Abstract
Extracellular vesicles (EVs) are nanometer-sized, membrane-bound vesicles released by cells into the extracellular milieu. EVs are now recognized to play a critical role in cell-to-cell communication. EVs contain important cargo in the form of proteins, lipids and nucleic acids and serve as vectors for delivering this cargo from donor to acceptor or target cell. The EVs are released both under physiologic and pathologic conditions, including liver diseases, and exert a wide range of effects on target cells. This review provides an overview on EV biogenesis, secretion, cargo, and target cell interactions in the context of select liver diseases. Specifically, the diverse roles of EVs in nonalcoholic steatohepatitis, alcoholic liver disease, viral hepatitis, cholangiopathies and hepatobiliary malignancies are emphasized. Liver diseases often result in an increased release of EVs and/or in different cargo sorting into these EVs. Either of these alterations can drive disease pathogenesis. Given this fact, EVs represent a potential target for therapeutic intervention in liver disorders. Because altered EV composition may reflect the underlying disease condition, circulating EVs can be exploited for diagnostic and prognostic purposes as a liquid biopsy. Furthermore, ex vivo modified or synthesized EVs can be engineered as therapeutic nano-shuttles. Finally, we highlight areas that merit further investigation relevant to understanding how EVs regulate liver disease pathogenesis.
Keywords: exosome, microvesicle, microparticle
INTRODUCTION
Intercellular communication is vital for multicellular organisms. It is now well recognized that cells communicate not only via direct contact and soluble factors, but also by membrane-derived nanometer-sized vesicles termed extracellular vesicles (EVs). EVs are released by normal, diseased and transformed cells in vitro and in vivo, both basally and under stress conditions. They have also been identified in all major bodily fluids, including blood, urine, bile, saliva, semen, cerebrospinal fluid, as well as in cirrhosis-associated ascites.(1) Although EVs were first identified a couple of decades ago, only recently have they received enhanced scientific scrutiny. Recent studies have revealed that EVs are released in a regulated, and in the case of polarized epithelia domain-specific, manner and that they participate in many important biological processes as well as in disease pathogenesis.(2, 3) Emerging studies have also identified various important roles for EVs in liver diseases. Thus, it is both timely and topical to review this information and emphasize diagnostic and therapeutic opportunities with respect to liver diseases.
DEFINITION AND CLASSIFICATION OF EVs
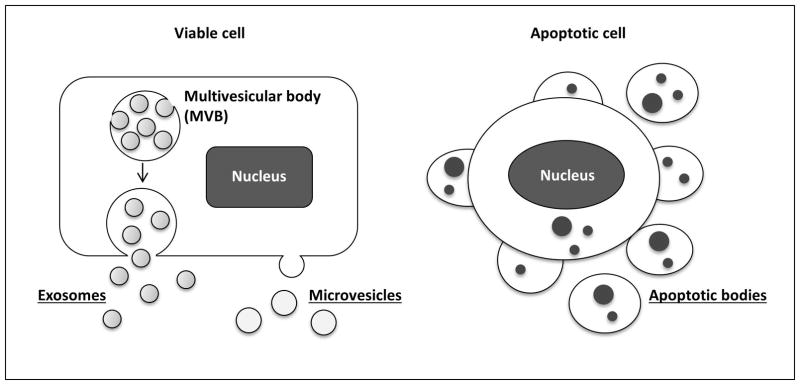
The term EV comprises vesicles enclosed by a lipid bilayer membrane that are released outside the cell. Currently, EVs are classified into three groups based on their cellular biogenesis: exosomes, microvesicles and apoptotic bodies (Figure 1).(1) EVs derived from intracellular trafficking via the endolysosomal pathway are termed exosomes. The size of exosomes is estimated to range between 40–150 nm in diameter. Their size is likely constrained by their intracellular packaging in multivesicular bodies (see below). EVs termed microvesicles bud directly from the plasma membrane and range usually from 50–1000 nm in diameter. Virtually any size of EVs can be released from the plasma membrane including very small EVs. In addition, certain cancer cells have been demonstrated to shed atypically large (1–10 μm diameter) vesicles from the plasma membrane; these vesicle were termed large oncosomes.(4) Thus, there is a considerable size overlap between EVs that originate from completely disparate pathways. A recent comprehensive, unbiased proteomic study highlights the heterogeneity of isolated populations, and commonality of shared protein markers between EVs isolated on the basis of size alone.(5) Furthermore, the heterogeneity of isolated EVs is heightened by the lack of standardized isolation techniques, which include differential ultracentrifugation, density gradient separation, immunoaffinity purification and size-exclusion chromatography.(6) Lastly, apoptotic bodies, formed by large-scale plasma membrane blebbing during apoptosis, also fall in the category of EVs. Apoptotic bodies are usually greater than 500 nm in diameter as they represent cell fragments.(7, 8) Apoptotic bodies are not the focus of this review wherein we focus on EVs released under sub-lethal pathophysiologic conditions. Lastly, in this review we have used the inclusive term “extracellular vesicle” for exosomes and microvesicles, unless discussing a specific type of EVs.
Figure 1. Current EV classification.
EVs can be divided into three main subgroups based on their cellular biogenesis: exosomes, microvesicles and apoptotic bodies. Exosomes originate from the multivesicular bodies (MVBs) which later fuse with the cellular membrane to release exosomes into the extracellular milieu. Microvesicles bud directly from the plasma membrane. Apoptotic bodies are formed by large-scale plasma membrane blebbing during apoptosis.
BIOGENESIS AND CELLULAR RELEASE OF EVs
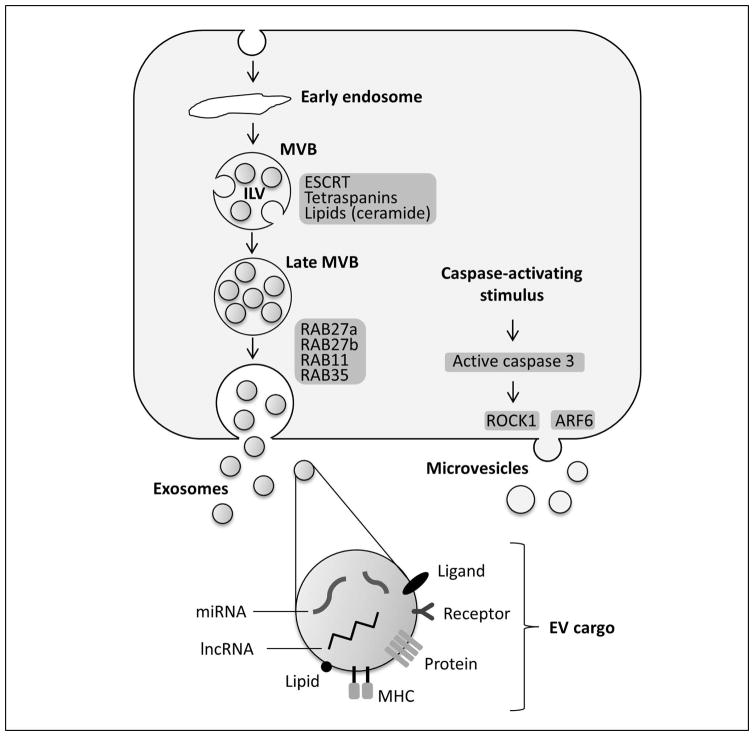
Current concepts distinguish exosomes and microvesicles based on their biogenesis and cellular release into the extracellular milieu; however, the understanding of biogenesis and release mechanisms is still incomplete. Exosomes begin as intraluminal vesicles (ILVs) formed from inward membrane protrusions within intracellular endosomal bodies, referred to as multivesicular bodies (MVBs). These MVBs may have several fates; when MVBs fuse with the plasma membrane, they can release the ILVs contained within their lumen into the extracellular environment, which are now termed exosomes (Figure 2). Exosome biogenesis is mechanistically defined by two distinct intracellular trafficking systems: ESCRT (endosomal sorting complex required for transport)-dependent and ESCRT-independent vesicular pathways.(9)
Figure 2. EV biogenesis, release, and cargo.
