Abstract
Lipopolysaccharide (LPS)-mediated activation of Toll-like receptors (TLRs) in hepatic macrophages and injury to hepatocytes are major contributors to the pathogenesis of alcoholic liver disease (ALD). However, the mechanisms by which TLR-dependent inflammatory responses and alcohol-induced hepatocellular damage coordinately lead to ALD are not completely understood. In this study, we found that mice deficient in IRAKM, a proximal Toll-like receptor pathway molecule typically associated with inhibition of TLR signaling, were actually protected from chronic ethanol-induced liver injury. In bone marrow derived macrophages challenged with low concentrations of LPS, which reflect the relevant pathophysiological levels of LPS in both alcoholic patients and ethanol-fed mice, the IRAKM Myddosome was preferentially formed. Further, the IRAKM Myddosome mediated the up-regulation of Mincle, a sensor for cell death. Mincle-deficient mice were also protected from ethanol-induced liver injury. The endogenous Mincle ligand, SAP130 is a danger signal released by damaged cells; culture of hepatocytes with ethanol increased the release of SAP130. Ex vivo studies in bone marrow derived macrophages suggested that the endogenous Mincle ligand, SAP130, and LPS synergistically activated inflammatory responses, including inflammasome activation. Conclusion: This study reveals a novel IRAKM-Mincle axis that contributes to the pathogenesis of ethanol-induced liver injury.
Keywords: Toll-like receptor, C-type lectin receptor, innate immune response
Introduction
Alcoholic liver disease (ALD) ranges from simple steatosis to alcoholic hepatitis, fibrosis, cirrhosis and hepatocellular carcinoma. Alcohol exposure induces endoplasmic reticulum (ER) stress and mitochondrial dysfunction in hepatocytes, which leads to hepatocyte apoptosis, necrosis, necroptosis and inflammation (1). Alcohol disrupts the balance of gut microflora associated with an increased intestinal permeability, resulting in increased translocation of bacterial products into the circulation (2, 3). Increased levels of lipopolysaccharide (LPS) are indeed detected in the serum of alcoholic patients (4, 5) and alcohol-treated experimental animals, ranging from 80–130 pg/ml (5–9). The activation of hepatic macrophages (Kupffer cells) in response to portal endotoxin/LPS plays a key role in the early pathogenesis of alcohol-induced liver injury. LPS recognition by Toll-like receptor 4 (TLR4) on hepatic macrophages results in the release of inflammatory cytokines, such as TNF and IL-1, that can in turn impact hepatocyte function. Mice deficient in TLR4 or interlukin-1 receptor (IL-1R) are resistant to ethanol-induced liver disease (9, 10). Conversely, previous studies have shown that signals released from hepatocytes injured by alcohol are also critical to the activation of hepatic macrophages. Thus, one important question is how TLR-dependent inflammatory responses and alcohol-induced cellular damage coordinately lead to the pathogenesis of ALD.
TLRs transduce signals through the adaptor molecule MyD88 and IL-1R-associated kinase (IRAK) family members, which include: IRAK1, IRAK2, IRAKM (also known as IRAK3) and IRAK4 (11). We recently reported the co-existence of two parallel TLR-mediated NFκB activation pathways: TAK1-dependent and MEKK3-dependent, respectively (12–14). IRAK4 kinase activity and its substrates, IRAK1 and IRAK2, are necessary for TAK1-dependent NFκB activation and mRNA stabilization of chemokines and cytokines, but not for MEKK3-dependent NFκB activation (15–17). IRAKM is able to interact with MyD88-IRAK4 to form IRAKM Myddosome, allowing it to control TAK1-independent MEKK3-dependent NFκB activation (18). This IRAKM-dependent pathway is necessary for the second wave of TLR-induced NFκB activation in the company of IRAK1/IRAK2. Notably, the IRAKM-dependent pathway only induces expression of the inhibitory molecules SOCS1, SHIP1, A20 and IκBα. Thus, IRAKM exerts an overall inhibitory effect on inflammatory response.
In this study, we were surprised to discover that IRAKM-deficient mice were protected from ethanol-induced liver injury and inflammation. Mechanistically, IRAKM-dependent NFκB activation was the dominant pathway in hepatic macrophages challenged with low dose of LPS (100 pg/ml). Analysis of TLR4-induced IRAKM-dependent genes revealed one gene of particular interest: Mincle, a C-type lectin receptor, that senses non-homeostatic cell death. Induction of Mincle in hepatic macrophages was ablated in IRAKM-deficient mice and Mincle-deficient mice were protected from ethanol-induced liver injury. Spliceosome-associated Protein 130 (SAP130, also known as SF3B3), the endogenous ligand of Mincle, was released from hepatocytes after ethanol exposure, while recombinant SAP130 synergized with low dose LPS to robustly induce inflammatory gene expression and activate the inflammasome in macrophages. Since Mincle is a sensor for cell death and its expression is IRAKM-dependent, our results suggest that TLR-induced IRAKM-dependent Mincle up-regulation in hepatic macrophages critically links alcohol-induced cell death to subsequent inflammatory responses, contributing to the pathogenesis of ALD.
Materials and methods
Details regarding materials, details of the animal procedures and basic biochemical methods are given in the Supporting Information.
Mouse models
IRAKM deficient and Mincle deficient mice were previously described (19, 20). All procedures using animals were approved by the Cleveland Clinic Institutional Animal Care and Use Committee. KO and wild-type (WT) mice in the ethanol-fed groups were allowed free access to an ethanol containing diet: 1% (vol/vol) ethanol for 2d followed by 2% ethanol for 2d, 4% ethanol for 1w, 5% ethanol for 1w followed by 6% ethanol (32% of total calories) for the final week. Control mice were pair-fed a control diet which iso-calorically substituted maltose dextrins for ethanol over the entire feeding period.
Inflammasome activation
Bone marrow derived macrophages (BMDMs) were plated in 6-well plates at a concentration of 2.0 × 106 cells per well the day before the experiment. The day of the experiment, cells were stimulated with low concentration of LPS (100 pg/ml), recombinant SAP130 (5 µg/ml) or TDB (10 µg/ml) for the indicated time. Cell free supernatants were then prepared as described in Supporting information.
Immunoblotting
Cells and tissues were harvested and lysed in a Triton-containing lysis buffer (0.5% Triton X-100, 20 mM HEPES (pH 7.4), 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM dithiothreitol, 1 mM sodium orthovanadate, 2mM EGTA, 1 mM phenylmethylsulfonyl fluoride and complete protease inhibitor cocktail from Roche). Cell lysates were then separated by 10% SDS-PAGE, transferred to Immobilon-P membranes (Millipore), and subjected to immunoblotting.
Data analysis and Statistics
Date were normally distributed, if not, non-parametric statistical analysis was applied to all data sets. Data are expressed as mean ± SEM. Differences were analyzed by Student t test and one-way ANOVA were used to compare normally distributed data set. Mann-Whitney U test and Kruskal-Wallis test were used for non-parametric analysis. P < 0.05 was considered significant.
Results
IRAKM is required for low dose LPS-mediated NFκB activation
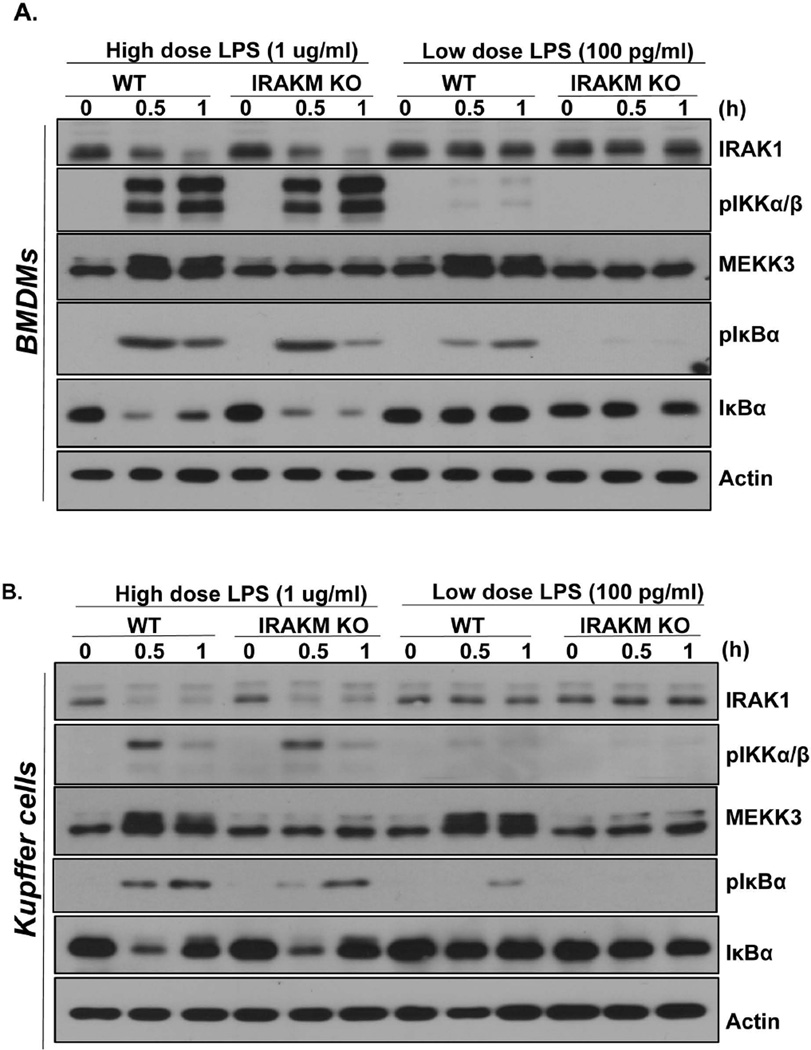
Recently, we reported a novel role for IRAKM in TLR signaling: IRAKM interacts with MyD88-IRAK4 to mediate a MEKK3-dependent second wave NFκB activation (18). In response to typical doses of LPS (10 ng/ml-1 µg/ml), MEKK3 modification and second wave IκBα phosphorylation are ablated in IRAKM-deficient macrophages, whereas LPS-induced IRAK1 modification/degradation, TAK1 activation and IKKα/β phosphorylation are not affected ((18), Fig. 1A and Suppl. Fig. 1). This IRAKM-dependent second wave of NFκB activation in response to LPS (10 ng/ml–1 µg/ml) induces expression of genes that are not regulated at the posttranscriptional level (including inhibitory molecules SOCS-1, SHIP-1, A20 and IκBα).
