexed mass spectrometry assay for hotspot alterations (Sequenom) based on matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS). DNA from the tumor samples was used to interrogate presence of single nucleotide variation (SNV) in 91 hot-spots in 8 oncogenes: EGFR, KRAS, BRAF, PIK3CA, AKT1, NRAS, MEK1, and ERBB2 as previously described 19. Immunohistochemistry studies and FISH were performed to investigate the status of PTEN in these tumors.
Results
A total of 8 patients were identified from the MSKCC pathology database, fulfilling the inclusion criteria stated above. All eight cases harbored poorly differentiated predominantly non-keratinizing squamous cell carcinoma, among which four exhibited focal abrupt keratinization (Figure 1). All eight tumors studied were infiltrative and were devoid of lymphoid cuff. The squamous differentiation was confirmed by diffuse and strong immunopositivity for p63 (7/8, 88%), cytokeratin 34βE12 (5/5, 100%), Cytokeratin 5/6 (4/4, 100%) and/or p40 (8/8, 100%); as well as immuno-negativity for androgen receptor (0/8), calponin (0/3), smooth muscle actin (0/4), and S100 (0/4).
A positive HPV status, as confirmed using CISH positivity for HR-HPV, was identified in 3 tumors (38%). All tumors that were positive for HR-HPV on CISH analysis showed strong nuclear and cytoplasmic p16 immunostain in > 70% of cells; while only one of the five HR-HPV negative cases were positive for p16 immunostain. The demographic, clinical and pathological features of HPV-positive and HPV-negative carcinomas are summarized in Table 2. In our series, primary parSCC affected predominantly elderly male patients. The median age of presentation was 68 years and the male to female ratio was 7:1. None of the patients had an identifiable history of previous radiation to the head and neck region. Three patients were ex-smokers (10 to 70 pack-years), while the remaining were non-smokers. No noticeable difference was identified between the HPV-positive and HPV-negative carcinomas in terms of age, gender, and risk factors. HPV-positive carcinomas were associated with a higher risk of lymphovascular invasion and lymph node metastases, compared to their HPV-negative counterparts. The remaining histological features, including tumor size, mitotic index, tumor necrosis, perineural invasion, AJCC pT and pN staging, were indistinguishable between HPV-positive and negative tumors.
Table 2.
Clinicopathologic characteristics of high risk human papillomavirus (HR-HPV)-negative and positive squamous cell carcinoma in the parotid gland a.
| Overall | HR-HPV (-) | HR-HPV (+) | ||
|---|---|---|---|---|
| N | 8 | 5 | 3 | |
| Clinical characteristics | ||||
| Age, median ( range) | 68 (51-87) | 67(51 – 87) | 70 (58 – 81) | |
| Gender | ||||
| Male | 7 | 5 | 2 | |
| Female | 1 | 0 | 1 | |
| Smoking status | ||||
| Non-smoker | 5 | 3 | 2 | |
| Ex-Smoker | 3 | 2 | 1 | |
| Clinical staging | ||||
| II | 4 | 4 | 0 | |
| III | 1 | 1 | 0 | |
| IVa | 3 | 0 | 3 | |
| Follow-up period (months)a | 61 (8 – 150) | 78 (21 – 150) | 31 (8 – 69) | |
| Disease status at last F/U | ||||
| No evidence of disease | 7 | 5 | 2 | |
| Dead of disease | 1 | 0 | 1 | |
| Pathological characteristics | ||||
| Tumor Size (cm) | 3.7 (2.1 – 5.0) | 3.1 (2.1 – 4.5) | 4.7 (2.0 – 6.0) | |
| Mitotic index (/10 HPFs) | 12 (2 – 38) | 11 (2 – 38) | 13 (9 – 19) | |
| Surgical Margin Status | ||||
| Negative | 4 | 3 | 1 | |
| Close (<0.1 cm) | 1 | 1 | 0 | |
| Positive | 3 | 1 | 2 | |
| Lymphovascular invasion | ||||
| Yes | 4 | 1 | 3 | |
| No | 4 | 4 | 0 | |
| Perineural invasion | ||||
| Yes | 5 | 2 | 3 | |
| No | 3 | 3 | 0 | |
| Tumor necrosis | ||||
| Yes | 5 | 2 | 3 | |
| No | 3 | 3 | 0 | |
| AJCC pT stage | ||||
| T2 | 4 | 3 | 1 | |
| T3 | 3 | 2 | 1 | |
| T4a | 1 | 0 | 1 | |
| AJCC pN stage | ||||
| N0 | 5 | 5 | 0 | |
| N2b | 3 | 0 | 3 | |
| PIK3CA E545K mutation | ||||
| Yes | 1 | 0 | 1 | |
| No | 7 | 5 | 2 | |
| Loss of PTEN immunoexpression and PTEN deletion on FISH analysis |
||||
| Yes | 1 | 0 | 1 | |
| No | 7 | 5 | 2 | |
Values were expressed as N or mean (range).
