Abstract
Traditional medicinal literature and previous studies have reported the possible role of Cissus 43 quadrangularis (CQ) as an anti-osteoporotic agent. This study examines the effectiveness of CQ in 44 promoting osteoblast differentiation of the murine pre-osteoblast cell line, MC3T3-E1. Ethanolic 45 extract of CQ (CQ-E) was found to affect growth kinetics of MC3T3-E1 cells in a dosage dependent 46 manner. High concentrations of CQ-E (more than 10 µg / ml) have particularly adverse effects, while 47 lower concentrations of 0.1 and 1 µg / ml were non-toxic and did not affect cell viability. Notably, cell 48 proliferation was significantly increased at the lower concentrations of CQ-E. CQ-E treatment also 49 augmented osteoblast differentiation, as reflected by a substantial increase in expression of the early 50 osteoblast marker ALP activity, and at later stage, by mineralization of extracellular matrix compared 51 to the control group. These findings suggest dose-dependent effect of CQ-E with lower concentrations 52 exhibiting anabolic and osteogenic properties.
Keywords: Cissus quadrangularis, osteoblast differentiation, matrix mineralization, growth kinetics, MC3T3-E1
Introduction
Osteoporosis is a systemic skeletal disease that is characterized by low bone mass, microarchitectural deterioration of bone tissue and an increased risk of fracture (Lorentzon and Cummings, 2015; Raisz, 2005). Approximately 200 million people worldwide suffer from this condition (Kanis, 2007; IOF, 2014). Osteoporosis and associated fractures are a major public health concern because of related morbidity and mortality (Cummings and Melton, 2002). Because loss of estrogen decreases bone mineral density, osteoporosis is particularly prevalent in post-menopausal women (IOF, 2014).
Currently synthetic therapeutic agents like bisphosphonates, parathyroid hormone, hormone replacement therapy (HRT) and selective estrogen receptor modulators (SERMs) are used to treat osteoporosis with some success. However, these drugs can have deleterious side effects in post-menopausal women, including hypercalcemia, osteonecrosis of the jaw (Hamadeh et al., 2015), increased risk of osteosarcoma (Vahle et al., 2002; Nanes and Kallen, 2009), esophageal/gastric irritation and cancer (Abrahamsen, 2010; Pazianas and Abrahamsen, 2011), increased risk of breast cancer, stroke, coronary heart disease and venous thromboembolism in post-menopausal women (Rossouw et al., 2002).
The pharmaceutical industry is currently scrutinizing flora in its search for alternate medicines, and scientists are investigating for new molecules that have therapeutic potential (Parisuthiman et al., 2009). It is estimated that approximately 25% of contemporary medicines are derived, directly or indirectly, from plants (Robinson and Zhang, 2011). Plant based medicine has experienced a revival in recent times. Cissus quadrangularis (CQ) (syn. Vitis quadrangularis), a rambling shrub belonging to the family Vitaceae (Parisuthiman et al., 2009), is commonly known as Bone Setter, Veldt Grape or Winged Treebine. This plant is also referred to as “Hadjora” in hindi and “asthisanhari” in sanskrit based on its ability to join bones (Sivarajan and Balachandran, 1994). Indigenous to the hot, dry regions, CQ is found in South Asia, Africa, Arabian Peninsula, Malaysia and Thailand (Williamson, 2002; Jainu et al., 2006; Parisuthiman et al., 2009). This herb is enriched in phytosterols, anabolic steroidal substances (Adesanya et al., 1999; Chidambara et al., 2003), ascorbic acid, triterpenoids (Bhutani et al., 1984; Gupta and Verma, 1990), vitamin E (Chidambaram and Anuradha, 2010), β-carotene (Pluemaj and Saifah, 1986) and calcium (Jainu et al., 2006; Jainu and Mohan, 2008).
Various in vivo studies have described the use of CQ as an anti-osteoporotic agent (Shirwaikar et al., 2003; Potu et al., 2009b, 2010, 2011) and for the treatment of bone fractures in animals (Prasad and Udupa, 1972; Chopra et al., 1976; Deka et al., 1994). It has been reported that CQ can stimulate ossification during intra-uterine development (Rao et al., 2007; Potu et al. 2008). Recent human trials involving 12 subjects who were fed this herb for treating osteoporosis have shown promising results for osteoporotic symptoms compared to placebo control group (Gupta et al., 2012). Several in vitro studies have shown that CQ possesses varying degrees of osteogenic capability (Parisuthiman et al., 2009; Potu et al., 2009a; Muthusami et al., 2011a,b). Kumar et al. (2010) have isolated 6´-O-trans-cinnamoyl-catalpol, a potent osteogenic stimulant, from CQ. Recently Pathomwichaiwat et al. (2015) have isolated 29 compounds including triterpenes, fatty acids methyl esters, glycerolipids, steroids, phytols and cerebrosides from hexane extract of Cissus quadrangularis and demonstrated synergistic effects of these compounds on bone formation.
In this study we rigorously examined the effect of ethanolic extract of Cissus quadrangularis (CQ-E) on the growth kinetics, proliferation, osteoblast differentiation and mineralization of the murine pre-osteoblast cell line, MC3T3-E1.
Materials and Methods
Maintenance of calvarial derived pre-osteoblast cell line, MC3T3-E1 subclone 4
All the chemicals were obtained from Sigma Aldrich (St. Louis, MO) and cell culture reagents were purchased from Gibco (Carlsbad, CA), unless otherwise stated. Murine cell line, MC3T3-E1 subclone 4 (ATCC® CRL-2593™), which is competent to produce mineralizing bone-like matrix was purchased from American Type Culture Collection. These cells were grown at 1 ×105 cells /100mm plate in growth medium [αMEM (HyClone, Logan, UT) without ascorbic acid, 10% FBS (HyClone, Logan, UT), 1% penicillin/streptomycin, and 2 mM glutamine] (Wang et al., 1999). Cells were subcultured when they reached 50–60% confluence, usually after 3–4 days after plating. All the experiments were done in triplicate with cells of passage 8–12, at 37° C, under 5% CO2 and in humidified conditions.
Preparation of ethanolic extract of Cissus quadrangularis (CQ-E)
Dried CQ was ground to a fine powder. The powdered herb (100 g) was added to 1 L absolute ethanol and kept at room temperature for 48 h. It was filtered and solvent ethanol - extractant was evaporated at 45 °C (Heidolph Rotacool) until complete dryness was achieved. With this powder of soluble CQ-E extract a stock solution of CQ-E 400 mg/ml (w/v) was prepared in dimethyl sulfoxide (DMSO) and was further diluted to 250, 150, 80, 40, 20 and 0.2 mg/ml for the preparation of media having final concentration of CQ-E as 200, 100, 50, 25, 10, 1 and 0.1 µg/ml, respectively. DMSO-only was used as a vehicle control. Final concentration of DMSO in cell culture medium in any experiment did not exceed 0.06%. Negative control cells were treated with 0.06% DMSO.
