Abstract
Mcm10 is an essential protein that functions to initiate DNA replication after the formation of the replication fork helicase. In this manuscript, we identified a budding yeast Mcm10 mutant (Mcm10-m2,3,4) that is defective in DNA binding in vitro. Moreover, this Mcm10-m2,3,4 mutant does not stimulate the phosphorylation of Mcm2 by DDK in vitro. When we expressed wild-type levels of mcm10-m2,3,4 in budding yeast cells, we observed a severe growth defect and substantially decreased DNA replication. We also observed a substantially reduced RPA-ChIP signal at origins of replication, reduced levels of DDK-phosphorylated-Mcm2 and diminished GINS association with Mcm2-7 in vivo. mcm5-bob1 bypasses the growth defect conferred by DDK-phosphodead-Mcm2 in budding yeast. However, the growth defect observed by expressing mcm10-m2,3,4 is not bypassed by the mcm5-bob1 mutation. Furthermore, origin melting and GINS association with Mcm2-7 are substantially decreased for cells expressing mcm10-m2,3,4 in the mcm5-bob1 background. Thus, the origin-melting and GINS-Mcm2-7-interaction defects we observed for mcm10-m2,3,4 are not explained by decreased Mcm2 phosphorylation by DDK, since the defects persist in an mcm5-bob1 background. These data suggest that DNA binding by Mcm10 is essential for the initiation of DNA replication.
Keywords: DNA replication, yeast, helicase, DNA unwinding, CMG complex
Graphical abstract
Introduction
The replication fork helicase in eukaryotes is composed of Cdc45, the heterohexameric Mcm2-7 complex and the tetrameric GINS (Go, Ichi, Ni and San, Japanese for 5, 1, 2, 3 for Sld5, Psf1, Psf2 and Psf3) forming the CMG complex (1-4). The CMG complex assembles during S phase. The coordinated action of two S-phase-specific kinases: cyclin-dependent kinase (CDK) and the Dbf4-dependet kinase (DDK) is required for the replicative helicase CMG complex assembly (5-7) (8). The Mcm2-7 complex is loaded at origins of replication to encircle double-stranded (ds)-DNA in late M phase or G1 (9) (10). During S phase, single-stranded (ss)-DNA is extruded from the central channel of the Mcm2-7 ring (11). The Mcm2-Mcm5 interface acts as the gate for the DNA movement into the Mcm2-5 ring. When Mcm2-7 is loaded, the Mcm2-7 ring opens at the Mcm2-Mcm5 interface to allow the dsDNA enters the Mcm2-7 central channel (12). Binding of Cdc45 and GINS seal the Mcm2-Mcm5 gate (13). The essential role of DDK is the phosphorylation of Mcm2-7 (19) (20-22) (23) (24). DDK phosphorylation of Mcm2 reduces the affinity between Mcm2 and Mcm5, which may be important for the opening of the gate to allow the extrusion of the ssDNA from the Mcm2-7 complex (18), while DDK phosphorylation of Mcm4 is required for Cdc45 attachment to Mcm2 (22,25). An mcm5 mutant (mcm5-P83L) that bypasses the requirement of DDK in yeast (mcm5-bob1 mutation) has been described (26). This mutation reduces the affinity between Mcm2 and Mcm5, and may open the Mcm2-Mcm5 gate in a similar way that this gate is open when Mcm2 is phosphorylated by DDK, allowing the extrusion of ssDNA (18). While expression of the DDK-phosphodead mutant of Mcm2 (mcm2-S164A-S170A) confers a dominant negative, severe growth defect in budding yeast, this growth defect is nearly completely suppressed by the mcm5-bob1 mutation (20). Expression of the DDK-phosphodead mutant of Mcm2 (mcm2-S164A-S170A) at wild-type levels in the absence of wild-type Mcm2 also results in a severe growth defect that is nearly completely suppressed by the mcm5-bob1 mutation, suggesting that the essential function of DDK-phosphorylation of Mcm2 is nearly completely suppressed by the mcm5-bob1 mutation (18).
Mcm10 is also required for DNA replication initiation (3,4,27). Mcm10 was first identified in the same Saccharomyces cerevisiae genetic screen as Mcm2-7 subunits, but Mcm10 does not share sequence homology with the Mcm2-7 subunits (28-30). Mcm10 has an essential role during helicase activation (31-33). Mcm10 has been shown to interact with the loaded-Mcm2-7 complex during G1 and early S phase (32,34,35). Mcm10 has been shown to be necessary for Pol-α stabilization and loading onto chromatin (27,34,39,40). Mcm10 is a key component of the machinery responsible for the initiation of DNA replication after assembly of the CMG (31,35,41,42). Mcm10 also stimulates the DDK phosphorylation of Mcm2 during S phase (35). In addition to its interactions with different replisome proteins (34,44-46), Mcm10 is able to bind both single- (ss) and double-stranded (ds) DNA. The DNA-binding function of Mcm10 is localized in the highly conserved internal domain (ID) and in the C-terminal domain (CTD). The C-terminal domain is unique to higher eukaryotes and is not present in yeast. Mcm10 shows a preference for ssDNA versus dsDNA, and Mcm10-DNA interaction does not display any sequence specificity(47-49). In this manuscript we show using purified proteins from budding yeast that Mcm10 binds directly to ssDNA and different duplex-DNA structures containing extensions of ssDNA, such as bubble-shaped DNA, which may occur during origin melting. We previously showed that Mcm10 interacts with the Mcm2-7 complex and Cdc45 in vitro (35). We show here that in the presence of ssDNA, the interaction between Mcm10 and both Mcm2-7 and Cdc45 are disrupted.
In this manuscript we identified a mutant of Mcm10, Mcm10-m2,3,4, that is defective in DNA interaction in vitro. In addition, Mcm10-m2,3,4 is defective in stimulating DDK phosphorylation of Mcm2 in vitro. Expression of mcm10-m2,3,4 confers a severe growth defect as a result of a defective DNA replication. Furthermore, when mcm10-m2,3,4 is expressed in budding yeast we observed a reduced replication protein A (RPA-ChIP) signal at origins of replication, decreased Mcm2 phosphorylation by DDK and no GINS recruitment to the Mcm2-7 complex during S phase. When we expressed mcm10-m2,3,4 in the mcm5-bob1 genetic background the growth defect is not suppressed. Furthermore, origin melting and GINS association with Mcm2-7 are substantially decreased for cells expressing mcm10-m2,3,4 in the mcm5-bob1 background. Thus, the origin-melting and GINS-Mcm2-7-interaction defects we observed for mcm10-m2,3,4 are not explained by decreased Mcm2 phosphorylation by DDK, since the defects persist in an mcm5-bob1 background. These data suggest that DNA binding by Mcm10 is essential for the initiation of DNA replication.
Results
Mcm10 binds preferentially to ssDNA
The Mcm10 ID (aa 150-571) is the most conserved region of this protein across all eukaryotes from vertebrates to yeast. This high homology all across eukaryotes indicates an essential function for the Mcm10 ID domain. Mcm10 ID has been shown to interact with ssDNA and dsDNA (48). Previous studies showed that Mcm10 binds both ssDNA and dsDNA with a strong preference for ssDNA and in a sequence-independent manner (46,49). 10-12 nucleotides was the minimal length of ssDNA reported for binding Mcm10 in Xenopus laevis and budding yeast (48) (49). The most stable complex is formed with ssDNA of 20-50 nucleotides, sustaining the formation of a nucleoprotein complex with a ~3:1 stoichiometry of Mcm10 to ssDNA (49). We wanted to investigate the ability of Mcm10 to bind different structures of ssDNA and dsDNA. These are DNA molecules containing a bubble of 80 residues (bubble), duplex DNA with tails of 80 residues (fork), 3′- and 5′-tailed duplexes (3′-tail and 5′-tail), blunt DNA duplex and ssDNA oligonucleotide. The bubble DNA is partially unwound dsDNA, which might mimic the molecular structure that DNA adopts during origin melting, before DNA replication begins. Lengths and structures of these DNAs are shown in Figure 1A. We performed a GST pulldown assay in which we incubated GST-Tag alone and GST-Mcm10 with increasing amounts of radiolabeled ssDNA and dsDNA structures. GST-Mcm10 pulled down a substantial fraction of bubble, ssDNA and fork DNA compared to the GST control (Figure 1A and 1B). Furthermore, GST-Mcm10 pulled down a modest fraction of 5′-, 3′-tailed and blunt DNAs compared to the GST control (Figure 1A and 1B). These data confirm the higher affinity of Mcm10 for ssDNA compared to dsDNA. However, this data also supports that Mcm10 binds to a bubble-structure, the structure that may be found at a replication origin during origin melting (Figure 1A and 1B).
Figure 1. ssDNA and bubble DNA dislodge Mcm10 from Mcm2-7 and Cdc45.
A. 30 pmoles of GST-Mcm10 or GST tag was incubated with increasing concentrations of different radiolabeled DNA structures at 30°C for 10 minutes in a GST pulldown assay. DNA structures and lengths are shown. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Mcm2-7 has multiple bands because all six subunits are tagged with PKA. B. Results from similar experiments to those shown in (A) were quantified, averaged and plotted. C. 30 pmoles of GST-Mcm10 was used to pulldown 30 pmoles of radiolabeled PKA-Mcm2-7 in the presence of increasing amounts of unlabeled bubble DNA. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. D. similar to C, except ssDNA was used instead of bubble DNA. E. similar to C, except blunt dsDNA was used instead of bubble DNA. F. 30 pmoles of GST-Cdc45 was used to pulldown 30 pmoles of radiolabeled PKA-Mcm10 in the presence of increasing amounts of unlabeled bubble DNA. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. G. similar to F, except ssDNA was used instead of bubble DNA. H. similar to F, except blunt dsDNA was used instead of bubble DNA. Graphs from (B), (C), (D), (E), (F), (G) and (H) represents mean values from two independent experiments and error bars indicate the standard deviation of the mean.
