Abstract
Sirtuin1 (Sirt1, mammalian homolog of S. Cerevisiae enzyme Sir2) is a transcriptional and transactivational regulator of murine Fxr, which is the primary bile acid (BA) sensor, and critical regulator of BA metabolism in physiological and pathophysiological conditions. Previous studies have suggested compromised Sirt1 expression in rodent models of cholestatic liver injury. We hypothesized that Sirt1 could be potentially targeted to alleviate cholestatic liver injury. In cultured primary human hepatocytes, SIRT1 mRNA was downregulated after GCA treatment, potentially via induction of miR-34a, whereas TUDCA induced SIRT1 expression without affecting miR-34a expression. Sirt1 expression was also significantly downregulated in three mouse models of liver injury (bile duct ligation, 1% cholic acid (CA) fed and the Mdr2−/− mouse). Mice fed CA diet also demonstrated hepatic FXR hyperacetylation and induction of the Jnk/p53 pathway. Mice fed CA diet and concurrently administered the Sirt1 activator; SRT1720 (50mg/kg/day, orally), demonstrated 40% and 45% decrease in plasma ALT and BA levels respectively. SRT1720 increased hepatic BA hydrophilicity by increasing tri- and tetra-hydroxylated and decreasing the di-hydroxylated BA fraction. SRT1720 administration also inhibited hepatic BA synthesis potentially via ileal Fgf15 and Fxr mediated inhibition of Cyp7a1 and Cyp27a1, along with increased hepatic BA hydroxylation in association with Cyp2b10 induction. SRT1720 administration significantly induced renal Mrp2, Mrp4, Pgc1α and Car expression along with ~2 fold increase in urinary BA concentrations.
Conclusion
SRT1720 administration alleviates cholestatic liver injury in mice by increasing hydrophilicity of hepatic BA composition and decreasing plasma BA concentration via increased BA excretion into urine. Thus, use of small molecule activators of Sirt1 presents a novel therapeutic target for cholestatic liver injury.
Keywords: Sirt1, cholestasis, bile acid, SRT1720, FXR
Sirtuin1 (Sirt1) is a NAD+ dependent protein deacetylase and the human homolog of yeast Sir2 that has been shown to affect numerous processes including, but not limited to, growth and development, life span regulation and metabolism (1). Sirt1 directly or indirectly regulates important nuclear receptors such as Car (2), Fxr (3), Pxr, Pparα, Hnf4α, Lxrα, Errα, and Rxr, along with numerous coregulators (4). Pgc1α is considered to be the primary coactivator downstream of Sirt1 activation (5). Sirt1 expression is generally downregulated in metabolic disorders such as obesity (6) and steatosis (7). Mice overexpressing Sirt1 are protected against high fat diet induced metabolic damage while administration of small molecule activator of Sirt1 in mice decreases diet induced steatotic damage (8–10).
Genetic and patho-physiological interruptions in bile acid (BA) metabolism and transport result in altered bile flow and cholestatic liver injury. Primary biliary cholangitis (PBC) (11) and primary sclerosing cholangitis (PSC) (12) are two chronic cholestatic diseases that, if left untreated, result in liver failure. The lack of successful therapies in treating these diseases has led to research into novel therapeutic targets. Farnesoid X Receptor (Fxr) has been well established as the primary BA sensor and a crucial regulator of BA metabolism. Hepatic Sirt1 deficiency increases BA concentrations and decreases activity of Fxr along with its target genes such as short heterodimer partner (Shp) (13, 14). Lack of intestinal Sirt1 downregulates DCoH2-Hnf1α-Fxr signaling, and decreases hepatic bile acid levels as well as ileal bile acid metabolic genes such as Ostα, Asbt, and Ibabp (15). However, Sirt1 activator resveratrol degrades Asbt expression in vitro (16). Sirt1 increases hepatic Fxr mRNA expression, thus increasing Pgc1α/Hnf1α binding to the FXR promoter (3). Sirt1 increases hepatic Fxr/Rxr heterodimerization at FXRE by deacetylating Fxr primarily at Lys-217 and activates Shp and Bsep mRNA expression. Sirt1 has also been shown to inhibit hepatic bile acid synthesis via Shp/Lrh1 regulatory loop (17). Conversely, Fxr mediated inhibition of miR-34a inhibits Sirt1 degradation, conserving Sirt1 activity (18). Thus, Sirt1 is a critical regulator of Fxr, and Sirt1-Fxr together may form an interactive regulatory network that could be potentially targeted to reverse cholestatic syndromes.
Previous studies in primary rat hepatocytes have indicated that DCA downregulates Sirt1 mRNA expression via activation of Jnk/Mapk mediated induction of p53-miR-34a expression (19). In a recent study by Castro et al., ursodeoxycholic acid (UDCA), the frontline therapy for treating PBC, reversed steatosis mediated decrease in hepatic Sirt1 expression via miR-34a/pJnk dependent pathway (7). Other compounds that have shown promise in cholestatic liver diseases, such as fibrates and retinoic acid, have demonstrated induction of Sirt1 in different disease models of neuronal differentiation and myeloid leukemia (20, 21).
Despite the ability of Sirt1 in modulating bile acid metabolism, very few studies have investigated Sirt1 pathway in cholestasis. Bile duct ligated models of cholestasis demonstrate decreased hepatic Sirt1 protein expression (22, 23). However, no further studies have been performed to determine how this downregulation affects cholestatic liver injury and whether activation of Sirt1 can alleviate cholestatic liver injury. The current study aims to: (1) confirm the decrease in expression and activity of Sirt1 in mouse models of cholestasis, (2) determine whether activation of Sirt1 via oral administration of SRT1720 alleviates cholestatic liver injury and, if so (3) by what mechanism.
EXPERIMENTAL PROCEDURES
CHEMICALS
SRT1720 was purchased from Selleck Chemicals (Houston, TX, USA). 1% cholic acid supplemented chow was custom obtained from Harlan Teklad Laboratories (WI, USA).
