Abstract
Health effects following low doses of ionizing radiation are uncertain. Military veterans at the Nevada Test Site (NTS) during the SMOKY atmospheric nuclear weapons test in 1957 were reported to be at increased risk for leukemia in 1979, but this increase was not evaluated with respect to radiation dose. The SMOKY test was one of 30 tests in 1957 within the PLUMBBOB test series. These early studies led to public laws where atomic veterans could qualify for compensation for presumptive radiogenic diseases.
A retrospective cohort study was conducted of 12,219 veterans at PLUMBBOB test series, including 3,020 at the SMOKY nuclear test. Mortality follow-up was through 2010 and observed causes of death were compared with expected causes based on general population rates. Radiation dose to red bone marrow was based on individual dose reconstructions, and Cox proportional hazards models were used to evaluate dose response for all leukemias other than chronic lymphocytic leukemia (non-CLL leukemia).
Vital status was determined for 95.3% of the 12,219 veterans. The dose to red bone marrow was low (mean 3.2 mGy, maximum 500 mGy). Military participants at the PLUMBBOB nuclear test series remained relatively healthy after 53 years and died at a lower rate than the general population. In contrast, and in comparison with national rates, the SMOKY participants showed significant increases in all causes of death, respiratory cancer, leukemia, nephritis and nephrosis, and accidents, possibly related in part to lifestyle factors common to enlisted men who made up 81% of the SMOKY cohort.
Compared with national rates, a statistically significant excess of non-CLL leukemia was observed among SMOKY participants (Standardized Mortality Ratio=1.89, 95% 1.24–2.75, n=27) but not among PLUMBBOB participants after excluding SMOKY (SMR=0.87, 95% 0.64–1.51, n=47). Leukemia risk, initially reported to be significantly increased among SMOKY participants, remained elevated, but this risk diminished over time. Despite an intense dose reconstruction, the risk for leukemia was not found to increase with increasing levels of radiation dose to the red bone marrow. Based on a linear model, the estimated excess relative risk per mGy is −0.05 (95% CI −0.14, 0.04). An explanation for the observed excess of leukemia remains unresolved but conceivably could be related to chance due to small numbers, subtle biases in the study design and/or high tobacco use among enlisted men. Larger studies should elucidate further the possible relationship between fallout radiation, leukemia and cancer among atomic veterans.
Keywords: atomic veterans, SMOKY, PLUMBBOB, leukemia, nuclear weapons tests, Nevada Test Site, epidemiology
1. INTRODUCTION
Between 1945 and 1962, the United States conducted more than 230 atmospheric nuclear weapons tests primarily in Nevada and the Pacific Ocean. The detonation of a nuclear device was called a test and each test was given a name. An official grouping of nuclear weapons tests was called a test series. The 230 tests were grouped into 19 test series. On August 31, 1957, a nuclear weapon named SMOKY was detonated at the Nevada Test Site (NTS). SMOKY was one of 30 tests comprising the Operation PLUMBBOB test series. Twenty-two years later, a team from the Centers for Disease Control and Prevention (CDC) published observations of a significant excess of eight leukemia incident cases among 3,224 military participants present at the NTS during this test, but incompletely identified [1]. Attempts were made to locate and contact these participants. A second and a third study reported results from an intensive follow-up that located 95.5% of all SMOKY cohort members and identified ten leukemia incident cases and eight leukemia deaths through 1979 [2,3]. This subsequent follow-up also documented the health status of the veterans and obtained, where appropriate, their medical records and death certificates. These studies and a later fourth study [4], confirmed the statistically significant increase in predominantly myeloid leukemia and non-significant increases in melanoma of the skin, cancers of the genital system, eye and orbit, brain and nervous system, and polycythemia vera. When these studies were undertaken, the radiation exposure data were often limited to a single film badge reading, which may not have represented the true or complete exposure to radiation from nuclear weapons tests or other sources of radiation [3,4].
The original publications had brought attention to the scientific and medical communities the importance of low dose radiation research, spurring on other studies and eventually leading to public laws where military veterans who served during nuclear weapons testing could qualify for compensation for presumptive radiogenic diseases [5,6]. Further, it raised issues regarding the carcinogenic effectiveness of low dose radiation that was received gradually over time and not during a brief moment in time. Such concerns remain important societal issues today as reflected in the Low-Dose Radiation Research Act of 2015 currently before the U.S. Senate [7].
The current study updates the mortality follow-up of the SMOKY cohort from 1979 to 2010, i.e., 53 years after the 1957 test. All participants at the PLUMBBOB test series, of which SMOKY was one of 30 tests, were also similarly followed. PLUMBBOB is one of the test series included in the ongoing Eight Series Study of cancer among atomic veterans conducted by Vanderbilt University in cooperation with the National Council on Radiation Protection and Measurements [8,9]. There have been several studies of atomic veterans that combined participants at different test series. The ongoing Eight Series Study is investigating participants at eight nuclear weapons test series: CROSSROADS, GREENHOUSE, UPSHOT-KNOTHOLE, CASTLE, REDWING, PLUMBBOB, HARDTACK I and TRINITY, and is named accordingly. The importance of this study relates to the effects of chronic radiation exposures rather than acute exposures as experienced by Japanese atomic bomb survivors.
2. METHODS
Human subjects research approval was received from Vanderbilt University and CDC Institutional Review Boards. Consent was obtained from participants of the SMOKY follow-up study at the time of the original CDC studies and in accordance with protocol at the time.
2.1. Population identification
PLUMBBOB nuclear weapons test participants were identified using the Nuclear Test Review Program and Information System (NuTRIS) in cooperation with the Defense Threat Reduction Agency [10]. The NuTRIS database includes detailed information on unit attachment dates among military participants as well as film badge readings of individuals and representative veterans present at all nuclear test series. Participants of the Eight Series Study represented nearly half of all U.S. participants at above ground nuclear tests and included military personnel previously studied in an investigation of five series (which included the PLUMBBOB test series) and the CROSSROADS study conducted by the Medical Follow-up Agency [11–14].
The SMOKY cohort was identified by matching the original CDC population [2–4], to the Eight Series Study population on the first five matching criteria used by the National Death Index (based on the Social Security number, birth date, and name) [15]. Further probabilistic matching on key variables (last name, first name, birth year, and military service identification numbers) was done using the CDC LinkPlus probabilistic matching software [16]. We successfully matched 3,020 (94%) of the original 3,217 SMOKY test participants [2–4]. The small difference between the numbers in the original SMOKY study population and in the current study population resulted from changes in or the unavailability of identifying variables (Social Security Number, name, birthdate, and military service identification numbers).