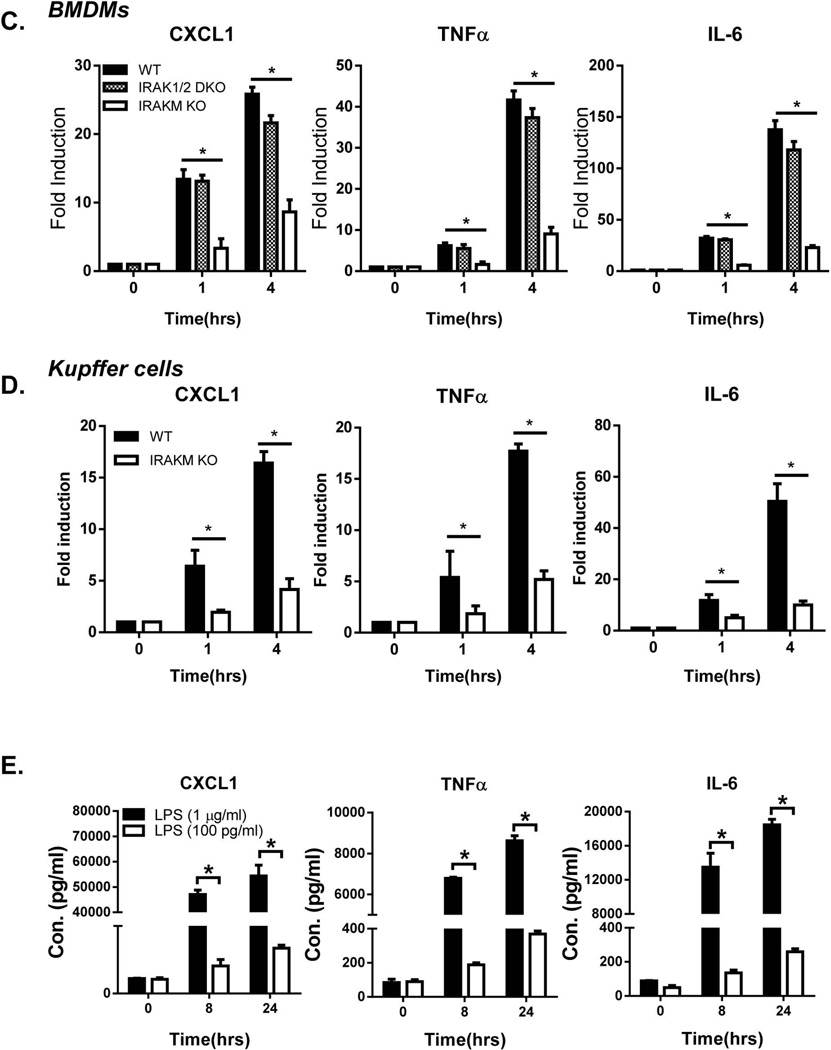
Fig. 1. IRAKM is required for low dose LPS-mediated NFκB activation.
Cell lysates were prepared from A. WT and IRAKM KO BMDMs and B. primary cultures of mouse Kupffer cells after they were untreated or treated with high dose LPS (1 µg/ml) and low dose LPS (100 pg/ml) for the indicated times and analyzed by Western blot analysis. C. Total mRNA from BMDMs of WT, IRAK1/2-DKO and IRAKM KO mice treated with low dose LPS (100 pg/ml) for the indicated times were subjected to RT-PCR analyses. D. Total mRNA from BMDMs of WT and IRAKM KO mice treated with low dose LPS (100 pg/ml) for the indicated times were subjected to RT-PCR analyses. E. BMDMs were treated with high dose LPS (1 µg/ml) or low dose LPS (100 pg/ml) for 24 hours. Cell-free supernatants were collected and cytokine concentrations were measured by ELISA assay. The experiments were repeated for five times with similar results. Data represent mean ± SEM; *, P<0.05.
Surprisingly, here we find that IRAKM-MEKK3-dependent NFκB activation was actually the dominant pro-inflammatory pathway in response to stimulation with low dose of LPS (100 pg/ml). In BMDMs (Fig. 1A) or primary Kupffer cells (Fig. 1B) treated with low dose LPS (100 pg/ml), IκBα phosphorylation – without IκBα degradation was observed, while IRAK1 modification/degradation and IKKα/β phosphorylation were absent, together indicating MEKK3-dependent NFκB activation. Importantly, IκBα phosphorylation and MEKK3 modification induced by low dose LPS were completely abolished in IRAKM-deficient cells (Fig. 1A & B). Based on these results, we hypothesized that IRAKM is dominantly activated to mediate MEKK3-dependent NFκB activation at low concentrations of LPS, whereas the IRAK1-TAK1-dependent pathway is not activated by low dose LPS. In support of this, we found that mRNA induction of the inflammatory genes CXCL1, TNF-α and IL-6 in response to low dose LPS was normal in IRAK1/2-deficient macrophages, but was greatly reduced in IRAKM-deficient cells (Fig. 1C). Similar results were also observed in cultures of primary Kupffer cells isolated from liver of WT and IRAKM-deficient mice (Fig. 1 D). Together, these data suggest that low dose LPS preferentially induces formation of the IRAKM Myddosome, leading to MEKK3-dependent NFκB activation.
Low-dose LPS preferentially induces the formation of an IRAKM Myddosome via the death domain of IRAKM
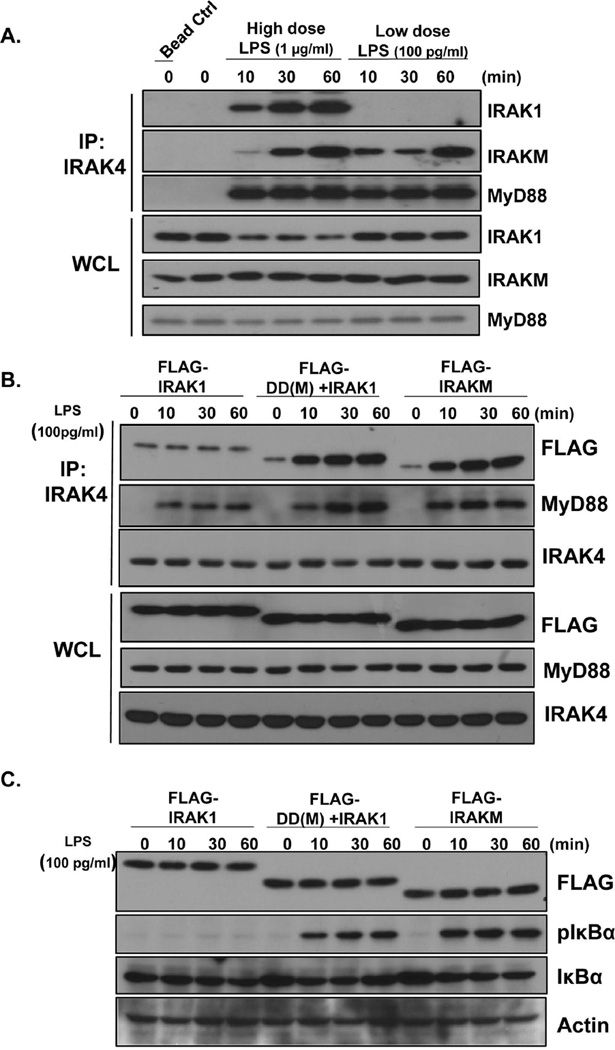
One important question is how low dose LPS activate the IRAKM-MEKK3 pathway. We previously reported that upon high dose ligand stimulation, the kinase activity of IRAK4 is required for TLR-induced IRAK1-TAK1-, but not MEKK3-, dependent NFκB activation (12–14). Further, IRAK1 and its phosphorylation are required for TLR-induced TAK1- but not MEKK3-, dependent NFκB activation in response to high doses of ligand (15, 16). Thus, we hypothesized that the TLR4 complex induced by low dose LPS fails to trigger the aggregation of IRAK4, leading to recruitment of IRAKM, but not IRAK1, and formation of the IRAKM Myddosome, with subsequent MEKK3-dependent NFκB activation. We indeed found that, upon low dose LPS stimulation (100 pg/ml), IRAKM, but not IRAK1, was recruited to the IRAK4-MyD88 complex, whereas both IRAK1 and IRAKM interacted with IRAK4 upon high dose LPS stimulation (1 µg/ml) (Fig. 2A). Furthermore, low dose LPS-induced MEKK3 modification and NFκB activation were unaffected in IRAK4 kinase inactive knock-in (KI) BMDMs (Suppl. Fig. 2). Taken together, these data suggest that IRAKM-dependent MEKK3-mediated NFκB activation in response to low dose LPS does not require IRAK4 kinase activity.