MATERIALS AND METHODS
Animals and treatments
Male C57Bl//6 (8–9 weeks old) were purchased from Jackson Labs (Bar Harbor, ME, USA) and maintained on 12hr dark/light cycle with food and water ad libitum. Animals were divided into 4 groups (n=6–8 per group): fed standard chow or chow supplemented with 1% cholic acid (CA) and concurrently administered vehicle (2% Hydroxypropyl methylcellulose (HPMC) +0.2% Sodium di(2-ethylhexyl) sulfosuccinate (DOSS)) or SRT1720 (50mg/kg/day, p.o.) via oral gavage once every day for a period of 5 days. Animals were euthanized after overnight fast between 8–11 am and blood, liver, kidney and intestines collected and stored at −80 until further use. In a separate experiment, liver and intestine were collected at the end of 5 days and total bile acid pool was quantified. All experiments were performed after obtaining protocol approval from Yale animal care and use committee.
Cells culture and treatments
Sandwich cultured primary human hepatocytes were maintained as described previously (24). Cells were treated with GCA or TUDCA (1, 2, 5, 10, 50 µM), CA (10 µM) or UDCA (50 µM) for 24 hours with PBS as control and collected for gene expression assays.
SIRT1 luciferase plasmid construct and transient transfection
The 5′-flanking regions of the human SIRT1 gene spanning ~2.5 kb upstream of the transcription start site (TSS) was amplified by polymerase chain reaction (PCR) using BAC clone RP11-57G10 (Chori BAPAC resource center, CA, USA) with Kapa Hi-Fi Taq Polymerase (Kapa Biosystems, MA, USA). The upstream primers contained an internal Nhe1 restriction site, the downstream primers an internal BamHI/BgIII site. Following an overnight sequential digestion, the PCR products were ligated into a pGL4.17 luciferase vector (Promega Corp, WI, USA). The resulting promoter luciferase construct was transiently transfected (0.5 µg/well) into Huh7 cells along with FXR (50ng), RXR (37.5 ng) and 0.5 ng pRL-CMV renilla luciferase as the transfection control using Fugene HD transfection reagent (Promega Corp, WI, USA). 20 hours after transfection, the cells were treated with DMSO, GCA (50 µM), TUDCA (50 µM), or TNFα (20ng/ml) for 24 hours. For JNK pathway inhibitor treatment, the cells were pretreated with 20uM SP600125 (Cayman chemicals, USA). After 24 hours, the cells were lysed using 1× passive lysis buffer and luciferase activity was measured using Dual Luciferase Assay kit (Promega Corp, WI, USA).
miR-34a quantification
Total RNA was isolated from control or primary human hepatocytes (n=3) treated with GCA (50uM) or TUDCA (50 uM) for 24 hours. 10ng total RNA was used for cDNA synthesis using TaqMan® MicroRNA Reverse Transcription Kit (Thermofisher Scientific, USA) and RT primers for miR-34a and U6sn RNA (control) (Applied Biosytems, Life Technologies Corp, USA) followed by qRT-PCR using TaqMan® Universal PCR Master Mix II, No UNG (Life Technologies, CA, USA).
Plasma Biochemistry and Liver Histology
Plasma liver alanine aminotransferase (ALT) and alkaline phosphatase (ALP) enzymes were analyzed by the Analytical Core in the Mouse Metabolic Phenotyping Center at Yale University (New Haven, CT). Plasma, urine, liver, ileal, fecal and total bile acid pool was measured as 3α-Hydroxy bile salt concentrations using a commercially available kit from Diazyme Laboratories (Poway, CA, USA).
Hepatic bile acid profiling
Liver tissue were dissolved in methanol-isopropanol mixture, centrifuged, and analyzed by nanoESI-MS using Perkin-Elmer Sciex API-III (Perkin-Elmer, Alberta, Canada) modified with a nanoelectrospray source from Protana A/S (Odense, Denmark). Palladium-coated borosilicate glass capillaries (Protana) were used for sample injection. Chemical identity of the peaks was confirmed by the fragmentation pattern of selected ion (Q3 mode) using argon collision gas. Presence of conjugated bile salts was confirmed by selection for glycine (m/z 74), sulfate (m/z 97) and taurine (m/z 124).
Gene expression analysis
Liver, kidney and intestinal gene expression was quantified using TaqMan real-time polymerase chain reaction in a LightCycler 480® (Roche Diagnostics, IN, USA) using Gapdh as the reference gene to normalize data. Information for TaqMan probes for transporters and BA metabolic genes have been previously described (Soroka et al., 2010; Boyer et al., 2006). Sirt1/SIRT1 and Pgc1α TaqMan probes were also obtained from Life Technologies Corporation, CA, USA.
Immunoprecipitation and Co-Immunoprecipitation
To determine acetylation status of Sirt1 and Fxr in liver and kidney homogenates, 1mg whole liver and kidney extracts were incubated with antibody to Sirt1 (Cell signaling, MA, USA) or Fxr (Santa Cruz, CA, USA) overnight under stringent conditions, immunopurified using Dynabeads Protein G beads (Life Technologies Corporation, CA, USA), and immunoblotted using Ac-Lysine (Cell signaling, MA, USA). For Co-immunoprecipitation assays to determine interaction of Sirt1 and Fxr proteins in liver; a similar protocol was followed and after immunopurification using protein G beads, the membranes were immunoblotted against Sirt1 and Fxr antibodies. Immunoblots presented in this study are representative of n=4–5 independent co-immunoprecipitation experiments.
Western blotting
Liver and kidney whole cell homogenates and membrane-enriched fractions were prepared as described previously. The antibody concentrations and sources for transporters have been described previously (25) and included in Supp Table 1. Unless specified, all observations were normalized to ShPTP1 expression in whole liver homogenates and Na+/K+ ATPase in membrane enriched liver fractions. Other antibodies used have been described in Supp Table 1. Immunoblotting data was quantified using ImageJ64 software.
Statistical analysis
Statistical significance of differences was determined by Tukey’s multiple comparisons ANOVA test. p < 0.05 was considered statistically significant. “*” denotes a significant difference between the CA groups with or without SRT1720 treatment.
