Abstract
Serine palmitoyltransferase (SPT) is the key enzyme in sphingolipid biosynthesis. Mice lacking SPT are embryonic lethal. We prepared liver-specific Sptlc2 deficient mice using an albumin-Cre approach, we found that the deficient mice have severe jaundice. Moreover, the deficiency impairs hepatocyte polarity, attenuates liver regeneration after hepatectomy, and promotes tumorigenesis. Importantly, we show that the deficiency significantly reduces sphingomyelin but not other sphingolipids in hepatocyte plasma membrane, greatly reduces cadherin, the major protein in adherens junctions, on the membrane and greatly induces cadherin phosphorylation, an indication for its degradation. The deficiency affects cellular distribution of β-catenin, the central component of the canonical Wnt pathway. Furthermore, such a defect can be partially corrected by sphingomyelin supplementation in vivo and in vitro.
CONCLUSION
Our results, for the first time, show that plasma membrane sphingomyelin level is one of the key factors in regulating hepatocyte polarity and tumorigenesis.
Keywords: Sptlc2 liver-specific knockout mice, Albumin-Cre recombinase, Adherens junctions, Lipid rafts, Cadherin, β-catenin, Cell polarity, Tumorigenesis, Sphingomyelin
Serine palmitoyltransferase (SPT) is the key enzyme involved in sphingolipid biosynthesis (1). The mammalian SPT holoenzyme is primarily a heterodimer composed of two subunits, Sptlc1 and Sptlc2, which share 20% sequence homology (2, 3). However, studies indicate the existence of a third subunit, Sptlc3, which has 68% homology to Sptlc2 (4). Two low-molecular-weight proteins, ssSPTa and ssSPTb, enhance enzyme activity and confer distinct acyl-CoA substrate specificities to mammalian SPT(5). A more recent discovery indicating that the yeast ORM (orosomucoid) 1/ORM2 proteins also associate with and negatively regulate SPT activity (6) has added an additional layer of complexity to sphingolipid biosynthesis.
SPT activity is required for normal cell function and alterations in SPT activity are associated with disease. Specific mutations identified in either Sptlc1 or Sptlc2 cause a rare genetic disorder called hereditary sensory and autonomic neuropathy type 1 (7–9). Mice that lack Sptlc1 or Sptlc2 are embryonic lethal (10). Tamehiro et al. reported that Sptlc1 binds ATP-binding cassette transporter (ABCA) 1 and negatively regulates trafficking and the cholesterol efflux activity of this transporter (11). Park et al. (12) and our group (13) reported that treatment with myriocin, a highly selective inhibitor of SPT activity, decreases plasma sphingomyelin levels and atherosclerosis in apoE KO mice. Recently, we found that macrophages from Sptlc2 haploinsufficient mice have significantly lower sphingomyelin levels in plasma membranes and lipid rafts (14). This reduction in sphingomyelin impairs inflammatory responses triggered by toll-like receptor 4 and its downstream pathways, enhances the reverse cholesterol transport, and reduces atherosclerosis in a mouse model (14).
Almost all cell types exhibit some sort of polarity, which enables them to carry out specialized functions. The apical-basal polarity of epithelial cells (15) and the propagation of signals from dendrites to axons in neurons (16) are classic examples of cell polarity. Adherens junctions, which consist of the transmembrane protein cadherin and the intracellular components β-catenin, α-catenin, and actin filaments (17), initiate cell-cell contacts and mediated the maturation and maintenance of the contact. Formation of the adherens junctions lead to assembly of the tight junction (18). Because all cell membranes that define cell boundaries and polarity contain lipid bi-layer structures, we hypothesized that the lipid, especially the sphingolipid, environment could influence polarity. Indeed the Sptlc1/2 complex can bind cell polarity factor Par3 and modulate monocyte polarity and migration (19).
Previously, we reported that adenovirus associated virus (AAV)-Cre-mediated liver Sptlc2 deficiency decreases liver SPT activity by more than 90% and significantly decreases plasma sphingomyelin levels, compared with controls (20). We expected the same results when we crossed Sptlc2-Flox with albumin-Cre transgenic mice. To our surprise, we observed that Sptlc2 deficiency in early life impairs hepatocyte polarity and promotes tumorigenesis. We explored the mechanisms in this study.
Materials and Methods
Liver-specific Sptlc2 KO Mouse Preparation
The overall strategy for gene targeting was to delete exon 1 of Sptlc2. Because exon 1 contains the translation codon, ATG, deletion of exon 1 is expected to create null Sptlc2 mouse model. The strategy of mouse generation (Supporting Fig. 1) and details were previously described (20). To prepare liver-specific Sptlc2 deficient mice, we crossed our Sptlc2-Flox mice with albumin-Cre transgenic mice (Jackson laboratory). Both mice have C57BL/6 genetic background.
Western Blot for Mouse Liver Sptlc1 and Sptlc2
The western blot procedure was similar as we did before (20).
Tissue SPT Activity Assay
The activity assay was similar as we did before (20).
Lipid and Lipoprotein Measurements
The procedure was similar as we did before (20).
Billirubin, Bile Acid Measurements
Billirubin and bile acid were measured using the bilirubin assay kits (BioAssay systems, DIBR-180) and total bile acids assay kit (Diazyme laboratories, DZ042A-K) according to manufacturer protocols.
Electronic Microscopy
Lipoprotein negative staining and examination: To prepare particles for negative staining, a small droplet of fresh plasma was placed on 200 mesh formvar & carbon-coated copper grids and stained with 1% sodium phosphotunigstenate (NaPTA) for 30 seconds. Then, the excess fluid was removed and the air dried grid was examined immediately by transmission electron microscopy (Jeol, JEM 100C).
Liver examination: Samples were immersed in modified Karnovsky’s fixative (2.5% glutaraldehyde and 2% paraformaldehyde in 0.15 M sodium cacodylate buffer, pH 7.4) for 4 hours, postfixed in 1% osmium tetroxide in 0.15 M cacodylate buffer for 1 hour. Samples were dehydrated in ethanol, embedded in epoxy resin (EMS), sectioned at 50 to 60 nm on a LKB ultramicrotome, picked up on 200 mesh copper grids, and then processed with positive staining with uranyl acetate and lead citrate. The samples were examined with transmission electron microscopy (Jeol, JEM 100C).
Primary Hepatocyte Isolation
Hepatocytes were isolated from 8-week-old mice as previously described (21).
Sphingolipid Analyses by LC/MS/MS
Sphingolipid levels were measured in WT and Sptlc2 KO hepatic plasma membrane by LC/MS/MS, as previously described (22, 23).
Hepatectomy
Mouse 2/3 partial hepatectomy was performed following a published protocol (24). Briefly, mouse abdominal organs were exposed with a 2 centimeter long abdominal midline incision and a 1.5 centimeter long horizontal incision below the bottom of ribcage and crossed with a midline incision at 1/3 of the length from the bottom of the incision. To remove the left lateral lobe of liver, a 4-0 silk thread was placed around the base of left lateral lobe to make a ligation, and then the lobe was resected above the suture knot with a microsurgery scissor. To remove the median lobe, a 4-0 silk thread was placed around the lobe at the level between gall bladder and vena cava. After ligation, the median lobe was resected above the suture knot. The peritoneum and skin were then closed with 5-0 vicryl sutures.