Cell Viability, metabolic activity and proliferation assays
Cell viability, active metabolism and proliferation are pre-requisites for osteogenesis. For cell viability assays, MC3T3-E1 cells were split and were cultured (2500 cells cm−2) in 12 well plates in growth medium for 24 h. Then fresh growth medium containing different concentrations (0.1, 1, 10, 25, 50, 100 and 200 µg/ml) of CQ-E were added. Medium was changed after 48 h with fresh CQ-E. Cells were washed and harvested using trypsin-EDTA 1×. Viable cells were counted on day 1, 3, 5 and 7 after treatment using hemacytometer and trypan blue exclusion assay. At least 95% cell viability was considered appropriate for healthy log-phase cultures.
The total number of cells was calculated in each well. Each treatment was performed in three wells in three independent experiments (total 9 wells). So average of number of cells from three independent replicates was taken. Results were plotted as a semi-log curve (log of total number of cells against day) to obtain growth curves.
To determine the metabolic activity of the cells, MC3T3-E1 cells (2500 cells cm−2) were grown in 24 well plates for 24 h and then treated with fresh growth medium containing the different concentrations of CQ-E used for cell viability assay or 0.06% DMSO-only (control). On days 1 and 3, cells were labeled with 0.12 mM MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; Invitrogen, Grand Island, NY; Cat No. M-6494] for 2 h at 37 °C. After labeling, medium was removed and 200 µl DMSO was added to each well. Plates were left at 37 °C for 10 min. Absorbance was read at 540 nm using Victor X4 multilabel plate reader (Perkin Elmer, Waltham, MA). The effect of CQ-E on metabolic activity of cells was expressed as percent metabolic activity compared with that of 0.06% DMSO-treated cultures (defined as 100%).
For cell proliferation assays, MC3T3-E1 cells were plated (1000 cells / well) in 96-well plates for 24 h and then treated with fresh growth medium containing the different concentrations of CQ-E used for cell viability assay or 0.06% DMSO-only (control). Effects of CQ-E extract on proliferation were assessed on days 1 and 3 using Cell Proliferation ELISA, BrdU kit (Roche, Mannheim, Germany) according to manufacturer’s instructions. Absorbance was read at 370 nm (sample) and 492 nm (reference wavelength, used as background control) using Victor ×4 multilabel plate reader. Cell proliferation of CQ-E of treated cultures was calculated in comparison to the cultures treated with 0.06% DMSO, defined as 100%.
Osteogenic differentiation
MC3T3-E1 cells were plated at 3,000 cells / cm2 in 6-well plates. At confluence, cells were fed with osteogenic (mineralizing) medium consisting of α-MEM without ascorbic acid, 10% FBS, 1% penicillin/streptomycin, 2 mM glutamine and 25 µg/ml ascorbic acid. For the second feeding, the same medium was used except that it contained 50 µg/ml ascorbic acid. For all later feedings, medium supplemented with 50 µg/ml\ of ascorbic acid and 5 mM β-glycerophosphate was added to promote the mineralization of the matrix. CQ-E extract was added to osteogenic medium as indicated for each experiment. The same amount of DMSO-only (0.06%) was added to cultures in osteogenic medium and indicated as CQ-E 0 concentration. During osteogenic differentiation, culture medium was changed every 48 h over the course of the experiment. Plates were incubated at 37 °C under 5% CO2 and humidified conditions for 3 weeks, collecting samples at 1, 2 and 3 weeks representing stages of osteoblast differentiation. This differentiation study was repeated three times.
Alkaline phosphatase (ALP) staining
Cells were washed three times with cold 1× PBS and fixed with 10% neutral buffered formalin for 15 min. Fixative was removed and the cells were washed with distilled water. Tris buffer (200 mM, pH 9.2) was added to the fixed cells for 15 min. Freshly prepared filtered ALP substrate (130 mg/ml of naphthol AS MX-PO4 dissolved in N, N-dimethylformamide was mixed with 0.1% Fast Red salt solution in 0.1 M Tris maleate buffer, (pH 8.3) was added to the cells at room temperature for 45 min. ALP substrate was removed and plates were rinsed three times with distilled water. Reddish pink color developed at areas that were rich in ALP activity.
Staining for mineralization-calcium deposition
Cells were washed three times with cold 1× PBS and were fixed with ice-cold 50% ethanol for 20 min. Fixative was removed, cells were washed three times with distilled water and were air dried. Cells were stained with 1% alizarin red stain (pH 4.0) at room temperature for 30 min. Stain was aspirated off and plates were extensively rinsed with distilled water to remove excess dye. Areas of calcium deposition in matrix manifest as bright red colored nodules that represent multilayered cells.
Von Kossa Staining for mineralization-phosphate deposition
Following ALP-staining, wells were washed with distilled water and silver nitrate (5%) solution was added, which detects phosphate in hydroxyapatite mineral deposits (black nodules). The plates were kept in bright light for 60 min, the solution was removed and the cell layer was washed three times with distilled water.
Quantification of osteoblast-related gene expression
Total RNA was isolated using TRIzol reagent (Invitrogen, Grand Island, NY) following the manufacturer’s instructions. RNA (5 µg) was treated with DNase I and then purified using the DNA-free RNA Kit™ (Zymo Research, Irvine, CA). The purified RNA (1 µg) was reverse transcribed to synthesize cDNA for RT-PCR using the SuperScript ™ First-strand synthesis system (Cat. No. 11904-018; Invitrogen, Grand Island, NY).
Quantitative PCR reaction was carried out using 2× iTaq™ SYBR® Green Supermix with ROX (Cat. No. 172-5850; BIO-RAD, Hercules, CA), 0.4 ng/µl of cDNA and 400 nM each of forward and reverse primers (see details of primers in Table 1). Amplification and quantification of the amplicon used a two-step cycling protocol (initial denaturation at 95 °C for 3 minutes, followed by 40 cycles each of denaturation at 95 °C for 15 seconds and annealing and extension at 60 °C for 1 min) on ViiA7 (Applied Biosystems, Foster City, CA). Uniformity of the PCR product was determined by dissociation curves performed at 60–90°C with every 0.5°C increments.
Table 1.
