Abstract
Dominant mutations in TRPV4, which encodes the Transient Receptor Potential Cation Channel Subfamily V Member 4 calcium channel, result in a series of musculoskeletal disorders that include a set of peripheral neuropathies and a broad phenotypic spectrum of skeletal dysplasias. The skeletal pheno-types range from brachyolmia, in which there is scoliosis with mild short stature, through perinatal lethal metatropic dysplasia. We describe a case with phenotypic findings consistent with metatropic dysplasia, but in whom no TRPV4 mutation was detected by Sanger sequence analysis. Exome sequence analysis identified a known lethal metatropic dysplasia mutation, TRPV4L618P, which was present at lower frequency than would be expected for a heterozygous change. The affected individual was shown to be a somatic mosaic for the mutation, providing an explanation for the milder than expected phenotype. The data illustrate that high-throughput sequencing of genomic DNA can facilitate detection of mosaicism with higher sensitivity than Sanger sequence analysis and identify a new genetic mechanism for metatropic dysplasia.
Keywords: metatropic dysplasia, TRPV4, somatic mosaicism, skeletal dysplasia
INTRODUCTION
Gain-of-function mutations in TRPV4, which encodes the Transient Receptor Potential Cation Channel Subfamily V Member 4 calcium channel, result in a series of musculoskeletal disorders that include a related set of peripheral neuropathies [Auer-Grumbach et al., 2010; Deng et al., 2010; Landoure et al., 2010] and a broad phenotypic spectrum of skeletal dysplasias [Schindler et al., 2014]. Among the skeletal disorders, autosomal dominant brachyolmia is at the mild end of the TRPV4 spectrum and is characterized by relatively mild short stature and scoliosis, with or without involvement of the appendicular skeleton [Rock et al., 2008]. More severe mutations result in spondylometaphyseal dysplasia, Kozlowski-type, presenting with marked platyspondyly, kyphoscoliosis, a flattened acetabular roof, and shortened long bones with epiphyseal and metaphyseal irregularity. The most severe mutations lead to metatropic dysplasia [Krakow et al., 2009], and lethal metatropic dysplasia [Camacho et al., 2010], with short stature involving the spine and long bones, and severe, progressive scoliosis. Radio-graphically, there is platyspondyly that is characterized by with a wafer-thin appearance to the vertebrae, shortened long bones with flared metaphyses, often resulting in a “dumbbell” appearance, and an irregular superior iliac mineralization pattern. Lethal metatropic dysplasia is differentiated by increased severity of the phenotype, leading to perinatal mortality. Here we report here a case of non-lethal metatropic dysplasia due to somatic mosaicism for a known lethal metatropic dysplasia TRPV4 mutation.
CLINICAL REPORT
The proband (International Skeletal Dysplasia Registry reference number R09-440A) was born to non-consanguineous parents (R09-440B and C) with no family history of skeletal disorders. The skeletal dysplasia was recognized at birth and radiographs taken at that time revealed odontoid hypoplasia, platyspondyly with anterior rounding, shortened long bones with a clubbed appearance, and flattened acetabular roofs. An MRI taken at 5 months of age revealed pronounced dextroscoliosis and kyphosis of the lumbosacral spine.
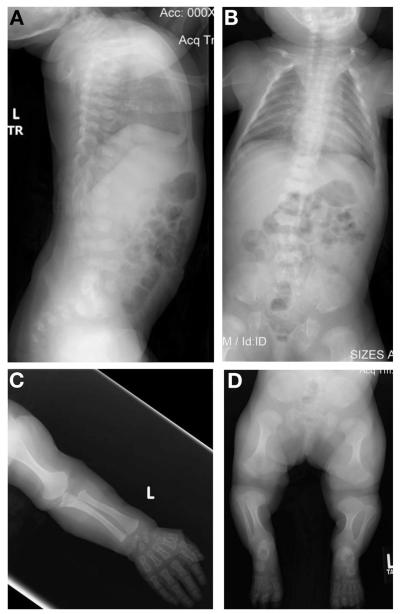
Clinical examination at 22 months of age revealed midface hypoplasia with frontal bossing and protuberant knees. Radiographic findings of the spine again showed flat, anteriorly rounded vertebral bodies, and scoliosis (Fig. 1A, B). There was significant metaphyseal widening of the long bones of the upper (Fig 1C) and lower (Fig 1D) extremities, halberd-shaped proximal femurs, wide ilia, and hypoplastic acetabular roofs. The epiphyses were flat and quite hypoplastic throughout and the phalanges were short and widened.
FIG. 1.