Sphingomyelin Supplemental Studies
For in vivo study: 2-week-old Sptlc2 KO mice were injected with vehicle or sphingomyelin mixture including one third C16:0, C24:1, and brain sphingomyelin, respectively, 10 μg sphingomyelin mixture/g body weight/day for 4 weeks, and then the plasma bilirubin levels were measured. For in vitro study: primary hepatocytes from Sptlc2 KO and WT mice were isolated and incubated with 20 μM sphingomyelin mixture or vehicle for 18 hours, and then plasma membrane, nucleus and cytoplasm were isolated and Western blots were performed.
Statistical Analysis
Each experiment was conducted at least five times. Data are typically expressed as mean ± S.D. Data between two groups were analyzed by Student’s t test. A p value of less than 0.05 was considered significant.
Results
Creation of Liver-specific Sptlc2-deficient Mice
We crossed Sptlc2-Flox mice with albumin-Cre transgenic mice (Supporting Fig. 1) and prepared liver-specific Sptlc2-deficient mice. Recombination of the floxed Sptlc2 alleles (and thereby inactivation of Sptlc2) was achieved in essentially 100% of in the liver but not in other tissues (Fig. 1A). We also found that Sptlc2 protein was undetectable in the liver, whereas Sptlc1 was dramatically increased compared with WT and the heterozygote (4.5-fold, P < 0.0001) (Fig. 1B–D). Moreover, Sptlc2 deficiency caused a 90% decrease in liver SPT activity compared with controls (Fig. 1E and 1F).
Figure 1. Albumin-Cre-mediated Liver-specific Sptlc2 KO Mouse Preparation.
(A) Sptlc2 mRNA (n = 7 per group). (B–D) Liver Sptlc1 and Sptlc2 protein levels measured by Western blots (n = 5 per group). (E,F) Liver SPT activity (n = 7 per group). Mouse liver (0.2 g) was homogenized in 0.5 ml of 50 mM Tris-HCl, pH 7.4, 5 mM EDTA, and 250 mM sucrose. SPT activity in the homogenates was measured with [14C]-serine and palmitoyl-CoA as substrates. The products 3-ketodihydrosphingosine (KDS) was detected on thin layer chromatograph. The molecular weight of Sptlc1 and Sptlc2 are 53 kDa and 63 kDa, respectively. Data are represented as mean ± SD, *P < 0.001.
Liver-specific Sptlc2 Deficiency Dramatically Increase Plasma Lipids
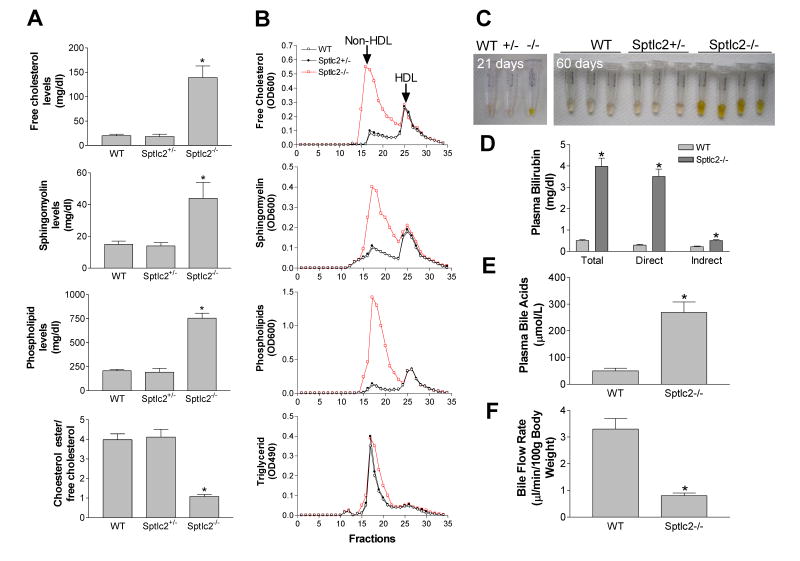
We next sought to measure plasma lipid levels. To our surprise, we found that all the lipids (including sphingomyelin, free cholesterol, and total phospholipids) except triglyceride were dramatically increased (2.7 to 3.7-fold) (Fig. 2A) (Supporting Table 1). Furthermore, we noticed that the ratio of cholesteryl ester (CE) to free cholesterol (FC) was 3.85 in wild-type mice, 4.11 in heterozygous, and 1.08 in homozygous, respectively (Fig. 2A) (Supporting Table 1), suggesting that FC was accumulated in the circulation. All these lipid phenotypes were not observed in mice with adenovirus associated virus (AAV)-Cre-mediated liver Sptlc2 deficiency (Supporting Table 1)(20).
Figure 2. Plasma Lipid and Bile Measurements.
(A) Plasma free cholesterol, sphingomyelin, total phospholipids, and choleteryl ester/free cholesterol ratio measurements (n = 7 per group). (B) Plasma lipoprotein distribution by fast protein liquid chromatography (FPLC). Free cholesterol, phospholipids, sphingomyelin and triglycerides were determined in each fraction. Plasms was pooled from 7 animals per group. (C) Plasma collected from 21-days old and 60-days old wild type, heterozygous (+/−), and homozygous (−/−) KO mice. (D) Fluorogram of plasma bilirubin levels (n = 7 per group). (E) Fluorogram of plasma total bile acid levels (n = 7 per group). (F) Fluorogram of bile flow rate (n = 7 per group). Data are represented as mean ± SD, *P < 0.01.
The distribution of lipids was determined by FPLC of pooled plasma. This confirmed that sphingomyelin (SM), free cholesterol, total phospholipid but not triglyceride levels were dramatically increased in non-HDL fractions from the KO mice compared with controls (Fig. 2B).
Liver-specific Sptlc2 Deficiency Significantly Reduced SM on the Plasma Membrane
We next isolated plasma membrane from primary hepatocytes of Sptlc2 KO mice and controls and measured sphingolipid levels. To our surprise, we found that although the deficiency significantly reduced almost all major measured SM in the plasma membrane (Table 1), it had a different effect on ceramide subspecies. Some major ceramides (C22:0 and C24:0) were significantly reduced, as expected, and some of them (C16:0 and C24:1) were significantly increased (Table 1). Moreover, Sptlc2 deficiency had a marginal effect on glucosylceramide and lactosylceramide, two glycosphingolipid levels (Table 1) and on sphingosine-1-phosphate and dihydroceramide in hepatocyte plasma membranes (Supporting Table 2).
Table 1.
