Abstract
Purpose
There is a lack of agreement regarding the types of lesions and clinical conditions that should be included in the term “geographic atrophy”. Varied and conflicting views prevail throughout the literature and are currently used by retinal experts and other health care professionals.
Methods
We reviewed the nominal definition of the term “geographic atrophy” and conducted a search of the ophthalmological literature focusing on preceding terminologies as well as the first citations of the term “geographic atrophy” secondary to age-related macular degeneration (AMD).
Results
According to the nominal definition, the term “geography” stands for a detailed description of the surface features of a specific region, indicating its relative position. However, it does not necessarily imply that the borders of the region must be sharply demarcated or related to any anatomical structures. The term “geographical areas of atrophy” was initially cited in the 1960s in the ophthalmological literature in the context of uveitic eye disease and shortly thereafter also for the description of variants of “senile macular degeneration”. However, no direct explanation could be found in the literature as to why the terms “geographical” and “geographic” were chosen. Presumably the terms were used as the atrophic regions resembled the map of a continent or well-defined country borders on thematic geographical maps. With the evolution of the terminology, the commonly used adjunct “of the retinal pigment epithelium” was frequently omitted and solely the term “geographic atrophy” prevailed for the non-exudative late-stage of AMD itself. Along with the quantification of atrophic areas, based on different imaging modalities and the use of both manual and semi-automated approaches, various and inconsistent definitions for the minimal lesion diameter or size of atrophic lesions have also emerged.
Conclusions
Reconsideration of the application of the term “geographic atrophy” in the context of AMD appears to be prudent given ongoing advances in multi-modal retinal imaging technology with identification of various phenotypic characteristics, and the observation of atrophy development in eyes under anti-angiogenic therapy.
Keywords: Age-related macular degeneration, geographic atrophy, retinal imaging, fundus grading, color fundus photography, spectral-domain optical coherence tomography, confocal scanning laser ophthalmoscopy
I. Introduction
The term “geographic atrophy” (GA) is commonly used in Ophthalmology although definitions vary and no generally accepted definition has been agreed upon with regard to the various phenotypic characteristics of atrophy at the level of the outer retina, the retinal pigment epithelium and the choriocapillaris. Consequently, there is substantial confusion among retina specialists, other health care professionals as well as in the scientific and lay press. It may be questioned whether the actual meaning of this term is an appropriate description of the addressed disease manifestation(s). The aim of this review is to discuss the development and the scope of the term “geographic atrophy” and how it has been used in the ophthalmological literature, particularly in the context of age-related macular degeneration (AMD). This review aims to aid ongoing efforts to develop a refined definition and classification for atrophy of the outer retina, the retinal pigment epithelium and the choriocapillaris.
The non-exudative, atrophic late-stage of AMD represents a common cause for severe visual loss1-3. There is an unmet need for an effective treatment of atrophic AMD and substantial efforts in basic and clinical research are in progress [for a review see Holz et al. 20144]. No exclusive dichotomy exists between late-stage non-exudative and neovascular AMD as they may be a continuum of the same disease process. Outer retinal atrophy develops or may preexist frequently in eyes with choroidal neovascularization (CNV). Furthermore, several lines of evidence point towards a high incidence of atrophy of the outer retina and the retinal pigment epithelium in eyes undergoing intravitreal anti-VEGF (vascular endothelial growth factor) therapy5, 6.
To elucidate the term “geographic atrophy”, it is important to note that some authors use the term exclusively for eyes with no signs and/or no history of exudation at all, while others use it to describe localized outer retinal and retinal pigment epithelium degeneration or atrophy in eyes that may also exhibit signs of present or previous exudation. It is noteworthy to mention that a history of exudation may later not be verifiable - even when applying state-of-art high-resolution in-vivo imaging.
Furthermore, there are many different pathways and factors that may contribute to the development of manifest atrophy of the outer retina and the retinal pigment epithelium. Presence of “pure atrophy” following collapse of retinal pigment epithelial detachments may be indistinguishable from development of atrophy de novo or in association with other phenomena (e.g. regression of soft drusen). Finally, it is debatable if peripheral atrophic lesions that have been described in non-exudative and neovascular stages of AMD shall also be subsumed under the term “geographic atrophy”7.
Another reason to challenge the term “geographic atrophy”, being coined 45 years ago, are major advances in the field of retinal imaging technology. High-resolution spectral-domain optical coherence tomography (SD-OCT) and confocal scanning laser ophthalmoscopy (cSLO) are now used in routine patient management. Hence it is of utmost importance to establish a new (or extended) classification system aiding ophthalmologists exactly to interpret and classify characteristic disease manifestations in various retinal disorders, including atrophy of the retina, the retinal pigment epithelium and the choriocapillaris secondary to AMD.
II. Semantic considerations
According to the American Heritage Dictionary of the English Language, the adjective “geographic” is related to “geography” and “concerns the topography of a specific region”. (Online Edition https://www.ahdictionary.com/). It gives a “detailed [and] precise description” of the topography “of a place or region” and the “graphic representation of the surface features of a place or region on a map, indicating their relative positions and elevation”. This definition of “topography” also includes the “study or description of an anatomical region or part”. Like geography, the word atrophy traces back to Greek and Latin origin and defines a progressive decline, “a wasting or decrease in size of a body organ, tissue, or part owing to disease, injury, or a lack of use”.
The advanced non-exudative form of AMD - as described by clinico-pathological correlations and high-resolution in-vivo imaging modalities - is typically characterized by well-defined patches lacking photoreceptors, by degeneration or complete loss of retinal pigment epithelium (RPE) as well as by loss or attenuation of the choriocapillaris layer8, 9. These observations are in accordance with a “decrease in size of tissue", i. e. the retina. With regard to the topography of atrophy, there is a high variability in the location of atrophic areas at the posterior pole as well as the number and shape of individual lesions. It is worthy to note that the phenomenon of “foveal sparing” (i. e. no involvement of the fovea by the atrophic process in the earlier stages of the disease), the predominant development of initial atrophic areas within the parafoveal macula and the typical bilateral symmetry of the distribution of atrophic patches in individual subjects, support the assumption that the location of occurrence of atrophic areas is not a random event10-12. Focusing on the word “geographic”, defined as a “detailed description of the surface features [of atrophic areas], indicating their relative positions”, it may be an adequate descriptive term for atrophic areas in individual eyes. However, the term “geographic” for the description of the disease manifestation in general appears suboptimal – when solely considering its nominal definition –as the disease itself would not be able to be “geographic” on its own. In contrast, it appears reasonable –based on the nominal definition – to describe “geographic areas of atrophy” or “geographic areas” in AMD patients as has been done in the 1970s by Gass, Blair and Sarks (see below). Finally, the definition of “geographic” does not imply “sharply-demarcated” or “clear-cut” edges of lesions nor anything about the orientation of lesions in relation to other anatomical structures.
In Medical Dictionaries, the word “geographic” is defined as “a term used in pathology referring to a pattern that is well demarcated, resembling the outline of a land mass against water on a map” or as “a general descriptor for lesions in which large areas of one color, histologic pattern, or radiologic density with variably scalloped borders sharply interface with another color, pattern or density, fancifully likened to national boundaries and/or coastlines”13- 15 . In addition to “geographic retinal atrophy” that is “most often associated with age-related macular degeneration”, “geographic tongue” and “geographic ulcer” are specifically mentioned as “geographic” disease entities (see below).
With regard to the word “areolar” which had been commonly used in the context of atrophic late AMD (“areolar atrophy” or similar), the primary definition of “areola” is a small ring of color around a center portion, as about the nipple of the breast or the part of the iris surrounding the pupil of the eye This term may be appropriate to describe the phenotype of monogenetically inherited “central areolar choroidal dystrophy” (CACD) which is typically characterized by a ring of pigmentary changes or speckled fundus autofluorescence changes around a large central patch of atrophy16. By contrast, the term would neither refer to the shape of atrophy itself nor appreciate the multifocality of atrophy commonly presented in late-stage atrophic AMD.
III. Review of the ophthalmological literature
A PubMed search on the 23rd of March 2016 of the keyword “geographic atrophy” revealed a total number of 973 publications. Using both “geographic atrophy” and “age-related macular degeneration”, 769 search results were identified of which 633 (82%) and 450 (59%) had been published in the last 10 and 5 years, respectively. The search of the ophthalmological literature focused on the first-time use of the term “geographic atrophy” in the context of age-related macular degeneration. In addition, we aimed to identify previous nomenclatures and to review the initial use of the term “geographic” or similar terms in order to understand better the development of the terminology. To this aim, we also reviewed historical textbooks and atlases.
1. Terminology before the introduction of the expression “geographic atrophy”
Before focusing on the term “geographic atrophy”, we will give some brief considerations on the historical use of the term “age-related macular degeneration” which may be found in the ophthalmological literature from 1984 onwards17-19. Before 1984, related terms such as “senile (disciform) macular degeneration”, “disciform degeneration”, “involutional macular degeneration”, “senile amblyopia” or “Kuhnt-Junius macular degeneration [in German: Junius-Kuhnt Makuladegeneration]” had been used. The latter term refers to the German ophthalmologists Hermann Kuhnt and Paul Junius who published the first monograph on the exudative form of the disease in 1926, also including several fundus drawings [Die scheibenförmige Entartung der Netzhautmitte (Degeneratio Maculae Luteae Disciformis)]20. While the authors described different features of exudative macular degeneration, they did not include a “pure atrophic” end-stage form of the disease. Even today, the term “Kuhnt-Junius macular degeneration” [Junius-Kuhnt Makuladegeneration] is used for disciform lesions with obvious fibrotic scar tissue. A “historical perspective” with an extensive review of the literature of “disciform macular degenerations” was published by Stephen J Ryan et al. in 198021.
