Abstract
Traditional methods of intraoperative human saphenous vein (SV) preparation for use as bypass grafts can be deleterious to the conduit. The purpose of this study was to characterize acute graft preparation injury, and to mitigate this harm via an improved preparation technique. Porcine saphenous veins were surgically harvested (unprepared controls, UnP) and prepared using the traditional (TraP) and improved preparations (ImP). The TraP used unregulated radial distension, marking with a surgical skin marker and preservation in heparinized normal saline. ImP used pressure-regulated distension, brilliant blue FCF-based pen marking and preservation in heparinized Plasma-Lyte A. Rings from each preparation were suspended on a muscle bath for characterization of physiologic responses to vasoactive agents and viscoelasticity. Cellular viability was assessed using the methyl thiazolyl tetrazolium (MTT) assay and the terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay for apoptosis. Contractile responses to potassium chloride (110 mM) and phenylephrine (10 μM), and endothelial -dependent and -independent vasodilatory responses to carbachol (0.5 μM) and sodium nitroprusside (1 μM), respectively, were decreased in TraP tissues compared to both UnP and ImP tissues (P ≤ .05). TraP tissues demonstrated diminished viscoelasticity relative to UnP and ImP tissues (P ≤ .05), and reduced cellular viability relative to UnP control (P ≤ .01) by the MTT assay. On TUNEL assay, TraP tissues demonstrated a greater degree of apoptosis relative to UnP and ImP tissues (P ≤ .01). In conclusion, an improved preparation technique prevents vascular graft smooth muscle and endothelial injury observed in tissues prepared using a traditional approach.
Keywords: vascular diseases, graft occlusion, vascular, veins, peripheral arterial disease, coronary artery bypass
Introduction
Over 300,000 grafts to the coronary and peripheral circulation are transplanted in the United States annually, and the human saphenous vein (SV) is the most common conduit used in these procedures.1–3 Despite wide utilization, human SV graft failure rates have been reported at 30–40% within one year.4, 5 Explantation, preparation and arterialization of human SV represents an autologous transplantation, necessitating the same tissue handling and attention to surgical technique given to solid organ allografts. The period of ex vivo graft preparation provides a critical window for the graft. While extracorporeal, the vein is highly prone to endothelial and smooth muscle injury due to handling, exacerbated by a relatively ischemic cellular environment.3, 6–8 Minimal attention has been given to intraoperative “back-table” preparation of human SV and commonly employed techniques include physical trauma and exposure to toxic elements within surgical skin markers and preservation media.7–9
Three particular ex vivo manipulations, comprising the traditional preparation (TraP), have been identified as candidates for potential optimization. Vein graft preparation first involves unregulated intraluminal distension to identify leaks and unligated branches, followed by marking with a standard surgical skin marker and finally, preservation in normal saline solution until implantation.10 Unregulated distension exposes the endothelium to supraphysiologic pressures, leading to denudation.6, 11, 12 Marking with a standard surgical skin marker exposes the smooth muscle cells to two cytotoxic components, gentian violet dye and isopropyl alcohol.8 Normal saline as a preservation medium is acidic, and its electrolyte composition is dissimilar to the plasma components normally seen by vein graft in vivo.7 Damage to the conduit, particularly the endothelial layer, may accelerate thrombosis and intimal hyperplasia.3, 6, 13–15 As their SV similar in caliber, composition and physiologic properties to human SV, a porcine model was chosen as a source of heterogeneous, readily obtainable tissue.6 The purposes of this investigation are two-fold. First, we aim to characterize acute conduit injury imposed by the TraP, as defined by impairment in smooth muscle function, biophysical properties and viability. Second, we aim to test our hypothesis that a rationally designed three-tiered technique that is more physiologically compatible with SV graft, the improved preparation (ImP), may reduce acute conduit injury.
Methods
Harvest of Porcine Saphenous Veins
Animal procedures followed the protocol approved for this study by the Vanderbilt Institutional Animal Care and Use Committee (IRB number M/11/123) and adhered to National Institute of Health guidelines for care and use of laboratory animals. Animals were prepared for surgery in the morning on the day of delivery, and were thus not housed or acclimated. Yorkshire/Landrace pigs weighing 43.0 ± 1.4 kg (Oak Hill Genetics, Ewing, IL; n = 28 in total) were anesthetized via the institutional protocol of ketamine (2.2 mg/kg)/xylazine (2.2 mg/kg)/telazol (4.4 mg/kg) induction, and isoflurane (1–5%) maintenance in the Vanderbilt animal operating theater. A licensed veterinary technician was present throughout all harvest procedures. Their hind legs were prepared and draped in the usual sterile fashion. Bilateral incisions were made, and the subcutaneous fat and fascia were carefully dissected to expose the porcine SVs from the ankle proximally to the sapheno-femoral junction, with care taken to minimize trauma to the vein in situ. Branches were ligated using 4-0 silk ties. Once exposed, both porcine SVs were clamped and explanted from the body. Control, unprepared tissue (UnP) was cut from these segments, after which they were immediately placed in heparinized (Hospira, 10 units/mL) Plasma-Lyte A (HP, Baxter). After harvest, the pigs were euthanized with sodium pentobarbital (125 mg/kg) overdose. Primary endpoints for these studies were physiologic responses, measures of viscoelastic conformity and cellular viability indices.
Traditional Preparation Technique
One of the two veins, determined by random draw to undergo TraP, was cannulated at the distal end with an olive-tipped cannula, and secured with a 4-0 silk suture. A 60 mL disposable syringe was filled with 10 mL heparinized (10 units/mL) 0.9% normal saline solution (HS, Baxter), and after occluding the opposite end of the vein with fingertip compression, the vein was flushed with HS three times. Unligated branches were identified and tied. After distension, the vein was marked in a continuous line using a standard surgical skin marker (Richard-Allan) containing gentian violet dye in isopropyl alcohol solvent. The TraP graft was then stored in HS with papaverine (1 mg/mL) for two hours, a period of time chosen to represent an extreme end of a practical preservation period that was thought, a priori, to pronounce the differences in conduit injury between solutions. Papaverine is a phosphodiesterase commonly used to prevent vasospasm.
