Abstract
Background
Muscle activity during REM sleep is markedly increased in people with REM sleep behavior disorder (RBD) and people with Parkinson’s disease (PD) who have freezing of gait. This study examined if individuals with RBD, who do not have a diagnosis of PD, show abnormalities in gait initiation that resemble the impairments observed in PD and whether there is a relationship between these deficits and the level of REM sleep without atonia.
Methods
Gait initiation and polysomnography studies were conducted in four groups of 10 subjects each: RBD, PD with and without freezing of gait and control subjects.
Results
Significant reductions were seen in the posterior shift of the center of pressure during the propulsive phase of gait initiation in the RBD and PD with freezing of gait groups compared with controls and PD non-freezers. These reductions negatively correlated with the amount of REM sleep without atonia. The duration of the initial dorsiflexor muscle burst during gait initiation was significantly reduced in both PD groups and the RBD cohort.
Conclusions
These results provide evidence that people with RBD, prior to a diagnosis of a degenerative neurologic disorder, show alterations in the coupling of posture and gait similar to those seen in PD. The correlation between increased REM sleep without atonia and deficits in forward propulsion during the push-off phase of gait initiation suggests that abnormities in the regulation of muscle tone during REM sleep may be related to the pathogenesis of freezing of gait.
Keywords: PD, RBD, freezing, gait, sleep
INTRODUCTION
Rapid eye movement (REM) sleep behavior disorder (RBD) is a parasomnia characterized by an abnormal increase in muscle activity during rapid eye movement (REM) sleep (termed REM sleep without atonia) in conjunction with dream enactment.1, 2 RBD often reflects a prodromal stage of parkinsonism or related synucleinopathy with up to 81% of patients developing Parkinson’s disease (PD), dementia with Lewy bodies or multiple system atrophy over the course of 5 to 29 years.3 Accordingly, the prevalence of RBD symptoms is high in PD with over one third of patients meeting the diagnostic criteria of RBD.4, 5 People who co-express PD and RBD are more likely to present with a nontremor-predominant subtype of disease, an increased frequency of falls and a higher incidence of freezing of gait (FOG).6, 7 Conversely, people with PD and FOG have been shown to have significantly increased REM sleep without atonia (RSWA) compared to patients without FOG.8 These findings suggest that RSWA is associated with dysfunction of systems that control posture and gait. We tested this hypothesis by examining: (i) if deficits in anticipatory postural adjustments (APAs) that precede and accompany gait initiation, a hallmark of PD9–12, can also be seen in patients with idiopathic RBD prior to a diagnosis of parkinsonism, and (ii) if there is an association between the level of RSWA and gait initiation impairment. Gait initiation was tested because it is a task that often provokes episodes of freezing13 and is associated with impairments in the coupling of posture and movement in people with PD.10–12 We hypothesized that participants with RBD would show significant impairment in gait initiation compared to control subjects, and the pattern of impairment would most resemble the deficits observed in PD patients with FOG. Also, the level of RSWA would be correlated with the magnitude of the gait initiation impairment across subjects.
METHODS
Subjects
Four groups of ten subjects were tested: (1) individuals with a polysomnography-confirmed RBD who did not have a diagnosis of PD, (2) individuals with PD with FOG (PD+FOG), (3) individuals with PD without FOG (PD-FOG), (4) a group of neurologically healthy adults who were age- (± 3 years) and sex-matched to the PD groups. The sample size for each group was chosen based on the results of our previous work that compared gait initiation in patients with PD versus matched controls.12 A comprehensive neurologic evaluation and Unified Parkinson’s Disease Rating Scaling (UPDRS) assessment were performed on all subjects. The inclusion criteria for PD group were a diagnosis of idiopathic PD14, Hoehn & Yahr stage 2 to 3, and the ability to ambulate independently without the use of an assistive device for 30 minutes. Subjects were assigned to PD+FOG group if they had a history of at least one FOG episode per week (a score of 1 on item 3 of the New Freezing of Gait Questionnaire, NFOG-Q)15 or if a FOG episode was observed by the assessing neurologist or investigators during testing. PD subjects were excluded if they had another neurological disorder, a tremor score of greater than 2 on items 20 and 21 of the UPDRS, a history of neurosurgery, a Mini Mental State Examination score of less than 26, any musculoskeletal disorder that affected walking. Experiments were performed in the morning after overnight withdrawal from all anti-parkinsonian medications.
Subjects with RBD were recruited if they had a diagnosis based on the International Classification of Sleep Disorders II and the ability to ambulate independently without an assistive device. RBD patients were excluded based on the criteria described above for the PD groups, significant sleep apnea (defined as an apnea-hypopnea index >15 events/hour of sleep on PSG), and or periodic limb movement disorder (defined as a periodic limb movement index >10 events/hour of sleep with awakening on PSG). RBD participants were allowed to take their RBD medications if they remained on a stable dose for two months prior to the screening. The same exclusion criteria were used for the matched control group. Participants gave written informed consent, and the study was approved by the Institutional Review Board at Northwestern University.