AJCC: American Joint Committee on Cancer, FISH: fluorescence in situ hybridization, F/U: clinical follow-up, HPFs: high power fields (400X), HPV: human papilloma virus.
The median follow-up period was 61 months (range: 8 to 150 months). All patients underwent parotidectomy, selective neck dissection, and adjuvant radiation therapy at initial presentation. Seven patients were disease-free at the time of last follow-up, including 5 HPV-negative and 2 HPV-positive cases. One patient who was diagnosed with pT3 pN2b HPV-positive poorly differentiated SCC developed a neck recurrence and possible metastases in the mediastinum and lungs 5 months post-surgery and died 4 months later.
While no mutation was identified using Sequenom assay from the tumors of the 7 patients who were disease-free at last follow-up, a missense somatic mutation of PIK3CA c.1633G>A (p.E545K) on exon 9 was detected in the patient with HPV-positive parSCC who suffered disease-specific death. Additionally, one HPV-positive parSCC demonstrated complete loss of PTEN protein expression, and a somatic hemizygous PTEN deletion on FISH assay (Figure 1E and 1F). The remaining tumors did not show PTEN deletion.
Discussion
Recent epidemiological data have implicated a key pathogenesis role of HR-HPV in head and neck squamous cell carcinoma, particularly those arising within the oropharynx 2, 20. In addition, HR-HPV has emerged as a prognostic and predictive biomarker in oropharyngeal SCC. A positive HR-HPV status has been associated with a favorable loco-regional control, overall survival, and disease specific survival, as well as an improved response to radiation therapy 5, 6. The roles of HR-HPV in head and neck cancer outside of the oropharynx, in particular within the salivary glands, remain unclear. Emerging yet scanty and controversial evidence has been published demonstrating the presence of HPV in certain but not all types of salivary gland epithelial neoplasms with a reported incidence ranging widely from nil to 100% (reviewed and summarized in Table 3) 7–15. Since the parotid gland directly communicates with the oral cavity through Stensen’s duct, it is possible that HR-HPV could theoretically gain access to the parotid gland via retrograde transportation. Using ISH or PCR techniques, HR-HPV positivity was detected in a variety of benign and malignant salivary gland epithelial lesions, including pleomorphic adenoma, oncocytoma, Warthin’s tumor, squamous cell carcinoma, acinic cell carcinoma, adenoid cystic carcinoma, adenocarcinoma, and mucoepidermoid carcinoma (Table 3) 7–15. However, most of these studies included only a limited number of cases, and the documented frequency of any particular entity was not always consistent across studies. For example, Vagali et al. (2007) 9 and Teymoortash et al. (2013) 14 showed that HPV DNA was absent in Warthin’s tumor, a benign salivary epithelial neoplasm. In contrast, Teng et al. (2014) 15 detected the presence of HR-HPVs, including HPV 16 and 18, in 4 of 12 (33%) Warthin’s tumors. Similarly, the reported incidence of HR-HPV in mucoepidermoid carcinoma, the most common malignant salivary gland epithelial tumor, varied from nil 7, 11 to 47 – 100% 12, 15. With regard to squamous cell carcinoma of the parotid gland, the only case with documented HPV status has been reported by Fischer and Von Winterfeld (2003) 8 who detected the presence of HPV in a single case of squamous cell carcinoma of the parotid gland. The authors employed a nested two step-PCR assay with customized primers targeting HPV 6, 11, 13, 16, 31 and 33. Thus, it was unclear whether this specific tumor harbored HR or low risk-HPV.