Improved Preparation Technique
The vein randomized for ImP was cannulated at the distal end using an olive-tipped cannula with an introducer for easier insertion into the graft lumen. The vein was then secured to the cannula with a 4-0 silk tie and the introducer was removed. A pressure release valve with adaptor tubing, engineered to limit distension pressure to 140 mmHg, was primed by placement of a finger over the outflow end of the valve with injection of HP to assess and confirm pressure release. The valve was then attached to the cannula, and a plastic bulldog clamp was placed on the opposite end of the vein for tamponade.16 The graft was distended with 10 mL HP three times, and unligated tributaries were identified and tied. After distension, the bulldog clamp was removed along with the segment of clamped vein, and the vein was marked in a continuous line using the marker containing FCF (Vasoprep, NJ). The ImP graft was then stored in HP with papaverine (1 mg/mL) for two hours at room temperature.
Determination of Physiologic Tissue Function
Rings ~1 mm. in width were cut from UnP, TraP and ImP vein graft segments after gentle dissection free from residual connective and adipose tissue. Tissues were suspended in a muscle bath, in duplicate, containing a bicarbonate buffer (120 mM sodium chloride, 4.7 mM potassium chloride, 1.0 mM magnesium sulfate, 1.0 mM monosodium phosphate, 10 mM glucose, 1.5 mM calcium chloride, and 25 mM sodium bicarbonate, pH 7.4), equilibrated with 95% O2/5% CO2 at 37°C. Tissues were sequentially stretched to the optimal resting tension (~1 g) and stretched 3–4 times the resting tension to determine the passive length-tension relationship, followed by maintenance at resting tension of 1g for an additional 1 hr.17, 18 Force measurements were obtained using the Radnoti force transducer (model 159901A) interfaced with a PowerLab data acquisition system and Chart software (AD Instruments).
Smooth muscle function was assessed by repeatedly contracting the tissues with 110 mM potassium chloride (KCl) until consistent maximal force was generated. To determine endothelial-dependent relaxation, porcine SV rings were pre-contracted with the alpha-1 agonist phenylephrine (10 μM), and relaxed with carbachol (0.5 μM), an acetylcholine analogue that induces nitric oxide release from viable endothelial cells. To determine endothelial-independent relaxation, porcine SV rings were again pre-contracted with phenylephrine (10 μM) and relaxed with sodium nitroprusside (1 μM), an exogenous nitrous oxide donor. Relaxation was reported as percent of maximal phenylephrine-induced contraction (figure 1). KCl, phenylephrine, carbachol and sodium nitroprusside were added directly to the muscle bath. After experimentation, all rings were weighed and their widths’ measured with calipers.
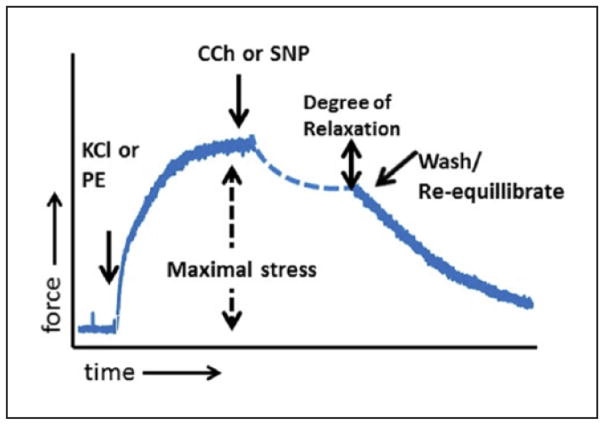
Figure 1. Representative force vs. time tracing.
A suspended ring of tissue was challenged with 110 mM KCl or 10 μM phenylephrine to cause contraction. Maximal contraction, normalized to the weight and length of the tissue, is represented by the dashed double-headed arrow. After phenylephrine pre-contraction, tissues were challenged with carbachol (0.5 μM) to assess endothelial-dependent relaxation, or sodium nitroprusside (1 μM) to assess endothelial-independent relaxation (solid line double-headed arrow).
KCl, potassium chloride; PE, phenylephrine; CCh, carbachol; SNP, sodium nitroprusside
Assessment of Viscoelasticity of PSV Graft
Data on tissue force generation upon contraction with 110 mM KCl as a function of time was recorded on LabChart and imported into Eureqa Equation Solver. The Eureqa Equation Solver uses symbolic regression to determine the best fit of the data as well as relevant fitting parameters. Viscoelastic conformity was assessed by fitting the tracings of each of the three conditions to the Hill viscoelastic model of the form F(t)=(a+rt)/(b+t) for isometric contractions. In the equation, t is the variable time and a, b and r are constants. Mean absolute error (MAE) was the primary measure used to analyze the goodness of fit of the model.
