Abstract
Prostate cancer is the most commonly diagnosed cancer, and the second leading cause of cancer-related death, for men in the United States. Despite the approval of several new agents for advanced disease, each of these has prolonged survival by only a few months. Consequently new therapies are sorely needed. For other cancer types, immunotherapy has demonstrated dramatic and durable treatment responses, causing many to hope that immunotherapies might provide an ideal treatment approach for advanced prostate cancer. However, apart from sipuleucel-T, prostate cancer has been conspicuously absent from the list of malignancies for which immunotherapies have received recent FDA approval. This has left some wondering if immunotherapy will “work” for this disease. In this review we describe current immunotherapy developments, including approaches to engage tumor-targeting T cells, disrupt immune regulation, and alter the immunosuppressive tumor microenvironment. We then describe the recent application of these approaches to the treatment of prostate cancer. Given the FDA approval of one agent, and the fact that several others are in advanced stages of clinical testing, we believe that immunotherapies offer real hope to improve patient outcomes for prostate cancer, especially as investigators begin to explore rational combinations of immunotherapies and combine these therapies with other conventional therapies.
Keywords: Prostate cancer, immunotherapy
Graphical abstract
Condensed Abstract: In this review we highlight the history of immunotherapeutic development for prostate cancer and many of the strategies currently being explored. We conclude that immunotherapies have promise for improving clinical outcomes, and that the greatest benefits will come as immunotherapy approaches begin to be rationally combined with other therapies.
Goals of Cancer Immunotherapy
The relationship between the human immune system and the development of cancer has been both well-studied, and hotly-debated, for over a century. From the foundational work by Paul Ehlrich in the early 1900s1, to Burnet and Thomas’s “cancer immunosurveillance” hypothesis of the 1950s2, to the most recently revised theory of “cancer immunoediting” by Schreiber and colleagues3, many have proposed a role for the immune system in controlling the development of cancer. However, until recently there was little clinical evidence demonstrating consistent anti-tumor responses following immune-based therapies. Many recent clinical trials, however, have demonstrated that the immune system can have potent anti-tumor activity in many cancer types. With recent trials demonstrating that CTLA-4 and PD-1 blockade can increase survival for patients with metastatic melanoma and other diseases4,7, to the current phenomenal results observed with CAR T cells for B-cell malignancies5, there is now no doubt that the immune system is a powerful anti-cancer tool. In fact, the designation of cancer immunotherapy as the 2013 scientific breakthrough of the year by Science effectively marked that cancer immunotherapy was no longer a theoretical possibility but a practical reality6. Given the recent momentum and interest in this field, many now believe that cancer immunotherapy will be a cornerstone of treatment for most cancers.
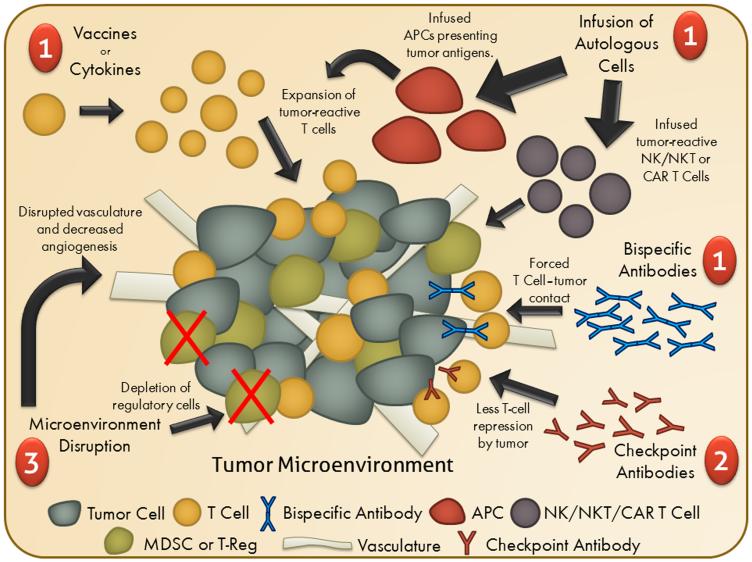
There is great diversity among the many cancer immunotherapies currently under investigation, but they can be loosely classified into three distinct categories based on their end goal: eliciting tumor-targeting cytolytic lymphocytes, disrupting immune regulation, and altering the tumor microenvironment (Figure 1). The first class of agents are designed to supply or augment the frequency of T cells in a patient specific for one or more tumor-associated antigens, or other non-antigen-specific anti-tumor effector cell populations such as NK cells. This can be carried out both in vivo, through the delivery of vaccines and cytokines, or ex vivo, through collecting, modifying/expanding and reinfusing these cells. Other cancer immunotherapies work by repressing the tumor’s ability to circumvent anti-tumor immunity. Because cancers derive from a patient’s own cells, they can maintain and exploit normal autoimmune defense mechanisms. Successfully disrupting these inhibitory pathways co-opted by cancers have proven to be remarkably effective in the case of checkpoint inhibitors. The last class of cancer immunotherapies work by altering the tumor microenvironment, turning what is often an unfavorable environment for productive anti-tumor immunity into one that is more favorable, typically by changing the types of cells that might be present at the tumor site or by disrupting the tumor vasculature, making the tumor environment more amenable to immune cell infiltration and destruction.
Figure 1. Schematic of Immunotherapy Classes.