Table 3.
Reported frequency of HPV in salivary gland epithelial neoplasms in English literature
| Ref. | Tumor tested (N) | HPV detection methods primers/probe) |
HPV N (%) |
HR- HPV N (%) |
HPV16 N (%) |
HPV18 N (%) |
|---|---|---|---|---|---|---|
| Atula, 1998 [7] |
N = 34 PA (19) ACC (4) SCC (3) MEC (3) Adenocarcinoma (2) AdCC (1) EMC (1) MC (1) |
PCR (GP5+/GP6+) | 0 | N/A | N/A | N/A |
| Fischer, 2003 [8] |
SCC of parotid (1) | Nested PCR (customized primers) |
1 (100%) |
N/A | N/A | N/A |
| Vageli, 2007 [9] |
N = 8 Oncocytoma (1) ACC (1) Warthin’s tumor (1) Adenocarcinoma HG (1) PA (2) Lymphoepithelial cyst (1) PLGA (1) |
Solution PCR (GP5+/GP6+) Multiplex qRT-PCR (E6/E7 of HPV16, 18, 31, 33, 35, 52, 58 and 67) In situ PCR |
6 (75%) | 6 (75%) |
5 (63%) | 4 (50%) |
| Boland, 2012 [10] |
AdCC (n = 25) | ISH for LR- and HR- HPV |
2 (8%) | 2 (8%) | N/A | N/A |
| Descamps, 2012 [11] |
N = 79 PA (40) AdCC (15) MEC (9) CAexPA (9) ACC (6) |
PCR (GP5+/GP6+) qRT-PCR (type specific sequences) |
4 (5%) PA (3) ACC (1) |
2 (3%) PA (1) ACC (1) |
2 (3%) | 0 |
| Isayeva, 2012 [12] |
MEC (89) | Nested RT-PCR (primers N/A) IF (C1P5 antibody) |
N/A | 42 (47%) |
N/A | N/A |
| Seethala 2012 [13] |
Lymphadenoma (7) | ISH (wide spectrum HPV-DNA probe) |
0 | N/A | N/A | N/A |
| Teymoortash, 2013 [14] |
Warthin’s tumor (40) | ISH for HR-HPV PCR (GP5+/GP6+ and E1 gene regions) |
13 (33%) episomal by FISH 0 by PCR |
N/A | N/A | N/A |
| Teng, 2014 [15] |
N = 59 PA (36) Adenoma (3) Warthin’s tumor (12) Myoepithelioma (1) Adenocarcinoma (3) AdCC (1) MEC (1) Others (2) |
PCR (37 LR and HR HPV type-specific probes) |
34 (58%) |
N/A | 11 (19%) |
14 (24%) |
ACC: acinic cell carcinoma, AdCC: adenoid cystic carcinoma, CAexPA: carcinoma ex-pleomorphic adenoma, EMC: epithelial myoepithelial carcinoma, HPV: human papillomavirus, HR-HPV: high risk-human papillomavirus, IF: immunofluorescence, ISH: in situ hybridization, LR-HPV: low risk-human papillomavirus, MC: myoepithelial carcinoma, MEC: myoepithelial carcinoma, N/A: not available, PA: pleomorphic adenoma, PCR: polymerase chain reaction, PLGA: polymorphous low-grade adenocarcinoma, qRT-PCR: quantitative real-time PCR, Ref. References, RT-PCR: reverse transcriptase PCR, SCC: squamous cell carcinoma.
