Abstract
Objective
To determine the longitudinal changes in functional outcome and compare ordinal outcome scale assessments in comatose cardiac arrest survivors.
Design
Prospective observational study of comatose cardiac arrest survivors. Subjects who survived to one-month were included.
Setting
Academic medical center ICU.
Patients
98 consecutive patients who remained comatose after resuscitation from cardiac arrest; 45 patients survived to one month.
Interventions
None
Measurements and Main Results
Patients’ functional neurologic outcomes were assessed by phone call or in-person clinic visit at 1, 3, 6, and 12 months post cardiac arrest using the modified Rankin Scale (mRS), Glasgow Outcome Scale (GOS), and Barthel Index (BI). A “good” outcome was defined as mRS 0–3, BI 70–100, and GOS 4–5. Changes in dichotomized outcomes and shifts on each outcome scale were analyzed. The mean age of survivors was 51±19 years and 18 (40%) were female. Five (19%) out of 26 patients with data available at all time-points improved to good mRS outcome and none worsened to poor outcome between post-arrest months 1 and 6 (p=0.06).Thirteen (50%) patients improved on the mRS scale by 1–3 points and 4 (15%) worsened by 1–2 points between months 1 and 6 (overall improvement by 0.5 points (95% CI 0–1), p=0.04). From post-arrest month 6 to 12, there was no change in the number of patients with good vs. poor outcomes. The mRS and BI were more sensitive to detecting changes in outcome than the GOS.
Conclusions
In initially comatose cardiac arrest survivors, improvements in functional status occur over the first six months after the event. There was no significant change in outcome between post-arrest months 6 to 12. The mRS is a sensitive outcome scale in this population.
Keywords: Outcomes, cardiac arrest, neurologic injury, post-resuscitation care, coma
Introduction
Over 600,000 patients suffer a cardiac arrest annually in the United States.1–3 Current survival rates with the use of therapeutic hypothermia vary from 30% to 60%, though even survivors classified as having “good” neurologic outcomes often experience significant long-term cognitive deficits or “post-resuscitation encephalopathy.”4–10 In the era of therapeutic hypothermia and targeted temperature management for comatose cardiac arrest survivors, increasing numbers of patients who remain comatose after resuscitation go on to have favorable neurologic outcomes.11–13
Given the historically grim prognosis of cardiac arrest, early resuscitation research used mortality and surrogate physiologic measures to define post-cardiac arrest outcomes.14, 15 As resuscitation and the management of post-cardiac arrest syndrome improved, the need for patient-focused outcomes that better assess neurologic function has developed. The optimal method and timing of neurological outcome assessment has not been established.14 The American Heart Association consensus statement recommends that a 90 day outcome be used “coupled with neurocognitive and quality-of-life assessments.” 14 The Cerebral Performance Category (CPC) or modified Rankin Scale (mRS) is suggested as a global outcome assessment of neurological function, though the authors acknowledge a significant lack of evidence to support a single scale or timepoint.14 Additionally, there is limited data characterizing the natural history of neurologic recovery after cardiac arrest, as most studies lack serial follow-up. One study showed that Mini-Mental State Examination scores improved initially after cardiac arrest but did not significantly change between 3 months and 1 year post-arrest.7 However, like much of the previous research in longer-term functional outcome after cardiac arrest, this work was performed prior to the era of therapeutic hypothermia.
Determining longitudinal changes in functional status in post-cardiac arrest survivors can provide valuable clinical information for care providers, patients and family members, and comparing three ordinal outcome scales used longitudinally at different time-points may help define and standardize research outcomes. Therefore, we sought to describe the functional neurologic outcome as measured by three performance scales over a 12 month period in patients who were initially comatose after resuscitation from cardiac arrest. The objective of the current study was to determine longitudinal changes in patients’ functional outcomes at 1, 3, 6, and 12 months after cardiac arrest and to compare performance of three functional outcomes scales (modified Rankin Scale (mRS), Barthel Index (BI), and Glasgow Outcome Score (GOS)).