The membrane of the early multivesicular body (MVB) bulges inward to form intraluminal vesicles (ILVs) which are later secreted as exosomes. During this process, proteins, nucleic acids, and lipids are packed into exosomes in a cell type-dependent manner. Several proteins have been implicated in ILV formation, including ECSRT proteins, tetraspanins and lipids, particularly ceramide. MVBs then can fuse with the cellular membrane to release ILVs/exosomes into the extracellular space. The fusion of MVBs with the plasma membrane has been demonstrated to be mediated by several members of the RAB family, such as RAB11, RAB27 and RAB35. Microvesicles bud directly from the plasma membrane, which appears to be mediated by ADP-ribosylation factor (ARF6) and Rho-associated protein kinase 1 (ROCK1) activity.
ESCRT proteins guide cargo selection, clustering and membrane fission to form ILVs. Protein ubiquitination modification is the critical signal to induce ESCRT-dependent sorting, internalization into early endosomes and intraluminal membrane budding.(9) ESCRT proteins are broadly classified into 4 categories (0, I, II, and III) with multiple proteins within each category. One major ESCRT protein includes ALIX (apoptosis-linked gene-2 interacting protein X), an ESCRT-III binding protein, which has been found to be directly involved with ILV formation and its packaging of cargo.(9) Studies reveal ALIX directly binds to charged multivesicular body protein 4 (CHMP4), a protein involved with the scission process of ILV from the endosomal membrane.(9) In epithelia, modification of ALIX by RNA interference (RNAi) can modulate domain-specific release of EVs from the apical and basolateral membrane domains (our unpublished data). The vacuolar protein sorting 4 (Vps4) complex is also an integral unit in the final generation of the MVB through the dissociation of the ESCRT-III complex and the recycling of other ESCRT proteins.(10) Using epidermal growth factor receptor (EGFR) trafficking and RNAi silencing the key ESCRT components, it has been shown that EGFR endosomal trafficking to ILVs is ESCRT-dependent, and a reduction in ILVs correlates also with reduced lysosomal degradation of the receptor.(11) Additionally, an RNAi screen for 23 ESCRT components demonstrated a decrease in EV secretion when ESCRT-0 or ESCRT-I was silenced, whereas an increase in EV secretion was observed when ESCRT-III was silenced.(12) Results from ESCRT component RNAi studies are not always in agreement with the observed effect on EV secretion for individual ESCRT proteins. Partially, this can be explained by differences in cell types studied: primary cells versus transformed cells; candidate cargo and markers used to define exosome populations; and also variations in isolation procedures. However, an increase in MVB size, altered morphology and the formation of large and heterogeneous ILV has been reported upon RNAi silencing of ESCRT components by several groups.(11)
ESCRT-independent pathways involve exosome generation via mechanisms that do not involve the ESCRT protein machinery. One particular pathway involves a lipid-driven mechanism. In this regard, ceramides are the most studied in their role in ILV formation. Using proteolipid protein (PLP) as a candidate cargo, it has been demonstrated that its trafficking to ILVs and subsequent secretion within the exosomes remained unchanged when ESCRT components were silenced.(11) This process was dependent on the generation of ceramide via the salvage pathway as pharmacologic or genetic inhibition of neutral sphingomyelinase reduced release of PLP-containing exosomes. Furthermore, ceramides in the exosome membrane were enriched by at least 3-fold as compared to total cellular membrane fraction. Exosomes were also enriched in cholesterol and sphingomyelin in comparison to total cellular membranes.(13, 14) The lipid-regulated concept of ILV formation has several unanswered mechanistic questions. For example, current research has not directly correlated a specific lipid to a specific function in vesicular formation. Some tetraspanins, e.g., CD63 and CD81, are enriched in EVs, and are implicated in exosome formation, especially in an ESCRT-independent manner.(15–17) In this context, interaction between specific cargo and the implicated tetraspanin determines its sorting into and formation of ILVs.(17)
Exosome release appears to be controlled by RAB GTPases involved with vesicular trafficking, endosome recycling and vesicular plasma membrane fusion.(18, 19) In exosome secretion during reticulocyte maturation, RAB11 may associate with vesicles derived from the trans-Golgi network, promoting MVB formation, and subsequent plasma membrane fusion.(20, 21) RAB27a inhibition decreased exosome secretion (22, 23) while RAB27b inhibition caused the MVBs to localize along the perinuclear region (24), suggesting RAB27a and RAB27b’s primary roles in late endosome docking and fusion to the plasma membrane. RAB35 may promote membrane docking of exosomes, as it increases the cell surface density of ILVs.(25)
In contrast to exosomes, studies focused on the mechanism of microvesicle generation are currently underdeveloped; however, current research defines microvesicles as plasma membrane-derived vesicles protruding into the extracellular space (Figure 2) followed by a scission step similar to the final stage of cytokinesis.(26) Plasma membrane release is currently correlated with the activation of acidic sphingomyelinase leading to ceramide generation, as observed in glial cells.(27) Additional mechanisms for microvesicle shedding include: (i) lipid aggregation and asymmetry, most notably with phosphatidylserine; (ii) membrane curvature proteins to force membrane bending, and (iii) intracellular calcium signaling.(26) ADP-ribosylation factor 6 (ARF6) is implicated in microvesicle shedding by tumor cells by extracellular signal-regulated kinase (ERK)-mediated activating phosphorylation of myosin light chain kinase.(28) ESCRT proteins are also involved with microvesicle release as observed by the direct association between the arrestin-related protein (ARRDC1) and ESCRT-I, which led to plasma membrane shedding.(9, 29) However, as endosomal trafficking is a primary function of ESCRT proteins, exosomal EVs are likely more affected by changes in ESCRT proteins as opposed to the plasma membrane budding of microvesicles. Tetraspanins are also located in the plasma membrane and found on microvesicles, though less is known about their role in microvesicle biogenesis.(15) Alternatively, the importance of plasma membrane protein aggregation in microvesicle biogenesis has been demonstrated by the use of engineered candidate proteins that express plasma membrane anchors. Overexpression leading to higher-order oligomerization in the plasma membrane is sufficient to increase protein targeting to microvesicles.(30) Stress responses in cells can activate the release of microvesicles. Increase in intracellular Ca2+ concentration is involved in microvesicle release in some cells. In others, release is based on the pre-apoptotic activation of caspase 3, which activates the Rho-associated protein kinase 1 (ROCK1) leading to actin-myosin contraction and microvesicle release.(31, 32)
CELL-TO-CELL COMMUNICATION VIA EV CARGO
Intercellular communication, which may be paracrine, endocrine, and possibly autocrine, defines the most critical function of EVs. Target cell communication involves two basic facets, the first is target cell recognition by EVs and the second is signal transfer to the target cell. EVs may recognize target cells by receptor- or membrane-mediated processes, which may be determined by protein or lipid cargo of EVs. EVs may communicate with target cells at the cell surface level, following internalization or membrane fusion with the cell membrane.(33) EV uptake can occur by clathrin-dependent endocytosis, caveolin-dependent pathway, macropinocytosis, phagocytosis, and lipid raft-mediated uptake, as reviewed elsewhere in detail.(33) Of these, clathrin-mediated and caveolin-dependent endocytosis appear to be the major pathways for EV uptake by cells. Further studies also describe specific surface targeting via protein-receptor interaction without EV internalization.(34) Few studies have demonstrated EV membrane fusion to the target cell plasma membrane using fluorescent lipid quenching to study lipid fusion interactions.(35) Furthermore, direct evidence has shown protein or nucleic acid delivery into target cells by EVs, with downstream changes induced within the recipient cells following EV internalization. Specific EV-dependent intercellular communication and the known cargos identified in influencing liver disease pathogenesis are discussed in the following sections.