Polysomnography
Electromyographic (EMG) recordings were obtained from the right and left ocular, submental and tibialis anterior (TA) muscles. Methods for the collection of the electroencephalographic recordings, scoring of sleep stages and phasic and tonic RSWA have been described previously.8
Gait initiation
A self-initiated forward stepping task was tested.12 Trials began with the subject standing stationary on two adjacent force platforms. Subjects were instructed to wait at least 3–5 seconds following a verbal instruction of “anytime”, then take at least three steps forward with their right or left leg “starting as fast as possible”. These instructions were designed to minimize the effects of external cueing and ensure that participants progressed forward toward stead-state gait on each trial. During the interval between the “anytime” instruction and initiation of gait, subjects were asked to stand steady and wait a prolonged time period prior to initiating the step. Trials in which subjects did not stand steady or leaned forward prior to gait initiation, or stepped with the wrong leg were discarded and repeated. A total of 10 trials for each leg were collected. The right leg was the most affected side (based on UPDRS scores and clinical examination) in both PD groups, therefore only right leg stepping trials were analyzed and compared across all groups.
Ground reaction forces (GRFs) and center of pressure (CoP) data were collected from force platforms beneath the right and left feet (AMTI, Dimensions: 464 × 508mm, Watertown, MA, USA). EMG signals (Delsys, Bagnoli-16 System, Natick, MA, USA) were recorded bilaterally from the tibialis anterior muscle (bandpass = 20–450 Hz). All kinetic and EMG signals were sampled at 1000 Hz (LabView 6.0, National Instruments, Austin, TX). The primary outcome variables were: the peak amplitude of the vertical loading force of the right leg (loading-GRF) and unloading force of the left leg (unloading-GRF), peak rightward excursion of the CoP (CoPRight), the two peaks of the posterior CoP (CoPPost-Peak1: typically occurs near the time of peak loading/unloading; CoPPost-Peak2: typically occurs near the time of step leg toe-off) and the duration of the initial TA EMG burst in the stepping leg (Figure 1). The incidence of trials with no anticipatory postural adjustments (APAs)12 before the step was also quantified. Data analysis was performed using customized software written in MATLAB R2012b (The MathWorks Inc., Natick, MA, USA). GRFs were normalized by expressing force as a percentage of total subject weight. Onset times of EMG, CoP and GRF changes were calculated based on two standard deviations from the mean signal recorded prior to the go-cue and were adjusted if necessary based on visual inspection.
Figure 1. Example of the primary outcome variables measured during gait initiation in a control subject.
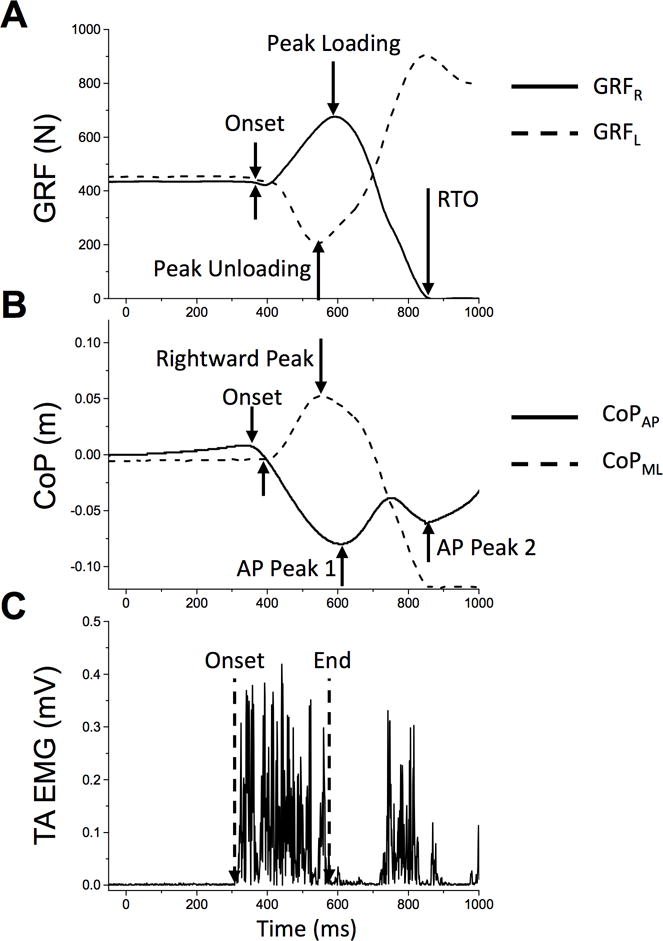
A. Vertical ground reaction forces (GRFs) generated by the stepping (right leg, solid line) and stance leg (left, dashed line). B. Center of pressure excursion (CoP) in the anterior-posterior (solid line) and rightward-leftward (dashed line) directions. Note there are often two distinct peaks in the posterior excursion. The first peak usually coincides with the peak loading and unloading of the step and stance legs respectively, and the second peak usually occurs near step leg toe-off. C. Example of the initial burst of EMG activity in the tibialis anterior (TA) muscle. RTO = right toe off.
Statistical analysis
Demographic and clinical variables were compared across groups using a univariate analysis of variance and t-tests for post-hoc pairwise comparisons. Since many of the gait initiation variables did not show a normal distribution, these data were compared between groups using the Kruskal-Wallis test. Associations between measures of RSWA and gait variables were tested using a partial correlation (two-tailed) while controlling for age. Differences between conditions and groups or associations between variables were considered significant at the p<0.05 level.