Materials and Methods
This is a single-center prospective observational study of functional outcomes in patients who initially remained comatose following resuscitation from cardiac arrest.
Subjects
Consecutive comatose post-cardiac arrest patients were prospectively enrolled. Adult patients who remained comatose after initial resuscitation for cardiac arrest were eligible if they met the following inclusion criteria: men and non-pregnant women at least 18 years old, resuscitation for primary and secondary cardiac arrest, and persistent coma defined as no eye opening to voice and inability to follow commands after return of perfusing cardiac rhythm. Patients who regained consciousness following return of spontaneous circulation (ROSC) were not included. Exclusion criteria were: pre-existing “do not resuscitate” status, pre-arrest modified Rankin scale of ≥3, receiving investigational drug or procedures, severe co-existing systemic disease limiting life expectancy, and brain death. The study was approved by the institutional review board and written informed consent was obtained from a legally authorized representative. The patients also gave written informed consent if they regained consciousness and sufficient cognitive status to allow for the informed consent process.
Clinical Care
All patients who remain comatose after resuscitation from cardiac arrest are co-managed by the neurocritical care team who work closely with the primary teams to provide post-resuscitation care. If patients met criteria for therapeutic hypothermia, they were cooled to a target temperature of 33±0.5 °C for 24 hours and then underwent controlled re-warming. Optimization of hemodynamics and work-up and treatment of an underlying cause of the arrest were performed per institutional protocol. Decisions regarding limitations of life-sustaining treatment were at the discretion of the treating team in conjunction with authorized patient representative and guided by the following framework: Decisions to limit life-sustaining treatment based on multi-organ failure, perceived poor prognosis from a non-neurologic standpoint, or patient’s/family’s wishes were accepted at any time point post-arrest. Decisions to limit maximal care based on perceived neurological prognosis were guided by an algorithm in which maximal care was continued for 72 hours post-arrest. After therapeutic hypothermia was completed, sedation was minimized to ensure patient comfort but preserve neurologic assessments as much as possible. If patients met historical predictors of poor prognosis at 72 hours post-arrest and after at least 24 hours of normothermia, then the team talked with family about likely poor prognosis and discussed options for limitations of care. Historical predictors of poor neurologic prognosis were considered any of the following: a motor score on the Glasgow Coma Scale ≤2, no pupillary reflexes, no corneal reflexes, burst suppression or electrocerebral silence on EEG in the absence of sedating medication, and absent N20 cortical response on somatosensory evoked potentials. If these findings were not present, then care was recommended to continue maximally until re-assessment at post-arrest day 7.
After the initial hospitalization, clinical care and decisions about changing overall goals of care was left to the discretion of the clinical treating team. The cause of death was recorded for all patients.
Outcome Assessment
Functional outcomes were measured by mRS, BI, and GOS, obtained via a structured telephone interview at 1, 3, and 12 months and an in-person clinic follow-up at 6 months. Patients who were unable to come to clinic at 6 months were assessed with structured telephone interviews. The outcomes scales were performed by a physician or research coordinator blinded to the clinical data and certified in the administration of these assessments. Outcomes were dichotomized to good vs. poor, and good outcomes were pre-defined as mRS 0–3, GOS 4–5 or Barthel Index of 70–100. Because dichotomized outcomes may not capture the clinical benefit associated with a shift of at least one grade on the mRS,16 we also determined the likelihood of transitioning between grades on the mRS between time-points (so called “shift analysis”)17–19.
The modified Rankin Scale is a seven point scale ranging from 0–6, in which a patient with a score of 0 has no residual symptoms and is able to carry out daily life activities independently while a 6 represents death.20–22 The Barthel Index is a scale that ranges from 0–100 with ten categories that assess independence in activities of daily living: feeding, bathing, grooming, dressing, bladder, toilet use, moving from bed to chair, ability to walk.22–24 The GOS is a five point scale that ranges from 1 (death) to 5 (good recovery, able to return to normal activities).25
Patients who were alive at 1-month post-arrest were included in this analysis. Patients with incomplete data sets had data carried backwards and forwards for sensitivity analyses, and these results are reported separately. For the patients to be included in the analyses, outcomes had to be assessed at least at one time-point. No patients were completely lost to follow-up.