Radiographs of the proband at age 22 months. (A and B) Lateral and anterior–posterior images of the spine. Platyspondyly with anterior rounding can be seen in both images with moderate scoliosis apparent in (B). (C) Upper extremity showing shortened long bones with wide metaphyses and short phalanges. (D) Lower extremities showing short long bones with widened metaphyses, halberd-shaped proximal femurs as well as the abnormal pelvis characteristic of metatropic dysplasia.
The clinical and radiographic findings were most consistent with a diagnosis of non-lethal metatropic dysplasia, but non-lethal fibrochondrogenesis [Tompson et al., 2010] and Kniest dysplasia [Siggers et al., 1974; Winterpacht et al., 1993] were considered to be secondary diagnostic possibilities. However, Sanger sequence analysis was negative for mutations in all of the coding exons of TRPV4, COL11A1, COL11A2, and COL2A1, the genes associated with the aforementioned phenotypes. Consequently, exome sequencing was carried out for the proband and parents, with an average coverage depth of 48. Analysis of the exome sequence was carried out using the standard Genome Analysis Toolkit [GATK, 2016] pipeline, modified by the use of the Exome Aggregation Consortium [ExAC, 2016] dataset for variant quality score recalibration. This analysis identified a point mutationin TRPV4 exon 12 which implied a leucine to proline change at residue 618 (p.L618P) in 16 out of 71 reads. The parental exomes were negative for the mutation, consistent with a de novo change. Heterozygosity for the mutation had previously been observed in a case of lethal metatropic dysplasia [Camacho et al., 2010]. The low frequency of reads with the mutation, combined with the decreased phenotypic severity, suggested that the affected individual might be a somatic mosaic for the variant.
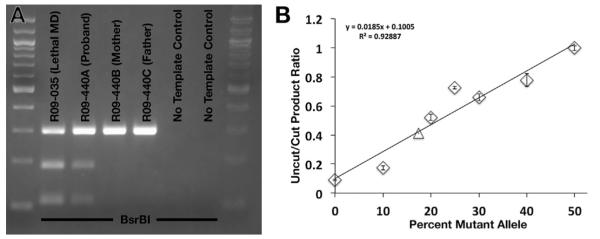
The p.L618P mutation created a BsrBI restriction endonuclease cleavage site so presence of the mutant allele in DNA from the proband was confirmed by PCR amplification TRPV4 exon 12 followed by cleavage with BsrBI (New England Biolabs). The primer sequences used for PCR were 5′-CACACTTATGCACCTGCAGACC-3′ and 5′-CCTATACATCATGGCTACTGTTCC-3′. As expected, the parental DNAs did not contain the BsrBI site, confirming that the mutation had occurred de novo (Fig. 2A).
FIG. 2.
Somatic mosaicism for the TRPV4 mutation. (A) Ethidium bromide-stained 2% agarose gel showing BsrBI-cleaved PCR products containing TRPV4 exon 12. The p.L618P mutation created a BsrBI site which was detected in both the heterozygous lethal MD control, R09-035 and the mosaic proband, R09-440A, but not in DNA from the parents of the mosaic proband. (B) Measurement of the level of somatic mosaicism in the proband (triangle). The level of mosaicism was matched to a standard curve (data points represented by diamonds). Error bars represent standard error.
To independently measure the level of the mutant allele in proband DNA, a standard curve was constructed by serial dilution of DNA from the lethal metatropic dysplasia case known to be heterozygous for the TRPV4 p.L618P mutation with DNA from an unaffected individual, to mimic different levels of mosaicism. Following PCR amplification and BsrBI cleavage, the intensity of the cleavage products in the dilution series were compared with proband sample (image analysis conducted using ImageJ, imagej.nih.gov). These data (Fig. 2B) showed that about 15% of the alleles contained the mutation, implying that about 30% of the cells in the affected individual would be expected to be heterozygous for the p.L618P allele.
DISCUSSION
In this report we describe a case of non-lethal metatropic dysplasia resulting from somatic mosaicism for a lethal metatropic dysplasia TRPV4 mutation. Lethal metatropic dysplasia, including the case heterozygous for the p.L618P mutation found in the mosaic case described here [Camacho et al., 2010], presents with severe platyspondyly with wafer-thin mineralization of the vertebrae, dramatically shortened long bones with very short diaphyses, widened metaphyses, and enlarged epiphyses. The appendicular skeleton of the p.L618P mosaic case was much milder and more characteristic of non-lethal metatropic dysplasia. The vertebral bodies, while exhibiting platyspondyly, were atypical for either non-lethal or lethal metatropic dysplasia, exhibiting a higher vertebral height. Thus the milder clinical and radiographic presentation in the affected individual likely reflects somatic mosaicism among chondrocytes. However, the level of mosaicism in the target tissue could not be assessed because a cartilage sample was not available.