RESULTS
Demographics and clinical assessment
A summary of the demographics, medications, and clinical characteristics of all participants have been reported previously8 and is provided in Table 1. The PD+FOG group had a significantly higher total UPDRS III score (p = 0.019) and longer disease duration (p = 0.033) compared with the PD-FOG group. The mean NFOG-Q score for PD+FOG group was 17 ± 5, whereas it was 0 for all other groups. There were no differences in age or MMSE scores between groups. One subject in the PD-FOG group and three subjects in the PD+FOG group had an established diagnosis of RBD. Note that the PD subjects were recruited based on a diagnosis of PD and the presence or absence of FOG, irrespective of a history of RBD. The mean UPDRS III score in the RBD group was 1.5 ± 1.9 but no subjects had a diagnosis of PD at the time of the study.
Table 1.
Demographics and disease characteristics of the participants
| PD-FOG (n = 10) | PD+FOG (n = 10) | RBD (n = 10) | Controls (n = 10) | Group ANOVA (p-value) | |
|---|---|---|---|---|---|
| Age (years) | 61.5 ± 9.4 | 65.9 ± 11.2 | 61.5 ± 8.6 | 62.7 ± 11.5 | 0.747 |
| Duration of disease since diagnosis (years) | 4.4 ± 2.5 | 7.2 ± 4.1 | 1.34 ± 0.94 | NA | 0.090 |
| Sex (M/F) | 8/2 | 8/2 | 6/4 | 8/2 | |
| UPDRS III-off meds | 24.8 ± 9.1 | 32.2 ± 9.8 | 1.5 ± 1.9 | 0.0 ± 0.0 | < 0.001* |
| UPDRS III-on meds | 18 ± 6.3 | 25.6± 10.5 | NA | NA | 0.066 |
| Hoehn and Yahr Score | 2.0 ± 0.2 | 2.2 ± 0.3 | NA | NA | 0.054 |
| Schwab & England | 89 ± 3 | 88 ± 4 | NA | NA | 0.360 |
| MMSE | 30 ± 1 | 30 ± 1 | 30 ± 0 | 30 ± 0 | 0.382 |
| NFOG-Q | 0 | 17 ± 5 | 0 | 0 | <0.001** |
| LED (mg) | 582 ± 436 | 727 ± 254 | – | – | 0.356 |
| Medications, n | |||||
| Antidepressants | 1 | 3 | 0 | 0 | |
| Melatonin | 1 | 0 | 3 | 0 | |
| Clonazepam | 1 | 3 | 5 | 0 |
Post-hoc t-tests: PD+FOG > PD−FOG, p = 0.047; PD+FOG > RBD, p < 0.001; PD−FOG > RBD, p = 0.033.
Post-hoc t-tests: PD+FOG > PD−FOG, p = 0.019, PD+FOG > controls and RBD, p < 0.001, PD−FOG > controls and RBD, p < 0.001.
Post-hoc t-tests: PD+FOG > PD−FOG, controls and RBD, p < 0.001.
Note: One subject in the PD−FOG group and 3 subjects in the PD+FOG group had an established diagnosis of RBD. Note that the PD subjects were recruited based on a diagnosis of PD and the presence or absence of FOG, irrespective of a history of RBD. The mean UPDRS III score in the RBD group was 1.5 ± 1.9 but no RBD subjects had a diagnosis of PD at the time of the study.
Sleep data
The findings of the PSG studies in these cohorts have been reported previously.8 There was a significant main effect of group for measures of tonic (χ2 = 23.19, df = 3, p < 0.001, η2 = 0.59) and phasic (χ2 = 22.71, df = 3, p < 0.001, η2 = 0.58) EMG activity during REM sleep. The RBD and PD+FOG groups had significantly higher tonic EMG compared to the control and PD-FOG groups (Control, mean = 3.5 ± 6.1; PD-FOG, mean = 10.9 ± 10.3; PD+FOG, mean = 31.5 ± 15.7; RBD, mean = 33.8 ± 15.8; χ2 > 7.41, p < 0.007, η2 > 0.38). Similarly, phasic EMG was significantly higher in the RBD and PD+FOG groups compared to the control group (Control, mean = 1.4 ± 1.9; PD-FOG, mean = 3.8 ± 4.0; PD+FOG, mean = 7.1 ± 4.0; RBD, mean = 11.1 ± 3.5; χ2 > 11.57, p < 0.002, η2 > 0.38), but there was no significant difference between the PD+FOG and PD-FOG groups (χ2 = 3.58, p = 0.059). Phasic EMG was significantly increased in the RBD group compared to both PD groups (χ2 > 4.79, p < 0.020, η2 > 0.25).
Gait initiation biomechanics
Examples of the vertical GRFs and CoP excursion profiles during five consecutive right leg stepping trials in individuals from each group are shown in Figure 2A. These examples demonstrate the consistency and smoothness of the GRF and CoP profiles observed in the control subjects compared to the variability across trials, reduced magnitudes and the presence of multiple inflections in the force and CoP profiles in the PD+FOG, PD-FOG and RBD subjects.
Figure 2.