During biogenesis, EV components are specifically sorted into vesicles, either into the ILVs or plasma membrane domains that shed microvesicles. The mechanisms of cargo selection and sorting into EVs remain, for the most part, critically undefined and elusive. Hepatocytes and cholangiocytes are polarized epithelial cells with distinct apical and basolateral membranes. The apical membranes release EVs into bile while the basolateral domains release EVs into the interstitial space, lymphatic and systemic circulation. It is likely that the cargo destined for these two distinct EV populations and their physiology and pathobiology is different. How this targeting occurs and the release mechanisms of EVs by liver cell types require further studies, although, as mentioned above, the ESCRT machinery and ceramides are likely involved. EV cargo comprises proteins, lipids, and various nucleic acid (Figure 2), which may serve as messengers and homing signals. These aspects are discussed in the following sections in the context of disease relevance, biomarkers and therapeutic utility of engineered cargo. Furthermore, identified EV proteins, mRNA, miRNA and lipids are cataloged and available at Vesiclepedia (www.microvesicles.org), ExoCarta (www.exocarta.org) or EVpedia (www.evpedia.info).
EVs IN LIVER DISEASE PATHOGENESIS
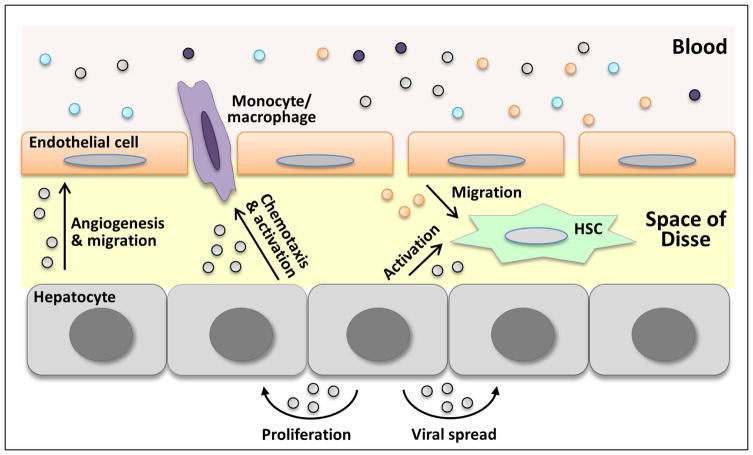
Recent literature suggests that EVs play an important role in liver physiology and pathophysiology. Multiple effects of EVs in cell-to-cell communication in the liver are illustrated in Figure 3 and summarized in Table 1.
Figure 3. EVs in liver biology and pathobiology.
EVs mediate intercellular communication between the liver cells as well as between other tissues and the liver via circulating EVs. EV-induced signaling can lead to a variety of biological responses including inflammation, angiogenesis, proliferation, and tissue remodeling.
Table 1.
Summary of reported EV functions in liver biology and pathobiology.
| Model | EV donor cell/source | EV target cell | Effect | EV cargo | Ref. |
|---|---|---|---|---|---|
| NAFLD/NASH | Hepatocyte | Macrophage | Chemotaxis | CXCL10, S1P | (39, 41) |
| Activation | TRAIL | (32) | |||
| Endothelial cell | Angiogenesis | Vanin-1 | (31) | ||
| HSC | Activation | miRNAs (e.g. miR-128-3p) | (43) | ||
| Serum | Immature myeloid cells | Chemotaxis | Not studied | (38) | |
| Alcoholic hepatitis | Hepatocyte | Macrophage | Activation | CD40L | (48) |
| Viral hepatitis | Hepatocyte | Hepatocyte | HCV transmission | full-length viral RNA, viral protein, and particles | (51) |
| Hepatectomy and ischemia/reperfusion injury | Hepatocyte | Hepatocyte | Proliferation | SK2 and S1P | (58) |
| Fibrosis | Endothelial cell | HSC | Migration | SK1 and S1P | (62) |
| HSC | HSC | Activation | miR-214, CTGF | (63, 64) | |
| Liver cancer | HCC cells | HCC cells | Tumor progression | miRNAs | (78) |
| HCC cells | Chemoresistance | lncRNA (e.g. linc-VLDLR) | (81) | ||
| NK cells | Antitumor response | Heat shock proteins | (80) | ||
| CCA cells | MSC | Fibroblastic differentiation | Not studied | (83) | |
| Cholangiocyte biology | Bile EVs | Cholangiocyte | Regulation of proliferation | Not studied | (70) |
Abbreviations: CCA, cholangiocarcinoma; CD40L, CD40 ligand; CXCL10, C-X-C motif chemokine 10; CTGF, connective tissue growth factor; EV, extracellular vesicle; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HSC, hepatic stellate cell; MSC, mesenchymal stem cell; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; NK, natural killer; S1P, sphingosine 1-phosphate; SK, SK, sphingosine kinase; TRAIL, tumor necrosis factor-like apoptosis inducing ligand.
Nonalcoholic steatohepatitis
EV release is increased in cell culture models of lipotoxicity as well as mouse models of nonalcoholic steatohepatitis (NASH). Using a cell culture model of saturated fatty acid-induced lipotoxicity an increase in hepatocyte EV release was described.(31) These vesicles were pro-angiogenic via vanin-1, a surface cargo protein, which mediated their internalization by endothelial cells. Utilizing the choline-deficient L-amino acid (CDAA) dietary murine NASH model, Povero et al. demonstrated an increase in serum EVs.(36) They found a progressive increase in liver-derived circulating EVs and a correlation with liver apoptosis, fibrosis and neoangiogenesis. The protein cargo of these EVs reflected the underlying disease process, and taxonomic analyses could segregate control mice-derived EVs from the NASH mice-derived EVs. Furthermore, miR-122, a liver-specific microRNA, was enriched in circulating EVs in CDAA-fed NASH mice. There was a shift in miR-122 from Ago2 to EVs in the diseased state. Furthermore, the authors go on to show an increase in circulating EVs in a high fat diet model of NASH. Mitochondrial DNA in hepatocyte-derived circulating EVs is increased in both mouse and human NASH, and implicated in the activation of toll-like receptor 9 (37), which in turn is important in activating the sterile inflammatory response associated with NASH. How mitochondrial DNA is targeted into EVs remains to be elucidated.