Statistical analysis
Changes in the dichotomized outcomes (good vs. poor) over time were analyzed with McNemar test. For the full range of each outcome scale, we estimated magnitude of the shifts in the patient outcome scores between assessment time-points using Hodges-Lehmann estimates for the median differences with 95% confidence intervals and then assessed them for significance using Wilcoxon Signed Rank test (for 2 time-points) or Friedman test (for multiple time-points). We also estimated the general odds ratio (ORG, a generalization of the odds ratio for ordinal data) for improvement vs. worsening by at least 1 point between assessments. Cases without change in the score were accounted as ties. Asymptotic 95% CI was estimated using logarithmic transformation to improve the normal approximation to the statistic ORG.26, 27 A p<0.05 was defined as significant. IBM SPSS Statistics version 22 software was used for all analyses.
Results
One hundred patients were enrolled between 2008 and 2014. One patient was ultimately determined to have been unlikely to have had a cardiac arrest and another patient withdrew from the study, leaving 98 patients included in the final analysis. The overall mortality rate for the entire duration of the one-year study was 59%. Fifty-three (54%) of the patients died during the initial hospitalization, and 4 survived to hospital discharge but died between 1 and 12 months post-arrest.
Of 45 patients who were alive at one month post-arrest, 26 patients (58%) completed follow-up at all time-points (1,3, 6, and 12 months). 19 patients had incomplete follow-up data: 38 (84%) were assessed at one month, 38 (84%) were assessed at 3 months, 40 (89%) were assessed at 6 months, and 35 (78%) were assessed at 12 months. Thirty-five patients had adequate data for a “last observation carried backwards” analysis and 43 patients had enough data for a “last observation carried forward” analysis.
The mean age was 51±19 years and 18 (40%) were female. Thirty-one patients (31/45, 69%) had out-of-hospital hospital cardiac arrest, and ventricular fibrillation was the most common underlying rhythm (N= 20, 44%). Thirty-eight (84%) were treated with therapeutic hypothermia. Seven patients did not undergo hypothermia due to developing clinical responsiveness despite early coma (n=3), refractory ventricular arrhythmia (n=1), severe coagulopathy (n=1), and hemodynamic instability (n=2). Additional patient characteristics are reported in Table 1.
Table 1.
Demographic and clinical factors
| Demographics | n= 45 | |
|---|---|---|
| Gender (female) n (%) | 18 (40) | |
| Age, mean±SD | 51±19 | |
| Race n (%) | ||
| White | 33 (73) | |
| Black | 5 (11) | |
| Asian | 5 (11) | |
|
Native Hawaiian/Pacific Islander |
2 (4) | |
| Ethnicity (Hispanic) n (%) | 10 (22) | |
|
Historic Rankin, median (IQR) |
1 (0–2) | |
| Cardiac Arrest Details | ||