MTT Assay
Viability of vascular smooth muscle cells was determined using the methyl thiazolyl tetrazolium (MTT) assay.19, 20 After preparation, rings were placed in 250 μL 0.01% thiazolyl blue tetrazolium bromide in phosphate-buffered saline for an additional one hour at 37°C. Rings were then transferred to 500 μL 2-ethoxyethanol solvent for 24 hrs to extract the resulting formazan dye. MTT viability indices were calculated, and represent spectrophotometric measurements (λ = 570 nm) of the 2-ethoxyethanol, normalized to both tissue mass and volume of solution; as such, these measurements are useful for comparison among other preparations, but do not reflect a percentage of viable smooth muscle cells.7, 19
TUNEL Assay
Extent of apoptosis of vascular smooth muscle cells was determined using the terminal deoxynucleotidyl transferase dUTP nick-end labelling (TUNEL) assay.21 Segments of the three tissue preparation groups of porcine SV were formalin-fixed immediately after preparation. Remaining segments were cultured in RPMI 1640 media with 30% FBS at 37°C in humidified incubator with 5% CO2 for 3 days. These tissues were then formalin-fixed, dehydrated with ethanol, embedded in paraffin. Apoptosis was detected using DeadEndTM Flourometric TUNEL system (Promega, CA) per manufacturer’s instructions and mounted with SlowFade® Gold anti-fade reagent with 4′6-diamidino-2-phenylindole (DAPI; Life Technologies, CA). Images were acquired on Zeiss Axiovert 200 Fluorescence microscope at 400x magnification. TUNEL signals were quantified by Image J with the plugin “color_pixel_counter.class” with minimum intensity value set at 100. Extent of apoptosis (apoptosis index) was represented as the proportion of DAPI-stained pixels (blue stain, representing nuclei) which subsequently stained green on TUNEL assay (TUNEL positivity).21
Statistical Analysis
Contractile responses were defined by stress, calculated using force generated by tissues as follows: stress [105 Newtons (N)/m2] = force (g) × 0.0987/area, where area is equal to the wet weight [(mg)/length (mm at maximal length)] divided by 1.055).22 Data were reported as mean ± standard error of the mean (SEM). One-way Analysis of Variance (ANOVA) with Tukey’s post-hoc analysis of multiple comparisons was conducted in order to determine statistical significance between treatment groups, at a level of evidence P ≤ .05. Statistical analysis was performed using GraphPad Prism 5 (La Jolla, CA) and Eureqa Equation Solver (Somerville, MA).
Results
Smooth Muscle Contractility of Porcine Saphenous Vein
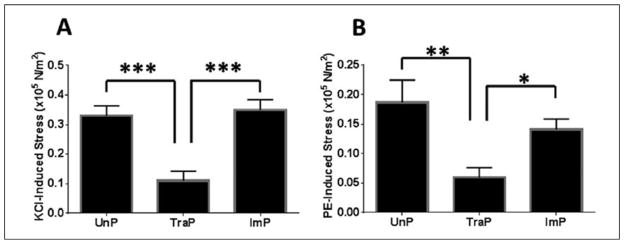
Differences in KCl-induced stress were found among treatment groups, upon one-way ANOVA (F = 20.4, P < .001, n = 15). Stress in TraP tissues was decreased relative to UnP tissues (0.11 ± 0.03 vs. 0.33 ± 0.03 ×105 N/m2, P ≤ .001; figure 2A) and ImP tissues (0.11 ± 0.03 vs. 0.35 ± 0.03 ×105 N/m2, P ≤ .001). Differences were not observed between UnP and ImP tissues (0.33 ± 0.03 vs 0.35 ± 0.03 ×105 N/m2, P > .05).
Figure 2. Contractile responses of porcine saphenous vein.
A- Stress induced by challenge with 110 mM KCl. B- Stress induced by challenge with 10 μM PE. *P ≤ .05, **P ≤ .01, ***P ≤ .001. Error bars represent standard error of the mean.
KCl, potassium chloride; PE, phenylephrine; UnP, unprepared tissue; TraP, traditional preparation; ImP, improved preparation
Similarly, differences in phenylephrine-induced stress were found among treatment groups (F = 7.7, P = .002, n = 15). Stress in TraP tissues was decreased relative to UnP tissues (0.059 ± 0.02 vs. 0.19 ± 0.04 ×105 N/m2, P ≤ .01; figure 2B) and ImP tissues (0.059 ± 0.02 vs. 0.14 ± 0.02 ×105 N/m2, P ≤ .05). Differences were not observed between UnP and ImP tissues (0.19 ± 0.04 vs. 0.14 ± 0.02 ×105 N/m2, P > .05).
Endothelial-Dependent and -Independent Relaxation of Porcine Saphenous Vein
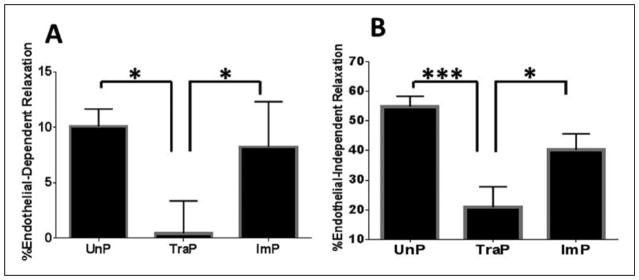
Differences in endothelial-dependent relaxation were found among treatment groups, upon one-way ANOVA (F = 6.3, P = .01, n = 7). Endothelial-dependent relaxation was decreased in TraP tissues relative to UnP tissues (0.42 ± 3.0% vs. 10.1 ± 1.6%, P ≤ .05; figure 3A) and ImP tissues (0.42 ± 3.0% vs. 8.2 ± 4.1%, P ≤ .05). Differences were not observed between UnP and ImP tissues (10.1 ± 1.6% vs. 8.2 ± 4.1%, P > .05).
Figure 3. Dilatory responses of porcine saphenous vein.
A- Endothelial-dependent relaxation induced by challenge with 0.5 μM carbachol. B- Endothelial-independent relaxation induced by challenge with 1 μM sodium nitroprusside. *P ≤ .05, ***P ≤ .001. Error bars represent standard error of the mean.
UnP, unprepared tissue; TraP, traditional preparation; ImP, improved preparation
Similarly, differences in endothelial-independent were found among treatment groups (F = 14.2, P ≤ .001, n = 10). Relaxation was decreased in TraP tissues relative to UnP tissues (20.8 ± 7.0% vs. 54.9 ± 3.6%, n = 10, P ≤ .001; figure 3B) and ImP tissues (20.8 ± 7.0% vs. 40.3 ± 5.4%, P ≤ .05). Differences were not observed between UnP and ImP tissues (54.9 ± 3.6% vs. 40.3 ± 5.4%, P > .05).
