Abstract
In the liver, CFTR regulates bile secretion and other functions at the apical membrane of biliary epithelial cells (i.e cholangiocytes). CF-related liver disease (CFLD) is a major cause of death in patients with CF. CFTR dysfunction affects innate immune pathways, generating a para-inflammatory status in the liver, and other epithelia. This study investigates the mechanisms linking CFTR to TLR4 activity. We found that CFTR is associated in a multi-protein complex at the apical membrane of normal mouse cholangiocytes, with proteins that negatively control Src activity. In CFTR-defective cholangiocytes, Src tyrosine kinase self-activates and phosphorylates TLR4, resulting in activation of NF-κB, and increased pro-inflammatory cytokines production in response to endotoxins. This Src/NF-κB-dependent inflammatory process attracts inflammatory cells, but also generates changes in the apical junctional complex and loss of epithelial barrier function. Inhibition of Src decreased the inflammatory response of CF-cholangiocytes to LPS, rescued the junctional defect in-vitro and significantly attenuated endotoxin-induced biliary damage and inflammation in vivo (Cftr-KO mice).
Conclusion
Our findings reveal a novel function of CFTR as regulator of TLR4 responses and cell polarity in biliary epithelial cells. This mechanism is pathogenetic, as shown by the protective effects of Src inhibition in vivo and maybe a novel therapeutic target in CFLD and other inflammatory cholangiopathies.
Keywords: Cystic Fibrosis, TLR4, cytokines, cytoskeleton, cell polarity
Cystic Fibrosis (CF) is a disease of secretory epithelia leading to chronic damage and insufficiency of several organs, including pancreas, lung and liver. CF is caused by mutations in the gene encoding for the cystic fibrosis transmembrane conductance regulator (CFTR), a cAMP-regulated epithelial chloride channel expressed at the apical membrane of most secretory epithelia (1, 2). More than twenty years after its discovery, the understanding of CFTR function(s) is still incomplete.
In addition of functioning as a channel, CFTR may also act as a “hub” protein that forms macromolecular complexes with several other proteins at the apical membrane, mostly through PDZ binding motifs (3). Through these interactions, CFTR may be involved in key cellular functions such as regulation of ATP release, membrane protein and vesicle trafficking and inflammation (4, 5). This multi-functional role of CFTR reflects the variety of manifestations associated with its defective function, and the diversity of the pathogenetic mechanisms involved in the target organs of CF, such as lung, pancreas, liver and intestine. Several defective inflammatory responses and altered innate immune pathways have been linked to CFTR deficiency in different organs affected by CF(6-8).
In the liver, CFTR is specifically expressed at the apical membrane of the epithelial cells lining the biliary epithelium (cholangiocytes) where it regulates chloride and bicarbonate secretion in response to cAMP/PKA stimulation (9). Defective CFTR function causes impaired biliary secretion of chloride and bicarbonate, and ductal cholestasis (10). A percentage of patients with Cystic Fibrosis develop liver disease (CFLD). CFLD is a chronic inflammatory cholangiopathy that can evolve into sclerosing cholangitis and focal biliary cirrhosis, and is the third leading cause of death in CF (11, 12). Severe CFLD affects only a minority of CF patients, suggesting that ductal cholestasis may be a predisposing factor to liver disease, which progresses only in the presence of a second insult (13, 14).
We have previously shown that the CFTR-defective biliary epithelium, when exposed to gut-derived bacterial endotoxins, produces high levels of cytokines/chemokines and attracts inflammatory cells (i.e macrophages and neutrophils) leading to peribiliary inflammation. In CF biliary epithelial cells TLR4/NF-κB-dependent innate immune response to endotoxins is upregulated (14).
In CFTR-defective cells, we have also found an increased activity of non-receptor tyrosine kinases belonging to the Src family (SFKs) (14). Src kinases are involved in a variety of signaling pathways including the regulation of inflammatory responses and phosphorylation of the cytoplasmic domain of TLRs (i.e TLR 2, 3, 4 and 5), which is involved in the activation of the downstream signaling cascade (15-17).
Members of the SFK family share a common and well-conserved regulatory mechanism. Src is able to self-activate, unless it is negatively regulated by phosphorylation mediated by the C-terminal Src kinase (Csk), a cytosolic protein that anchors to the membrane through the adaptor Cbp (Csk Binding Protein) (18, 19). Cbp is anchored to the cytoskeleton by the PDZ domains of EBP50 (Ezrin-radix-moesin binding protein 50) (20, 21). EBP50 is an adapter protein localized at the apical region of epithelial cells. EBP50 also binds CFTR favoring its association into macromolecular signaling complexes at the plasma membrane (22-24).
In this study, we address the hypothesis that CFTR regulates TLR4-dependent inflammatory responses in the biliary epithelium by modulating Src activity. Expression of CFTR at the apical membrane facilitates the assembly of a protein complex able to maintain the kinase inactive. In the absence of CFTR this complex does not assemble, resulting in self-activation of Src and subsequent increase in TLR4 phosphorylation and NF-κB–dependent cytokine production, in response to endotoxins. We also documented that epithelial cell-cell junctions and monolayer barrier function are impaired in CFTR-defective cells as a secondary effect of the NF-κB-dependent inflammatory process. The protective effects of SFKs inhibition in vitro and in vivo demonstrate the pathogenetic relevance of this mechanism and suggest that targeting SFK activity or NF-κB is a potential therapeutic target for CF-liver disease and possibly other cholangiopathies.
MATERIALS AND METHODS
Reagents and additional methods are detailed in the supplemental materials and methods.
Animals and Experimental Protocol
All procedures involving animals in this study were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee.
Congenic B6.129P2-Cftrtm1Unc mice, which possess the S489X mutation that blocks transcription of CFTR (25) and ΔF508-CFTR (B6-129-Cftrtm1Kth) (26) mice with targeted mutation corresponding to the ΔF508 mutation in human, were used for in vivo experiments and/or for the isolation of primary cholangiocytes cell lines. Animals were bred in our facility or provided by the CF Core Center Animal Core (Case Western Reserve University, Cleveland, OH) and were maintained as previously described (14, 27, 28).
Cftr-KO mice and WT littermates were exposed to DSS (14) alone or in combination with the tyrosine kinase inhibitor PP2 (1mg/Kg body weight/day) by i.p. At the end of the treatment mice were sacrificed and liver tissue was harvested formalin-fixed and paraffin-embedded for histochemical analysis.
Cell culture
Mouse cholangiocytes were isolated from WT, DeltaF508 and Cftr-KO mice as described (27). After the first passage cells were plated into 25-cm2 tissue culture flasks coated with rat-tail collagen as previously described (14, 27). Before selected experiments cells were cultured in transwell inserts with a 0.4 μm pore semipermeable membrane (Becton, Dickinson, and Co, Franklin Lakes, NJ); in this condition, cells grow as a polarized monolayer that can be accessed from both the apical and basolateral domain distinctly. Establishment of a confluent monolayer was routinely checked, measuring transepithelial resistance and membrane potential difference (Millicell ERS System; Millipore, Billerica, MA). One week after confluence, transepithelial resistance was >1000 Ω·cm2 and cells were ready for analysis.