2.2. Tracing
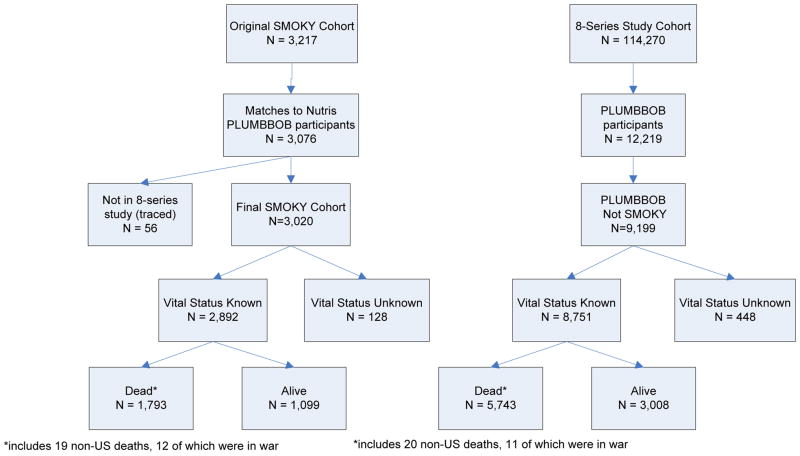
Mortality status as of December 31, 2010 was obtained by matching the study population against the Social Security Administration death master file as well as numerous state mortality data files. For those who had not died but were presumed to be alive, vital status as living was confirmed through linkages with the Social Security Administration Service for Epidemiological Researchers that also included information from the Internal Revenue Service. Deaths and alive status were also obtained using the Department of Veterans Affairs Beneficiary Identification Record Location System, where matching on military service identification numbers was possible (especially helpful because Social Security number was not available for many participants). Cause of death was obtained from the National Death Index, state mortality data, previously conducted epidemiologic studies [11–14], or death certificates obtained from state departments of health, regional offices of the Department of Veterans Affairs and the Federal Archives. Contributing causes of death, in addition to the primary cause of death, were also available from the National Death Index and death certificates. Credit bureaus and LexisNexis were used to confirm and correct key matching variables. Overall, these techniques confirmed the vital status of 95.3% of the PLUMBBOB test series population of whom 62% had died (Figure 1). For about 3% of those known to have died, cause of death was not available. Deaths outside the United States, including those during military action, are excluded from the standardized mortality analyses because national mortality rates used for comparison are based only on persons who died inside the United States.
Figure 1.
Vital status through 2010 of SMOKY and PLUMBBOB nuclear weapons test participants. SMOKY is not included in the PLUMBBOB tabulations.
2.3. Radiation dose assessment
Radiation dose assessment during nuclear weapons testing was based on data from the NuTRIS database, which included film badge measurements, and on a substantial number of historical documents related to the atmospheric nuclear testing program compiled to support estimates of dose for compensation programs for exposed veterans [5]. In accord with the case-cohort study design, dose reconstructions and categorization was for all cases of leukemia and a random sample of all veterans in the Eight Series Study. The detailed dose reconstruction methodology for nuclear weapons test participants was reported by Till and colleagues [17].
Available dosimetry film badge records specified the dates of badge issue and badge return but did not specify the nuclear test/s covered by the badge. The badge exposure period often spanned the dates of more than one test in the PLUMBBOB test series (most tests were just a few days apart). Therefore, selecting film badge doses that included the date of the SMOKY test (August 31, 1957) yielded more military personnel than the number of SMOKY participants included in the CDC investigations. Our definition of SMOKY participants is taken as the cohort from the original CDC studies.
Several military units, such as helicopter and transportation units, participated in SMOKY and also in other tests conducted during August and September 1957. Many members of the SMOKY cohort were in support units at Camp Desert Rock and did not participate directly in any test activities or receive any meaningful exposure.
For the radiation dose reconstructions for all leukemia deaths and the 1% random sample, radiation exposures that occurred at any of the 19 test series in which the veterans participated were included. To obtain as complete an assessment of occupational radiation exposure as possible, we also linked the rosters of atomic veterans with dosimetry registries available from the military services (Navy, Army, and Air Force), the U.S. Nuclear Regulatory Commission’s Radiation Exposure Information Reporting System, the U.S. Department of Energy’s Radiation Exposure Monitoring System, and a private dosimetry service (Landauer, Inc.). This supplemental linkage provided additional radiation exposure information for about 3 percent of the participants in the PLUMBBOB test series.
2.4. Statistical analysis
Standardized mortality ratios (SMR) analyses were conducted comparing the number of observed deaths with the number of expected deaths based on mortality rates in the general population using an approach similar to the University of Pittsburgh’s Occupational Cohort Mortality Analysis Program [18]. In brief, male population rates for specific causes of death by age and calendar year are applied to the corresponding person-years of follow-up to obtain the expected number of deaths had the veteran population experienced the same force of mortality as that of the general population. The start of follow-up is taken as the date of the first participation in the PLUMBBOB test series for PLUMBBOB test participants and the date of the SMOKY test (August 31, 1957) for the SMOKY test participants. The end of the follow-up is taken as the date of death, age 95, December 31, 2010, or whichever came first. The observed and expected numbers of deaths for selected causes were examined overall and by two time periods, 1957–1979 representing the follow-up period for the original SMOKY population [3], and 1980–2010 for the subsequent follow-up period. Statistical variability was evaluated by the 95% exact Poisson confidence interval (CI) of the SMR assuming that the observed number of deaths followed a Poisson distribution. A 95% CI that excludes 1.0 was considered as statistically significant at the 2-sided significance level of 0.05. Exact p-values are also presented when informative.
Because it would be prohibitively expensive to perform individual dose reconstructions on all test participants in the Eight Series Study, the case-cohort design was employed [19–22]. Cases are all test participants at the PLUMBBOB test series and the SMOKY test in whom leukemia developed, excluding chronic lymphocytic leukemia (CLL), which is not generally considered to be induced by radiation. The subcohort for comparison is a 1% random sample (n = 1,076) within defined strata from the overall cohort of 114,270 Eight Series Study participants. Rank (enlisted/officer) was considered a surrogate measure of socio-economic status (SES). Cox proportional hazards modeling was used to compute risks for non-CLL across categories of estimated radiation dose to the red bone marrow [23]. Adjustment was made for year of birth, year of first test participation, service and rank, and sampling fraction for the subcohort. To allow for a possible minimum latent period between radiation exposure and leukemia death, doses were lagged by 2 years, i.e., doses were excluded if they occurred 2 years or less before the date of death. Age was used as the timescale, and R version 3.02 was used for the analysis [24].
A cohort comparison with the entire 114,270 atomic veterans in the Eight Series Studies was also conducted based on the NuTRIS doses. As above, film badge results and NTPR generic dose reconstructions for each military unit were available as a starting point for estimating both external and internal exposure and organ doses for all atomic veterans. Much of the detailed information available, i.e., the NuTRIS doses, had been compiled to support estimation of dose for compensation programs for exposed veterans [5, 6]. The subcohort of 1,076 randomly selected participants formed the comparison group for which comprehensive dose reconstruction was performed for the case-cohort analyses [17]. During the dose reconstruction [17], it was found that the NuRIS dose [5], available for the entire cohort, could be adjusted with a scaling factor to bring them into close alignment with the dose estimates for organs where internal exposures from weapons fallout were negligible. The NuTRIS doses available for the entire cohort of atomic veterans are for external radiation and do not consider internal radiation, but since any intakes of radionuclides would contribute only a negligible dose to red bone marrow they would be of little consequence. These adjusted NuTRIS dose estimates are used in a full cohort analysis for comparison with the case-cohort analyses. Specifically, Cox proportional hazards modeling was used to compute risks of leukemia across categories of the adjusted NuTRIS dose estimates to the red bone marrow for the entire cohort of 114,270 veterans. Adjustment was made for year of birth, year of first test participation, test area and rank. Age was used as the timescale. All statistical analyses where conducted using R version 3.02 for both the Cox and linear models [24].