Somatic mosaicism resulting in a milder manifestation of a more severe genetic disorder has been previously documented for several dominantly inherited skeletal dysplasias [Cohn et al., 1990; Wallis et al., 1990; Takagi et al., 2012] and calcium channel disorders [Etheridge et al., 2011]. For the mosaic TRPV4 case described herein, because mosaicism was detected in blood and implied in cartilage, the mutational event must have occurred very early in embryonic development in a somatic progenitor cell that at least gave rise to these two somatic tissues, but likely most other tissues as well. The non-lethal metatropic dysplasia phenotype in this case is consistent with a cell autonomous effect at the level of cartilage tissue, with amelioration of the expected lethal phenotype due to the presence of wild-type cells. While the data demonstrate that post-zygotic mutations which result in a proportion of cells carrying the mutation can have phenotypic consequences, the level of mosaicism necessary for a TRPV4 mutation to produce a phenotypic effect is unknown, and is likely dependent on the level of TRPV4 activity that results from the specific mutation. Since levels of somatic mosaicism can vary significantly among tissues [Qin et al., 2016], it is not possible to extrapolate from the measured level of mosaicism in blood to other tissues, including cartilage, and no other tissues from the patient were available. Also unknown is whether the proportion of mutant cells in cartilage could vary due to selection in terms of either increased survival or proliferation of mutant versus wild-type cells conferred by the expression of activated TRPV4 [Camacho et al., 2010; Weinstein et al., 2014].
An important clinical implication of our findings is that Sanger sequence analysis of candidate genes in dominant disorders cannot unequivocally rule out presence of a mutation in all instances, as low-level mosaicism would fall below the detection threshold [Qin et al., 2016]. High-throughput sequencing approaches with high depth of coverage could rule out such false negatives, but settings for automatic variant calling programs may need to be adjusted so that mutations present at the low levels present in mosaic individuals can be observed. In addition, the level of mosaicism detectable will also depend on the depth of coverage. In a clinical diagnostic setting, this could make identification of mosaic cases challenging. For somatic mosaics, genetic counseling can take into account the likelihood that these individuals are also mosaic in their germline [Campbell et al., 2014; Qin et al., 2016], adjusting risk accordingly, and considering the possibility that their heterozygous offspring could manifest a more severe form of the disorder [Wallis et al., 1990]. In males, characterizing the presence and level of germline mosaicism could make such counseling more precise, a benefit not generally available for females. For sporadic cases of dominant genetic disorders, the possibility of parental somatic and germline mosaicism could be considered when evaluating cases and estimating recurrence risk [Cohn et al., 1990; Wallis et al., 1990; Campbell et al., 2014; Qin et al., 2016]. For the TRPV4 disorders in particular, the results of this study suggest that clinical evaluation of patients with musculoskeletal disorders similar to the peripheral neuropathies or skeletal dysplasias with scoliosis that have been ascribed to the TRPV4 spectrum could consider whether the phenotypes might result from somatic mosaicism in the target tissues affected in these phenotypes.
ACKNOWLEDGMENTS
We thank the family for their participation. This work was supported in part by grants from the National Institutes of Health (NIAMS R01AR062651 and RO1AR066124). Sequencing was provided by the University of Washington Center for Mendelian Genomics (UW CMG) which is funded by the National Human Genome Research Institute and the National Heart, Lung, and Blood Institute Grant 1U54 HG006493. We thank the Joseph Drown Foundation and the March of Dimes for their support of the International Skeletal Dysplasia Registry. MMW was supported in part by a Collaboratory fellowship from the UCLA Quantitative and Computational Biosciences Institute.
Footnotes
Conflict of interest: None.