Determination of cytokine secretion
Polarized WT and Cftr-KO cholangiocytes were untreated or treated with PP2 (10 μM), LPS (100 ng/ml) or their combination for 12 h. At the end of the treatment, apical and basolateral media were collected and processed for cytokines/chemokines quantification analysis by using the Milliplex Mouse Cytokines/Chemokines Kit EMD Millipore (Billerica,MA) coupled with BioPlex Luminex platform (Bio-Rad Laboratories, Inc, Hercules, CA) following the manufacturer’s instructions. Data were normalized for the cell protein content as previously described (14).
Co-immunoprecipitation
Polarized WT and Cftr-KO cholangiocytes were lysated in ODG buffer (50 mM Tris-HCl, pH 7.4, 1 mM EDTA, 2% Octyl-D-glucoside, 5mM βmercaptoethanol, 0.25 M NaCl, 1mM Na3VO4, 20 mM NaF, 5% glycerol, 100μl Triton X-100). To immuno-precipitate Csk or Cbp, 3 mg of proteins were incubated with the proper primary antibody for 1 h at 4°C under rotation (see supplemental table 1). Samples were then incubated with 30 μl of Protein A/G PLUS-Agarose for 3 h at 4°C under rotation. After several washing, the beads were resuspended in 50 μl of 4x NuPAGE Lithium Dodecyl Sulphate (LDS) sample buffer. Western blot with antibodies against CFTR, CSK, Cbp or EBP50 was performed on the immune-precipitated lysates (see supplementary table 1).
Immunofluorescence and confocal microscopy
Polarized WT and Cftr-KO cholangiocytes were processed for immunofluorescence staining. Briefly, cells were fixed with either a) 100% methanol and washed with PBS; b) or 3.7% PFA, washed using PBS/10mM glycine, and permeabilized with PBS/0.2%TritonX-100. Unspecific binding sites were blocked with Normal Horse Serum (1:20) or with 3% non-fat milk for 45 minutes at RT. Cells were incubated overnight at 4°C with specific primary antibody in blocking buffer (supplementary table 1) and for 1 hour at RT with the proper secondary antibody (conjugated with Alexa Fluor 488, 555, 594 or 647 in PBS/BSA/Gly). Cells were then mounted using a Vectashield Kit (Vector Laboratories, Inc, Burlingame, CA) with 4’,6-diamidino-2-phenylindole (DAPI). Confocal analysis was performed using a Zeiss LSM 710 Duo confocal microscope or a Zeiss LSM 510 Meta with x63, 1.4 NA objective. Serial optical sections (0.5 μm thick) were collected for 3D analysis.
Proximity ligation assay (PLA)
PLA experiments were done using Duolink In Situ reagents (Olink Bioscience). WT and CFTR-KO cholangiocytes seeded on collagen I coated coverslips were fixed with 3.7% PFA, washed using PBS/10mM glycine, and permeabilized with PBS/TritonX-100 0.2%. Cells were incubated with blocking solution (Olink Bioscience) for 30 minutes and then with one (negative control) or two primary antibodies for 1h at RT.
Coverslips were then incubated with secondary antibodies linked to PLA oligonucleotide probes PLUS and MINUS (Olink Bioscience) for 1 h at 37 °C. The samples were then incubated with the ligation ligase solution for 30 min at 37 °C to hybridize oligonucleotides tagged on probes. The coverslips were then incubated with the amplification polymerase solution for 100 min at 37 °C to amplify hybridized oligonucleotides and fluorescently label (Alexa-Fluor 488) the amplification products. Cells were then stained with rhodamine conjugate phalloidin for 20 minutes and coverslips were mounted on slides with Duolink In Situ Mounting Medium with DAPI. Imaging was done using a Zeiss LSM 710 Duo confocal microscope.
Immunohistochemistry
Paraffin-embedded 4 μm liver slides were processed and stained with the cholagiocyte-specific marker K19 to visualize the ductular reaction, with the leukocyte specific marker CD45 and the macrophage marker F4/80 to analyze the inflammatory cell infiltrate. Quantification of the K19, CD45 and F4/80 positive areas by morphometric analysis was performed as described (14).
Epithelial permeability assay
Epithelial permeability was assessed in polarized monolayers of WT and Cftr-KO cholangiocytes by measuring transepithelial flux of 10-kilodalton FITC-labelled dextrans as previously described (29). Briefly, epithelial monolayers were equilibrated with PBS at 37°C, then the fluorescent marker (400 μl diluted in PBS at a concentration of 200 mg/mL) was added to the apical chamber. Samples were removed at 1-hour intervals for 4 hours. The volume removed (100 μl) was replaced with 37°C PBS. In experiments with SFK or NF-κB inhibitors, PP2 (0.1, 1 or 10 μM) or Bay 11- 7082 (5 μM) were added to the apical chamber for the last 2 hours and epithelial permeability was compared at the end of the 4 hours time point. At the end of the treatment proteins were extracted for Western Blot processing. After sampling, fluorescence intensity (excitation, 485nm; emission, 530nm) was measured on a fluorescent plate reader Sinergy2 (Biotek Instruments, Winooski, VT). Tracer concentrations were determined by linear regression using dilutions of FITC-dextran in PBS.
Measurement of trans-epithelial resistance (TER)
WT and Cftr-KO cholangiocytes were seeded in transwell inserts with a 0.4 μm pore semipermeable membrane (Becton, Dickinson, and Co, Franklin Lakes, NJ). After four days in culture TER was measured daily for 9 days using a Millicell ERS System (Millipore, Billerica, MA). TER was calculated as ohms/cm2 multiplying it by the surface area of the monolayer (0.33 cm2).
Statistics
Results are shown as mean ± SD. Statistical comparisons were made using one-way analysis of variance or the Wilcoxon–Mann–Whitney 2-sample rank sum test, where appropriate, using Prism GraphPad. P values less than .05 were considered significant.
RESULTS
Src activity and TLR4 phosphorylation are increased in CFTR-KO and DeltaF508 mutated cholangiocytes
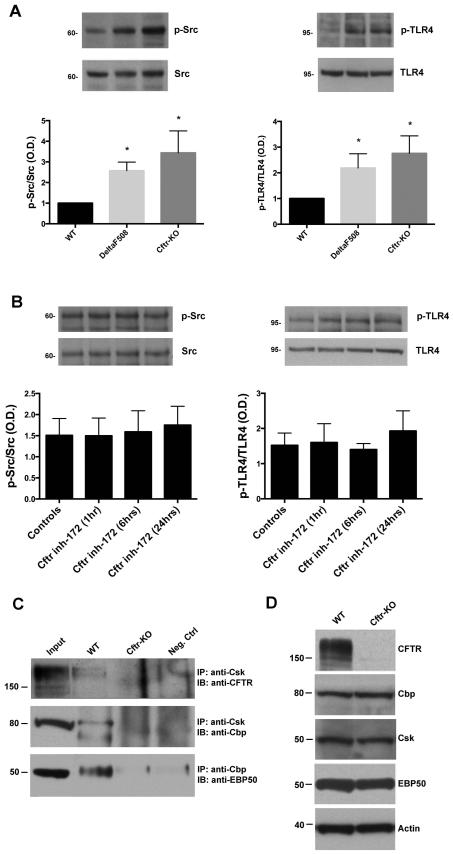
Cholangiocytes isolated from CFTR-null mice (CFTR-KO; Cftrtm1Unc) have higher Src activity and TLR4 phosphorylation at tyrosine 674 (see figure 1A), as previously reported by our group (14). CFLD occurs in patients with severe CFTR mutations (class I-III), ΔF508 being the most frequent (about 80% of CF patients) (30). To understand if Src signaling is enhanced also in the most common mutation, we isolated cholangiocytes from DeltaF508;Cftrtm1Kth mice (corresponding to the ΔF508 mutation in humans) (26) and tested Src activity, measuring the phosphorylation at the tyrosine 419 of the catalytic domain and TLR4 phosphorylation at the tyrosine 674. As shown in figure 1A, similarly to Cftr-KO cholangiocytes, DeltaF508 cells have significant increased Src tyrosine kinase activity (pY419) and increased TLR4 phosphorylation compared to WT cholangiocytes.