3. RESULTS
The Eight Series Study cohort consisted of 114,270 male veterans, of whom 12,219 participated in the PLUMBBOB test series and 3,020 at the SMOKY test. Vital status as of December 31, 2010 was determined for 95.3% of all participants in the PLUMBBOB test series (Figure 1).
Compared with other PLUMBBOB participants, SMOKY participants were more likely to be Army personnel (99% vs. 44%), enlisted men (81% vs. 53%), 24 years of age or younger (57% vs. 39%), and to have participated in only one test series (98% vs. 88%) or to have received a radiation dose of < 5 mSv (71% vs. 64%) (Table 1).
Table 1.
Characteristics of participants at the PLUMBBOB nuclear test series and the SMOKY detonation, and the respective non CLL leukemia cases.
| Characteristic | SMOKY Participants (n=3,020) | PLUMBBOB excluding SMOKY Participants (n=9,199) | SMOKY non-CLL Leukemias (n=27) | PLUMBBOB excluding SMOKY non-CLL Leukemias (n=47) | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Number | Percent | Number | Percent | Number | Percent | Number | Percent | |
| Year of Birth | ||||||||
| 1874–1919 | 418 | 13.8% | 2,400 | 26.1% | 2 | 7.4% | 18 | 38.3% |
| 1920–1924 | 259 | 8.6% | 1,443 | 15.7% | 3 | 11.1% | 11 | 23.4% |
| 1925–1929 | 469 | 15.5% | 1,436 | 15.6% | 4 | 14.8% | 6 | 12.8% |
| 1930–1934 | 898 | 29.7% | 1,642 | 17.8% | 11 | 40.7% | 7 | 14.9% |
| 1934–1941 | 976 | 32.3% | 2,277 | 24.8% | 7 | 25.9% | 5 | 10.6% |
| missing | - | 0.0% | 1 | 0.0% | ||||
| Age at First Test Participation | ||||||||
| 16–20 | 419 | 13.9% | 1,110 | 12.1% | 3 | 11.1% | 4 | 8.5% |
| 20–24 | 1,302 | 43.1% | 2,447 | 26.6% | 13 | 48.1% | 7 | 14.9% |
| 25–29 | 514 | 17.0% | 1,461 | 15.9% | 3 | 11.1% | 7 | 14.9% |
| 30–71 | 785 | 26.0% | 4,181 | 45.5% | 8 | 29.6% | 29 | 61.7% |
| Service | ||||||||
| Air Force | 8 | 0.3% | 2,444 | 26.6% | 1 | 3.7% | 8 | 17.0% |
| Army | 3,003 | 99.4% | 4,086 | 44.4% | 26 | 96.3% | 21 | 44.7% |
| Marines | 1 | 0.0% | 2,158 | 23.5% | 0 | 0.0% | 15 | 31.9% |
| Navy | 8 | 0.3% | 504 | 5.5% | 0 | 0.0% | 3 | 6.4% |
| Other | - | 0.0% | 7 | 0.1% | 0 | 0.0% | 0 | 0.0% |
| Rank (Pay Type) | ||||||||
| Enlisted | 2,445 | 81.0% | 4,904 | 53.3% | 20 | 74.1% | 23 | 48.9% |
| Officer | 575 | 19.0% | 4,295 | 46.7% | 7 | 25.9% | 24 | 51.1% |
| Number of Test Series of Participation | ||||||||
| 1 | 2,974 | 98.5% | 8,057 | 87.6% | 27 | 100.0% | 45 | 95.7% |
| 2 | 43 | 1.4% | 823 | 8.9% | - | 0.0% | 2 | 4.3% |
| 3 or more | 3 | 0.1% | 319 | 3.5% | - | 0.0% | 0 | 0.0% |
| NuTRISa Radiation Dose | ||||||||
| less than 5 mSv | 2,136 | 70.7% | 5,890 | 64.0% | 20 | 74.1% | 34 | 72.3% |
| 5 – 19 mSv | 723 | 23.9% | 2,287 | 24.9% | 5 | 18.5% | 12 | 25.5% |
| 20 – 908 mSv | 160 | 5.3% | 771 | 8.4% | 2 | 7.4% | 0 | 0.0% |
| missing | 1 | 0.0% | 251 | 2.7% | 0 | 0.0% | 1 | 2.1% |
| Vital Status as of Dec 31, 2010 | ||||||||
| Confirmed dead | 1,793 | 59.4% | 5,743 | 62.4% | 27 | 100.0% | 47 | 100.0% |
| Confirmed alive | 1,099 | 36.4% | 3,008 | 32.7% | ||||
| Lost to follow-up | 128 | 4.2% | 448 | 4.9% | ||||
NuTRIS denotes Nuclear Test Review Information System maintained by the US Defense Threat Reduction Agency, Department of Defense [5]
For participants in the PLUMBBOB nuclear test series excluding the SMOKY cohort, the SMRs over all follow-up periods (1957–2010) for a number of causes were statistically significantly less than 1.0, including all causes of death, all heart disease, all malignant neoplasms, diabetes mellitus, cirrhosis of the liver, suicides, non-malignant respiratory and kidney diseases, and tuberculosis (Table 2).
Table 2.
Standardized mortality ratios (SMR) and 95% confidence intervals (CI) for cancer and other causes of death among participants at the PLUMBBOB Nuclear Test Series and the SMOKY detonation by calendar years of follow-up.