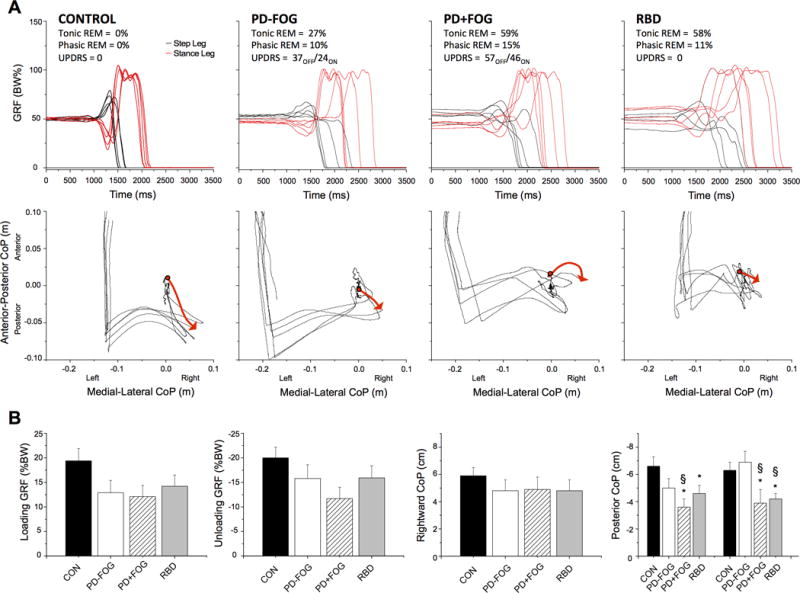
A. Examples of five consecutive trials of self-initiated gait in a representative subject from each group. The top row shows right (step leg) and left (stance leg) vertical ground reaction forces (GRFs). Each trial has been aligned to the onset of the initial increase in the right GRF at 1000ms. Note the consistency and smoothness of the profiles in the control subject compared with the presence of multiple inflections, hesitations and reduced magnitude of the profiles in the PD-FOG, PD+FOG and RBD subjects. The bottom row shows the anterior-posterior and medial-lateral excursions of the net CoP. The red line with an arrow highlights the initial trajectory of the CoP during the APA phase for one trial. B. Changes in the magnitude of the vertical GRFs and the net center of pressure (CoP) excursion across groups. Summary of the mean peak amplitudes of the dependent variables across groups during self-initiated gait: (A) stepping leg loading force, (B) stance leg unloading force, (C) peak rightward excursion of the center of pressure, (D) first and second peaks of the posterior excursion of the CoP. * = significantly different from controls at the p<0.05 level, § = significantly different from the PD-FOG group at the p<0.05 level. Error bars are one standard error. (CON = control subjects, PD-FOG = PD without FOG, PD+FOG = PD with FOG, RBD = REM sleep behavior disorder).
The group average values of the primary gait initiation variables are presented in Figure 2B. Significant main effects of group were observed in the amplitudes of the CoPPost-Peak1 (Chi2 = 9.5, p = 0.023) and CoPPost-Peak2 (Chi2 = 10.5, p = 0.014) excursion. Post-hoc comparisons between groups showed that the CoPPost-Peak1 was reduced in the PD+FOG (Chi2 = 7.4, p = 0.007) and RBD (Chi2 = 3.1, p = 0.049) groups compared with the Control group, and between the PD groups (Chi2 = 3.9, = 0.048). Similarly, the CoPPost-Peak2 excursion was reduced in the PD+FOG and RBD groups compared with the Control (Chi2 = 3.9, p = 0.048 & Chi2 = 6.3, p = 0.012, respectively) and PD-FOG groups (Chi2 = 4.7, = 0.030 & Chi2 = 6.7, p = 0.010, respectively). There was no significant difference in CoPPost-Peak2 excursion between the PD+FOG and RBD groups (p > 0.692). There was no significant main effect of group in the loading or unloading GRFs or CoPRight excursion (p > 0.165), or in measures of the time between the onset of gait initiation and toe-off of the stepping or stance legs (p > 0.242).
Trials without an APA (no initial loading and unloading of the step and stance legs respectively, and no posterior and rightward shift of CoP) were observed in four subjects in the PD+FOG group, three subjects in the RBD group, and two subjects in the PD-FOG group, but never in the Control group. The average incidence of trials with no APA was 15%, 15%, 6%, and 0%, in the PD+FOG, RBD, PD-FOG, control groups respectively. There was no significant group effect in the incidence of trials without an APA across groups (p = 0.192).
EMG activity
A distinct feature of step initiation in the PD and RBD groups was the presence of multiple short-duration bursts in the TA muscle (Figure 3). The duration of the first burst of TA EMG activity was significantly reduced in the PD-FOG (Chi2 =14.286, p < 0.001), PD+FOG (Chi2 =15.010, p < 0.001) and RBD (Chi2 =7.542, p = 0.010) groups compared with Control subjects.
Figure 3.