| OHCA | 31 (69) | |
| Therapeutic Hypothermia | 38 (84) | |
| Type of Cardiac Arrest | ||
| V-Fib | 20 (44) | |
| V-tach | 1 (2) | |
| PEA | 16 (36) | |
| Asystole | 4 (9) | |
| Other | 4 (9) | |
| Coma Duration >3 days n(%) | 14 (31) | |
| ROSC (min) | 22±15 | |
OHCA = Out-of-hospital cardiac arrest; ROSC = Return of spontaneous circulation
mRS
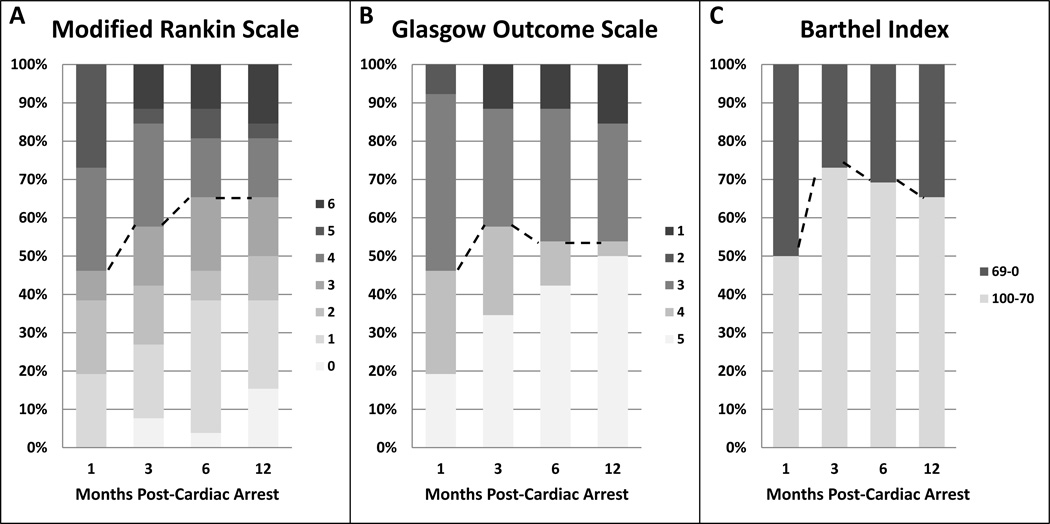
In the dichotomized analysis, 12 (46%) of the 26 patients with data available from all time-points had a good functional outcome (mRS 0–3) at one month. There were no statistically significant differences in dichotomized outcome between month 1–3 and month 3–6. However, between 1 month and 6 months, five (36%, 95% CI 16–61%) patients improved sufficiently to be re-classified from poor to good functional outcome leading to an increase in the percentage of patients with good functional outcome from 46% to 65% (strong trend for significance with p=0.063). While four patients worsened (15%, 95% CI 6–34%) on the overall mRS between post-arrest months 1 and 6, the worsening did not result in re-classified into a different primary outcome group (0%, 95% CI 0–24%). Three of the four patients with worsening on the mRS died, going from a mRS of 5 to 6 (n=2) or mRS of 4 to 6 (n=1). Causes of death for the three patients who died between post-arrest month 1 and 6 were non-neurologic: acute renal failure, acute respiratory failure and acute systolic heart failure. From month 6 to 12, there was no change in the number of patients with good vs. poor outcomes: one patient improved from poor to good outcome and one patients worsened from good to poor outcome, leaving overall 17 patients (65%) remaining in the good outcome category (p=1.0).
On the full mRS scale, shift analysis showed thirteen (50%) patients improved between post arrest month 1 to 6 (Figure 1). 10 patients improved by one point, 2 patients improved by 2 points, and 1 patient improved by 3 points (Table 2). Overall, there was a median 0.5 (95% CI 0–1) shift in mRS toward improvement between months 1 and 6 (p=0.04), with ORG for improvement by at least 1 point was 2.06 (95% CI 0.91–4.67). Between months 6 to 12 post-arrest, individual patients continued to improve on the full mRS scale. 5 out of 23 alive patients at 6 months post-arrest improved further by month 12 (22%, 95% CI 10–42%): 3 patients with a score of 1 improved to 0, 1 patient improved from mRS of 3 to 1, and 1 patient improved from 4 to 3. In the same time period (between 6–12 months post-arrest), 3 patients (13%, 95% CI 5–32%) worsened on the full scale: one patient worsened from 1 to 2, one from 3 to 4, and one patient with a mRS of 5 at 6 months had died by 12 months. There was no overall shift in the mRS scores between 6–12 months: median (95% CI) difference of 0 (0–0.5), p=0.366; ORG for improvement by at least 1 point was 1.17 (95% CI 0.54–2.52).