| Calendar Years of Follow-up | 1957–1979
|
|||||
|---|---|---|---|---|---|---|
| SMOKY | PLUMBBOB excluding SMOKY | |||||
|
|
||||||
| Number of Participants | 3,020 | 9,199 | ||||
| Person-years at Risk | 64,718 | 199,946 | ||||
|
| ||||||
| Cause of Death (ICD9) | Obs | SMR | 95% CI | Obs | SMR | 95% CI |
| All Causes of Death (001–999) | 301 | 1.05 | 0.94–1.18 | 934 | 0.76* | 0.71–0.81 |
| All Malignant Neoplasms (140–208) | 59 | 1.10 | 0.84–1.42 | 177 | 0.71* | 0.61–0.82 |
| Buccal Cavity & Pharynx (140–149) | 1 | 0.58 | 0.01–3.23 | 1 | 0.12* | 0.00–0.65 |
| Digestive Organs & Peritoneum (150–159) | 14 | 1.11 | 0.60–1.86 | 43 | 0.70* | 0.51–0.94 |
| Respiratory System (160–165) | 17 | 0.92 | 0.53–1.47 | 61 | 0.67* | 0.51–0.86 |
| Bone (170) | 0 | 0.00 | 0.00–10.5 | 2 | 1.47 | 0.16–5.30 |
| Connective & Other Soft Tissue (171) | 0 | 0.00 | 0.00–10.1 | 2 | 1.45 | 0.16–5.23 |
| Melanoma of Skin (172) | 4 | 2.74 | 0.74–7.02 | 7 | 1.32 | 0.53–2.73 |
| Breast (175) | 0 | 0.00 | 0.00–46.5 | 0 | 0.00 | 0.00–9.99 |
| Bladder & Other Urinary (188, 189.3–189.9) | 0 | 0.00 | 0.00–3.74 | 3 | 0.59 | 0.12–1.72 |
| Testes & Other Male Genital Organs (186–187) | 1 | 1.13 | 0.01–6.31 | 1 | 0.38 | 0.01–2.14 |
| Eye (190) | 0 | 0.00 | 0.00–73.3 | 0 | 0.00 | 0.00–15.9 |
| Brain & CNS (191–192) | 5 | 1.85 | 0.60–4.31 | 11 | 1.01 | 0.50–1.81 |
| Thyroid & Other Endocrine Glands (193–194) | 0 | 0.00 | 0.00–13.6 | 0 | 0.00 | 0.00–3.32 |
| Non-Hodgkin Lymphoma (200,202) | 1 | 0.69 | 0.01–3.82 | 2 | 0.42 | 0.05–1.50 |
| Multiple Myeloma (203) | 0 | 0.00 | 0.00–5.98 | 3 | 0.98 | 0.20–2.88 |
| Chronic Lymphocytic Leukemia (204.1) | 1 | 3.43 | 0.04–19.1 | 0 | 0.00 | 0.00–2.50 |
| Leukemia other than CLL | 9 | 3.74* | 1.71–7.10 | 10 | 1.09 | 0.52–2.00 |
| Tuberculosis (010–018) | 1 | 0.93 | 0.01–5.16 | 2 | 0.40 | 0.04–1.44 |
| Diabetes (250) | 5 | 1.27 | 0.41–2.97 | 5 | 0.30* | 0.10–0.70 |
| All Heart Disease (390–398, 404, 410–429) | 76 | 0.79* | 0.62–0.98 | 284 | 0.61* | 0.54–0.68 |
| Non-malignant Respiratory Disease (460–519) | 12 | 0.98 | 0.50–1.71 | 22 | 0.38* | 0.24–0.57 |
| Cirrhosis of Liver (571) | 14 | 1.26 | 0.69–2.11 | 29 | 0.62* | 0.41–0.89 |
| Nephritis & Nephrosis (580–589) | 3 | 1.54 | 0.31–4.51 | 4 | 0.53 | 0.14–1.36 |
| Accidents (850–949) | 59 | 1.33* | 1.02–1.72 | 127 | 0.93 | 0.77–1.10 |
| Suicides (950–959) | 13 | 0.97 | 0.52–1.67 | 28 | 0.62* | 0.41–0.90 |
| Homicides & Other External Causes (960–978, 980–999) a | 13 | 1.90* | 1.01–3.26 | 18 | 0.90 | 0.54–1.43 |
| Calendar Years of Follow-up | 1980–2010 | Total (1957–2010) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| SMOKY | PLUMBBOB excluding SMOKY | SMOKY | PLUMBBOB excluding SMOKY | |||||||||
|
|
||||||||||||
| Number of Participants | 2,700 | 8,245 | 3,020 | 9,199 | ||||||||
| Person-years at Risk | 64,970 | 192,079 | 129,577 | 391,830 | ||||||||
|
| ||||||||||||
| Cause of Death (ICD9) | Obs | SMR | 95% CI | Obs | SMR | 95% CI | Obs | SMR | 95% CI | Obs | SMR | 95% CI |
| All Causes of Death (001–999) | 1,468 | 1.07* | 1.01–1.12 | 4,743 | 0.84* | 0.81–0.86 | 1,769 | 1.06* | 1.02–1.11 | 5,677 | 0.82* | 0.80–0.84 |
| All Malignant Neoplasms (140–208) | 470 | 1.15* | 1.05–1.26 | 1,408 | 0.91* | 0.87–0.96 | 529 | 1.14* | 1.05–1.25 | 1,585 | 0.89* | 0.84–0.93 |
| Buccal Cavity & Pharynx (140–149) | 11 | 1.40 | 0.70–2.51 | 30 | 1.10 | 0.74–1.57 | 12 | 1.26 | 0.65–2.20 | 31 | 0.86 | 0.59–1.23 |
| Digestive Organs & Peritoneum (150–159) | 99 | 1.02 | 0.83–1.24 | 330 | 0.91 | 0.81–1.01 | 113 | 1.03 | 0.85–1.24 | 373 | 0.88* | 0.79–0.97 |
| Respiratory System (160–165) | 183 | 1.19* | 1.02–1.37 | 493 | 0.89* | 0.81–0.97 | 200 | 1.16* | 1.00–1.33 | 554 | 0.86* | 0.79–0.93 |
| Bone (170) | 0 | 0.00 | 0.00–5.81 | 3 | 1.30 | 0.26–3.80 | 0 | 0.00 | 0.00–3.75 | 5 | 1.36 | 0.44–3.18 |
| Connective & Other Soft Tissue (171) | 0 | 0.00 | 0.00–1.69 | 8 | 1.04 | 0.45–2.06 | 0 | 0.00 | 0.00–1.45 | 10 | 1.11 | 0.53–2.03 |
| Melanoma of Skin (172) | 4 | 0.57 | 0.15–1.46 | 20 | 0.83 | 0.51–1.29 | 8 | 0.94 | 0.41–1.86 | 27 | 0.92 | 0.61–1.34 |
| Breast (175) | 2 | 4.15 | 0.47–15.0 | 1 | 0.55 | 0.01–3.08 | 2 | 3.57 | 0.40–12.88 | 1 | 0.46 | 0.01–2.56 |
| Bladder & Other Urinary (188, 189.3–189.9) | 14 | 1.19 | 0.65–2.00 | 37 | 0.75 | 0.52–1.03 | 14 | 1.10 | 0.60–1.85 | 40 | 0.73* | 0.52–1.00 |
| Testes & Other Male Genital Organs (186–187) | 0 | 0.00 | 0.00–7.10 | 0 | 0.00 | 0.00–2.01 | 1 | 0.72 | 0.01–3.98 | 1 | 0.23 | 0.00–1.26 |
| Eye (190) | 0 | 0.00 | 0.00–18.6 | 0 | 0.00 | 0.00–5.15 | 0 | 0.00 | 0.00–14.85 | 0 | 0.00 | 0.00–3.89 |