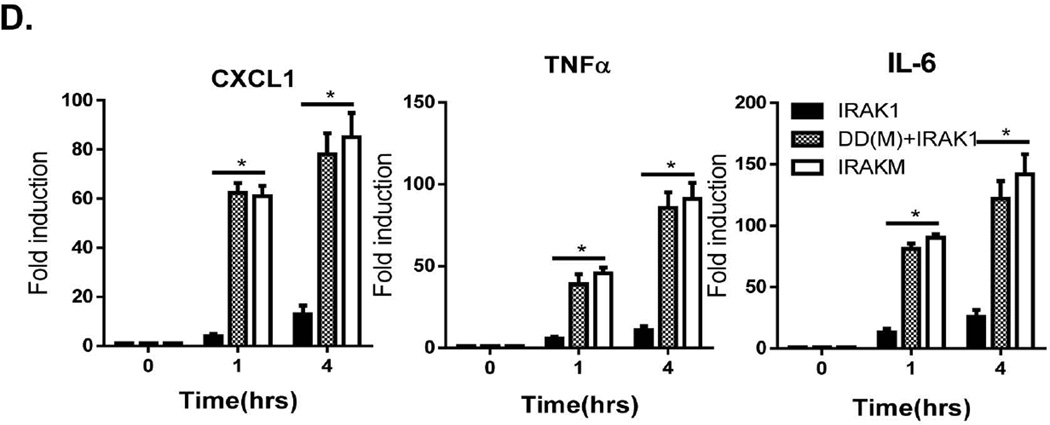
Fig. 2. Low-doses LPS preferentially induces the formation of IRAKM Myddosome via the death domain of IRAKM.
A. WT BMDMs were treated with high dose LPS (1 µg/ml) and low dose LPS (100 pg/ml) for the indicated times, followed by immunoprecipitation (IP) with anti-IRAK-4 antibody and analyzed by Western blot analysis. B–D. IRAK-1/2/M-triple deficient immortalized BMDMs infected with adenovirus expressing FLAG-tagged IRAK1, FLAG-tagged chimeric IRAKM death domain with IRAK1 kinase domain (DD(M)+IRAK1) and FLAG-tagged IRAKM were treated with low dose LPS (100 pg/ml) for indicated times. B. immunoprecipitates and C. cell lysates were analyzed by Western blot analysis. D. Total mRNA from transfected cells was subjected to RT-PCR analyses.
To further elucidate the mechanism for the specific activation of the IRAKM-MEKK3-dependent pathway by low dose LPS, we compared IRAKM versus IRAK1 in their differential recruitment to the TLR-MyD88-IRAK4 complex. The crystal structure of the Myddosome complex suggests that assembly of MyD88-IRAK4 with IRAK1, IRAK2 or IRAKM occurs via death domain (DD) interactions (21). We thus hypothesized that differential recruitment of IRAKM versus IRAK1 in response to low versus high dose LPS stimulation is determined by their respective death domains. We generated a chimeric protein by replacing the death domain of IRAK1 with that of IRAKM (DD(M)-IRAK1). IRAK1/2/M-deficient macrophages were then retrovirally infected with Flag-tagged wild-type IRAK1, wild-type IRAKM, and DD(M)-IRAK1, followed by stimulation with low dose LPS. Indeed, we found that interaction of both wild-type IRAKM and DD(M)-IRAK1, but not IRAK1, with MyD88-IRAK4 was induced in response to low dose LPS stimulation (Fig. 2B). The weak constitutive interaction of between IRAK1 and IRAK4 was probably due to the overexpression of IRAK1 (Fig. 2B). Similarly, IRAKM and DD(M)-IRAK1, but not IRAK1, restored low dose LPS-induced IκBα phosphorylation (Fig. 2C) and target gene expression (Fig. 2D). Together, these data suggest that the IRAKM death domain mediates the specific recruitment of IRAKM to the TLR-MyD88-IRAK4 complex in response to low dose LPS and promotes MEKK3-dependent NFκB activation.
IRAKM-dependent pathway is required for the development of chronic ethanol-induced liver disease
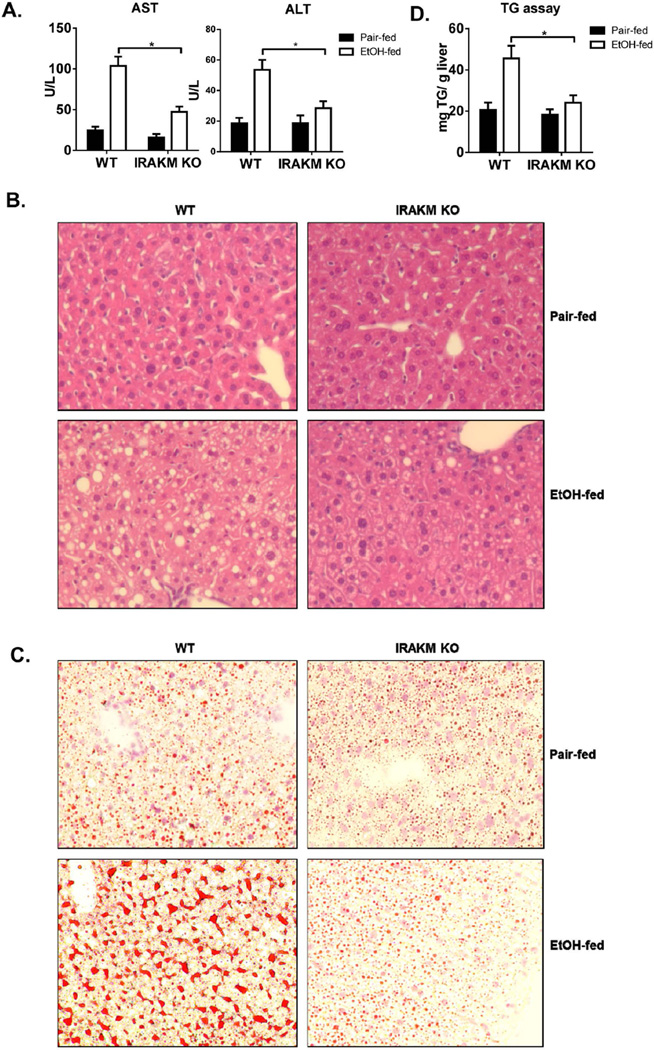
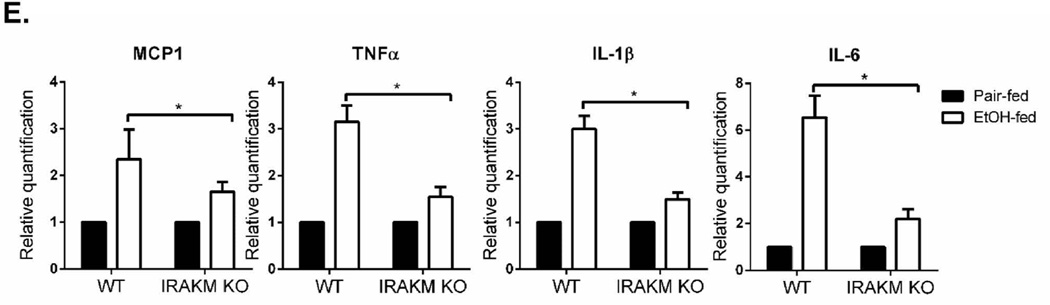
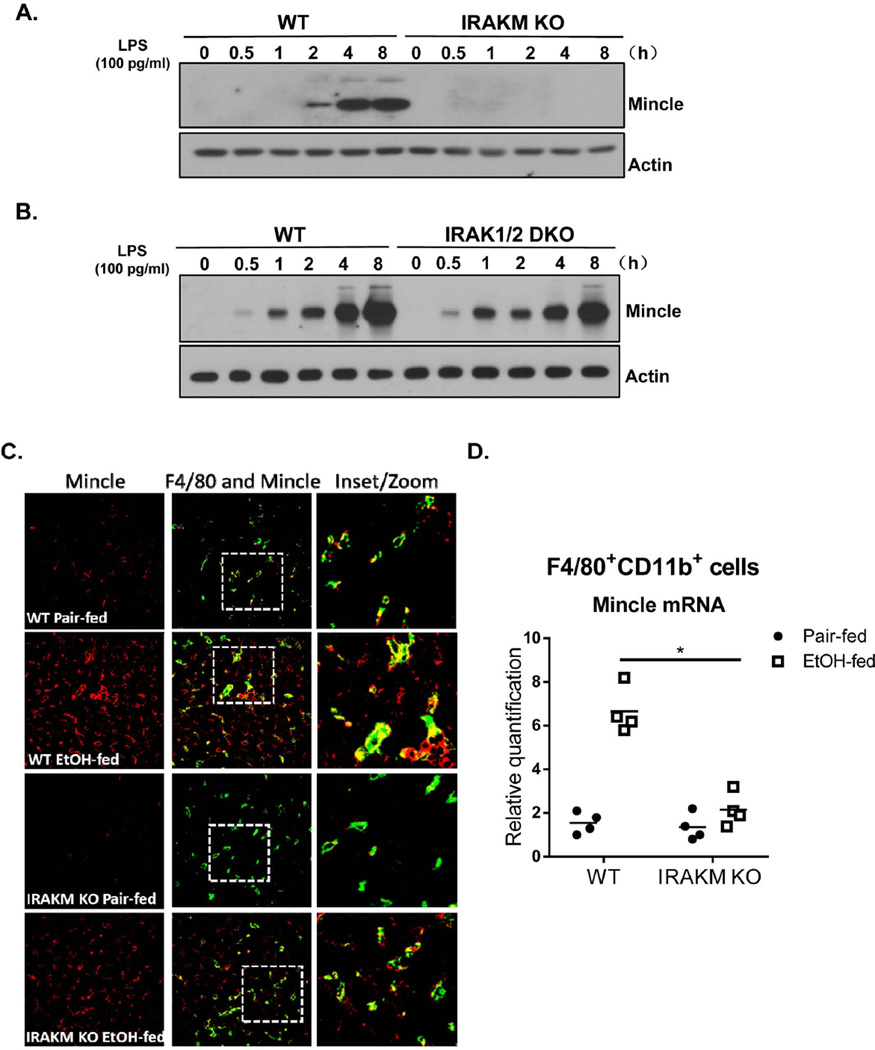
Alcohol intake increases gut permeability, allowing accumulation of low levels of TLR ligands in the circulation. Activation of hepatic macrophages (Kupffer cells) in response to portal endotoxin/LPS plays a key role in the early pathogenesis of ALD (22). Considering the reported low concentrations of LPS in serum of alcoholic patients and ethanol-treated experimental animals (ranging from 80–130 pg/ml), our findings led us to hypothesize that IRAKM-dependent signaling induced at low concentrations of LPS might be the dominant TLR-activated pathway in ALD. To test this hypothesis, we subjected female IRAKM-deficient and littermate control WT mice to chronic ethanol feeding via the Lieber-DeCarli liquid diet to model steatosis and mild inflammation. Compared to pair-fed control mice, chronic ethanol-fed WT mice had increased hepatocyte injury, as assessed by serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Fig. 3A) and steatosis, characterized by the presence of lipid droplets in hepatocytes by H&E and Oil Red O staining, (Fig. 3B and 3C) and hepatic triglycerides (Fig. 3D). Compared to WT mice, IRAKM-deficient mice had reduced serum AST and ALT levels (Fig. 3A) and hepatic steatosis (Fig. 3B–D). Moreover, IRAKM deficiency significantly reduced the chronic ethanol-induced upregulation of TNF-α, IL-6, pro-IL-1β and MCP-1 mRNA in liver of ethanol-fed mice (Fig. 3E). Together, these data support the crucial role of IRAKM in the pathogenesis of chronic ethanol-induced inflammatory responses in the liver.