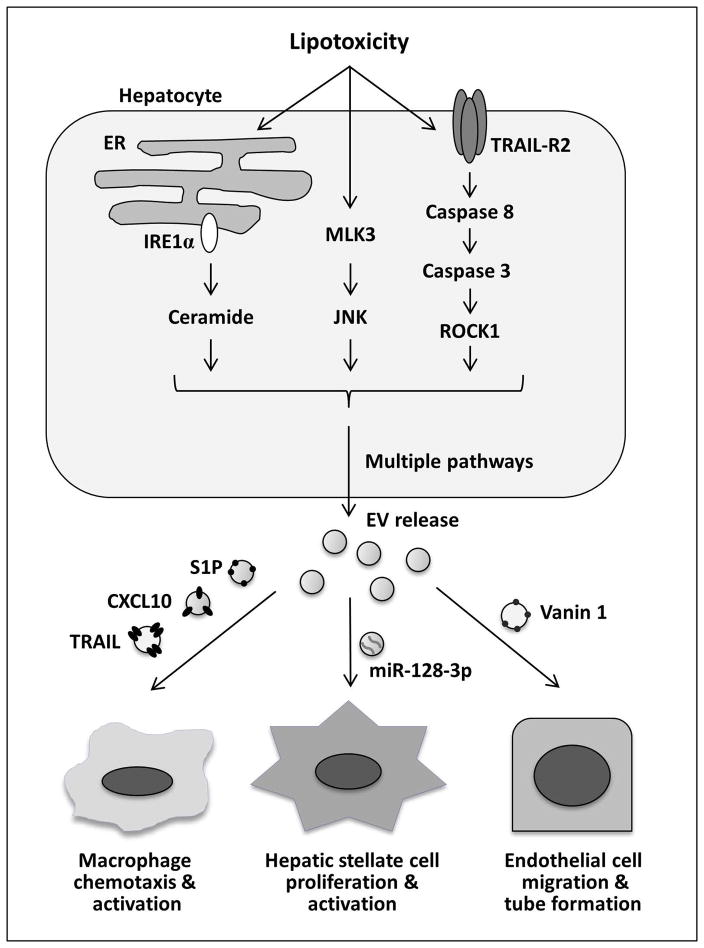
Hepatocyte-derived EVs are implicated in liver inflammation in NASH pathogenesis. Adoptive transfer of peripheral blood-derived EVs from high fat diet-fed mice markedly enhanced liver homing of immature myeloid cells.(38) This was associated with increased levels of proinflammatory markers. Recent studies have defined protein and lipid EV cargo released from steatotic hepatocytes, and how these EVs attract and activate macrophages to the liver. Palmitate and lysophosphatidylcholine (LPC) both stimulated EV release in mouse, rat and human hepatocytes, demonstrating conservation of a toxic-lipid induced EV response across species in hepatocytes.(32) Furthermore, the release of EVs by LPC was dependent on tumor necrosis factor-like apoptosis inducing ligand (TRAIL) receptor 2 (TRAIL-R2) signaling cascade, comprising TRAIL-R2, caspase 8 and caspase 3.(32) The release of EVs from hepatocytes was mediated by ROCK1 activity, as its pharmacological and genetic inhibition ameliorated EV release both in vitro and in vivo. Furthermore, LPC-induced hepatocyte-derived EVs contained TRAIL, which subsequently activated proinflammatory macrophage signaling via TRAIL-R2-induced activation of receptor interacting kinase 1 (RIP1). Another interesting protein cargo present on LPC-induced EVs is the C-X-C motif chemokine 10 (CXCL10).(39) The release of CXCL10-containing EVs is dependent on mixed lineage kinase 3 (MLK3) activation. CXCL10-enriched EVs were chemoattractive toward macrophages in vitro, and MLK3−/− mice were protected against the development of dietary steatohepatitis (39, 40). This was associated with a reduction in the circulating EV number and CXCL10 EV content. This study also identified CYP2E1 within EVs, a potentially specific marker for hepatocyte-derived EVs. Interestingly, palmitate-induced EVs are enriched in C16:0 ceramide.(41) The release of EVs in this model was mediated by the endoplasmic reticulum stress sensor, inositol-requiring protein 1 alpha (IRE1α). Palmitate-induced EVs were chemoattractive toward macrophages by ceramide-derived sphingosine 1-phosphate (S1P), which in turn activated its receptors on macrophages (Figure 4). Similarly to these concepts, adipocyte-derived EVs displayed potent chemotactic properties, which may contribute to macrophage recruitment into adipose tissue during obesity (42).
Figure 4. EV release during hepatocyte lipotoxicity.
Toxic lipids, such as palmitate and its metabolite lysophosphatidyl choline, promote EV release by hepatocytes. The enhanced EV release during lipotoxicity is mediated by the ER stress sensor IRE1α (inositol-requiring protein 1 alpha), stress kinase MLK3 (mixed lineage kinase 3) and TRAIL-R2 (tumor necrosis factor-like apoptosis inducing ligand receptor 2) signaling cascade. Recent in vitro and in vivo studies have defined multiple roles of lipotoxic EVs in NASH pathogenesis. C-X-C motif ligand 10 (CXCL10) and ceramide-enriched EVs mediate monocyte/macrophage chemotaxis to the liver while TNF-related apoptosis-inducing ligand (TRAIL)-enriched EVs contribute to macrophage activation. Vanin-1-bearing EVs mediate endothelial cell migration and tube formation and neovascularization, while miR-128-3p-laden EVs enhance hepatic stellate cells proliferation and activation.
EVs released from lipid-laden hepatocytes may drive liver fibrosis not only by promoting inflammation but also via direct effect on HSCs. Lipotoxic hepatocyte-derived EVs have been demonstrated to induce HSC profibrogenic activation (43). This effect was mediated by EV miRNA cargo, miR-128-3p in particular, which suppressed PPAR-γ expression in EV-treated HSCs.
Circulating EVs are heterogeneous and can be derived from diverse cell types. Using immune cell-derived EVs as markers, patients with chronic hepatitis C could be differentiated from patients with NASH.(44, 45) Furthermore, circulating EVs in chronic hepatitis C were CD4+ and CD8+ T cell-derived, whereas, in NASH were invariant natural killer T cell-derived and monocyte/macrophage-derived, corresponding with the immune cells implicated in liver inflammation and disease pathogenesis.
Alcoholic hepatitis
Several recent studies have focused on EVs in alcoholic hepatitis. Both hepatocyte- and monocyte-derived EVs have been postulated to regulate macrophage phenotype, thereby promoting inflammation in alcoholic hepatitis. Several microRNAs have been proposed to be responsible for EV-mediated cell-to-cell signaling, including miR-122 and miR-155.(46, 47) Recent studies have also focused on protein cargo within EVs that connects ethanol-exposed hepatocytes with macrophage activation. CD40L was proposed as an EV cargo that could promote macrophage activation in vitro and in vivo in experimental models of alcoholic hepatitis.(48) These studies in total indicate that EVs may be a mechanism by which hepatocytes injured by alcohol can stimulate the innate immune response.
Studies on EVs have also been conducted using human specimens with a focus on EVs as potential biomarkers in alcoholic hepatitis. Indeed, multiple studies have shown that EVs are increased in patients with alcoholic hepatitis and even in patients consuming excess ethanol.(49, 50) Further studies will be required to ascertain if EV number or content can serve as biomarkers for alcoholic hepatitis diagnosis and/or prognosis.
Viral hepatitis
EVs play multiple roles in pathogenesis of viral hepatitis, including viral spread, host immunity regulation, and manipulation of the microenvironment. EVs derived from hepatitis C virus (HCV)-infected hepatocytes transmit HCV infection in vitro and are partially resistant to antibody neutralization.(51) HCV transmission via EVs thus serves as an immune evasion strategy. Furthermore, EVs isolated from sera of chronic HCV-infected patients contain HCV RNA and mediate viral receptor-independent transmission of HCV to primary human hepatocytes.(52) HCV RNA in EVs is associated with Ago2, HSP90 and miR-122, which all are known to enhance HCV replication. EVs loaded with inhibitors of either miR-122 or HSP90, significantly suppressed EV-mediated HCV transmission to naïve cells. Disrupting the endocytosis pathway by neutralizing the pH of early endosomes prevented HCV infection of Huh7.5 cells. These studies have identified an important role of EVs in HCV infection propagation between hepatocytes.
Hepatitis viruses may hijack the exosome biogenesis machinery to promote viral spread. Hepatitis E virus particles are trafficked and released through the MVBs (53); similarly, hepatitis B virus (HBV) release also requires the endosomal cellular machinery that generates ILVs and MVBs. Perturbation of the MVB machinery arrested virus particle maturation by entrapping the viral core and large and small envelope proteins in detergent-insoluble membrane structures, suggesting that blocking EV release may abrogate viral propagation.(54) On the other hand, EVs released from the liver nonparenchymal cells, including Kupffer cells, liver sinusoidal endothelial cells and lymphocytes, and from circulating lymphocytes can abrogate hepatitis progression by limiting viral replication. For example, patients with active hepatitis C have increased activated T lymphocyte-derived EVs in their circulation compared with patients with quiescent disease or healthy controls.(55) EV uptake by hepatic stellate cells (HSCs) activates nuclear factor kappa B and ERK 1/2 and upregulates fibrolytic genes in these cells. This may be due to the CD147 cargo on CD8+ T cell-derived EVs. Thus, induction of T lymphocyte-derived EVs is proposed as a novel strategy to induce fibrosis regression in hepatitis C.(55)
HCV is also taken up by liver sinusoidal endothelial cells. Although the virus does not replicate in these cells, it causes the induction of type I and type III interferons, and upregulation of an array of classical interferon-stimulated genes, which inhibit HCV replication.(56) Endothelial cells were able to modulate the replication of HCV in co-cultured hepatocytes through secretion of interferons (IFN) in a conventional manner, but more importantly through the release of antiviral EVs. Li et al. demonstrated that EVs can mediate the transfer of IFN-α-induced antiviral molecules from liver nonparenchymal cells to HBV-infected hepatocytes in vitro and in vivo, and result in suppression of HBV replication.(57) IFN-α-induced STAT1 signaling pathway was required for the antiviral activity of these EVs. Hence, identification and delivery of specific antiviral molecules via EVs is a potential therapeutic strategy for chronic hepatitis B and C.