| Brain & CNS (191–192) | 6 | 0.62 | 0.23–1.34 | 34 | 1.06 | 0.73–1.48 | 11 | 0.88 | 0.44–1.58 | 45 | 1.05 | 0.76–1.40 |
| Thyroid & Other Endocrine Glands (193–194) | 3 | 2.66 | 0.54–7.78 | 2 | 0.51 | 0.06–1.84 | 3 | 2.15 | 0.43–6.28 | 2 | 0.40 | 0.04–1.44 |
| Non-Hodgkin Lymphoma (200,202) | 3 | 3.18 | 0.64–9.30 | 2 | 0.61 | 0.07–2.21 | 4 | 1.67 | 0.45–4.27 | 4 | 0.50 | 0.13–1.27 |
| Multiple Myeloma (203) | 9 | 1.23 | 0.56–2.33 | 33 | 1.19 | 0.82–1.67 | 9 | 1.13 | 0.52–2.15 | 36 | 1.17 | 0.82–1.62 |
| Chronic Lymphocytic Leukemia (204.1) | 3 | 0.83 | 0.17–2.42 | 12 | 0.81 | 0.42–1.42 | 4 | 1.02 | 0.28–2.62 | 12 | 0.74 | 0.38–1.29 |
| Leukemia other than CLL | 18 | 1.51 | 0.90–2.39 | 37 | 0.82 | 0.58–1.13 | 27 | 1.89* | 1.24–2.75 | 47 | 0.87 | 0.64–1.15 |
| Tuberculosis (010–018) | 1 | 1.54 | 0.02–8.57 | 0 | 0.00 | 0.00–1.42 | 2 | 1.16 | 0.13–4.19 | 2 | 0.26* | 0.03–0.95 |
| Diabetes (250) | 40 | 1.13 | 0.81–1.54 | 113 | 0.84 | 0.69–1.01 | 45 | 1.15 | 0.84–1.54 | 118 | 0.78* | 0.64–0.93 |
| All Heart Disease (390–398, 404, 410–429) | 444 | 0.99 | 0.90–1.09 | 1,414 | 0.73* | 0.70–0.77 | 520 | 0.95 | 0.87–1.04 | 1,698 | 0.71* | 0.68–0.74 |
| Non-malignant Respiratory Disease (460–519) | 134 | 1.01 | 0.85–1.20 | 467 | 0.79* | 0.72–0.86 | 146 | 1.01 | 0.85–1.19 | 489 | 0.75* | 0.68–0.82 |
| Cirrhosis of Liver (571) | 25 | 1.05 | 0.68–1.55 | 62 | 0.83 | 0.64–1.06 | 39 | 1.12 | 0.79–1.53 | 91 | 0.75* | 0.60–0.92 |
| Nephritis & Nephrosis (580–589) | 27 | 1.53* | 1.01–2.23 | 58 | 0.73* | 0.55–0.94 | 30 | 1.53* | 1.03–2.19 | 62 | 0.71* | 0.54–0.91 |
| Accidents (850–949) | 48 | 1.25 | 0.92–1.66 | 134 | 0.97 | 0.81–1.14 | 107 | 1.30* | 1.06–1.57 | 261 | 0.95 | 0.84–1.07 |
| Suicides (950–959) | 14 | 0.79 | 0.43–1.33 | 53 | 0.93 | 0.69–1.21 | 27 | 0.87 | 0.57–1.27 | 81 | 0.79* | 0.63–0.99 |
| Homicides & Other External Causes (960–978, 980–999) a | 6 | 1.07 | 0.39–2.34 | 11 | 0.67 | 0.33–1.19 | 19 | 1.53 | 0.92–2.39 | 29 | 0.80 | 0.53–1.15 |
23 deaths due to war (E990–E999) outside the US were excluded
p< 0.05
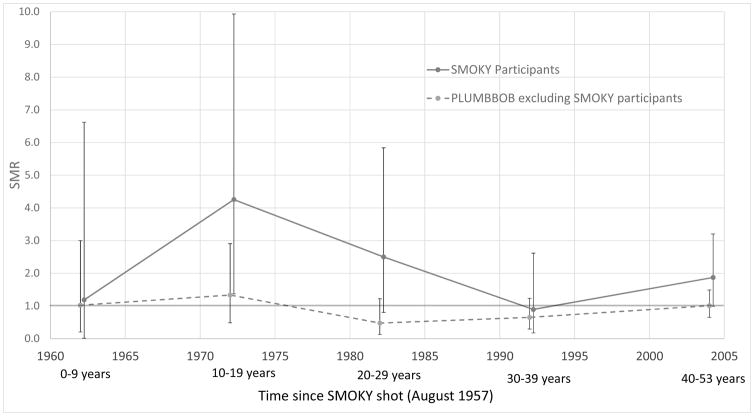
In contrast, for participants at the SMOKY test, the SMRs over all follow-up periods (1957–2010) were statistically significant greater than 1.0 for all causes of death (SMR = 1.06, 95% CI 1.02–1.11, p < 0.001), all malignant neoplasms (SMR = 1.14, 95% CI 1.05–1.25, p = 0.002), respiratory cancer (SMR = 1.16, 95% CI 1.0–1.33, p = 0.038), leukemia other than CLL (SMR = 1.89, 95% CI 1.24–2.75, p < 0.001), non-malignant kidney disease (nephritis and nephrosis) (SMR = 1.53, 95% CI 1.03–2.19, p = 0.018), and accidents (SMR = 1.30, 95% CI 1.06–1.57, p = 0.007) (Table 2). The non-CLL leukemias were predominantly myeloid leukemia (67% in the first follow-up period and 61% in the second follow-up period). The excess of non-CLL leukemia was most apparent in the first follow-up period and then declined but persisted through 2010 (Figure 2).
Figure 2.
Leukemia risk (other than CLL) over time for participants at the 1957 SMOKY test and PLUMBBOB series. 95% confidence limits are presented.
The participants at SMOKY where younger than participants at the other tests in the PLUMBBOB series. Age at participation was evaluated to learn whether the excess leukemias among SMOKY participants might have been concentrated in the younger soldiers, but there was little statistical evidence of heterogeneity by age. The SMRs for leukemia other than CLL were 2.28 (n=3), 2.39 (n=13), 1.19 (n=3), and 1.60 (n=8) for ages at participation under 20 y, 20–24 y, 25–29 y, and over 30 y, respectively.
An intra-cohort dose-response evaluation (internal analysis) was performed among the 27 non-CLL cases among the SMOKY participants and a comparison sample of 1,076 atomic veterans in the Eight Series cohort (Table 3). The 27 non-CLL cases were distributed over categories of dose to the red bone marrow, and increasing dose estimates did not increase risk for non-CLL. A cohort comparison with the entire 114,270 atomic veterans in the Eight Series Studies was also conducted based on the NuTRIS doses. A comparison between the NuTRIS doses and the subcohort doses (which were based on a comprehensive dose reconstruction) [17] showed a strong correlation with only a scaling factor of about 0.64 needed to bring them into close alignment. The pattern of leukemia risk over categories of NuTRIS bone marrow dose is similar to that seen for the case-cohort analysis (Table 3). Based on a linear model, the estimated excess relative risk per mGy is −0.05 (95% CI −0.14 to 0.04).
Table 3.