Figure 1. Change in outcome distribution over 12 months.
A) Modified Rankin Scale B) Glasgow Outcome Scale C) Barthel Index. n=26
Table 2.
Individual patient changes in mRS between post-arrest month 1 and 6
| mRS at 6 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | Total | ||
| mRS at 1 month | 1 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 5 |
| 2 | 0 | 4 | 0 | 1 | 0 | 0 | 0 | 5 | |
| 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | |
| 4 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 7 | |
| 5 | 0 | 0 | 0 | 1 | 2 | 2 | 2 | 7 | |
| Total | 1 | 9 | 2 | 5 | 4 | 2 | 3 | 26 | |
Changes in mRS between post-arrest month 1 and 6. Numbers to the left of the shaded boxes denote number of patients who improved, while numbers to the right of the shaded boxes denote number of patients who worsened.
When data were not available for all follow-up time-points, but were available for at least one time-point, data were carried forward or backward. Using the inferred data, the overall results were similar to the results from patients with a complete data set. On the dichotomized scale, the proportion of patients with good outcome again did not change significantly between 1 and 3 months (51% to 63%, p=0.063, carried forward data; 57% to 66%, p=0.250, carried backward data) but significantly increased from 1 to 6 months in the carried forward/backward datasets: from 57% to 71% (p=0.063) when carried backward; and from 51% to 72% (p=0.004) when carried forward. Meanwhile, the proportion of patients with good outcome remained unchanged from 6 to 12 months in both datasets (p=1.0). There was overall improvement on mRS scale between 1–6 months by 0.5 (0–1.0) (median (95% CI)) (p=0.012) for carried backward and 0.5 (0–1) (p=0.008) for carried forward analyses. There was still no change between 1–3 months (p=0.262 carried forward, p=0.084 carried backward) and 6–12 months (p=0.366 carried backward and carried forward).
GOS
When GOS outcomes were assessed in dichotomized analyses, there was no difference in the proportion of patients with good and poor outcomes at 1, 3, 6, and 12 months (46%, 58%, 54%, and 54% respectively with good outcome) (Figure 1). Similarly, there was no significant difference in shift in the GOS scores between all 4 time-points, p=0.44. At an individual level, between months 1 and 3, 3 patients worsened and 6 patients improved (median shift 0, 95%CI 0–0.5). Between months 1 and 6, 8 patients (31%, 95% CI 17–50%) had improvements in their GOS, and 4 patients ((15%, 95% CI 6–34%) worsened on the GOS scale, but there was no significant overall shift (p=0.356 for overall GOS shift between 1–6 months). In the patients who did improve, five patients improved from a 4 (moderate disability) to 5 (mild disability), 2 patients from 3 (severe disability) to 4 and one patient from 3 to 5. Of the four patients who worsened, one patient worsened from 4 to 3, and one patient with GOS of 3 and two with GOS of 2 (persistent vegetative state) died. Between months 6 to 12, only two patients (8%) improved from GOS of 4 to 5 and one patient with GOS of 3 died, and these changes were not statistically significant. Results did not change when patients with carried-forward or backward data were included.
Barthel Index
When functional status was assessed using the Barthel Index, the proportion of patients with good outcome increased between months 1 and 3 (13 (50%) to 19 (73%), p=0.03). There were no additional changes in the dichotomized outcome groups between months 3–6 and months 6–12. In the shift analysis, when looking at the continuous scale, there was significant improvement between months 1 and 3 on the full Barthel scale: index increased by 7.5 (95% CI 0–30) points, p=0.021. There was no change in the overall index between 3–6 months or 6–12 months. Using carried forward and carried backwards data, the results were similar in that there was a significant shift towards improvement from 1–3 months but no significant changes after: 5 points (95% CI 0–20), p=0.01, carried forward; 2.5 points (0–12.5), p=0.05, carried backwards.