Figure 1. CFTR expression at the membrane is essential to modulate Src activity and TLR4 phosphorylation.
A) WT, DeltaF508 and Cftr-KO cholangiocytes were cultured in polarized conditions on transwell inserts. Src activity and TLR4 phosphorylation were determined by western blot using antibodies respectively against the active form of Src (pY418) or against the TLR4 tyrosine phosphorylation site (Y674). Bar graphs represent the optical density ratio of p-Src (pY418) vs total Src and p-TLR4 (pY674) vs total TLR4. A statistical significant increase in Src activity and TLR4 phosphorylation is shown in DeltaF508 and Cftr-KO cholangiocytes. Data represent mean ± SD of n=3 independent experiments (*p<0.05 vs WT). Representative western blot images were cropped from the same gel. B) Polarized WT cholangiocytes were treated with CFTR-inh172 for 1, 6 or 24 hours to inhibit the channel function activity. Src activity and TLR4 phosphorylation were determined by western blot. Bar graphs represent the optical density ratio of p-Src (pY418) vs total Src and p-TLR4 (pY674) vs total TLR4. No statistical differences were shown between the different treatments. Data represent mean ± SD of n=4 different experiments. C) Lysates from WT and Cftr-KO cells were immunoprecipitated with antibodies against Csk or Cbp and immunoblotted with specific antibodies against CFTR, Cbp and EBP50. The Western blot analysis shows that CFTR, Cbp, Csk and EBP50 are physically interacting in WT cells but not in Cftr-KO cells. Nuclear fraction lysates were used as negative controls. CFTR input is displayed from a shorter exposure of the same blot. D) Expression of CFTR, Cbp, Csk and EBP50 was evaluated by Western blot in total cell lysates from WT and Cftr-KO cholangiocytes.
Increased Src activation in Cftr-KO cholangiocytes is caused by lack of CFTR expression at the membrane
To investigate if the increased Src kinase activity depends on the defective CFTR channel function, we treated WT cells for 1h, 6hrs and 24hrs with a specific CFTR blocker (CFTR inh-172, 10 μM), known to alter the channel gating function (31). Abolishment of cAMP-mediated chloride efflux (32) confirmed the effective inhibition of CFTR activity in WT cholangiocytes treated with CFTR inh-172 (Supplementary figure 2). As shown in figure 1B, both basal levels of Src and TLR4 phosphorylation were unaffected, suggesting that the localization of CFTR at the membrane, rather than its channel function is important for the regulation of Src activity.
Thus, we hypothesized that the membrane expression of CFTR might be necessary for the assembly and stabilization of a protein complex able to regulate Src. As discussed in the introduction, Csk and Cbp could interact with CFTR through the PDZ domains of the scaffold protein EBP50, to maintain Src in an inactive state. To demonstrate the interaction of the endogenous proteins CFTR-EBP50-Cbp-Csk in a complex, we used three different approaches. Co-immunoprecipitation experiments showed that in WT cholangiocytes, Csk co-immunoprecipitates with Cbp and CFTR, and Cbp co-immunoprecipitates with EBP50 (figure 1C). Interestingly, although by Western blot the expression level of EBP50, Csk and Cbp was comparable in WT and CF cells (figure 1D), we found no co-immunoprecipitation between Cbp, Csk and EBP50 in CFTR-defective cells.
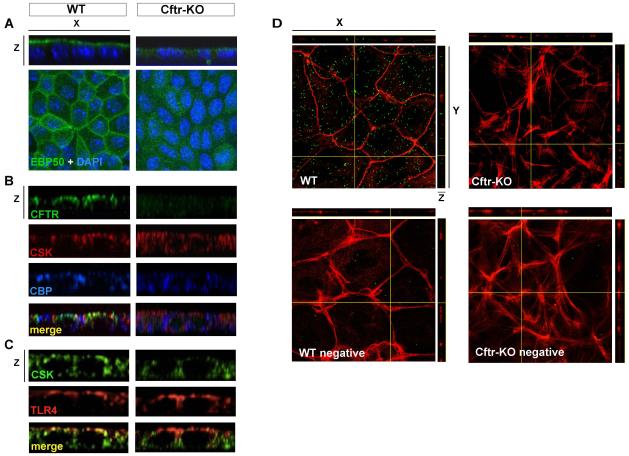
By confocal microscopy, we visualized the apical distribution of CFTR, EBP50, Cbp and Csk and their interactions in the polarized monolayer. As shown in panel 2A, in WT cells expressing CFTR, EBP50 staining is restricted to the apical membrane and co-localizes with CFTR, Cbp and Csk at the apical membrane (figure 2B). Csk also appears to be localized in close proximity to TLR4 at the apical membrane (figure 2C). On the contrary, in CFTR-defective cells, EBP50 staining is diffuse and mislocalized in the cytosol (figure 2A). Furthermore, co-localization of Cbp and Csk at the apical membrane is lost (figure 2B). TLR4 on the other hand, appears to be normally expressed at the apical membrane also in Cftr-KO cells (figure 2C), suggesting that the different susceptibility of CF cells to LPS does not depend on an altered membrane distribution of the TLR4 receptor.
Figure 2. Proteins involved in Src regulation co-localize at the apical membrane of WT but not Cftr-KO cholangiocytes.
Confocal images of polarized WT and Cftr-KO cells single stained for EBP50 (A) or co-stained for CFTR-Cbp-Csk (B) or TLR4-Csk (C). A) EBP50 is localized on the apical membrane in WT cells while appears diffused and mislocalized in Cftr-KO cells. B) Z-stack sections show that CFTR, Cbp and Csk co-localize on the apical membrane in WT cells but not in CF cells. C) TLR4 shows the same distribution in WT and CF cells but does not co-localize with Csk in CF cells. D) Confocal imaging of in-situ proximity ligation assay, using EBP50 and Cbp antibodies, indicates interaction of the two proteins (green dots) in WT cells but not in CFTR-KO cells. Phalloidin staining (red) was used to show the localization of the interaction in proximity of F-actin fibers in x-z and y-z images. In the negative control the assay was performed using EBP50 antibody only.