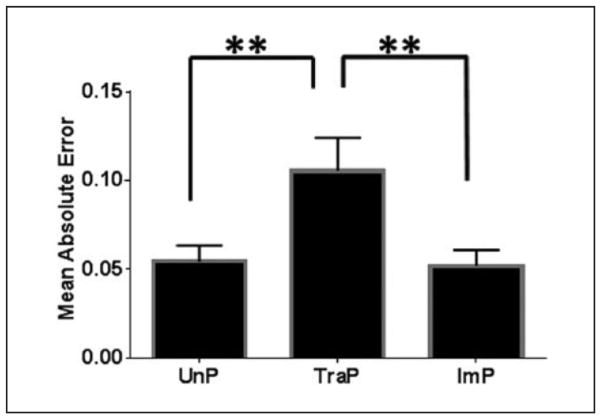
Viscoelasticity of Porcine Saphenous Vein Tissues
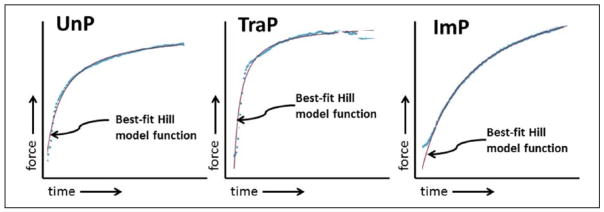
The fitting of representative tracings is illustrated in figure 4. Differences in MAE among treatment groups were found using one-way ANOVA (F = 11.2, P = .003, n = 6). MAE was significantly greater in TraP tissues relative to UnP tissues (0.11 ± 0.02 vs. 0.054 ± 0.009, P ≤ .01; figure 5A) and ImP tissues (0.11 ± 0.02 vs. 0.052 ± 0.009, P ≤ .01). Differences between UnP and ImP tissues were not significantly different (0.054 ± 0.009 vs. 0.052 ± 0.009, P > .05).
Figure 4. Representative force vs. time tracings.
Tracings for UnP, TraP and ImP tissues are shown with their best-fit viscoelastic functions of the form F(t)=(a+rt)/(b+t), where F = force, and t = time. UnP and ImP tissues conformed almost perfectly, while the TraP tissues were of poorer fit.
UnP, unprepared tissue; TraP, traditional preparation; ImP, improved preparation
Figure 5. Viscoelastic conformity of porcine saphenous vein.
Tissues were isometrically contracted with 110 mM KCl and fit to the Hill model of the form F(t)=(a+rt)/(b+t). Mean absolute error (MAE) is plotted among UnP, TraP and ImP tissues. **P ≤ .01. Error bars represent standard error of the mean.
UnP, unprepared tissue; TraP, traditional preparation; ImP, improved preparation
Cellular Viability of Porcine Saphenous Vein
Differences in MTT viability indices were found among treatment groups, upon one-way ANOVA (F = 8.4, P = .007, n = 6). Relative to UnP tissues, the MTT viability index was reduced in TraP tissues, (0.080 ± 0.02 OD50 mg−1 mL−1 vs. 0.034 ± 0.008 OD50 mg−1 mL−1, P ≤ .01; figure 6), but not in ImP tissues (0.080 ± 0.02 OD50 mg−1 mL−1 vs. 0.050 ± 0.01 OD50 mg−1 mL−1, P > .05). The difference in viability indices between TraP and ImP tissues were not significant (0.034 ± 0.008 OD50 mg−1 mL−1 vs. 0.050 ± 0.01 OD50 mg−1 mL−1, P > .05).
Figure 6. MTT cellular viability assay.