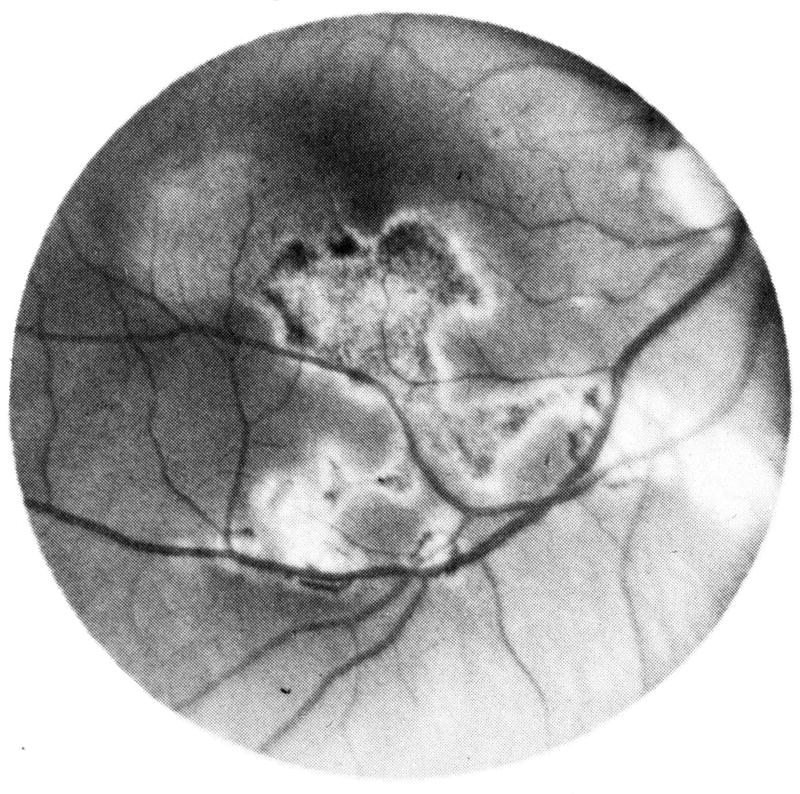
In 1885, Otto Haab reported on “atrophy of the pigment epithelium” in “senile macular disease”22. Subsequent authors sometimes used the nomenclature “Senile Macular Degeneration of Haab” or variations of this term for describing what we call today “geographic atrophy” secondary to AMD23. In 1884, and just one year before Haab, Edward Nettleship reported on a clinical case of “central senile areolar atrophy of choroid” as a variant of “senile disease of the choroid”24. He described a bilateral occurrence of large areas of atrophied choroid in the central region of the fundus with circular appearance in a 60 year old female whose father had gone blind “six years before he died” (Figure 1 A). Nettleship explained that the case was different from “central guttate choroiditis” previously described by Tay and Hutchinson. At that time, in the 1880s, neither Haab nor Nettleship used the term “geographic”.
Figure 1. Prior to the introduction of the term “geographic atrophy”.
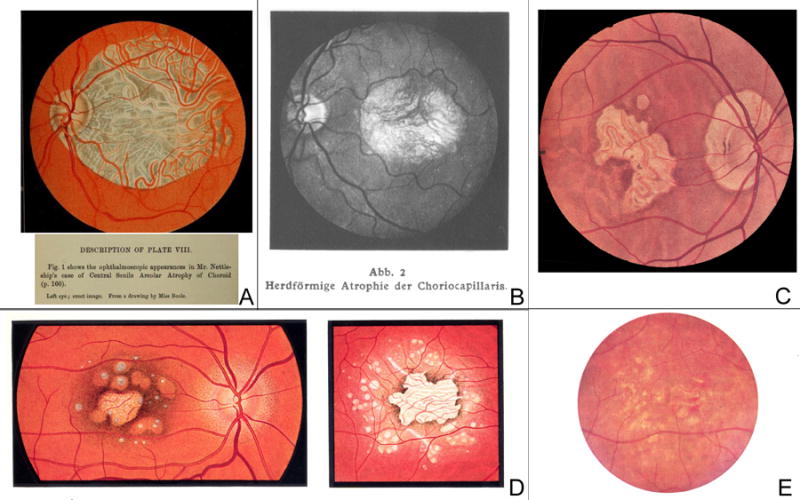
A. This illustrative fundus drawing was published in Nettleship’s case report of 1884 on “central senile areolar atrophy of choroid” in a 60 year old female whose father had gone blind six years before his death [Plate VIII, fig. 1]24. B. In the first atlas of ophthalmic photography (1927), Pillat and Dimmer included a case of focal atrophy of the choriocapillaris [“Herdförmige Atrophie der Choriokapillaris”] in a 69 year old female [Plate 75]91. C. Fundus drawing of “senile macular degeneration” in 1943 [Plate 23, 3]26. D. Two fundus drawings in the Atlas of the Ocular Fundus by Marchesani and Sautter (1957) which were called an example of heredodegeneration of the central retina [Plate 104, c and d, p. 209. Copyright with friendly permission by Elsevier]92. E. Color photograph of “senile macular degeneration” with multiple areas of depigmentation in a 70 year old male, by Hollwich in “Der Augenarzt” (1963) [Fig. 95, p. 685]46.
Up to the 1960s, it had not been clearly stated that atrophic macular degeneration in the elderly is closely related to exudative macular degeneration in the elderly (Kuhnt-Junius macular degeneration). For example, in the “Kurzes Handbuch der Ophthalmologie” [Short Handbook on Ophthalmology], published in 1930, the “Kuhnt-Junius macular degeneration” was described in detail, while – in the same chapter “Erkrankungsformen der Netzhautmitte” [“Diseases of the Central Retina”] - several “macular heredodegenerations” were listed separately25. These also include “pre-senile and senile heredodegenerations”; the descriptions are in accordance with the late form of atrophic AMD, but were not linked to the “Kuhnt-Junius macular degeneration”, described on the opposite page. This reflects the uncertainty and the debates as to whether these manifestations of “senile heredodegenerations” were in fact hereditary like “Stargardt’s” and “Best” disease26. This latter hypothesis and the term “Heredodegeneration der Makula” had been introduced by Carl Behr in 192027. “Behr disease” - a term used in the 1960s - neither showed sharply-demarcated areas of atrophy nor shared similar features with end-stage AMD28. Clemens Harms in 1904 –which probably represents the first histopathological report - had already outlined the sharp borders of the lesions by describing that “one had almost the impression at low magnification” that they “were cut off with a sharp instrument”29. In the first atlas of ocular fundus photography (1927), Friedrich Dimmer and Arnold Pillat included several cases of choroidal atrophy; however, they did not clearly associate the clinical cases with “senile macular degeneration” but rather described them as “focal atrophy” [“herdformige Atrophie”] or “peculiar atrophy” [“eigentümliche Atrophie”] (Figure 1 B).
In 1952, Jules François and J.-P. Dewer discussed that senile macular degeneration (“heredodegenerative”) frequently occurred in families with “Kuhnt-Junius macular degeneration” and pointed out the hereditary association as well as the association between senile degenerative and exudative Kuhnt-Junius macular degeneration30. In addition to the discussion about heredity, a long-standing and controversial debate about the specific layers primarily affected by the disease can be traced in the literature ranging from the vitreous, to the retinal vessels, the retinal neuroepithelium, the retinal pigment epithelium, the choriocapillaris and finally to the choroid31-33. In the multi-volume publication “Der Augenarzt” [“The Ophthalmologist”] (1963), Fritz Hollwich included “senile macular degeneration” in the group of “Heredodegenerationen der Makula” (heredodegenerations of the macula). The depicted illustration corresponds well with the current understanding of atrophic end-stage AMD (Figure 1 E). Accordingly, the “System of Ophthalmology”, edited by Sir Stewart Duke-Elder (1966+67), refers to “Behr’s Disease” as “adult heredo-macular dystrophy” and summarizes “pre-senile and senile heredo-macular dystrophies”34, 35. The latter was characterized by “the presence of degenerative changes, usually punctate in nature, occurring bilaterally, limited to the region of the macula.” Furthermore, Duke-Elder mentioned that the “general degenerative and non-exduative - the “dry” type of degenerative senile changes [stands] in contrast to the exudative form typified by disciform degeneration”, referring to publications by Hans Sautter, Abraham L. Kornzweig et al. and Alson E. Braley36-38. These three latter publications included detailed descriptions of epidemiology, pathogenesis and disease clinico-pathological manifestations, but still did not utilize the term “geographic atrophy”. For example, Kornzweig et al. also used in the context the word “abiotrophy” to describe an accumulation of metabolites in a given cell which the cell was unable to discard. Eventually the process would destroy the ability of the cell to function properly and the cell then would atrophy and die. In the mid-1960s, increasing efforts were made to obtain differential diagnoses of macular degenerations as well as a classification system. Bertha A. Klien [in German “Klein”] reported in 1964 on “some aspects of classification and differential diagnosis of senile macular degeneration” and a symposium (1966) for the differential diagnosis of retinal diseases was held at the University of California Medical Center39, 40.