Discussion
The results of this prospective study of 45 survivors of cardiac arrest who were initially comatose after resuscitation shows functional outcomes improve over the first 6 months post-arrest. On the mRS scale, a strong trend toward improvement was seen between post-arrest months 1 to 6 using a dichotomized outcome, and there was a significant difference in outcomes between 1 and 6 months post-arrest when assessing outcome changes on the full mRS scale. Interestingly, there was not a significant change between the shorter intervals of months 1–3 and 3–6, but the improvement became significant when outcomes were compared between month 1 and 6. From post-arrest month 6–12, there was no significant change in the total number of patients in the dichotomized outcome groups by mRS, but individuals within the good outcome group continued to see improvements in mRS during this time period. Other outcomes scales also supported this finding of longitudinal improvements in functional outcome. There were significant improvements in functional outcomes by Barthel Index seen by 3 months post arrest, though these improvements stabilized and did not show significant further improvements at month 6 or 12. When outcomes were assessed by the GOS, there was a non-significant trend towards improved outcomes between post-arrest months 1 and 6.
While the majority of long-term disability in survivors of cardiac arrest is due to neurologic dysfunction, there is little data about optimal timing or methodology of assessing functional outcome. This study is significant because patients were followed prospectively over one year after cardiac arrest and functional outcomes were assessed at multiple time-points with multiple assessment scales. It provides information on the chances of longer-term improvement beyond the acute injury period, which can offer valuable prognostic information to survivors and their families. It also provides critical information about the trajectory of recovery and supports previous studies in patients with brain injury due to other types of insults (trauma, stroke), showing that the majority of functional improvement occurs during the first 6 months after injury, but there is still potential for longer-term recovery.28 Most patients who attain a good outcome will do so within the first 6 months. The results also suggest that the mRS and the BI are more sensitive for detecting improvements than the GOS. The GOS may not be an adequately refined outcome scale to assess functional status in this population.
Several limitations are important to address. Despite enrolling 100 patients in a consecutive prospective sample, only 45 (45%) patients survived to one month follow-up and were eligible for inclusion. This relatively low patient number from a single center limits generalizability. We also utilized structured phone interviews for the majority of the follow-ups and performed in-person evaluations at the 6-month follow-up if patients could come in to clinic. While structured phone interviews have been validated as a reliable assessment methodology,29, 30 in-person assessments likely provide the best opportunity for evaluation. Future research should include longitudinal in-person evaluations and make use of technology for tele-medicine evaluation if travel to the clinical center is not feasible.
Finally, while outcomes were assessed with three different outcomes scales at each time-point, other commonly used outcomes measures such as the Cerebral Performance Category (CPC) were not used. However, the GOS has essentially the same number, description, and categories of outcome as the CPC, and in fact the CPC was adapted for hypoxic-ischemic brain injury patients based on the originally described GOS.31 As such, the results seen here using the GOS would also likely be replicated if the CPC were used, but further research is needed. It is also important to note that recovery that is meaningful to a patient or family member occurs in a more nuanced manner than what may be measurable by course outcomes scales. Recovery in cognition, independence, and other areas that lead to improvement in a patient’s quality of life are also important to assess, and future studies should include more subjective quality-of-life assessments.
This study provides important information about the timing and trajectory of functional neurologic recovery in survivors of cardiac arrest who initially remain comatose. Given that a significant proportion of patients see functional improvements through post-arrest month 6, future research should consider following patients to at least a 6 month outcome assessment. Additionally, there were differences in the results between assessments with the GOS versus the mRS, despite overall similar trends. Assessments using the mRS showed significant changes in outcome while those using the GOS did not. Additional work is necessary to identify the optimal assessment tool(s) to quantify neurologic recovery after hypoxic-ischemic brain injury, and more nuanced scales are likely to prove beneficial.
Conclusions
In this prospective study of long-term functional outcome in initially comatose cardiac arrest survivors, improvements in functional status may occur over the first six months after the event. There is little evidence for significant changes in outcome between post-arrest months 6 to 12. The mRS may be a more sensitive ordinal outcome scale than the GOS or CPC in this patient population, but additional research is needed. Future resuscitation research should incorporate a 6 month outcome assessment of functional neurologic status.