RESULTS
TUDCA induces, while GCA inhibits, SIRT1 mRNA expression in primary human hepatocytes
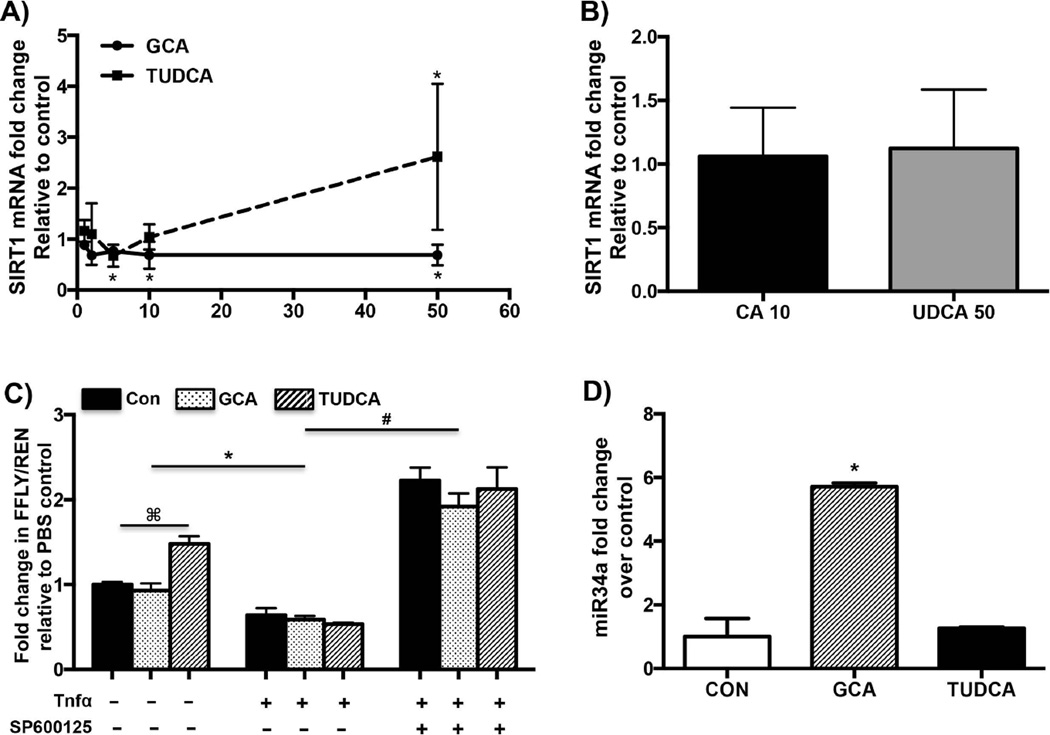
To determine whether BAs alter SIRT1 expression, SIRT1 mRNA expression was quantified in sandwich cultured human hepatocytes treated with different concentrations of GCA or TUDCA for 24 hours. Consistent with earlier observations made in rat hepatocytes, GCA (50 µM) inhibited SIRT1 mRNA expression by about 30% (Fig. 1A) relative to control (19). As seen in Fig. 1A, TUDCA induced SIRT1 mRNA expression by ~2.5 fold in human hepatocytes. However, as seen in Fig. 1A, these changes were not dose dependent. Interestingly, non-conjugated BA did not alter SIRT1 mRNA expression (Fig. 1B). To determine the mechanism of GCA/TUDCA mediated downregulation/induction of SIRT1 mRNA, SIRT1-luciferase reporter constructs were transfected into Huh7 cells and treated with GCA or TUDCA (50 µM) for 24 hours. SIRT1-luciferase activity was not significantly affected by treatment with GCA, whereas treatment with TUDCA induced SIRT1 luciferase activity slightly (Fig. 1C). Bile acid mediated activation of the JNK pathway has been established as an important mechanism of downstream activation of apoptotic genes and inhibition of BA synthetic genes (26, 27). In line with these observations, induction of JNK pathway by TNFα (20ng/ml) along with the BA treatment inhibited SIRT1 luciferase activity significantly (~40–50%, Fig. 1C) over controls. To determine whether inhibition of JNK pathway would reverse inhibition of SIRT1 luciferase activity, Huh7 cells were pre-treated with a selective JNK inhibitor SP600125 (20 µM) for 1 hour before treatment with BA and Tnfα. This pretreatment inhibited JNK/BA downregulation on SIRT1 luciferase activity (Fig. 1C). Further, inhibition of JNK pathway significantly upregulated SIRT1-luciferase activity indicating that the JNK pathway is an important mediator of BA mediated changes in altering SIRT1 expression.
FIG. 1. Therapeutic BA induces SIRT1 while GCA inhibits SIRT1 mRNA expression in human.
Sandwich cultured primary human hepatocytes (n=4–6) were treated with (A) GCA or TUDCA (1,2, 5, 10 or 50 µM) and (B) CA (10 µM) or UDCA (50 µM) for 24 hours with PBS as control. SIRT1 mRNA expression was quantified by qPCR. (C) Luciferase activity measured from Huh7 cells transiently transfected with SIRT1-luc plasmid, FXR-RXR and treated with control, GCA (50 µM), TUDCA (50 µM) with or without TNFα (20ng/ml) and SP600125 (20 µM) for 24 hours. Renilla activity was used as the transfection control and normalized to the pcDNA/pGL4.17 controls. (D) miR-34a expression quantified in Huh7 cells treated with DCA or TUDCA (50 µM) by qRT-PCR using U6 SnRNA as control. A,B and D-Data is presented as mean ± SD (n=4–6 replicates) fold change in mRNA expression over control. p< 0.05 was considered to be significant. “*” denotes a significant difference between PBS treated and bile acid treated human hepatocytes. C-Data is represented as mean ± SD (n=3 separate experiments in triplicate). “⌘” denotes a significant difference between PBS treated and bile acid treated Huh7 cells, “*” denotes a significant difference between TNFα co-treated vs the non-cotreated Huh7 cells, “#” denotes a significant difference between SP600125 pretreated-TNFα-cotreated vs TNFα-cotreated Huh7 cells.
Recent studies have also indicated that BA downregulate SIRT1 mRNA expression via JNK mediated activation of miR-34a (19). GCA induced expression of miR-34a by over 5 fold relative to controls, whereas TUDCA treatment had no effect on miR-34a expression in primary human hepatocytes (Fig. 1D).
The above results indicate that GCA mediated downregulation of SIRT1 mRNA expression in primary human hepatocytes involves JNK mediated induction of miR-34a, and induction of SIRT1 mRNA expression by TUDCA is potentially due to its inability to induce miR-34a.