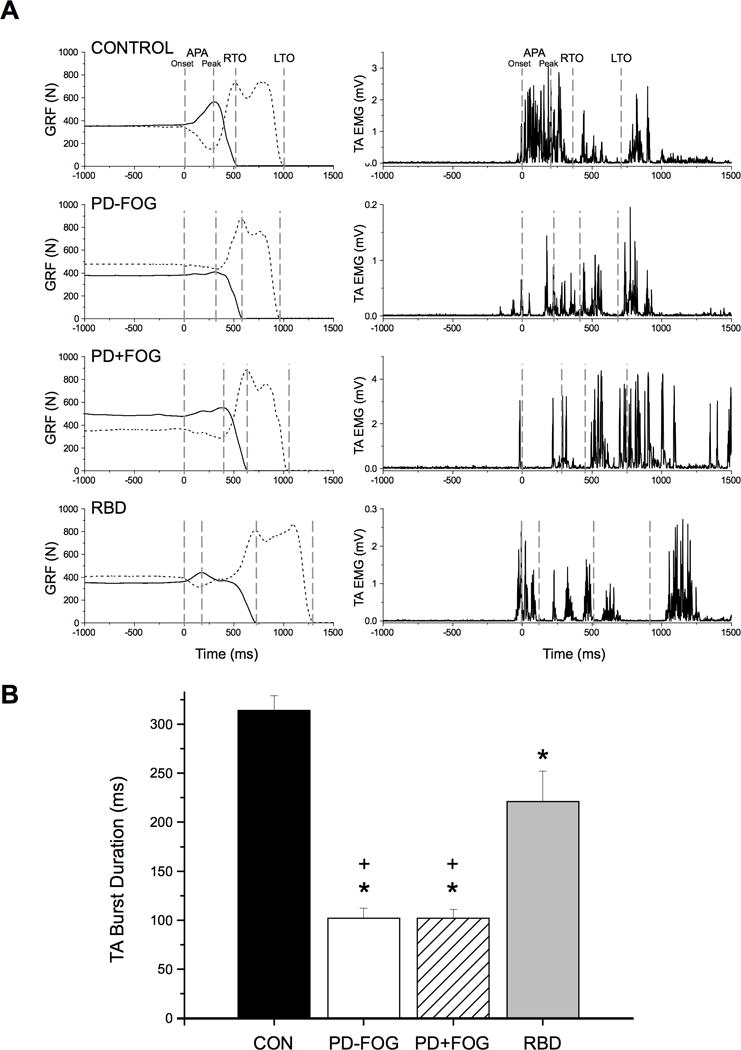
A. Muscle activation patterns in the tibialis anterior (TA) muscle during gait initiation. Representative examples of vertical ground reaction forces (left column) on the stepping (solid line) and stance (dashed line) legs and TA EMG (right column) in a control, PD-FOG, PD+FOG and RBD subject. Plots have been aligned to the start of the APA at time = 0 ms. Note the fused and prolonged first burst of activity in the TA muscle in the control subject compared to an initial short duration burst, followed by addition bursts in all other groups. RTO = right toe-off, LTO = left toe-off. B. Group averages of the mean duration of the first burst in the TA muscle. The first burst duration was significantly reduced in the PD-FOG, PD+FOG and RBD groups compared with controls, and both PD groups compared with the RBD group. * = significantly different from controls at the p<0.05 level, + = significantly different from the RBD group at the p<0.05 level. Error bars are one standard error. (CON = control subjects, PD-FOG = PD without FOG, PD+FOG = PD with FOG, RBD = REM sleep behavior disorder)
Correlation between RSWA and gait initiation
Our previous study showed that tonic and phasic RSWA were significantly increased in RBD and people with PD with FOG, but not those without FOG, compared to Controls.8 Correlation analysis of the RSWA versus gait data across subjects (all groups combined) showed that tonic EMG during REM sleep was negatively correlated with the CoPPost-Peak1 (r = −0.535, p < 0.001) and CoPPost-Peak2 (r = −0.463, p = 0.004) excursion and the unloading-GRF (r = −0.436, p = 0.008). Phasic EMG was also negatively correlated with the CoPPost-Peak1 (r = −0.383, p = 0.021) and the CoPPost-Peak2 (r = −0.436, p = 0.008) but not correlated with other gait variables. In contrast, tonic and phasic EMG were not significantly correlated with the CoPRight excursion (r < 0.210, p > 0.220).
DISCUSSION
There were three main findings from this experiment. First, the participants with RBD (without any clinical motor symptom of PD) showed impairments in biomechanical measures of self-initiated stepping similar to those seen in PD+FOG patients. These impairments were characterized by significant reductions in the posterior excursion of the CoP during both the anticipatory (peak 1) and propulsive (peak 2, immediately prior to toe-off) phases of gait initiation. In some RBD subjects, APAs for gait initiation were absent, a phenomenon commonly observed in PD patients.16 Moreover, the reduction in the capacity to generate posterior movement of the CoP late in the stepping cycle in the RBD group was a distinct feature of gait initiation in the PD+FOG group. Second, the duration of the first burst of TA muscle activity was significantly reduced and fractionated muscle activity was present in both PD groups and the RBD cohort. Third, we found a strong negative correlation between the level of RSWA and deficits in the capacity to generate a posterior excursion of the CoP during gait initiation, suggesting that pathophysiological changes in control of muscle tone during REM sleep are linked to alterations in the capacity to couple posture and movement during gait initiation.