2. Coining of the term “geographic atrophy” in the context of “senile macular degeneration”
In 1967, J. Donald M. Gass published “Pathogenesis of disciform detachment of the neuroepithelium” which included a section on the pathogenesis and etiology of “senile disciform macular degeneration”41. In this article, Gass did not connect the end-stage manifestation without signs of exudation with the spectrum of senile macular degenerations. One explanation might be that the publication focused on different exudative detachments of the retina, not limited to senile macular degeneration, and hence did not include any “dry” manifestations.
Three years later in the first edition of the “Stereoscopic Atlas of Macular Diseases” (1970), Gass described the “central areolar pigment epithelial atrophy secondary to senile macular choroidal degeneration” as a “not infrequently” occurring manifestation in patients with senile choroidal degeneration42. Although not yet using the term “geographic atrophy”, he described the development of “sharply circumscribed geographic areas of atrophy of the pigment epithelium at the posterior pole”, exposing the large underlying choroidal vessels. The accompanying illustration with color fundus photograph, also provided as a stereoscopic color slide, and the arteriovenous stage of fluorescein angiography, is well in accordance with present-day understanding of “geographic atrophy secondary to AMD” (Figure 2 A). Gass also described the presence of multiple drusen surrounding the areas of atrophy and the typically similar pattern of atrophy in the fellow eye. With regard to the etiology, he pointed out that the pathogenesis was “not fully understood”. Also providing histopathological findings from one patient, he suggested that marked atrophy of the pigment epithelium may result from diffuse deposition of subpigment epithelial material and secondary to severe atrophy of the choriocapillaris. He also noted that this disease phenotype could develop following prolonged exudative detachment of the pigment epithelium or following the cleavage of a thin hemorrhagic detachment of the pigment epithelium, thereby leading to atrophy, but with only minimal or no scar tissue at all. Gass also pointed out that “central areolar pigment epithelial atrophy secondary to senile macular choroidal degeneration" differed from Sorsby’s central areolar choroidal sclerosis in “several respects”, including a lack of “family history of similar difficulty”. In addition, patients with atrophy secondary to “senile macular choroidal degeneration” had quite good visual acuity until they were beyond 50 years and presented with “drusen surrounding the geographic areas of pigment epithelial atrophy“. Indeed, in 1953, Arnold Sorsby and Ronald P. Crick already had reported that “central areolar choroidal sclerosis” was an inherited disease which could occur even in 20-year-olds43. Sorsby and Crick did not use the term “geographic” nor did they consider senile macular degeneration as an etiological possibility. The term “choroidal sclerosis” in general appears to have already been used at the time of Nettleship and Haab in the 1880s and later on as a descriptive term for the appearance of prominent white choroidal vessels in which the blood columns were not seen or were sheathed by white lines, also implying a angiosclerotic, and/or cardiovascular association for the presence of atrophy21, 22, 24, 44. Finally, it is important to note that Gass stressed another entity in the differential diagnosis of “central areolar pigment epithelial atrophy secondary to senile macular choroidal degeneration” in the first edition of the atlas, namely “central areolar choroidal sclerosis” as part of a spectrum of “non-exudative heredodegenerative diseases of the choroid”42. This latter disease appears to be what is now called “central areolar choroidal dystrophy” (CACD) (see below).
Figure 2. Coining of the term “geographic atrophy” in the context of “senile macular degeneration”.
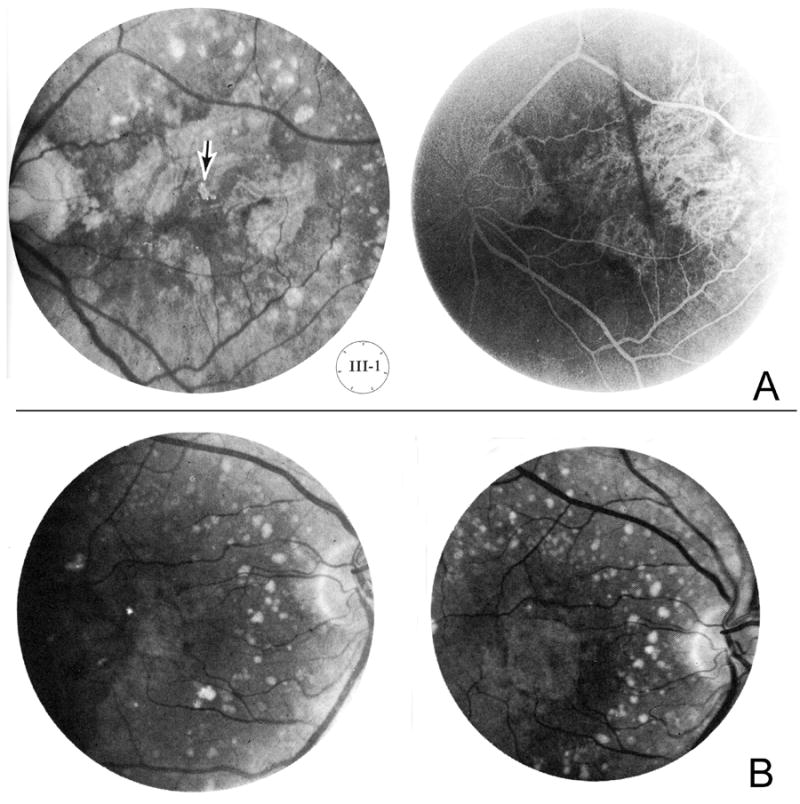
A. Initial description by Gass of “sharply circumscribed geographic areas of atrophy of the pigment epithelium at the posterior pole”, exposing the large underlying choroidal vessels, in a 67-year old woman with “central areolar pigment epithelial atrophy secondary to senile macular choroidal degeneration “, published in 1970 in the first edition of the “Stereoscopic Atlas of Macular Diseases” (left: fundus photograph, right: arteriovenous stage of fluorescein angiography) [Fig. 2-17, A and B, p. 37. Copyright with friendly permission by Elsevier]33. B. Development and progression of geographic atrophy by Gass, reported in 1972/73 (middle row: imaged 1964 and 1968, lower row: 1969 and 1971) [Fig. 4, p. 209. Copyright with friendly permission by American Ophthalmological Society]33, 45.
It was only two years later that Gass used in his article “Drusen and disciform macular detachment” for the first time the precise expression “geographic atrophy” in association with “senile macular degeneration”33, 45. This report was published in 1972 in the Transactions of the American Ophthalmological Society and in 1973 in Archives of Ophthalmology and also referred to the first edition of the Stereoscopic Atlas of Macular Diseases42. While Gass described the morphological and clinical features as well as the precursors of “geographic atrophy”, he did not explain whether he used the term “geographic” as a new concept or whether this very term had been used by other authors before. According to Gass, “geographic or areolar atrophy of the pigment epithelium and retina usually [showed] no evidence of defects in Bruch’s membrane”. The corresponding illustration, labelled as “development and progression of geographic atrophy” correlates to our present-day understanding of two typical eyes that had initially shown confluent drusen, pigmentary changes and refractile deposits and later had developed the atrophic end-stage of AMD (Figure 2 B). Although similar characteristics had been previously reported e.g. by Duke-Elder, Hollwich and Schieck, the publications by Gass included much more in depth discussion. It seems that Gass was the first to use the terminology of “geographic” in the English ophthalmological literature and in the context of “senile macular degeneration”25, 34, 35, 46.
In the second and third edition of the Stereoscopic Atlas of Macular Diseases, published in 1977 and 1987, Gass did not use the term “geographic atrophy” exclusively for “senile macular degeneration”, but also described “geographic atrophy” from other causes, including Sorsby’s dystrophy, dominant macular drusen and juvenile foveal dystrophy, dominant macular drusen associated with senile macular dystrophy and disciform detachment, Stargardt’s disease, atrophy secondary to myopia, atrophy secondary to serous and hemorrhagic disciform detachment and serpiginous choroiditis47, 48. In patients with macular drusen, he employed the term “geographic atrophy” or “central areolar atrophy” without clear distinction. In addition, these terms were always used by Gass with the adjunct “of the retinal pigment epithelium”, “of the retinal pigment epithelium and choroid” or even “of the retinal pigment epithelium and retina”.
The first publication using the term “geographic atrophy” in its title was an article by Charles J. Blair (Bascom Palmer Eye Institute in Miami) published in 197549. He described 11 patients with “geographic atrophy of the retinal pigment epithelium secondary to senile macular degeneration”. According to his description, “areas of geographic atrophy of the retinal pigment epithelium were circular to oval and were usually sharply demarcated”. He pointed out that “geographic atrophy of the retinal pigment epithelium” was a manifestation of “senile macular degeneration”, differentiating from other entities such as serous detachments of the retinal pigment epithelium, disciform lesions or hemorrhagic detachments. Although “morphologically similar” he further emphasized that “geographic atrophy of the retinal pigment epithelium” was “strikingly” different from “central areolar choroidal sclerosis” (=Sorsby’s disease) in age of onset, family history, association with senile macular degeneration and pathologic features. Like Gass, Blair did not mention the origin of the terminology “geographic atrophy of the retinal pigment epithelium” in this article, although he referred to other publications, including those by Nettleship and Gass24, 42.