Fig. 3. IRAKM-dependent pathway is required for the development of chronic ethanol-induced liver disease.
WT and IRAKM KO mice were allowed free access to ethanol (EtOH) (n=6) or pair-fed control diets (n=4). A. AST and ALT activity was determined in plasma. B. Paraffin-embedded liver sections were stained with hematoxylin and eosin. C. Frozen liver sections were subjected to Oil Red O staining. All images were acquired using a 10× objective. D. Hepatic triglyceride content was measured in whole liver homogenates. E. Total mRNAs from livers of WT and IRAKM KO mice (pair-fed and EtOH-fed) were subjected to RT-PCR analysis.
IRAKM is required for TLR-mediated Mincle expression
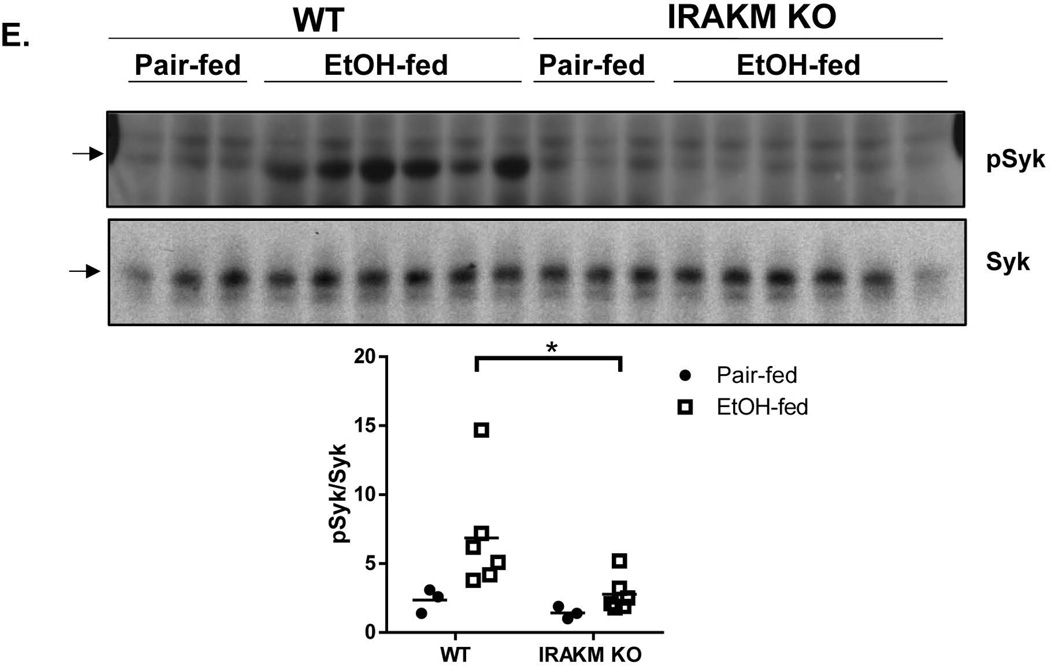
Given that low dose of LPS induced only modest levels of inflammatory cytokines and chemokines in wild-type BMDMs (Fig. 1E), we suspected that the impact of IRAKM on ALD might not be solely due to its direct role in induction of canonical inflammatory genes. Interestingly, we found that Mincle, a C-type lectin (CLR) membrane receptor that senses cell death, was strongly induced by low dose of LPS in WT, but not in IRAKM-deficient, BMDMs (Fig. 4A) and primary Kupffer cells (Suppl. Fig. 3A). It is important to note that induction of Mincle was not affected in IRAK1/2-double deficient macrophages (Fig. 4B).
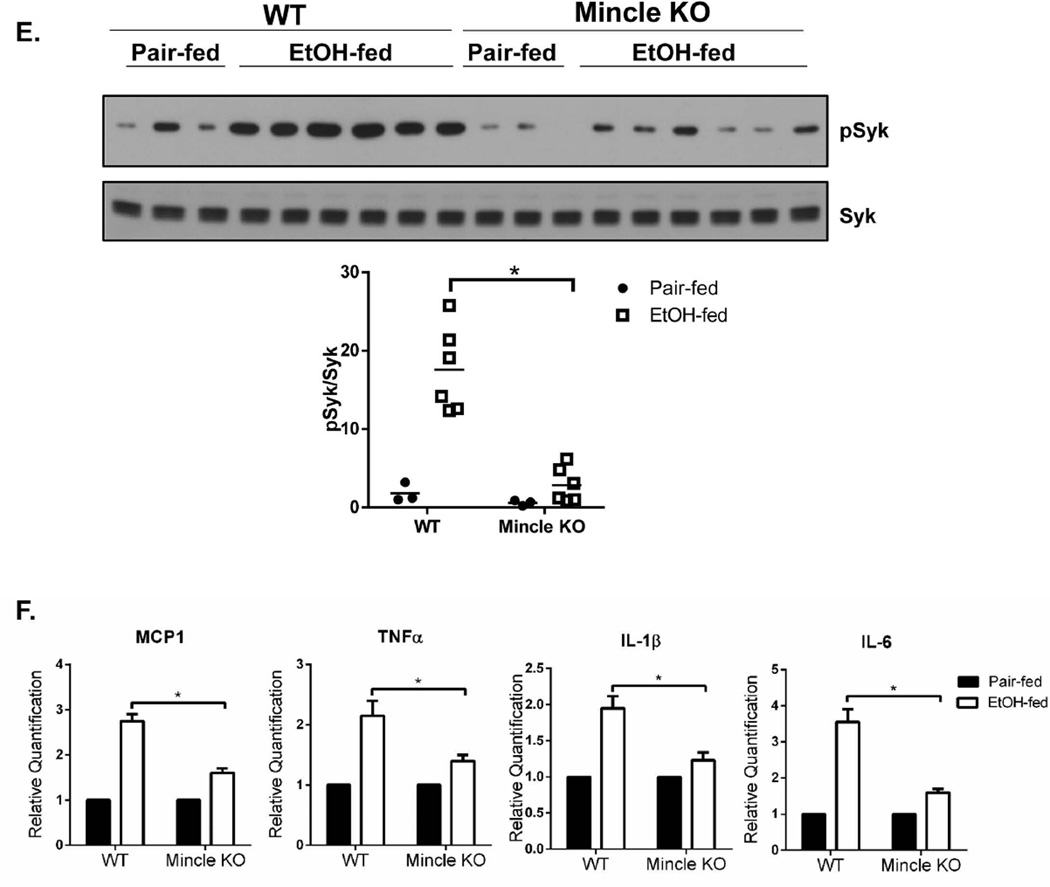
Fig. 4. IRAKM is required for TLR and chronic ethanol-induced Mincle upregulation.