Sirt1 expression and activity are compromised in animal models of cholestasis
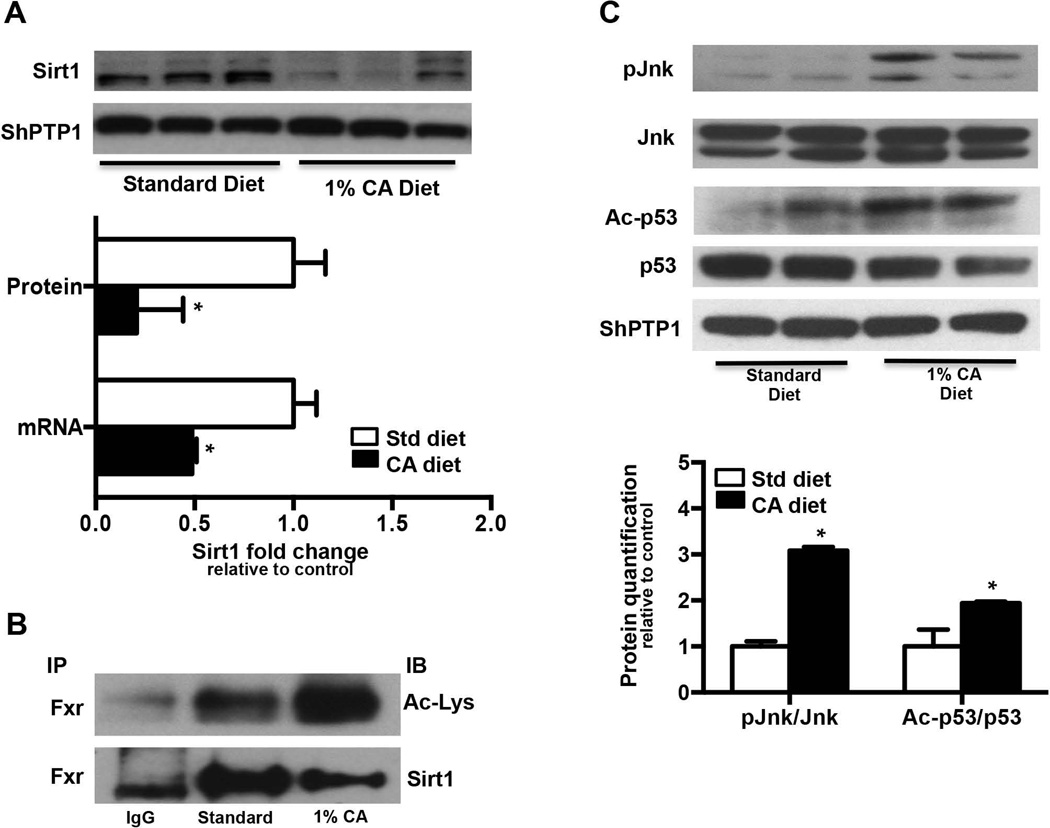
Upon determining that GCA inhibits SIRT1 mRNA expression in isolated human hepatocytes, hepatic Sirt1 mRNA, protein and activity were determined in livers from 1% cholic acid diet fed (CA, 5 days) mice to establish the effect of cholestatic liver injury on Sirt1 in rodents. Sirt1 mRNA expression was significantly downregulated in CA fed mice by 55%, while Sirt1 protein expression was decreased by 80% in CA fed mouse livers relative to rodent chow (standard diet, SD) fed controls (Fig. 2A).
FIG. 2. Sirt1 expression and activity is compromised in mouse models of cholestasis.
(A) Sirt1 mRNA and protein expression, (B) Fxr was immunoprecipitated from standard chow and 1% CA supplemented diet livers overnight, and acetylation status measured by immunoblotting against Ac-Lysine or Sirt1 antibody. Immunoblots are representative of n=4 independent coimmunoprecipitation experiments, (C) p-jnk/jnk, Ac-p53/p53 protein expression in standard chow or 1% CA supplemented chow fed (5 days, n=3) mouse livers. Data is presented as mean ± SD (n=3) fold change in mRNA or protein expression over standard chow fed controls. p< 0.05 was considered to be significant. “*” denotes a significant difference between standard diet (SD) fed group as compared to the CA supplemented diet fed group.
Immunoprecipitation assays using Fxr antibodies demonstrated that FXR was hyperacetylated in livers from CA fed mice relative to their controls, along with reduced Sirt1 recruitment to Fxr, indicating that Sirt1 mediated deacetylation of Fxr decreases in cholestatic livers (Fig. 2B). Similarly, co-immunoprecipitation assay of Sirt1 in the CA fed liver homogenates demonstrates a significantly lower pull down of Sirt1 (Fig. 2B), which corresponds to the increased Fxr acetylation in these livers.
Similar to observations in CA fed mouse livers, Sirt1 protein expression was downregulated by 80% in 7-day BDL mouse livers relative to sham controls with no change in Sirt1 mRNA expression. As with CA fed mice, FXR was hyperacetylated in these livers (Supp. Fig. 2A). In Mdr2−/− mouse livers, Sirt1 protein expression was inversely correlated with plasma ALT values at 6 weeks (Supp. Fig. 2B), previously suggested by Cai et al, 2014 as the point of most pronounced liver injury in this model of cholestasis.
Activation of Jnk/p53 pathway contributes to downregulation of Sirt1 in cholic acid fed mouse liver
Similar to previous observations, 1% CA feeding indeed increased liver p-Jnk 3 fold over standard diet as measured by immunoblotting (Fig. 2C). Along with Jnk activation, CA fed livers also showed increased p53 acetylation (1.8 fold) (Fig. 2C). Studies have shown that BA mediated induction of jnk/Mapk decreases Sirt1 expression in vitro (19). These observations suggest that p-Jnk mediated activation of p53 potentially decreases Sirt1 mRNA and protein in CA fed mouse livers.