Details of primers for quantification of RT-PCR detection
| Primer ID |
Gene | Primer Sequence (5′→3′) | Reference |
|---|---|---|---|
| Bglap-2-F | Osteocalcin | CTGACAAAGCCTTCATGTCCAA | Lengner et al., 2004 |
| Bglap-2-R | GCGCCGGAGTCTGTTCACTA | ||
| Col 1α1-F | Procollagen, type IA1 | GCTCCTCTTAGGGGCCACT | MGH Primerbank |
| Col 1α1-R | CCACGTCTCACCATTGGGG | ||
| HPRT1-F | Hypoxanthine-guanine phosphoribosyltransfer ase |
CAGGCCAGACTTTGTTGGAT | Mitra et al., 2009 |
| HPRT1-R | TTGCGCTCATCTTAGGCTTT | ||
| Ibsp-F | Bone sialoprotein | CAGGGAGGCAGTGACTCTTC | MGH Primerbank |
| Ibsp-R | AGTGTGGAAAGTGTGGCGTT | ||
| Runx2-F | Runt related transcription factor 2 |
CGGCCCTCCCTGAACTCT | Lengner et al., 2005 |
| Runx2-R | TGCCTGCCTGGGATCTGTA |
For quantification of target gene transcripts (BGLAP2, COL1A1, IBSP and RUNX2) Ct values were normalized using values for the housekeeping gene hypoxanthine-guanine phosphoribosyl transferase (HPRT1) in the same experimental conditions. The relative change in the expression (n-fold) of target genes was calculated as the ratio of relative quantity of target gene transcript under given experimental condition (normalized to HPRT1 in given experimental conditions) to the relative quantity of target gene transcript under control conditions (normalized to HPRT1 in control conditions).
Statistical analysis
For each experiment, three independent experimental sets were conducted. Quantitative data were represented as mean ± SD. Statistical analyses of the data, where required, were conducted by one way analysis of variance (ANOVA) followed by Tukey multiple-comparison test using GraphPad InStat 3 software. The results were considered significant if p < 0.05.
Results
Effect of CQ-E on growth kinetics of MC3T3-E1
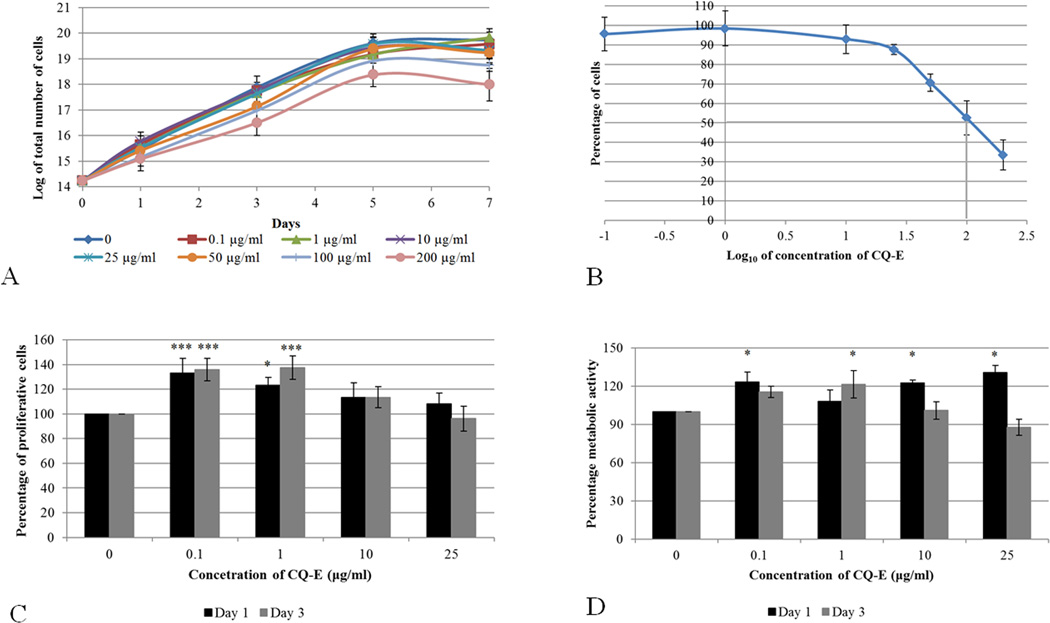
We first examined the growth of osteoblastic cells in the presence of CQ-E. The murine pre-osteoblast cell line, MC3T3-E1, was cultured in the presence of different concentrations of CQ-E (0, 0.1, 1, 10, 25, 50, 100 and 200 µg/ml). The cells were harvested on days 1, 3, 5 and 7, counted and growth curves were plotted (Fig. 1A). Cells that were treated with 100 and 200 µg/ml of CQ-E lagged behind in growth and did not reach the density of cells treated with 0.06% DMSO (vehicle control). At lower doses, the cells’ growth pattern was comparable to that of the vehicle control treated cultures.
Fig. 1.
Effect of different concentrations of CQ-E on growth curve (A), dose-response curve (B) proliferation (C) and metabolic activity (D) of MC3T3-E1.
For growth curve cells were trypsinized at each time point, viable cells were counted using trypan blue.
For dose response curve MC3T3-E1 cells were treated with CQ-E for three days (log phase culture). Cells were harvested and viable cells were counted using trypan blue. Percentage of viable cells in each treatment group was calculated with reference to the DMSO-only control. Grey vertical line presents the LC50.
Proliferations of cells were assessed by BrdU-ELISA assay on days 1 and 3. Percentage of proliferative cells in each treatment group was calculated as percentage of DMSO-only control.
Metabolic activity of cells were assessed by MTT assay on day 1 and 3. Metabolic activity of cells in each treatment group was presented as percentage of DMSO-only control.
Data has been presented as Mean ± SD: Student’s “t” test; *P< 0.05, **P< 0.01,***P< 0.001.
LC50, defined as the concentration of CQ-E at which MC3T3-E1 cells had 50% viability (LC50) with reference to control cells (0.06% DMSO), was determined from the cell count on day 3 (log phase) (Fig. 1C). It was found to be ~109 µg/ml (anti-log 2.04). At lower concentrations (≤ 50 µg/ml) of CQ-E, 85–100% of the cells survived the treatment.
These results, therefore, indicate that lower concentrations (≤ 50 µg/ml) of CQ-E did not have a significant effect on growth kinetics of pre-osteoblast cells, whereas higher concentrations (100 and 200 µg/ml) of CQ-E adversely affected the growth kinetics of MC3T3-E1 cells.
Effect of CQ-E on metabolism and proliferation of MC3T3-E1 cells
Because lower doses of CQ-E (≤ 50 µg/ml) resulted in more than 80% cell survival, CQ-E doses of 0.1, 1, 10 and 25 µg/ml were used to elucidate the metabolic activity and proliferation of cells using MTT and BrdU assays, respectively. The MTT assay determines the metabolic activity of cells based on the activity of the cellular NAD(P)H dependent-oxidoreductase enzyme that reduces the tetrazolium salt (MTT reagent) to formazan (coloured product), which is directly correlated with metabolic activity of the cell. The metabolic activity of MC3T3-E1 cells grown in the presence of CQ-E (except for 1 µg/ml of CQ-E) was significantly higher than that of the control (0.06% DMSO) after a day of treatment (Fig. 1D). A significant increase in metabolic activity was, however, observed in the cultures that were treated with 1 µg/ml of CQ-E for 3 days. All concentrations tested showed similar growth characteristics (Figs. 1A, 1B) compared to the control-treated cultures. No detrimental effect on metabolic activity of MC3T3-E1 cells was observed at these concentrations.