Acknowledgments
Financial Support: NIH NHLBI 1 R01 HL089116-01; Stanford University Department of Neurology departmental funding
Footnotes
Conflicts of Interest: None
Copyright form disclosures: Dr. Hirsch received support for article research from the National Institutes of Health (NIH). Her institution received funding from the NIH, NHLBI and Stanford University Department of Neurology. Dr. Tong received support for article research from the NIH. Her institution received funding from the NIH, NHLBI and Stanford University Department of Neurology. Dr. Eyngorn disclosed work for hire. Dr. Mlynash disclosed work for hire. Dr. Albers received support for article research from the NIH and received funding from iSchemaView, Covidien, and Lundbeck. His institution received funding from the NHLBI and the NIH.
References
- 1.Lown B. Sudden cardiac death -- 1978. Circulation. 1979;60:1593–1599. doi: 10.1161/01.cir.60.7.1593. [DOI] [PubMed] [Google Scholar]
- 2.Cummins RO, Ornato JP, Thies WH, Pepe PE. Improving survival from sudden cardiac arrest: The "chain of survival" concept. A statement for health professionals from the advanced cardiac life support subcommittee and the emergency cardiac care committee, american heart association. Circulation. 1991;83:1832–1847. doi: 10.1161/01.cir.83.5.1832. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiainen M, Poutiainen E, Kovala T, Takkunen O, Happola O, Roine RO. Cognitive and neurophysiological outcome of cardiac arrest survivors treated with therapeutic hypothermia. Stroke; a journal of cerebral circulation. 2007;38:2303–2308. doi: 10.1161/STROKEAHA.107.483867. [DOI] [PubMed] [Google Scholar]
- 5.Moulaert VR, Verbunt JA, van Heugten CM, Wade DT. Cognitive impairments in survivors of out-of-hospital cardiac arrest: A systematic review. Resuscitation. 2009;80:297–305. doi: 10.1016/j.resuscitation.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 6.van Alem AP, de Vos R, Schmand B, Koster RW. Cognitive impairment in survivors of out-of-hospital cardiac arrest. American heart journal. 2004;148:416–421. doi: 10.1016/j.ahj.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Roine RO, Kajaste S, Kaste M. Neuropsychological sequelae of cardiac arrest. JAMA : the journal of the American Medical Association. 1993;269:237–242. [PubMed] [Google Scholar]
- 8.Sauve MJ, Doolittle N, Walker JA, Paul SM, Scheinman MM. Factors associated with cognitive recovery after cardiopulmonary resuscitation. American journal of critical care : an official publication, American Association of Critical-Care Nurses. 1996;5:127–139. [PubMed] [Google Scholar]
- 9.Maramattom BV, Wijdicks EF. Postresuscitation encephalopathy. Current views, management, and prognostication. The neurologist. 2005;11:234–243. doi: 10.1097/01.nrl.0000159985.07242.22. [DOI] [PubMed] [Google Scholar]
- 10.Cronberg T, Lilja G, Horn J, Kjaergaard J, Wise MP, Pellis T, Hovdenes J, Gasche Y, Aneman A, Stammet P, Erlinge D, Friberg H, Hassager C, Kuiper M, Wanscher M, Bosch F, Cranshaw J, Kleger GR, Persson S, Unden J, Walden A, Winkel P, Wetterslev J, Nielsen N. Neurologic function and health-related quality of life in patients following targeted temperature management at 33 degrees c vs 36 degrees c after out-of-hospital cardiac arrest: A randomized clinical trial. JAMA neurology. 2015;72:634–641. doi: 10.1001/jamaneurol.2015.0169. [DOI] [PubMed] [Google Scholar]