Shown is a schematic of the classes of immunotherapy approaches being investigated for the treatment of prostate cancer including (1) approaches to increase tumor-targeting cytolytic lymphocytes (e.g. vaccines, cytokines, adoptive transfer of cytolytic anti-tumor cells, or bispecific antibodies); (2) approaches to disrupt immune regulation; and (3) approaches to disrupt the immunosuppressive tumor microenvironment.
Prostate cancer is one malignancy for which there has been much exploration of immunotherapeutic agents. Due to its typically slow progression, abundance of tissue-specific target antigens, a reliable serum marker to assess clinical responses, and the non-essential nature of the target tissue (reducing concerns about autoimmune destruction of normal prostate cells), prostate cancer is in many ways an ideal malignancy for evaluating new immunotherapy treatments. And because prostate cancer remains the most commonly diagnosed cancer and second leading cause of cancer-related death for men in the United States, new therapies are sorely needed7. However, apart from sipuleucel-T, prostate cancer has been conspicuously absent from the list of malignancies for which new immunotherapies have been recently FDA approved, leaving many wondering if immunotherapy can provide any real hope for improving patient outcomes. In this review we highlight the history of immunotherapeutic development for prostate cancer and many of the strategies currently being explored. We conclude that immunotherapies have real promise for improving clinical outcomes, and that the greatest benefits are yet to come as immunotherapy approaches begin to be rationally combined with other therapies.
History of Immunotherapy for Prostate Cancer
Cytokines
The first class of immunotherapies are agents designed to increase the frequency or activity of T cells specific for one or more targets overexpressed by tumors (tumor-associated antigens, TAAs). Some of these first attempts were through the delivery of cytokines, as prior work had shown that the delivery of IL-2 could successfully expand tumor-reactive T-cell populations and elicit anti-tumor immune responses in patients with melanoma or renal cell cancer8,9. A phase I trial explored the intratumoral delivery of IL-2 in prostate cancer patients with either locally advanced or recurrent disease following prostatectomy10. Although the treatment was well tolerated and they observed increased T-cell infiltration into tumors, only modest changes in prostate-specific antigen (PSA) levels were observed. Another group examined the safety and efficacy of an IL-2 immunocytokine, EMD 273066 (huKS-IL2), a human EpCAM-targeting antibody fused with IL-2. This treatment was also well tolerated, but also showed little signs of anti-tumor activity11. Another group examined the efficacy of subcutaneous IL-2 in combination with interferon-alpha (IFNα) in patients with metastatic prostate cancer and again observed no improvements in regards to PSA levels or survival12. Together, all of these trials demonstrated that, although well tolerated, IL-2 cytokine therapy (in various formats) was not able to elicit a meaningful anti-tumor immune response as a monotherapy, and thus its evaluation in prostate cancer therapy has essentially been discontinued.
Similarly, phase I trials treating patients with either intratumoral IFNα, or with intratumoral tumor necrosis factor-alpha (TNFα) along with systemic IFNα2b, demonstrated that these treatments again were well tolerated but exhibited little clinical activity13,14. Conversely, clinical trials examining systemic treatment with GM-CSF as a monotherapy have shown some signs of efficacy (Table 1-line 1, Table-L1). In a Phase II trial examining treatment of patients with CRPC with GM-CSF in combination with thalidomide, nearly all patients experienced a transient decrease in PSA levels, and a trial employing similar treatment in patients with non-castrate resistant prostate cancer also demonstrated a marked decrease in PSA levels in nearly 90% of patients15,16. Another trial also demonstrated that long-term treatment of GM-CSF in patients with recurrent disease was well tolerated and that a substantial fraction of patients experienced long-term disease control17. Taken together, these findings suggest that, while well tolerated, the systemic or intratumoral delivery of cytokines seems able to elicit only marginal anti-tumor responses in prostate cancer patients when given as single agents. There may be promise for GM-CSF as a monotherapy, but there has been more interest in the combination of cytokines with other immunotherapies.
Table 1.
Summary of Immunotherapies Approved or in Clinical Testing for Prostate Cancer
| # | Drug Name | Description | Current Phase | Results | Ongoing Trials |
References | |
|---|---|---|---|---|---|---|---|
| Cytokines | 1 | GM-CSF | Systemic recombinant GM-CSF (sometimes with thalidomide) |
II | Δa in PSA levels No SAEa,b Some SDa |
NCT00678054 (and many combinations) |
15 – 17 |
| 2 | F16IL2 | Immunocytokine, antibody specific for tenascin- C fused with recombinant IL-2 |