BrdU, a thymidine analogue, gets incorporated in the DNA during replication, and hence acts as an indicator of proliferation. The incorporated BrdU is then measured using an ELISA based assay A significant increase in incorporated BrdU was estimated in the MC3T3-E1 preosteoblast cells grown in the presence of 0.1 and 1 µg/ml of CQ-E compared to cells that were grown only in 0.06% DMSO only (Fig. 1E). This result indicates that the aforementioned CQ-E concentrations had a stimulatory effect on proliferation of MC3T3-E1 cells. However, similar growth curve and no significant decrease in doubling time was seen Fig. 1A–1B) as a result of increased proliferation at 0.1 and 1 µg/ml of CQ-E with reference to vehicle control.
CQ-E at doses, (0.1, 1, 10 and 25 µg/ml) compatible with good growth kinetics of MC3T3-E1 cells does not have an unfavorable effect on metabolism; however, only 0.1 and 1 µg/ml exhibited a mitogenic effect on the cells.
Effect of CQ-E on alkaline phosphatase activity and mineralization
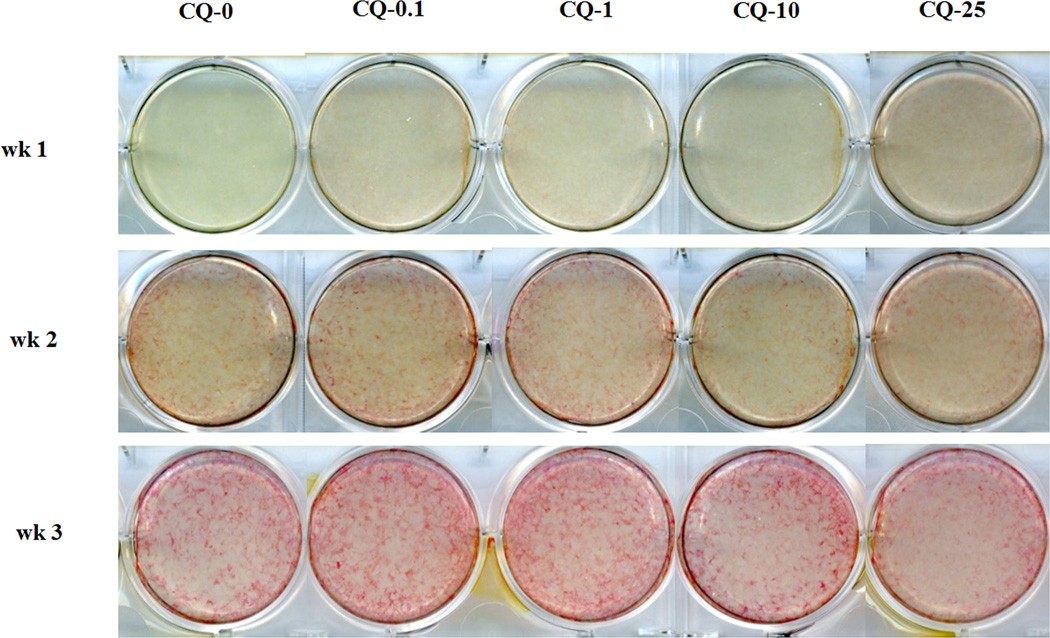
The unique bone extra-cellular matrix with competency for mineralization is the most characteristic feature of bone formation. Alkaline phosphatase (ALP) is involved in this process and its activity corresponds with the formation of hydroxyapatite. Based on viability, metabolic and proliferation results, MC3T3-E1 cells at confluence were treated without CQ-E (0) or with 0.1, 1, 10 and 25 µg/ml of CQ-E and cultured in mineralizing medium (see Materials & Methods) for 3 weeks (Fig. 2). At week 1, CQ-E compared to control with DMSO and no CQ-E (0), showed a slight enhancement of ALP starting at 0.1 and at 25 µg/ml, ALP was more robust than at lower doses. However, by the second week, only the 0.1 dose showed increased ALP over all other concentrations and the control. After 3 weeks, the high dose CQ-25 µg/ml had the least ALP which is consistent with its decreased cell number at this dose. In contrast the strongest ALP staining was observed in cultures treated with 0.1 and 1 µg/ml CQ-E, compared to positive control, 10 and 25 µg/ml CQ-E.
Fig. 2.
Effect of CQ-E treatment on ALP activity. Confluent cells were treated with CQ-E at the indicated concentrations in mineralizing medium (having ascorbic acid and β-glycerophosphate) for 3 weeks. ALP staining was done every week. Bright red areas/ spots indicate ALP positive cells. CQ-0, 0.1, 1, 10 and 25 represent concentration of CQ-E in µg/ml.
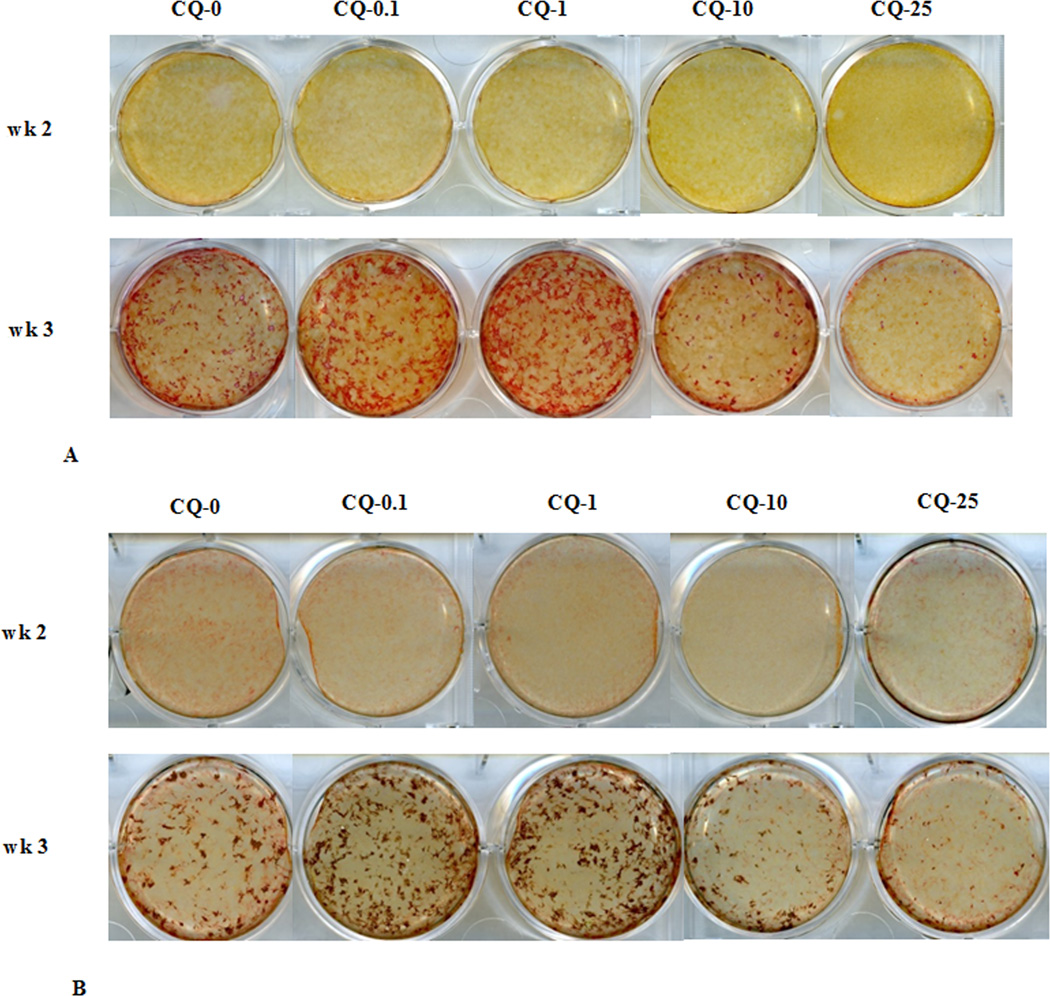
Alizarin red (Fig. 3A) and von Kossa (Fig. 3B) staining were carried out to elucidate the effect of CQ-E on mineralization of extracellular matrix. After 3 weeks, more pronounced mineralization was observed in the case of cultures treated with 0.1 or 1 µg/ml CQ-E compared to cultures treated with osteogenic medium alone (CQ-E not added, 0 dose). The high doses of 10 and 25 µg/ml exhibited a dose related inhibition of mineralization compared to the control group.