Using an in-situ proximity ligation assay, based on the formation of fluorescent spots when two proteins of interest are located within a distance of 40nm, we confirmed that EBP50 and Cbp strictly interact in normal cholangiocytes, whereas no signal was detected in Cftr-KO cells (Figure 2D). For negative control, detection was performed in WT and Cftr-KO cells when one of the two antibodies was omitted.
Altogether, these results indicate that in WT cells, CFTR physically interacts with Cbp and Csk through EBP50, and that this interaction is missing in CF cells. In CF cells, the negative regulation of Src is lost and the kinase is free to self-activate and to phosphorylate TLR4, increasing its responsiveness to TLR4 agonists.
Inhibition of Src activity decreases LPS-induced activation of NF-κB and cytokine secretion in CF cholangiocytes
To study if inhibition of Src kinase decreases the response of the CF biliary epithelium to endotoxins, polarized WT and Cftr-KO cholangiocytes were exposed to LPS (100 ng/ml) for 6 hours in the presence or absence of the SFK family inhibitor PP2 (10 μM) (14). PP2 treatment did not alter the PKA signaling as shown by secretin receptor gene expression analysis and intracellular cAMP production (supplementary figure 3). NF-κB activation was assessed by Western blot for the p65 subunit of NF-κB in nuclear fractions and by measuring the levels of phospho-IkBα. As shown in figure 3A, in Cftr-KO cholangiocytes, as compared to WT cells, NF-κB activation was significantly increased, both in basal conditions and after LPS stimulation. In CF cells not exposed to LPS, treatment with PP2 restored NF-κB to levels similar to WT cells. When cells were treated with LPS in the presence of PP2, NF-κB activation was significantly inhibited. Moreover, the levels of phospho-IkBα were decreased after treatment with PP2 correlating with the decreased p65 nuclear expression (supplementary figure 4).
Figure 3. Treatment with the SFK inhibitor PP2 decreases LPS-induced NF-κB activation (A) and cytokine secretion (B) in Cftr-KO cholangiocytes.
Polarized cholangiocytes were treated with PP2 (10 μM), LPS (100 ng/ml) or their combination. A) NF-κB activity was assessed by western blot using an antibody against p65 in nuclear fractions. Histone-3 was used to normalize for the protein content. Bar graphs represent optical density quantification of n=4 experiments. B) Apical and basolateral media were analyzed by Luminex and data were normalized for the cell protein content. Treatment with PP2 significantly inhibited LPS-stimulated cytokine secretion in Cftr-KO cells. The plots represent the means of 5 independent experiments (*p<0.05; **p<0.01).
A similar experimental approach was used to study the effects of Src inhibition on the secretion of NF-κB-dependent cytokines previously shown to be increased in CF-cholangiocytes (i.e. G-CSF, CXCL1, LIX and CXCL2)(14, 33). The concentration of cytokines was quantified in the apical and basolateral media by Luminex assay in CF-cholangiocytes exposed to LPS and compared with CF-cholangiocytes exposed to LPS and PP2 (10μM). As shown in figure 3B, LPS-stimulated secretion of G-CSF, CXCL1, LIX and CXCL2 was significantly increased in Cftr-KO cholangiocytes as compared to WT. However, secretion of these inflammatory mediators was significantly decreased by treatment with PP2, consistent with the hypothesis that Src controls TLR4/NF-κB response to LPS in CFTR-defective cells.
The distribution of apical junction proteins and actin cytoskeleton is altered in Cftr-KO cholangiocytes.
While performing the in-situ proximity assay, we noticed significant changes in the actin cytoskeleton in CFTR-defective cholangiocytes that lead us to investigate it further.
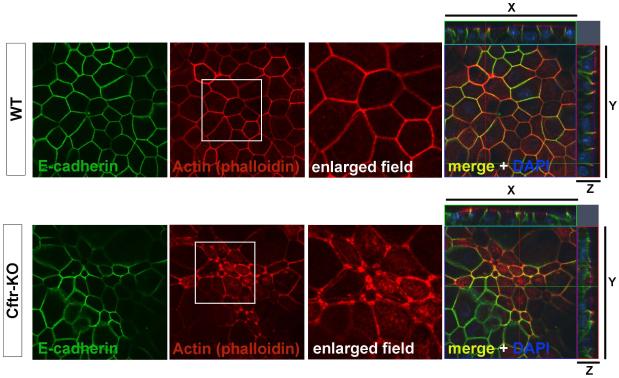
Phalloidin staining revealed stark differences in the structure of the actin cytoskeleton in CFTR-defective cells both in not polarized (see figure 2D) and polarized culture conditions (figure 4). As shown in figure 4, while cadherin-catenin complexes accumulated normally at areas of cell-cell contact in WT cells, their association with circumferential actin fibers was largely abolished in cells lacking CFTR. Apical actin filaments were still present, but unlike WT cells where the subcortical actin ring co-localized closely with cell-cell junctions, in Cftr-KO cells the actin appeared split in multiple fibers and failed to co-localize with E-cadherin at the adherens junctions. Interestingly, we observed increased actin accumulation at the tricellular junctions (structures where three cells meet).
Figure 4. F-actin cytoskeleton defect in Cftr-KO cells.
Confocal images of confluent monolayers of WT and Cftr-KO cholangiocytes were acquired with the same imaging setting. Distibution of phalloidin (F-actin) staining reveals a defect in the proper formation of the cortical actin ring with accumulation of F-actin at the tri-cellular junctions in Cftr-KO cells compared with WT cells. E-cadherin was used to define the junctional and lateral cell areas.
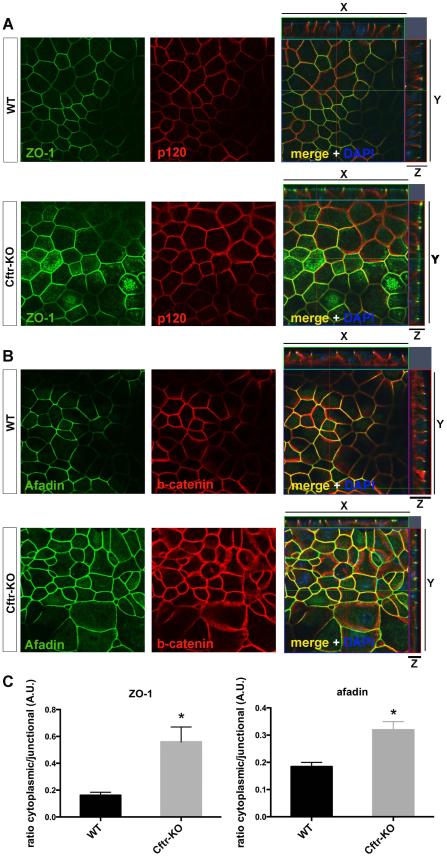
Since actin filaments are directly connected with cell junction structures (34, 35), we investigated if the defective organization of actin filaments reflected a loss of tight junctions (TJs) and adherens junctions (AJs) integrity. Using confocal microscopy, we analyzed the distribution of proteins of the apical junction complex formed by TJs (i.e ZO-1, zona occludens-1) and AJs (i.e afadin). As shown in figure 5A,B while in WT cells ZO-1 and afadin staining are strictly localized at the cellular junctions, in CF cells both proteins appear diffusely distributed in the cytoplasm. The analysis of the fluorescence intensity confirmed a significant increase of the cytoplasmic/junctional ratio in CF as compared to WT cells suggesting a destabilization of the cellular junctions (figure 5C).