I/II | N/A | NCT01134250 | ||
| Vaccines | 3 | Sipuleucel-T | Autologous APCs loaded with PAP-GM-CSF fusion protein |
FDA Approved | IRa elicited Few SAE Δ in OSa |
Many Combinations | 23,63,64 |
| 4 | PSA-TRICOM | Vaccina/Fowlpox virus-based vaccine encoding PSA |
III | IR elicited Few SAE Δ in OS |
NCT01322490 NCT02326805 (and many combinations) |
24 – 26 | |
| 5 | DCVAC | Autologous APCs loaded with LNCaP tumor cell lysate |
III | IR elicited No SAE Δ in OS |
NCT02111577 | 65 | |
| 6 | Ad/PSA | Adenovirus-based vaccine encoding PSA | II | IR elicited No SAE Δ in PSA DTa |
NCT00583752 NCT00583024 |
66 | |
| 7 | pTVG-hPAP | DNA vaccine encoding PAP | II | IR elicited No SAE Δ in PSA DT |
NCT01341652 (and combinations) |
27 | |
| 8 | Natural DCs | Autologous dendritic cells loaded with NY- ESO-1 and MUC1 peptides |
II | N/A | NCT02692976 | ||
| 9 | ME TARP | Autologous dendritic cells loaded with TARP peptides |
II | N/A |
NCT02362451 NCT02362464 |
||
| 10 | GX301 | Synthetic multi-peptide vaccine targeting telomerase |
II | N/A | NCT02293707 | ||
| 11 | Tecemotide | Synthetic lipopeptide vaccine targeting MUC-1 | II | N/A | NCT01496131 | ||
| 12 | UV1/hTERT2012P | Synthetic peptide vaccine | II | N/A | NCT01784913 | ||
| 13 | RNActive | mRNA vaccine targeting multiple antigens | II | N/A | NCT02140138 | ||
| 14 | INO-5150 | DNA vaccine encoding PSA and PSMA | I | N/A | NCT02514213 | ||
| 15 | JNJ-64041809 | Live attenuated double deleted (LADD) Listeria
monocytogenes encoding PAP, SSX2, and NKX3.1 |
I | N/A | NCT02625857 | ||
| 16 | ADXS31-142 | Live attenuated Listeria monocytogenes
encoding PSA |
I | N/A | NCT02325557 | ||
| 17 | alpha-DC1 | Autologous dendritic cells loaded with allogenic prostate cell lines |
I | N/A | NCT00970203 | ||
| 18 | BPX-201 | Autologous dendritic cells with DeCIDe activation technology |
I | N/A | NCT01823978 | ||
| 19 | pTVG-AR | DNA vaccine encoding AR LBD | I | N/A | NCT02411786 | ||
| 20 | VANCE | ChAdOx1 and MVA virus-based vaccine encoding 5T4 |
I | N/A | NCT02390063 | ||
| 21 | ProscaVax | A PSA/IL-2/GM-CSF encoding vaccine | I | N/A | NCT02058680 | ||
| 22 | DRibble | Tumor-derived autophagosome-based vaccine | I | N/A | NCT02234921 | ||
| 23 | Ad-sig-hMUC-1/ecdCD40L | Adenovirus-based vaccine encoding MUC- 1/CD40L fusion |
I | N/A | NCT02140996 | ||
| 24 | PrCa VBIR | Heterologous prime/boost vaccination platform | I | N/A | NCT02616185 | ||
| 25 | GVAX-PCa | Irradiated PC3 and LNCaP cell line with GM- CSF |
Discontinued as monotherapy (combinations still being explored) |
IR elicited Few SAE No Δ in OS |
NCT01696877 | 20,21 | |
| Autologous Cell Infusion | 26 | CryoIT DC | Autologous dendritic cells administered to cryoablated tumor region |
I | N/A | NCT02423928 | |
| 27 | Autologous NKT cells |
Autologous natural killer T cell isolation, expansion, and reinfusion |
I | N/A | NCT01801852 | ||
| 28 | Autologous NK cells |
Autologous natural killer cell isolation, expansion, and reinfusion |
I | N/A | NCT00720785 | ||
| CAR-T | 29 | CAR+ T cells/PSMA |
Autologous T cells engineered to express a chimeric antigen receptor (CAR) specific for PSMA |
I | N/A | NCT01140373 | |
| Bispecific T cell engager | 30 | BAY2010112 | Bispecific T cell engager (BiTE) specific for CD3 and PSMA |
I | N/A | NCT01723475 | |
| 31 | MOR209/ES414 | Bispecific T cell engager (BiTE) specific for CD3 and PSMA |
I | N/A | NCT02262910 | ||
| 32 | MT110 | Bispecific T cell engager (BiTE) specific for CD3 and EpCAM |
I | N/A | NCT00635596 | ||
| Checkpoint Antibody |
33 | Pembrolizumab | Monoclonal antibody blocking PD-1 ligation by PD-L1 and PD-L2 |
II | No OR Some SAE |
NCT02312557 (and many combinations) |
43 |
| 34 | Ipilimumab | Monoclonal antibody blocking CTLA-4 ligation by CD80 or CD86 |
Previous Phase III. Still under testing as monotherapy and in combination |
Δ in PSA levels Some SAE No Δ in OS |
NCT02279862 (and many combinations) |
40,41 | |
| 35 | Tremelimumab | Monoclonal antibody blocking CTLA-4 ligation by CD80 or CD86 |
Discontinued as monotherapy (combinations still being explored) |
Δ in PSA DT Some SAE |
NCT02616185 NCT02643303 |
67 | |
| 36 | Nivolumab | Monoclonal antibody blocking PD-1 ligation by PD-L1 and PD-L2 |
Discontinued as monotherapy (combinations still being explored) |
No ORa
Few SAE |
NCT02601014 | 42 | |
| Microenvironment Disruption | 37 | LY3022855 | Monoclonal antibody specific for CSF1R, depletes tumor-associated macrophages |
I | N/A | NCT02265536 | |
| 38 | Sunitinib | Tyrosine kinase inhibitor that depletes MDSCs and inhibits tumor angiogenesis |
Discontinued as monotherapy (combinations still being explored) |
Δ in PFSa
Common SAE No Δ in OS |
NCT01803503 NCT00329043 |
45 – 47 | |
| 39 | Tasquinimod | Engages with S100A9 and depletes MDSCs and inhibits tumor angiogenesis |
Discontinued as monotherapy (combinations still being explored) |
Δ in PFS Common SAE No Δ in OS |
NCT01513733 NCT02159950 |
51 |
Δ = Change. SAE = Serious Adverse Event. SD = Stable Disease. IR = Immune Response. OS = Overall Survival. DT = Doubling Time. OR = Objective Response. PFS = Progression-Free Survival.