It has previously shown that Mcm10 is able to interact with the Mcm2-7 complex in vitro (35,57). We next determined whether ssDNA, bubble or blunt DNAs releases Mcm10 from the Mcm2-7 complex. For that purpose, we incubated GST-Mcm2-7 with radiolabeled PKA-Mcm10 and increasing amounts of ssDNA, bubble or blunt dsDNA. We found that ssDNA and bubble DNA are able to disrupt the binding between Mcm10 and Mcm2-7 complex. As the amount of ssDNA or bubble DNA was increased, there was a progressive decrease in the amount of Mcm10 bound to GST-Mcm2-7 (Figure 1C, 1D and S2). In contrast, blunt dsDNA does not influence the interaction between Mcm10 and Mcm2-7 (Figure 1E and S2). These data suggest that during the origin-melting step, the generation of ssDNA may dislodge Mcm10 from the Mcm2-7 complex since Mcm10 has high affinity for ssDNA and bubble DNA (Figure 1A and 1B). This displacement must be transient, however, since Mcm10 stays bound to Mcm2-7 throughout S phase (34,35). Another possibility could be that Mcm2-7 is the complex that interacts with the ssDNA in this situation.
It has previously shown that Mcm10 binds directly Cdc45 in vitro (35). We next assessed whether ssDNA, bubble or blunt dsDNA releases Mcm10 from Cdc45, analogous to the observation for Mcm2-7. Using a GST pulldown assay, we found that ssDNA and bubble DNA dislodge Mcm10 from Cdc45 in a concentration-dependent manner (Figure 1F and 1G). However, blunt dsDNA does not disrupt the interaction between Mcm10 and Mcm2-7 (Figure 1E). These data suggest that during the origin-melting step, the generation of ssDNA may also dislodge Mcm10 from Cdc45 and the Mcm2-7 complex.
Mcm10-m2,3,4 shows a defective DNA binding activity in vitro
To determine the role of Mcm10-DNA interaction in DNA replication, we sought to identify a mutant that is specifically defective for DNA binding. The DNA binding surface of the Mcm10 ID forms an oligonucleotides/oligosaccharide-binding-fold (OB-fold) cleft and a CCCH zinc finger (ZnF1) motif that contain hydrophobic residues and clusters of polar/charged residues (48). It has previously been described the functional importance of several basic residues belonging to X. laevis Mcm10 ID (Lys293, Lys385 and Lys386) for in vitro DNA binding (48). We wanted to study the corresponding mutations in Saccharomyces cerevisiae Mcm10 (His215, Lys216, Asn313 and Lys314) since these residues are highly conserved (Figure 2A) and the double mutant Asn313Glu/Lys314Glu exhibits decreased cell viability in the presence of hydroxyurea (48). We reversed the charge of the histidine, lysines and asparagine generating mutants H215E (Mcm10-m1), K216E (Mcm10-m2), K314E (Mcm10-m4) and N313E-K314E (Mcm10-m3,4).
Figure 2. Mcm10-m2,3,4 is defective in DNA binding.
A. Sequence alignment of homologous regions of Mcm10 intermediate domain from Homo sapiens (Hs), Mus musculus (Mm), Drosophila melanogaster (Dm), Caenorhabditis elegans (Ce), Sacchraromyces cerevisiaes (Sc) and Schizosaccharomyces pombe (Sp) (48). Residues 207-228 and 305-325 from S. cerevisiae are shown. His215, Lys216, Asn313 and Lys314 were targeted for site-directed mutagenesis. B and C. Mcm10-m1 is Mcm10-H215E, Mcm10-m2 is Mcm10-K216E, Mcm10-m4 is Mcm10-K314E, Mcm10-m3,4 is Mcm10-N313E-K314E and Mcm10-m2,3,4 is K216E-N313E-K314E. B. 30 pmoles of wild-type GSTMcm10, GST-Mcm10-m1, GST-Mcm10-m2, GST-Mcm10-m4, GSTMcm10-m3,4, GST-Mcm10-m2,3,4 or GST tag was incubated with increasing concentrations of radiolabeled ssDNA at 30°C for 10 minutes in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. C. 30 pmoles of wild-type GST-Mcm10, GST-Mcm10-m1, GST-Mcm10-m2, GST-Mcm10-m4, GSTMcm10-m3,4, GST-Mcm10-m2,3,4 or GST tag was incubated with increasing concentrations of radiolabeled bubble DNA at 30°C for 10 minutes in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. Graphs from (B) and (C) represent mean values from two independent experiments and error bars indicate the standard deviation of the mean.
To study the effect of Mcm10-DNA binding, we identified mutations that disrupt both Mcm10-bubble DNA and Mcm10-ssDNA interaction. We performed a GST pulldown assay in which we incubated wild-type Mcm10, Mcm10-m1, Mcm10-m2, Mcm10-m4 or Mcm10-m3,4 with either radiolabeled ssDNA or bubble DNA. We found that mutants Mcm10-m4 and Mcm10-m3,4 were each slightly defective and Mcm10-m2 was modestly defective in DNA binding (Figure 2B and 2C). Mutant Mcm10-m1 was slightly defective in binding bubble DNA and showed a wild-type binding for ssDNA (Figure 2B and 2C). In order to find an Mcm10 mutant that is substantially defective in DNA binding, we combined mutations K216E and N313E-K314E to form a triple mutant Mcm10-K216E-N313E-K314E (Mcm10-m2,3,4). Mcm10-m2,3,4 binds both bubble and ssDNAs at background levels, indicating that Mcm10-m2,3,4 is substantially defective in DNA binding, as measured by this assay (Figure 2B and 2C).
We also wanted to test two more residues from the OB-fold domain (Phe230 and Phe231), which were previously shown to be important for cell viability in the presence of hydroxyurea, and the corresponding X. laevis residue Phe306 was suggested to bind DNA in a structural model (48). For that purpose, we made the Mcm10 double mutant (Mcm10-F230A-F231A), purified the protein and tested it for the interaction with DNA, Mcm2-7 and Cdc45. We found that this mutant was substantially defective in binding to Mcm2-7, Cdc45, ssDNA and bubble DNA (Figure S3). Since this mutant is substantially defective in binding to all of these substrates, and we were looking for a separation-of-function mutation, we did not pursue it further.
Mcm10-m2,3,4 is slightly defective in Mcm2-7 interaction in vitro
Mcm10 binds to the Mcm2-7 complex in vivo and in vitro (31,35,43,45,57). We purified Mcm2-7, wild-type Mcm10 and Mcm10-m2,3,4 to investigate the interaction between these proteins in vitro. We performed a GST pulldown assay, in which wild-type and mutant GST-Mcm10 were incubated with increasing amounts of radiolabeled PKA-Mcm2-7. We found that Mcm10-m2,3,4 was slightly defective in Mcm2-7 binding (Figure 3A).
Figure 3. Mcm10-m2,3,4 mutant binds and recruits Cdc45 to the Mcm2-7 complex.
A. 30 pmoles of wild-type GST-Mcm10, GST-Mcm10-m2,3,4 or GST tag was incubated with increasing concentrations of radiolabeled PKA-Mcm2-7 at 30°C for 10 minutes in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. B. 30 pmoles of GST tag or GST-Cdc45 was incubated with varying amounts of either radiolabeled wild-type PKA-Mcm10 or PKA-Mcm10-m2,3,4 in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. C. 30 pmoles of GST-Cdc45 was incubated with 10 pmoles of radiolabeled PKA-Mcm2-7 and increasing amounts of either radiolabeled wild-type PKA-Mcm10 or PKA-Mcm10-m2,3,4 in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. D. 30 pmoles of GST-Cdc45 was used to pulldown 30 pmoles of radiolabeled PKA-Mcm10-m2,3,4 in the presence of increasing amounts of unlabeled ssDNA. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. E. 30 pmoles of GST-Tag, wild-type GST-Mcm10 or GST-Mcm10-m2,3,4 was incubated with increasing amounts of radiolabeled PKA-GINS in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. Graphs from (A), (B), (C), (D) and (E) represent mean values from two independent experiments and error bars indicate the standard deviation of the mean.