In 1976, Shirley H. Sarks used the term “geographical atrophy” instead of “geographic atrophy” in her landmark article published in the British Journal of Opthhalmology”9. This slight difference in terminology appears to be more related to British versus American English which was indeed confirmed by Sarks in 2016 (personal communication). Sarks did not use the adjunct “of the retinal pigment epithelium” (Blair 1975) or “of the pigment epithelium and choroid” (Gass 1977)47, 49. In this article, Sarks described a clinico-pathological study on 378 eyes of subjects who had been admitted to a hospital for aged homeless men in Australia. This study had been commenced in the mid-1960s after she had visited Kornzweig who had published his clincopathologic observations at a similar institution in the United States (see above). In the publication, Sarks focused on normal ageing changes versus manifestation of changes in “senile macular degeneration”. Dividing the eyes in six progressive groups according to the degree of abnormality, eyes in group V showed “areas of geographical atrophy”. Sarks did not define the meaning of “geographical atrophy” in this article. According to her, “geographical atrophy” was “clinically visible”. In the corresponding figure, she described the gradual development of an “area of depigmentation”. In fact, the report is more detailed about histological findings including distinct features at the edge of atrophy such as ending of the retinal pigment epithelium, disappearance of photoreceptors, and ending of the external limiting membrane. At the site of atrophy itself, the outer plexiform layer rested directly on basal material (due to tissue loss of the outer retina) and atrophy of the choroid was observed. Finally, she differentiated between “geographical atrophy” and “disciform degeneration”, the latter representing changes along with exudation. This is in contrast to Gass’s report in 1972/73 in which the term “disciform detachment and degeneration” was used to refer to lesions with (1) serous exudation, (2) neovascular ingrowth through breaks in Bruch membrane and (3) geographic atrophy with usually no evidence of defects in Bruch membrane33, 45.
In a second publication “Drusen patterns predisposing to geographic atrophy of the retinal pigment epithelium” (1982) Sarks altered the term “geographical atrophy” to “geographic atrophy of the retinal pigment epithelium”50. According to her description, “geographic atrophy” was “usually a later development in eyes believed to have survived the period during which they were most susceptible to neovascularization”. The manuscript focused on the development of “geographic atrophy” from drusenoid lesions and did not explain the term “geographic atrophy”. In a third publication (1988) on the “evolution of geographic atrophy of the retinal pigment epithelium”, Sarks et al. wrote that the term “geographic atrophy of the retinal pigment epithelium” had been applied by Gass to “one or more circumscribed areas of atrophy which slowly enlarge and coalesce such that the spreading lesion is often irregular”, giving him the merit for introducing the terminology51. Hereby, Sarks et al. referred to Gass’s publication of 1973 that had also been published in a very similar version in 1972 (see above)33, 45. Sarks et al. also stated in 1988 that Green and Key had “proposed the term areolar”32. While the term “areolar” had been already used by Nettleship in 1884, the landmark article by Green and Key of 1977 represented an extensive histopathological study on “senile macular degeneration”, including many aspects that are still discussed today, such as the role of drusen (at that time often called “colloid bodies”) in the pathogenesis, the role of changes in the choroid as well as the coexistence and association of drusen, “areolar retinal pigment atrophy”, “subretinal pigment epithelial neovascularization and disciform degeneration”. Throughout this publication the term “areolar atrophy” is frequently used without mentioning the term “geographic atrophy”. From this, it is presumed that the term “geographic atrophy” was not yet fully established in 1977.
Today, the term “areolar” is more or less restricted to “central areolar choroidal dystrophy” or CACD which represents a well-defined retinal disease with mutations in the PRPH2 gene16, 52 With the development of molecular genetic technologies and the wide availability of genetic testing, we now have a much better understanding of the phenotype and manifestations of monogenetic diseases of the retina and choroid. For example, typical characteristics of macular dystrophies mimicking AMD have been recently summarized by Sakens et al.53.
3. The further development of the term “geographic atrophy”, longitudinal case series and the quantification of atrophic areas
Initially, the term “geographic atrophy” had been used with the adjunct “of the retinal pigment epithelium (and choroid)” or “of retinal pigment epithelium and retina”, indicating somehow a lack of knowledge as to which layer was the first to be involved in the disease process. Later, the term was often just shortened to “geographic atrophy”. In addition, other terms were also applied to describe the end-stage of non-exudative age-related macular degeneration, such as “age-related geographic atrophy of the macula”, “geographic atrophy in advanced AMD”, “the geographic atrophy form of age-related macular degeneration” and “geographic atrophy of the retinal pigment epithelium associated with age-related macular degeneration”51, 54, 55. Beyond the description of the manifestation, a growing knowledge about disease progression, particularly with regards to enlargement of atrophic patches and the corresponding impact on visual disabilities, evolved from the 1980s onwards.
One of the first longitudinal reports on the natural progression of geographic atrophy and its resulting effects on visual acuity was written in 1986 by Paul Maguire and Andrew K. Vine 11. This retrospective chart review was based on 29 eyes (18 patients) with a follow-up between 10 to 60 months (average, 22 months). The main finding was the identification of three different disease phases based on foveal involvement by the atrophy and visual function. The authors stated in the discussion that “the term geographic atrophy of the retinal pigment epithelium [did] not adequately describe the histopathologic findings of affected eyes” as not only the retinal pigment epithelium but also the outer retina and the underlying choriocapillaris showed abnormalities.
In 1989, Howard Schatz and H. Richard MacDonald reported about the rate of spread of geographic atrophy and visual loss over time (observational time 2 to 6 years). The average rate per year – defined as the change in the widest horizontal diameter of the largest area as seen on fundus photographs – was 139 μm in one direction. The baseline size of the widest horizontal diameter in this cohort varied between 200 to 5300 μm. Interestingly, they also gave an explanation for the term “geographic atrophy” in the introduction of the report: “The atrophic form of macular degeneration has also been called “geographic” because the areas of RPE [retinal pigment epithelium] atrophy tend to form well-defined demarcated borders that do not seem related to specific anatomic structures”. In fact, this is the only explanation – found in the current literature search – which refers to the origin of the term “geographic atrophy” in the context of AMD. However, as we have noted, there is nothing inherent in the term “geography” to imply that a structure had to be “well-demarcated” or that it had not be related to a “specific structure.”
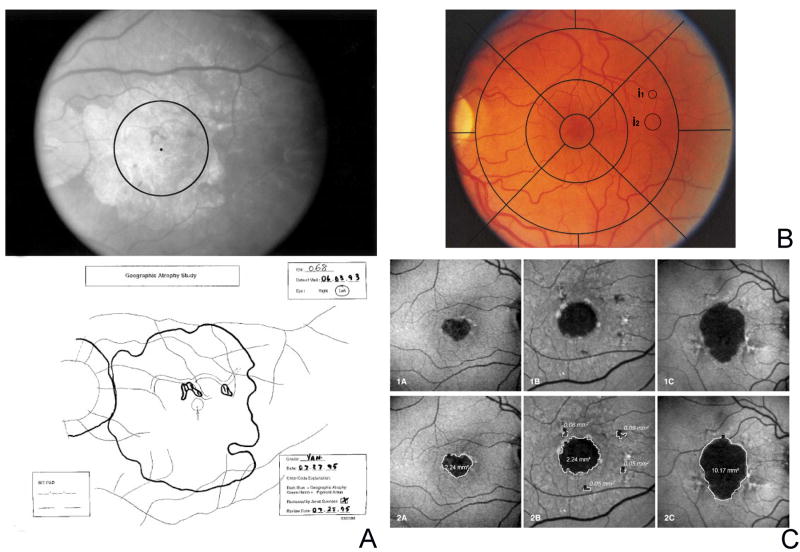
Early attempts to measure lesion size of atrophic areas in square millimeters began in the late 1980s. Based on the method used by the Macular Photocoagulation Study, frames of fluorescein angiographs or color fundus photographs were projected by means of a microfilm reader onto a digitizing pad in order to manually outline areas of atrophy with a stylus51, 56. Sarks et al. (1988) required one single area measuring ≥1 mm across and included all areas ≥ 0.3 mm across. The total atrophy was then expressed as the sum of all individual lesions51. The definition of “geographic atrophy” as a sharply defined area of “dropout of the RPE [retinal pigment epithelium], exposing choroidal blood vessels” as assessed by stereoscopic color fundus photographs, was used by the Wisconsin Age-related Maculopathy Grading System, published in 199157. The minimal size had to be as large as 1/8 disc diameter (defined as circle I1). This classification was developed for the systematic analysis of large imaging data sets in a reading center setting. The Age-Related Eye Disease Study (AREDS) System for Classifying Age-related macular degeneration (AREDS Report No. 6 in 2001), also based on stereoscopic color fundus photographs, and representing an extension of the Wisconsin Age-Related Maculopathy Grading System, defined “geographic atrophy” as “a sharply demarcated, usually circular zone of partial or complete depigmentation of the retinal pigment epithelium, typically with exposure of underlying large choroidal bloods vessels that must be as large as the circle I1 (1/8 disc diameter)” (Figure 3 B)58. This classification specifically distinguished “geographic atrophy” from “areas of depigmentation” which were defined as an area that did not meet the requirements for geographic atrophy, being less well defined, less regular in shape and/or less severe, including subretinal fibrous scars. The grading system specifically mentioned that “areas of RPE atrophy adjacent to disciform scars […] may have an appearance very similar to that of geographic atrophy”, but had not “conventionally been described as geographic atrophy” (http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?phd=15&study_id=phs00000 1.v1.p1). Therefore, these areas should be included in the category “RPE depigmentation, with a grade of questionable assigned to geographic atrophy if an appearance typical of geographic atrophy [is] present in such a location”. Furthermore, the areas of “geographic atrophy” were not quantified directly, but grouped in categories (such as area size ≥ 1 DA (disc area) but < 2 DA). In 2009, an analysis of a subset of fundus photographs from AREDS participants with a cumulative area of geographic atrophy greater or equal to 0.5 DA (translated by the authors to 1.33 mm2) within 1500 μm of the fovea at baseline or with development over time was performed59. For this analysis, slides were scanned in order to determine the area by using planimetric measurements. Hereby, the grader outlined the affected area and created a closed polygon. A similar approach was used by Klein and coworkers in 2008 to assess the progression of “pure geographic atrophy” in the Beaver Dam Eye Study60. In 2013, the AREDS2 Study group reported that the minimal area of a definite GA area had not to be greater than or equal to circle I1 (as previously – see above), but circle I2 (which was translated to the area size of 0.146 mm2 or the diameter of 433 μm)61. At the same time, the AREDS2 Study group discussed the challenge of translating the size of disc areas in the metric system, outlining that the classic assumption equaled a standard disc diameter to 1500 μm, while the calibration to 1800 μm would be more correct.