“No SAE” = 0 patients, “Few SAE” = 1-10% of patients, “Some SAE” = 10-25%, “Common SAE” = 25+%
Vaccines
As a more specific means of amplifying tumor-specific T cells, others have explored the use of anti-tumor vaccines. This approach is especially relevant to prostate cancer given the abundance of target proteins that are nearly exclusively expressed in prostate tissue, dampening concerns about off-target side effects. Previous data have also shown that T cells (and antibodies) specific for several of these prostate-specific targets can exist in the peripheral blood of prostate cancer patients, suggesting that vaccines may be useful to augment prostate-specific T-cell populations18,19.
An early vaccine to enter clinical testing for prostate cancer was GVAX-PCa, a mixture of irradiated PC-3 and LNCaP cell lines engineered to overexpress GM-CSF, with the goal of eliciting T cells specific for one or more TAAs20. Phase I/II trials indicated that the treatment was well tolerated and induced antibody responses to various proteins in the cell lysates, suggesting the vaccine was eliciting antigen-specific immune responses20,21. Higher doses of treatment appeared to be associated with prolonged survival compared to lower doses. However, two independent phase III trials were closed prematurely due to lack of superior clinical efficacy compared to chemotherapy in one trial, and an increase in patient mortality observed in the other trial (hazard ratio, 1.03 [95% C.I. 0.83-1.28], P=0.78)22.
Other groups have explored the use of vaccines encoding one or more specific prostate cancer TAA, such as sipuleucel-T (Provenge®, Dendreon Corporation), an autologous antigen-presenting cell (APC) vaccine in which a patient’s peripheral blood APC are isolated, pulsed with recombinant GM-CSF fused to the TAA prostatic acid phosphatase (PAP), and then re-infused 72 hours later (Table-L3). In a phase III trial, patients receiving sipuleucel-T had a greater median overall survival (25.8 months) versus patients receiving placebo (21.7 months), leading to its FDA approval in 2010 (hazard ratio, 0.78 [95% C.I. 0.61-0.98]; P=0.03)23. This made sipuleucel-T the first FDA-approved vaccine for the treatment of any cancer, and provided the first solid evidence that vaccines could provide a real benefit in disease outcome for prostate cancer patients.
Many other groups have explored different vaccine platforms and target antigens. A highly anticipated vaccine currently under development is PSA-TRICOM (Prostvac®, Bavarian Nordic), a vaccinia and fowlpox viral vector approach encoding PSA (Table-L4)24. Early phase trials demonstrated the tolerability and immunological activity of PSA-TRICOM and two independent phase II studies reported an increase in overall survival for patients receiving PSA-TRICOM compared to placebo or historical controls25,26. A phase III approval trial is currently underway in patients with mCRPC (NCT01322490).
Other groups have explored different vaccine constructs targeting these or similar antigens. Both PAP and PSA have been targeted using DNA-based vaccination, with a DNA vaccine encoding PAP currently being evaluated in a randomized phase II trial (Table-L7, NCT01341652)27,28. These and other trials have demonstrated the tolerability of DNA immunization and their ability to elicit antigen-specific T cells, using a simpler platform than those of either Prostvac or sipuleucel-T. Still others are exploring the use of Listeria monocytogenes as a potentially more potent means of antigen delivery, particularly given evidence of clinical activity of listeria-based vaccines for pancreatic cancer29. Specifically, recombinant listeria encoding PSA, PAP, and other TAAs are under investigation for treating advanced prostate cancer (Table-L15,16; NCT02625857, NCT02325557). While the approval of sipuleucel-T suggests that tumor vaccines have a place in the treatment of prostate cancer, it is not currently known if one vaccine approach is superior to another in terms of anti-tumor effects. Trials comparing different vaccine strategies, as well as trials combining vaccines with other immune-modulating agents, are eagerly anticipated.
CAR T cells and Bispecific Antibodies
As a more direct means of providing tumor-reactive T cells, others have explored the use of adoptive cell therapy using ex vivo expansion of tumor-reactive T cells, or T cells engineered to be specific for a particular TAA by modifying their T cell receptors (TCRs). Recent studies have demonstrated dramatic anti-tumor activity using T cells engineered to express a chimeric antigen receptor (CAR) that permits recognition of a cell-surface protein using an antibody-recognition domain fused to the TCR signaling domain30. Specifically, CAR T cells targeting CD19 have led to complete responses in some B cell malignancies, prompting exploration of CAR T cells for other malignancies31. The availability of tissue-specific membrane proteins has limited development of this approach for many solid tumors. However, for prostate cancer some groups are exploring targeting prostate-specific membrane antigen (PSMA) using CAR T cell approaches32,33. A phase I dose-escalation trial evaluating PSMA-specific CAR T cells is currently underway (Table-L29, NCT01140373).
Another means to increase the reactivity of T cells to tumor cells is through the use of bispecific antibodies (e.g. BiTEs®, Amgen). These consist of the binding domain of two antibodies, one specific for the T cell, such as CD3, and the other specific for a desired membrane-associated TAA, fused together34. These dual antibodies then force the physical encounter of tumor cells by T cells. Work in preclinical models has demonstrated that a CD3xPSMA bispecific antibody was able to efficiently direct T cells toward tumors and could initiate cytolytic responses35. The one major benefit of these over CAR T cells is that they are effectively an “off-the-shelf” product, as they do not require the collection and reinfusion of a patient’s autologous T cells. This could allow bispecific antibodies to be a more cost-effective, and therefore hopefully more widely accessible, treatment option. However, like CAR T cells, they carry the same concerns regarding off-target toxicity for targets that are not completely tumor-specific, including PSMA. These concerns will be more thoroughly understood following the completion of two currently underway phase I trials examining the safety and efficacy of CD3xPSMA or CD3xEpCAM bispecific antibodies in patients with CRPC (Table-L30-32; NCT01723475, NCT00635596).