Liver regeneration
Compared to other liver diseases, there are only few studies on EVs in liver regeneration. First studies have shown that isolated primary hepatocyte-derived EVs promote hepatocyte proliferation in vitro, and liver regeneration after partial hepatectomy or ischemia/reperfusion injury in vivo.(58) These effects were mediated by sphingosine-1-phosphate (S1P) produced by sphingosine kinase (SK) 2, which is contained within the vesicles. It remains to be explored whether mesenchymal stem cell-derived EVs are capable of promoting liver repair and regeneration as demonstrated in other organs.
Drug-induced liver injury (DILI)
Similar to other liver diseases, DILI promotes release and changes composition of hepatocyte-derived EVs, which may be exploited for diagnostic purposes (59). For example, acetaminophen toxicity enhances release of miR-122 associated with EVs in vitro and in vivo.(46, 60) Whether circulating EVs will prove to be useful biomarkers for DILI remains to be explored.
Liver fibrosis
Fibrogenic pathways are triggered in chronic liver diseases as a response to chronic injury and often lead to deposition of insoluble collagen leading to fibrosis. HSCs are the major effector cells for excess matrix deposition in liver fibrosis, and the main precursor of liver myofibroblasts.(61) One key component of HSC activation is enhanced migration. In a recent study by Wang et al., endothelial cell-derived EVs were found to regulate pathological HSC migration during liver fibrosis.(62) The study provides evidence that EV-induced HSC migration is dependent on EV adhesion, which is mediated by EV-bound fibronectin binding with αVβ1-integrin on target cells. Additionally, adhesion facilitates EV entry into the target cell through dynamin-dependent endocytosis facilitating HSC migration. At the molecular level, the enzyme SK1 was identified as the mediator of the migratory effect induced by EVs. Both SK1 and its product S1P were present on the EVs and were required for their chemotactic effects. Thus, EVs mediate paracrine crosstalk between endothelial cells and HSC in fibrosis. On the other hand, connective tissue growth factor (CTGF) drives fibrogenesis in HSCs. In a recent study, CTGF upregulation in fibrotic livers, or in cultured activated primary mouse HSCs, was associated with a downregulation of microRNA-214 (miR-214).(63) Interestingly HSCs produced miR-214-enriched EVs which, when taken up by nearby HSC or hepatocytes, suppressed CTGF 3′-UTR activity and subsequent expression of CTGF downstream targets, e.g., alpha smooth muscle actin and collagen. Nonetheless, the same group demonstrated afterward that CTGF is also packaged into EVs and transferred between HSCs causing an increase in alpha smooth muscle expression in the recipient HSCs.(64) Hence EVs act as fibrosis modulators and may serve as a biomarker of hepatic fibrosis.
In addition, platelet-derived growth factor-stimulated cholangiocytes and HSCs release hedgehog ligands-bearing EVs.(65) Likewise, EVs isolated from the plasma or bile of rats after bile duct ligation were enriched in hedgehog ligands. These ligands were biologically active as they induced expression of activation markers in hepatic sinusoidal endothelial cells. Furthermore, angiogenesis is proposed to promote fibrogenesis in the liver and hepatic fibrosis usually decreases when angiogenesis is pharmacologically inhibited.(66) Moreover, EVs derived from platelets or granulocytes in patients with sepsis have procoagulant properties and demonstrated thrombin generation, in proportion to the severity of the disseminated intravascular coagulopathy.(67, 68) Multiple studies have suggested that coagulation activation promotes liver fibrosis (69), and thus EVs might also promote fibrogenesis. Hence, EVs appear to be a key mediator in fibrosis through enhancing HSC migration and activation, in addition to their known procoagulant and angiogenic role that enhance their profibrogenic potential.
Cholangiopathies
Cholangiopathies define a subset of liver diseases directly targeting the bile duct epithelia, cholangiocytes. Understanding of the role of EVs in cholangiopathies has been advanced by the discovery of EVs in bile. Previous data detected EVs circulating within the lumen of the intrahepatic bile ducts in normal and cystic livers.(70) While exploring the pathophysiological relevance of EVs in bile, studies were performed to test the functional outcomes of exosome interaction with cholangiocyte cilia on the apical membrane. Biliary exosomes interacted with ciliated cholangiocytes and inhibited cholangiocyte proliferation, decreased phospho-ERK and increased the expression of miR-15a.(70) These data show EV-induced targeting effects specific to cholangiocytes via interactions with the primary cilia. Through primary cilia interactions, exosomes could potentially be involved with regulating the proper cholangiocyte cellular maintenance.
Hepatobiliary malignancies
There has been a recent explosion of publications implicating EVs in cancer pathobiology including, but not limited to, cancer progression, angiogenesis, drug resistance, immune escape, metastasis promotion and tumor-associated thrombotic events.(71–76) Here, we focus on hepatocellular carcinoma and cholangiocarcinoma, the two most frequent primary hepatobiliary malignancies.
Hepatocellular carcinoma (HCC)
An emerging body of literature examines the role of EVs in HCC pathogenesis, progression and metastasis. For example, integrin αMβ2, an adhesion receptor that mediates immune cell migration, is enriched in EVs derived from innate immune cells.(77) These EVs when taken up by HCC cells promote their migration, invasion, attachment to the endothelium, and metastasis. Furthermore, EV-mediated miRNA transfer between HCC cells was identified as an important contributor to local spread, and multifocal growth.(78) Loss of transforming growth factor β activated kinase-1 (TAK1) has been implicated in HCC pathogenesis and HCC-derived EVs (through their miRNA cargo) can modulate TAK1 expression and associated signaling, and enhance anchorage-independent growth in the recipient cells. Furthermore Vps4A, a key regulator of exosome biogenesis, is down-regulated in HCC tissues.(79) The reduction of Vps4A in HCC tissues was associated with tumor progression and metastasis. In vitro studies revealed that Vps4A repressed the growth, colony formation, migration, and invasion of HCC cells. Vps4A facilitated the release of oncogenic miRNAs in EVs as well as accumulation and uptake of tumor suppressor miRNAs in cells. These observations indicate that EV-mediated miRNA transfer is an important mechanism of self-modulation of the miRNA expression profiles in HCC cells, and that Vps4A may function as a tumor suppressor that utilizes EVs to regulate the release and uptake of miRNAs in HCC cells. HCC is characterized by intra-tumoral heterogeneity. These studies unveil mechanisms by which clones can affect behavior of neighboring malignant clones.