This experiment provides quantitative evidence that people with RBD can present with subliminal changes in the kinetics and EMG patterns of gait initiation prior to the clinical expression of parkinsonian symptoms.6, 17–19 Distinct deficits in the posterior shift of the CoP were observed during both the anticipatory and propulsive phases of gait initiation. Comparable deficits in the posterior CoP shift during the propulsive phase were only seen in the PD+FOG group. The posterior shift in the CoP is a component of the anticipatory postural adjustments necessary to accelerate the center of mass forward and towards the single-stance leg for the regulation of whole body balance prior to, and during, the first step.20 Failure to sufficiently shift the CoP backward can result in reduced step velocity, shortening of the initial step or, in the extreme, termination/failure of the gait initiation cycle.9 Previous studies have shown that people with PD and freezing of gait have a significantly reduced capacity to shift weight in the anterior-posterior plane during standing21 and gait initiation16 compared with PD non-freezers. This distinct impairment may explain why falls are common in people with FOG22 and why episodes of freezing often occur during attempts to generate forward progression such as during gait, gait initiation or the propulsive phase of turning.13, 23
The observation of a reduced capacity to shift the CoP backward during gait initiation in participants with RBD suggests that this disorder is associated with early changes in neuronal systems that integrate postural adjustments with movement initiation. Disordered coupling of posture and gait has been hypothesized to contribute to episodes of FOG24 and may explain why the co-expression of PD and RBD is associated with an increased frequency of falls and FOG.6, 7, 25 This may occur at the level of the brainstem, including regions of the mesencephalic locomotor region [pedunculopontine nucleus (PPN) and cuneiform nucleus] and pontomedullary reticular formation that are involved in the spatial and temporal coupling of posture and movement.26, 27 In this case, impaired postural control (reduced posterior shift in the CoP) would result in the reduced step length and velocity that characterizes gait initiation and steady-state gait in people with PD+FOG. Alternatively, attentional or scaling deficits that result in an attenuated supraspinal command for the first step may also explain the reduced posterior excursion of the center of pressure. Both RBD and PD with FOG are associated with impaired executive function, including deficits in attentional set shifting.28, 29 Thus, while our assessment of cognitive status did not show differences between groups (based on Mini Mental Status Exam scores) and all participants were effectively able to follow instructions, we cannot rule out the possibility that attentional deficits in the RBD and PD+FOG groups may have contributed to differences in the APAs generated during gait initiation.
We also observed that the onset of gait initiation was accompanied by multiple short-duration bursts of dorsiflexor activity in the RBD subjects, similar to our PD subjects. Muscle activation patterns during voluntary movement in PD patients are characterized by multiple low-amplitude, short-duration bursts.30, 31 This pattern of fractionated muscle activity contributes to slow movement and impaired scaling of the intended response.32 Currently the mechanisms contributing to the fractionation of muscle activity in PD are poorly understood. The fact that dopamine replacement therapies33 and deep brain stimulation34, 35 can improve muscle activation patterns suggests that dopaminergic denervation contributes to this abnormality, although non-dopaminergic systems at the level of the brain stem (e.g. noradrenergic neurons of the locus coeruleus; serotonergic neurons of the raphe nucleus) that control postural muscle tone and motor neuron activation may also contribute.36
High levels of RSWA were correlated with low-amplitude posterior excursions of the CoP. We have previously shown that RSWA, one of the hallmarks of RBD1, is significantly increased in people with PD+FOG compared with those without FOG.8 Moreover, the amount of tonic EMG during REM sleep in the PD+FOG group was comparable to that seen in the RBD cohort. The present study extends these findings to show that abnormal RSWA is associated with deficits in gait initiation. Postmortem studies have shown that RBD is associated with neurodegeneration in regions (e.g. locus coeruleus/subcoeruleus, PPN, substantial nigra pars compacta) considered to play a critical role in the regulation of RSWA and also, the control of posture and locomotion.2 The PPN is considered to be central to the pathogenesis of FOG based on its connectivity with the basal ganglia and evidence that cholinergic neurons in this region degenerate in PD.37, 38 The loss of cholinergic neurons of the PPN, in conjunction with increased inhibitory output from the basal ganglia in the parkinsonian state, likely results in suppression of mesencephalic locomotor region function and contributes to postural instability, reduced gait speed and falls.39–42 Cholinergic neurons of the PPN have also been implicated in the control of the REM sleep atonia2, 43, 44 and may play an important role in the regulation of muscle tone during REM sleep, posture and gait.
Several limitations of this study are recognized. First, some participants in the study (RBD, n = 5; PD+FOG, n = 3) were being treated with oral clonazepam for their sleep disorder. Clonazepam has been shown to reduce the symptoms of RBD45 in conjunction with a suppression of phasic, but not tonic, RSWA.46 It can sometimes be associated with morning sedation (approximately 10% of cases)45, 47, 48 and this may have impacted performance during gait initiation testing. However, tolerance to the sedative effects of this medication usually develops over one or two weeks after starting the medication.49 Since participants who were taking clonazepam had been on a stable dose for two months or more prior to the study, the sedative effects of the medication were likely minimal. Increased early morning incoordination has also been reported with clonazepam, but this is relatively rare (seen in only 2 of 93 cases reported by Olson et al., 2000). Another potential limitation of this study was the relatively small sample size per group, which may have affected the effect sizes and extrapolation of the findings to the larger PD and RBD populations.
Taken together, the findings of the present study suggest that synucleinopathy that affects the control of muscle tone during REM sleep in prodromal stages of PD, as seen in some patients with RBD, also affects the function of systems involved in the coupling of posture and gait prior to the expression of motor symptoms associated with nigral degeneration. This naturally leads to the hypothesis that RBD patients with these abnormalities will eventually convert to a subtype of PD dominated by postural instability, gait disturbances, falls and FOG. Proof of this latter hypothesis will require a longitudinal study of RBD patients with quantitative evaluations of motor systems.