Risk of non-CLL among participants at the SMOKY nuclear detonation by radiation dose.
| Red Bone Marrow Dose (mGy)
|
|||||
|---|---|---|---|---|---|
| 0 - | 2.5 - | 5- | 10+ | Total | |
| Leukemia cases | 14 | 8 | 3 | 2 | 27 |
| SubCohort a | 484 | 235 | 163 | 194 | 1,076 |
| Relative Risk b | 1.0^ | 0.59 | 0.62 | 0.58 | |
| 95% Confidence Interval | 0.24 – 1.45 | 0.17 – 2.21 | 0.12 – 2.72 | ||
|
| |||||
| Leukemia cases by adjusted NuTRIS Dose to red bone marrow | 16 | 5 | 4 | 2 | 27 |
|
| |||||
| Full Cohort by adjusted NuTRIS Dose to red bone marrow | 58,793 | 19,031 | 17,068 | 19,378 | 114,270 |
|
| |||||
| Relative Risk b | 1.0^ | 0.67 | 0.85 | 0.53 | |
|
| |||||
| 95% Confidence Interval | 0.24–1.86 | 0.28–2.58 | 0.12–2.38 | ||
Referent Category
The subcohort is an approximate 1% random sample of all participants in the Eight Series Study for which comprehensive dose reconstruction was performed [17]. The full cohort is everyone in the Eight Series Study with a NuTRIS dose, adjusted to be comparable with the subcohort dose.
Adjusted for service, rank (enlisted/officer), year of birth and year of first participation at a weapons test (and sampling fraction for the case-cohort analyses).
Similar dose-response evaluations for all PLUMBBOB participants also showed little evidence for a relationship between leukemia other than CLL and radiation dose estimates. Comparisons using different subcohorts (i.e., the Eight Series subcohort, the NTS subcohort and the PLUMBBOB subcohort) yielded essentially the same null results (data not presented).
4. DISCUSSION
The continued concern about the risks from low level radiation exposure remains today despite increased knowledge of radiation effects and continued efforts to minimize unnecessary radiation exposures in occupation, medical, and environmental circumstances [25]. Veterans and other populations exposed to radiation remain concerned about their potential risks and the specific health conditions that might be related to their past exposure.
This 53-year mortality follow-up of soldiers at one large Nevada nuclear test series and of an intensively studied cohort of soldiers present at one test (SMOKY) shows that mortality in the PLUMBBOB test series participants, other than those at SMOKY, is statistically significantly less than that expected from all causes of death and from specific grouped causes of death, including all heart disease, all malignant neoplasms, all non-malignant respiratory diseases, diabetes mellitus, liver cirrhosis, nephritis and nephrosis, suicide, and tuberculosis. The deficit particularly in heart disease deaths demonstrates the healthy warrior effect due to selection of a healthier than normal population group for entry into and persistence during military service [26,27]. Interestingly, deaths due to heart disease were significantly reduced only during the first 22 years of follow-up but not later. The selection factors associated with the healthy worker effect, particularly for heart disease, often diminish significantly with time since initial selection for employment [28].
Follow-up studies of other participants at nuclear tests from Australia, New Zealand and the United Kingdom have reported increases in leukemia but not in relation to radiation dose, nor have there been consistent reports of increases in other malignancies [29–32]. Participants at the British nuclear tests also reflect the healthy warrior effect in that the SMR for all causes of death was significantly below 1.0 (SMR = 0.86) [30].
Compared with other occupationally-exposed populations the SMOKY cohort and the other veterans in the PLUMBBOB tests received relatively lower radiation exposures. Nonetheless, approximately 5% of participants received >50 mSv from all sources of identified occupational radiation exposure.
Besides estimating radiation doses to individuals, the dosimetry team investigated activities of the various military units in the SMOKY cohort to determine if any unusual maneuvers occurred that may have led to increased radiation dose or exposure to other environmental hazards. We could not find any particular activity or type of exposure unique to the SMOKY cohort. One activity known as Task Force Warrior [11], involved members of the SMOKY cohort taken closer to areas near the bomb test site. Three of these men were reported to have died from non-CLL leukemia [11]. While no film badge indication of excess radiation exposure was found [11], the increase in Task Force Warrior activities among cohort members in whom leukemia developed is noteworthy. Dosimetry uncertainty was also evaluated and the effect of shared Berkson (classical) error on inferences was determined to depend on both the strength of the dose response and the extent of sharing of dosimetry errors.32 Since the sharing of errors was negligible [17,33], accounting for uncertainty in the dose-response analyses also has a negligible effect.
As in our initial papers [2–4], and other studies that included SMOKY participants [11,12], we can only speculate why the SMOKY participants show increases in leukemia, certain cancers, and nonmalignant diseases. Given our dose reconstruction estimates and the absence of a dose response, we have little evidence that radiation is the cause. Furthermore, the absence of any such excesses in the other PLUMBBOB participants suggests that the SMOKY veterans had some characteristics, currently unknown, that increased their mortality risks, overall and for selected causes of death. Interestingly, the SMOKY participants differed from other atomic veterans (and the general population it seems) in surrogate measures of socio-economic status, e.g., more SMOKY participants were enlisted men (81% vs. 53%). Because enlisted men, similar to blue collar workers, are more likely to smoke cigarettes than officers or white collar workers, such differences might explain the elevations in lung cancer and nonmalignant kidney disease [34–37].
An intriguing finding in our study is the apparent absence of the healthy warrior effect in the SMOKY cohort, unlike that seen among other PLUMBBOB nuclear test participants. Again, the increase in lung cancer and kidney disease suggests that smoking cigarettes might have been more prevalent in the SMOKY cohort with a larger percentage of enlisted personnel, and possibly related to the general availability of cigarettes in military rations during and after World War II and the Korean conflict [38]. The increase in accidental deaths also suggests that SMOKY cohort members may have been risk takers or employed in dangerous occupations. Since we compared many causes of death over two time periods, the leukemia excess in the SMOKY cohort, one of 30 tests during the PLUMBBOB test series, could be a chance finding. Finally, the possibility of an unusual and undetermined bias on how the SMOKY cohort was selected might also have been a factor.
The full cohort analysis of all 114,270 veterans in the Eight Series Study applied the adjusted NuTRIS doses and the cohort results were nearly identical with those from the case-cohort analysis. The NuTRIS doses were only for external exposures and did not take into account any internal intakes of radionuclides from weapons fallout. However, any intakes of radionuclides would contribute only a negligible dose to bone marrow and thus would be of little consequence for leukemia. The strong correlation had not been anticipated because the NuTRIS doses had been developed to be “high-sided” and for compensation purposes. One implication of these nearly identical results for leukemia is that many other cancers can now be evaluated inexpensively when it is unlikely that any internal intakes would contribute to organ dose. Specifically, the NuTRIS adjustment factor of 0.64 for red bone marrow doses can be scaled by the respective ratios of exposure-to-dose coefficients for gamma rays for male breast, testes, brain, heart and other organs to red bone marrow [39] to estimate the corresponding organ absorbed doses from external exposure.