Mcm10-m2,3,4 is able to interact and recruit Cdc45 to the Mcm2-7 complex in vitro
Cdc45 binds weakly to Mcm2-7 in the absence of accessory factors (52). It has previously shown that Mcm10 interacts with Cdc45 in in vitro (35). Furthermore, budding yeast Mcm10 is required for the timely recruitment of Cdc45 to the Mcm2-7 complex during S phase (35,38). To determine whether the Mcm10-Cdc45 interaction was disrupted in our mutant, we performed a GST pulldown assay. Increasing amounts of either radiolabeled wild-type PKA-Mcm10 or PKA-Mcm10-m2,3,4 were incubated with GST-Cdc45 or GST-Tag alone. We observed a slightly reduced Cdc45 interaction, indicating that Mcm10-m2,3,4 is slightly defective in binding Cdc45 (Figure 3B). To test whether Mcm10-m2,3,4 is able to recruit Cdc45 to Mcm2-7, we next incubated GST-Cdc45 with radiolabeled PKA-Mcm2-7 and increasing amounts of either wild-type PKA-Mcm10 or PKA-Mcm10-m2,3,4. We observed a weak interaction between Cdc45 and Mcm2-7 as expected. As increasing amounts of wild-type Mcm10 were added to the reaction, the amount of Cdc45 bound to Mcm2-7 substantially increased. We found that Mcm10-m2,3,4 is slightly defective in Cdc45 recruitment to Mcm2-7 (Figure 3C). We observed that ssDNA was able to dislodge Mcm10 from Cdc45 in vitro (Figure 1F). Next, we determined whether ssDNA was able to disrupt the interaction between Cdc45 and Mcm10-m2,3,4. ssDNA does not affect the interaction between Mcm10-m2,3,4 and Cdc45 (Figure 3D) as expected, since Mcm10-m2,3,4 is a mutant specifically defective in DNA binding and DNA cannot compete with Cdc45 for Mcm10 binding.
Next, we wanted to study the interaction between both Mcm10 wild-type and Mcm10-m2,3,4 with GINS in vitro. We performed a GST-pulldown assay in which we incubated GST-Mcm10, GST-Mcm10-m2,3,4 or GST-Tag with increasing amounts of radiolabeled PKA-GINS. We found that both wild-type Mcm10 and Mcm10-m2,3,4 bind to GINS at background levels (Figure 3E), indicating that Mcm10 interacts very weakly with GINS in vitro.
Mcm10-m2,3,4 is defective in binding and stimulating DDK in vitro
It is known that DDK phosphorylates residues Ser164 and Ser170 of the Mcm2 N-terminal region (20). DDK phosphorylates Mcm2 weakly in vitro (20). Work from our laboratory has previously shown that both Sld3 and Mcm10 interact with DDK in vitro and are able to stimulate the DDK phosphorylation of Mcm2 in budding yeast (35,61). We first wanted to study the interaction between Mcm10-m2,3,4 with both DDK subunits: the catalytic Cdc7 and the regulatory Dbf4. For that purpose, we performed a GST pulldown assay, in which we incubated both GST-Dbf4 and GST-Cdc7 with either wild-type PKAMcm10 or PKA-Mcm10-m2,3,4. We found that Mcm10-m2,3,4 is slightly defective in Dbf4 binding, but substantially defective in Cdc7 binding (Figures 4A and 4B). These data indicate that residues belonging to Mcm10 ID may be important not only for DNA binding, but also for the interaction between Mcm10 and DDK in vitro.
Figure 4. Mcm10-m2,3,4 is defective in stimulating Mcm2 phosphorylation by DDK.
A. 30 pmoles of GST-Dbf4 or GST tag was incubated with increasing concentrations of either radiolabeled wild-type PKA-Mcm10 or PKA-Mcm10-m2,3,4 at 30°C for 10 minutes in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. B. 30 pmoles of GST-Cdc7 or GST tag was incubated with increasing concentrations of either radiolabeled wild-type PKA-Mcm10 or PKAMcm10-m2,3,4 at 30°C for 10 minutes in a GST pulldown assay. The products of the pulldown were analyzed by SDS-PAGE followed by phosphorimaging. Results from similar experiments were quantified, averaged and plotted. C. 5 μg of Mcm2 was incubated with 50 ng of DDK and varying amounts of either wild-type Mcm10 or Mcm10-m2,3,4 in a volume of 25 μl at 30°C for 1 h. The reactions were then analyzed by Western blot for expression of phospho-Mcm2. Graphs from (A) and (B) represent mean values from two independent experiments and error bars indicate the standard deviation of the mean.
We next determined the capacity of Mcm10-m2,3,4 to stimulate Mcm2 phosphorylation by DDK in vitro. For that purpose, we performed an in vitro kinase assay in which Mcm2 was incubated with DDK in the presence of increasing amounts of either wild-type Mcm10 or Mcm10-m2,3,4. We analyzed those samples by Western-blot using an antibody directed against the phosphorylated version of Mcm2 (anti-Mcm2-phospho-Ser164-phospho-Ser170)(18,20). We detected a substantial increase in Mcm2 phosphorylation as wild-type Mcm10 was added to the reaction (Figure 4C, left panel). In contrast, Mcm10-m2,3,4 had no effect on the phosphorylation state of Mcm2 (Figure 4C, right panel). We detected some phosphoMcm2 signal at the no-DDK point for wild-type Mcm10 (Figure 4C, left panel). It may be the result of some weak cross-reactivity between the phospho-antibody and unphosphorylated Mcm2. These data indicate that Mcm10-m2,3,4 is defective in stimulating DDK phosphorylation of Mcm2, possibly as a result of a substantially defective Cdc7 interaction.
These data suggest that residues Lys216, Asn313 and Lys314 from Mcm10 ID are also involved in the ability of Mcm10 to bind and stimulate DDK phosphorylation of Mcm2.
Expression of mcm10-m2,3,4 at wild-type levels results in a severe growth defect and slowed progression through S-phase
To investigate the in vivo role of Mcm10-m2,3,4 we constructed plasmids with wild-type and MCM10 mutant mcm10-m2,3,4 under the control of GalS promoter, a low-copy inducible expression system (pRS415 vector) (62). These plasmids were transformed into cells harboring an indole-3-acetic (IAA)-inducible degron (aid) for MCM10 (mcm10-1-aid) (41). In these cells, Mcm10 degradation is induced by the addition of IAA before releasing cells from G1. At the permissive conditions (in the absence of IAA) and in the absence of galactose only the genomic copy of MCM10 is expressed. Under the restrictive conditions (in the presence of IAA) and in the presence of galactose, only the plasmid copy of mcm10 is expressed and is expressed at wild-type levels. We varied levels of galactose to achieve equal Mcm10 expression levels in our plasmid containing cells (degron on) compared to wild-type cells (degron off) (Figure S4).
We studied these strains for cell growth by performing a 10-fold serial dilution and spotting on agar plates. At the permissive conditions and in the absence of galactose, cells harboring plasmids with mcm10-m2,3,4 and vector control all grow like MCM10-WT (Figure 5A, left panel). However, at the restrictive conditions there is a severe growth defect in the mcm10-m2,3,4 and vector control compared to the MCM10-WT (Figure 5A, right panel).
Figure 5. Expression of mcm10-m2,3,4 in budding yeast results in a severe growth defect and reduced RPA binding at replication origins.
A. 10-fold serial dilution analysis of budding yeast mcm10-1-aid cells expressing MCM10-WT, vector and mcm10-m2,3,4 from the GAL-S plasmid inducible promoter system (pRS415). Plates were incubated for 3 days at 25°C. B. mcm10-1-aid cells expressing MCM10-WT and mcm10-m2,3,4 were grown as described in Material and Methods. Cells were analyzed by FACS with propidium iodide staining for DNA content. C. mcm10-1-aid cells expressing MCM10-WT and mcm10-m2,3,4 were grown and chromatin immunoprecipitation was performed as described in Material and Methods. We used an antibody against RPA. Radioactive PCR bands were quantified, averaged and plotted. D. mcm10-1-aid cells expressing MCM10-WT and mcm10-m2,3,4 were grown as described in Material and Methods. D, upper panel. Mcm2 IP samples were analyzed by Western blot for expression of Mcm2. D, lower panel. Mcm2 IP samples were analyzed by Western blot for expression of phospho-Mcm2. Results from similar experiments were quantified, averaged and plotted. Graphs from (C) and (D) represent mean values from two independent experiments and error bars indicate the standard deviation of the mean.
To determine whether the growth defect of mcm10-m2,3,4 cells was the result of a DNA replication defect, we performed FACS analysis. We found normal progression through S phase in wild-type cells, whereas a slow S phase progression for cells expressing mcm10-m2,3,4 was observed (Figure 5B). This result indicates that cells expressing mcm10-m2,3,4 show a defect in DNA replication.
Expression of mcm10-m2,3,4 results in a decreased RPA signal at origins of replication
The binding of yeast ssDNA binding protein RPA to origins of replication occurs as a result of origin melting and the initiation of helicase bidirectional movement. We next determined whether cells expressing mcm10-m2,3,4 at the restrictive conditions exhibit a defect in RPA signal at origins of replication. For this experiment, we synchronized cells in G1 with α-factor in the presence of IAA and then released them into medium lacking α-factor for 0, 15, 30, 45 and 60 minutes to assess the recruitment of RPA to DNA during S phase. We performed chromatin immunoprecipitation with antibodies against RPA (RPA-ChIP). We then subjected the immunoprecipitates to quantitative PCR using oligonucleotides directed against the early origin ARS306 and a non-origin region located midway origins ARS305 and ARS306. RPA-ChIP signal at an origin increases in wild-type cells after 15 min of releasing cells into S phase, coincident with the formation of ssDNA at origins of replication. At 60 min we observed a decrease in RPA-ChIP signal, indicating that cells are entering G2. In contrast, cells expressing mcm10-m2,3,4 exhibit a substantially decreased RPA-ChIP signal at origin of replication at all time points (Figure 5C).