On the other hand, HCC-derived EVs may enhance the host antitumor immune responses. The stress-induced heat shock proteins (HSP) are known “danger signals” that activate natural killer (NK) cells and promote tumor immunogenicity.(80) HSP-bearing EVs released by HepG2 cells under heat shock stress or anticancer drug treatment efficiently stimulated NK cell cytotoxicity. Moreover, EVs derived from HepG2 cells treated with a chemotherapeutic agent to which they were resistant, conferred superior immunogenicity in inducing HSP-specific NK cell responses, suggesting a role for modulating EVs in HCC immunotherapy. Nonetheless, EVs can also mediate chemoresistance in HCC. Exposure to diverse anticancer agents increased the expression of the long noncoding RNAs (lncRNAs) linc-VLDLR in HCC cells and their derived EVs.(81) Incubation with these EVs reduced chemotherapy-induced cell death and also increased linc-VLDLR expression in the recipient cells. RNAi-mediated knockdown of linc-VLDLR decreased cell viability; thus linc-VLDLR was identified as an EV-enriched lncRNA that mediates HCC cells chemoresistance. Collectively, the above studies define important and multifactorial roles for EVs in HCC pathobiology (Table 3). In addition, EV-contained RNA species could be exploited for noninvasive diagnosis of HCC.(82)
Table 3.
Non-coding RNAs implicated in EV-mediated HCC biology.
| Non-coding RNA | Function | Ref. |
|---|---|---|
| miR-584, miR-517c, miR-378, miR-520f, miR-142-5p, miR-451, miR-518d, miR-215, miR-376a, miR-133b, miR-367 | Inhibition of TAK1 expression and signaling leading to enhanced HCC cell growth | (78) |
| lincRNA-VLDLR | Promotes chemoresistance | (82) |
| linc-ROR | Promotes tumor cell survival during hypoxia | (82) |
| TUC339 | Modulates tumor cell growth and adhesion | (82) |
Abbreviations: EV, extracellular vesicle; HCC, hepatocellular carcinoma; TAK1, transforming growth factor β activated kinase-1.
Cholangiocarcinoma (CCA)
EVs are also implicated in the pathogenesis and metastases of CCA, the second most common primary hepatobiliary malignancy. Haga et al. have demonstrated how CCA-derived EVs may modulate cellular behavior of tumor stroma to promote tumor cell growth. Specifically, CCA-derived EVs suspended in culture medium induced fibroblastic differentiation of mesenchymal stem cells, which, in turn, displayed enhanced secretion of soluble factors that promoted CCA cell proliferation.(83) Interestingly, recent studies show a miRNA-based panel specific for CCA, generated from miRNA extracted from human bile EVs, suggesting the possibility of using biliary EVs as a diagnostic modality.(84) Greater focus on how EVs impact the pathogenesis of the CCA could improve clinical treatment options for patients stricken with these diseases.
Hepatic metastases
Recent studies have implicated tumor-derived EVs in the formation of a metastatic niche within the liver. Two notable studies have examined how pancreatic cancer-derived EVs educate Kupffer cells in the liver, leading to subsequent favorable engraftment of metastatic tumor cells, and defined the exosomal integrins that mediate EV organ tropism.(85, 86) In one study pancreatic ductal adenocarcinoma (PDAC)-derived EV-associated macrophage migration inhibitory factor (MIF) led to EV uptake by hepatic macrophages.(85) These macrophages secreted transforming growth factor β which then led to fibronectin production by HSCs, creating thus a pre-metastatic niche for PDAC. More generally, EVs derived from tumor cells that exhibit metastatic organ tropism mirrored the organ tropism of the originating tumor cells.(86) This was linked to EV integrins, e.g., αVβ5 was linked to hepatic metastases.
EVs play multifaceted roles in tumor biology. Not only are tumor-derived EVs involved in promoting tumor growth, angiogenesis and metastasis, they truly are a double-edged sword, as they also promote tumor immune surveillance. These observations provoke several questions on how tumor-derived EVs influence intercellular communication, e.g., cargo-specific effects or number-driven effects. These are open questions for future research.
EVs AS BIOMARKERS
A great interest has recently been directed toward using EVs as biomarkers for disease diagnosis and prognostication since EVs can encapsulate known and novel biomarkers, as demonstrated for CK18, miRNAs and other biomarkers.(82, 84, 87) Serum EVs may serve as a “liquid biopsy”, thus reducing the need for invasive and more risky tissue procurement and analysis. In liver diseases, EVs isolated from several biological fluids, including urine, bile, and serum, have been characterized via unbiased proteomic, genomic and lipidomic approaches, in addition to the observation that specific populations of EVs can be elevated in liver diseases, such as T lymphocyte-derived EVs in HCV infection, as discussed above. Disease-specific EV cargo that could be potential biomarkers in liver diseases are summarized in Table 2. Despite the potential advantages and opportunities of EVs as biomarkers, a number of challenges remain. Isolation of EVs and their quantitative analysis either by enumeration or interrogation of their cargo is challenging due to requirement of sophisticated instrumentation with still evolving technologies. Nonetheless, the concept of EVs as biomarkers in liver diseases warrants further clinical studies.
Table 2.
Potential EV-associated biomarkers of liver injury.
| Type of study | Injury/Disease | EV source | EV cargo | Ref. |
|---|---|---|---|---|
| Clinical | Alcoholic hepatitis | Plasma | ↑ CD40L, ↑ MIF | (48) |
| CCA | Bile | A panel of 11 miRNAs | (84) | |
| HCV | Serum/plasma | ↑ CD4 and CD8 | (44) | |
| Serum | A panel of 9 miRNAs | (45) | ||
| NAFLD/NASH | Serum/plasma | ↑ CD14 | (45) | |
| Plasma | ↑ Ceramide, ↑ S1P | (41) | ||
| Preclinical | Acetaminophen-induced liver injury | Serum | ↑ miR-122 | (46) |
| Alcoholic hepatitis | Serum | ↑ miR-122 and miR-155 | (46) | |
| NASH | Plasma | ↑ miR-122, ↑ miR-192, ↑ ASGPR1 | (36) | |
| Plasma | ↑ CXCL10 | (39) | ||
| Plasma | ↑ Ceramide and ↑ S1P | (41) | ||
| Serum | ↑ Cyp2e1 | (32, 39) |
Abbreviations: ASGPR1, asialoglycoprotein receptor 1; CCA, cholangiocarcinoma; CD, cluster of differentiation; CD40L, CD40 ligand; CXCL10, C-X-C motif chemokine 10; Cyp2e1, cytochrome P450 2e1; EV, extracellular vesicle; HCV, hepatitis C virus; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; S1P, sphingosine 1-phosphate.
THERAPEUTIC APPLICATIONS
Due to their important roles in disease pathogenesis, EVs represent a potential target for therapeutic interventions. EV-driven pathological processes can be disrupted by several strategies including, but not limited to: i) inhibition of EV biogenesis or release; ii) inhibition of specific cargo sorted into EV; iii) inhibition of EV interaction with or uptake by the target cell.
Recent studies have revealed crucial molecules involved in EV biogenesis and release, which could be targeted by small-molecule inhibitors. Several studies have demonstrated that drugs such as amiloride, fasudil or inhibitors of neutral sphingomyelinase 2 can inhibit EV release in vitro and in vivo to attenuate disease pathogenesis.(11, 32, 63, 88) Multiple proteins from RAB GTPases family may also be targeted to prevent EV release. Though, several crucial components of EV biogenesis and release are known, specific inhibition strategies still remain largely unexplored. Theoretically, the effect of EVs can also be modulated by inhibiting the sorting processes of a specific signaling molecule into EVs. For example, MLK3 inhibitors prevent a lipotoxicity-induced enrichment of chemokine CXCL10 in hepatocyte-derived EVs (39), which can potentially decrease immune cell infiltration of the liver during NASH. Finally, EVs can exert their effects by interaction with the target cell at the level of the plasma membrane as well as upon cellular uptake by the target cell. The interaction with target cell can be inhibited by blocking specific EV components or their interacting counterparts on the acceptor cells as has been shown by neutralization of Fas ligand-containing vesicles in a melanoma model or by blocking fibronectin-integrin interaction on the surface of HSCs.(62, 89) In the context of NASH, decreased uptake of lipotoxic hepatocyte-derived EVs by endothelial cells prevented their proangiogenic effects.(31) The biggest challenge of the earlier-mentioned strategies will be finding very specific targets that would not interfere with other physiological functions of EVs. Also, the proteins involved in EV biogenesis and release are crucial for other cellular processes, for example endolysosomal trafficking.