Fig. 3.
Effect of CQ-E treatment on mineralization: (A) Alizarin red staining (B) Von kossa staining. Confluent cells were treated with CQ-E at the indicated concentrations in mineralizing medium (having ascorbic acid and β-glycerophosphate) for 3 weeks. Mineralized areas appear as bright red spots (A) and brown-black spot (B) in alizarin red (A) and von kossa (B) staining, respectively. CQ-0, 0.1, 1, 10 and 25 represent concentration of CQ-E in µg/ml.
Together these findings from ALP staining and mineralization assays demonstrate a stimulatory effect of 0.1 and 1 µg/ml of CQ-E on pre-osteoblast differentiation.
Effect of CQ-E on expression of osteoblast related genes
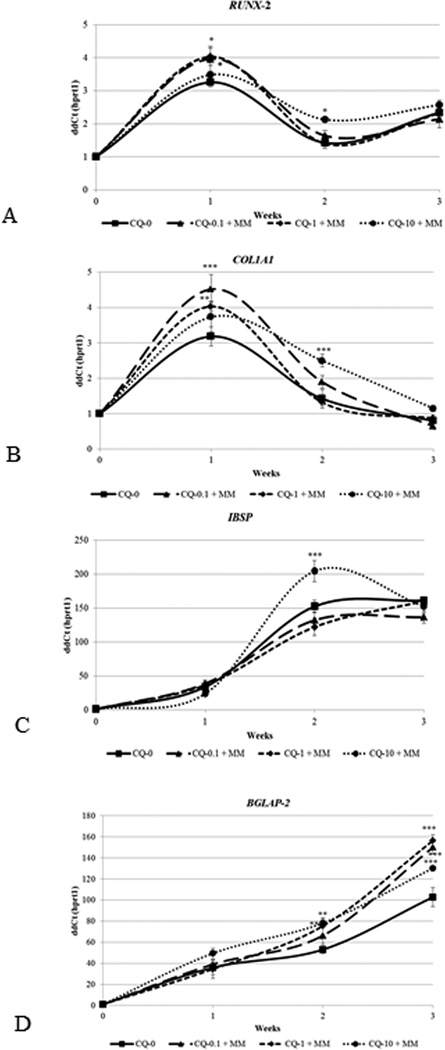
Any change in cellular activity during osteogenesis is accompanied by changes in expression of the genes involved in osteoblast differentiation. Relative expression of osteoblast related genes such as bone γ-carboxyglutamic acid-containing protein or osteocalcin (BGLAP2), Procollagen type 1A1 (COL1A1), integrin binding sialoprotein or bone sialoprotein (IBSP) and runt related transcription factor 2 (RUNX2) was determined to elucidate the effect of plant extract on osteoblast differentiation of mouse pre-osteoblasts (MC3T3-E1). Quantification of expression of osteoblast related genes was conducted using RNA isolated from cultures treated with no CQ-E or with 0.1, 1.0 or 10 µg/ml CQ-E to elucidate its effect on osteoblast differentiation at the molecular level (Fig. 4). Expression of early phase genes, Runx2 and collagen IAI (Fig. 4 A, B), was significantly enhanced after one week of exposure to 0.1 and 1 µg/ml of CQ-E compared to the cells that were grown only in the presence of mineralizing medium. For both Runx2 and Col1A1, the highest dose (10 µg/ml) exhibited the lowest levels. After collagen reaches its peak synthesis and accumulate a matrix, a mid stage marker gene, bone sialoprotein, peaked at 2 weeks with the highest dose (10 µg/ml) stimulating IBSP to the greatest extent above the control and or lower dose groups (Fig. 4C). CQ-E treatment did not have any significant effect on the expression of a late phase differentiation marker gene, osteocalcin, known to be correlated with mineral deposition, continuously increases with more rising levels from 2 to 3 weeks (Fig. 4D). During this time mineral is being actively deposited (shown in Figures 2 and 3). Each of the 3 doses of CQ-E (0.1, 1.0 and 10 µg/ml) followed the same profile showing a striking increase over the control group.
Fig. 4.
Effect of CQ-E treatment on expression of Runx 2 RNA (A), Col1A1 RNA (B), bone sialoprotein RNA (C) and osteocalcin RNA (D). Confluent cells were treated with CQ-E at indicated concentrations in mineralizing medium (having ascorbic acid and β-glycerophosphate) for 3 weeks. Gene expression was analyzed every week. CQ-0, 0.1, 1 and 10 represents concentration of CQ-E in µg/ml.
*P< 0.05, **P< 0.01, ***P< 0.001.
Taken together, these gene expression results indicate that 0.1 or 1 µg/ml CQ-E has an anabolic effect on the normal temporal sequence of osteoblast differentiation and mineralization.
Discussion
Bone is an active and versatile connective tissue and a hub of mineral homeostasis. Osteogenesis or bone formation is a multi-step process that commences with the proliferation of mesenchymal stem cells and their subsequent differentiation into pre-osteoblasts. These pre-osteoblasts then mature into osteoblasts that synthesize extracellular matrix proteins, which promote bone mineralization (Lian et al., 2006).