Expression of mcm10-m2,3,4 results in a decreased Mcm2 phosphorylation during S phase
DDK phosphorylates Mcm2 during S phase in yeast (19). We found that Mcm10-m2,3,4 is defective in DDK binding and DDK stimulation of Mcm2 phosphorylation in vitro (Figure 4). To determine whether Mcm10-m2,3,4 has any effect on Mcm2 phosphorylation by DDK in vivo, we synchronized cells in G1 with α–factor under restrictive conditions and then released them into S phase for 0, 15, 30 and 45 minutes. We made whole cell extracts from cells expressing MCM10-WT and mcm10-m2,3,4 and studied phospho-Mcm2 levels by Western-blot. In cells expressing MCM10-WT, we detected an increase in DDK phosphorylation of Mcm2 as cells entered and progressed through S phase (Figure 5D, lower left panel). We detected some phosphoMcm2 signal at the 0 time point corresponding to G1. This may be the result of either some weak cross-reactivity between the phospho-antibody and unphosphorylated Mcm2, or that maybe some Mcm2 is phosphorylated at serines 164 and 170 in G1. In cells expressing mcm10-m2,3,4, we observed a substantially reduced DDK-phosphorylated-Mcm2 signal compared to normal cells (Figure 5C, lower right panel), indicating that DDK phosphorylation of Mcm2 is decreased in vivo when mcm10-m2,3,4 is expressed, supporting our data in which Mcm10-m2,3,4 is defective in DDK stimulation in vitro (Figure 4C).
Expression of mcm10-m2,3,4 results in a severe reduced GINS binding to the Mcm2-7 complex
To assess whether CMG is assembled in cells expressing mcm10-m2,3,4, cells were arrested in G1 with α–factor and incubated under restrictive conditions for 3 hours. Then, cells were released into S phase for 0, 15, 30 and 45 minutes. No crosslinking agent or hydroxyurea were added to these experiments. We made whole cell extracts and probed them with antibodies against Mcm10. Western analysis revealed similar levels of MCM10-WT and mcm10-m2,3,4 (Figure 6A, left panel). These data suggest that the mutation does not alter the expression levels of the Mcm10 protein. We next probed whole cell extracts for Cdc45 and GINS and found similar levels of these two proteins in cells expressing MCM10-WT and mcm10-m2,3,4 (Figure 6A, left panel).
Figure 6. GINS binding is substantially defective in cells expressing mcm10-m2,3,4.
A. mcm10-1-aid cells expressing MCM10-WT and mcm10-m2,3,4 were grown as described in Material and Methods. A, left panel. Whole cell extracts were analyzed by Western blot for the expression of the indicated proteins. A, right panel. Cells were immunoprecipitated with antibodies directed against Mcm2 as described in Materials and Methods, followed by Western analysis with antibodies directed against Mcm2, Mcm10, Cdc45 and GINS. Whole cell extracts were analyzed by Western blot for Arp3 expression as a loading control. B. Results from experiments similar to those shown in (A) were quantified, averaged and plotted. Graphs represent mean values from three independent experiments and error bars indicate the standard deviation of the mean.
We then immunoprecipitated cells with an antibody against Mcm2 in order to isolate loaded Mcm2-7 complexes on chromosomal DNA as described (31). For cells expressing MCM10-WT and mcm10-m2,3,4, the interaction between Mcm10 and Mcm2-7 is clearly visible at G1 and S phase (Figure 6A, right panel and 6B). These data suggest that expression of mcm10-m2,3,4 does not affect the interaction between Mcm10 and Mcm2-7 in vivo.
We also found that the interaction between Cdc45 and Mcm2-7 was similar for cells expressing MCM10-WT and mcm10-m2,3,4 (Figure 6A, right panel and 6B), indicating that Cdc45 recruitment by mcm10-m2,3,4 is not affected, supporting our in vitro data (Figure 3C and 3D). Although there is no effect of mcm10-m2,3,4 on the loading of Cdc45 to Mcm2-7 during S phase, there is a substantially diminished signal for GINS-Mcm2-7 interaction in cells expressing mcm10-m2,3,4 (Figure 6A, right panel and Figure 6B).
We next performed Mcm10-ChIP to study whether cells expressing mcm10-m2,3,4 at the restrictive conditions exhibit a defect in Mcm10 signal at origins of replication during G1. For this experiment, we arrested cells in G2/M with nocodazole and then cells were washed and released into fresh medium containing α-factor. We performed chromatin immunoprecipitation with antibodies against Mcm10. We then subjected the immunoprecipitates to quantitative PCR using oligonucleotides directed against the early origins ARS305 and ARS306 and a non-origin region located midway origins ARS305 and ARS306. Cells expressing mcm10-m2,3,4 exhibit similar origin Mcm10-ChIP signal in G1 phase compared to cells expressing MCM10-WT (Figure S5). We detected very little Mcm10 signal during G2/M or at the non-origin region in cells expressing wild-type or mutant Mcm10 (Figure S5). These data indicate that the expression of mcm10-m2,3,4 does not affect the localization of Mcm10 at origins of replication during G1 in vivo. The localization of Mcm10 at sites of replication initiation occurs by direct interaction with the DNA-loaded Mcm2-7 complex (57).
The presence of mcm5-bob1 mutation does not suppress the growth defect observed in mcm10-m2,3,4
The mcm5-bob1 mutation is able to bypass the requirement for Cdc7 during S phase in budding yeast (26). mcm5-bob1 also suppresses the severe growth defect conferred by a mutant of Mcm2 (mcm2-S164A-S170A) that is not phosphorylated by DDK (18). We wanted to study the effect of the expression of mcm10-m2,3,4 in mcm10-1-aid cells harboring the mcm5-bob1 genetic background. We transformed mcm10-1-aid mcm5-bob1 cells with our galactose inducible MCM10-WT and mcm10-m2,3,4. The growth defect observed when mcm10-m2,3,4 mutant is expressed under restrictive conditions (Figure 7A) was not at all suppressed by the presence of mcm5-bob1 mutation (Figure 7B). As a control, we repeated with this strain the observation that mcm5-bob1 suppresses the severe growth defect conferred by a mutant of Mcm2 (mcm2-S164A-S170A) that is not phosphorylated by DDK (Figure S5).
Figure 7. The growth defect observed in cells expressing mcm10-m2,3,4 is not suppressed by the presence of mcm5-bob1 mutation.
A. 10-fold serial dilution analysis of budding yeast mcm10-1-aid cells expressing MCM10-WT and mcm10-m2,3,4 from the GAL-S plasmid inducible promoter system (pRS415). Plates were incubated for 3 days at 25°C. B. similar to A, except the cells harbored the mcm5-bob1 mutation. C. mcm10-1-aid mcm5-bob1cells expressing MCM10-WT and mcm10-m2,3,4 were grown as described in Material and Methods. C, left panel. Whole cell extracts were analyzed by Western blot for the expression of the indicated proteins. C, right panel. Cells were immunoprecipitated with antibodies directed against Mcm2 as described in Materials and Methods, followed by Western analysis with antibodies directed against Mcm10, Cdc45, GINS, RPA, Mcm2 and phospho-Mcm2.
Next, we determined the assembly of CMG in cells expressing mcm10-m2,3,4 in the mcm5-bob1 background. We arrested cells in G1 with α–factor and incubated under restrictive conditions for 3 hours. Then, cells were released into S phase for 0, 15, 30 and 45 minutes. We made whole cell extracts and immunoprecipitated cells with an antibody against Mcm2 as described for Figure 6. For cells expressing MCM10-WT and mcm10-m2,3,4, the interaction between Mcm10 and Mcm2-7 is clearly visible at G1 and S phase (Figure 7C, right panel). These data suggest that the presence of mcm5-bob1 mutation does not affect the interaction between either wild-type or mutant Mcm10 and Mcm2-7 in vivo. We also found that the interaction between Cdc45 and Mcm2-7 was similar for cells expressing MCM10-WT and mcm10-m2,3,4 (Figure 7C, right panel), indicating that Cdc45 recruitment by MCM10-WT and mcm10-m2,3,4 is not affected by the presence of mcm5-bob1 (Figure 7C, right panel). In contrast, there is a substantially reduced signal for GINS-Mcm2-7 interaction in cells expressing mcm10-m2,3,4 (Figure 7C, right panel), similar to the result observed in cells harboring MCM5-WT (Figure 6A, right panel and 6B).
In cells expressing mcm10-m2,3,4 in the MCM5-WT background, we observed a substantially reduced DDK-phosphorylated-Mcm2 and a diminished RPA signal at origins of replication compared to normal cells (Figure 5C and 5D). We next examined levels of phospho-Mcm2 and RPA in mcm5-bob1 cells expressing either MCM10-WT or mcm10-m2,3,4. We observed that levels of both RPA and phospho-Mcm2 are substantially reduced in mcm5-bob1 cells expressing mcm10-m2,3,4 compared to MCM10-WT (Figure 7C, right panel), suggesting that origin melting is defective. The expression of mcm10-m2,3,4 in the mcm5-bob1 background results in following defects: reduced phosphorylation of Mcm2 by DDK, diminished RPA signal at origins of replication and defective GINS-Mcm2-7 interaction.
Discussion
ssDNA-Mcm10 Interaction Disrupts the Interaction with Mcm2-7 and Cdc45
The Mcm10 ID displays high sequence similarity among species. It is involved in DNA binding (47-49), but also involved in protein-protein interactions with Pol-α, the Mcm2-7 complex, PCNA and Dbf4 (34,44-46), reflecting the adaptability of this domain to bind different molecules belonging to the replication fork.