Figure 3. Quantification of atrophy and definition of minimal lesion size.
A. Measuring geographic atrophy in advanced age-related macular degeneration, as developed by Sunness et al. in 1999, based on stereoscopic color fundus photograph, projected on a microfilm reader, followed by manual outlining of atrophic borders and retinal landmarks [Fig. 1, p. 1762. Copyright with friendly permission by ARVO]62. B. The Early Treatment Diabetes Retinopathy Study (ETDRS) grid and the size of the circles I1 (1/8 disc diameters) and I2 (1/4 disc diameters) are drawn in a normal fundus photograph. The former represents the minimal requirement in terms of lesion size for an atrophic lesion according to the Age-Related Eye Disease Study (AREDS) System for Classifying Age-related macular degeneration [modified according to fig. 1, p.670. Copyright with friendly permission by Elsevier]58. In 2013, AREDS2 suggested to use circle I2 instead of I1 as the minimal area of a definite GA area61. C. Detection and quantification of atrophy based on fundus autofluorescence imaging as reported by Schmitz-Valckenberg et al. in 2002 [Fig. 1 and 2, p. 74]66. Rather than manually outlining atrophic areas, they were defined using a semi-automated segmentation algorithm.
In 1995, Janet Sunness and coworkers defined geographic atrophy as at least one area of discrete retinal pigment epithelial atrophy of at least 500 μm in diameter within one disc diameter of the fovea54. Four years later, in 1999, Sunness et al. dedicated one original article solely to the topic of “Measuring geographic atrophy in advanced age-related macular degeneration” (Figure 3 A)62. Following “the general methods used for drawing choroidal neovascularization in the Macular Photocoagulation Study except that [stereoscopic] color fundus photographs were used rather than fluorescein angiograms”, retinal landmarks including the visible parts of the optic disc and areas of atrophy were drawn on a white paper taped to the viewing surface of a microfilm reader. In a second step, the areas of atrophy as manually outlined on the projector were than traced again on a digitizing tablet in order to compute the area in square millimeters (that was based on the revised standard DA (as opposed to the MPS DA) with one DA equivalent to 2.54 mm2). The process included considering spared islands within atrophic areas and several quality assurance procedures including at least two independent measurements (whose difference had to be less than 0.05 DA, or less than 0.5% of the total area) and confirmation of measurement results by a second grader. In this publication Sunness et al. also reported about the potential sources of errors in case of peripapillary atrophy and when the atrophy extended to or beyond the edge of the photograph. The authors also noted the challenges of color fundus photographs particularly with regard to color differentiation from the atrophic areas to choroidal vessels, hypopigmentations, (confluent) drusen at the edge of atrophy and overlying calcifications. This method for measuring atrophic areas was then used in a longitudinal observational study in patients with geographic atrophy by Sunness et al., published in 2009.10
From 1995 onwards the introduction of confocal scanning laser ophthalmoscopy fundus autofluorescence (FAF) imaging opened the door for an alternative approach to detect and quantify areas of atrophy as compared to color fundus photographs and fluorescein angiographs63, 64. The approach to delineate atrophic areas - based on FAF images - was developed following the observation that the FAF signal was markedly reduced at the site of atrophy compared to the background signal. The absence of the FAF signal over the degenerated retinal pigment epithelium was explained by the loss of intrinsic fluorophores which had been identified for being mainly responsible for the FAF signal in the normal state65. The high-contrast difference between atrophy and non-atrophic retina by FAF imaging also allowed – beyond pure and time-consuming manual outlining - quantification of atrophic areas by semi-automated image analysis software using segmentation and region-growing algorithms, initially reported in 2002 and used in the Fundus autofluorescence in Age-related Macular degeneration (FAM)-study (Figure 3 C)66-68.
4. The last decade
Within the last 10 years, we have seen an extensive acceleration in research activities and publications on atrophic age-related macular degeneration. The term “geographic atrophy” has gained increasing “popularity”; 83% of all reports found in PubMed search were published within this period (see above). Several large-scale natural history studies and the results of the first interventional studies have been reported4, 69-72. Important progress in automated image analysis has been achieved, including robust and reliable approaches to quantify lesion size, not only based on FAF imaging, but also using spectral-domain optical coherence tomography en-face imaging73-75. With regard to quantification of lesion characteristics, the variability of definitions has also increased. Variability within different studies has not been limited to the minimum size or diameter of an area of “geographic atrophy”, but also to the total area size of atrophy (sum of all atrophic lesions present within one eye) and – finally – to the minimal size or diameter of the single largest atrophic spot within one eye that had to be present to meet the definition of “geographic atrophy”. Phenotyping and genetic analysis have given more insight in the differential diagnosis of AMD53. At the same time, there is still a lack of agreement if “geographic atrophy” only implies “pure atrophy” without any signs of current or previous exudation – as prominently outlined by the AREDS classification. In contrast to the AREDS classification, the term “geographic atrophy” has recently been used by the CATT (Comparison of Age-related macular degeneration Treatment Trials) study group in the context of atrophic lesions associated with successfully treated choroidal neovascularization76.
5. The use of the term “geographic” beyond age-related (or ”senile”) macular degeneration
As discussed in section II (“semantic consideration”), medical dictionaries specifically list the term “geographic” in the context of “geographic tongue”. This benign and usually asymptomatic condition, also called – among others – “wandering rash of the tongue”, is characterized by oval red areas, surrounded by a well-defined white rim and caused by desquamation of the superficial epithelium of the dorsum of the tongue77, 78. Historically, it is reported to have been described as early as 1831, whereas the terminology “landkartenartig” (in English: “map-like appearance”) and “inselformig” (“island-shaped”) appears to be initially used by Santlus in 1854, i. e. well before the use of the term “geographic” in Ophthalmology79, 80.
The word “geographic” is also well-established in herpetic corneal disease. Particularly with the use of topical corticosteroids, areas of dendritic keratitis may coalesce further and enlarge into a more expansive “geographic ulcer”, showing a epithelial lesion that extends through the basement membrane in the sense of a true ulcer81. These lesions are described as having swollen epithelial borders that contain live virus. Interestingly, the “geographic lesions” in ocular herpes have been also been described by similar terms that have been also used in parallel in the context of AMD (see above), such as “maplike” or “areolar ulcers”, while “disciform” keratitis has been distinguished as a deep stromal manifestation from the more superficial (=epithelial + stromal) “geographic ulcer” form of herpetic keratitis. In addition, “scalloped” is another adjective that has been applied to describe the borders of the “geographic” lesions in herpetic keratitis. Even further, “geographic-shaped” discrete gray lesions, together with ”broad bands with scalloped edges” have been described in “posterior polymorphous dystrophy” (PPMD), a spectrum of corneal and anterior segment abnormalities82. An extensive search for the origin of the term “geographic” in the context of corneal eye disease would be beyond the scope of the current review. However, it is noteworthy to mention that the very term “geographic ulcer” in the context of ocular herpes can already be found in the textbook “Manual of Diseases of the Cornea”, published in 196983. Even earlier, Michael J. Hogan et al. reported in 1956 that the “so-called ‘geographic’ or ‘geographic map’ ulcer” was “the best example of certain variations of the dendritic figure” seen in herpetic keratitis84. They explained that it had been “so named because its irregular outline resembles the map of a continent”. A direct reference for the use of this terminology was not given by the authors. Noteworthy, this is the earliest use for description of ocular lesions as “geographic” as identified in the current literature search and also included a direct explanation why the term “geographic” had been applied.