SRT1720 reverses CA feeding induced liver injury by increasing hepatic hydrophilic BA fraction and inhibition of BA synthesis
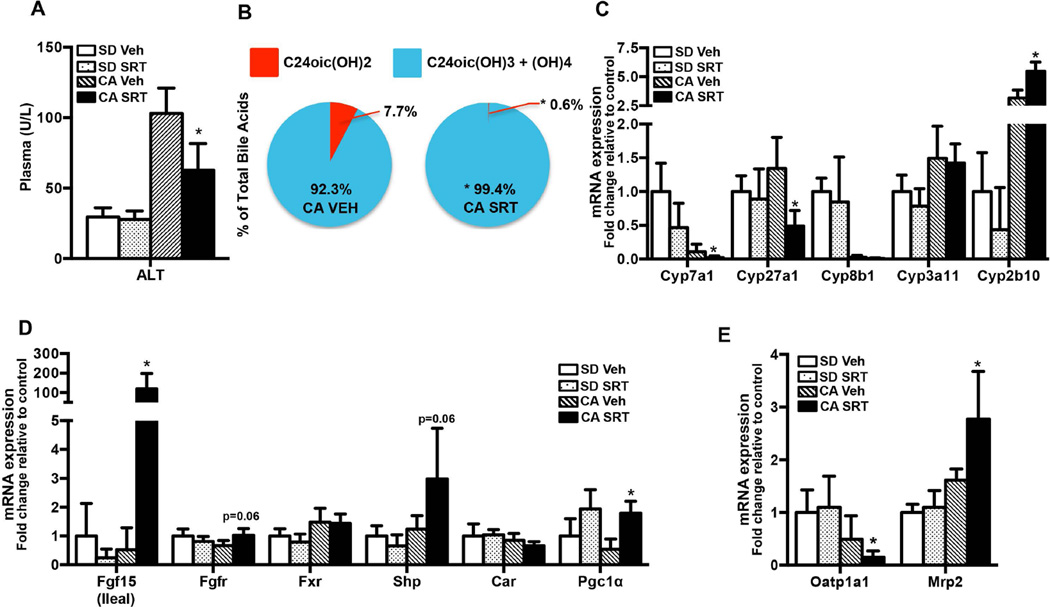
To determine whether SRT1720 could reverse cholestatic liver injury, C57Bl/6 mice were fed a standard rodent chow (SD) or chow supplemented with 1% cholic acid (CA) for 5 days with concurrent oral administration of vehicle (Veh) or the Sirt1 activator, SRT1720 (50 mg/kg/day). CA feeding caused liver injury as evidenced by a 4-fold increase in plasma ALT values over SD fed mice (Fig. 3A) without any effect on plasma ALP levels (Supp. Fig. 3). Upon SRT1720 administration, plasma ALT levels significantly decreased by 40% relative to vehicle fed mice (Fig. 3A) indicating an attenuation of liver injury.
FIG. 3. SRT1720 administration decreases cholestatic liver injury in CA fed mice.
C57Bl/6 mice (n= 5–7/group) were fed standard chow or 1% CA supplemented chow for concurrent oral vehicle or SRT1720 (50mg/kg body wt) for 5 days. (A) Plasma ALT (U/L), (B) hepatic di-, tri- and tetra-hydroxylated BA composition, (C) hepatic BA metabolic genes, (D) hepatic BA metabolic regulators, and (E) hepatic BA transporter mRNA expression as measured by quantitative PCR. Data is presented as mean ± SD (n=5–7), Units/L, µM, or fold change in mRNA/protein expression relative to standard chow fed controls. p< 0.05 was considered to be significant. “*” denotes a significant difference between CA SRT1720 treated group as compared to the CA vehicle fed group.
CA feeding increased plasma and hepatic bile acid concentrations by 4–5 fold over SD fed mice, along with increases in the total bile acid pool (Supp Table 2). SRT1720 decreased hepatic di-hydroxylated BA fraction by 30% as compared to SD-Veh fed mice (Supp. Fig. 3A). While hepatic BA levels remained unchanged (Supp Table 2), SRT1720 administration shifted the BA profile to being more hydrophilic as evidenced by >99.5% of the total BA fraction constituted by tri- and tetra-hydroxylated BA compared to ~92% in vehicle treated mice on a CA diet (Fig. 3B). SRT1720 administration also decreased total di-hydroxylated BA fraction to 0.6% as compared to 7.7% in CA fed vehicle treated mice (Fig. 3B). Notably, even on SD, SRT1720 administration decreased the di-hydroxylated BA fraction by 30% and increased tetra-hydroxylated BA fraction over 4 fold compared to SD fed vehicle treated mice (Supp. Fig. 4A).
CA feeding significantly inhibited Cyp7a1 and Cyp8b1 expression by 90% relative to SD fed mouse livers (Fig. 3C). SRT1720 administration almost completely abolished Cyp7a1, Cyp8b1 expression and inhibited hepatic Cyp27a1 expression relative to CA fed vehicle treated controls (Fig. 3C). Increased hydrophilic BA composition indicates a higher cytochrome p450-mediated hydroxylation of BAs. SRT1720 administration increased Cyp2b10 expression while Cyp3a11 (Fig. 3C), Sult2a1 and Ugt1a1 (Supp. Fig. 4B) expression remained unchanged or decreased, indicating a Cyp2b10 mediated increase in BA hydroxylation and hydrophilicity in these livers.
Previous studies have demonstrated that hepatic Cyp7a1 is downregulated by both Fxr and Fgf15 mediated pathways. Consistent with these observations, SRT1720 administration dramatically induced expression of ileal Fgf15 (~30 fold), as well as Fgfr4 (~1.6 fold) and Shp mRNA expression (Fig. 3D), along with Fxr protein (1.5 fold) (see Fig. 5A). These results indicate that SRT1720 inhibition of Cyp7a1 expression in CA fed mouse liver could be mediated by ileal Fgf15-Fgfr4 and Fxr-Shp.