We found that budding yeast Mcm10 has distinct affinity for ssDNA and dsDNA binding in vitro (Figure 1A and Figure 1B). DNA competition experiments presented in this manuscript demonstrate that ssDNA and bubble DNA, but not blunt dsDNA, disrupt the interaction between Mcm10 and Mcm2-7 in vitro (Figure 1C, 1D and 1E). ssDNA and bubble DNA are present at a replication origin during origin melting. Thus, during the origin-melting step, ssDNA or bubble DNA may dislodge Mcm10 from Mcm2-7. However, this disruption must be transient, since Mcm10 remains associated with Mcm2-7 throughout S phase (34,35). Mcm10 binds and recruits Cdc45 to Mcm2-7 in an in vitro assay (35,38). Furthermore, ssDNA and bubble DNA, but not dsDNA, also disrupts the interaction between Mcm10 and Cdc45 (Figure 1F, 1G and 1H).
The Growth Defect Shown by the Expression of mcm10-m2,3,4 is the Result of a Diminished Mcm10-ssDNA Interaction
We identified point mutations in the Mcm10 ID that are specifically defective for DNA interaction in vitro (Figure 2) and DDK phosphorylation of Mcm2 in vitro and in vivo (Figure 4 and 5D). Expression of mcm10-m2,3,4 in an auxin-inducible degron (mcm10-1-aid) at the restrictive conditions resulted in a severe growth defect as a result of a defective DNA replication (Figure 5A and 5B). These data suggest that the interaction of Mcm10 with either DNA and/or DDK is required for DNA synthesis in vivo. To distinguish whether the defect in DNA replication exhibited by expressing mcm10-m2,3,4 is the result of defective Mcm10-DNA binding or defective Mcm10-stimulation of DDK phosphorylation of Mcm2, we expressed mcm10-m2,3,4 in an mcm5-bob1 background. The mcm5-bob1 mutation (mcm5-P83L) suppresses the DNA replication defect conferred by the expression of the Mcm2 mutant that cannot be phosphorylated by DDK (mcm2-S164A-S170A)(18). Furthermore, mcm5-bob1 suppresses the DNA replication defect conferred by deleting cdc7 from the genome (53). We found that in the mcm5-bob1 background, expression of mcm10-m2,3,4 resulted in a severe growth defect, similar to that observed in the MCM5-WT background (Figure 7A and 7B). These data suggest that the severe cell growth defect observed due to expression of mcm10-m2,3,4 is the result of a diminished Mcm10-DNA interaction, and not diminished DDK phosphorylation of Mcm2. Another possibility is that the growth defect observed in Mcm10 mutant is the result of some altered Mcm10-Mcm2-7 interaction in cells expressing mcm10-m2,3,4 since this mutant is slightly defective in Mcm2-7 binding in vitro (Figure 3A).
We next determined the mechanism for the defect in cell growth due to expression of mcm10-m2,3,4 in an mcm5-bob1 background. We found that Cdc45 interaction with Mcm2-7 is similar to MCM10-WT (Figure 7C, right panel), suggesting that disrupting Mcm10-DNA interaction does not reduce Cdc45 recruitment to Mcm2-7. We also found that Mcm2-RPA interaction during S phase is substantially reduced in cells expressing mcm10-m2,3,4 (Figure 7C, right panel), suggesting that origin melting is defective in the mutant cells. These data suggest that Mcm10-DNA interaction is required for origin melting in budding yeast. Mcm10 does not have any ATPase activity, and it thus does not function as a helicase. It is likely that the ATPase activity of Mcm2-7 or ORC (63) is required for the catalytic melting of origin DNA. Nevertheless, Mcm10 may play an accessory role in this process of origin melting. Mcm10 may stabilize the bubble structure during origin melting, since purified Mcm10 has high affinity for bubble DNA (Figures 1A and 1B). Furthermore, GINS interaction with Mcm2-7 is substantially decreased in cells expressing mcm10-m2,3,4 compared to MCM10-WT, indicating that GINS does not assemble with Mcm2-7 in mutant cells. These data suggest that Mcm10-DNA interaction is required for GINS assembly with Mcm2-7 in budding yeast. Mcm10 does not bind directly to GINS (Figure 3E), and DNA does not directly affect the interaction between GINS and Mcm2-7; thus, the mechanism for how loss of Mcm10-DNA interaction results in diminished GINS recruitment to Mcm2-7 is likely an indirect mechanism. One possibility to explain this indirect effect is that Sld3 and Sld2 block the interaction between GINS and Mcm2-7. Once the origin is melted, however, Sld3 and Sld2 are sequestered by ssDNA, allowing GINS to bind Mcm2-7 by a passive, sequestration mechanism (52,55,56,64). The lack of DNA melting at origins of replication could explain the defective GINS-Mcm2-7 interaction during S phase in cells expressing mcm10-m2,3,4. Other mechanisms connecting Mcm10-DNA interaction with GINS-Mcm2-7 interaction are possible as well, since the observed effect is indirect.
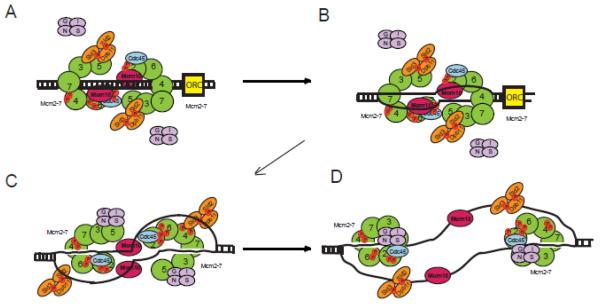
Model for the Initiation of DNA Replication in Budding Yeast
Mcm2-7 loads as a double hexamer during late M phase and G1 (Figure 8A). This reaction is catalyzed by ORC, Cdc6, Cdt1, and ATP (9,10,12,65). In S phase, Mcm10 and Sld3 stimulate DDK phosphorylation of Mcm2. Both Mcm10 and Sld3 are required for efficient DDK phosphorylation of Mcm2 in vivo (35,61). In addition, DDK phosphorylates Mcm4 and Mcm6 (22) (23,24) (66) (67) (Figure 8B). DDK phosphorylation of Mcm4 stimulates Sld3 binding to Mcm4 (25) (not shown) and DDK phosphorylation of Mcm4 is required for Sld3 recruitment of Cdc45 to Mcm2-7 (8,21,22,68-70) (35). CDK is also active at this time, catalyzing the phosphorylation of Sld2 and Sld3. CDK-phosphorylated Sld2 and Sld3 from a ternary complex with Dpb11 (71-73). Furthermore, Sld2 and Sld3 block the premature interaction between GINS and Mcm2-7 prior to origin melting, by a direct-competition mechanism for binding Mcm5 and Mcm3 (52,56,64).
Figure 8. Mcm10 promotes origin melting in this speculative model for DNA replication initiation.
A. The Mcm2-7 complex is loaded as a double hexamer to encircle dsDNA during late M and G1 phase. DDK phosphorylates Mcm2, Mcm4 and Mcm6. DDK phosphorylation of Mcm2 promotes opening of the Mcm2-Mcm5 gate. B. Mcm10 binds to ssDNA to promote origin melting. C. Once the origin DNA is melted, Sld3/Sld2/Dpb11 dissociate from Mcm2-7, gripping the melted DNA. D. The CMG helicase is assembled around ssDNA to unwind DNA bidirectionally.
Once DDK phosphorylates Mcm2, the Mcm2-7 ring may open (Figure 8A). This ring-opening mechanism allows for the extrusion of ssDNA from the central channel of Mcm2-7 (18) (Figure 8B). Origin melting is probably catalyzed by ORC and Mcm2-7, since these proteins are ATPases (63) (not shown). Nevertheless, origin melting may also depend upon Mcm10-interaction with ssDNA, as revealed in this manuscript, possible because Mcm10 stabilizes the melted DNA structure (Figure 8B). Mcm10 may release from Mcm2-7 during origin melting, gripping the single-stranded DNA as the double-stranded DNA is partially unwound. Mcm10 binding to partially melted origin DNA may prevent the two origin strands from reannealing, and thus Mcm10 may promote origin melting. The Sld3-Sld2-Dpb11 ternary complex has high affinity for ssDNA and ssDNA sequesters Sld3-Sld2-Dpb11 from binding to Mcm3/Mcm5 (56,64) (Figure 8C). This allows GINS to bind to Mcm5/Mcm3 interface of Mcm2-7 by a passive, sequestration mechanism (Figure 8C). The CMG complex is now completely assembled to encircle ssDNA (Figure 8D). Mcm10 binding to ssDNA is transient (Figure 8D), since Mcm10 interaction with Mcm2-7 is observed throughout S phase (34,35). Thus, Mcm10, unlike Sld2-Sld3-Dpb11, returns to bind CMG after origin melting is complete.
Materials and Methods
Cloning and purification of proteins
Full length Mcm10 PCR product was cloned into SpeI/XhoI sites of pET-41 vector and NdeI/XhoI sites of pET-33 vector to contain an N-terminal GST tag or a PKA tag respectively as described (50). The cloning of Mcm10 into pET-41 generates two-tagged protein (a GST tag at the N-terminus and a His tag at the C-terminus). We subcloned Mcm10 from pET-41-Mcm10 into pRS415 plasmid. We used pET-41-Mcm10, pET-33b-Mcm10 and pRS415-Mcm10 plasmids as templates to generate mutations on Mcm10 gene using Quick Change Site-directed Mutagenesis Kit (Agilent Technologies). Purification of wild-type and mutant GST-Mcm10 uses sequential nickel and glutathione resins. The details of GST-Mcm10 and PKA-Mcm10 purification are described (50). GST-Cdc45 was purified as described (51). Mcm2-7 proteins were purified and Mcm2-7 complex was assembled from recombinant subunits as described (14,20). PKA-GINS was purified as described (52). GST-Dbf4 and GST-Cdc7 were purified a described (20). Protein kinase A was a generous gift from Susan Taylor. Silver stained-gels of all of the proteins used in this study are shown in Figure S6.