T-Cell Checkpoint Blockade
The second class of immunotherapies works by disrupting the tumor cells’ ability to repress anti-tumor immunity. Because cancer cells derive from a patient’s own cells, they retain and can exploit defense mechanisms that cells have developed to avoid autoimmune destruction. These mechanisms include interference with molecules on T cells that regulate their expansion and function, known as immune checkpoints. Early work in this field identified the first of these T-cell checkpoint molecules, CTLA-4, as a major inhibitor of cytolytic anti-tumor T-cell responses36. Preclinical and subsequent clinical work demonstrated that antibodies blocking CTLA-4 (preventing its ligation by CD80/CD86) could prevent this T-cell repression from occurring, ultimately leading to the approval of ipilimumab (Yervoy®, Bristol-Myers Squibb) for the treatment of metastatic melanoma4. Subsequent work has identified many other checkpoint molecules similar, but not redundant, to CTLA-4 including most notably PD-1, TIM-3, and LAG-3. Ligation of these molecules by tumor-expressed molecules also leads to decrease in T-cell effector function. Antibodies blocking PD-1 have recently received FDA approval for the treatment of melanoma, non-small cell lung cancer, and renal cell cancer37–39.
In the case of prostate cancer, an early phase I/II trial treating mCRPC patients with ipilimumab (Table-L34) as either a monotherapy or in combination with radiotherapy demonstrated that some patients receiving the combination had a decrease in PSA levels and stable disease (with one complete response)40. This led to a randomized phase III trial in patients with mCRPC receiving either ipilimumab or placebo after radiotherapy41. This trial, although demonstrating a difference in progression-free survival between the two groups, did not demonstrate a significant difference in overall survival (ipilimumab: 11.2 months; placebo: 10.0 months; hazard ratio, 0.85 [95% C.I. 0.72-1.00]; P=0.053).
More recently, groups have also examined the treatment of prostate cancer with PD-1 blockade (Table-L33,36). Two independent phase I trials conducted using PD-1 blockade in patients with many types of solid tumors included those with mCRPC42,43. No objective responses were observed in the 25 mCRPC patients who were treated in both of these trials. A phase II trial is currently underway more thoroughly examining the anti-tumor efficacy of PD-1 blockade in patients with mCRPC (NCT02312557). However, results to date examining either CTLA-4 or PD-1 blockade alone have suggested little role for these treatments as monotherapy for prostate cancer. It remains to be seen if other checkpoint inhibitors will be more effective in prostate cancer, or if CTLA-4 or PD-1 blockade will be more effective when used in combination, as is currently underway (NCT01420965).
Microenvironment Disruptors
The last class of immunotherapy agents is those designed to disrupt or otherwise modify the immunosuppressive tumor microenvironment, making it more amenable to a cytolytic immune response. Many tumors are infiltrated by regulatory T cells and/or myeloid-derived suppressor cells (MDSCs), which have been shown to repress anti-tumor immune responses by either direct cell-cell interactions or secretion of inhibitory molecules such as IL-10, nitric oxide or indoleamine 2,3-dioxygenase (IDO). Tumors are also known to have altered or disorganized vasculature, often not expressing the appropriate ligands necessary for immune cell trafficking. Agents designed to disrupt the tumor vasculature and/or deplete tumor-infiltrating regulatory cells have been shown to have antitumor activity in many cancer types. Among several of the approved agents targeting the vascular endothelial growth factor receptors, one agent, sunitinib (Sutent®, Pfizer), has been shown to inhibit tumor angiogenesis and also deplete MDSCs from tumors (Table-L38)44. Several independent phase II trials examining sunitinib as monotherapy for patients with mCRPC demonstrated signs of efficacy, as marked by PSA declines and objective responses, leading to a randomized, placebo-controlled phase III trial of sunitinib in patients with mCRPC45,46. This trial revealed that sunitinib increased progression-free survival, but did not impact overall survival compared to placebo (sunitinib: 13.1 months; placebo: 11.8 months; hazard ratio, 0.914 [95% C.I. 0.762-1.097]; P=0.17)47. Combinations of VEGFR-targeting agents with chemotherapy have similarly not demonstrated significant benefit in prostate cancer48. Nonetheless, there remains interest in combination treatment using these agents specifically with immune-targeted therapies. In addition, chemotherapy and radiation therapy, agents already used in the management of prostate cancer that can disrupt tumor vasculature, are also being explored in combination with immune-targeted therapies (NCT02649855).
Another immunotherapy shown to impede the recruitment of MDSCs and to have antiangiogenic activity, tasquinimod49,50, has been evaluated in patients with recurrent prostate cancer. Early trials demonstrated it was well tolerated and led to a significant increase in progression-free survival and overall disease control (stable disease and objective responses) compared to placebo51. An international double-blind, placebo-controlled phase III trial in men with mCRPC, however, showed no significant increase in overall survival (hazard ratio, 1.097 [95% C.I. 0.938-1.282])52. Despite this, there remains interest in the use of tasquinimod in combination with other immunotherapies.