Sphingolipid levels on liver plasma membrane.
| Mice | C16:0 | C18:0 | C18:1 | C24:0 | C24:1 | C20:0 | C22:0 |
|---|---|---|---|---|---|---|---|
| (pmol/mg protein) | |||||||
| Sphingomyelin | |||||||
| WT | 163±23 | 32±4 | 5±1 | 83±6 | 175±53 | --- | 26±9 |
| Sptlc2 KO | 76±12** | 22±2* | 4±1 | 22±5* | 62±16** | --- | 10±3* |
| Ceramide | |||||||
| WT | 17±3 | 4±1 | --- | 36±9 | 38±6 | 4±1 | 33±5 |
| Sptlc2 KO | 37±6* | 7±1* | --- | 19±7* | 63±11* | 3±1 | 11±4* |
| GlcCer | |||||||
| WT | 35±5 | --- | --- | 40±10 | 88±21 | 9±2 | 23±8 |
| Sptlc2 KO | 30±6 | --- | --- | 37±7 | 110±26 | 8±1 | 26±5 |
| LacCer | |||||||
| WT | 22±4 | --- | --- | --- | 50±10 | --- | --- |
| Sptlc2 KO | 30±9 | --- | --- | --- | 61±11 | --- | --- |
Liver-specific Sptlc2 KO Mice Have Severe Jaundice
The Sptlc2 KO mice developed jaundice and the severity was related to age (Fig. 2C). Measurement of plasma bilirubin levels showed a dramatic increase of conjugated (direct) bilirubin (Fig. 2D). Plasma bile acid levels were also dramatically increased in Sptlc2 KO mice (Fig. 2E). We then evaluated bile flow rate by bile duct canalization and found Sptlc2 KO mice had a significantly slower flow rate than that of controls (Fig. 2F). Moreover, we measured plasma alanine transaminase (ALT) and aspartate transaminase (AST) activities and found that ALT but not AST was significantly increased (Supporting Fig. 2).
Lipoprotein-X is a low-density lipoprotein that is enriched with free cholesterol (25). Normally, lipoprotein-X is excreted by hepatocytes into the bile canaliculi (26), but it is found in the blood of jaundiced subjects (25, 27). Based on the observation that free cholesterol, phospholipids, and conjugated bilirubin accumulated in Sptlc2 KO mouse plasma, we speculated that bile is directly released into the blood and that lipoprotein-X (a rod-like lipoprotein) would be present in the plasma of the KO mice. Indeed, negative staining and electron microscopy revealed normal lipoproteins with different sizes in control mouse plasma (Fig. 3A) and a dramatic accumulation of lipoprotein-X particles with lamellar structures that masked the presence of normal lipoproteins in Sptlc2 KO mouse plasma (Fig. 3A).
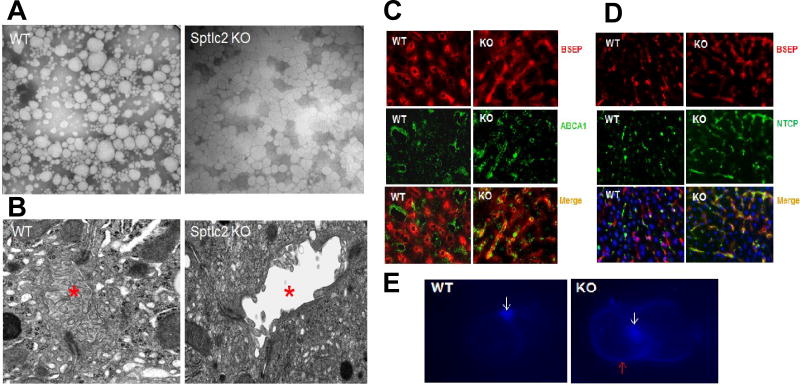
Figure 3. Impairment of Hepatocyte Apical-basal Polarity.
(A) Electronic microscope images of mouse plasma lipoproteins which were negative stained. (B) Electronic microscope images of mouse liver sections. Bile canaliculus was indicated by red asterisks. (C) Bile salt export pump (BSEP) and ABCA1 were immuno-stained on liver sections. (D) BSEP and sodium-taurocholate co-transporting polypeptide (NTCP) were immuno-stained on liver sections. (E) Freshly isolated hepatocyte couplets were stained with Filipin. White arrows indicate bile canaliculus and red arrow indicates basal membrane. The pictures are the representatives of three mice/group.
Sptlc2 Deficiency Impairs Hepatocyte Polarity, Attenuates Liver Regeneration, and Promotes Tumorigenesis
Similar to epithelial cells with apical-basal polarity (15), hepatocytes also have an apical and a basal surface. The apical surface forms the bile canaliculus, whereas the basal surface contacts the wall of the sinusoids. We next visualized mouse liver bile canaliculi with electron microscopy and found that the microvilli of the canaliculi were dramatically reduced in the KO mice compared with controls (Fig. 3B), suggesting that inhibiting SPT activity in early life impaired cell polarity and, thus, the bile canaliculus structure. If this is the case, then the distribution of proteins on the apical bile canaliculi membrane and basolateral plasma membrane should be altered. The bile salt export pump (BSEP) is located on the bile canaliculi membrane (28), whereas ABCA1 and sodium-taurocholate co-transporting polypeptide (NTCP) are located in the basolateral membranes of hepatocytes (29, 30). To investigate whether liver-specific Sptlc2 KO impacts the distribution of these proteins, we immunohistochemically stained liver sections. Control liver sections showed no overlap in the distribution of BSEP and ABCA1 (Fig. 3C) or BSEP and NTCP (Fig. 3D). However, in Sptlc2 KO liver sections, these proteins showed considerable overlap (Figs. 3C and D). These results suggested that Sptlc2 deficiency causes impaired hepatocyte polarity.
To further investigate cell polarity changes, we next isolated hepatocyte couplets and stained them with filipin, a cholesterol-staining dye. The bile canaliculi membrane was strongly stained by filipin in both control and Sptlc2 KO hepatocytes (Fig. 3E). However, staining of the basolateral plasma membrane was much stronger in Sptlc2 KO hepatocytes than controls, suggesting that impaired cell polarity alters the distribution of free cholesterol on apical and basal membranes of hepatocytes.
At age 2 months, we found that Sptlc2 KO mice had significantly bigger livers (Fig. 4A) and hepatic fibrosis (Fig. 4B). At age 6 months, we not only observed the bigger liver but also tumors all over the liver (Fig. 4C). Moreover, at age 10 months, almost all the KO mice had much larger tumors, while control mice had no tumors (Fig. 4C).
Figure 4. Pathology of Sptlc2 KO Mice.
(A) Liver weight of 2-months old mice. Values are mean ± SD, n = 7, *P < 0.01. (B) 2-month old mouse liver section was stained with sliver for reticulum. (C) 6- and 10-months old Sptlc2 KO mouse livers and 10-months old WT mouse liver. Tumors were indicated by arrows as well as a cycle. The pictures are the representatives of five mice/group.