DNA substrates
The sequences of the synthetic oligonucleotides used to prepare the DNA substrates are reported in Table S1 (Supplemental Data). Oligonucleotides were end labeled with T4 polynucleotide kinase (New England Biolabs) and radiolabeled ssDNA was purified over G-25 Sephadex Columns for Radiolabeled DNA Purification (Roche) according to manufacturer’s instructions. To make double stranded (ds) DNA substrates, 4 μl of 500 nM radiolabeled ssDNA was incubated with 4 μl of 1 μM complementary DNA (1:2 molar ratio) and 4 μl of reaction buffer (20 mM Tris-HCl, 4% glycerol, 0.1 mM EDTA, 40 μg/ml BSA, 5 mM DTT and 10 mM magnesium acetate) in a final volume of 12 μl. The reaction was incubated 5 min at 95°C in a water bath, and then allowed to gradually cool down to 25°C. The reaction was then diluted with TE (20 mM Tris-HCl pH 8, 0.1 mM EDTA) to a final volume of 40 nM radiolabeled DNA.
Kinase labeling of proteins
Kinase labeling was performed as described (50). Proteins with a PKA tag at the N-terminus (wild-type and mutant Mcm10, Mcm2, Mcm2-7, GINS) were labeled in a reaction volume of 100 μl that contain 20 μM of PKA-tagged protein with 5 μg PKA in kinase reaction buffer (25 mM Tris-HCl pH7.5, 5mM DTT, 50 mM MgCl2 and 500 μCi [γ-32 P]-ATP). The reaction was incubated at 30°C for 1 hour.
GST pulldown assay
The GST pulldown assays were performed as described (50). GST-tagged protein attached to Glutathione Sepharose (GE Healthcare) previously equilibrated with GST binding buffer (40 mM Tris-HCl pH 7.5, 100 mM NaCl, 0.1 mM EDTA, 10% glycerol, 0.1% Triton X-100, 1 mM DTT, 0.7 μg/ml pepstatin, 0.1 μg/ml leupeptin, 0.1 mM PMSF and 0.1 mg/ml BSA) was incubated with varying concentrations of radiolabeled protein in GST binding buffer in a final reaction volume of 100 μl. The reactions were incubated at 30°C for 10 minutes with gentle mixing every few minutes. When the binding was complete, the reactions were shifted to room temperature and glutathione beads were allowed to settle. The supernatant was removed and the beads were washed two times with 500 μl GST binding buffer. After the last wash, beads were heated at 90°C for 10 minutes in SDS-sample buffer (2% SDS, 4% glycerol, 4 mM Tris-HCl, 2 mM DTT and 0.01% bromophenol blue). The reactions were then analyzed by SDS-PAGE followed by phosphorimaging and quantification.
DDK kinase assay
Kinase reactions were performed in a volume of 25 μl and contained 25 mM HEPES-NaOH pH 7.5, 1 mM DTT, 10 mM magnesium acetate, 75 mM sodium acetate, 5 mM ATP, 0.1 mg/ml BSA, 1 mM sodium fluoride, 0.1 mM Na3VO4, 5 μg Mcm2 and 50 ng DDK. The amount of Mcm10 added in each reaction is described in the figure. Reactions were incubated at 30°C for 1 h. Reactions were stopped by addition of SDS-sample buffer and analyzed by Western blot using an antibody against Mcm2-164-phosphoserine-170-phosphoserine.
Yeast strains and cell growth
All yeast strains used in this study are shown in Table S2 (Supplemental Data). The mcm10-1-aid strains were obtained from Yeast Genetic Resource Center. The mcm5-bob1 strain was obtained from Robert A. Sclafani (53). The mcm10-1-aid mcm5-bob1 strain was constructed by yeast mating (using strains BY25926 and RSY728), sporulation and tetrad analysis. The presence of mcm5-bob1 mutation was confirmed by survival at the restrictive temperature of 37°C after Cdc7 deletion. The cdc7 deletion mutation cdc7Δ::Trp1 was created by PCR-based gene disruption technique and confirmed by PCR. The strain that grew at 37°C after Cdc7 deletion was then sequenced to confirm the presence of mcm5-bob1. Yeast transformation was carried out by either electroporation (plasmid DNA) or the lithium acetate procedure (linear DNA) (54). In all experiments cells were grown at 25°C to slow down replication dynamics as described before (27). mcm10-1-aid and mcm10-1-aid mcm5-bob1 strains carrying plasmids for exogenous expression of wild-type mcm10 and mcm10-K216E-N313E-K314E were grown overnight in CSM-Leu media supplemented with 2% raffinose. Wild-type and mutant Mcm10 proteins were overexpressed in YPGal media from galactose-inducible promoter in the presence of 500 μM IAA (Sigma) to induce the degradation of endogenous MCM10 gene. mcm10-1-aid and mcm10-1-aid mcm5-bob1 strains carrying plasmids for exogenous expression of MCM2-WT and mcm2-S164A-S170A were grown overnight in CSM-Leu media supplemented with 2% raffinose. MCM2-WT and mcm2-S164AS170A proteins were overexpressed in YPGal media from galactose-inducible promoter.
Plasmids
The following plasmids were used in this study: pPP13 (pRS415 CEN6/ARSH4 GALS::MCM10 LEU2), pPP23 (pRS415 CEN6/ARSH4 GALS::mcm10K216E N313E K314E LEU2), pIB302 (pRS415 CEN6/ARSH4 GALS::MCM2 LEU2), pIB305 (pRS415 CEN6/ARSH4 GALS::mcm2S164A S170A LEU2).
Yeast serial dilution analysis
Serial dilution was performed as described (55). 10-fold serial dilution was performed and spotted on the indicated media and incubated at 25°C for 3 days.
Co-immunoprecipitation
Cells were grown overnight in CSM-Leu containing raffinose (2%) at 25°C. When the cell density reached 6×106 cells, the cells were spun down and resuspended in YPGal (0.15% galactose) in the presence of 500 μM IAA and α-factor (Zymo Research). The cells were grown for 3 hours at 25°C, spun down, washed three times and resuspended in fresh, pre-warmed YPGal with 500 μM IAA. Cells were further incubated at 25°C for the indicated times. Co-immunoprecipitation was performed as described (56). Cells were collected and lysed at 4°C with acid-washed glass beads (Sigma) in IP buffer [100 mM HEPES-KOH pH 7.9, 100 mM potassium acetate, 10 mM magnesium acetate, 2 mM sodium fluoride, 0.1 mM Na3VO4, 20 mM β-glycerophosphate, 1% Triton X-100, 0.7 μg/ml pepstatin, 0.1 μg/ml leupeptine, 0.1 mM PMSF, 1x complete protease inhibitor cocktail without EDTA (Roche)] using a BeadBeater. Lysed material was treated with 200U of Benzonase (Novagen) at 4°C for 30 minutes. Clarified extracts were then mixed with 2 μl of specific antibody and rotated for 2 hours at 4°C. Following this, 50 μl of Protein G Sepharose beads (GE Healthcare) equilibrated in IP buffer were added to the extracts and further rotated for 1 hour at 4°C. Beads were washed twice with IP buffer and finally resuspended in SDS-sample buffer. Western analysis was performed and blots were scanned using the LI-COR Odyssey Infrared Imager and analyzed and quantified in the Image Studio 4.0 Software.
Chromatin immunoprecipitation
Cells initially cultured in CSM-Leu were treated with α-factor and 500 μM IAA in YPGal for 3 hours at 25°C. Following extensive washes, cells were further incubated and time course samples were taken at the indicated points. Chromatin immunoprecipitation was performed as described (51). Formaldehyde cross-linked cells were lysed with acid-washed glass beads in a BeadBeater. DNA was fragmented by sonication (Branson 450, 5 cycles of 15 s. each). RPA (2 μl) or Mcm10 (2 μl) antibody and magnetic protein A beads (Dynalbeads Protein A, Invitrogen) were added to the cleared lysate to immunoprecipitate DNA. Immunoprecipitates were then washed extensively to remove nonspecific DNA. Eluted DNA was subjected to PCR analysis using primers directed against ARS305, ARS306 or a midway between ARS305 and ARS306 as described (31). We performed PCR with [32P-α]-dCTP as a component of the PCR reaction to quantify the amplified product. The radioactive band in the native gel, representing specific PCR amplified DNA product, was quantified by phosphorimaging and normalized by a reference standard (a PCR reaction with a known quantity of template DNA) run in the same gel.
Fluorescence Activated Cell Sorting
Samples were fixed with 70% ethanol and FACS was performed as described previously (55). Cells initially cultured in CSM-Leu were treated with α-factor and 500 μM IAA in YPGal for 3 hours at 25°C. After extensive washes, cells were incubated for the indicated times and stained with propidium iodide. Cell cycle progression data were obtained using BD FACS Canto Ruo Special Order System and analyzed using FACS Diva software.
Antibodies
Antibodies against Mcm10, Mcm2 and Mcm2-phosphoserine-164-phosphoserine-170-phosphoserine, Cdc45 and GINS were supplied by Open Biosystems (we supplied the antigens). Crude serum was purified against immobilized antigen to remove nonspecific antibodies. The specificity of each antibody was analyzed by Western blot of purified proteins and yeast extract. Antibodies directed against RPA (Thermo Scientific), FLAG epitope (Sigma) and Arp3 (Santa Cruz Biotechnology) were commercially purchased.