Strengths of the study include the cohort design, the near complete follow-up and mortality ascertainment over a 53-year period, the comprehensive dose reconstructions and linkages with other dosimetry data bases to estimate cumulative radiation doses, and the access to unique military and veteran data bases that enhanced the quality and completeness of the data collected. Limitations include the low radiation doses received during the atmospheric weapons tests, the relatively small number of leukemia deaths, the inability to control directly for potential confounders such as smoking, and reliance on death information and not incidence. Over the years of study, however, myeloid leukemia was associated with a high fatality rate so that mortality would reflect incidence fairly closely. On the other hand, there have been recent improvements in survival for CML in the United States, approaching 64% (http://seer.cancer.gov/statfacts/html/cmyl.html) so that persons who developed CML after 2000 would have been less likely to have died than in earlier years. We did search death notifications for contributing causes of death and found only one mention of CML, suggesting that there were few persons with CML who died of other causes. Further the study of atomic bomb survivors indicates a strong decline in the excess of CML over time and with attained age [40] suggesting that few radiation-related cases would have occurred in these later years. Nonetheless, a small number of cases of CML conceivably could have been missed and an incidence study would have had more statistical power to detect an excess had there been one.
Rank (enlisted/officer) was used as the measure of SES in the internal analyses as a surrogate control for smoking and other unknown factors. Cigarette smoking has been associated with a relatively small risk of myelogenous leukemia among military veterans [41], but it is unlikely that large differences in smoking by dose categories exist that might distort the dose-response analyses, particularly after adjusting for SES. Conceivably, smoking might have contributed to the unexplained excess of leukemia deaths among SMOKY veterans.
The conundrum of the SMOKY cohort remains. The radiation dose reconstruction and environmental records do not indicate that SMOKY participants experienced greater or different radiation exposure than other test participants. Was the SMOKY cohort somehow different or less fit than participants at the other PLUMBBOB nuclear tests? Where SMOKY participants heavy smokers which contributed to their excess of lung cancer and conceivably of leukemia? Or is this cohort finding simply a chance observation? Nothing we have found so far suggests that radiation exposure in the SMOKY cohort differed from those in the other PLUMBBOB participants.
5. CONCLUSION
Military participants at the PLUMBBOB nuclear test series remained relatively healthy after 53 years and continued to die at a lower rate than the general population. In contrast, the SMOKY cohort showed significant increases in all causes of death, respiratory cancer, nephritis and nephrosis, and accidents, possibly related to lifestyle factors common to enlisted men who made up 81% of the cohort. Leukemia risk, initially reported to be significantly increased among SMOKY participants, remained elevated, but this risk diminished over time. Despite an intense dose reconstruction, the risk for leukemia was not found to increase with increasing levels of radiation dose to the red bone marrow. Historically, the SMOKY studies have importance in recognizing that environmental exposure from atmospheric testing may have contributed to subsequent health effects, and Public Laws were enacted to compensate atomic veterans for their service. The larger Eight Series study of atomic veterans, which includes Plumbbob as one of the eight test series, should elucidate further the possible relationship between fallout radiation and leukemia among atomic veterans [8,9].
Acknowledgments
This research was supported in part by contracts and grants from the National Cancer Institute (Grant No. U01 CA137026); the U.S. Department of Energy (Grant No. DE-SC0008944 awarded to the National Council on Radiation Protection and Measurements), which included interagency support from the U.S. Nuclear Regulatory Commission, the U.S. Environmental Protection Agency and the National Aeronautics and Space Administration; and a Discovery Grant from the Vanderbilt-Ingram Cancer Center (Center No. 404-357-9682).
We also acknowledge Dr. Paul Blake, Chief, Nuclear Test Personnel Review, Defense Threat Reduction Agency, U.S. Department of Defense and his staff for their technical support of the project, and Hanson Gaugler at L-3 Services for his critical contributions to our accessing and interpreting the comprehensive data sources necessary for study conduct. Similarly, Han Kang and Tim Bullman, Environmental Epidemiology Service, U.S. Department of Veterans Affairs, were instrumental in providing support and assistance throughout the conduct of the Eight Series Atomic Veteran Study. We also are indebted to: the Department of Energy (Nimi Rao), the Nuclear Regulatory Commission (Doris Lewis), Oak Ridge Associated Universities (Derek A. Hagemeyer), Landauer, Inc. (Craig Yoder, PhD), the U.S. Army Dosimetry Center (William S. Harris, Jr., CHP), the U.S. Air Force Radiation Dosimetry Laboratory (Ms. Linda Wilson), and the Naval Dosimetry Center (CDR Anthony Williams and Lt Selena Hayes) for facilitating linkages with their respective dosimetry files. The findings and conclusions in this paper are those of the authors. Its publication does not necessarily represent the official position of or imply endorsement by the Centers for Disease Control and Prevention, National Council on Radiation Protection and Measurements, Vanderbilt University, University of Kentucky or any of the acknowledged agencies.
References
- 1.Centers for Disease Control and Prevention. Leukemia among persons present at an atmospheric nuclear test (SMOKY) MMWR. 1979;28:361–362. [Google Scholar]
- 2.Caldwell GG, Kelley DB, Heath CW., Jr Leukemia among participants in military maneuvers at a nuclear bomb test (SMOKY) a preliminary report. JAMA. 1980;244:1575–1578. [PubMed] [Google Scholar]
- 3.Caldwell GG, Kelley DB, Zack MM, Falk H, Heath CW., Jr Mortality and cancer frequency among military nuclear test (SMOKY) participants, 1957–1979. JAMA. 1983;250:620–624. [PubMed] [Google Scholar]
- 4.Caldwell GG, Kelley DB, Heath CW, Jr, Zack M. Polycythemia vera among nuclear test participants. JAMA. 1984;252:3027–3028. [PubMed] [Google Scholar]
- 5.National Research Council. A review of the Dose Reconstruction Program of the Defense Threat Reduction Agency. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Blake PK, Komp GR. Radiation exposure of U. S. military individuals. Health Phys. 2014;106:272–278. doi: 10.1097/HP.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 7.HR [Accessed May 2, 2016];Low-Dose Radiation Research Act of 2015. 2015 https://www.congress.gov/bill/114th-congress/house-bill/35.
- 8.Boice JD., Jr Atomic bombs, asbestos, and healthy warriors. [Accessed May 2, 2016];Health Phys News. 2014 XLII:21–23. http://ncrponline.org/wp-content/themes/ncrp/PDFs/BOICE-HPnews/20_Atomic_Veterans_Jan2014.pdf. [Google Scholar]
- 9.Bouville A, Toohey RE, Boice JD, Jr, Beck HL, Dauer LT, Eckerman KF, Hagemeyer D, Leggett RW, Mumma MT, Napier B, Pryor KH, Rosenstein M, Schauer DA, Sherbini S, Stram DO, Thompson JL, Till JE, Yoder C, Zeitlin C. Dose reconstruction for the million worker study: status and guidelines. Health Phys. 2015;108:206–220. doi: 10.1097/HP.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Defense Threat Reduction Agency. Operation PLUMBBOB. [Accessed May 2, 2016];Fact Sheet. http://www.dtra.mil/Portals/61/Documents/NTPR/1-Fact_Sheets/19_PLUMBBOB.pdf.