Figure 5. Cell junction defect in Cftr-KO cells.
Confocal images of confluent monolayers of WT and Cftr-KO cholangiocytes were acquired with the same imaging setting. Staining for ZO-1 (B), a protein of tight-junctions and afadin (C), a protein of adherens junctions, shows that in Cftr-KO cells both proteins have lost their junctional restriction and appear diffusely distributed in the cytoplasm. Quantification of the fluorescence intensity revealed an increased cytoplasmic/junctional ratio in Cftr-KO cells of both proteins. Fluorescence intensity was measured in 10 cells per field and in 3 fields per sample (*p<0.05). P120 (B) and β-catenin (C) were used to define the entire lateral areas of cell-cell contact.
Barrier function is impaired in Cftr-KO cell monolayer.
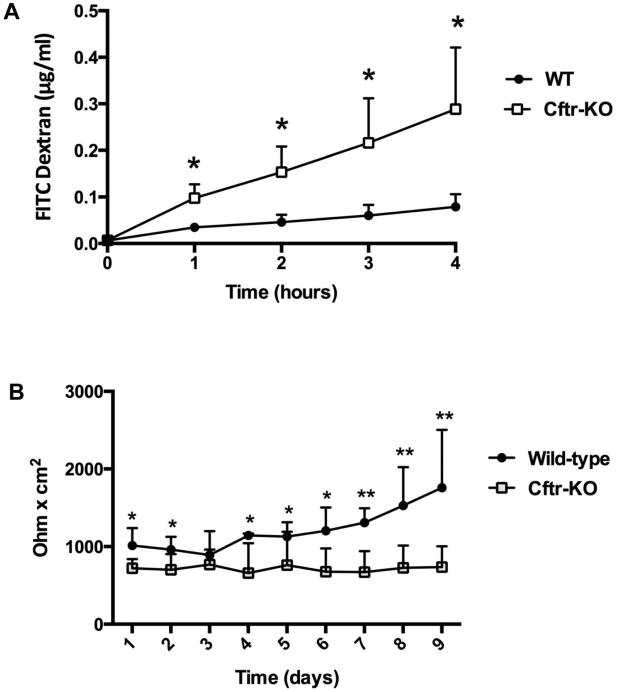
The apical junction complex is a functional unit formed by tight junctions (TJs), adherens junctions (AJs) and the perijunctional F-actin cytoskeleton that functionally interact to preserve the barrier function of the epithelium. To determine whether the junctional defect affected the barrier function of Cftr-KO cholangiocyte monolayers, we measured the trans-epithelial movement of FITC-labeled dextrans (10 KDa) across the epithelial monolayer over time (29) and the trans-epithelial resistance (TER) in polarized WT and CFTR-defective cholangiocytes. As shown in figure 6A, the passage of FITC-dextrans across the paracellular compartment was significantly increased in Cftr-KO compared to WT monolayers, whereas TER measurements were significantly decreased in Cftr-KO monolayers (figure 6B), indicating that paracellular permeability and barrier function is altered in Cftr-KO cells.
Figure 6. Impaired epithelial barrier function in Cftr-KO monolayers.
A) Polarized monolayers of WT and Cftr-KO cholangiocytes were assayed for paracellular permeability to 10KDa FITC-dextrans. Concentration of dextrans diffused from the apical to the basolateral compartment was determined by linear regression (4-hour assay period) using known standard concentrations. Results represent the mean ± SD of 3 independent experiments in triplicate. B) WT and Cftr-KO cholangiocytes were seeded in transwell inserts. After reaching confluence TER was recorded daily for 9 days. Results represent the mean ± SD of 5 independent experiments.
Cytoskeletal and paracellular changes are due to Src- and NF-κB-dependent inflammatory responses
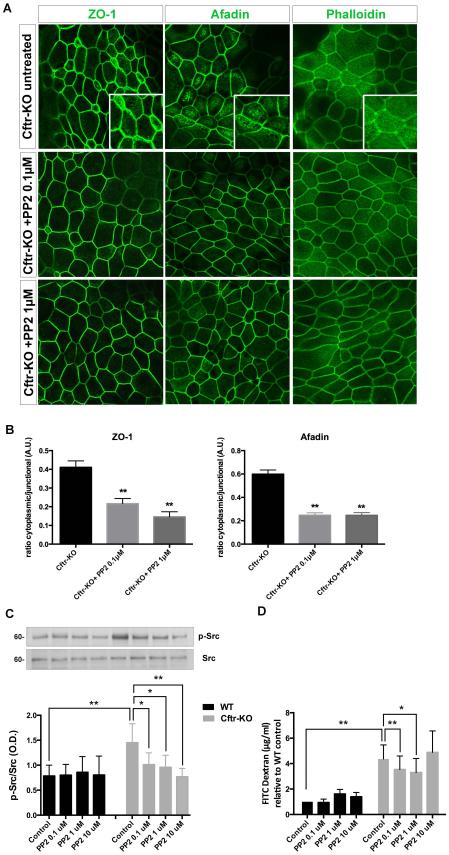
To test the effects of Src inhibition on the cytoskeletal and paracellular changes, we treated CF cholangiocytes with the Src inhibitor PP2 (0.1,1 and 10μM, not shown). As shown in figure 7A, treatment with PP2 significantly decreased the junctional defect (i.e afadin and ZO-1 cytoplasmic mislocalization) and rescued the single fiber organization of actin.
Figure 7. Cytoskeletal and paracellular defects are rescued by Src inhibition in Cftr-KO cholangiocytes.
A) Confluent monolayers of Cftr-KO cholangiocytes were treated with SFK inhibitor PP2 (0.1-1μM) for 2 hours and stained for ZO-1, afadin and phalloidin (F-actin) and analyzed at the confocal microscope. Images show the re-localization and restriction of ZO-1 and afadin to the junctions and the rescue of the normal subcortical distribution of F-actin after treatment with PP2. B) Quantification of the fluorescence intensity revealed a decreased cytoplasmic/junctional ratio in Cftr-KO cells, after PP2 treatment, of both proteins. Fluorescence intensity was measured in 10 cells per field and in 3 fields per sample (**p<0.01). C) Polarized monolayers of WT and Cftr-KO cholangiocytes were exposed to different concentrations of PP2 that significantly decreased Src activity in Cftr-KO cells as shown by the quantification of p-Src (pY418) vs total Src by Western Blot. D) Bar graph shows the quantification of FITC-dextrans paracellular permeability at the end of the treatment. Results represent the mean ± SD of 4 independent experiments in triplicate; (*p<0.05; **p<0.01).
Consistent with the rescue of cell junction defects by Src inhibition, treatment with PP2 significantly influenced the paracellular permeability of Cftr-KO cells with a dose-dependent effect. As shown in figure 7, as PP2 progressively inhibited Src (Y416) phosphorylation (figure 7C), paracellular permeability of Cftr-KO cells decreased (figure 7D).