Supplementary Material
Highlights.
-
-
Mcm10 is an essential protein for DNA replication initiation in eukaryotes.
-
-
The interaction between Mcm10 and DNA is essential for the function of Mcm10 during DNA replication initiation.
Acknowledgments
We thank Cheryl Pye and Brian Washburn from FSU Molecular Cloning Facility for helping with cloning and mutant constructs. We thank Ryan Higgins and Dr. Yanchang Wang for their help in the construction of mcm10-1-aid mcm5-bob1 strain. We also thank Dr. Irina Bruck for DDK proteins and for performing DDK kinase assay in vitro and Mcm10-ChIP. We thank Yeast Genetic Resource Center for providing mcm10-1-aid reagents, Dr. Robert A. Sclafani for mcm5-bob1 strain, Dr. Susan Taylor for supplying purified PKA and Dr. Mike O’Donnell for providing expression constructs for GINS. We also thank Ruth Didier for help with FACS analysis and Dr. Yoichi Kato for the use of his sonicator. We also thank Max Colbert for helping with reagent preparation. This work has been supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R15GM113167. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Footnotes
Conflict of Interest
We have no conflicts of interest to disclose.
Author Contribution
D. L. K. and P. P. A. designed the experiments. P. P. A performed the experiments. D. L. K. and P. P. A. analyzed the data and wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ilves I, Petojevic T, Pesavento JJ, Botchan MR. Activation of the MCM2-7 helicase by association with Cdc45 and GINS proteins. Molecular cell. 2010;37:247–258. doi: 10.1016/j.molcel.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moyer SE, Lewis PW, Botchan MR. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10236–10241. doi: 10.1073/pnas.0602400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pacek M, Tutter AV, Kubota Y, Takisawa H, Walter JC. Localization of MCM2-7, Cdc45, and GINS to the site of DNA unwinding during eukaryotic DNA replication. Molecular cell. 2006;21:581–587. doi: 10.1016/j.molcel.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nature cell biology. 2006;8:358–366. doi: 10.1038/ncb1382. [DOI] [PubMed] [Google Scholar]
- 5.Labib K. How do Cdc7 and cyclin-dependent kinases trigger the initiation of chromosome replication in eukaryotic cells? Genes & development. 2010;24:1208–1219. doi: 10.1101/gad.1933010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki H. Cyclin-dependent kinase-dependent initiation of chromosomal DNA replication. Current opinion in cell biology. 2010;22:766–771. doi: 10.1016/j.ceb.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Sclafani RA, Holzen TM. Cell cycle regulation of DNA replication. Annual review of genetics. 2007;41:237–280. doi: 10.1146/annurev.genet.41.110306.130308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller RC, Kang S, Lam WM, Chen S, Chan CS, Bell SP. Eukaryotic origin-dependent DNA replication in vitro reveals sequential action of DDK and S-CDK kinases. Cell. 2011;146:80–91. doi: 10.1016/j.cell.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JF. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719–730. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evrin C, Clarke P, Zech J, Lurz R, Sun J, Uhle S, Li H, Stillman B, Speck C. A double-hexameric MCM2-7 complex is loaded onto origin DNA during licensing of eukaryotic DNA replication. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20240–20245. doi: 10.1073/pnas.0911500106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu YV, Yardimci H, Long DT, Ho TV, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Scharer OD, Walter JC. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell. 2011;146:931–941. doi: 10.1016/j.cell.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samel SA, Fernandez-Cid A, Sun J, Riera A, Tognetti S, Herrera MC, Li H, Speck C. A unique DNA entry gate serves for regulated loading of the eukaryotic replicative helicase MCM2-7 onto DNA. Genes & development. 2014;28:1653–1666. doi: 10.1101/gad.242404.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa A, Ilves I, Tamberg N, Petojevic T, Nogales E, Botchan MR, Berger JM. The structural basis for MCM2-7 helicase activation by GINS and Cdc45. Nature structural & molecular biology. 2011;18:471–477. doi: 10.1038/nsmb.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey MJ, Indiani C, O'Donnell M. Reconstitution of the Mcm2-7p heterohexamer, subunit arrangement, and ATP site architecture. The Journal of biological chemistry. 2003;278:4491–4499. doi: 10.1074/jbc.M210511200. [DOI] [PubMed] [Google Scholar]
- 15.Bochman ML, Schwacha A. Differences in the single-stranded DNA binding activities of MCM2-7 and MCM467: MCM2 and MCM5 define a slow ATP-dependent step. The Journal of biological chemistry. 2007;282:33795–33804. doi: 10.1074/jbc.M703824200. [DOI] [PubMed] [Google Scholar]
- 16.Bochman ML, Schwacha A. The Mcm2-7 complex has in vitro helicase activity. Molecular cell. 2008;31:287–293. doi: 10.1016/j.molcel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Bochman ML, Schwacha A. The Saccharomyces cerevisiae Mcm6/2 and Mcm5/3 ATPase active sites contribute to the function of the putative Mcm2-7 'gate'. Nucleic acids research. 2010;38:6078–6088. doi: 10.1093/nar/gkq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruck I, Kaplan DL. The Dbf4-Cdc7 kinase promotes Mcm2-7 ring opening to allow for single-stranded DNA extrusion and helicase assembly. The Journal of biological chemistry. 2015;290:1210–1221. doi: 10.1074/jbc.M114.608232. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Lei M, Kawasaki Y, Young MR, Kihara M, Sugino A, Tye BK. Mcm2 is a target of regulation by Cdc7-Dbf4 during the initiation of DNA synthesis. Genes & development. 1997;11:3365–3374. doi: 10.1101/gad.11.24.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruck I, Kaplan D. Dbf4-Cdc7 phosphorylation of Mcm2 is required for cell growth. The Journal of biological chemistry. 2009;284:28823–28831. doi: 10.1074/jbc.M109.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeeles JT, Deegan TD, Janska A, Early A, Diffley JF. Regulated eukaryotic DNA replication origin firing with purified proteins. Nature. 2015;519:431–435. doi: 10.1038/nature14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheu YJ, Stillman B. Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Molecular cell. 2006;24:101–113. doi: 10.1016/j.molcel.2006.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheu YJ, Stillman B. The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature. 2010;463:113–117. doi: 10.1038/nature08647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masai H, Taniyama C, Ogino K, Matsui E, Kakusho N, Matsumoto S, Kim JM, Ishii A, Tanaka T, Kobayashi T, et al. Phosphorylation of MCM4 by Cdc7 kinase facilitates its interaction with Cdc45 on the chromatin. The Journal of biological chemistry. 2006;281:39249–39261. doi: 10.1074/jbc.M608935200. [DOI] [PubMed] [Google Scholar]
- 25.Deegan TD, Yeeles JT, Diffley JF. Phosphopeptide binding by Sld3 links Dbf4-dependent kinase to MCM replicative helicase activation. The EMBO journal. 2016 doi: 10.15252/embj.201593552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy CF, Dryga O, Seematter S, Pahl PM, Sclafani RA. mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3151–3155. doi: 10.1073/pnas.94.7.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ricke RM, Bielinsky AK. Mcm10 regulates the stability and chromatin association of DNA polymerase-alpha. Molecular cell. 2004;16:173–185. doi: 10.1016/j.molcel.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Maine GT, Sinha P, Tye BK. Mutants of S. cerevisiae defective in the maintenance of minichromosomes. Genetics. 1984;106:365–385. doi: 10.1093/genetics/106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumas LB, Lussky JP, McFarland EJ, Shampay J. New temperature-sensitive mutants of Saccharomyces cerevisiae affecting DNA replication. Molecular & general genetics : MGG. 1982;187:42–46. doi: 10.1007/BF00384381. [DOI] [PubMed] [Google Scholar]
- 30.Merchant AM, Kawasaki Y, Chen Y, Lei M, Tye BK. A lesion in the DNA replication initiation factor Mcm10 induces pausing of elongation forks through chromosomal replication origins in Saccharomyces cerevisiae. Molecular and cellular biology. 1997;17:3261–3271. doi: 10.1128/mcb.17.6.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Deursen F, Sengupta S, De Piccoli G, Sanchez-Diaz A, Labib K. Mcm10 associates with the loaded DNA helicase at replication origins and defines a novel step in its activation. The EMBO journal. 2012;31:2195–2206. doi: 10.1038/emboj.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thu YM, Bielinsky AK. Enigmatic roles of Mcm10 in DNA replication. Trends in biochemical sciences. 2013;38:184–194. doi: 10.1016/j.tibs.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thu YM, Bielinsky AK. MCM10: one tool for all-Integrity, maintenance and damage control. Seminars in cell & developmental biology. 2014;30:121–130. doi: 10.1016/j.semcdb.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricke RM, Bielinsky AK. A conserved Hsp10-like domain in Mcm10 is required to stabilize the catalytic subunit of DNA polymerase-alpha in budding yeast. The Journal of biological chemistry. 2006;281:18414–18425. doi: 10.1074/jbc.M513551200. [DOI] [PubMed] [Google Scholar]