The marked variation in the reported frequency of HPV in salivary gland tumors might be due to the small number of cases included in these studies, but could also be attributed to the different detection methods used. Although HPV infection is nearly ubiquitous in humans, the vast majority are transient and not carcinogenic. Thus, the ultimate goal of HPV detection is to recognize oncogenic HR-HPV infection. PCR-based assays, in particular nested PCR, are extremely sensitive. Therefore, a positive PCR result using GP5+/6+ primers detects the presence of the virus genome, even at a very low copy number, and may not represent clinically relevant HPV infection. Indeed, HPV is only implicated in tumorigenesis when E6/E7 mRNA is transcriptionally activated, while a significant proportion (14–50%) of carcinomas with detected HR-HPV genomic DNA using PCR do not contain transcribed E6/E7 mRNA 1, 21–23. Moreover, PCR techniques do not distinguish between episomal and integrated HPV DNA, or between non-neoplastic and neoplastic tissue, leading to a further increase in the detection of clinically insignificant HPV infection. In situ hybridization (ISH), on the other hand, is a highly specific method allowing reliable topographical visualization of HPV within the nuclei of tumor cells. Additionally, the ISH technique allows discrimination between the integrated and the episomal state of HPV. Compared with the diffuse nuclear staining for episomal virus, a punctuated nuclear signal indicates integration of the viral DNA into the host DNA, which would then trigger carcinogenesis in the host cell 1, 21–23. P16 protein overexpression, detected by immunohistochemistry, is an indirect consequence of E7 oncogene transcription, and can serve as a cost-effective surrogate marker with 94–100% sensitivity and 79 – 82% of specificity for tumorigenic HR-HPV infection 1, 21–23. The majority of previously published studies have adopted PCR-based assays 7–9, 11, 12, 14, 15. Hence, the detection of HPV in these studies did not necessarily indicate a pathogenic role of HR-HPV in these tumors. Only three groups have studied the status of HPV in salivary gland tumor using ISH techniques 10, 13, 14. While Seethala et al. (2012) 13 did not detect HR-HPV in 7 cases of lymphadenoma Boland et al. (2012) 10 identified HR-HPV in 2 of 25 (8%) cases of adenoid cystic carcinoma; and Teymoortash et al. (2013) 14 reported HR-HPV positivity in 13 of 40 (33%) Warthin’s tumors. Interestingly, the ISH staining pattern in HPV-positive Warthin’s tumors was episomal 14, suggesting that the HR-HPV may not be pathogenic in Warthin’s tumor. The current study is the first study showing that some primary SCCs of the parotid gland (38%) may be driven by HR-HPV as demonstrated by positive CISH studies and confirmed by immunohistochemistry for p16. One potential weakness of the present study was that the CISH probe utilized only detected the most common HR-HPV types, namely 16, 18, 31, 33, and 51, but not other rare types of HR-HPVs. The current study is also the only study with a long-term clinical follow-up allowing evaluation of the clinical relevance and prognostic value of HR-HPV in these tumors. We intentionally excluded cases from our consult service, and included only those patients who received surgical resection and clinical follow-up at our center. This approach minimized selection bias, ensured comparable and consistent results of HPV detection, and allowed collection of reliable long term clinical data. Unlike SCC of the oropharynx which is associated with an improved clinical outcome 2, 20, our study showed that HPV positivity in SCC of the parotid gland had no significant impact on clinical and pathological staging and prognosis. However, the small number of cases is a limiting factor, and additional larger scale studies are needed.
The facts that HR-HPV related parotid squamous cell carcinomas was associated with a propensity for lymphovascular invasion and metastases to neck lymph nodes may raise concerns that these cases are parotid metastases rather than primary squamous cell carcinoma of the parotid gland. However, this possibility was excluded on the basis of an extensive clinico-radiological examination and/or multiple benign biopsies of the oropharynx. Additionally, two of three patients with HR HPV-related ParSCC showed no evidence of disease 23 and 66 months after the initial resection, which also argues against the metastatic nature of their disease.