Inhibition of NF-κB using Bay 11-7082 had a similar rescue effect on the actin distribution and the paracellular permeability (supplementary figure 1A,B), suggesting that the apical junction changes are mediated by the increased Src-dependent NF-κB activation in CF cells. Furthermore, to exclude a potential effect of NF-κB on Src activation in Cftr-KO cholangiocytes, we measure Src phosphorylation at the tyrosine 419 of the catalytic domain by western blot in CF cells treated with the NF-κB inhibitor Bay 11-7082 (5 μM). As shown in supplementary figure 1C, phosphorylation level of Src was unchanged by Bay 11-7082 treatment, suggesting that Src activation is upstream of NF-κB.
In vivo inhibition of SFK activity reduces biliary damage and inflammation in Cftr-KO mice treated with DSS
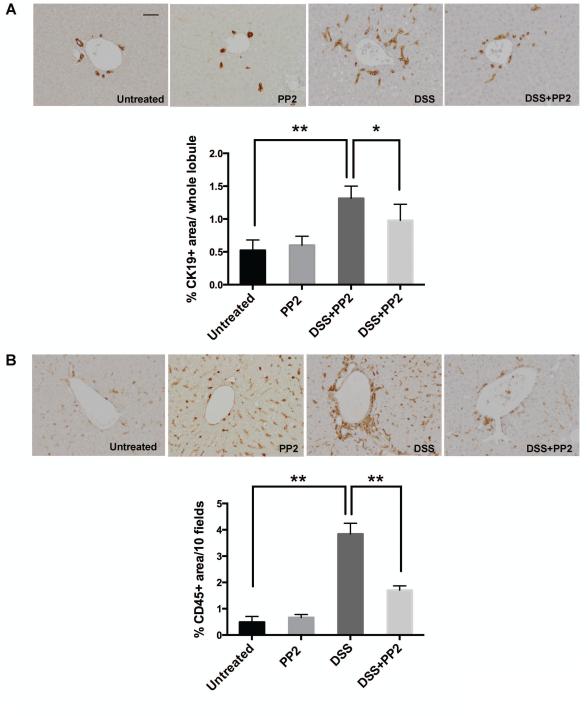
To validate in vivo the role of Src inhibition and its possible use as a therapeutic target in CFLD, we treated Cftr-KO mice with DSS, in the presence or the absence of the specific SFK inhibitor PP2. It has been previously established (13, 14) that the increased intestinal permeability caused by DSS induced colitis favors the translocation of gut-derived endotoxins to the liver, causing damage and inflammation in the liver of Cftr-KO mice. At the end of the treatment, liver tissues were harvested and stained with K19, to quantify the biliary and progenitor cell compartment expansion as a marker of biliary damage and with CD45, to quantify the amount of infiltrating leukocytes in the portal space as a marker of inflammation. DSS-treated Cftr-KO mice developed the expected biliary damage with expansion of a small cholangiocyte ductular reactive component (36) and portal inflammatory reaction and extensive infiltration of mononuclear cells whereas no toxicity was shown in the group treated with PP2 alone. As shown in figure 8, in Cftr-KO mice treated with DSS in combination with PP2 (1 mg/Kg by i.p. daily), bile duct proliferation (A) and inflammatory cell infiltration (B) were significantly reduced as determined by computer-assisted morphometric analysis of K19 and CD45 positive areas respectively, suggesting a protective effect of Src inhibition. In addition, treatment with PP2 significantly reduced the amount of macrophages in DSS-treated Cftr-KO mice as shown by staining for macrophage marker F4/80 (supplementary figure 5).
Figure 8. PP2 treatment reduces biliary damage and inflammation in Cftr-KO mice treated with DSS.
Cftr-KO mice were untreated (n=6) or either treated with DSS (n=5) or with DSS and PP2 (1 mg/Kg by i.p. daily)(n=5). At the end of the treatment, liver tissues were harvested and stained with the cholangiocyte-specific marker K19 (A) or the leukocyte specific marker CD45 (B). Computer-assisted morphometric analysis of K19 and CD45 positive areas shows that PP2 treatment significantly reduced the bile duct proliferation and inflammatory cell infiltration in Cftr-KO mice treated with DSS (*p<0.05 vs DSS only). Bar scale=50 um.
DISCUSSION
Epithelial cells represent the first line of defense against external pathogens thanks to the innate immune system and the presence of a number of pattern recognition receptors (such as Toll-like receptors). This system must be tightly controlled to avoid excessive inflammation and damage that may occur even in the presence of physiological amounts of bacterial components (37, 38). In this study, we demonstrate a novel mechanism by which the protein CFTR controls TLR4 activation and inflammation in secretory epithelia. Our findings provide a mechanistic explanation to the observation that CFTR dysfunction affects innate immune pathways, in the liver, as well as in other epithelia in Cystic Fibrosis (39).
We have recently shown that in CFTR-defective cholangiocytes, TLR4 phosphorylation at tyrosine 674 is increased as compared to normal cells (14) and that TLR4 signaling is up-regulated. Tyrosine phosphorylation of TLRs cytoplasmic domain takes part in the recruitment process of cytoplasmic adaptor proteins during activation of TLR4 downstream signaling (15). In immune cells (i.e. macrophages), members of the Src protein tyrosine kinase family (SFK) phosphorylate the TIR domain of TLR4 and increase the recruitment of downstream signal-competent adapter proteins (e.g MyD88) and kinases (e.g. IRAK-4 and IRAK-1). As a counter-regulatory mechanism, TLR4 tyrosine phosphorylation is reduced to avoid uncontrolled inflammation during continuous exposure to LPS (LPS tolerance)(15, 17).
In this study we sought to understand whether activation of Src signaling plays a role in the pathologic inflammatory responses seen in CF cholangiocytes and to clarify the mechanism linking CFTR-deficiency to Src activation and cell dysfunction. Our findings indicate that CFTR associates in an apical membrane complex with proteins acting as constitutive negative regulators of Src (i.e Cbp and Csk). In CF, decreased CFTR protein at the membrane prevents the formation of this multiprotein complex, and consequently Src is able to self-activate and to phosphorylate TLR4, thereby sustaining an increased inflammatory response to LPS and other TLR agonists (Fiorotto/Strazzabosco, unpublished data). Interestingly, pharmacologic inhibition of Src in vitro reduces the LPS-induced NF-κB activation and cytokine secretion in CF biliary cells. CFTR is known to interact directly or indirectly with several proteins that regulate its channel activity (i.e AKAP, PKA), integrate signaling pathways (i.e adenosine 2b receptors, β2-adrenergic receptor) or coordinate other transport activities (i.e ENaC, ROMK) (3-5). Here we demonstrate that CFTR independently from its channel activity participates in the regulation of epithelial innate immunity by controlling the activity of Src. In fact, pharmacological inhibition of CFTR channel function in WT cells has no significant effects on Src activation and TLR4 phosphorylation. Conversely, chemical inhibition of Src significantly decreases the activation of NF-κB, downstream to TLR4 signaling and the dependent secretion of cytokines.