Acknowledgments
The authors thank the study participants for their time, Dr. Mark Rogers for his help in designing the project, Claire Marlin at department of Physical Therapy of Northwestern University for her invaluable assistance with recruiting and scheduling all participants, Lan Ly Manalo and Rodney Neely at the Center for Sleep Medicine at Northwestern University for their assistance with arranging for and conducting polysomnograms.
Financial Disclosures
L. Alibiglou received grant support from the Michael J. Fox Foundation for Parkinson’s Research. A. Videnovic received grant support from the NIH (K23 NS072283) and the Michael J. Fox Foundation for Parkinson’s Research. P. Planetta received grant support from the NIH (RO1 NS052318). D. Vaillancourt received grant support from the NIH (R01 NS058487, R01 NS052318, R01 NS075012), the Michael J. Fox Foundation for Parkinson’s Research, and is a consultant for projects at the Department of Defense and the Great Lakes NeuroTechnologies. C. MacKinnon received grant support from the Michael J. Fox Foundation for Parkinson’s Research and the NIH (RO1 NS070264).
Funding Agency:
The study was funded and supported by a grant from the Michael J. Fox Foundation (MJFF) for Parkinson’s Research.
Footnotes
Relevant Conflicts of Interest:
Nothing to report.
Authors’ Roles
Dr. Alibiglou: study concept and design; acquisition of data; analysis and interpretation; critical revision of the manuscript for important intellectual content; study supervision.
Dr. Videnovic: study concept and design; acquisition of data; analysis and interpretation; critical revision of the manuscript for important intellectual content; study supervision.
Dr. Planetta: study concept and design; analysis and interpretation; critical revision of the manuscript for important intellectual content.
Dr. Vaillancourt: study concept and design; analysis and interpretation; critical revision of the manuscript for important intellectual content.
Dr. MacKinnon: study concept and design; analysis and interpretation; critical revision of the manuscript for important intellectual content; study supervision.
References
- 1.Schenck CH, Bundlie SR, Ettinger MG, Mahowald MW. Chronic behavioral disorders of human REM sleep: a new category of parasomnia. Sleep. 1986;9:293–308. doi: 10.1093/sleep/9.2.293. [DOI] [PubMed] [Google Scholar]
- 2.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 3.Schenck CH, Boeve BF, Mahowald MW. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series. Sleep Med. 2013;14:744–748. doi: 10.1016/j.sleep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Wetter TC, Trenkwalder C, Gershanik O, Hogl B. Polysomnographic measures in Parkinson’s disease: a comparison between patients with and without REM sleep disturbances. Wien Klin Wochenschr. 2001;113:249–253. [PubMed] [Google Scholar]
- 5.Gagnon JF, Bedard MA, Fantini ML, et al. REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease. Neurology. 2002;59:585–589. doi: 10.1212/wnl.59.4.585. [DOI] [PubMed] [Google Scholar]
- 6.Postuma RB, Gagnon JF, Vendette M, Charland K, Montplaisir J. REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J Neurol Neurosurg Psychiatry. 2008;79:1117–1121. doi: 10.1136/jnnp.2008.149195. [DOI] [PubMed] [Google Scholar]
- 7.Romenets SR, Gagnon JF, Latreille V, et al. Rapid eye movement sleep behavior disorder and subtypes of Parkinson’s disease. Mov Disord. 2012;27:996–1003. doi: 10.1002/mds.25086. [DOI] [PubMed] [Google Scholar]
- 8.Videnovic A, Marlin C, Alibiglou L, Planetta PJ, Vaillancourt DE, Mackinnon CD. Increased REM sleep without atonia in Parkinson disease with freezing of gait. Neurology. 2013;81(12):1030–5. doi: 10.1212/WNL.0b013e3182a4a408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delval A, Tard C, Defebvre L. Why we should study gait initiation in Parkinson’s disease. Neurophysiol Clin. 2014;44:69–76. doi: 10.1016/j.neucli.2013.10.127. [DOI] [PubMed] [Google Scholar]
- 10.Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalogr Clin Neurophysiol. 1996;101:110–120. doi: 10.1016/0924-980x(95)00253-h. [DOI] [PubMed] [Google Scholar]
- 11.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 12.Rogers MW, Kennedy R, Palmer S, et al. Postural preparation prior to stepping in patients with Parkinson’s disease. J Neurophysiol. 2011;106:915–924. doi: 10.1152/jn.00005.2010. [DOI] [PubMed] [Google Scholar]
- 13.Giladi N, Tal J, Azulay T, et al. Validation of the freezing of gait questionnaire in patients with Parkinson’s disease. Mov Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- 14.Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 15.Nieuwboer A, Rochester L, Herman T, et al. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- 16.Delval A, Moreau C, Bleuse S, et al. Auditory cueing of gait initiation in Parkinson’s disease patients with freezing of gait. Clin Neurophysiol. 2014;125:1675–1681. doi: 10.1016/j.clinph.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 17.Postuma RB, Lang AE, Massicotte-Marquez J, Montplaisir J. Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder. Neurology. 2006;66:845–851. doi: 10.1212/01.wnl.0000203648.80727.5b. [DOI] [PubMed] [Google Scholar]