According to the National Organization for Rare Disorders (http://rarediseases.org/rare-diseases/choroiditis-serpiginous/), synonyms of serpiginous choroiditis are – amongst other – “Geographic Choroiditis”, “Geographic Choroidopathy”, “Geographic Helicoid Peripapillary Choroidopathy (GHPC)” and “Geographic Serpiginous Choroiditis”. Several reports using the terminology “geographic” in association with choroiditis and “serpiginious” were published in the 1970s. Gass too used, in the first edition of his atlas (1970), the term “geographic area of atrophy” not only in the context of “senile macular choroidal degeneration”, but also in association with “serpiginous peripapillary choroiditis” (see above)42. In 1976, G. Seerp Baarsma and August F. Deutman referred to Theodore F. Schlaegel Jr. who described “serpiginous or geographic choroiditis” as a nongranulomatous uveitis in his book “Essentials in Uveitis”(1969) (Figure 4)85, 86. Already Schlaegel himself referred to a presentation by A. Edward Maumenee and Lea Hyvärinen at the 27th Wilmer Residents Association Meeting in Baltimore the year before (April 25, 1968) about “Geographic choroiditis vs. choroidal vascular abiatrophy” (There might be a slight misspelling, it should rather read “abiotrophy”) . This is the first mention of the term “geographic” in the context of sharply-demarcated areas of atrophy at the posterior pole in the English language as identified in the current literature research (outside “senile macular degeneration, but more related to the current understanding of “serpiginous choroiditis”). Previously, other names had been introduced for the disease or similar variants, such as “Chorioditis areata” (Kristijan Sveinsson 1939, referring to a discussion with Henning Rønne – today considered different from serpiginous choroidopathy –, “Chorioretinitis striata” (Georg Kraffel 1955) and “Helicoid peripapillar chorioretinal degeneration” (Adolphe Franceschetti 1962) – referring to an airplane propeller (helix)87-90. It is noteworthy that sharply-demarcated borders of atrophy had been already noted by these earlier publications. Particularly the publication by Kraffel (1955) which was written in German used the expression “map-like choroidal atrophy” [“landkartenartige Chorioidalatrophie”]. However, he did not use the term “geographic” or the German adjective “geographisch”.
Figure 4.
“Serpiginous or geographic choroiditis – a mild disease of the choriocapillaris and pigment epithelium”, published in the textbook “Essentials in Uveitis” by Schlaegel in 1969 [Fig. 5-9, p.101. Copyright with friendly permission by Elsevier]86.
Lea Hyvarinen stated in 2016 (personal communication) that the name “geographic choroiditis” was related to an angiogram where the chroroiditis area looked like a peninsula. She could not remember exactly who had first seen that angiographic finding as geographic. Most probably, this had been during the weekly fluorescein angiographic conferences, after Dick Green’s pathology meeting and before the Grand Rounds held at the Wilmer Eye Institute, Johns Hopkins University, Baltimore, USA. She also mentioned that those had been the early days of fluorescein angiography when “we learned something new every single day”.
IV. Summary and outlook
The adjective “geographic” in combination with “choroiditis” was initially used for the disease manifestation of what is now commonly called “serpigionous choroiditis” and probably first mentioned by Maumenee and Hyvarinen (1968). Before, “landkartenartige Chorioidalatrophie” [“map-like choroidal atrophy”] was described by Kraffel (1955) in “Chorioretinitis striata” and “geographic map ulcer” (1956) was applied – beyond macular diseases – to describe a variation of the dendritic figure seen in herpetic keratitis. Gass used the terminology “geographic areas of atrophy of the pigment epithelium” in 1970 for the first time with regard to “senile macular degeneration” to describe that areas of atrophy were “sharply circumscribed” as seen by funduscopy. Neither Gass nor any other author explained the reason why they chose this terminology. They also did not refer to corneal or uveitis posterior eye diseases in which the same terminology had been applied earlier. It is conceivable that ophthalmologists used the adjective “geographic” since the atrophic areas resemble the map of a continent or sharply-demarcated country borders (as on thematic maps in geography) and not because of any associations to a specific disease or etiology. Similar terminology outside Ophthalmology had been introduced in the mid-1900 for description of the condition of “geographical tongue”. This is actually beyond the exact definition of “geography” which is a detailed description of the surface features of a specific region, also indicating its relative position and not implying that the borders must be sharply demarcated nor stating anything about the orientation of lesions in relation to other anatomical structures. Within one decade, the terminology evolved from a pure description of the disease manifestation as “geographic areas of atrophy” to naming the disease itself “geographic atrophy”, also abandoning the adjunct “of the retinal pigment epithelium”. In contrast to previous nomenclatures such as “macular heredodegeneration”, “disciform lesions” and “areolar atrophy”, the term “geographic atrophy” emphasizes the importance of the morphological features of the disease. It moved away from the long-standing debate of a possible genetic factor, clearly describing atrophic “senile macular degeneration” as being different from exudative manifestations and finally appreciating the characteristic appearance of atrophic lesions and also their common multifocality. The emphasis on morphology may have also been largely driven by the advances in retinal imaging, including the introduction of fluorescein angiography, which stimulated discussions regarding distinct structural changes amongst ophthalmologists since the 1960s.
The term “geographic atrophy” is a concise term. From the 1980s onwards, it has been constantly used by more or less all key experts in the field. “Geographic atrophy” has been firmly established for the description of the end-stage of AMD – perhaps also because a better term was lacking. However, recent advances in retinal imaging, in the field of genetics and the therapeutic breakthrough with anti-VEGF therapy for neovascular AMD have clearly shown a number of limitations associated with this term. Even today ophthalmologists use the expression “geographic atrophy” with various meanings. The term does not clearly imply that the atrophy is attributable to AMD or another etiology, or if active or regressed exudation is additionally present. Further, it gives no insights as to which anatomical retinal layers are affected or any information to the extent of their atrophy. The term “geographic atrophy” does not indicate with which diagnostic tools the lesions have been detected, e.g. clinical examination or specific imaging modalities such as fundus autofluorescence or spectral-domain optical coherence tomography. Moreover, it does not give an exact definition with respect to the minimal diameter or lesion area of individual atrophic spots, nor does the term clearly define the single largest spot and/or of the total size of atrophy within one eye. Finally, lesions with loss of the retinal pigment epithelium and photoreceptors but without an absolutely sharp demarcation can also be observed. Like the term “age-related macular degeneration”, the term “geographic atrophy” without further description appears to be currently used all too frequently rather than to further classify the degeneration in question. The need for a more rational approach to the nomenclature and classification of atrophic AMD is clearly evident not only for research and the development of new therapeutic strategies but also for patient management in routine clinical patient care.
Lewis Carroll, Through the Looking Glass
“When I use a word,’ Humpty Dumpty said in rather a scornful tone, ‘it means just what I choose it to mean — neither more nor less.’
’The question is,’ said Alice, ‘whether you can make words mean so many different things.’
’The question is,’ said Humpty Dumpty, ‘which is to be master — that’s all.”
Supplementary Material
Figure 5.
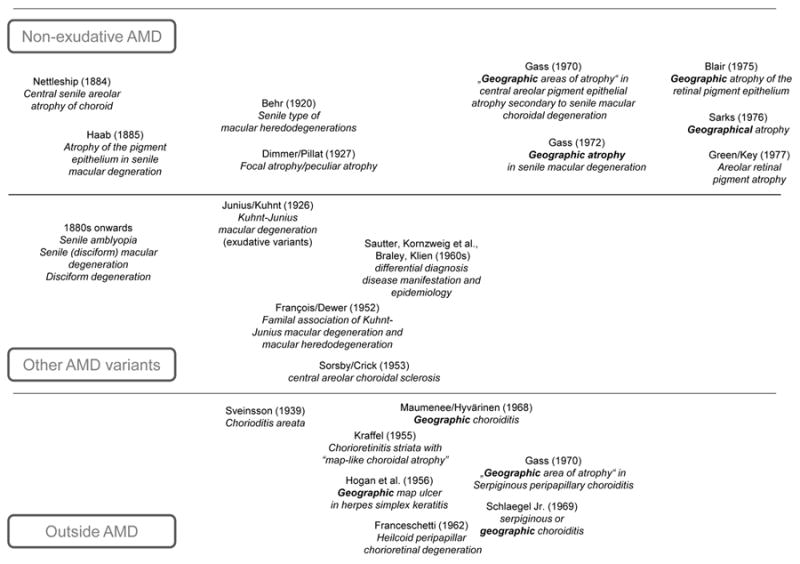
Timeline of the development of the term “geographic atrophy” in the ophthalmological literature
Acknowledgments
The authors thank Alan C. Bird for critically reading the manuscript and for excellent discussion. In addition, the authors are grateful for critical review by Frauke Kersten and Suzan Hunt.
Financial support: None
Footnotes
None of the authors has any proprietary interest in the content of the manuscript.