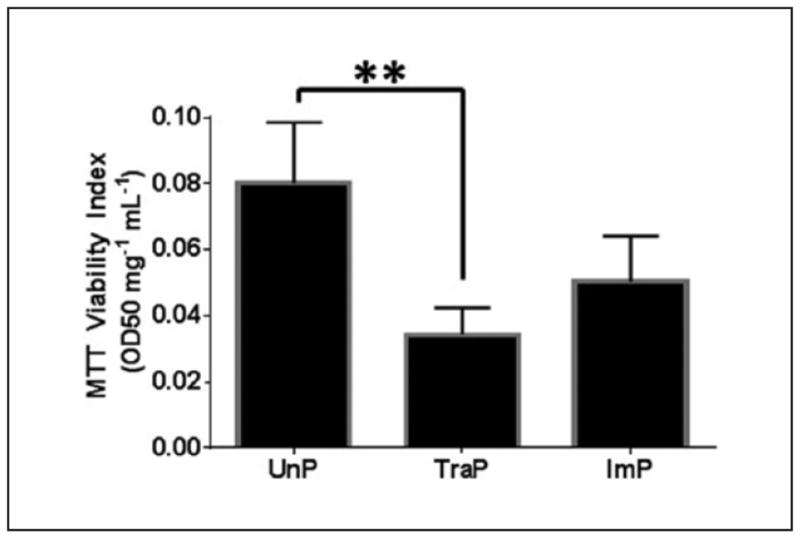
**P ≤ .01. Error bars represent standard error of the mean.
MTT, methyl thiazolyl tetrazolium; UnP, unprepared tissue; TraP, traditional preparation; ImP, improved preparation
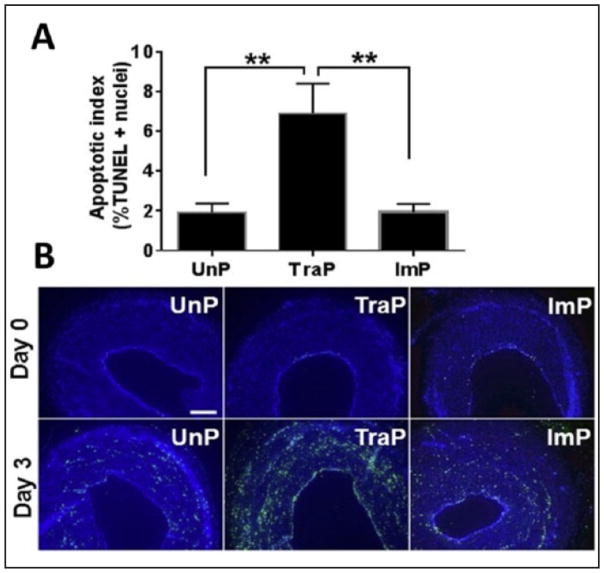
Differences in %TUNEL positive nuclei were also found among treatment groups, upon one-way ANOVA (F = 9.8, P = .002, n = 6). Relative to UnP tissues, there was a greater percentage of TUNEL positive nuclei in TraP tissues (1.9 ± 0.4% vs. 6.9 ± 1.5%, P ≤ .01; figure 7A), but not in ImP tissues (1.9 ± 0.4% vs. 1.9 ± 0.4%, P > .05). There was also a greater percentage of TUNEL positive nuclei in TraP tissues relative to ImP tissues (6.9 ± 1.5% vs. 1.9 ± 0.4%, P ≤ .01). Representative TUNEL stains are illustrated in figure 7B.
Figure 7. TUNEL apoptosis assay.
A- **P ≤ .01. Error bars represent standard error of the mean. B- Representative images of TUNEL staining. TUNEL-positive cells appear in green. Top row: Day 0; bottom row: Day 3. Scale bars = 200μm.
TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labelling; UnP- unprepared tissue; TraP- traditional preparation; ImP- improved preparation
Discussion
Our data demonstrate that traditional intraoperative preparation of harvested porcine SV cause injury to the conduit. The collective effects of the TraP method caused both decreased contractile and dilatory responses to all agents, including KCl and phenylephrine-induced vasoconstriction, endothelial-dependent relaxation with carbachol and endothelial-independent relaxation with sodium nitroprusside, a pattern of physiologic impairment mitigated by the ImP technique. These injuries correlated with reduced cellular viability as well, as TraP tissues had a reduced MTT viability index and greater TUNEL positive nuclei relative to UnP tissues, a finding not observed with ImP tissues.
In cardiac and peripheral bypass operations, there are several properties of conduit that are highly desirable. The conduit must have adequate biocompatibility, blood compatibility and biostability, all properties intrinsic to an autologous vascular transplantation. In addition, porosity, low thrombogenicity, the ability to stretch and the ability to resist kinking are also considered optimal parameters, particularly in the rational design of a successful synthetic conduit.23 The property of viscoelasticity is an important biophysical characteristics in a successful vascular graft, necessary for adaptation to the blood flow of the arterial circulation.24 In its simplest definition, a viscoelastic tissue has a recoverable (elastic) and a non-recoverable (viscous) component. In the case of a purely elastic vessel, application of a load (e.g., KCl) would continuously deform the wall in linear fashion. A viscous component allows for a dissipation of energy over the course of the load application. Healthy soft tissues, including arteries and muscles are intrinsically viscoelastic.25 Loss of viscoelasticity has been identified as a marker of diseased human carotid artery, associated with pulmonary hypertension in mice and contributory to cardiac dysfunction in pre-term infants.26–30 Consistent with the injury patterns observed in assessments of physiology and cellular viability, our data revealed diminished viscoelastic conformity in TraP tissues that was not observed in ImP tissues.
The first component of traditional vein graft preparation, hand-held pressure distension of the conduit is known to lead to deleterious biochemical and cellular changes in vascular smooth muscle cells, including release of extracellular ATP, accumulation of cholesterol, greater expression of inflammatory markers and increased proliferation.12, 31–36 The impact of unregulated distension on the endothelial layer is more pronounced. Peak distension pressure is proportional to endothelial loss and dysfunction in human SV.6, 35, 37–39 Unregulated distension leads to intraluminal pressures in excess of 600 mmHg.3, 6 Pressures as low as 300 mmHg also demonstrated a 91% endothelial cell loss, as determined by CD31-immunostaining.40 As an intact endothelial monolayer is critical to human SV patency,13, 41, 42 methods have been developed to minimize distension pressures of human SV. In 1980, a balloon device interposed between the syringe and cannula was developed to lower infusion pressure.43 In 1992, Angelini et al. used a distension system in which a cannulated human SV was connected to the side-arm of an arterial cannula previously inserted and secured into a CABG patient’s ascending aorta. This allowed for the patient’s own arterial pulsation to dilate the vein, and led to preservation of medial and endothelial function.44, 45 The pressure release valve utilized in this report represents a simple, effective modality that fits in line with the distension tubing array to alleviate supraphysiologic distension. Pressure release valve use preserves endothelial functional responses and prevents endothelial denudation.6 Physiologic distension pressure does not prevent the effective identification of graft leaks.44, 45
In 2011, Eagle et al. showed that both smooth muscle contractility and endothelial-dependent relaxation were decreased with topical application of standard surgical skin markers containing gentian violet dye (10%) and isopropyl alcohol solvent (50%).9 Moreover, at subclinical concentrations, brief exposures to both gentian violet dye and isopropyl alcohol solvent have been reported to be cytotoxic to cultured human umbilical venous smooth muscle cells as assessed histologically by trypan blue staining, as well as the CytoTox-Glo assay.8 Topical application of standard surgical skin markers has been shown to harm other human tissue as well, including hamstring tendon and endothelium in the anterior lens and cornea of the eye.46–48
FCF was chosen as a non-toxic, water soluble dye with which to mark conduit on the basis of several recent reports. Topical FCF treatment, or preservation in a balanced salt solution containing FCF has been shown to maintain physiologic functions of human SV.8, 49 In contrast to gentian violet and isopropyl alcohol treatment, topical FCF is also not acutely cytotoxic to cultured human umbilical venous smooth muscle cells.8, 49 Interestingly, FCF has been found to enhance the contractile responses of damaged human SV to KCl depolarization and enhance endothelial-dependent relaxation suggesting that FCF has beneficial pharmacologic properties.49 The mechanism of prevention and amelioration of acute cellular injury has been linked to vascular cell P2X7 receptors by FCF.8, 15, 49, 50 These receptors are activated by ATP released during injury, leading to downstream effects such as increased cytosolic calcium flux, migration, inflammation, proliferation and apoptosis.8, 49, 51
Among patients in the Project of Ex-Vivo vein graft Engineering via Transfection-IV (PREVENT-IV) cohort, normal saline was the most common media for graft preservation prior to implantation.14 Normal saline was also identified as an independent risk factor for vein graft failure at one year among patients in this same cohort.14 Tissues preserved in normal saline for two hours demonstrated decreased responses to vasoconstrictors, as well as to carbachol and sodium nitroprusside, along with a concomitant reduction in MTT viability index. These acute changes were minimized via preservation with buffered salt solutions such as University of Wisconsin solution or Plasma-Lyte A.7 Mechanistically, the significant acidity of normal saline relative to the physiologic pH of 7.4 is the most probable cause for this damage.7 Thus, a simple balanced salt solution of a physiologic pH, Plasma-Lyte A, was chosen as a more optimal preservation solution.