A. Cell lysates from WT and IRAKM KO BMDMs untreated or treated with low dose LPS (100 pg/ml) for the indicated times were analyzed by Western blot analysis B. Cell lysates from WT and IRAK1/2 DKO BMDMs untreated or treated with low dose LPS (100 pg/ml) for the indicated times were analyzed by Western blot analysis. C. Immunostaining of F4/80 (green) and Mincle (red) was performed on frozen sections of liver from of WT and IRAKM KO mice (pair-fed and EtOH-fed). Confocal images are representative of five mice per group. D. Resident macrophages (F4/80+CD11b+) from livers of pair-fed and ethanol-fed mice were isolated by flow cytometry-based sorting. Total RNA was subjected to RT-PCR analysis. E. Tissue lysates from the whole liver of WT and IRAKM KO mice (pair-fed and EtOH-fed) were analyzed by Western blot analysis.
We next examined Mincle expression in liver in pair-fed and ethanol-fed WT and IRAKM-deficient mice. Interestingly, immunofluorescent staining showed that Mincle expression was increased in liver of ethanol-fed wild-type mice. Mincle expression co-localized with F4/80, a marker of resident macrophages (Fig. 4C). In contrast, chronic ethanol-induced expression of Mincle was attenuated in IRAKM-deficient mice. To further confirm this finding, we performed flow cytometry-based sorting of macrophages (F4/80+CD11b+) from the livers of pair-fed and ethanol-fed mice. Mincle mRNA expression was induced by ethanol-feeding in resident macrophages of WT mice; this response was attenuated in resident macrophages from IRAKM-deficient mice (Fig. 4D). Consistent with an induction of Mincle, the phosphorylation of Syk, a tyrosine kinase known to be activated CLR-mediated signaling(23, 24), was increased in the liver of ethanol-fed WT mice, but not in IRAKM-deficient mice (Fig. 4E). Taken together, these data implicate the induction of Mincle as a possible effector in mediating the impact of IRAKM in the pathogenesis of ethanol-induced liver injury.
Mincle-mediated pathway is required for the development of chronic ethanol-induced liver disease
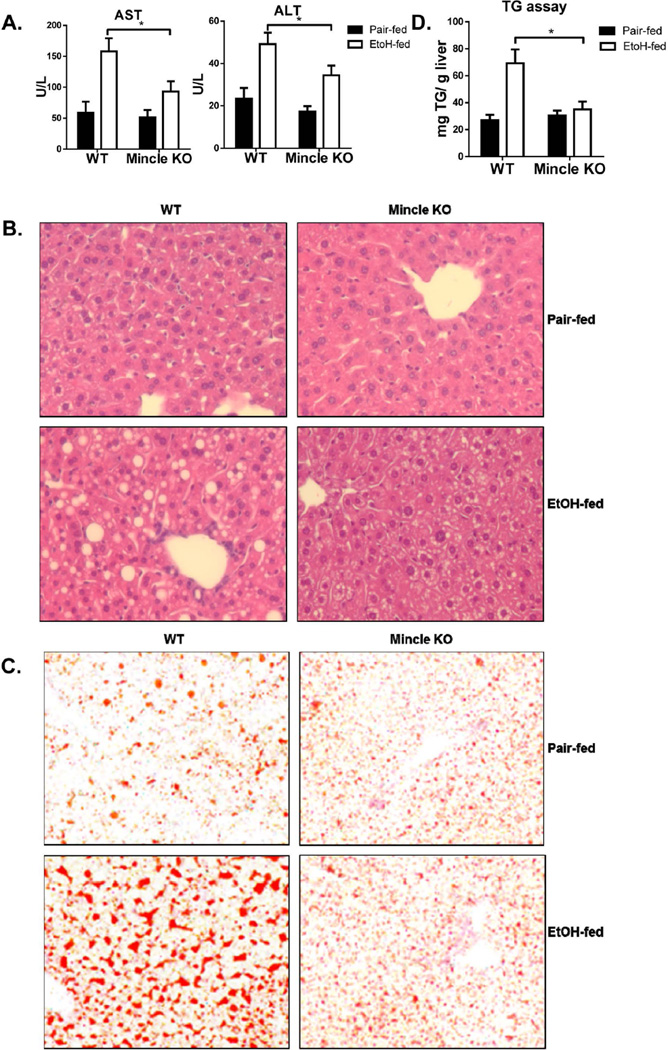
Given that ethanol feeding induced Mincle expression in hepatic macrophages in an IRAKM-dependent manner, we hypothesized that this upregulation of Mincle might contribute to the pathogenesis of ALD. To test this hypothesis, we examined the impact of genetic deletion of Mincle on ethanol-induced liver injury. Compared to WT, Mincle-deficient mice indeed showed reduced chronic ethanol-induced hepatocyte injury and steatosis, demonstrated by lower levels of ALT/AST in the plasma, reduced lipid droplet formation in hepatocytes and reduced hepatic triglyceride concentrations (Fig. 5A–D). Further, phosphorylation of Syk was upregulated in liver of ethanol-fed WT mice, but not Mincle-deficient mice (Fig. 5E). Mincle deficiency also attenuated the upregulation of TNF-α, IL-6, pro-IL-1β and MCP-1 gene expression in the livers of ethanol-fed mice, suggesting a crucial role for Mincle in the pathogenesis of chronic ethanol-induced inflammatory responses in the liver (Fig. 5F).
Fig. 5. Mincle-dependent pathway is required for the development of chronic ethanol-induced liver disease.
WT and Mincle KO mice were allowed free access to ethanol (EtOH) (n=6) or pair-fed control diets (n=4). A. AST and ALT activity was determined in plasma. B. Paraffin-embedded liver sections were stained with hematoxylin and eosin. C. Frozen liver sections were subjected to Oil Red O staining. All images were acquired using a 10× objective. D. Hepatic triglyceride content was measured in whole liver homogenates. E. Tissue lysates from the whole liver of WT and Mincle KO mice (pair-fed and EtOH-fed) were analyzed by Western blot analysis. The black arrows point out the specific bands of pSyk. F. Total mRNAs from livers of WT and Mincle KO mice (pair-fed and EtOH-fed) were subjected to RT-PCR analysis.
SAP130 activates Mincle and induces inflammasome activity following priming with low dose LPS
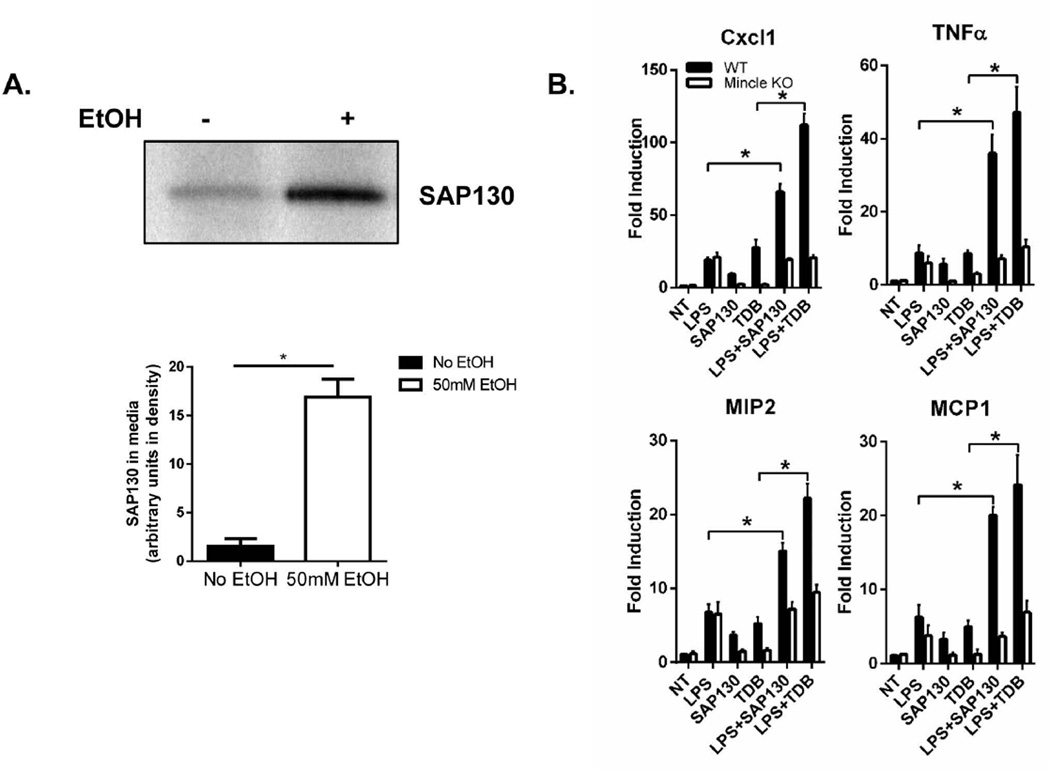
Mincle was originally discovered based on its strong induction in macrophages by inflammatory stimuli, including TLR ligands (25). Interestingly, a known endogenous ligand for Mincle is SAP130, a component of the small nuclear ribonucloprotein, which diffuses out of dying cells and initiates an inflammatory response (19). Alcohol exposure is known to induce endoplasmic reticulum (ER) stress and mitochondrial dysfunction in hepatocytes, resulting in hepatocyte apoptosis, necroptosis and necrosis. Exposure of primary hepatocyte cultures to ethanol results in both apoptotic and necroptotic cell death (26) (Suppl. Fig. 4). Interestingly, we observed that hepatocytes challenged with ethanol released SAP130 into the culture supernatant (Fig. 6A), suggesting the possibility that Mincle might mediate chronic ethanol-induced inflammatory responses in the liver in response to SAP130. We indeed found that, while individually low dose LPS or SAP130 only induced very low levels of inflammatory gene expression in BMDMs, SAP130 was able to induce much higher levels of inflammatory gene expression in wild-type BMDMs primed by low dose of LPS (Fig. 6B). This synergistic impact of low LPS with SAP130 was abolished by Mincle deficiency, indicating low LPS-induced Mincle expression allows SAP130, a ligand associated with dying cells, to induce a robust inflammatory response (Fig. 6B).