High bone turnover with a loss of bone mass leads to osteoporosis (Stewart and Ralston, 2000; Raisz, 2005). It has been estimated that by 2050 more than half of all osteoporotic fractures will occur in Asia (Woolf and Pfleger, 2003; Mithal et al., 2009). Plant based medicine can provide a cost-effective alternative approach to treat and manage this condition.
This study was carried out to evaluate the osteogenic potential of ethanolic extract of Cissus quadrangularis using the murine pre-osteoblast cell line, MC3T3-E1. We observed that high concentrations of CQ-E adversely affect growth kinetics of cells whereas lower concentrations do not have any effect on growth kinetics, metabolic activity and viability of these cells. CQ-E at 0.1 and 1 µg/ml concentration increased the proliferation of MC3T3-E1 cell line. These observations are in accordance with another study by Parisuthiman et al. 2009) that has found a dosage-dependent cytotoxic effect of CQ-E on viability of cells, with lower concentration being non-cytotoxic. We did not observe significant difference in the doubling time among non-cytotoxic concentrations, however a significant increase was observed in proliferation at 0.1 and 1 µg/ml. Similar to the observations made in this study, mitogenic effect of petroleum ether extract (Potu et al., 2009a) of CQ has been reported on rat mesenchymal stem cells. Muthusami et al. (2011b) reported comparable results and have shown dose-dependent mitogenic effect of CQ-E on SaOS-2 at lower concentrations that declines with an increase in CQ-E concentration, though these concentrations did not have any effect on viability or metabolic activity. This stimulatory effect on proliferation can be attributed to the presence of anabolic steroidal substances in the CQ-E (Widyowati et al., 2010; Wu et al., 2010). The dosage-dependent effect of CQ-E on growth kinetics of MC3T3-E1 cells can be attributed to the presence of resveratrol as described by Peltz et al. (2012). This study has elucidated the discrete action of CQ-E on differentiation and mineralization of osteoblasts. CQ-E at concentration of 0.1 and 1 µg/ml, up-regulated ALP activity, enhanced mineralization and stimulated the expression of early- (Runx-2 and Col1α1) and late- Bglap2) phase genes of osteoblast differentiation. These results reveal the possible role of CQ-E in enhancing the differentiation process.
At 10 µg/ml CQ-E, there was up-regulation of osteocalcin and bone sialoprotein gene expression. After a week of treatment, early phase genes did not show a significant difference in expression compared to cells grown in mineralizing medium alone (positive control) and their expression was not down regulated after two weeks, as in positive control cultures. Consequently, cells at this concentration have less ALP activity and did not mineralize well. These findings indicate that 10/µg ml CQ-E was not entirely inhibitory to differentiation and mineralization but it somewhat impeded these physiological processes.
Parisuthiman et al. (2009) has reported some interesting results. The authors found that treatment of MC3T3-E1 cells with CQ-E, dissolved in polyvinylpyrrolidone (PVP), resulted in enhanced ALP activity and biomineralization but this treatment did not affect differentiation as the expression of osteoblast related genes (Runx-2, Sp7 and Bglap2) were not altered. This enhanced biomineralization was attributed to the up-regulation of ALP activity via MAPK dependent pathway. In the present study, not only enhanced ALP activity and biomineralization were observed, but also osteogenesis in cells treated with CQ-E dissolved in DMSO. Differences between Parisuthiman studies and ours could be related to the solubility of CQ-E in PVP and DMSO (Muthusami et al., 2011b).
In another study CQ-E treatment of osteoblast like SaOS-2 cells had anabolic effects on differentiation, ALP activity and mineralization of extracellular matrix. This treatment also resulted in the upregulation of expression of ALP, Col1α1 and Runx-2 also demonstrated increased transcriptional activity of Runx-2 at osteocalcin promoter in CQ-E treated cultures. It has also been reported that CQ-E mediates its effect on osteoblast differentiation by upregulating the IGF (insulin-like growth factor) system (Muthusami et al., 2011a). A study using rat mesenchymal stem cells (MSCs) reported that petroleum ether extract of CQ enhanced ALP activity and mineralization (Potu et al., 2009b). Thus, together these studies strongly support anabolic effects of CQ on osteoblast gene.
In our studies, anabolic effects of CQ on proliferation, differentiation, ALP activity and mineralization have been described that can be attributed to the presence of beta carotene, phytoestrogens and flavonols (e.g., resveratrol, kaempferol and quercetin) in CQ extract (Pluemaj and Saifah, 1986; Adesanya et al., 1999; Chidambara et al., 2003). Similar types of effects of these compounds have been reported from other laboratories that demonstrate dosage dependent increases in osteogenic differentiation of hMSCs after treatment of cells with lower doses of resveratrol (Prouillet et al., 2004; Wong and Rabie., 2008; Miyake et al., 2003; Mizutani et al., 1998; Park et al., 1997). Peltz et al. (2012). Thus, regenerative progenitor cells also have an osteogenic anabolic effect; however higher concentrations of resveratrol were, inhibitory to proliferation and osteogenic differentiation.
The flavanols (resveratrol, quercetin and kaempferol) present in CQ have been reported to promote osteoblast differentiation by activating ER-dependent ERK pathway (Prouillet et al., 2004; Dai et al., 2007; Guo et al., 2012). Both stimulatory (Prouillet et al., 2004; Wong and Rabie, 2008) and inhibitory (Notoya et al., 2004) effects of quercetin on osteoblast differentiation have been documented. Activated NF-κB pathway inhibits osteogenesis, whereas either the activated TGFβ or BMP2 pathway promotes osteogenesis. Quercetin acts as an antagonist of NF-κB pathway, thus having a stimulatory effect on osteogenesis. Also quercetin acts as an antagonist for TGFβ and BMP-2 induced Smad activation, leading to inhibition of osteogenesis. The net effect of quercetin on osteoblast therefore depends upon equilibrium among these signaling pathways viz. ER dependent ERK, NF-κB, TGFβ and BMP-2 pathways (Yamaguchi and Weitzmann, 2011).
The advantage of combinatorial effect of components present in the herbal extract is that concentration and availability of each chemical constituent in the CQ-E dictates the outcome of the differentiation pathway. As mentioned above, these chemical constituents of CQ-can affect multiple signaling pathways of cells. Coordination among these pathways results in either the amalgamation of signal at Runx-2 that directs the osteoblast pathway quenching of the signal before it up-regulates Runx-2 (Yamaguchi et al., 2000). Thus the effect of different concentrations of CQ-E on osteoblast differentiation and mineralization that had been observed in our study can be ascribed to the net effect of these chemical constituents on the signaling pathways that regulate osteoblast differentiation. It would also be interesting to study the effect of this plant extract on the differentiation of osteoclasts vitro, a distinct but parallel pathway that also contributes to osteoporosis.
This work strengthens the claim by traditional medicinal literature that Cissus quadrangularis influences bone formation and thus CQ-E has the potential to be used for healing fractures and to prevent and treat osteoporosis. While many components of CQ-E have been described, further studies are needed to scrutinize the active chemical constituent(s) of CQ-E and to decipher the molecular pathways that are regulated by this herb.