References
- 1.Klein R, Chou CF, Klein BE, et al. Prevalence of age-related macular degeneration in the US population. Arch Ophthalmol. 2011;129:75–80. doi: 10.1001/archophthalmol.2010.318. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarthy U, Augood C, Bentham GC, et al. Cigarette Smoking and Age-Related Macular Degeneration in the EUREYE Study. Ophthalmology. 2007;114:1164–1169. doi: 10.1016/j.ophtha.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Tomany SC, Wang JJ, Van Leeuwen R, et al. Risk factors for incident age-related macular degeneration: pooled findings from 3 continents. Ophthalmology. 2004;111:1280–1287. doi: 10.1016/j.ophtha.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Holz FG, Strauss EC, Schmitz-Valckenberg S, et al. Geographic Atrophy: Clinical Features and Potential Therapeutic Approaches. Ophthalmology. 2014;121:1079–1091. doi: 10.1016/j.ophtha.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Bhisitkul RB, Mendes TS, Rofagha S, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA and HORIZON studies (SEVEN-UP Study) Am J Ophthalmol. 2015;159:915–924. doi: 10.1016/j.ajo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 6.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 7.Duisdieker V, Fleckenstein M, Zilkens KM, et al. Long-Term Follow-Up of Fundus Autofluorescence Imaging Using Wide-Field Scanning Laser Ophthalmoscopy. Ophthalmologica. 2015;234:218–226. doi: 10.1159/000439358. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz-Valckenberg S, Fleckenstein M, Gobel AP, et al. Optical coherence tomography and autofluorescence findings in areas with geographic atrophy due to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:1–6. doi: 10.1167/iovs.10-5619. [DOI] [PubMed] [Google Scholar]
- 9.Sarks SH. Ageing and degeneration in the macular region: a clinico-pathological study. Br J Ophthalmol. 1976;60:324–341. doi: 10.1136/bjo.60.5.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunness JS, Gonzalez-Baron J, Applegate CA, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999;106:1768–1779. doi: 10.1016/S0161-6420(99)90340-8. [DOI] [PubMed] [Google Scholar]
- 11.Maguire P, Vine AK. Geographic atrophy of the retinal pigment epithelium. Am J Ophthalmol. 1986;102:621–625. doi: 10.1016/0002-9394(86)90535-0. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz-Valckenberg S, Fleckenstein M, Helb HM, et al. In-vivo imaging of foveal sparing in geographic atrophy secondary to age-related macular degeneration. Invest Ophthalmol Vis Sci. 2009;50:3915–3921. doi: 10.1167/iovs.08-2484. [DOI] [PubMed] [Google Scholar]
- 13.Dorland’s Illustrated Medical Dictionary. Dorland’s Medical Dictionary. Philadelphia: Elsevier Saunders; 2011. [Google Scholar]
- 14.Mosby’s Dictionary of Medicine, Nursing & Health Professions. Missouri: Elsevier/Mosby; 2013. [Google Scholar]
- 15.Segen J. Concise Dictionary of Modern Medicine. Enfield, United Kingdom: McGraw- Hill; 2006. [Google Scholar]
- 16.Smailhodzic D, Fleckenstein M, Theelen T, et al. Central areolar choroidal dystrophy (CACD) and age-related macular degeneration (AMD): differentiating characteristics in multimodal imaging. Invest Ophthalmol Vis Sci. 2011;52:8908–8918. doi: 10.1167/iovs.11-7926. [DOI] [PubMed] [Google Scholar]
- 17.Age-related macular degeneration. The Macular Photocoagulation Study. Am J Ophthalmol. 1984;98:376–377. doi: 10.1016/0002-9394(84)90332-5. [DOI] [PubMed] [Google Scholar]
- 18.Laser therapy for age-related macular degeneration. Med Lett Drugs Ther. 1984;26:69–70. [PubMed] [Google Scholar]
- 19.Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102:1640–1642. doi: 10.1001/archopht.1984.01040031330019. [DOI] [PubMed] [Google Scholar]
- 20.Junius P, Kuhnt H. Die scheibenförmige Entartung der Netzhautmitte (Degeneratio Maculae Luteae Disciformis) Berlin: S Karger; 1926. [Google Scholar]
- 21.Ryan SJ, Mittl RN, Maumenee AE. The disciform response: an historical perspective. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215:1–20. doi: 10.1007/BF00413392. [DOI] [PubMed] [Google Scholar]
- 22.Haab O. Erkrankungen der Macula Lutea. Centralblat Augenheilkd. 1885;9:384–391. [Google Scholar]
- 23.Falls HF. Genetics and Management - senile macular degeneration. In: Kimura SJ, Caygill WM, editors. Retinal diseases symposium on differential diagnostic problems of posterior uveitis. Philadelphia: Lea & Febiger; 1966. pp. 227–228. [Google Scholar]
- 24.Nettleship E. Central senile areolar choroidal atrophy. Trans Ophthalmol Soc U K. 1884;4:165–167. [Google Scholar]
- 25.Schieck F. Die Erkrankungen der Netzhaut. In: Schieck F, Brückner A, editors. Kurzes Handbuch der Ophthalmologie. Berlin: Julius Springer; 1930. pp. 563–583. [Google Scholar]
- 26.Fuchs AE. Die Erkrankungen des Augehintergrundes. Wien: Franz Deuticke; 1943. Zentrale Retinaerkrankungen. 4. Senile Makuladegeneration; pp. 112–114. [Google Scholar]
- 27.Behr C. Die Heredodegeneration der Makula. Monatsblätter für Augenheilkunde. 1920;65:465–505. [Google Scholar]
- 28.Wessing A. Fluorescein angiography of the retina - textbook and atlas. Saint Louis: The C. V. Mosby Company; 1969. [Google Scholar]
- 29.Harms C. Anatomisches über die senile Makulaaffektion. Monatsblätter für Augenheilkunde. 1904;42(1):448–461. [Google Scholar]
- 30.Francois J, Deweer JP. Hereditary senile degenerescence of the macula. Ann Ocul (Paris) 1952;185:136–154. [PubMed] [Google Scholar]
- 31.Steinbach PD. Involvement of the retinal capillaries in senile macular degeneration (fluorescein-angiographic study) Klinische Monatsblatter fur Augenheilkunde. 1970;156:710–715. [PubMed] [Google Scholar]
- 32.Green WR, Key SN., 3rd Senile macular degeneration: a histopathologic study. Trans Am Ophthalmol Soc. 1977;75:180–254. [PMC free article] [PubMed] [Google Scholar]
- 33.Gass JD. Drusen and disciform macular detachment and degeneration. Trans Am Ophthalmol Soc. 1972;70:409–436. [PMC free article] [PubMed] [Google Scholar]
- 34.Duke-Elder S, Perkins ES. Diseases of the uveal tract. In: Duke-Elder S, editor. System of Ophthalmology. London: Henry Kimpton; 1966. pp. 603–629. [Google Scholar]
- 35.Duke-Elder S. Diseases of the Retina. In: Duke-Elder S, editor. System of Ophthalmology. London: Henry Kimpton, Dobree, J H; pp. 638–641. [Google Scholar]
- 36.Sautter H. Korrelation zwischen Augenbefund und allgemeiner Pathologie bei der Arteriosklerose. Ber dtsch ophth Ges. 1959;63:3–10. [Google Scholar]
- 37.Kornzweig AL, Feldstein M, Schneider J. The pathogenesis of senile macular degeneration. Am J Ophthalmol. 1959;48:22–28. doi: 10.1016/0002-9394(59)90238-7. [DOI] [PubMed] [Google Scholar]
- 38.Braley AE. Dystrophy of the macula. Am J Ophthalmol. 1966;61:1–24. doi: 10.1016/0002-9394(66)90741-0. [DOI] [PubMed] [Google Scholar]
- 39.Kimura SJ, Caygill WM. Retinal diseases symposium on differential diagnostic problems of posterior uveitis. Philadelphia: Lea & Febiger; 1966. [Google Scholar]
- 40.Klien BA. Some aspects of classification and differential diagnosis of senile macular degeneration. Am J Ophthalmol. 1964;58:927–939. doi: 10.1016/0002-9394(64)90001-7. [DOI] [PubMed] [Google Scholar]
- 41.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium III. Am J Ophthalmol. 1967;63:617. [PubMed] [Google Scholar]
- 42.Gass JD. Stereoscopic atlas of macular diseases. St Louis: The C V Mosby Company; 1970. [Google Scholar]
- 43.Sorsby A, Crick RP. Central areolar choroidal sclerosis. Br J Ophthalmol. 1953;37:129–139. doi: 10.1136/bjo.37.3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klien BA. The heredodegeneration of the macula lutea - diagnostic and differential diagnostic considerations and a histopathologic report. Am J Ophthalmol. 1950;33:371–379. [Google Scholar]
- 45.Gass JD. Drusen and disciform macular detachment and degeneration. Arch Ophthalmol. 1973;90:206–217. doi: 10.1001/archopht.1973.01000050208006. [DOI] [PubMed] [Google Scholar]
- 46.Hollwich F. Erkrankungsformen der Netzhautmitte. In: Velhagen K, editor. Der Augenarzt. Leipzig: Verlag für Kunst und Wissenschaft; 1963. pp. 683–700. [Google Scholar]
- 47.Gass JD. Stereoscopic atlas of macular diseases. St Louis: The C V Mosby Company; 1977. [Google Scholar]
- 48.Gass JD. Stereoscopic atlas of macular diseases. St Louis Washington, D.C., Toronto: The C V Mosby Company; 1987. [Google Scholar]
- 49.Blair CJ. Geographic atrophy of the retinal pigment epithelium. A manifestation of senile macular degeneration. Arch Ophthalmol. 1975;93:19–25. doi: 10.1001/archopht.1975.01010020023003. [DOI] [PubMed] [Google Scholar]