Fig. 6. SAP130-mediated mincle activation is required for low concentration LPS induced inflammation through inflammasome activation.
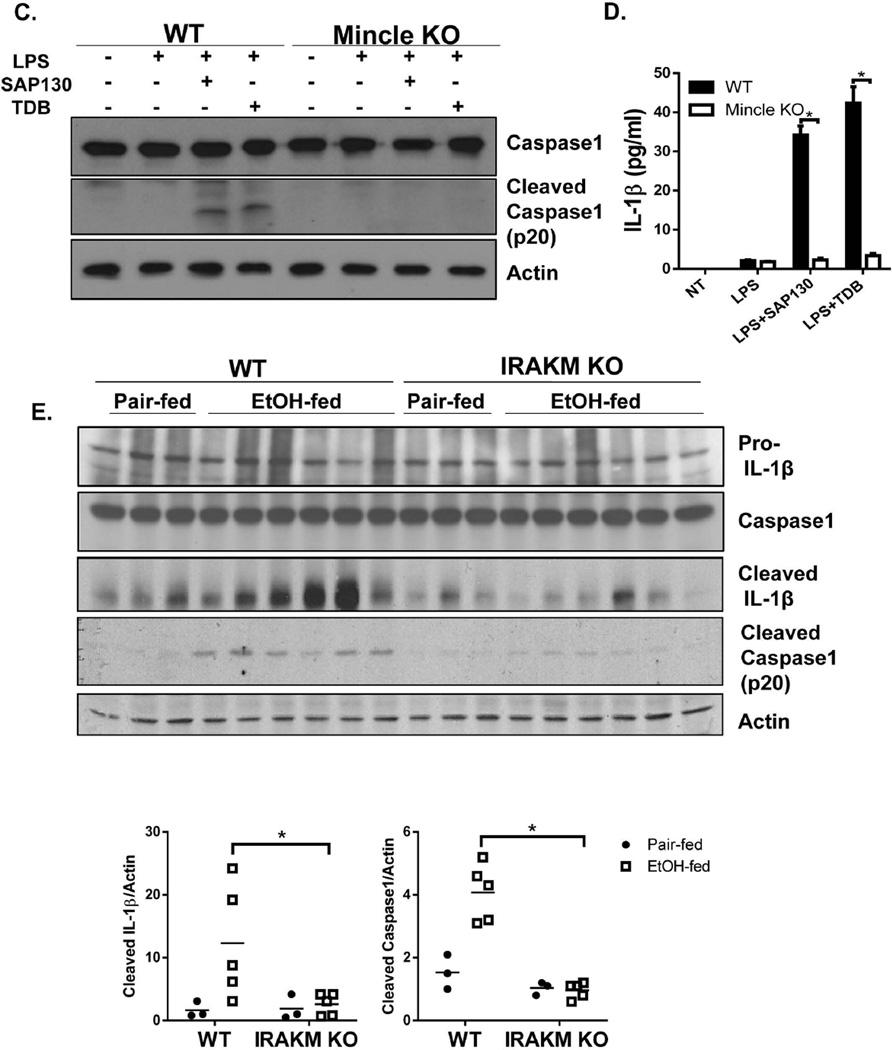
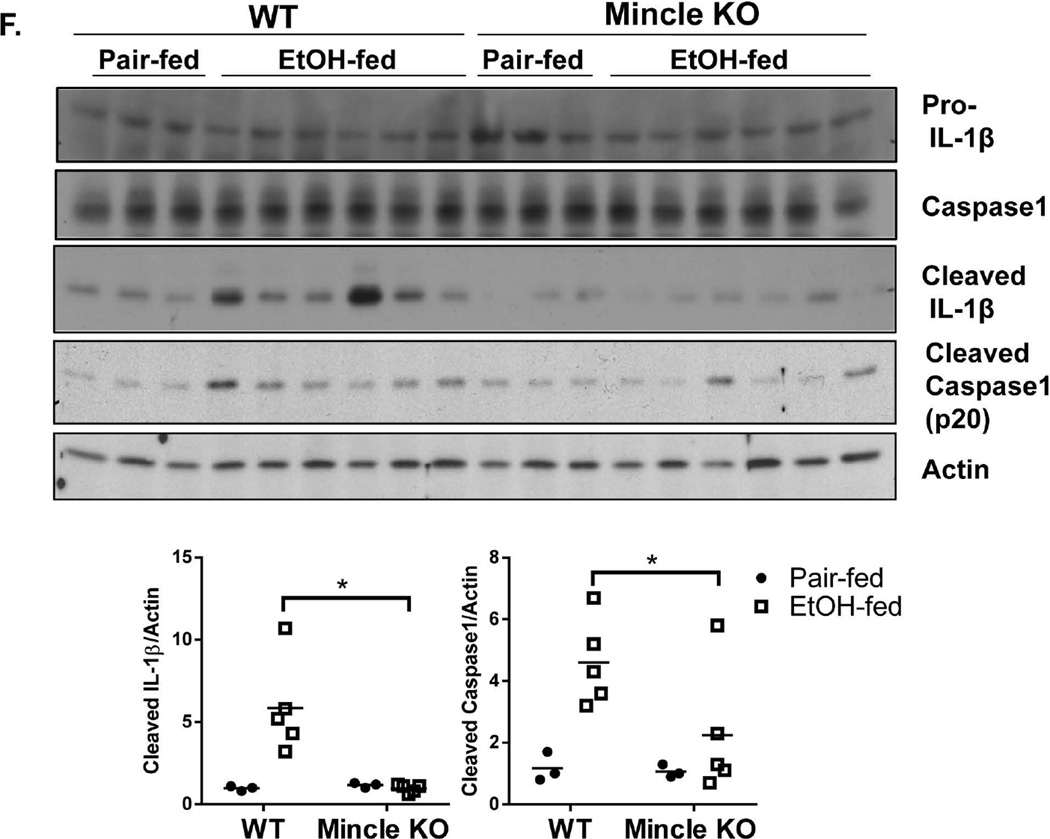
A. Primary hepatocytes from WT mice were treated with EtOH (50 mM) for 24 hours. Cell-free supernatants were collected and SAP130 was measured by Western blot analysis. B. BMDMs from WT and Mincle KO mice were treated with PBS, LPS (100 pg/ml) for 24 hours, SAP130 (5 ug/ml) for 2 hours, TDB (100 µg/ml, 2 hours), LPS (100 pg/ml, 24 hours) + SAP130 (5 µg/ml, 2 hours) or LPS (100 pg/ml, 24 hours) + TDB (100 µg/ml, 2 hours). Total mRNA was subjected to RT-PCR analyses. C. BMDMs from WT and Mincle KO mice were treated with PBS, LPS (100 pg/ml) for 24 hours, LPS (100 pg/ml, 24 hours) + SAP130 (5 µg/ml, 6 hours) or LPS (100 pg/ml, 24 hours) + TDB (100 µg/ml, 6 hours). Cell lysates and supernatants were collected together and analyzed by Western blot. D. IL-1β was measured in Cell-free supernatants by ELISA. E. Tissue lysates from the whole liver of WT and IRAKM KO mice (pair-fed and EtOH-fed) were analyzed by Western blot. F. Tissue lysates from the whole liver of WT and Mincle KO mice (pair-fed and EtOH-fed) were analyzed by Western blot analysis.
In addition to ligands released during necrotic cell death, fungal and mycobacterial ligands for Mincle have also been identified. Mincle is essential for recognition of the mycobacterial cord factor and its synthetic analog Trehalose-Dibehenate (TDB) (27). Interestingly, while TDB has been shown to activate the NLRP3 inflammasome (28, 29), Dectin-1, another member of C-type lectin receptors, activates processing of IL-1β via a noncanonical caspase-8 inflammasome (30). Thus, we tested the possibility that Mincle might also mediate inflammasome activation in response to SAP130. Indeed, recombinant SAP130 induced caspase-1 cleavage and IL-1β production in macrophages primed with low dose LPS (to up-regulate Mincle). This response was abolished in Mincle-, ASC- and NLRP3-deficient macrophages (Fig. 6 C–D & Suppl. Fig. 5). These results suggest that Mincle ligation by SAP130 in macrophages results in activation of ASC-dependent inflammasomes.
Recent studies have shown that mice deficient in inflammasome components, such as the adaptor ASC and caspase 1, or IL-1R are protected from ethanol-induced liver injury (10). However, it has remained unclear how the inflammasome is activated in the liver of ethanol-fed mice. Based on the ability of recombinant SAP130 to activate the inflammasome in vitro, we hypothesized that in vivo Mincle senses SAP130 released during chronic ethanol-induced hepatocyte necrosis or necroptosis. SAP130 ligation of Mincle then activates an NLRP3/ASC-dependent inflammasome, leading to caspase1 activation and IL-1β production. Indeed, we detected increased caspase-1 activity (shown as cleaved caspase 1 and processed IL-1β) in the liver of ethanol-fed mice, which was greatly reduced in IRAKM- and Mincle-deficient mice (Fig. 6 E–F).