Acknowledgments
This research work was financially supported through research grants from NIH (USA) –AR039588 (GSS, JBL), R37 DE 012538 (JBL,JLS), and funds from Deanship of Scientific Research at King Saud University through Prolific Research Group Project No. -1436-011 KAAG, ARS) and University of the Punjab (ARS).
Footnotes
Conflict of interest statement
Authors have no conflict of interest to declare.
Literature Cited
- Abrahamsen B. Adverse effects of bisphosphonates. Calcif Tissue Int. 2010;86:421–435. doi: 10.1007/s00223-010-9364-1. [DOI] [PubMed] [Google Scholar]
- Adesanya SA, Nia R, Martin M, Boukamcha N, Montagnac A, Pas M. Stilbene derivatives from Cissus quadrangularis. J Nat Prod. 1999;62:1694–1695. [Google Scholar]
- Bhutani KK, Kapoor R, Atal CK. Two unsymmetric tetracyclic triterpenoids from Cissus quadrangularis. Phytochemistry. 1984;23:407–410. [Google Scholar]
- Chidambara MKN, Vanitha A, Mahadeva SM, Ravishankar GA. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J Med Food. 2003;6:99–105. doi: 10.1089/109662003322233495. [DOI] [PubMed] [Google Scholar]
- Chidambaram J, Anuradha CK. Cissus quadrangularis stem alleviates insulin resistance, oxidative injury and fatty liver disease in rats fed high fat plus fructose diet. Food Chem Toxicol. 2010;48:2021–2029. doi: 10.1016/j.fct.2010.04.044. [DOI] [PubMed] [Google Scholar]
- Chopra SS, Patel MR, Awadhiya RP. Studies of Cissus quadrangularis in experimental fracture repair : a histopathological study. Indian J Med Res. 1976;64(9):1365–1368. [PubMed] [Google Scholar]
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359:1761–1767. doi: 10.1016/S0140-6736(02)08657-9. [DOI] [PubMed] [Google Scholar]
- Dai Z, Li Y, Quarles LD, Son T, Pan W, Zhou H, Xiao Z. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806–814. doi: 10.1016/j.phymed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Deka DK, Lahon DC, Saikia J, Mukit A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius-ulna of dog, a preliminary study. Indian J Pharmacol. 1994;26:44–45. [Google Scholar]
- Guo AJ, Choi RC, Zheng KY, Chen VP, Dong TT, Wang ZT, Vollmer G, Lau DT, Tsim KW. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin Med. 2012;7:10. doi: 10.1186/1749-8546-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta AK, Shah N, Thakar AB. Effect of Majja Basti (therapeutic enema) and Asthi Shrinkhala (Cissus quadrangularis) in the management of Osteoporosis (Asthi-Majjakshaya) Ayu. 2012;33(1):110–113. doi: 10.4103/0974-8520.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MM, Verma RK. Unsymmetric tetracyclic triterpenoid from Cissus quadrangularis. Phytochemistry. 1990;29:336–337. [Google Scholar]
- Hamadeh IS, Ngwa BA, Gong Y. Drug induced osteonecrosis of the jaw. Cancer Treat Rev. 2015;41:455–464. doi: 10.1016/j.ctrv.2015.04.007. [DOI] [PubMed] [Google Scholar]
- IOF (International Osteoporosis Foundation) Facts and statistics. [Accessed on 01-18-2014];International Osteoporosis Foundation Website. 2014 Available at http://www.iofbonehealth.org/facts-statistics. [Google Scholar]
- Jainu M, Mohan KV. Protective role of ascorbic acid isolated from Cissus quadrangularis on NSAID induced toxicity through immunomodulating response and growth factors expression. Int Immunopharmacol. 2008;8:1721–1727. doi: 10.1016/j.intimp.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Jainu M, Mohan KV, Devi CS. Protective effect of Cissus quadrangularis on neutrophil mediated tissue injury induced by aspirin in rats. J Ethnopharmacol. 2006;104:302–305. doi: 10.1016/j.jep.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Kanis JA. UK: University of Sheffield; 2007. WHO Technical Report; p. 66. [Google Scholar]
- Kumar M, Rawat P, Dixit P, Mishra D, Gautam AK, Pandey R, Singh D, Chattopadhyay N, Maurya R. Anti-osteoporotic constituents from Indian medicinal plants. Phytomedicine. 2010;17(13):993–999. doi: 10.1016/j.phymed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Hassan MQ, Serra RW, Lepper C, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280:15872–15879. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- Lengner CJ, Lepper C, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Primary mouse embryonic fibroblasts: a model of mesenchymal cartilage formation. J Cell Physiol. 2004;200:327–333. doi: 10.1002/jcp.20118. [DOI] [PubMed] [Google Scholar]
- Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, Hassan MQ, Gaur T, Lengner CJ, Young DW. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- Lorentzon M, Cummings SR. Osteoporosis the evolution of diagnsosis. J Intern Med. 2015;277(6):650–661. doi: 10.1111/joim.12369. [DOI] [PubMed] [Google Scholar]
- Mithal A, Dhingra V, Lau E. In: The Asian Audit: Epidemiology, costs and burden of osteoporosis in Asia. Stenmark J, Nauroy L, editors. Switzerland: International Osteoporosis Foundation (IOF); 2009. pp. 6–10.pp. 40–42. [Google Scholar]
- Mitra P, Ghule PN, van der Deen M, Medina R, Xie RL, Holmes WF, Ye X, Nakayama KI, Harper JW, Stein JL, Stein GS, van Wijnen AJ. CDK inhibitors selectively diminish cell cycle controlled activation of the histone H4 gene promoter by p220NPAT and HiNF-P. J Cell Physiol. 2009;219:438–448. doi: 10.1002/jcp.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake M, Arai N, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Promoting effect of kaempferol on the differentiation and mineralization of murine pre-osteoblastic cell line MC3T3-E1. Biosci Biotechnol Biochem. 2003;67(6):1199–1205. doi: 10.1271/bbb.67.1199. [DOI] [PubMed] [Google Scholar]
- Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253(3):859–863. doi: 10.1006/bbrc.1998.9870. [DOI] [PubMed] [Google Scholar]
- Muthusami S, Ramachandran I, Krishnamoorthy S, Govindan R, Narasimhan S. Cissus quadrangularis IGF system components in human osteoblast like SaOS-2 cells. Growh Hormone IGF Res. 2011a;21:343–348. doi: 10.1016/j.ghir.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Muthusami S, Senthilkumar K, Vignesh C, Ilangovan R, Stanley J, Selvamurugan N, Srinivasan N. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J Cell Biochem. 2011b;112:1035–1045. doi: 10.1002/jcb.23016. [DOI] [PubMed] [Google Scholar]