Recent genomic evidence has shown that alteration in PTEN-PIK3CA-AKT-mTOR pathway is the most frequent genomic alteration detected in 48 to 53% of HPV-positive head and neck squamous cell carcinomas 24–28. Such alterations include PTEN inactivation by mutation and deletion, and PIK3CA mutation or amplification. Using Sanger sequencing, Chiosea et al. (2013) 24 have detected PIK3CA alteration in 31% of oropharyngeal SCC, the most common one being p.E545K missense mutation. Similarly, using massive parallel high throughout DNA sequencing, three recent studies have shown that PIK3CA and PTEN alterations are present in 37% and 11% of HPV-positive head and neck squamous cell carcinomas, respectively 26, 27, 29. Consistent with what has been reported in the literature, we identified PIK3CA E545K mutation (1/3, 33%) and PTEN hemizygous deletion (1/3, 33%) in HPV-positive parSCCs. Although previous studies reported that the presence of PIK3CA alteration did not alter clinical prognosis of head and neck squamous cell carcinoma 24, the patient with HPV-associated SCC harboring PIK3CA somatic mutation in our series was the only patient who suffered adverse outcomes, including distant recurrence and ultimately disease specific death. As SCC is a rare malignancy of salivary gland, future collaborative studies across different tertiary centers are needed to collect sufficient evidence in order to clarify the prognostic value of PIK3CA somatic mutation in HPV-positive and HPV-negative SCCs of parotid gland.
Conclusions
To the best of our knowledge, our study is the first to report the presence of high risk human papillomavirus in a proportion of primary squamous cell carcinoma of the parotid gland suggesting that HPV may contribute to the pathogenesis of parSCCs. PTEN hemizygous deletion and PIK3CA somatic mutation were detected in HPV-positive primary SCCs, suggesting that alteration in PTEN-PIK3CA-AKT-mTOR pathway may play a role in HPV-related parSCC. Unlike oropharyngeal squamous cell carcinoma in which HPV is associated with an improved survival, HPV in parotid SCC may not correlate with better outcome. However, future studies with larger number of cases are needed to confirm such findings.
Acknowledgments
Grant numbers and sources of support: Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure/conflict of interest: No competing financial interests exist for all contributory authors.
Author contributions:
Bin Xu: Organized the database, reviewed the immunohistochemistry and histologic slides, and drafted the manuscript.
Lu Wang: Performed the fluorescence in situ hybridization studies.
Laetitia Borsu: Performed the sequenom (mass spectrum) studies.
Ronald Ghossein: Reviewed the pathology, and provided feedbacks on the study design and manuscript.
Nora Katabi: Reviewed the pathology, and provided feedbacks on the study design and manuscript.
Ian Ganly: Provide clinical aspect of the study and feedbacks on the manuscript.
Snjezana Dogan: Designed the study, reviewed all pathology and molecular aspects of the study, and finalized the manuscript.
References
- 1.Groves IJ, Coleman N. Pathogenesis of human papillomavirus-associated mucosal disease. The Journal of pathology. 2015;235:527–538. doi: 10.1002/path.4496. [DOI] [PubMed] [Google Scholar]
- 2.Woods R, Sr., O'Regan EM, Kennedy S, Martin C, O'Leary JJ, Timon C. Role of human papillomavirus in oropharyngeal squamous cell carcinoma: A review. World journal of clinical cases. 2014;2:172–193. doi: 10.12998/wjcc.v2.i6.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stein AP, Saha S, Yu M, Kimple RJ, Lambert PF. Prevalence of human papillomavirus in oropharyngeal squamous cell carcinoma in the united states across time. Chemical research in toxicology. 2014;27:462–469. doi: 10.1021/tx500034c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young D, Xiao CC, Murphy B, Moore M, Fakhry C, Day TA. Increase in head and neck cancer in younger patients due to human papillomavirus (hpv) Oral oncology. 2015 doi: 10.1016/j.oraloncology.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. European journal of cancer. 2014;50:2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Petrelli F, Sarti E, Barni S. Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: An updated systematic review and meta-analysis of 30 trials. Head & neck. 2014;36:750–759. doi: 10.1002/hed.23351. [DOI] [PubMed] [Google Scholar]