As opposed to inhibition of “bad” vesicles driving the disease pathogenesis, “good” therapeutic vesicles have been used to stimulate tissue regeneration or modulate immune responses. For instance, EVs derived from multipotent stem cells decrease myocardial infarction size or ischemia-reperfusion injury in several preclinical models.(90) Of note, the beneficial effects observed during stem cell therapy might be actually largely attributed to the EVs and soluble factors released by the stem cells.(91) In regards to liver disease, human mesenchymal stem cell-derived EVs were quite effective in alleviating liver fibrosis in tetrachloride-induced mouse model.(92) Compared to cell-based therapies, EV-based therapy might have several advantages. EVs, as opposed to cells, are less likely to change their ‘phenotype’ upon administration and are presumably safer for use in humans. First clinical trials with EV have demonstrated that they are safe for use in patients.(93) EVs also have a great potential to modulate immune responses and have been tested, for example, for cancer vaccination. In these studies, cancer cell-derived EVs bearing cancer antigens are used to educate dendritic cells.(93)
Finally, EVs represent an ideal vector for both macromolecule and small-molecule drug delivery. Both unmodified and engineered vesicles can deliver nucleic acids, proteins, lipids, peptides and chemotherapeutics to the target cells. For instance, EVs could be loaded with macromolecular drugs or chemotherapeutic agents and engineered to target surface proteins of intended recipient cells to deliver these EVs specifically to these cells. This would be of great potential for cancer therapy with minimal toxicity. These carrier vesicles could be obtained from diverse sources ranging from patient-derived cultured cells to various plant-derived exosome-like nanoparticles. For example, grapefruit-derived nanoparticles were able to deliver chemotherapeutics, siRNA, DNA expression vectors and proteins to varied target cell types and alter their functions.(94) Thus, EVs have a great potential to be translated into clinical practice as therapeutics or vectors for targeted therapy.
CONCLUSIONS AND FUTURE DIRECTIONS
Our knowledge about EVs has been rapidly growing in the recent years. As with any other relatively new field, EVs are an exciting biological phenomenon with a lot of promise as well as questions. It may be quite likely that many molecules that mediate intercellular communication and were considered as soluble factors may actually be associated with EV. We also do not know what the concentrations of EVs in tissues are and what proportion of specific tissue/cell type-derived EVs appear in the circulation. It remains an open question to which extent EVs exhibit their effect on their target cells by paracrine, autocrine or endocrine manner.
As of now, three different EV subpopulations are recognized based on their cellular origin. It is quite likely that other subpopulations of vesicles with distinct modes of biogenesis exist. For example, EVs enriched with autophagic proteins are released from cells undergoing apoptosis and autophagy, suggesting that autophagic vacuoles were rerouted for extracellular release.(95) Similarly, several viruses, e.g. enterovirus, are able to hijack the autophagosome machinery for their viral particle release.(96) In this scenario, enterovirus-containing autophagosomes are trafficked to the cell periphery where their outer membranes fuse with the plasma membrane, and their inner membranes, carrying multiple viral particles, are released into the extracellular space.(97) Future studies will elucidate mechanisms of extracellular release, especially the factors that determine whether the autophagosome or MVBs are destined for degradation or extracellular release.
The future technical challenges lie in developing techniques that would allow isolation of different subsets of EVs and finding their specific markers, though recent advances have been made in this field.(5) Also, standardization of isolation protocols and methods is urgently needed for a better comparison between current studies and for their clinical translation. Due to the nanometer size of EVs, there are many technical obstacles for their direct visualization. The field eagerly awaits new imaging techniques with higher resolution and improvement in monitoring the EV release and function in vivo. Finally, EVs have a great potential to be translated into clinical practice as a form of liquid biopsy and vectors of targeted therapy for diagnostic and therapeutic purposes, respectively. One can envision the scenario wherein engineered or patient’s own EVs are loaded with a therapeutic agent and a targeting peptide, which would address them directly and specifically to target cells.
Acknowledgments
Grant Support: This work was supported by NIH grants DK41876 (to GJG), KL2TR000136-09 (to SHI), DK97178 and DK107402 (to HM), DK59615 (to VHS), DK24031 and DK57993 (to NFL), P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology), and the Mayo Clinic. Support was also provided to Petra Hirsova by the American Liver Foundation.
Abbreviations
- EV
extracellular vesicle
- ILV
intraluminal vesicle
- MVB
multivesicular body
- ESCRT
endosomal sorting complex required for transport
- ALIX
apoptosis-linked gene-2 interacting protein X
- RNAi
RNA interference
- Vps4
vacuolar protein sorting 4
- EGFR
epidermal growth factor receptor
- PLP
proteolipid protein
- ARF6
ADP-ribosylation factor 6
- ERK
extracellular signal-regulated kinase
- ROCK1
Rho-associated protein kinase 1
- NASH
nonalcoholic steatohepatitis
- CDAA
choline-deficient L-amino acid
- LPC
lysophosphatidylcholine
- TRAIL
tumor necrosis factor-like apoptosis inducing ligand
- TRAIL-R2
tumor necrosis factor-like apoptosis inducing ligand receptor 2
- CXCL10
C-X-C motif chemokine 10
- MLK3
mixed linage kinase 3
- IRE1α
inositol-requiring protein 1 alpha
- HCV
hepatitis C virus
- HBV
hepatitis B virus
- HSC
hepatic stellate cell
- IFN
interferon
- SK
sphingosine kinase
- S1P
sphingosine 1-phosphate
- DILI
drug-induced liver injury
- CTGF
connective tissue growth factor
- HCC
hepatocellular carcinoma
- TAK1
transforming growth factor β activated kinase-1
- HSP
heat shot protein
- NK
natural killer
- CCA
cholangiocarcinoma
- PDAC
pancreatic ductal adenocarcinoma
- MIF
macrophage migration inhibitory factor
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sato K, Meng F, Glaser S, Alpini G. Exosomes in liver pathology. Journal of hepatology. 2016;65:213–221. doi: 10.1016/j.jhep.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Seminars in cell & developmental biology. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of extracellular vesicles. 2013:2. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gould SJ, Raposo G. As we wait: coping with an imperfect nomenclature for extracellular vesicles. Journal of extracellular vesicles. 2013:2. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowal J, Tkach M, Thery C. Biogenesis and secretion of exosomes. Current opinion in cell biology. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Hurley JH, Odorizzi G. Get on the exosome bus with ALIX. Nature cell biology. 2012;14:654–655. doi: 10.1038/ncb2530. [DOI] [PubMed] [Google Scholar]
- 10.Babst M. MVB vesicle formation: ESCRT-dependent, ESCRT-independent and everything in between. Current opinion in cell biology. 2011;23:452–457. doi: 10.1016/j.ceb.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 12.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of cell science. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 13.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. The Biochemical journal. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ. Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic. 2003;4:222–231. doi: 10.1034/j.1600-0854.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 15.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, Ossendorp F, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10:1528–1542. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 17.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Developmental cell. 2011;21:708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature reviews Molecular cell biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 20.Fader CM, Savina A, Sanchez D, Colombo MI. Exosome secretion and red cell maturation: Exploring molecular components involved in the docking and fusion of multivesicular bodies in K562 cells. Blood cells, molecules & diseases. 2005;35:153–157. doi: 10.1016/j.bcmd.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6:131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- 22.Li W, Hu Y, Jiang T, Han Y, Han G, Chen J, Li X. Rab27A regulates exosome secretion from lung adenocarcinoma cells A549: involvement of EPI64. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2014;122:1080–1087. doi: 10.1111/apm.12261. [DOI] [PubMed] [Google Scholar]