Discussion
Alcohol intake increases gut permeability and leads to accumulation of low levels of LPS in the circulation. Here we have identified a novel TLR4-IRAKM-dependent signaling pathway induced by low dose LPS that contributes to the pathogenesis of ALD. We found that low dose LPS preferentially induces the formation of an IRAKM Myddosome resulting in MEKK3-dependent NFκB activation. Importantly, whereas the low dose LPS-activated IRAKM Myddosome induced only very modest induction of inflammatory gene expression, it mediated strong up-regulation of Mincle, a sensor of cell death. SAP130, the endogenous ligand of Mincle, was released from hepatocytes after ethanol exposure, while recombinant SAP130 synergized with low dose LPS to robustly induce inflammatory gene expression and inflammasome activation in macrophages. Consistent with the IRAKM-Mincle synergy observed ex vivo, upregulation of Mincle was ablated in the liver of IRAKM-deficient mice and deficiency of either IRAKM or Mincle was strongly protective from chronic ethanol-induced liver injury (Fig. 7). Further, phosphorylation of Syk, a hallmark of Mincle signaling, was increased in the liver of ethanol-fed WT mice, but not in IRAKM-deficient mice. These results suggest that the IRAKM-Mincle axis may represent a critical and previously missing link between ethanol-induced hepatocellular damage and innate immune activation during the pathogenesis of ALD.
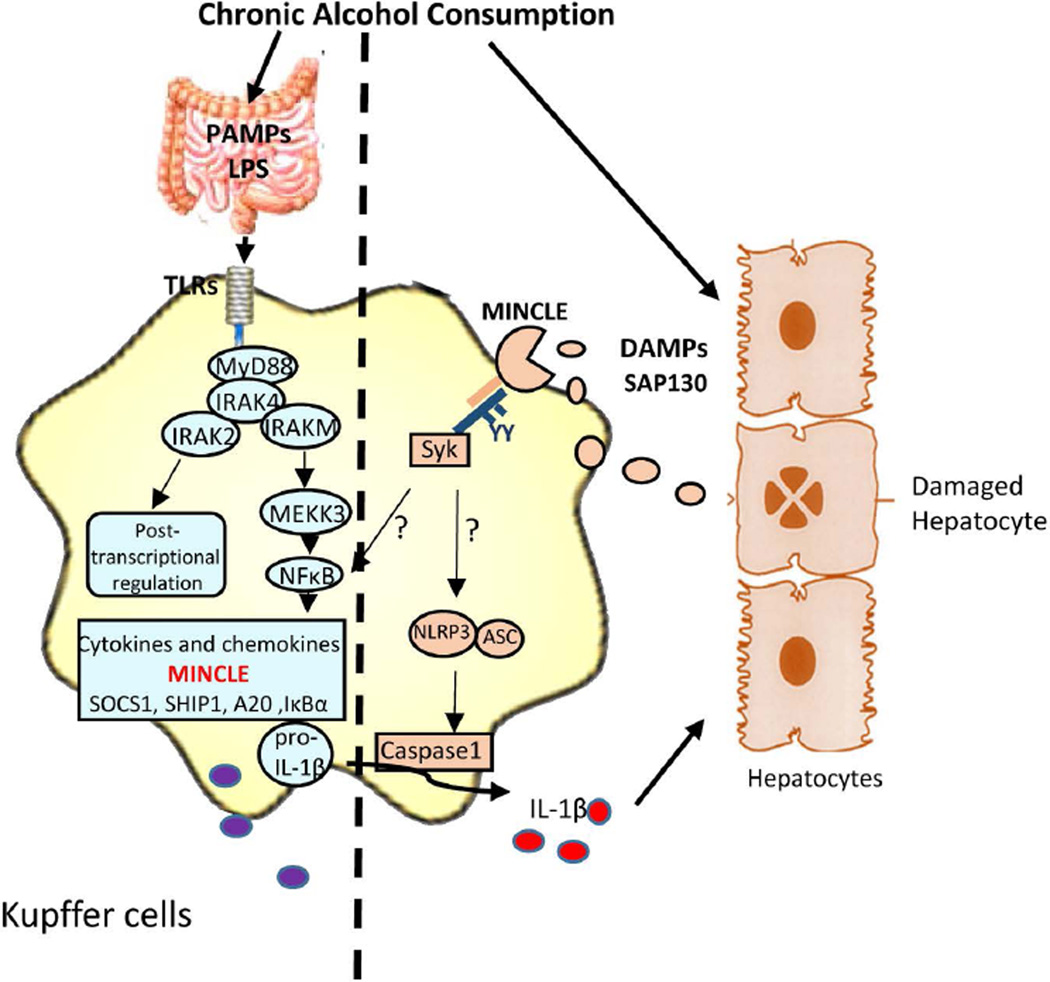
Fig. 7. IRAKM-Mincle axis contributes to the pathogenesis and development of ALD.
Chronic alcohol consumption results in increased intestinal permeability and changes in bacterial microflora increase levels of bacterial products in alcoholic patients and animal models of ALD. Further, alcohol exposure can induce endoplasmic reticulum (ER) stress and mitochondrial dysfunction in hepatocytes, contributing to hepatocellular injury and death. TLR4- induced IRAKM-mediated MEKK3-dependent NFκB activation is required for the up-regulation of Minlce in hepatic macrophages. Mincle sense the necroptotic hepatocytes-released nuclear protein, SAP130, which in turn activates the inflammasome activation in macrophages. Secreted IL-1β may further act on hepatocytes inducing pyroptosis or on Stellate cells which leads to fibrosis.
Low concentrations of LPS have been detected in alcoholic patients and in ethanol-treated experimental animals. Importantly, mice deficient in TLR4 show a reduced ALD phenotype (9, 31). To our knowledge, this is the first study to show that the MyD88 downstream component IRAKM-Mincle axis is required for the development and pathogenesis of chronic ethanol-induced liver injury. Interestingly, Wang et al reported that IRAKM deficiency worsened liver injury in mice in response to an acute challenge with alcohol; in their study, mice were treated with 10% alcohol in drinking water for 7 day then gavaged with alcohol (6 g/Kg body weight) on day 7 (32). Furthermore, Mandrekar et al, making use of primary human monocytes in culture, found that IRAKM differentially contributes to the acute and chronic effects of alcohol on LPS-induced inflammation (33). Taken together with our study, these results suggest that the IRAKM-Mincle axis may differentially contribute to acute versus chronic effects of ethanol. Another important model to consider is the acute on chronic (Gao-binge) model of ethanol exposure that induces a more severe liver damage (34). In preliminary studies, we found that Mincle expression was not induced in Kupffer cells isolated from mice exposed to the Gao-binge protocol (unpublished data, Li and Nagy), in contrast to the strong induction observed in response to the Lieber-DeCarli chronic ethanol feeding protocol used here (Fig. 4C & D). Future studies are required to carefully compare and contrast the role of IRAKM-Mincle axis in these different models of ethanol-induced liver injury, likely leading to a better understanding of the multiple pathways by which different drinking patterns (acute, chronic and binge) can differentially lead to liver injury.
We and others have previously shown that high doses of LPS lead to rapid clustering/aggregation of the TLR-MyD88-IRAK4 complex and activation of IRAK4, which then recruits and phosphorylates IRAK1 to activate TAK1-dependent NFκB activation (14). In support of this, we reported that IRAK4 dimerization is required for both IRAK4 auto-phosphorylation and activation of kinase activity (35). Importantly, the IRAKM Myddosome, via the MEKK3-dependent pathway, is required for the late phase of NFκB activation which upregulates the inhibitory molecules SOCS-1, SHIP-1, A20 and IκBα. Furthermore, IRAKM, through interaction with IRAK2, inhibits TLR-mediated production of cytokines and chemokines at the levels of translational. Thus, IRAKM is known to function as a net negative regulator of overt microbial infection (modeled in vitro by high-doses LPS) (20). However, the function of IRAKM in chronic inflammatory diseases, in which LPS concentrations are much lower than in response to infections, has not been carefully studied. Here, our data suggest that the low dose LPS-induced IRAKM Myddosome-dependent pathway plays a pathogenic pro-inflammatory role in ALD. Mechanistically, low dose LPS-induced IRAKM-dependent MEKK3-mediated NFκB activation does not require IRAK4 kinase activity, but does require IRAK4 as a structural adaptor for recruitment of IRAKM to proximal TLR-MyD88 complexes. Furthermore, the death domain of IRAKM specifically directs IRAKM recruitment to the TLR-MyD88-IRAK4 complex in response to low dose of LPS, leading to MEKK3-dependent NFκB activation.