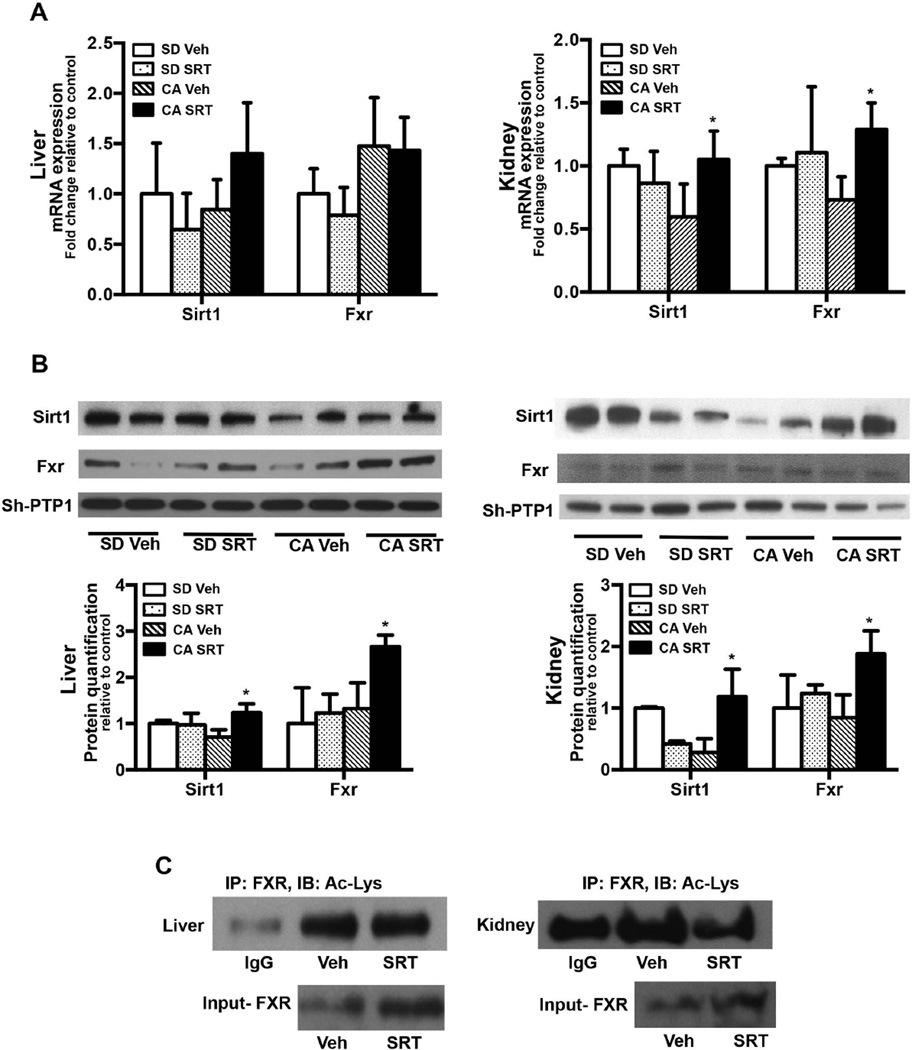
FIG. 5. SRT1720 administration induces hepatic and renal Sirt1 mRNA and protein expression in CA fed mice.
C57Bl/6 mice (n= 5–7/group) were fed standard chow or 1% CA supplemented chow for concurrent oral vehicle or SRT1720 (50mg/kg body wt) for 5 days. Sirt1 and Fxr mRNA and whole cell lysate protein expression was quantified in (A) liver and (B) kidney. Data is presented as mean ± SD (n=5–7) fold change in mRNA or protein expression relative to standard chow fed controls. p< 0.05 was considered to be significant. “*” denotes a significant difference between CA SRT1720 treated group as compared to the CA vehicle fed group. (C) Liver and kidney whole cell lysates from CA fed mice treated with vehicle or SRT1720 were immunoprecipated with Fxr antibody and immunoblotted using Ac-Lys antibody. Immunoblots are representative of three separate coimmunoprecipitation experiments.
In contrast, Cyp2b10 is a prototypical target gene of Constitutive androstane receptor (Car) co-activated by Pgc1α. Prior studies in hepatocytes show that Car activation is, in part, Sirt1-Pgc1α-Hnf4α mediated (28). Here, SRT1720 significantly induced Pgc1α mRNA expression alone (~2.5 fold, Fig. 3D), while Car and Hnf4α remained unchanged, suggesting a potential post-transcriptional activation of Car by Hnf4α/Pgc1α (Fig. 3D).
SRT1720 administration also significantly inhibited Oatp1a1 and induced Mrp2 mRNA expression; along with a minor induction of Bsep (1.2 folds, Supplementary figure 4) protein without any effect on other BA uptake and efflux transporters (Fig. 3E) relative to CA fed controls.
SRT1720 administration decreases plasma BA and increases renal excretion of BAs
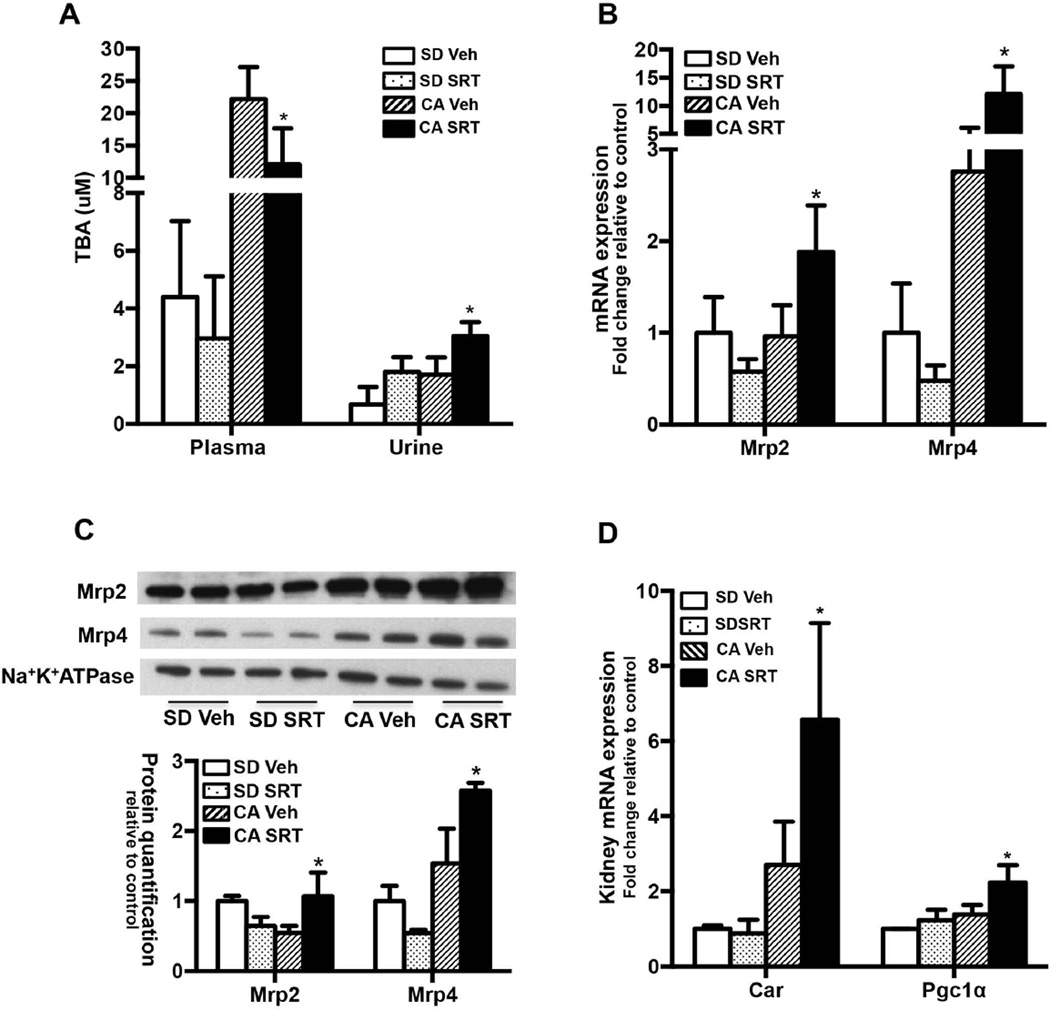
CA feeding increased plasma and urinary BA concentrations by 5 and 2.5 fold over SD fed controls respectively (Fig. 4A). When SRT1720 was administered concurrent with CA feeding, plasma BA decreased by 45% while urinary BA concentrations increased 1.8 fold over the vehicle fed controls (Fig. 4A).