- 23.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer research. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 24.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nature cell biology. 2010;12:19–30. doi: 10.1038/ncb2000. sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 25.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, et al. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. Journal of cell science. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11–19. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’SouzaSchorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang Y, Wu N, Gan X, Yan W, Morrell JC, Gould SJ. Higher-order oligomerization targets plasma membrane proteins and HIV gag to exosomes. PLoS biology. 2007;5:e158. doi: 10.1371/journal.pbio.0050158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, Berk M, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6:ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirsova P, Ibrahim SH, Krishnan A, Verma VK, Bronk SF, Werneburg NW, Charlton MR, et al. Lipid-Induced Signaling Causes Release of Inflammatory Extracellular Vesicles From Hepatocytes. Gastroenterology. 2016;150:956–967. doi: 10.1053/j.gastro.2015.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. Journal of extracellular vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep. 2016;6:20254. doi: 10.1038/srep20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De Milito A, Coscia C, et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. The Journal of biological chemistry. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PloS one. 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, Shlomchik MJ, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. J Clin Invest. 2016;126:859–864. doi: 10.1172/JCI83885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng ZB, Liu Y, Liu C, Xiang X, Wang J, Cheng Z, Shah SV, et al. Immature myeloid cells induced by a high-fat diet contribute to liver inflammation. Hepatology. 2009;50:1412–1420. doi: 10.1002/hep.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63:731–744. doi: 10.1002/hep.28252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibrahim SH, Gores GJ, Hirsova P, Kirby M, Miles L, Jaeschke A, Kohli R. Mixed lineage kinase 3 deficient mice are protected against the high fat high carbohydrate diet-induced steatohepatitis. Liver international: official journal of the International Association for the Study of the Liver. 2014;34:427–437. doi: 10.1111/liv.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. Journal of lipid research. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D, Gornicka A, et al. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PloS one. 2015;10:e0123110. doi: 10.1371/journal.pone.0123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, Pinatel EM, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cellular and molecular gastroenterology and hepatology. 2015;1:646–663. e644. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, Schuppan D. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murakami Y, Toyoda H, Tanahashi T, Tanaka J, Kumada T, Yoshioka Y, Kosaka N, et al. Comprehensive miRNA expression analysis in peripheral blood can diagnose liver disease. PloS one. 2012;7:e48366. doi: 10.1371/journal.pone.0048366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Scientific reports. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. Journal of hepatology. 2016;64:651–660. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA Cargo of Extracellular Vesicles from Alcohol-exposed Monocytes Signals Naive Monocytes to Differentiate into M2 Macrophages. The Journal of biological chemistry. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. Journal of translational medicine. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS pathogens. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagashima S, Jirintai S, Takahashi M, Kobayashi T, Tanggis, Nishizawa T, Kouki T, et al. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. The Journal of general virology. 2014;95:2166–2175. doi: 10.1099/vir.0.066910-0. [DOI] [PubMed] [Google Scholar]
- 54.Lambert C, Doring T, Prange R. Hepatitis B virus maturation is sensitive to functional inhibition of ESCRT-III, Vps4, and gamma 2-adaptin. Journal of virology. 2007;81:9050–9060. doi: 10.1128/JVI.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, Mitchell A, et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:392–402. e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, et al. Exosomes mediate the cell-to-cell transmission of IFN-alpha-induced antiviral activity. Nature immunology. 2013;14:793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 58.Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, Gulbins E, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. Journal of hepatology. 2016;64:60–68. doi: 10.1016/j.jhep.2015.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Suarez E, Gonzalez E, Hughes C, Conde-Vancells J, Rudella A, Royo F, Palomo L, et al. Quantitative proteomic analysis of hepatocyte-secreted extracellular vesicles reveals candidate markers for liver toxicity. Journal of proteomics. 2014;103:227–240. doi: 10.1016/j.jprot.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holman NS, Mosedale M, Wolf KK, LeCluyse EL, Watkins PB. Subtoxic Alterations in Hepatocyte-Derived Exosomes: An Early Step in Drug-Induced Liver Injury? Toxicological sciences: an official journal of the Society of Toxicology. 2016;151:365–375. doi: 10.1093/toxsci/kfw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiological reviews. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, Hirsova P, Cao S, et al. Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate dependent migration. The Journal of biological chemistry. 2015 doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, Tsukamoto H, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, Brigstock DR. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, Choi SS, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. e322. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thabut D, Shah V. Intrahepatic angiogenesis and sinusoidal remodeling in chronic liver disease: new targets for the treatment of portal hypertension? Journal of hepatology. 2010;53:976–980. doi: 10.1016/j.jhep.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 67.Owens AP, 3rd, Mackman N. Microparticles in hemostasis and thrombosis. Circulation research. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nieuwland R, Berckmans RJ, McGregor S, Boing AN, Romijn FP, Westendorp RG, Hack CE, et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood. 2000;95:930–935. [PubMed] [Google Scholar]
- 69.Valla DC. Thrombosis and anticoagulation in liver disease. Hepatology. 2008;47:1384–1393. doi: 10.1002/hep.22192. [DOI] [PubMed] [Google Scholar]
- 70.Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, Radtke B, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. American journal of physiology Gastrointestinal and liver physiology. 2010;299:G990–999. doi: 10.1152/ajpgi.00093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nature cell biology. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 72.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaiswal R, Gong J, Sambasivam S, Combes V, Mathys JM, Davey R, Grau GE, et al. Microparticle-associated nucleic acids mediate trait dominance in cancer. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2012;26:420–429. doi: 10.1096/fj.11-186817. [DOI] [PubMed] [Google Scholar]
- 74.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. Journal of immunology. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 76.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? Journal of thrombosis and haemostasis: JTH. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 77.Ma J, Cai W, Zhang Y, Huang C, Zhang H, Liu J, Tang K, et al. Innate immune cell-derived microparticles facilitate hepatocarcinoma metastasis by transferring integrin alpha(M)beta(2) to tumor cells. Journal of immunology. 2013;191:3453–3461. doi: 10.4049/jimmunol.1300171. [DOI] [PubMed] [Google Scholar]
- 78.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54:1237–1248. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, et al. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61:1284–1294. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. The Journal of biological chemistry. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Molecular cancer research: MCR. 2014;12:1377–1387. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mohankumar S, Patel T. Extracellular vesicle long noncoding RNA as potential biomarkers of liver cancer. Briefings in functional genomics. 2016;15:249–256. doi: 10.1093/bfgp/elv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. Journal of extracellular vesicles. 2015;4:24900. doi: 10.3402/jev.v4.24900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li L, Masica D, Ishida M, Tomuleasa C, Umegaki S, Kalloo AN, Georgiades C, et al. Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis. Hepatology. 2014;60:896–907. doi: 10.1002/hep.27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature cell biology. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, Cavallini L, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. Journal of immunology. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 90.Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circulation research. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA. Microvesicles and exosomes for intracardiac communication. Cardiovascular research. 2014;102:302–311. doi: 10.1093/cvr/cvu022. [DOI] [PubMed] [Google Scholar]
- 92.Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem cells and development. 2013;22:845–854. doi: 10.1089/scd.2012.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, Chaput N, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. Journal of extracellular vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Q, Zhuang X, Mu J, Deng ZB, Jiang H, Zhang L, Xiang X, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nature communications. 2013;4:1867. doi: 10.1038/ncomms2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pallet N, Sirois I, Bell C, Hanafi LA, Hamelin K, Dieude M, Rondeau C, et al. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics. 2013;13:1108–1120. doi: 10.1002/pmic.201200531. [DOI] [PubMed] [Google Scholar]
- 96.Altan-Bonnet N, Chen YH. Intercellular Transmission of Viral Populations with Vesicles. Journal of virology. 2015;89:12242–12244. doi: 10.1128/JVI.01452-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, et al. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell. 2015;160:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]