Microscopic examination of the liver mass from WT mice (Supporting Fig. 3A) and Sptlc2 KO mice (Supporting Fig. 3B–D) shows that the KO liver has a well-differentiated hepatic neoplasm that lacks bile ducts, morphologically consistent with a hepatocellular adenoma. The tumor does not show any cytological atypia or mitotic activity. Reticulin framework is also largely preserved. Patchy myxoid areas are also seen, primarily surrounding the central veins, with marked capillary proliferation and a few residual degenerated hepatocytes. These changes are most likely due to ischemia and degeneration secondary to the enlarged mass outgrowing its blood supply. No other gross macroscopic abnormalities were noted in the KO mice. Interestingly, although the KO mice had significantly higher cholesterol levels (about 300 mg/dl) (Supporting Table 1), they had no atherosclerotic lesions in the aorta (Supporting Fig. 4).
It has been reported that lose hepatocyte polarity attenuates liver regeneration(31), a unique feature for the liver. To investigate whether Sptlc2 deficiency has an effect on liver regeneration, Sptlc2 KO and control mice were subjected to 70% hepatectomy. We found that the regeneration ratio (regenerated liver weight/original liver weight) was significantly smaller in the KO mice compared with the WT (Fig. 5A), suggesting again that Sptlc2 deficiency mediates an impairment of hepatocyte polarity. Also, severe jaundice and accumulation plasma bilirubin were observed in the KO mice (Supporting Fig. 5).
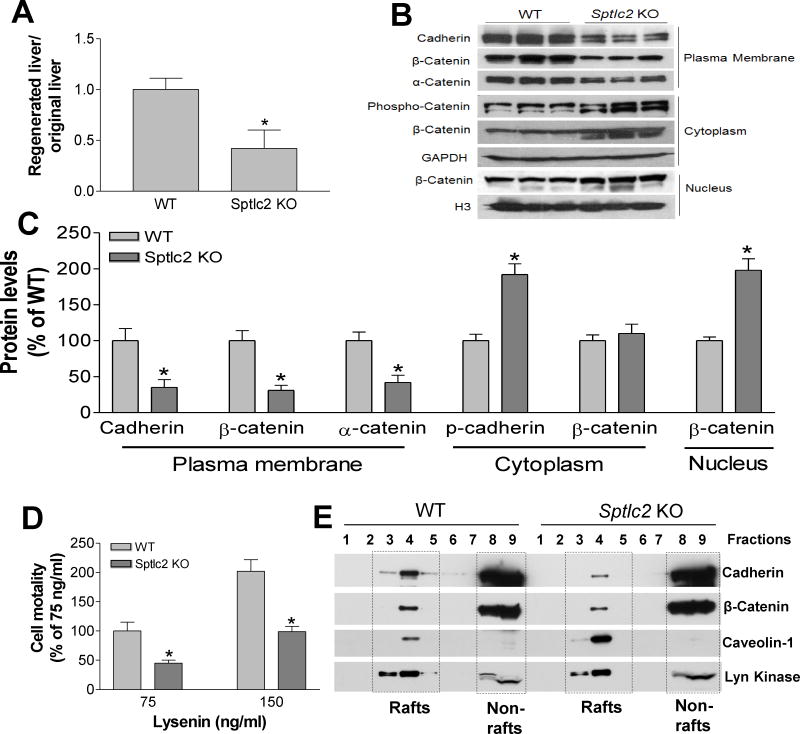
Figure 5. Liver Regeneration and β-catenin Cellular Distribution.
(A) Liver regeneration. Mouse 2/3 partial hepatectomy was performed and at the day 7 after the treatment, the mice were sacrificed and the liver and plasma were collected. Liver weight and plasma bilirubin levels were measured. Liver regeneration ratio was compared between Sptlc2 KO and WT mice. (B) Western blots of cadherin, β-catenin, and α-catenin on plasma membrane (PM), cytoplasma, and nucleus from WT and Sptlc2 KO mice. (C) Fluorogram of cadherin, β-catenin, and α-catenin levels. Data are represented as mean ± SD, n=5, *P < 0.01. (D) Cell surface SM measurement by lysenin-mediated cell lysis assay represented by % cell mortality. Data are represented as mean ± SD, n=5, *P < 0.01. (E) Lipid rafts were isolated from mouse livers (pooled from 4 mice) using sucrose density gradient centrifugation and fractions were collected. Western blots were performed for caveolin-1 (Cav-1, raft marker), CD71 (non-raft marker), cadherin and β-catenin with respective antibodies. The molecular weight of cadherin, β-catenin, α-catenin, caveiolin-1, and Lyn kinase are 120/130, 92, 100, 22, and 53/56 kDa, respectively. Data are representative of three independent experiments.
We next sought to determine the mechanism by which hepatocyte polarity is lost. Since adherens junctions initiate cell-cell contacts which are mediated by extracellular cadherin domains (18), thus, they play a crucial role in cell polarity formation. We utilized liver homogenates to isolate plasma membrane, cytosol, and nucleus. We found that Sptlc2 deficiency significantly reduced plasma-membrane cadherin (pan) levels (70%, P<0.01) (Figs. 5B and C) and significantly induced phosphorylated cadherin, an indication for its degradation (32), in cytosol (Figs. 5B and C). We also found that significant changes in the subcellular distribution of β-catenin, with significantly less on the plasma membrane (75%, P<0.01) and more in the nucleus (190%, P<0.01) (Figs. 5B and C). We also found that plasma-membrane α-catenin, a linking protein between cadherin/β-catenin and actin-containing filaments of the cytoskeleton (33), was significantly reduced (Figs. 5B and C). Moreover, Sptlc2 deficiency–mediated cadherin/β-catenin regulation occurs post-transcriptionally because E-cadherin, N-cadherin, and β-catenin mRNA levels were unchanged (Supporting Fig. 6). Thus, Sptlc2 deficiency–mediated reduction of cadherin on plasma membrane could be the molecular basis for the observed loss of hepatocyte polarity.
To further evaluate plasma membrane cadherin decrease is related with Sptlc2 deficiency-mediated membrane SM reduction, we performed lysenin assay. Lysenin is a SM-specific cytotoxin, which binds to SM-enriched microdomains in plasma membranes, causing lysis of the cells. Therefore, lysenin-mediated cell mortality indirectly reflects SM levels on cell surfaces. As shown in Figure 5D, Sptlc2 KO primary hepatocytes showed significantly less sensitivity to lysenin-mediated cytolysis than control cells. This indicates that Sptlc2 deficiency significantly decreases SM levels on hepatocyte surfaces.
It is known that the interaction of SM, cholesterol, and glycosphingolipid drives the formation of plasma membrane rafts (34) and cadherin is well located in the lipid rafts (35, 36). Thus, we next sought to isolate lipid raft and non-raft regions using cold detergent treated liver lysates which were fractionated in sucrose density gradients. The lipid rafts containing fractions were identified by a raft-specific marker, Caveolin-1(Cav-1), while the others were confirmed with a non-raft marker, Lyn kinase. We found that both cadherin and β-catenin in Sptlc2 deficient lipid rafts are dramatically reduced, compared to control (Fig. 5E). Interestingly, Cav-1 is dramatically increased in the deficient lipid rafts (Fig. 5E). This finding clearly indicates that Sptlc2 deficiency results in an altered raft arrangement and influence adherens junctional complex formation.