Likelihood of Success
Of all immunotherapy approaches currently being pursued for prostate cancer, the most successful to date have been vaccines. Vaccines have been shown to be well tolerated, able to elicit both antibodies and cytolytic T cells specific for TAAs, and to prolong overall survival in prostate cancer patients. This is intriguing given the relatively disappointing results vaccines have shown for most other malignancies. Prostate cancer is currently the only malignancy for which a vaccine is FDA approved and for which another vaccine is currently in phase III approval testing. In contrast, T-cell checkpoint inhibitors to date have shown less activity in the treatment of prostate cancer, at least as monotherapies, relative to other solid tumors. These findings suggest there could be differences in the immunogenicity of prostate tumors relative to other cancer types. In fact it has been suggested that prostate tumors have a lower frequency of infiltrating immune cells compared with many other solid tumor types53. Consequently, it is conceivable that anti-tumor vaccines have demonstrated activity for this disease simply by increasing the number of tumor-specific infiltrating T cells, compared with other tumors in which there may already be abundant T-cell infiltration.
Even with the potential that vaccines have shown in treating prostate cancer patients, the benefit shown to date by sipuleucel-T is fairly modest in terms of overall survival. This treatment has struggled to gain widespread use, possibly due to high cost, or median survival benefit of only 4 months, or because it is a first-in-class drug with which clinicians are less familiar. This has prompted many to study other simpler vaccines, study vaccines in combination with other therapies, or study vaccines at different stages of disease. In modeling the treatment effect of PSA-TRICOM, Madan and colleagues have suggested that vaccines may work to slow disease progression. In this case, vaccines may have their greatest effect in earlier stages of disease or combined with therapies to reduce tumor burden54. Numerous trials are currently underway examining the efficacy of either sipuleucel-T or PSA-TRICOM at delaying disease progression in patients with earlier stages of disease (Table-L4, NCT02326805, NCT01431391, NCT00779402).
As described above, the T-cell checkpoint inhibitors that have been investigated to date, while active as monotherapies for many solid tumors, have been relatively disappointing as treatments for prostate cancer. However, as checkpoint inhibitors work by enhancing the activity of tumor-reactive T cells otherwise repressed by the tumor, and as prostate cancer may have fewer of these infiltrating T cells, these findings are perhaps not surprising. Other groups have also shown that the malignancies for which checkpoint blockade tends to be most effective are those with the highest mutational loads, presumably because T cells that can recognize these aberrantly expressed high-affinity neo-epitopes have high levels of checkpoint receptors and are otherwise dysfunctional in the absence of checkpoint blockade55. Prostate tumors are known to have a lower mutational burden than many other tumor types, decreasing the frequency with which T cells might recognize a mutated neo-epitope antigen leading to tumor-infiltrating T cells56. These findings suggest that checkpoint blockade may be more effective for prostate cancer when combined with vaccines or other therapies that augment tumor-specific T cells. In fact, it has recently been demonstrated that treating patients with either sipuleucel-T or another prostate cancer vaccine led to the upregulation of the checkpoint ligand PD-L1 on the surface of tumor cells, and that antigen-specific immune responses could be enhanced when combined with PD-1 blockade57. Groups have also demonstrated in pre-clinical models that anti-tumor vaccine efficacy could be enhanced when combined with checkpoint blockade58,59. Many groups have therefore begun exploring the combination of anti-tumor vaccines with checkpoint blockade in clinical trials. One recently reported trial which examined GVAX-PCa combined with ipilimumab for patients with mCRPC found that the combination treatment was generally well tolerated and was able to elicit anti-tumor responses (as measured by PSA decline) in some patients60. Another trial combining PSA-TRICOM with ipilimumab had similar findings61. Many other clinical trials examining these combination approaches are currently underway (Table-L16, NCT02499835, NCT02325557, NCT02506114, NCT01804465).
Radiation therapy, chemotherapy, and androgen deprivation therapy are all standard treatments in the management of prostate cancer, and all also have immune modulating activities. All three treatments can cause tumor cell death, potentially leading to release of prostate tumor antigens. Androgen deprivation has distinct immune-modulating activities by leading to thymic release of naïve T cells and can specifically lead to T-cell infiltration of prostate tumors62. Radiation therapy and chemotherapy can also disrupt tumor vasculature, raising the possibility that these treatments may make the tumor microenvironment more amenable to the development of an immune response. For all of these reasons, there is a strong rationale for combining these standard therapies with immunotherapies. On the other hand, both chemotherapy and radiation therapy can have immunosuppressive effects, underscoring the importance of careful planning and trials needed to determine optimal treatment strategies for patients with various stages of disease.
A Model for Success – Rational Combination Approaches
As recurrent prostate cancer is one of the leading causes of cancer-related death in the United States, there is a great need for the development of novel therapies. Within the last five years several targeted agents have been approved for prostate cancer, but each has demonstrated a median prolongation of survival of only a few months. The field of cancer immunotherapy continues to grow and several agents have demonstrated dramatic successful anti-tumor activity for some diseases, including responses that continue after treatment has been discontinued. To date, while different immunotherapy approaches have been investigated for prostate cancer, including vaccines, checkpoint inhibitors, and tumor microenvironment disrupting agents, the results from each of these treatments as monotherapies has been more modest. Notwithstanding, clinical signals have been observed with cytokine-based therapies, CTLA-4 blockade, and with treatments that affect the immune regulatory populations within the tumor microenvironment. And prostate cancer is a disease for which vaccines have demonstrated clinical activity as single agents, with sipuleucel-T being the first vaccine to receive FDA approval for the treatment of any malignancy. These findings and observations suggest that optimal treatment effect may be observed when immunotherapy agents will be used in combination, and specifically combining treatments aimed at increasing the frequency tumor-reactive T cells (e.g. by vaccination, androgen deprivation, radiation therapy, or chemotherapy) with agents to increase their effectiveness (e.g., cytokines, checkpoint blockade, or regulatory cell function blockade). Many clinical trials evaluating these approaches are currently underway, and we believe that the rational combination of immunotherapies with other standard cancer therapies will lead to markedly improved treatments for patients with prostate cancer over the next decade.