- 18.McDade EM, Boot BP, Christianson TJ, et al. Subtle gait changes in patients with REM sleep behavior disorder. Mov Disord. 2013;28(13):1847–53. doi: 10.1002/mds.25653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen TZ, Xu GJ, Zhou GA, Wang JR, Chan P, Du YF. Postural sway in idiopathic rapid eye movement sleep behavior disorder: a potential marker of prodromal Parkinson’s disease. Brain Res. 2014;1559:26–32. doi: 10.1016/j.brainres.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol. 1991;437:635–653. doi: 10.1113/jphysiol.1991.sp018616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vervoort G, Nackaerts E, Mohammadi F, et al. Which Aspects of Postural Control Differentiate between Patients with Parkinson’s Disease with and without Freezing of Gait? Parkinsons Dis. 2013;2013:971480. doi: 10.1155/2013/971480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson’s disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 23.Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18:149–154. doi: 10.1016/j.parkreldis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10(8):734–44. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sixel-Döring F, Trautmann E, Mollenhauer B, Trenkwalder C. Associated factors for REM sleep behavior disorder in Parkinson disease. Neurology. 2011;77(11):1048–54. doi: 10.1212/WNL.0b013e31822e560e. [DOI] [PubMed] [Google Scholar]
- 26.Schepens B, Stapley PJ, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol. 2008;100:2235–2253. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- 27.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57(1):192–8. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 28.Massicotte-Marquez J1, Décary A, Gagnon JF, Vendette M, Mathieu A, Postuma RB, Carrier J, Montplaisir J. Executive dysfunction and memory impairment in idiopathic REM sleep behavior disorder. Neurology. 2008;70(15):1250–7. doi: 10.1212/01.wnl.0000286943.79593.a6. [DOI] [PubMed] [Google Scholar]
- 29.Shine JM, Naismith SL, Palavra NC, Lewis SJG, Moore ST, Dilda V, Morris TR. Attentional set-shifting deficits correlate with the severity of freezing of gait in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:388–90. doi: 10.1016/j.parkreldis.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- 31.Robichaud JA, Pfann KD, Leurgans S, Vaillancourt DE, Comella CL, Corcos DM. Variability of EMG patterns: a potential neurophysiological marker of Parkinson’s disease? Clin Neurophysiol. 2009;120:390–397. doi: 10.1016/j.clinph.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Mov Disord. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- 33.Robichaud JA, Pfann KD, Comella CL, Corcos DM. Effect of medication on EMG patterns in individuals with Parkinson’s disease. Mov Disord. 2002;17:950–960. doi: 10.1002/mds.10218. [DOI] [PubMed] [Google Scholar]
- 34.Vaillancourt DE, Prodoehl J, Verhagen Metman L, Bakay RA, Corcos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- 35.Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RA, Metman LV, Corcos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Mov Disord. 2006;21:50–58. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimbergen YA, Langston JW, Roos RA, Bloem BR. Postural instability in Parkinson’s disease: the adrenergic hypothesis and the locus coeruleus. Expert Rev Neurother. 2009;9:279–290. doi: 10.1586/14737175.9.2.279. [DOI] [PubMed] [Google Scholar]
- 37.Jellinger K. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1988;51:540–543. doi: 10.1136/jnnp.51.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL. The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol. 1989;26:41–46. doi: 10.1002/ana.410260106. [DOI] [PubMed] [Google Scholar]
- 39.Grabli D, Karachi C, Folgoas E, et al. Gait disorders in parkinsonian monkeys with pedunculopontine nucleus lesions: a tale of two systems. J Neurosci. 2013;33:11986–11993. doi: 10.1523/JNEUROSCI.1568-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohnen NI, Frey KA, Studenski S, et al. Gait speed in Parkinson disease correlates with cholinergic degeneration. Neurology. 2013 doi: 10.1212/WNL.0b013e3182a9f558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73:1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller ML, Albin RL, Kotagal V, et al. Thalamic cholinergic innervation and postural sensory integration function in Parkinson’s disease. Brain. 2013 doi: 10.1093/brain/awt247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Torontali ZA, Grace KP, Horner RL, Peever JH. Cholinergic involvement in control of REM sleep paralysis. J Physiol. 2014;592:1425–1426. doi: 10.1113/jphysiol.2014.271304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schenck CH, Mahowald MW. Polysomnographic, neurologic, psychiatric, and clinical outcome report on 70 consecutive cases with REM sleep behavior disorder (RBD): sustained clonazepam efficacy in 89.5% of 57 treated patients. Cleve Clin J Med. 1990;57(suppl):S9–S23. [Google Scholar]
- 46.Lapierre O, Montplaisir J. Polysomnographic features of REM sleep behavior disorder: development of a scoring method. Neurology. 1992;42(7):1371–4. doi: 10.1212/wnl.42.7.1371. [DOI] [PubMed] [Google Scholar]
- 47.Schenck CH, Mahowald MW. Long-term, nightly benzodiazepine treatment of injurious parasomnias and other disorders of disrupted nocturnal sleep in 170 adults. Am J Med. 1996 Mar;100(3):333–7. doi: 10.1016/S0002-9343(97)89493-4. [DOI] [PubMed] [Google Scholar]
- 48.Olson EJ, Boeve BF, Silber MH. Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases. Brain. 2000 Feb;123(Pt 2):331–9. doi: 10.1093/brain/123.2.331. [DOI] [PubMed] [Google Scholar]
- 49.Ashton H. Toxicity and adverse consequences of benzodiazepine use. Psychiatric Annals. 1995;25:158–165. [Google Scholar]