SM Supplementation Partially Corrects Sptlc2-deficiency-mediated Defect
Because the Sptlc2 deficiency significantly reduced almost all major measured SM (Table 1) in plasma membrane and significantly reduced blood SM (C16:0, C18:1, C18:0, and C24:1, P < 0.01) at day 14 after birth (Supporting Fig. 7), we decided to use a SM mixture, including one third C16:0, C24:1, and brain sphingomyelin, respectively, to correct the defect in polarity starting from day 14 after birth. First, we injected the deficient mice with SM mixture or vehicle. Four weeks after the treatment, we measured plasma bilirubin levels and found SM supplementation reduces the severity of jaundice (Fig. 6A) and significantly reduces plasma bilirubin levels (Fig. 6A). We then isolated primary hepatocytes from Sptlc2 KO and WT mice and incubate them with SM mixture or vehicle. We observed that Sptlc2 KO hepatocytes have a significant reduction of cadherin and dramatic induction of phosphorylated cadherin on the plasma membrane and cytosol (Figs. 6B–D). We also observed that the KO hepatocytes have a significant reduction of β-catenin on the plasma membrane and a significant induction of β-catenin in nucleus compared to WT mice (Figs. 6B, C, and E). More importantly, SM treatment significantly increased cadherin but decreased phosphorylated cadherin on the plasma membrane (Figs. 6B and C). Same treatment also increased β-catenin levels on the plasma membrane, and deceased β-catenin levels in the nucleus in Sptlc2 KO hepatocytes (Figs. 6B, C, and E). Phosphorylated cadherin and β-catenin levels in the cytosol was also dramatically accumulated in the KO mice compared with controls, however, with or without SM treatment did not show significant differences (Figs. 6B and D). We also used primary hepatocytes from the KO and control mice and examined the effect of SM supplementation on two oncogens, c-Myc and Cyclin D1, which are downstream of β-catenin (37). We found that both oncogene protein levels in the nucleus of Sptlc2 KO hepatocytes are significantly higher than that of controls. Importantly, SM supplementation significantly reduces both protein levels in the nucleus (Fig. 6F), suggesting exogenous SM can partially but significantly suppress the tumorigenecity mediated by Sptlc2 deficiency. We also utilized sphinganine, ceramide, and ganglioside supplementation to correct the defect of both cadherin and β-catenin on the plasma membrane of primary hepatocytes of the KO mice and we did not find significant changes (Supporting Fig. 8A). Moreover, we measured sphingomyelin synthase activity in the liver and did not find significant difference between WT and Sptlc2 KO mice (Supporting Fig. 8B).
Figure 6. Sphingomyelin Supplementation.
(A) in vivo study. Two weeks old Sptlc2 KO mice (n=4) were injected with sphingomyelin mixture or vehicle for 4 weeks, the plasma were collected. Sphingomyelin supplementation reduces the severity of the Jaundice (insert) and plasma bilirubin levels. (B–E) in vitro study. Primary hepatocytes were isolated from Sptlc2 KO and WT mice. The cells were then incubated with sphingomyelin mixture or vehicle for 18 hr. Plasma membrane, cytoplasm, and nucleus were isolated from the hepatocytes. (B) Western blots of cadherin, phosphorylated cadherin, and β-catenin on plasma membrane; phosphorylated cadherin, and β-catenin in cytoplasm; and β-catenin in nucleus from WT and Sptlc2 KO hepatocytes. Fluorogram of cadherin, phosphorylated cadherin, and β-catenin on plasma membrane (C); phosphorylated cadherin and β-catenin in cytoplasm (D); β-catenin in nucleus (E). (F) Western blots and quantification of c-Myc and Cyclin D1. The molecular weight of cadherin, β-catenin, c-Myc, and cyclin D1 are 120/130, 92, 65, and 36 kDa, respectively. Data are represented as mean ± SD, n=6, *P < 0.01.
Discussion
In this study, we prepared liver-specific Sptlc2 deficient mice by crossing Sptlc2-Flox mice with albumin-Cre recombinase transgenic mice. We demonstrated that disruption of liver Sptlc2 gene in early life resulted in: 1) significant increase of total phospholipids, free cholesterol, billirubin, and bile acids in the blood; 2) accumulation of lipoprotein-X in the blood; 3) impairment of hepatocyte apical-basal polarity; 4) promotion of liver tumorigenesis; 5) attenuation of liver regeneration; 6) reduction of plasma membrane cadherin and induction of phosphorylation of cadherin; and 7) alteration of the cellular distribution of β-catenin, the central component of the canonical Wnt pathway. Importantly, SM supplementation can partially correct the phenotype mediated by Sptlc2 deficiency.
One of the key findings in this study is that SPT activity is a critical factor for establishing postnatal hepatocyte polarity. Albumin-Cre expression levels was low at the later stage of embryo and early stage of life (about 40% at day 1 after birth)(38). Also, it is known that during embryonic development, early fetal hepatocytes are not polarized (39, 40). In fetal mice and rats, infrequent small canaliculi are present, but do not attain an adult appearance until several days postpartum (41). Moreover, we found that the heterozygous Sptlc2 KO mice (with 50% depletion) had the same phenotype as that of WT mice (Figs. 1A and B). Thus, albumin-Cre-mediated Sptlc2 depletion should have marginal effect on embryo development and early post-natal period. However, the first 6 weeks after birth is characterized by a post-natal spurt in hepatic growth and development (42). During this period of time, albumin-Cre expression levels, then depletion of Sptlc2, reached to the peak (60% at day 7 and 90% at day 45) (38). The levels of the Cre expression could be maintained since (90% at day 90) (38). It appeared that the Cre expression level was correlated with severity of jaundice observed in the KO mice (Fig. 2C), suggesting that Sptlc2 deficiency gradually attenuated hepatocyte polarity during the later post-natal period. To support the notion that SPT activity requirement for polarity in hapatocytes is restricted to the early stages of development, we used siRNA to knockdown Sptlc2 (more than 90%) in HepG2 cells. We neither observed cell morphological changes, nor cadherine and β-catenin changes (Supporting Fig. 9).
On the other hand, we found that depletion of Sptlc2 (through adenovirus associated virus-Cre) in adult mouse livers did not alter cholesterol or bile metabolism (Supporting Table 1) (20). One possible reason for the absence of the phenotype could be that SPT activity was not completely abolished in that model, and that this might reduce the severity of the subsequent phenotype. However, the heterozygous mice argue against that the differing phenotypes are due to severity of SPT deficiency. An alternative explanation is that adult (10 weeks or older) livers, after the establishment of cell polarity, are stable and resistant to Sptlc2 deficiency-mediated cell polarity changes (20). This is not an uncommon phenomenon. Liver kinase B1 (LKB1) is a primary upstream kinase of adenine monophosphate-activated protein kinase (AMPK), a necessary element in cell metabolism (43) and cell polarity (44, 45). Albumin-Cre-mediated liver-specific deletion of Lkb1 in mice leads to defective canaliculi and bile duct formation, and leads to jaundice and lipoprotein-X accumulation in the blood (46). However, Lkb1 depletion in adult mouse liver had no such effect (43).