Human SV injury during preparation may contribute to the subsequent development of intimal hyperplasia and graft failure. In 2014, Osgood and colleagues reported that intraoperatively prepared human SV generated increased intimal thickness relative to unprepared control, in an organ culture model.3 In 2012, Li et al. demonstrated that unregulated distension led to physiologic impairment in response to KCl, carbachol and sodium nitroprusside, patchy endothelial denudation and increases in neointimal growth in organ culture which were prevented with distension with an in line pressure release valve.6 FCF treatment has recently been reported to prevent neointimal formation in a human SV organ culture model.49 Additionally, ex vivo treatment of rabbit external jugular conduit in a 50 μM solution of FCF in Plasma-Lyte A for one hour prior to implantation led to decreased neointimal growth in an in vivo carotid interposition model.15 Taken together, these data suggest that current methods used to prepare human SV for transplantation into the coronary or peripheral circulation lead to significant damage to the conduit that may result in vein graft failure.
There are several limitations to this study. First, the data collected in this study reflected acute changes in tissue physiology. Upon implantation into the arterial circulation, human SV has the capacity to undergo adaptive remodeling, including generation of a thicker smooth muscle layer to accommodate the rise in flow and shear stress of the arterial circulation, regeneration of denuded endothelium and a strengthening of the extracellular matrix.52–54 Next, while ideal for size and wall thickness, porcine SV has properties that are different from human SV. The porcine specimens were uniformly healthy, in contrast to the heterogenous human cohort of aged CABG patients. Finally, this study did not discern the relative contributions of each of the three components of graft preparation on the measures of injury assessed. It is most likely that each subsequent injury incrementally impairs the conduit, however, each component itself may be enough to fully abrogate smooth muscle and endothelial function. Nonetheless, minimization of intraluminal distension, graft marking with a non-toxic alternative surgical skin marker and preservation in a buffered salt solution have been shown, collectively and individually, to maintain saphenous vein explant function. Together, they comprise an improved preparation technique for human SV that may plausibly abrogate neointimal growth.
Conclusions
The profile of acute graft injury seen with traditional preparation includes physiologic functional impairment, diminished viscoelasticity and reduced cellular viability. Minimization of acute tissue damage has been correlated with decreased neointimal growth. A rationally designed improved technique provides a feasible alternative approach to conduit preparation while addressing three of the key harmful intraoperative manipulations.
Acknowledgments
We would like to acknowledge the staff of the Vanderbilt University Animal Operating Room for their assistance.
Grant Support
This study was supported in part with resources and materials from the VA Tennessee Valley Healthcare System; NIH R01HL70715-09 and a Biomedical Laboratory Research and Development Grant to CB for design and conduct of the study, collection, management, analysis, and interpretation of the data, and drafting and approval of the manuscript; and NIH R01HL105731-01A1 to JC for design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, and drafting and approval of the manuscript. Research materials and additional data may be obtained by contacting the corresponding author.
Footnotes
Declaration of Conflicting Interests
Drs. Hocking, Komalavilas, Cheung-Flynn and Brophy disclose a commercial relationship with Vasoprep Surgical Inc.
Any further data or supporting materials related to this study may be made available by contacting the corresponding author of the manuscript.