Acknowledgments
Funding Acknowledgements: BTR and DGM were supported by NIH R01 CA142608 and by DOD W81XWH-15-1-0492.
Footnotes
COI Disclosures: DGM has ownership interest, receives research support, and serves as consultant to Madison Vaccines, Inc., that has licensed material described in this report. BTR has no potential competing interests.
Author Contributions: BTR and DGM both contributed to the planning, organization, data collection, and writing of the manuscript. DGM is responsible for the overall content.
References
- 1.Ehrlich P. Ueber den jetzigen stand der Karzinomforschung. Ned Tijdschr Geneeskd. 1909;5:273–290. [Google Scholar]
- 2.Burnet M. Cancer; a biological approach. I. The processes of control. Br Med J. 1957;1(5022):779–786. doi: 10.1136/bmj.1.5022.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 4.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342(6165):1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 7.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 8.Rosenberg SA, Lotze MT, Muul LM, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 9.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 10.Belldegrun A, Tso CL, Zisman A, et al. Interleukin 2 gene therapy for prostate cancer: phase I clinical trial and basic biology. Hum Gene Ther. 2001;12(8):883–892. doi: 10.1089/104303401750195854. [DOI] [PubMed] [Google Scholar]
- 11.Ko Y-J, Bubley GJ, Weber R, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother. 2004;27(3):232–239. doi: 10.1097/00002371-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Maffezzini M, Simonato A, Fortis C. Salvage immunotherapy with subcutaneous recombinant interleukin 2 (rIL-2) and alpha-interferon (A-IFN) for stage D3 prostate carcinoma failing second-line hormonal treatment. Prostate. 1996;28(5):282–286. doi: 10.1002/(SICI)1097-0045(199605)28:5<282::AID-PROS2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 13.Kramer G, Steiner GE, Sokol P, et al. Local intratumoral tumor necrosis factor-alpha and systemic IFN-alpha 2b in patients with locally advanced prostate cancer. J Interferon Cytokine Res. 2001;21(7):475–484. doi: 10.1089/10799900152434349. [DOI] [PubMed] [Google Scholar]
- 14.Emerson L, Morales A. Intralesional recombinant alpha-interferon for localized prostate cancer: a pilot study with follow-up of >10 years. BJU Int. 2009;104(8):1068–1070. doi: 10.1111/j.1464-410X.2009.08482.x. [DOI] [PubMed] [Google Scholar]
- 15.Song L, Zhou X, Li X. Phase II trial of granulocyte-macrophage colony-stimulating factor plus thalidomide in older patients with castration-resistant prostate cancer. Mol Clin Oncol. 2015;3(4):865–868. doi: 10.3892/mco.2015.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amato RJ, Hernandez-McClain J, Henary H. Phase 2 study of granulocyte-macrophage colony-stimulating factor plus thalidomide in patients with hormone-naïve adenocarcinoma of the prostate. Urol Oncol. 2009;27(1):8–13. doi: 10.1016/j.urolonc.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Rini BI, Fong L, Weinberg V, Kavanaugh B, Small EJ. Clinical and immunological characteristics of patients with serologic progression of prostate cancer achieving long-term disease control with granulocyte-macrophage colony-stimulating factor. J Urol. 2006;175(6):2087–2091. doi: 10.1016/S0022-5347(06)00261-8. [DOI] [PubMed] [Google Scholar]
- 18.Olson BM, Frye TP, Johnson LE, et al. HLA-A2-restricted T-cell epitopes specific for prostatic acid phosphatase. Cancer Immunol Immunother. 2010;59(6):943–953. doi: 10.1007/s00262-010-0820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164(5):1825–1829. [PubMed] [Google Scholar]
- 20.Sanda MG, Ayyagari SR, Jaffee EM, et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J Urol. 1994;151(3):622–628. doi: 10.1016/s0022-5347(17)35032-2. [DOI] [PubMed] [Google Scholar]
- 21.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor--secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin Cancer Res. 2007;13(13):3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 22.Higano C, Saad F, Somer B, et al. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC) GU ASCO 2009. Abstract: LBA150. [Google Scholar]
- 23.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 24.Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009;18(7):1001–1011. doi: 10.1517/13543780902997928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNeel DG, Becker JT, Eickhoff JC, et al. Real-Time Immune Monitoring to Guide Plasmid DNA Vaccination Schedule Targeting Prostatic Acid Phosphatase (PAP) in Patients with Castration-Resistant Prostate Cancer. Clin Cancer Res. 2014;20(14):3692–704. doi: 10.1158/1078-0432.CCR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavlenko M, Roos A-K, Lundqvist A, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91(4):688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le DT, Brockstedt DG, Nir-Paz R, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res. 2012;18(3):858–868. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jena B, Dotti G, Cooper LJN. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slovin SF, Wang X, Borquez-Ojeda O, et al. Targeting castration resistant prostate cancer (CRPC) with autologous PSMA-directed CAR+ T cells. J Clin Oncol (Meeting Abstracts) 2012;30(15):TPS4700. [Google Scholar]
- 33.Junghans RP, Ma Q, Rathore R, et al. Phase I trial of anti-PSMA designer T cells in advanced prostate cancer. Cancer Res (Meeting Abstracts) 2012;72(C13) [Google Scholar]