- Nanes MS, Kallen CB. Clinical assessment of fracture risk and novel therapeutic strategies to combat osteoporosis. Fertil Steril. 2009;92(2):403–412. doi: 10.1016/j.fertnstert.2009.05.049. [DOI] [PubMed] [Google Scholar]
- Notoya M, Tsukamoto Y, Nishimura H, Woo JT, Nagai K, Lee IS, Hagiwara H. Quercetin, a flavonoid, inhibits the proliferation, differentiation, and mineralization of osteoblasts in vitro. Eur J Pharmacol. 2004;485(1–3):89–96. doi: 10.1016/j.ejphar.2003.11.058. [DOI] [PubMed] [Google Scholar]
- Parisuthiman D, Singhatanadgit W, Dechatiwongse T, Koontongkaew S. Cissus quadrangularis extract enhances biomineralization through up-regulation of MAPK-dependent alkaline phosphatase activity in osteoblasts. In Vitro Cell Dev Biol–Animal. 2009;45(3–4):194–200. doi: 10.1007/s11626-008-9158-1. [DOI] [PubMed] [Google Scholar]
- Park CK, Ishimi Y, Ohmura M, Yamaguchi M, Ikegami S. Vitamin A and carotenoids stimulate differentiation of mouse osteoblastic cells. J Nutr Sci Vitaminol. 1997;43(3):281–296. doi: 10.3177/jnsv.43.281. [DOI] [PubMed] [Google Scholar]
- Pathomwichaiwat T, Ochareon P, Soonthornchareonnon N, Ali Z, Khan IA, Prathanturarug S. Alkaline phosphatase activity–guided isolation of active compounds and new dammarane-type triterpenes from Cissius quadrangularis hexane extract. J Ethnopharmacol. 2015;160:52–60. doi: 10.1016/j.jep.2014.11.026. http://dx.doi.org/10.1016/Jep 2014.11.026. [DOI] [PubMed] [Google Scholar]
- Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49:103–110. doi: 10.1016/j.bone.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Peltz L, Gomez J, Marquez M, Alencastro F, Atashpanjeh N, Quang T, Bach T, Zhao Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One. 2012;7(5):e37162. doi: 10.1371/journal.pone.0037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluemaj T, Saifa E. Constituents of Cissus quadrangularis Linn. J Pharm Sci. 1986;11:205–211. [Google Scholar]
- Potu BK, Bhat KMR, Rao MS, Nampurath GK, Chamallamudi MR, Nayak SR, Muttigi MS. Petroleum ether extract of Cissus quadrangularis (LINN) enhances bone marrow mesenchymal stem cell proliferation and fascilitates osteoblastogenesis. Clinics. 2009a;64(10):993–998. doi: 10.1590/S1807-59322009001000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potu BK, Nampurath GK, Rao MS, Bhat KM. Effect of Cissus quadrangularis Linn on the development of osteopenia induced by ovariectomy in rats. Clin Ter. 2011;162(4):307–312. [PubMed] [Google Scholar]
- Potu BK, Rao MS, Nampurath GK, Bhat KMR, Chamallamudi MR, Nayak SR. Anti-osteoporotic activity of the petroleum ether extract of Cissus quadrangularis Linn in ovariectomized wistar rats. Chang Gung Med J. 2010;33:252–257. [PubMed] [Google Scholar]
- Potu BK, Rao MS, Nampurath GK, Bhat KMR, Chamallamudi MR, Nayak SR. Petroleum ether extract of Cissus quadrangularis (LINN) stimulates the growth of fetal bone during intrauterine developmental period: a morphometric analysis. Clinics. 2008;63(6):815–820. doi: 10.1590/S1807-59322008000600018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potu BK, Rao MS, Nampurath GK, Chamallamudi MR, Prasad K, Nayak SR, Dharmavarapu PK, Kedage V, Bhat KMR. Evidence-based assessment of antiosteoporotic activity of petroleum-ether extract of Cissus quadrangularis Linn. on ovariectomy-induced osteoporosis. Ups J Med Sci. 2009b;114(3):140–148. doi: 10.1080/03009730902891784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad GC, Udupa KN. Pathways and site of action of phytogenic steroids from Cissus quadrangularis. J Res Indian Med. 1972;7:29. [Google Scholar]
- Prouillet C, Mazière JC, Mazière C, Wattel A, Brazier M, Kamel S. Stimulatory effect of naturally occurring flavonols quercetin and kaempferol on alkaline phosphatase activity in MG-63 human osteoblasts through ERK and estrogen receptor pathway. Biochem Pharmacol. 2004;67(7):1307–1313. doi: 10.1016/j.bcp.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Kumar B, Swamy N, Kutty G. Cissus quadrangularis plant extract enhances the development of cortical bones and trabeculae in the fetal femur. Pharmacology online. 2007;3:190–202. [Google Scholar]
- Robinson MM, Zhang X. Geneva: World Health Organization (WHO); 2011. The world medicines situations 2011: Traditional medicines global situation, issues and challenges (document WHO/EMP/MIE/2011.2.3) pp. 1–9. [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the women's health Initiative randomized controlled trial. J. Am Med Assoc. 2002;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Shirwaikar A, Khan S, Malini S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. J Ethnopharmacol. 2003;89:245–250. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Sivarajan VV, Balachandran I. Ayurvedic drugs and their plant sources. New Delhi: Oxford and India Book House Publishing Co. Pvt. Ltd; 1994. p. 496. [Google Scholar]
- Stewart TL, Ralston SH. Role of genetic factors in the pathogenesis of osteoporosis. J Endocrinol. 2000;166:235–245. doi: 10.1677/joe.0.1660235. [DOI] [PubMed] [Google Scholar]
- Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt A, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1–34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in virtro and in vivo differentiation/mineralization potential. J Bone Min Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- Widyowati R, Tezuka Y, Miyahara T, Awale S, Kadota S. Alkaline phosphatase (ALP) enhancing iridoid glucosides from the Indonesian medicinal plant Barleria lupulina. Nat Prod Commun. 2010;5:1711–1716. [PubMed] [Google Scholar]
- Williamson EM, editor. Major herbs of Ayuerveda. China: Churchill Livingstone; 2002. pp. 106–109. [Google Scholar]
- Wong RWK, Rabie ABM. Effect of quercetin on preosteoblasts and bone defects. The Open Orthopaed J. 2008;2:27–32. doi: 10.2174/1874325000802010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Hlth Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- Wu M, Zhao S, LI E, Bai X. Effects of catalpol from Radix rehmanniae on proliferation, differentiation and matrix mineralization of MC3T3-E1 cells. Bull Wld Hlth Organ. 2010;81:646–656. [Google Scholar]
- Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Weitzmann MN. Quercetin, a potent suppressor of NF-κB and Smad activation in osteoblasts. Int J Mol Med. 2011;28(4):521–525. doi: 10.3892/ijmm.2011.749. [DOI] [PubMed] [Google Scholar]