- 11.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. New England Journal of Medicine. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 12.Holzer MSF, Darby JM, et al. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. New England Journal of Medicine. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Åneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Køber L, Langørgen J, Lilja G, Møller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. Targeted temperature management at 33°c versus 36°c after cardiac arrest. New England Journal of Medicine. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 14.Becker LB, Aufderheide TP, Geocadin RG, Callaway CW, Lazar RM, Donnino MW, Nadkarni VM, Abella BS, Adrie C, Berg RA, Merchant RM, O'Connor RE, Meltzer DO, Holm MB, Longstreth WT, Halperin HR. Primary outcomes for resuscitation science studies: A consensus statement from the american heart association. Circulation. 2011;124:2158–2177. doi: 10.1161/CIR.0b013e3182340239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clancy CM, Eisenberg JM. Outcomes research: Measuring the end results of health care. Science (New York, NY) 1998;282:245–246. doi: 10.1126/science.282.5387.245. [DOI] [PubMed] [Google Scholar]
- 16.Lai SM, Duncan PW. Stroke recovery profile and the modified rankin assessment. Neuroepidemiology. 2001;20:26–30. doi: 10.1159/000054754. [DOI] [PubMed] [Google Scholar]
- 17.Savitz SI, Lew R, Bluhmki E, Hacke W, Fisher M. Shift analysis versus dichotomization of the modified rankin scale outcome scores in the ninds and ecass-ii trials. Stroke; a journal of cerebral circulation. 2007;38:3205–3212. doi: 10.1161/STROKEAHA.107.489351. [DOI] [PubMed] [Google Scholar]
- 18.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: Novel derivation method and application to thrombolytic therapy for acute stroke. Archives of neurology. 2004;61:1066–1070. doi: 10.1001/archneur.61.7.1066. [DOI] [PubMed] [Google Scholar]
- 19.Howard G, Waller JL, Voeks JH, Howard VJ, Jauch EC, Lees KR, Nichols FT, Rahlfs VW, Hess DC. A simple, assumption-free, and clinically interpretable approach for analysis of modified rankin outcomes. Stroke; a journal of cerebral circulation. 2012;43:664–669. doi: 10.1161/STROKEAHA.111.632935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: Use of a structured interview to assign grades on the modified rankin scale. Stroke; a journal of cerebral circulation. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 21.Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified rankin scale across multiple raters: Benefits of a structured interview. Stroke; a journal of cerebral circulation. 2005;36:777–781. doi: 10.1161/01.STR.0000157596.13234.95. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe CD, Taub NA, Woodrow EJ, Burney PG. Assessment of scales of disability and handicap for stroke patients. Stroke; a journal of cerebral circulation. 1991;22:1242–1244. doi: 10.1161/01.str.22.10.1242. [DOI] [PubMed] [Google Scholar]
- 23.Collin C, Wade DT, Davies S, Horne V. The barthel adl index: A reliability study. International disability studies. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney FI, Barthel DW. Functional evaluation: The barthel index. Maryland state medical journal. 1965;14:61–65. [PubMed] [Google Scholar]
- 25.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 26.A A. Generalized odds ratios for ordinal data. Biometrics. 1980;36:59–67. [Google Scholar]
- 27.Kung-Jong L. Notes on estimation of the general odds ratio and the general risk difference for paired-sample data. Biometrics Journal. 2002;44:957–968. [Google Scholar]
- 28.Wade DT, Hewer RL. Functional abilities after stroke: Measurement, natural history and prognosis. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:177–182. doi: 10.1136/jnnp.50.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savio K, Pietra GL, Oddone E, Reggiani M, Leone MA. Reliability of the modified rankin scale applied by telephone. Neurology international. 2013;5:e2. doi: 10.4081/ni.2013.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified rankin scale. Cerebrovascular diseases (Basel, Switzerland) 2010;29:137–139. doi: 10.1159/000262309. [DOI] [PubMed] [Google Scholar]
- 31.Wijdicks EFM. Famous first papers for the neurointensivist. New York: Springer; 2013. [Google Scholar]