- 34.Suryadevara CM, Gedeon PC, Sanchez-Perez L, et al. Are BiTEs the “missing link” in cancer therapy? Oncoimmunology. 2015;4(6):e1008339. doi: 10.1080/2162402X.2015.1008339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedrich M, Raum T, Lutterbuese R, et al. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-Bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther. 2012;11(12):2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 36.Lee KM, Chuang E, Griffin M, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282(5397):2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 37.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ko JS, Zea AH, Rini BI, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15(6):2148–2157. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 45.Dror Michaelson M, Regan MM, Oh WK, et al. Phase II study of sunitinib in men with advanced prostate cancer. Ann Oncol. 2009;20(5):913–920. doi: 10.1093/annonc/mdp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonpavde G, Periman PO, Bernold D, et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann Oncol. 2010;21(2):319–324. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 47.Michaelson MD, Oudard S, Ou Y-C, et al. Randomized, placebo-controlled, phase III trial of sunitinib plus prednisone versus prednisone alone in progressive, metastatic, castration-resistant prostate cancer. J Clin Oncol. 2014;32(2):76–82. doi: 10.1200/JCO.2012.48.5268. [DOI] [PubMed] [Google Scholar]
- 48.Kelly WK, Halabi S, Carducci M, et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30(13):1534–1540. doi: 10.1200/JCO.2011.39.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Björk P, Björk A, Vogl T, et al. Identification of human S100A9 as a novel target for treatment of autoimmune disease via binding to quinoline-3-carboxamides. PLoS Biol. 2009;7(4) doi: 10.1371/journal.pbio.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsson A, Björk A, Vallon-Christersson J, Isaacs JT, Leanderson T. Tasquinimod (ABR-215050), a quinoline-3-carboxamide anti-angiogenic agent, modulates the expression of thrombospondin-1 in human prostate tumors. Mol Cancer. 2010;9:107. doi: 10.1186/1476-4598-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pili R, Häggman M, Stadler WM, et al. Phase II randomized, double-blind, placebo-controlled study of tasquinimod in men with minimally symptomatic metastatic castrate-resistant prostate cancer. J Clin Oncol. 2011;29(30):4022–4028. doi: 10.1200/JCO.2011.35.6295. [DOI] [PubMed] [Google Scholar]
- 52.Sternberg C, Armstrong AJ, Pili R, Nederman T, Carducci MA. A Phase 3, randomized, double-blind, placebo-controlled study of tasquinimod (TASQ) in men with metastatic castration-resistant prostate cancer (mCRPC), secondary endpoints. J Clin Oncol. 2016;34(suppl 2S) doi: 10.1200/JCO.2016.66.9697. abstr 239. [DOI] [PubMed] [Google Scholar]
- 53.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic Cancer Vaccines in Prostate Cancer: The Paradox of Improved Survival Without Changes in Time to Progression. Oncologist. 2010;15(9):969–975. doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grasso CS, Wu Y-M, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rekoske BT, Olson BM, McNeel DG. Anti-tumor vaccination of prostate cancer patients elicits PD-1/PD-L1 regulated antigen-specific immune responses. OncoImmunology. 2016 doi: 10.1080/2162402X.2016.1165377. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rekoske BT, Smith HA, Olson BM, Maricque BB, McNeel DG. PD-1 or PD-L1 Blockade Restores Antitumor Efficacy Following SSX2 Epitope-Modified DNA Vaccine Immunization. Cancer Immunol Res. 2015;3(8):946–955. doi: 10.1158/2326-6066.CIR-14-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu J, Malm I-J, Kadayakkara DK, Levitsky H, Pardoll D, Kim YJ. Preclinical evidence that PD1 blockade cooperates with cancer vaccine TEGVAX to elicit regression of established tumors. Cancer Res. 2014;74(15):4042–4052. doi: 10.1158/0008-5472.CAN-13-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Santegoets SJAM, Stam AGM, Lougheed SM, et al. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer Immunol Immunother. 2013;62(2):245–256. doi: 10.1007/s00262-012-1330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madan RA, Mohebtash M, Arlen PM, et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2012;13(5):501–508. doi: 10.1016/S1470-2045(12)70006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71(5):496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 64.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 65.Podrazil M, Horvath R, Becht E, et al. Phase I/II clinical trial of dendritic-cell based immunotherapy (DCVAC/PCa) combined with chemotherapy in patients with metastatic, castration-resistant prostate cancer. Oncotarget. 2015;6(20):18192–18205. doi: 10.18632/oncotarget.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lubaroff DM, Konety BR, Link B, et al. Phase I clinical trial of an adenovirus/prostate-specific antigen vaccine for prostate cancer: safety and immunologic results. Clin Cancer Res. 2009;15(23):7375–7380. doi: 10.1158/1078-0432.CCR-09-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNeel DG, Smith HA, Eickhoff JC, et al. Phase I trial of tremelimumab in combination with short-term androgen deprivation in patients with PSA-recurrent prostate cancer. Cancer Immunol Immunother. 2012;61(7):1137–1147. doi: 10.1007/s00262-011-1193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]