FIG. 4. SRT1720 administration decreases plasma BA by increasing urinary BA excretion through induction of Mrp2 and Mrp4 expression in CA fed mouse livers.
C57Bl/6 mice (n= 5–7/group) were fed standard chow or 1% CA supplemented chow for concurrent oral vehicle or SRT1720 (50mg/kg body wt) for 5 days. (A) Plasma and urinary bile acid concentration, (B) kidney Mrp2 and Mrp4 mRNA expression, (C) kidney Mrp2 and Mrp4 protein expression and quantification in membrane enriched fraction and (D) Car and Pgc1α mRNA expression. Data is presented as mean ± SD (n=5–7) fold change in mRNA or protein expression relative to standard chow fed controls. p< 0.05 was considered to be significant. “*” denotes a significant difference between CA SRT1720 treated group as compared to the CA vehicle fed group.
SRT1720 administration increased mRNA and protein expression of renal Mrp2 (2 fold) and Mrp4 (4.4 fold) relative to vehicle treated controls (Fig. 4B and 4C), whereas Mrp3 (Supp. Fig 5), Asbt, Oatp1a1, and Ostα/β expression remained unchanged (data not shown). Previous studies have demonstrated that renal Mrp2 and Mrp4 expression is largely independent of Fxr, but mediated instead by Car (29). SRT1720 administration induced renal Car mRNA expression (~2.4 fold) along with Pgc1α (Fig. 4D). Thus, SRT1720 stimulated increases in urinary bile acid excretion (Fig. 4A) are potentially mediated via Car/Pgc1α induction of Mrp2 and Mrp4.
SRT1720 reverses hepatic and renal Sirt1 protein expression and Fxr activity in cholic acid fed mice
To determine whether the improvement in cholic acid induced cholestatic liver injury occurs via SRT1720 induction of Sirt1 expression and downstream activity, Sirt1 mRNA and protein were quantified. While hepatic expression remained unchanged, SRT1720 administration significantly induced renal Sirt1 mRNA expression (Fig. 5A). As shown in Fig. 5B, SRT1720 administration reversed the downregulation of hepatic and renal Sirt1 protein seen with CA feeding. SRT1720 also induced Fxr protein expression through Sirt1 mediated activation as suggested by previous studies (30). SRT1720 administration also reversed hepatic and renal Fxr acetylation (Fig. 5C) indicating that activation of Sirt1 mediates protective effects of SRT1720 in this model of injury.
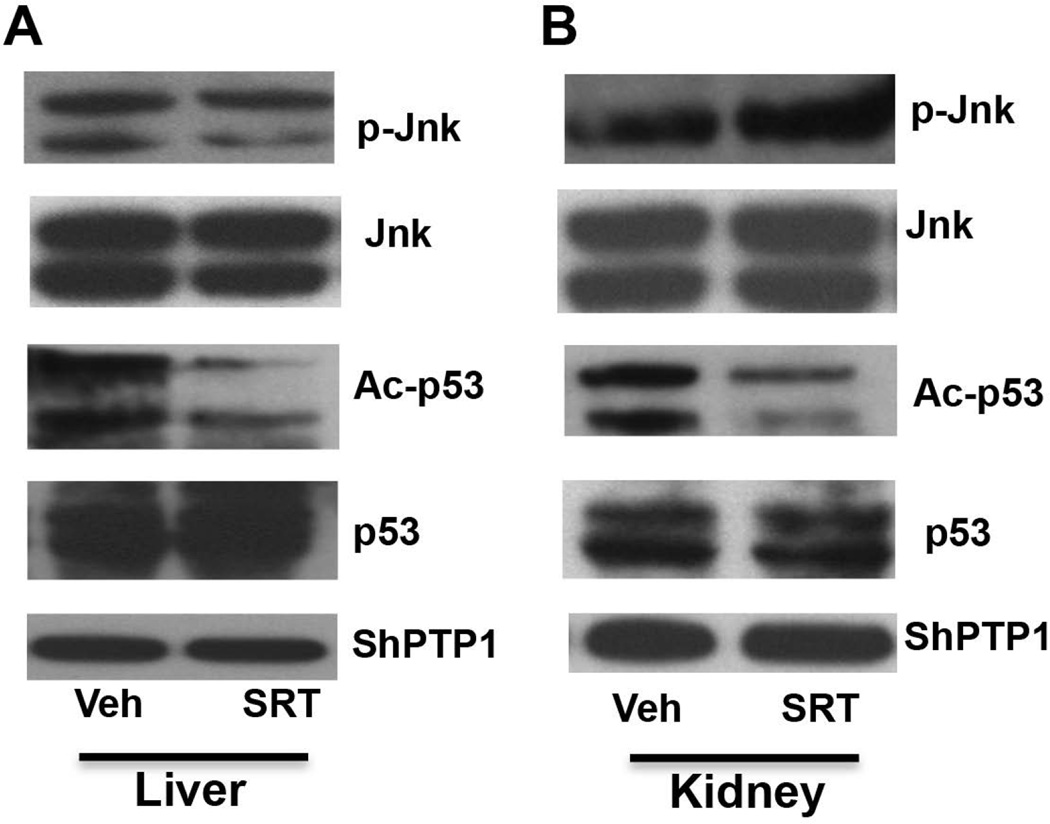
SRT1720 reverses hepatic and renal Jnk and p53 activation in cholic acid fed mice
To determine whether SRT1720 mediates reversal of Jnk (phosphorylation) and p53 (acetylation) activation, p-Jnk and Ac-p53 protein levels were quantified in liver and kidney whole cell lysates from SRT1720 treated CA fed mice. SRT1720 administration in CA fed mice reversed Jnk and p53 activation in liver (Fig. 6A). However, reversal of Jnk phosphorylation was less pronounced as compared to reversal of p53 acetylation in the kidney (Fig. 6B). Increase in Sirt1 mRNA and protein expression via inhibition of Jnk and p53 pathway is also reflected in the decreased Fxr acetylation as determined by immunoprecipitation (Fig. 5C).