One important question is how the IRAKM-dependent pathway mediates the pathogenesis of ALD. Since low dose of LPS induced only modest levels of inflammatory cytokines and chemokines in wild-type BMDMs, we suspect that the impact of IRAKM on ALD is not solely due to its direct role in TLR-mediated inflammatory gene expression. Through a search for novel IRAKM-dependent genes, we found that Mincle, a CLR membrane receptor that senses microbial products and cell death, is strongly induced by low dose of LPS and this induction was greatly reduced in IRAKM-deficient cells. Mincle-mediated responses are critical for anti-fungal and anti-mycobacterial host defense (36–39). Importantly, Mincle also functions as a sensor of non-homeostatic cell death in which the cellular contents are released (e.g. necroptosis and pyroptosis), thus promoting sterile inflammation. SAP130 was identified as an endogenous ligand recognized by Mincle following necrotic cell death (19). In this study, we found that TLR-induced Mincle expression is dependent on IRAKM in macrophages. In addition, Mincle is upregulated in hepatic macrophages from of ethanol-fed wild-type mice, but not in IRAKM-deficient mice. Both IRAKM- and Mincle-deficient mice were protected from chronic ethanol-induced hepatocyte injury, steatosis and liver inflammation. Since IRAKM’s expression is restricted to myeloid cells (Suppl. Fig. 3A–C), these results suggest that IRAKM-dependent Mincle expression in myeloid cells likely contributes to the pathogenesis of ALD. Mincle is commonly thought to be expressed mainly in myeloid lineage cells, such as macrophages. Notably, some Mincle positive cells in the liver of ethanol-fed wild-type and IRAKM-deficient mice did not co-localize with F4/80+ cells. We indeed found that Mincle expression was induced in primary hepatocytes by IL-1β (Suppl. Fig. 3B–C). Cell-specific deletion will help to determine the impact of Mincle in different cell populations on the pathogenesis of ALD. In the future, we will also test whether restoration of Mincle expression in IRAKM-deficient mice via generation of Mincle-transgenic mice would be sufficient to drive ethanol-induced liver injury.
Recent studies have shown that mice with genetic deletion of either inflammasome components or IL-1R are protected from ALD (10). In this study, we found that low dose LPS upregulates Mincle expression in macrophages and subsequent treatment with recombinant SAP130 was able to activate the ASC/NLRP3/caspase-1 inflammasome. Chronic ethanol-feeding induced caspase-1 and IL-1β activation in the liver; this response was dramatically reduced in IRAKM- and Mincle-deficient mice. However, future studies are required to elucidate the precise molecular mechanism by which the SAP130-induced Mincle-dependent pathway activates the inflammasome. Interestingly, Tanaka el al reported that resident macrophages in adipose tissue express Mincle, which is activated by an endogenous ligand released from dying adipocytes. This Mincle signaling was shown to be required for macrophages to form crown-like structures (CLS), as well as for the development of adipose tissue fibrosis (40). Additionally, Mincle was shown to play a pivotal role in a model of ischemic stroke (41). These studies thus underline the potentially wide-ranging importance of the IRAKM-Mincle axis and suggest this novel pathway as a possible therapeutic target for the treatment of diverse disease states.
Supplementary Material
Acknowledgments
This work was supported by grants from NIH (1R01AA023722 to X.L. and L.E.N. (MPI); 2PO1HL029582-26A1 & PO1CA062220-16A1 to X.L; R01AA011975 & 5P20AA017837 to L.E.N).
We thank Dr. Richard A Flavell (Department of Immunobiology, Yale University School of Medicine, New Haven, Connecticut) for providing the IRAKM-deficient mice. We thank Dr. Christine A. Wells (The Australian Institute for Bioengineering and Nanotechnology, University of Queensland, Brisbane, Australia) for providing anti-Mincle antibody for immune fluorescence staining.
Abbreviations
- LPS
lipopolysaccharide
- TLRs
Toll-like receptors
- ALD
alcoholic liver disease
- IL-1R
Interlukin-1 receptor
- IRAK
IL-1R-associated kinase
- KO
knockout
- WT
wild-type
- TDB
trehalose-dibehenate
- DD
death domain
- SAP130
Spliceosome-associated protein 130
- AST
Aspartate transaminase
- ALT
Alanine transaminase
Reference
- 1.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638–644. doi: 10.1002/hep.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- 4.Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 5.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michelena J, Altamirano J, Abraldes JG, Affo S, Morales-Ibanez O, Sancho-Bru P, Dominguez M, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- 9.Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. 2001;34:101–108. doi: 10.1053/jhep.2001.25350. [DOI] [PubMed] [Google Scholar]
- 10.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Fraczek J, Kim TW, Xiao H, Yao J, Wen Q, Li Y, Casanova JL, et al. The kinase activity of IL-1 receptor-associated kinase 4 is required for interleukin-1 receptor/toll-like receptor-induced TAK1-dependent NFkappaB activation. J Biol Chem. 2008;283:31697–31705. doi: 10.1074/jbc.M804779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim TW, Staschke K, Bulek K, Yao J, Peters K, Oh KH, Vandenburg Y, et al. A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med. 2007;204:1025–1036. doi: 10.1084/jem.20061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, et al. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
- 15.Cui W, Xiao N, Xiao H, Zhou H, Yu M, Gu J, Li X. beta-TrCP-mediated IRAK1 degradation releases TAK1-TRAF6 from the membrane to the cytosol for TAK1-dependent NF-kappaB activation. Mol Cell Biol. 2012;32:3990–4000. doi: 10.1128/MCB.00722-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TW, Yu M, Zhou H, Cui W, Wang J, DiCorleto P, Fox P, et al. Pellino 2 is critical for Toll-like receptor/interleukin-1 receptor (TLR/IL-1R)-mediated post-transcriptional control. J Biol Chem. 2012;287:25686–25695. doi: 10.1074/jbc.M112.352625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan Y, Xiao H, Affolter J, Kim TW, Bulek K, Chaudhuri S, Carlson D, et al. Interleukin-1 receptor-associated kinase 2 is critical for lipopolysaccharide-mediated post-transcriptional control. J Biol Chem. 2009;284:10367–10375. doi: 10.1074/jbc.M807822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H, Yu M, Fukuda K, Im J, Yao P, Cui W, Bulek K, et al. IRAK-M mediates Toll-like receptor/IL-1R-induced NFkappaB activation and cytokine production. The EMBO journal. 2013;32:583–596. doi: 10.1038/emboj.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 21.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–368. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Z, Ma S, Zhou H, Zang A, Fang Y, Li T, Shi H, et al. Tyrosine phosphatase SHP-2 mediates C-type lectin receptor-induced activation of the kinase Syk and anti-fungal TH17 responses. Nat Immunol. 2015;16:642–652. doi: 10.1038/ni.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strasser D, Neumann K, Bergmann H, Marakalala MJ, Guler R, Rojowska A, Hopfner KP, et al. Syk kinase-coupled C-type lectin receptors engage protein kinase C-sigma to elicit Card9 adaptor-mediated innate immunity. Immunity. 2012;36:32–42. doi: 10.1016/j.immuni.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol. 1999;163:5039–5048. [PubMed] [Google Scholar]
- 26.Bakhautdin B, Das D, Mandal P, Roychowdhury S, Danner J, Bush K, Pollard K, et al. Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice. J Hepatol. 2014;61:1029–1037. doi: 10.1016/j.jhep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H, Takeuchi O, Kinoshita T, et al. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–2888. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schweneker K, Gorka O, Schweneker M, Poeck H, Tschopp J, Peschel C, Ruland J, et al. The mycobacterial cord factor adjuvant analogue trehalose-6,6'-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology. 2013;218:664–673. doi: 10.1016/j.imbio.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, Oland S, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol. 2013;190:5722–5730. doi: 10.4049/jimmunol.1203343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nat Immunol. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 31.Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224–1231. doi: 10.1002/hep.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Hu Y, Chao C, Yuksel M, Colle I, Flavell RA, Ma Y, et al. Role of IRAK-M in alcohol induced liver injury. PLoS One. 2013;8:e57085. doi: 10.1371/journal.pone.0057085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–1823. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrao R, Zhou H, Shan Y, Liu Q, Li Q, Shaw DE, Li X, et al. IRAK4 dimerization and trans-autophosphorylation are induced by Myddosome assembly. Mol Cell. 2014;55:891–903. doi: 10.1016/j.molcel.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wells CA, Salvage-Jones JA, Li X, Hitchens K, Butcher S, Murray RZ, Beckhouse AG, et al. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J Immunol. 2008;180:7404–7413. doi: 10.4049/jimmunol.180.11.7404. [DOI] [PubMed] [Google Scholar]
- 37.Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E, Sakuma M, Tateno H, et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A. 2009;106:1897–1902. doi: 10.1073/pnas.0805177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feinberg H, Jegouzo SA, Rowntree TJ, Guan Y, Brash MA, Taylor ME, Weis WI, et al. Mechanism for recognition of an unusual mycobacterial glycolipid by the macrophage receptor mincle. J Biol Chem. 2013;288:28457–28465. doi: 10.1074/jbc.M113.497149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishikawa T, Itoh F, Yoshida S, Saijo S, Matsuzawa T, Gonoi T, Saito T, et al. Identification of distinct ligands for the C-type lectin receptors Mincle and Dectin-2 in the pathogenic fungus Malassezia. Cell Host Microbe. 2013;13:477–488. doi: 10.1016/j.chom.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka M, Ikeda K, Suganami T, Komiya C, Ochi K, Shirakawa I, Hamaguchi M, et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat Commun. 2014;5:4982. doi: 10.1038/ncomms5982. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki Y, Nakano Y, Mishiro K, Takagi T, Tsuruma K, Nakamura M, Yoshimura S, et al. Involvement of Mincle and Syk in the changes to innate immunity after ischemic stroke. Sci Rep. 2013;3:3177. doi: 10.1038/srep03177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.