Another key finding of this study is that SPT activity is involved in the adherens junction formation. Adherens junctions are mediated by extracellular cadherin domains (18). We found that plasma membrane associated-adherins are dramatically reduced while phosphorylated adherins are dramatically induced (Figs. 5B and C). There are at least two types of cadherin, E-cadherin and N-cadherin, the former is expressed in the periportal zones of the liver, while the latter is expressed in the perivenous zones of the liver (47). We utilized anti-cadherin (pan) antibody to do the experiment on both liver and primary hepatocytes.
Adherens junction impairment could also influence Wnt/β-catenin pathway, since adherens junctions consist of the transmembrane protein cadherin and the intracellular components β-catenin, α-catenin, and actin filaments (17). β-catenin is the central protein in the Wnt pathway (48). The relationship between sphingolipids and the Wnt/β-catenin pathway has been described in different systems (49–52). The effects of sphingolipids in the Wnt/β-catenin pathway are particularly important in cancer cells, since β-catenin is one of the most important proteins regulating development and homeostasis (53). It has been reported that dietary sphingolipids, especially sphingosines and long-chain ceramides, not only decreased the number of adenomas in a mouse colon cancer model, but also resulted in redistribution of β-catenin which was mainly localized to the cellular membranes, similar to WT mice (54). These results were confirmed in vitro using two colon cancer cell lines (55). The effect of sphingolipids (SM in this case) on the redistribution of β-catenin was also observed in CF1 mice treated with dimethylhydrazine (carcinogen) (55). The mechanism behind the redistribution of β-catenin by sphingolipid supplementation is not very well known. It could be a post-transcriptional or post-translational modulation, since the mRNA levels of β-catenin showed no changes (55). We also did not see changes of β-catenin mRNA in Sptlc2 KO mouse liver compared with control (Supporting Fig. 6).
Why does Sptlc2 deficiency impair hepatocyte polarity and promote tumorigenesis? Our studies indicated that SM could be the mediator. We found that Sptlc2 deficiency reduced the levels of almost all SM (Table 1), which are a major component of lipid rafts, in the plasma membrane (14). We also found that primary hepatocyte surface contains significantly less SM, thus resisting lysenin-mediated cell lysate (Fig. 5D). It is known that cadherin stabilization at cell-cell junctions requires raft microdomains, rafts (35, 36). Thus, the reduction of SM could decrease cadherin and cadherin-associated β-catenin in the lipid rafts and could promote more β-catenin entering the nucleus. Indeed, SM supplementation can partially correct the Sptlc2-deficiency-mediated phenotype (Figs. 6A–E). The contribution of other lipid molecules to the phenotype was also considered. Ceramide could be one of the potential mediators. It is known that ceramide has anti-cancer properties (56) and a loss of ceramide from plasma membranes could theoretically promote tumorigenesis. Our studies showed that ceramide subspecies C22:0 and C24:0 were reduced, but C16:0 and C24:1 were induced on the plasma membrane of hepatocytes (Table 1) and total ceramide had no significant changes. Without an overall loss, it is unlikely that the findings are due to changes in the ceramide subspecies. Sphingosine-1-phosphate (S1P), a sphingolipid, could be another mediator. S1P regulates processes such as inflammation, which can drive tumorigenesis (57). However, we did not observe a significant change of S1P, so S1P is unlikely to explain the phenotype. We also measured two glycosphinglipid, glucosylceramide and lactosylceramide, levels in the plasma membrane of primary hepatocytes (Table 1) and we did not find difference between Sptlc2 deficient and control mice. Thus, it is not likely that glycosphinglipids would play some roles in the observed phenotype. We also utilized sphinganine and ceramide supplementation to correct the defect of both cadherin and β-catenin on the plasma membrane of primary hepatocytes of the KO mice and we did not find significant changes (Supporting Fig. 8A). We also measured liver sphingomyelin synthase (SMS) activity and found no difference between the KO and control mice (Supporting Fig. 8B). It is likely that there is a technical difficulty to reverse the SM level by exogenous sphinganine or ceramide. Moreover, no correction was observed when exogenous ganglioside was used (Supporting Fig. 8A), again, suggesting that SM is a crucial factor.
To show the importance of sphingomyelin, liver-specific disruption of sphingomyelin synthase (SMS) should be a more appropriate approach. We are in the middle of preparing preparing liver-specific SMS1/SMS2 doulble KO mice. SM-mediated hepatocyte polarity establishment in the early stages of development deservers further investigation.
Since the Sptlc2 deficiency destroys the polarity of hepatocytes, it would be possible that the mis-localization of bile salt export pump (BSEP) (Fig. 3C) throughout the liver might cause inflammation, and such inflammation would in turn promote tumorigenesis. We measured IL-6 and TNFα mRNA levels in the liver of 10 weeks old KO and WT mice and found that both are significantly increased (Supporting Fig. 10).
In summary, deficiency of liver SPT at the early stage of life leads to loss of cell polarity, severe jaundice, and initial tumorigenesis. The alterations is, or partially, due to a direct influence of plasma membrane sphingomyelin-reduction on cadherin degradation, then β-catenin nuclear accumulation, as proposed in our model (Fig. 7).
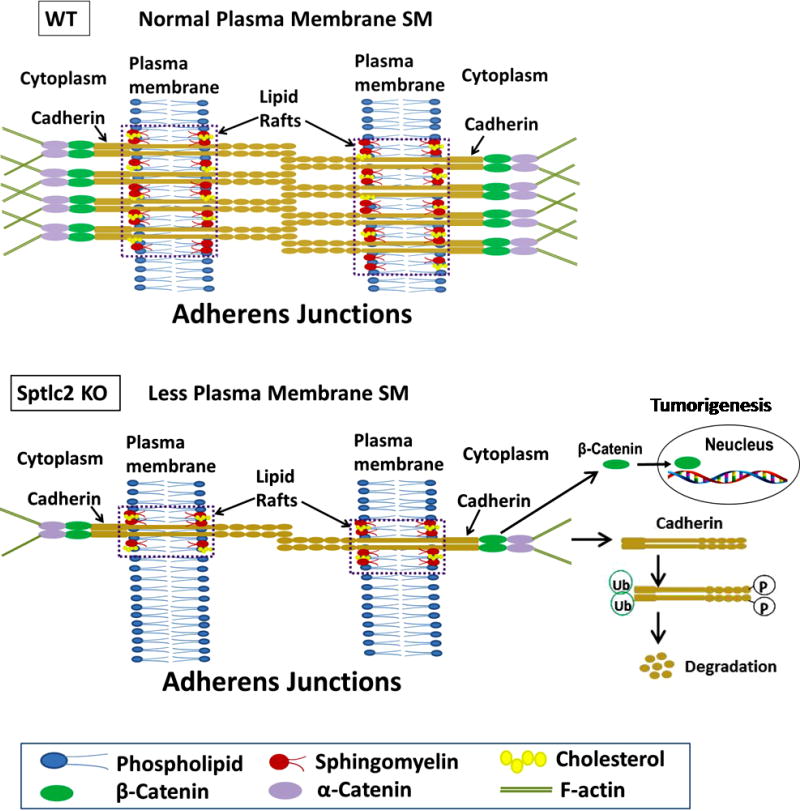
Figure 7. A proposed model.