References
- 1.Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. Journal of vascular surgery. 2015;61:203–16. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhasin M, Huang Z, Pradhan-Nabzdyk L, et al. Temporal network based analysis of cell specific vein graft transcriptome defines key pathways and hub genes in implantation injury. PloS one. 2012;7:e39123. doi: 10.1371/journal.pone.0039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osgood MJ, Hocking KM, Voskresensky IV, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. Journal of vascular surgery. 2014;60:202–11. doi: 10.1016/j.jvs.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conte MS, Bandyk DF, Clowes AW, et al. Results of PREVENT III: a multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. Journal of vascular surgery. 2006;43:742–51. doi: 10.1016/j.jvs.2005.12.058. discussion 51. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JH, Hafley G, Harrington RA, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA: the journal of the American Medical Association. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 6.Li FD, Eagle S, Brophy C, et al. Pressure control during preparation of saphenous veins. JAMA surgery. 2014;149:655–62. doi: 10.1001/jamasurg.2013.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise ES, Hocking KM, Eagle S, et al. Preservation solution impacts psychiologic function and cellular viability of human saphenous vein graft. Surgery. 2015;158:537–46. doi: 10.1016/j.surg.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hocking KM, Luo W, Li FD, Komalavilas P, Brophy CM, Cheung-Flynn J. Brilliant blue FCF is a nontoxic dye for saphenous vein graft marking that abrogates response to injury. Journal of vascular surgery. 2015 doi: 10.1016/j.jvs.2014.12.059. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eagle S, Brophy CM, Komalavilas P, et al. Surgical Skin Markers Impair Human Saphenous Vein Graft Smooth Muscle and Endothelial Function. Am Surgeon. 2011;77:922–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Schanzer A, Hevelone N, Owens CD, et al. Technical factors affecting autogenous vein graft failure: observations from a large multicenter trial. Journal of vascular surgery. 2007;46:1180–90. doi: 10.1016/j.jvs.2007.08.033. discussion 90. [DOI] [PubMed] [Google Scholar]
- 11.Chello M, Mastroroberto P, Frati G, et al. Pressure distension stimulates the expression of endothelial adhesion molecules in the human saphenous vein graft. The Annals of thoracic surgery. 2003;76:453–8. doi: 10.1016/s0003-4975(03)00433-8. discussion 8. [DOI] [PubMed] [Google Scholar]
- 12.Khaleel MS, Dorheim TA, Duryee MJ, et al. High-pressure distention of the saphenous vein during preparation results in increased markers of inflammation: a potential mechanism for graft failure. The Annals of thoracic surgery. 2012;93:552–8. doi: 10.1016/j.athoracsur.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 13.Thatte HS, Khuri SF. The coronary artery bypass conduit: I. Intraoperative endothelial injury and its implication on graft patency. The Annals of thoracic surgery. 2001;72:S2245–52. doi: 10.1016/s0003-4975(01)03272-6. discussion S67–70. [DOI] [PubMed] [Google Scholar]
- 14.Harskamp RE, Alexander JH, Schulte PJ, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA surgery. 2014;149:798–805. doi: 10.1001/jamasurg.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osgood MJ, Sexton KW, Voskresensky IV, et al. Use of Brilliant Blue FCF During Vein Graft Preparation Inhibits Intimal Hyperplasia. Journal of vascular surgery. 2015 doi: 10.1016/j.jvs.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wise ES, Hocking KM, Feldman D, Komalavilas P, Cheung-Flynn J, Brophy CM. An Optimized Preparation Technique for Saphenous Vein Graft. The American surgeon. 2015;81:E274–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Bai TR, Bates JH, Brusasco V, et al. On the terminology for describing the length-force relationship and its changes in airway smooth muscle. J Appl Physiol. 2004;97:2029–34. doi: 10.1152/japplphysiol.00884.2004. [DOI] [PubMed] [Google Scholar]
- 18.Herlihy JT, Murphy RA. Length-tension relationship of smooth muscle of the hog carotid artery. Circ Res. 1973;33:275–83. doi: 10.1161/01.res.33.3.275. [DOI] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Berridge MV, Tan AS. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Archives of biochemistry and biophysics. 1993;303:474–82. doi: 10.1006/abbi.1993.1311. [DOI] [PubMed] [Google Scholar]
- 21.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of cell biology. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li FD, Sexton KW, Hocking KM, et al. Intimal thickness associated with endothelial dysfunction in human vein grafts. The Journal of surgical research. 2013;180:e55–62. doi: 10.1016/j.jss.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai M, Seifalian AM, Hamilton G. Role of prosthetic conduits in coronary artery bypass grafting. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2011;40:394–8. doi: 10.1016/j.ejcts.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 24.Balocco S, Basset O, Courbebaisse G, et al. Estimation of the viscoelastic properties of vessel walls using a computational model and Doppler ultrasound. Physics in medicine and biology. 2010;55:3557–75. doi: 10.1088/0031-9155/55/12/019. [DOI] [PubMed] [Google Scholar]
- 25.Gunther M, Rohrle O, Haeufle DF, Schmitt S. Spreading out muscle mass within a Hill-type model: a computer simulation study. Computational and mathematical methods in medicine. 2012;2012:848630. doi: 10.1155/2012/848630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taniguchi R, Hosaka A, Miyahara T, et al. Viscoelastic Deterioration of the Carotid Artery Vascular Wall is a Possible Predictor of Coronary Artery Disease. Journal of atherosclerosis and thrombosis. 2015;22:415–23. doi: 10.5551/jat.24513. [DOI] [PubMed] [Google Scholar]
- 27.Cheng KS, Tiwari A, Baker CR, Morris R, Hamilton G, Seifalian AM. Impaired carotid and femoral viscoelastic properties and elevated intima-media thickness in peripheral vascular disease. Atherosclerosis. 2002;164:113–20. doi: 10.1016/s0021-9150(02)00042-4. [DOI] [PubMed] [Google Scholar]