REFERENCES
- Auer-Grumbach M, Olschewski A, Papic L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Frohlich E, Balint Z, Tang B, Strohmaier H, Lochmuller H, Schlotter-Weigel B, Senderek J, Krebs A, Dick KJ, Petty R, Longman C, Anderson NE, Padberg GW, Schelhaas HJ, van Ravenswaaij-Arts CM, Pieber TR, Crosby AH, Guelly C. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho N, Krakow D, Johnykutty S, Katzman PJ, Pepkowitz S, Vriens J, Nilius B, Boyce BF, Cohn DH. Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am J Med Genet Part A. 2010;152A:1169–1177. doi: 10.1002/ajmg.a.33392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IM, Stewart JR, James RA, Lupski JR, Stankiewicz P, Olofsson P, Shaw CA. Parent of origin, mosaicism, and recurrence risk: Probabilistic modeling explains the broken symmetry of transmission genetics. Am J Hum Genet. 2014;95:345–359. doi: 10.1016/j.ajhg.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn DH, Starman BJ, Blumberg B, Byers PH. Recurrence of lethal osteogenesis imperfecta due to parental mosaicism for a dominant mutation in a human type I collagen gene (COL1A1) Am J Hum Genet. 1990;46:591–601. [PMC free article] [PubMed] [Google Scholar]
- Deng HX, Klein CJ, Yan J, Shi Y, Wu Y, Fecto F, Yau HJ, Yang Y, Zhai H, Siddique N, Hedley-Whyte ET, Delong R, Martina M, Dyck PJ, Siddique T. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat Genet. 2010;42:165–169. doi: 10.1038/ng.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge SP, Bowles NE, Arrington CB, Pilcher T, Rope A, Wilde AA, Alders M, Saarel EV, Tavernier R, Timothy KW, Tristani-Firouzi M. Somatic mosaicism contributes to phenotypic variation in Timothy syndrome. Am J Med Genet Part A. 2011;155A:2578–2583. doi: 10.1002/ajmg.a.34223. [DOI] [PubMed] [Google Scholar]
- ExAC. Exome Aggregation Consortium [Accessed April 2016]; URL: http://exac.broadinstitute.org.
- GATK. Genome Analysis Toolkit [Accessed April 2016]; URL: http://www.broadinstitute.org/gatk.
- Krakow D, Vriens J, Camacho N, Luong P, Deixler H, Funari TL, Bacino CA, Irons MB, Holm IA, Sadler L, Okenfuss EB, Janssens A, Voets T, Rimoin DL, Lachman RS, Nilius B, Cohn DH. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, Gaudet R, Kleta R, Fischbeck KH, Sumner CJ. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wang J, Tian X, Yu H, Truong C, Mitchell JJ, Wierenga KJ, Craigen WJ, Zhang VW, Wong LC. Detection and quantification of mosaic mutations in disease genes by next-generation sequencing. J Mol Diagn. 2016;18:446–453. doi: 10.1016/j.jmoldx.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, Lachman RS, Wilcox WR, Reyno S, Quadrelli R, Vaglio A, Owsianik G, Janssens A, Voets T, Ikegawa S, Nagai T, Rimoin DL, Nilius B, Cohn DH. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler A, Sumner C, Hoover-Fong JE. GeneReviews at GeneTests Medical Genetics Information Resource (database online) Copyright, University of Washington; Seattle: [Accessed 8/12/2016]. 2014. TRPV4-associated disorders. 1997–2013. Available at http://www.genetests.org. [Google Scholar]
- Siggers CD, Rimoin DL, Dorst JP, Doty SB, Williams BR, Hollister DW, Silberberg R, Cranley RE, Kaufman RL, McKusick VA. The Kniest syndrome. Birth Defects Orig Artic Ser. 1974;10:193–208. [PubMed] [Google Scholar]
- Takagi M, Kaneko-Schmitt S, Suzumori N, Nishimura G, Hasegawa T. Atypical achondroplasia due to somatic mosaicism for the common thanatophoric dysplasia mutation R248C. Am J Med Genet Part A. 2012;158A:247–250. doi: 10.1002/ajmg.a.34358. [DOI] [PubMed] [Google Scholar]
- Tompson SW, Bacino CA, Safina NP, Bober MB, Proud VK, Funari T, Wangler MF, Nevarez L, Ala-Kokko L, Wilcox WR, Eyre DR, Krakow D, Cohn DH. Fibrochondrogenesis results from mutations in the COL11A1 type XI collagen gene. Am J Hum Genet. 2010;87:708–712. doi: 10.1016/j.ajhg.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis GA, Starman BJ, Zinn AB, Byers PH. Variable expression of osteogenesis imperfecta in a nuclear family is explained by somatic mosaicism for a lethal point mutation in the alpha 1(I) gene (COL1A1) of type I collagen in a parent. Am J Hum Genet. 1990;46:1034–1040. [PMC free article] [PubMed] [Google Scholar]
- Weinstein MM, Tompson SW, Chen Y, Lee B, Cohn DH. Mice expressing mutant Trpv4 recapitulate the human TRPV4 disorders. J Bone Miner Res. 2014;29:1815–1822. doi: 10.1002/jbmr.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterpacht A, Hilbert M, Schwarze U, Mundlos S, Spranger J, Zabel BU. Kniest and Stickler dysplasia phenotypes caused by collagen type II gene (COL2A1) defect. Nat Genet. 1993;3:323–326. doi: 10.1038/ng0493-323. [DOI] [PubMed] [Google Scholar]