- 7.Atula T, Grenman R, Klemi P, Syrjanen S. Human papillomavirus, epstein-barr virus, human herpesvirus 8 and human cytomegalovirus involvement in salivary gland tumours. Oral oncology. 1998;34:391–395. doi: 10.1016/s1368-8375(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 8.Fischer M, von Winterfeld F. Evaluation and application of a broad-spectrum polymerase chain reaction assay for human papillomaviruses in the screening of squamous cell tumours of the head and neck. Acta oto-laryngologica. 2003;123:752–758. doi: 10.1080/00016480310001420. [DOI] [PubMed] [Google Scholar]
- 9.Vageli D, Sourvinos G, Ioannou M, Koukoulis GK, Spandidos DA. High-risk human papillomavirus (hpv) in parotid lesions. The International journal of biological markers. 2007;22:239–244. doi: 10.1177/172460080702200401. [DOI] [PubMed] [Google Scholar]
- 10.Boland JM, McPhail ED, Garcia JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 11.Descamps G, Duray A, Rodriguez A, et al. Detection and quantification of human papillomavirus in benign and malignant parotid lesions. Anticancer research. 2012;32:3929–3932. [PubMed] [Google Scholar]
- 12.Isayeva T, Li Y, Maswahu D, Brandwein-Gensler M. Human papillomavirus in non-oropharyngeal head and neck cancers: A systematic literature review. Head and neck pathology. 2012;6(Suppl 1):S104–S120. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seethala RR, Thompson LD, Gnepp DR, et al. Lymphadenoma of the salivary gland: Clinicopathological and immunohistochemical analysis of 33 tumors. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2012;25:26–35. doi: 10.1038/modpathol.2011.135. [DOI] [PubMed] [Google Scholar]
- 14.Teymoortash A, Bohne F, Jonsdottir T, et al. Human papilloma virus (hpv) is not implicated in the etiology of warthin's tumor of the parotid gland. Acta oto-laryngologica. 2013;133:972–976. doi: 10.3109/00016489.2013.797603. [DOI] [PubMed] [Google Scholar]
- 15.Teng WQ, Chen XP, Xue XC, et al. Distribution of 37 human papillomavirus types in parotid gland tumor tissues. Oncology letters. 2014;7:834–838. doi: 10.3892/ol.2013.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Kim GE, Park CS, et al. Primary squamous cell carcinoma of the parotid gland. American journal of otolaryngology. 2001;22:400–406. doi: 10.1053/ajot.2001.28068. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, Li H, Yang Z, Chen W, Zhang Q. Outcomes of primary squamous cell carcinoma of major salivary glands treated by surgery with or without postoperative radiotherapy. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 2015 doi: 10.1016/j.joms.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. Ajcc cancer staging manual. Springer; 2009. [Google Scholar]
- 19.Arcila M, Lau C, Nafa K, Ladanyi M. Detection of kras and braf mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-pcr sequencing and broad-spectrum mass spectrometry genotyping. The Journal of molecular diagnostics : JMD. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Mofty SK. Hpv-related squamous cell carcinoma variants in the head and neck. Head and neck pathology. 2012;6(Suppl 1):S55–S62. doi: 10.1007/s12105-012-0363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirghani H, Amen F, Moreau F, et al. Human papilloma virus testing in oropharyngeal squamous cell carcinoma: What the clinician should know. Oral oncology. 2014;50:1–9. doi: 10.1016/j.oraloncology.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Mirghani H, Amen F, Moreau F, Lacau St Guily J. Do high-risk human papillomaviruses cause oral cavity squamous cell carcinoma? Oral oncology. 2014 doi: 10.1016/j.oraloncology.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Venuti A, Paolini F. Hpv detection methods in head and neck cancer. Head and neck pathology. 2012;6(Suppl 1):S63–S74. doi: 10.1007/s12105-012-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiosea SI, Grandis JR, Lui VW, et al. Pik3ca, hras and pten in human papillomavirus positive oropharyngeal squamous cell carcinoma. BMC cancer. 2013;13:602. doi: 10.1186/1471-2407-13-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chun SH, Jung CK, Won HS, Kang JH, Kim YS, Kim MS. Divergence of p53, pten, pi3k, akt and mtor expression in tonsillar cancer. Head & neck. 2014 doi: 10.1002/hed.23643. [DOI] [PubMed] [Google Scholar]
- 26.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung CH, Guthrie VB, Masica DL, et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann Oncol. 2015;26:1216–1223. doi: 10.1093/annonc/mdv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lechner M, Frampton GM, Fenton T, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in hpv+ and hpv− tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seiwert TY, Zuo Z, Keck MK, et al. Integrative and comparative genomic analysis of hpv-positive and hpv-negative head and neck squamous cell carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]