- 11.Robinette CD, Jablon S, Preston TL. Mortality of Nuclear Weapons Test Participants. Washington, DC: National Academies Press; 1985. [Google Scholar]
- 12.Thaul S, Page WF, Crawford H, O’Maonaigh H. The Five Series Study: Mortality of Military Participants in U.S. Nuclear Weapons Tests. Washington, DC: National Academies Press; Institute of Medicine; 2000. [PubMed] [Google Scholar]
- 13.Johnson JC, Thaul S, Page WF, Crawford H. Mortality of Veteran Participants in the CROSSROADS Nuclear Test. Washington, DC: National Academies Press; 1996. [PubMed] [Google Scholar]
- 14.Watanabe KK, Kang HK, Dalager NA. Cancer mortality risk among military participants of a 1958 atmospheric nuclear weapons test. Am J Public Health. 1995;85:523–527. doi: 10.2105/ajph.85.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Death Index. Hyattsville, MD: CDC, National Center for Health Statistics; [Accessed May 2, 2016]. http://www.cdc.gov/nchs/data/ndi/ndi_users_guide_chapter4.pdf. [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed May 2, 2016];National Program of Cancer Registries (NPCR). Registry Plus™ Link Plus. http://www.cdc.gov/cancer/npcr/tools/registryplus/lp.htm.
- 17.Till JE, Beck HL, Aanenson JW, Grogan HA, Mohler HJ, Mohler SS, Voillequé PG. Military participants at U.S. atmospheric nuclear weapons testing -- Methodology for estimating dose and uncertainty. Radiat Res. 2014;181:471–484. doi: 10.1667/RR13597.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh GM, Youk AO, Stone RA, Sefcik S, Alcorn C. OCMAP-PLUS: a program for the comprehensive analysis of occupational cohort data. Occup Environ Med. 1998;40:351–362. doi: 10.1097/00043764-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Wacholder S. Practical considerations in choosing between the case-cohort and nested case-control designs. Epidemiology. 1991;2:155–158. doi: 10.1097/00001648-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Barlow WF, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52:1165–1172. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 21.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data Anal. 2007;51:3737–3748. [Google Scholar]
- 22.Breslow NE, Lumley T, Ballantyne CM, Chambless LE, Kulich M. Using the whole cohort in the analysis of case-cohort data. Am J Epidemiol. 2009;169:1398–1405. doi: 10.1093/aje/kwp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 24.R Development Core Team. The R project for statistical computing. Vienna, Austria: R Foundation for Statistical Computing;; [Accessed May 2, 2016]. http://www.R-project.org. [Google Scholar]
- 25.Boice JD., Jr Implications of radiation dose and exposed populations on radiation protection in the 21st century. Health Phys. 2014;106:313–328. doi: 10.1097/HP.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 26.McLaughlin R, Nielson L, Waller M. An evaluation of the effect of military service on mortality: quantifying the healthy soldier effect. Ann Epidemiol. 2008;18:928–936. doi: 10.1016/j.annepidem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Seltzer CC, Jablon S. Effects of selection on mortality. Am J Epidemiol. 1974;100:367–372. doi: 10.1093/oxfordjournals.aje.a112047. [DOI] [PubMed] [Google Scholar]
- 28.Howe GR, Chiarelli AM, Lindsay JP. Components and modifiers of the healthy worker effect: evidence from three occupational cohorts and implications for industrial compensation. Am J Epidemiol. 1988;128:1364–1375. doi: 10.1093/oxfordjournals.aje.a115089. [DOI] [PubMed] [Google Scholar]
- 29.Muirhead CR, Bingham D, Haylock R, O’Hagan JA, Goodill AA, Berridge GL, English MA, Hunter N, Kendall GM. Follow up of mortality and incidence of cancer 1952–98 in men from the UK who participated in the UK’s atmospheric nuclear weapon tests and experimental programs. Occup Environ Med. 2003;60:165–172. doi: 10.1136/oem.60.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muirhead CR, Kendall GM, Darby SC, Doll R, Haylock RG, O’Hagan JA, Berridge GL, Phillipson MA, Hunter N. Epidemiological studies of UK test veterans: II. mortality and cancer incidence. J Radiol Prot. 2004;24:219–241. doi: 10.1088/0952-4746/24/3/002. [DOI] [PubMed] [Google Scholar]
- 31.Gun RT, Parsons J, Crouch P, Ryan P, Hiller JE. Mortality and cancer incidence of Australian participants in the British nuclear tests in Australia. Occup Environ Med. 2008;65:843–848. doi: 10.1136/oem.2007.034652. [DOI] [PubMed] [Google Scholar]
- 32.Pearce N, Prior I, Methven D, Culling C, Marshall S, Auld J, de Boer G, Bethwaite P. Follow up of New Zealand participants in British atmospheric nuclear weapons tests in the Pacific. BMJ. 1990;300:1161–1166. doi: 10.1136/bmj.300.6733.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stram DO, Preston DL, Sokolnikov M, Napier B, Kopecky KJ, Boice J, Beck H, Till J, Bouville A. Shared dosimetry error in epidemiological dose-response analyses. PLoS One. 2015;10:e0119418. doi: 10.1371/journal.pone.0119418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seltzer CC, Jablon S. Army rank and subsequent mortality by cause: 23-year follow-up. Am J Epidemiol. 1977;105:559–566. doi: 10.1093/oxfordjournals.aje.a112420. [DOI] [PubMed] [Google Scholar]
- 35.Lodge LH. Tri-service Health Questionnaire-1989. J R Army Med Corps. 1991;137:80–83. doi: 10.1136/jramc-137-02-05. [DOI] [PubMed] [Google Scholar]
- 36.Lee DJ, LeBlanc W, Fleming LE, Gómez-Marín O, Pitman T. Trends in US smoking rates in occupational groups: the National Health Interview Survey 1987–1994. J Occup Environ Med. 2004;46:538–548. doi: 10.1097/01.jom.0000128152.01896.ae. [DOI] [PubMed] [Google Scholar]
- 37.Williams JO, Bell NS, Amoroso PJ. Drinking and other risk taking behaviors of enlisted male soldiers in the US Army. Work. 2002;18:141–150. [PMC free article] [PubMed] [Google Scholar]
- 38.Koehler FA. Special Rations for the Armed Forces, 1946–53. Chapter 1. Washington DC: Historical Branch, Office of the Quartermaster General; 1958. [Accessed May 2, 2016]. QMC Historical Studies, Series II, No. 6. http://archiveis/u9eRO. [Google Scholar]
- 39.ICRP Publication 116. Conversion coefficients for radiological protection quantities for external radiation exposures. Ann ICRP. 2010;40:1–257. doi: 10.1016/j.icrp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, Iwanaga M, Miyazaki Y, Cullings HM, Suyama A, Ozasa K, Shore RE, Mabuchi K. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001. Radiat Res. 2013;179:361–382. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLaughlin JK, Hrubec Z, Linet MS, Heineman EF, Blot WJ, Fraumeni JF., Jr Cigarette smoking and leukemia. J Natl Cancer Inst. 1989;81:1262–1263. doi: 10.1093/jnci/81.16.1262. [DOI] [PubMed] [Google Scholar]