- 50.Sarks SH. Drusen patterns predisposing to geographic atrophy of the retinal pigment epithelium. Aust J Ophthalmol. 1982;10:91–97. doi: 10.1111/j.1442-9071.1982.tb00366.x. [DOI] [PubMed] [Google Scholar]
- 51.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 52.Hughes AE, Lotery AJ, Silvestri G. Fine localisation of the gene for central areolar choroidal dystrophy on chromosome 17p. Journal of medical genetics. 1998;35:770–772. doi: 10.1136/jmg.35.9.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saksens NT, Fleckenstein M, Schmitz-Valckenberg S, et al. Macular dystrophies mimicking age-related macular degeneration. Prog Retin Eye Res. 2014;39:23–57. doi: 10.1016/j.preteyeres.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Sunness JS, Bressler NM, Maguire MG. Scanning laser ophthalmoscopic analysis of the pattern of visual loss in age-related geographic atrophy of the macula. Am J Ophthalmol. 1995;119:143–151. doi: 10.1016/s0002-9394(14)73866-8. [DOI] [PubMed] [Google Scholar]
- 55.Sunness JS. The natural history of geographic atrophy, the advanced atrophic form of age-related macular degeneration. Mol Vis. 1999;5:25. [PubMed] [Google Scholar]
- 56.Macular Photocoagulation Study: Manual of Procedures. Baltimore, Maryland: 1986. pp. 14–18. [Google Scholar]
- 57.Klein R, Davis MD, Magli YL, et al. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 58.AREDS. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study Report Number 6. Am J Ophthalmol. 2001;132:668–681. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 59.Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol. 2009;127:1168–1174. doi: 10.1001/archophthalmol.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein R, Meuer SM, Knudtson MD, et al. The Epidemiology of Progression of Pure Geographic Atrophy: The Beaver Dam Eye Study. Am J Ophthalmol. 2008;146:692–699. doi: 10.1016/j.ajo.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Danis RP, Domalpally A, Chew EY, et al. Methods and reproducibility of grading optimized digital color fundus photographs in the Age-Related Eye Disease Study 2 (AREDS2 Report Number 2) Invest Ophthalmol Vis Sci. 2013;54:4548–4554. doi: 10.1167/iovs.13-11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sunness JS, Bressler NM, Tian Y, et al. Measuring geographic atrophy in advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1761–1769. [PubMed] [Google Scholar]
- 63.von Ruckmann A, Fitzke FW, Bird AC. Distribution of fundus autofluorescence with a scanning laser ophthalmoscope. Br J Ophthalmol. 1995;79:407–412. doi: 10.1136/bjo.79.5.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holz FG, Bellman C, Staudt S, et al. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2001;42:1051–1056. [PubMed] [Google Scholar]
- 65.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- 66.Schmitz-Valckenberg S, Jorzik J, Unnebrink K, et al. Analysis of digital scanning laser ophthalmoscopy fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2002;240:73–78. doi: 10.1007/s00417-001-0413-3. [DOI] [PubMed] [Google Scholar]
- 67.Holz FG, Bindewald-Wittich A, Fleckenstein M, et al. Progression of geographic atrophy and impact of fundus autofluorescence patterns in age-related macular degeneration. Am J Ophthalmol. 2007;143:463–472. doi: 10.1016/j.ajo.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 68.Fleckenstein M, Schmitz-Valckenberg S, Adrion C, et al. Progression of age-related geographic atrophy: role of the fellow eye. Invest Ophthalmol Vis Sci. 2011;52:6552–6557. doi: 10.1167/iovs.11-7298. [DOI] [PubMed] [Google Scholar]
- 69.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. Natural History of Geographic Atrophy Progression Secondary to Age-Related Macular Degeneration (Geographic Atrophy Progression Study) Ophthalmology. 2016;123:361–368. doi: 10.1016/j.ophtha.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 70.Jaffe GJ, Schmitz-Valckenberg S, Boyer D, et al. Randomized Trial to Evaluate Tandospirone in Geographic Atrophy Secondary to Age-Related Macular Degeneration: The GATE Study. Am J Ophthalmol. 2015;160:1226–1234. doi: 10.1016/j.ajo.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 71.Mata NL, Lichter JB, Vogel R, et al. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina. 2013;33:498–507. doi: 10.1097/IAE.0b013e318265801d. [DOI] [PubMed] [Google Scholar]
- 72.Yehoshua Z, de Amorim Garcia Filho C Alexandre, Nunes RP, et al. Systemic Complement Inhibition with Eculizumab for Geographic Atrophy in Age-Related Macular Degeneration: The COMPLETE Study. Ophthalmology. 2014;121:693–701. doi: 10.1016/j.ophtha.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deckert A, Schmitz-Valckenberg S, Jorzik J, et al. Automated analysis of digital fundus autofluorescence images of geographic atrophy in advanced age-related macular degeneration using confocal scanning laser ophthalmoscopy (cSLO) BMC Ophthalmol. 2005;5:8. doi: 10.1186/1471-2415-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yehoshua Z, de Amorim Garcia Filho CA, Nunes RP, et al. Comparison of Geographic Atrophy Growth Rates Using Different Imaging Modalities in the COMPLETE Study. Ophthalmic Surg Lasers Imaging Retina. 2015;46:413–422. doi: 10.3928/23258160-20150422-03. [DOI] [PubMed] [Google Scholar]
- 75.Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52:7640–7646. doi: 10.1167/iovs.11-7457. [DOI] [PubMed] [Google Scholar]
- 76.Grunwald JE, Pistilli M, Ying GS, et al. Growth of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2015;122:809–816. doi: 10.1016/j.ophtha.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klausner E. Über Lingua geographica hereditaria. Archiv für Dermatologie und Syphilis. 1910;103:103–122. [Google Scholar]
- 78.Varoni E, Decani S. IMAGES IN CLINICAL MEDICINE. Geographic Tongue. The New England journal of medicine. 2016;374:670. doi: 10.1056/NEJMicm1502932. [DOI] [PubMed] [Google Scholar]
- 79.Santlus Zur Lehre von der Zungenhäutung (Zungenfratt) Journal für Kinderkrankheiten. 1854;XXIII:161–167. [Google Scholar]
- 80.Prinz H. Wandering rash of the tongue. The Dental Cosmos. 1927;Ixix:272–275. [Google Scholar]
- 81.Holland EJ, Brilakis HS, Schwartz GS. Herpex Simplex Keratitis. In: Krachmer JH, Mannis MJ, Holland EJ, editors. Cornea. Philadephia Edinburgh London New York Oxford St Louis Sydney Toronto: Elsvier Mosby; 2005. pp. 1050–1051. [Google Scholar]
- 82.Rezende RA, Cohen EJ, Uchoandro CU, et al. Congenital Corneal Opacities. In: Krachmer JH, Mannis MJ, Holland EJ, et al., editors. Cornea. Philadephia Edinburgh London New York Oxford St Louis Sydney Toronto: Elsvier Mosby; 2005. p. 330. [Google Scholar]
- 83.Grayson M, Keates RH. Manual of Diseases of the Cornea. Boston: Little, Brown and Company; 1969. [Google Scholar]
- 84.Hogan MJ, Kimura SJ, Thygeson P. Observations on herpetic keratitis and keratoconjunctivitis. AMA Arch Ophthalmol. 1956;56:375–388. doi: 10.1001/archopht.1956.00930040383006. [DOI] [PubMed] [Google Scholar]
- 85.Baarsma GS, Deutman AF. Serpiginous (geographic) choroiditis. Doc Ophthalmol. 1976;40:269–285. doi: 10.1007/BF00155041. [DOI] [PubMed] [Google Scholar]
- 86.Schlaegel TF. Essentials in Uveitis. London: J & A Churchill Ltd; 1969. [Google Scholar]
- 87.Jonasson F, Sander B, Eysteinsson T, et al. Sveinsson chorioretinal atrophy: the mildest changes are located in the photoreceptor outer segment/retinal pigment epithelium junction. Acta Ophthalmol Scand. 2007;85:862–867. doi: 10.1111/j.1600-0420.2007.01002.x. [DOI] [PubMed] [Google Scholar]
- 88.Sveinsson K. Chrioiditis areata. Acta Ophthalmol. 1939;17:73–80. [Google Scholar]
- 89.Kraffel G. Contribution on chorioretinitis striata. Klinische Monatsblatter fur Augenheilkunde und fur augenarztliche Fortbildung. 1955;127:664–669. [PubMed] [Google Scholar]
- 90.Franceschetti A. A curious affection of the fundus oculi: helicoid peripapillar chorioretinal degeneration. Its relation to pigmentary paravenous chorioretinal degeneration. Doc Ophthalmol. 1962;16:81–110. doi: 10.1007/BF00146721. [DOI] [PubMed] [Google Scholar]
- 91.Dimmer F, Pillat A. Atlas photographischer Bilder des menschlichen Augenhintergrundes. Leipzig Wien: Franz Deuticke; 1927. Tafel 75. Abb.72. [Google Scholar]
- 92.Marchesani O, Sautter H. Atlas des Augenhintergrundes. München Berlin: Urban & Schwarzenberg; 1957. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.