- 28.Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension. 1995;26:48–54. doi: 10.1161/01.hyp.26.1.48. [DOI] [PubMed] [Google Scholar]
- 29.Tauzin L. Alterations in viscoelastic properties following premature birth may lead to hypertension and cardiovascular disease development in later life. Acta paediatrica. 2015;104:19–26. doi: 10.1111/apa.12815. [DOI] [PubMed] [Google Scholar]
- 30.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. The Journal of pathology. 2000;190:292–9. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 31.Angelini GD, Breckenridge IM, Butchart EG, et al. Metabolic damage to human saphenous vein during preparation for coronary artery bypass grafting. Cardiovascular research. 1985;19:326–34. doi: 10.1093/cvr/19.6.326. [DOI] [PubMed] [Google Scholar]
- 32.Angelini GD, Breckenridge IM, Psaila JV, Williams HM, Henderson AH, Newby AC. Preparation of human saphenous vein for coronary artery bypass grafting impairs its capacity to produce prostacyclin. Cardiovascular research. 1987;21:28–33. doi: 10.1093/cvr/21.1.28. [DOI] [PubMed] [Google Scholar]
- 33.Boerboom LE, Olinger GN, Bonchek LI, et al. The relative influence of arterial pressure versus intraoperative distention on lipid accumulation in primate vein bypass grafts. The Journal of thoracic and cardiovascular surgery. 1985;90:756–64. [PubMed] [Google Scholar]
- 34.Desai M, Mirzay-Razzaz J, von Delft D, Sarkar S, Hamilton G, Seifalian AM. Inhibition of neointimal formation and hyperplasia in vein grafts by external stent/sheath. Vascular medicine. 2010;15:287–97. doi: 10.1177/1358863X10366479. [DOI] [PubMed] [Google Scholar]
- 35.Chester AH, Buttery LD, Borland JA, et al. Structural, biochemical and functional effects of distending pressure in the human saphenous vein: implications for bypass grafting. Coronary artery disease. 1998;9:143–51. [PubMed] [Google Scholar]
- 36.Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovascular research. 1993;27:1961–7. doi: 10.1093/cvr/27.11.1961. [DOI] [PubMed] [Google Scholar]
- 37.Okon EB, Millar MJ, Crowley CM, et al. Effect of moderate pressure distention on the human saphenous vein vasomotor function. The Annals of thoracic surgery. 2004;77:108–14. doi: 10.1016/j.athoracsur.2003.06.007. discussion 14–5. [DOI] [PubMed] [Google Scholar]
- 38.Viaro F, Carlotti CG, Jr, Rodrigues AJ, et al. Endothelium dysfunction caused by acute pressure distension of human saphenous vein used for myocardial revascularization. Revista brasileira de cirurgia cardiovascular: orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2007;22:169–75. doi: 10.1590/s0102-76382007000200004. [DOI] [PubMed] [Google Scholar]
- 39.LoGerfo FW, Quist WC, Crawshaw HM, Haudenschild C. An improved technique for preservation of endothelial morphology in vein grafts. Surgery. 1981;90:1015–24. [PubMed] [Google Scholar]
- 40.Stigler R, Steger C, Schachner T, et al. The impact of distension pressure on acute endothelial cell loss and neointimal proliferation in saphenous vein grafts. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery. 2012;42:e74–9. doi: 10.1093/ejcts/ezs402. [DOI] [PubMed] [Google Scholar]
- 41.Poston RS, Kwon MH, Gu J. Role of procurement-related injury in early saphenous vein graft failure after coronary artery bypass surgery. Future cardiology. 2006;2:503–12. doi: 10.2217/14796678.2.4.503. [DOI] [PubMed] [Google Scholar]
- 42.Chong CF, Ong PJ, Moat N, Collins P. Effects of hydrostatic distention on in vitro vasoreactivity of radial artery conduits. The Journal of thoracic and cardiovascular surgery. 2004;128:609–14. doi: 10.1016/j.jtcvs.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Bonchek LI. Prevention of endothelial damage during preparation of saphenous veins for bypass grafting. The Journal of thoracic and cardiovascular surgery. 1980;79:911–5. [PubMed] [Google Scholar]
- 44.Angelini GD, Bryan AJ, Hunter S, Newby AC. A surgical technique that preserves human saphenous vein functional integrity. The Annals of thoracic surgery. 1992;53:871–4. doi: 10.1016/0003-4975(92)91455-i. [DOI] [PubMed] [Google Scholar]
- 45.Waters DJ, Thomsen TA. Saphenous vein preparation for coronary artery bypass grafting using a cardioplegia delivery set. The Annals of thoracic surgery. 1993;56:385–6. doi: 10.1016/0003-4975(93)91189-t. [DOI] [PubMed] [Google Scholar]
- 46.Franklin SL, Jayadev C, Poulsen R, Hulley P, Price A. An ink surgical marker pen is damaging to tendon cells. Bone & joint research. 2012;1:36–40. doi: 10.1302/2046-3758.13.2000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andjelic S, Zupancic G, Hawlina M. The effect of gentian violet on human anterior lens epithelial cells. Current eye research. 2014;39:1020–5. doi: 10.3109/02713683.2014.894077. [DOI] [PubMed] [Google Scholar]
- 48.Stoeger C, Holiman J, Davis-Boozer D, Terry MA. The endothelial safety of using a gentian violet dry-ink “S” stamp for precut corneal tissue. Cornea. 2012;31:801–3. doi: 10.1097/ICO.0b013e31823f7571. [DOI] [PubMed] [Google Scholar]
- 49.Voskresensky IV, Wise ES, Hocking KM, et al. Brilliant blue FCF as an alternative dye for saphenous vein graft marking: effect on conduit function. JAMA surgery. 2014;149:1176–81. doi: 10.1001/jamasurg.2014.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peng W, Cotrina ML, Han X, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:12489–93. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piscopiello M, Sessa M, Anzalone N, et al. P2X7 receptor is expressed in human vessels and might play a role in atherosclerosis. International journal of cardiology. 2013;168:2863–6. doi: 10.1016/j.ijcard.2013.03.084. [DOI] [PubMed] [Google Scholar]
- 52.Lu DY, Chen EY, Wong DJ, et al. Vein graft adaptation and fistula maturation in the arterial environment. The Journal of surgical research. 2014;188:162–73. doi: 10.1016/j.jss.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramos JR, Berger K, Mansfield PB, Sauvage LR. Histologic fate and endothelial changes of distended and nondistended vein grafts. Annals of surgery. 1976;183:205–28. doi: 10.1097/00000658-197603000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao VK, Nightingale G, O’Brien BM. Scanning electron microscope study of microvenous grafts to artery. Plastic and reconstructive surgery. 1983;71:98–106. doi: 10.1097/00006534-198301000-00023. [DOI] [PubMed] [Google Scholar]