- 35.Perez-Arnaiz P, Bruck I, Kaplan DL. Mcm10 coordinates the timely assembly and activation of the replication fork helicase. Nucleic acids research. 2016;44:315–329. doi: 10.1093/nar/gkv1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen TW, Tye BK. Drosophila MCM10 interacts with members of the prereplication complex and is required for proper chromosome condensation. Molecular biology of the cell. 2003;14:2206–2215. doi: 10.1091/mbc.E02-11-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Perna R, Aria V, De Falco M, Sannino V, Okorokov AL, Pisani FM, De Felice M. The physical interaction of Mcm10 with Cdc45 modulates their DNA-binding properties. The Biochemical journal. 2013;454:333–343. doi: 10.1042/BJ20130059. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer SL, Cheng IH, Chai W, Tye BK. Mcm10 and Cdc45 cooperate in origin activation in Saccharomyces cerevisiae. Journal of molecular biology. 2004;340:195–202. doi: 10.1016/j.jmb.2004.04.066. [DOI] [PubMed] [Google Scholar]
- 39.Fien K, Cho YS, Lee JK, Raychaudhuri S, Tappin I, Hurwitz J. Primer utilization by DNA polymerase alpha-primase is influenced by its interaction with Mcm10p. The Journal of biological chemistry. 2004;279:16144–16153. doi: 10.1074/jbc.M400142200. [DOI] [PubMed] [Google Scholar]
- 40.Chattopadhyay S, Bielinsky AK. Human Mcm10 regulates the catalytic subunit of DNA polymerase-alpha and prevents DNA damage during replication. Molecular biology of the cell. 2007;18:4085–4095. doi: 10.1091/mbc.E06-12-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watase G, Takisawa H, Kanemaki MT. Mcm10 plays a role in functioning of the eukaryotic replicative DNA helicase, Cdc45-Mcm-GINS. Current biology : CB. 2012;22:343–349. doi: 10.1016/j.cub.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 42.Kanke M, Kodama Y, Takahashi TS, Nakagawa T, Masukata H. Mcm10 plays an essential role in origin DNA unwinding after loading of the CMG components. The EMBO journal. 2012;31:2182–2194. doi: 10.1038/emboj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quan Y, Xia Y, Liu L, Cui J, Li Z, Cao Q, Chen XS, Campbell JL, Lou H. Cell-Cycle-Regulated Interaction between Mcm10 and Double Hexameric Mcm2-7 Is Required for Helicase Splitting and Activation during S Phase. Cell reports. 2015;13:2576–2586. doi: 10.1016/j.celrep.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das-Bradoo S, Ricke RM, Bielinsky AK. Interaction between PCNA and diubiquitinated Mcm10 is essential for cell growth in budding yeast. Molecular and cellular biology. 2006;26:4806–4817. doi: 10.1128/MCB.02062-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Seo YS, Hurwitz J. The Cdc23 (Mcm10) protein is required for the phosphorylation of minichromosome maintenance complex by the Dfp1-Hsk1 kinase. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2334–2339. doi: 10.1073/pnas.0237384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren EM, Huang H, Fanning E, Chazin WJ, Eichman BF. Physical interactions between Mcm10, DNA, and DNA polymerase alpha. The Journal of biological chemistry. 2009;284:24662–24672. doi: 10.1074/jbc.M109.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robertson PD, Warren EM, Zhang H, Friedman DB, Lary JW, Cole JL, Tutter AV, Walter JC, Fanning E, Eichman BF. Domain architecture and biochemical characterization of vertebrate Mcm10. The Journal of biological chemistry. 2008;283:3338–3348. doi: 10.1074/jbc.M706267200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warren EM, Vaithiyalingam S, Haworth J, Greer B, Bielinsky AK, Chazin WJ, Eichman BF. Structural basis for DNA binding by replication initiator Mcm10. Structure. 2008;16:1892–1901. doi: 10.1016/j.str.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eisenberg S, Korza G, Carson J, Liachko I, Tye BK. Novel DNA binding properties of the Mcm10 protein from Saccharomyces cerevisiae. The Journal of biological chemistry. 2009;284:25412–25420. doi: 10.1074/jbc.M109.033175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanter DM, Kaplan DL. Sld2 binds to origin single-stranded DNA and stimulates DNA annealing. Nucleic acids research. 2011;39:2580–2592. doi: 10.1093/nar/gkq1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bruck I, Kaplan DL. Cdc45 protein-single-stranded DNA interaction is important for stalling the helicase during replication stress. The Journal of biological chemistry. 2013;288:7550–7563. doi: 10.1074/jbc.M112.440941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruck I, Kaplan DL. GINS and Sld3 compete with one another for Mcm2-7 and Cdc45 binding. The Journal of biological chemistry. 2011;286:14157–14167. doi: 10.1074/jbc.M111.218305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sclafani RA, Tecklenburg M, Pierce A. The mcm5-bob1 bypass of Cdc7p/Dbf4p in DNA replication depends on both Cdk1-independent and Cdk1-dependent steps in Saccharomyces cerevisiae. Genetics. 2002;161:47–57. doi: 10.1093/genetics/161.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gietz RD, Woods RA. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods in enzymology. 2002;350:87–96. doi: 10.1016/s0076-6879(02)50957-5. [DOI] [PubMed] [Google Scholar]
- 55.Bruck I, Kanter DM, Kaplan DL. Enabling association of the GINS protein tetramer with the mini chromosome maintenance (Mcm)2-7 protein complex by phosphorylated Sld2 protein and single-stranded origin DNA. The Journal of biological chemistry. 2011;286:36414–36426. doi: 10.1074/jbc.M111.282822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bruck I, Kaplan DL. The replication initiation protein sld2 regulates helicase assembly. The Journal of biological chemistry. 2014;289:1948–1959. doi: 10.1074/jbc.M113.532085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douglas ME, Diffley JF. Recruitment of Mcm10 to Sites of Replication Initiation Requires Direct Binding to the MCM Complex. The Journal of biological chemistry. 2015 doi: 10.1074/jbc.M115.707802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregan J, Lindner K, Brimage L, Franklin R, Namdar M, Hart EA, Aves SJ, Kearsey SE. Fission yeast Cdc23/Mcm10 functions after pre-replicative complex formation to promote Cdc45 chromatin binding. Molecular biology of the cell. 2003;14:3876–3887. doi: 10.1091/mbc.E03-02-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wohlschlegel JA, Dhar SK, Prokhorova TA, Dutta A, Walter JC. Xenopus Mcm10 binds to origins of DNA replication after Mcm2-7 and stimulates origin binding of Cdc45. Molecular cell. 2002;9:233–240. doi: 10.1016/s1097-2765(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 60.Im JS, Ki SH, Farina A, Jung DS, Hurwitz J, Lee JK. Assembly of the Cdc45-Mcm2-7-GINS complex in human cells requires the Ctf4/And-1, RecQL4, and Mcm10 proteins. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15628–15632. doi: 10.1073/pnas.0908039106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruck I, Kaplan DL. Conserved mechanism for coordinating replication fork helicase assembly with phosphorylation of the helicase. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:11223–11228. doi: 10.1073/pnas.1509608112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mumberg D, Muller R, Funk M. Regulatable promoters of Saccharomyces cerevisiae: comparison of transcriptional activity and their use for heterologous expression. Nucleic acids research. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annual review of biophysics and biomolecular structure. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- 64.Bruck I, Kaplan DL. Origin single-stranded DNA releases Sld3 protein from the Mcm2-7 complex, allowing the GINS tetramer to bind the Mcm2-7 complex. The Journal of biological chemistry. 2011;286:18602–18613. doi: 10.1074/jbc.M111.226332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frigola J, Remus D, Mehanna A, Diffley JF. ATPase-dependent quality control of DNA replication origin licensing. Nature. 2013;495:339–343. doi: 10.1038/nature11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheu YJ, Kinney JB, Lengronne A, Pasero P, Stillman B. Domain within the helicase subunit Mcm4 integrates multiple kinase signals to control DNA replication initiation and fork progression. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E1899–1908. doi: 10.1073/pnas.1404063111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Francis LI, Randell JC, Takara TJ, Uchima L, Bell SP. Incorporation into the prereplicative complex activates the Mcm2-7 helicase for Cdc7-Dbf4 phosphorylation. Genes & development. 2009;23:643–654. doi: 10.1101/gad.1759609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamimura Y, Tak YS, Sugino A, Araki H. Sld3, which interacts with Cdc45 (Sld4), functions for chromosomal DNA replication in Saccharomyces cerevisiae. The EMBO journal. 2001;20:2097–2107. doi: 10.1093/emboj/20.8.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Natsume T, Muller CA, Katou Y, Retkute R, Gierlinski M, Araki H, Blow JJ, Shirahige K, Nieduszynski CA, Tanaka TU. Kinetochores coordinate pericentromeric cohesion and early DNA replication by Cdc7-Dbf4 kinase recruitment. Molecular cell. 2013;50:661–674. doi: 10.1016/j.molcel.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka S, Nakato R, Katou Y, Shirahige K, Araki H. Origin association of Sld3, Sld7, and Cdc45 proteins is a key step for determination of origin-firing timing. Current biology : CB. 2011;21:2055–2063. doi: 10.1016/j.cub.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 71.Zegerman P, Diffley JF. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature. 2007;445:281–285. doi: 10.1038/nature05432. [DOI] [PubMed] [Google Scholar]
- 72.Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature. 2007;445:328–332. doi: 10.1038/nature05465. [DOI] [PubMed] [Google Scholar]
- 73.Tak YS, Tanaka Y, Endo S, Kamimura Y, Araki H. A CDK-catalysed regulatory phosphorylation for formation of the DNA replication complex Sld2-Dpb11. The EMBO journal. 2006;25:1987–1996. doi: 10.1038/sj.emboj.7601075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.