Sptlc2 deficiency decreases sphingomyelin levels in hepatocyte plasma membranes, especially in lipid rafts, where cadherin is located, thus diminishing the formation of adherens junctional complexes, which contain cadherin, β-catenin, α-catenin, and actin filaments. This would result in more phosphorylated cadherin, then degradation, and results in accumulation of β-catenin into the nucleus, where it can initiate tumorigenesis.
Supplementary Material
Acknowledgments
This work was supported by grants VA Merit 000900-01 to XCJ and NIH-R56HL121409 to XCJ.
Abbreviations used
- SPT
Serine palmitoyltransferase
- Sptlc2
Serine palmitoyltransferase long chair base subunit 2
- SM
sphingomyelin
- KO
gene knockout
Footnotes
AUTHOR CONTRIBUTIONS
Z.L. performed 80% of the experiments, analyzed data and modified the manuscript. I.K., H.J., H.Z., K.R.L., and S.Z. performed some Western blots and lipid analysis. J.L. and J.Z. performed sectioning and staining of liver tumor, and edited the manuscript. P.O. and A.S. performed liver immunostaining; T-S.P., T.S.W., and B.K. measured sphingolipids using LC/MS/MS, and modified the manuscript. H.H.B. and M-S.K. measured sphingolipids using LC/MS/MS. C.H. conceived the ideas and performed hepatectomy experiment. X.C.J. conceived the ideas, designed and discussed experiments, supervised progress and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
Author names in bold designate shared co-first authorship
- 1.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in Chinese hamster overy cells. Biochim Biophys Acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 2.Weiss B, Stoffel W. Human and murine serine-palmitoyl-CoA transferase--cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur J Biochem. 1997;249:239–247. doi: 10.1111/j.1432-1033.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- 3.Hanada K, Hara T, Nishijima M. Purification of the serine palmitoyltransferase complex responsible for sphingoid base synthesis by using affinity peptide chromatography techniques. J Biol Chem. 2000;275:8409–8415. doi: 10.1074/jbc.275.12.8409. [DOI] [PubMed] [Google Scholar]
- 4.Hornemann T, Richard S, Rutti MF, Wei Y, von Eckardstein A. Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J Biol Chem. 2006;281:37275–37281. doi: 10.1074/jbc.M608066200. [DOI] [PubMed] [Google Scholar]
- 5.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, Brown RH, Jr, et al. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc Natl Acad Sci USA. 2009;106:8186–8191. doi: 10.1073/pnas.0811269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A, Ejsing CS, et al. Orm family proteins mediate sphingolipid homeostasis. Nature. 2010;463:1048–1053. doi: 10.1038/nature08787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bejaoui K, Wu C, Scheffler MD, Haan G, Ashby P, Wu L, de Jong P, et al. SPTLC1 is mutated in hereditary sensory neuropathy, type 1. Nat Genet. 2001;27:261–262. doi: 10.1038/85817. [DOI] [PubMed] [Google Scholar]
- 8.Gable K, Han G, Monaghan E, Bacikova D, Natarajan M, Williams R, Dunn TM. Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy type I mutations, dominantly inactivate serine palmitoyltransferase. J Biol Chem. 2002;277:10194–10200. doi: 10.1074/jbc.M107873200. [DOI] [PubMed] [Google Scholar]
- 9.Rotthier A, Auer-Grumbach M, Janssens K, Baets J, Penno A, Almeida-Souza L, Van Hoof K, et al. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am J Hum Genet. 2010;87:513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hojjati MR, Li Z, Jiang XC. Serine palmitoyl-CoA transferase (SPT) deficiency and sphingolipid levels in mice. Biochim Biophys Acta. 2005;1737:44–51. doi: 10.1016/j.bbalip.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Tamehiro N, Zhou S, Okuhira K, Benita Y, Brown CE, Zhuang DZ, Latz E, et al. SPTLC1 binds ABCA1 to negatively regulate trafficking and cholesterol efflux activity of the transporter. Biochemistry. 2008;47:6138–6147. doi: 10.1021/bi800182t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park TS, Panek RL, Mueller SB, Hanselman JC, Rosebury WS, Robertson AW, Kindt EK, et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2004;110:3465–3471. doi: 10.1161/01.CIR.0000148370.60535.22. [DOI] [PubMed] [Google Scholar]
- 13.Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J Biol Chem. 2005;280:10284–10289. doi: 10.1074/jbc.M412348200. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty M, Lou C, Huan C, Kuo MS, Park TS, Cao G, Jiang XC. Myeloid cell-specific serine palmitoyltransferase subunit 2 haploinsufficiency reduces murine atherosclerosis. J Clin Invest. 2013;123:1784–1797. doi: 10.1172/JCI60415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–562. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–612. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamehiro N, Mujawar Z, Zhou S, Zhuang DZ, Hornemann T, von Eckardstein A, Fitzgerald ML. Cell polarity factor Par3 binds SPTLC1 and modulates monocyte serine palmitoyltransferase activity and chemotaxis. J Biol Chem. 2009;284:24881–24890. doi: 10.1074/jbc.M109.014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Z, Li Y, Chakraborty M, Fan Y, Bui HH, Peake DA, Kuo MS, et al. Liver-specific deficiency of serine palmitoyltransferase subunit 2 decreases plasma sphingomyelin and increases apolipoprotein E levels. J Biol Chem. 2009;284:27010–27019. doi: 10.1074/jbc.M109.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silver DL, Wang N, Tall AR. Defective HDL particle uptake in ob/ob hepatocytes causes decreased recycling, degradation, and selective lipid uptake. J Clin Invest. 2000;105:151–159. doi: 10.1172/JCI8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hailemariam TK, Huan C, Liu J, Li Z, Roman C, Kalbfeisch M, Bui HH, et al. Sphingomyelin synthase 2 deficiency attenuates NFkappaB activation. Arterioscler Thromb Vasc Biol. 2008;28:1519–1526. doi: 10.1161/ATVBAHA.108.168682. [DOI] [PubMed] [Google Scholar]