The finding that inhibition of Src in vivo improves the inflammatory liver phenotype in Cftr-KO mice exposed to gut-derived endotoxins, provides a proof of concept that Src has a pathogenetic role in CF and is a possible target for treatment. In physiological conditions, Src activity is tightly regulated by Csk, (C-terminal Src kinase), a kinase that holds Src in an inactive conformation by phosphorylating its Y529 residue. To inactivate Src, Csk must translocate from the cytoplasm to the plasma membrane and be anchored in close proximity to the kinase, by the membrane adaptor Csk Binding Protein (Cbp)/PAG which resides in the lipid rafts and is anchored to the cytoskeleton through PDZ domains of ezrin-radix-moesin binding protein 50 (EBP50) (18, 19). EBP50 is an adapter protein localized at the apical region of epithelial cells (22, 23). EBP-50 acts as a scaffold protein that binds CFTR and cooperates in the formation of multiprotein complexes (40). Interestingly, CFTR and EBP50 are mislocalized in cholangiocytes of ezrin defective mice, which also present a cholestatic phenotype (41, 42).
Co-immunoprecipitation, confocal microscopy and proximity ligation assay experiments show that in normal cholangiocytes, Cbp is physically linked to Csk and CFTR, and EBP50 to Csk and Cbp. These interactions are lost in Cftr-KO cholangiocytes, suggesting that CFTR insertion at the membrane regulates the assembly of a multi-protein complex that controls Src activation. Confocal microscopy experiments confirmed that EBP50 has a clear distribution on the apical membrane in the normal epithelium but is mislocalized and diffusely distributed in Cftr-KO cholangiocytes. This is consistent with the observation that in bile ducts of CF patients, EBP50 immunoreactivity is not localized at the apical membrane, but distributed throughout the cytoplasm (43). In addition to EBP50, also Cbp and Csk are mislocalized in CF cells suggesting a defect of the apical compartmentalization of these proteins.
We also found evidence that lack of CFTR affects the apical junction complex of the biliary epithelium. In fact, the subcortical and peri-junctional F-actin cytoskeleton fails to form properly and ZO-1 and afadin, two key members of the tight and adherens junctions respectively, loose their junctional restriction and appear diffusely distributed in the cytoplasm of CFTR-defective cells. Consistent with the presence of junctional defects, epithelial permeability is significantly increased while transepithelial resistance is decreased in monolayers of Cftr-KO cells.
In the biliary epithelium, the integrity of the barrier function is essential to prevent the back-diffusion of toxic bile acids that would cause peribiliary inflammation and fibrogenesis and aggravate the cholestasis. Noteworthy, barrier defects have been associated with several human inflammatory diseases, including cholestatic diseases (44, 45).
Normal cell polarity requires epithelial barrier function and apical junctions integrity (34, 35). These structures are anchored into cytoskeletal components such as actin and myosin filaments (46). Several studies have shown that inflammation and production of inflammatory mediators affect the epithelial barrier function. Our group has previously shown that treatment with pro-inflammatory cytokines (i.e IL6, TNFα, IFNγ and IL1) increases the paracellular permeability in normal cholangiocytes (29). Here we show that treatment with Src or NF-kB inhibitors restores the F-actin distribution and normalizes trans-epithelial permeability in Cftr-KO cells, suggesting that these structural defects may be secondary to the increased NF-kB-dependent inflammatory response caused by Src activation.
Our findings suggest that targeting tyrosine kinase activation and inflammation could be of potential therapeutic value in CF. In fact, administration of an Src inhibitor to our previously used model of CF cholangitis (Cftr-KO mice with DSS-induced colitis and portal endotoxemia (14)), significantly reduces biliary damage and inflammation, as compared to Cftr-KO mice treated only with DSS.
In conclusion, this study provides strong evidence that CFTR regulates TLR-mediated responses in secretory epithelia, by controlling the activation of Src tyrosine-kinase. When CFTR is defective, the negative regulation of Src is lost and the tyrosine kinase is free to target TLR4/NFkB and increase its response to endotoxins. Moreover, aberrant TLR4 activation decreases the epithelial barrier function by destabilizing actin microfilaments and cell-junctional complexes (see cartoon in supplementary figure 6). In the biliary epithelium, decreased barrier function would increase the back-diffusion of toxic bile acids, resulting in further peribiliary inflammation and fibrogenesis. Given the effects of PP2 administration in vivo, and the availability of tyrosine kinase inhibitors already approved for use in several other human conditions (47), targeting SFK activity in the liver may prove to be a useful strategy for the treatment of CFLD.
Supplementary Material
Acknowledgements
We thank Dr. Ruslan Medzithov, Yale School of Medicine, for helpful discussions, Dr. Nadia Ameen, Yale School of Medicine, for the CFTR antibody (AME4991) and Dr. John Riordan, University of North Carolina – Chapel Hill and Cystic Fibrosis Foundation Therapeutics, for the CFTR antibody (A596).
Financial support
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number RO1 DK096096 to MS, P30 DK034989-Silvio O. Conte Digestive Diseases Research Core Centers to MS and RF, R01 NS069753 to P.Z.A. and partially RO1DK101528 to C.S.; by Fondazione Fibrosi Cistica (Grant #18-2012) and by Telethon (Grant #GGP12133) to M.S. and partially supported by PSC Partners Seeking a Cure Foundation (to RF). A.K. is supported by the Jay and Deanie Stein CDA for Cancer Research, Mayo Clinic.
List of abbreviations
- CFTR
cystic fibrosis transmembrane conductance regulator
- CF
cystic fibrosis
- TLR
toll-like receptor
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- Src
Rous sarcoma oncogene cellular homolog
- LPS
lipopolysaccharide
- cAMP
Cyclic adenosine monophosphate
- ATP
Adenosine triphosphate
- PKA
Protein kinase A
- CFLD
cystic fibrosis-associated liver disease
- SFK
Src family kinases
- Csk
Carboxy-terminal Src kinase
- Cbp
Csk-binding protein/PAG
- PAG
phosphoprotein associated with GEMs
- PDZ
post synaptic density protein (PSD95)
- EBP50
ERM binding protein 50
- G-CSF
granulocyte colony-stimulating factor
- CXCL1
Chemokine (C-X-C motif) ligand 1
- LIX
LPS-induced CXC chemokine
- CXCL2
chemokine (C-X-C motif) ligand 2
- ZO-1
zona occludens-1 protein
- AJ
adherens junction
- TJ
tight junction
- TER
trans-epithelial resistance
- DSS
dextran sodium sulfate
- K19
cytokeratin19
- TIR
toll-IL-1 receptor
- MyD88
Myeloid differentiation factor 88
- IRAK
Interleukin 1 receptor-associated kinase 1
- AKAP
A-kinase anchor protein
- ENaC
epithelial sodium channel
- ROMK
renal outer medullary potassium channel
Footnotes
Potential conflict of interests: Nothing to disclose
Author names in bold designate shared co-first authorship.
REFERENCES
- 1.Colombo C, Battezzati PM. Liver involvement in cystic fibrosis: primary organ damage or innocent bystander? J Hepatol. 2004;41:1041–1044. doi: 10.1016/j.jhep.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Naren AP. CFTR chloride channel in the apical compartments: spatiotemporal coupling to its interacting partners. Integr Biol (Camb) 2010;2:161–177. doi: 10.1039/b924455g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guggino WB. The cystic fibrosis transmembrane regulator forms macromolecular complexes with PDZ domain scaffold proteins. Proc Am Thorac Soc. 2004;1:28–32. doi: 10.1513/pats.2306011. [DOI] [PubMed] [Google Scholar]
- 5.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 6.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, et al. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. Journal of immunology. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sturges NC, Wikstrom ME, Winfield KR, Gard SE, Brennan S, Sly PD, et al. Monocytes from children with clinically stable cystic fibrosis show enhanced expression of Toll-like receptor 4. Pediatr Pulmonol. 2010;45:883–889. doi: 10.1002/ppul.21230. [DOI] [PubMed] [Google Scholar]
- 8.John G, Yildirim AO, Rubin BK, Gruenert DC, Henke MO. TLR-4-mediated innate immunity is reduced in cystic fibrosis airway cells. Am J Respir Cell Mol Biol. 2010;42:424–431. doi: 10.1165/rcmb.2008-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn JA, Strong TV, Picciotto MR, Nairn AC, Collins FS, Fitz JG. Localization of the cystic fibrosis transmembrane conductance regulator in human bile duct epithelial cells. Gastroenterology. 1993;105:1857–1864. doi: 10.1016/0016-5085(93)91085-v. [DOI] [PubMed] [Google Scholar]
- 10.Colombo C, Battezzati PM, Strazzabosco M, Podda M. Liver and biliary problems in cystic fibrosis. Semin Liver Dis. 1998;18:227–235. doi: 10.1055/s-2007-1007159. [DOI] [PubMed] [Google Scholar]
- 11.Feranchak AP, Sokol RJ. Cholangiocyte biology and cystic fibrosis liver disease. Semin Liver Dis. 2001;21:471–488. doi: 10.1055/s-2001-19030. [DOI] [PubMed] [Google Scholar]
- 12.Strazzabosco M, Fabris L, Spirli C. Pathophysiology of cholangiopathies. J Clin Gastroenterol. 2005;39:S90–S102. doi: 10.1097/01.mcg.0000155549.29643.ad. [DOI] [PubMed] [Google Scholar]
- 13.Blanco PG, Zaman MM, Junaidi O, Sheth S, Yantiss RK, Nasser IA, et al. Induction of colitis in cftr−/− mice results in bile duct injury. Am J Physiol Gastrointest Liver Physiol. 2004;287:G491–496. doi: 10.1152/ajpgi.00452.2003. [DOI] [PubMed] [Google Scholar]
- 14.Fiorotto R, Scirpo R, Trauner M, Fabris L, Hoque R, Spirli C, et al. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chattopadhyay S, Sen GC. Tyrosine phosphorylation in Toll-like receptor signaling. Cytokine Growth Factor Rev. 2014;25:533–541. doi: 10.1016/j.cytogfr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 17.Medvedev AE, Piao W, Shoenfelt J, Rhee SH, Chen H, Basu S, et al. Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J Biol Chem. 2007;282:16042–16053. doi: 10.1074/jbc.M606781200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong YP, Mulhern TD, Cheng HC. C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)--endogenous negative regulators of Src-family protein kinases. Growth Factors. 2005;23:233–244. doi: 10.1080/08977190500178877. [DOI] [PubMed] [Google Scholar]
- 19.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim Biophys Acta. 2008;1784:56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Hrdinka M, Horejsi V. PAG--a multipurpose transmembrane adaptor protein. Oncogene. 2014;33:4881–4892. doi: 10.1038/onc.2013.485. [DOI] [PubMed] [Google Scholar]
- 21.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 22.Bezprozvanny I, Maximov A. PDZ domains: More than just a glue. Proc Natl Acad Sci U S A. 2001;98:787–789. doi: 10.1073/pnas.98.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouassier L, Duan CY, Feranchak AP, Yun CH, Sutherland E, Simon F, et al. Ezrin-radixin-moesin-binding phosphoprotein 50 is expressed at the apical membrane of rat liver epithelia. Hepatology. 2001;33:166–176. doi: 10.1053/jhep.2001.21143. [DOI] [PubMed] [Google Scholar]
- 24.Moyer BD, Denton J, Karlson KH, Reynolds D, Wang S, Mickle JE, et al. A PDZ-interacting domain in CFTR is an apical membrane polarization signal. J Clin Invest. 1999;104:1353–1361. doi: 10.1172/JCI7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–1088. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 26.Zeiher BG, Eichwald E, Zabner J, Smith JJ, Puga AP, McCray PB, Jr., et al. A mouse model for the delta F508 allele of cystic fibrosis. J Clin Invest. 1995;96:2051–2064. doi: 10.1172/JCI118253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 28.Spirli C, Fiorotto R, Song L, Santos-Sacchi J, Okolicsanyi L, Masier S, et al. Glibenclamide stimulates fluid secretion in rodent cholangiocytes through a cystic fibrosis transmembrane conductance regulator-independent mechanism. Gastroenterology. 2005;129:220–233. doi: 10.1053/j.gastro.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 29.Spirli C, Nathanson MH, Fiorotto R, Duner E, Denson LA, Sanz JM, et al. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Tana A, Shankar A. Cystic fibrosis--what are the prospects for a cure? Eur J Intern Med. 2014;25:803–807. doi: 10.1016/j.ejim.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeer NA, Anandalingam K, Fields RJ, Caputo C, Kopic S, Gupta A, et al. Nanoparticles that deliver triplex-forming peptide nucleic acid molecules correct F508del CFTR in airway epithelium. Nat Commun. 2015;6:6952. doi: 10.1038/ncomms7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scirpo R, Fiorotto R, Villani A, Amenduni M, Spirli C, Strazzabosco M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology. 2015;62:1551–1562. doi: 10.1002/hep.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–524. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng F, Francis H, Glaser S, Han Y, DeMorrow S, Stokes A, et al. Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology. 2012;55:209–221. doi: 10.1002/hep.24673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sansonetti PJ, Medzhitov R. Learning tolerance while fighting ignorance. Cell. 2009;138:416–420. doi: 10.1016/j.cell.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi O, Akira S. Toll-like receptors; their physiological role and signal transduction system. Int Immunopharmacol. 2001;1:625–635. doi: 10.1016/s1567-5769(01)00010-8. [DOI] [PubMed] [Google Scholar]
- 39.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehon RG, McClatchey AI, Bretscher A. Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol. 2010;11:276–287. doi: 10.1038/nrm2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fouassier L, Fiorotto R. Ezrin finds its groove in cholangiocytes. Hepatology. 2014 doi: 10.1002/hep.27675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatano R, Akiyama K, Tamura A, Hosogi S, Marunaka Y, Caplan MJ, et al. Knockdown of ezrin causes intrahepatic cholestasis by the dysregulation of bile fluidity in the bile duct epithelium. Hepatology. 2014 doi: 10.1002/hep.27565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fouassier L, Rosenberg P, Mergey M, Saubamea B, Claperon A, Kinnman N, et al. Ezrin-radixin-moesin-binding phosphoprotein (EBP50), an estrogen-inducible scaffold protein, contributes to biliary epithelial cell proliferation. Am J Pathol. 2009;174:869–880. doi: 10.2353/ajpath.2009.080079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrotta M, Strautnieks S, Papouli E, Rushton P, Clark BE, Parry DA, et al. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326–328. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.