- 23.Liu JY, Lo PC, Jiang XJ, Fong WP, Ng DK. Synthesis and in vitro photodynamic activities of di-alpha-substituted zinc(ii) phthalocyanine derivatives. Dalton Trans. 2009:4129–4135. doi: 10.1039/b817940a. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- 25.Seidel D, Alaupovic P, Furman RH. A lipoprotein characterizing obstructive jaundice. I.Method for quantitative separation and identification of lipoproteins in jaundiced subjects. J Clin Invest. 1969;48:1211–1223. doi: 10.1172/JCI106085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elferink RP, Ottenhoff R, van Marle J, Frijters CM, Smith AJ, Groen AK. Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse. J Clin Invest. 1998;102:1749–1757. doi: 10.1172/JCI3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidel D, Alaupovic P, Furman RH, McConathy WJ. A lipoprotein characterizing obstructive jaundice. II. Isolation and partial characterization of the protein moieties of low density lipoproteins. J Clin Invest. 1970;49:2396–2407. doi: 10.1172/JCI106459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noe J, Stieger B, Meier PJ. Functional expression of the canalicular bile salt export pump of human liver. Gastroenterology. 2002;123:1659–1666. doi: 10.1053/gast.2002.36587. [DOI] [PubMed] [Google Scholar]
- 29.Neufeld EB, Demosky SJ, Jr, Stonik JA, Combs C, Remaley AT, Duverger N, Santamarina-Fojo S, et al. The ABCA1 transporter functions on the basolateral surface of hepatocytes. Biochem Biophys Res Commun. 2002;297:974–979. doi: 10.1016/s0006-291x(02)02274-x. [DOI] [PubMed] [Google Scholar]
- 30.Hagenbuch B, Meier PJ. Molecular cloning, chromosomal localization, and functional characterization of a human liver Na+/bile acid cotransporter. J Clin Invest. 1994;93:1326–1331. doi: 10.1172/JCI117091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan H, Zhang H, Wu X, Zhang Z, Du D, Zhou W, Zhou S, et al. Hepatocyte-specific deletion of Cdc42 results in delayed liver regeneration after partial hepatectomy in mice. Hepatology. 2009;49:240–249. doi: 10.1002/hep.22610. [DOI] [PubMed] [Google Scholar]
- 32.Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, et al. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- 33.Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, et al. alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol. 1998;142:847–857. doi: 10.1083/jcb.142.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 35.Causeret M, Taulet N, Comunale F, Favard C, Gauthier-Rouviere C. N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol Biol Cell. 2005;16:2168–2180. doi: 10.1091/mbc.E04-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boscher C, Zheng YZ, Lakshminarayan R, Johannes L, Dennis JW, Foster LJ, Nabi IR. Galectin-3 protein regulates mobility of N-cadherin and GM1 ganglioside at cell-cell junctions of mammary carcinoma cells. J Biol Chem. 2012;287:32940–32952. doi: 10.1074/jbc.M112.353334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ripple MJ, Parker Struckhoff A, Trillo-Tinoco J, Li L, Margolin DA, McGoey R, Del Valle L. Activation of c-Myc and Cyclin D1 by JCV T-Antigen and beta-catenin in colon cancer. PLoS One. 2014;9:e106257. doi: 10.1371/journal.pone.0106257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postic C, Magnuson MA. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 39.Kingsbury JW, Alexanderson M, Kornstein ES. The development of the liver in the chick. Anat Rec. 1956;124:165–187. doi: 10.1002/ar.1091240204. [DOI] [PubMed] [Google Scholar]
- 40.Feracci H, Connolly TP, Margolis RN, Hubbard AL. The establishment of hepatocyte cell surface polarity during fetal liver development. Dev Biol. 1987;123:73–84. doi: 10.1016/0012-1606(87)90429-5. [DOI] [PubMed] [Google Scholar]
- 41.Kanamura S, Kanai K, Watanabe J. Fine structure and function of hepatocytes during development. J Electron Microsc Tech. 1990;14:92–105. doi: 10.1002/jemt.1060140204. [DOI] [PubMed] [Google Scholar]
- 42.Cadoret A, Ovejero C, Saadi-Kheddouci S, Souil E, Fabre M, Romagnolo B, Kahn A, et al. Hepatomegaly in transgenic mice expressing an oncogenic form of beta-catenin. Cancer Res. 2001;61:3245–3249. [PubMed] [Google Scholar]
- 43.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA. 2007;104:819–822. doi: 10.1073/pnas.0610157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proc Natl Acad Sci USA. 2011;108:1403–1408. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods A, Heslegrave AJ, Muckett PJ, Levene AP, Clements M, Mobberley M, Ryder TA, et al. LKB1 is required for hepatic bile acid transport and canalicular membrane integrity in mice. Biochem J. 2011;434:49–60. doi: 10.1042/BJ20101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doi Y, Tamura S, Nammo T, Fukui K, Kiso S, Nagafuchi A. Development of complementary expression patterns of E- and N-cadherin in the mouse liver. Hepatol Res. 2007;37:230–237. doi: 10.1111/j.1872-034X.2007.00028.x. [DOI] [PubMed] [Google Scholar]
- 48.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Pepperl J, Reim G, Luthi U, Kaech A, Hausmann G, Basler K. Sphingolipid depletion impairs endocytic traffic and inhibits Wingless signaling. Mech Dev. 2013;130:493–505. doi: 10.1016/j.mod.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Liu YY, Gupta V, Patwardhan GA, Bhinge K, Zhao Y, Bao J, Mehendale H, et al. Glucosylceramide synthase upregulates MDR1 expression in the regulation of cancer drug resistance through cSrc and beta-catenin signaling. Mol Cancer. 2010;9:145. doi: 10.1186/1476-4598-9-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchesini N, Jones JA, Hannun YA. Confluence induced threonine41/serine45 phospho-beta-catenin dephosphorylation via ceramide-mediated activation of PP1cgamma. Biochim Biophys Acta. 2007;1771:1418–1428. doi: 10.1016/j.bbalip.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuzaki E, Hiratsuka S, Hamachi T, Takahashi-Yanaga F, Hashimoto Y, Higashi K, Kobayashi M, et al. Sphingosine-1-phosphate promotes the nuclear translocation of beta-catenin and thereby induces osteoprotegerin gene expression in osteoblast-like cell lines. Bone. 2013;55:315–324. doi: 10.1016/j.bone.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Barros M, Coant N, Truman JP, Snider AJ, Hannun YA. Sphingolipids in colon cancer. Biochim Biophys Acta. 2014;1841:773–782. doi: 10.1016/j.bbalip.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmelz EM, Roberts PC, Kustin EM, Lemonnier LA, Sullards MC, Dillehay DL, Merrill AH., Jr Modulation of intracellular beta-catenin localization and intestinal tumorigenesis in vivo and in vitro by sphingolipids. Cancer Res. 2001;61:6723–6729. [PubMed] [Google Scholar]
- 55.Simon KW, Roberts PC, Vespremi MJ, Manchen S, Schmelz EM. Regulation of beta-catenin and connexin-43 expression: targets for sphingolipids in colon cancer prevention. Mol Nutr Food Res. 2009;53:332–340. doi: 10.1002/mnfr.200800102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rizzieri KE, Hannun YA. Sphingolipid metabolism, apoptosis and resistance to cytotoxic agents: can we interfere? Drug Resist Updat. 1998;1:359–376. doi: 10.1016/s1368-7646(98)80012-5. [DOI] [PubMed] [Google Scholar]
- 57.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.