FIG. 6. SRT1720 administration reverses pJnk activation in liver and kidney homogenates in CA fed mice.
C57Bl/6 mice (n= 5–7/group) were fed standard chow or 1% CA supplemented chow for concurrent oral vehicle or SRT1720 (50mg/kg body wt) for 5 days. p-jnk/jnk, Ac-p53/p53 protein expression (normalized to ShPTP1) in (A) liver and (B) kidney from CA fed Vehicle or SRT1720 administered mice was quantified by western blotting. Data is presented as immunoblots representative of three separate western blotting experiments. p< 0.05 was considered to be significant. “*” denotes a significant difference between CA SRT1720 treated group as compared to the CA vehicle fed group.
DISCUSSION
Standards of care for cholestatic liver disease are currently limited to the use of Ursodeoxycholic acid (UDCA) for primary biliary cholangitis (PBC), while there are no accepted treatments for primary sclerosing cholangitis and other adult cholestatic disorders (31). Even in PBC, ~40% of patients do not respond or are incomplete responders to UDCA treatment (32). Thus, there is a major need for alternative therapies. Recent studies suggest that Obeticholic acid (OCA), a potent synthetic FXR agonist, and fibrates, which are PPAR activators, might be useful. OCA was recently approved by the FDA for PBC while fibrates have yet to be tested in randomized trials (33).
In the present study we provide pre-clinical information that a synthetic activator of Sirt1 may be a promising new candidate. First we show that SIRT1 expression is impaired in human hepatocytes exposed in vitro to GCA, a hydophobic bile acid. In addition, using surgically induced, diet induced and transgenic rodent models of cholestasis, we show that Sirt1 expression and activity is also impaired in liver injury. Finally we demonstrate that activation of Sirt1 expression using a small molecule activator of Sirt1, SRT1720, reverses the liver injury produced by 1% cholic acid feeding of mice by hepatic and extra hepatic mechanisms. Together these findings provide evidence for a novel, potentially translatable and targetable, per-oral small molecule activator of Sirt1 as an alternative therapy for cholestatic liver diseases. The major protective effect of SRT1720 in this model of cholestasis is manifested by a significant reduction in plasma ALT levels (Fig. 3A) that is likely attributed to (1) a small but significant increase in the fraction of hepatic hydrophilic BAs and (2) a reduction in plasma BA concentrations that are associated with an increase in the concentrations of BAs in the urine (Fig. 4A). These changes may be attributed to SRT1720 acting to deacetylate Fxr, thus leading to a potentially “normalizing” effect on expression of downstream genes that have been deregulated in the BA induced liver injury, along with other mechanisms such as induction of Car target genes
Sirt1 and Fxr together form an interactive regulatory network. Studies have published that Fxr acetylation stabilizes the protein, but reduces its activity by decreasing Fxr/Rxr heterodimerization along with its ability to bind FXRE and trans-activate other genes (13). Fxr acetylation and activity has also been shown to be aberrant in disease states and in mouse models lacking Sirt1 activity (13, 14). Previous studies have demonstrated that Sirt1 downregulation in BA induced hepatocyte injury is mediated via pJnk/p53 mediated induction of miR34a expression (19). In primary human hepatocytes GCA, but not TUDCA induced miR-34a expression (Fig. 1D). This indicates that downregulation of SIRT1 mRNA expression in human hepatocytes is potentially driven by bile acids that can activate JNK pathway mediated miR-34a induction (Fig. 1). The increase in SIRT1 mRNA upon TUDCA treatment is observed due to its inability to induce miR-34a and hence, degradation of SIRT1 mRNA (Fig. 1). In line with these studies, our rodent models of cholestasis demonstrated significantly lower levels of Sirt1 along with increased Jnk and p53 activation (Fig. 2 and Supp. Fig. 2). In our study, administration of SRT1720, an agonist of Sirt1, reverses Fxr hyperacetylation in CA fed livers and potentially reinstates Fxr activity.
SRT1720 administration led to a small but significant increase in both hepatic tri- and tetra-hydroxylated BA fractions, thus decreasing the hydrophobicity of the bile acid pool. Abcb11−/− mice, generated on a mixed or a pure genetic background, also develop significant increases in their hepatic hydrophilic BA composition that is thought to represent the major adaptive response preventing these mice from developing cholestatic liver injury (34, 35). Similarly, Fxr−/− mice also demonstrate higher levels of hepatic polyhydroxylated BAs potentially mediated via induction of Cyp3a11 (36). norUDCA administration reverses BA induced liver injury in Mdr2−/− model of PSC by increasing the hydrophilicity of hepatic BA along with an induction of BA hydroxylation enzymes, including Cyp2b10 and Cyp3a11 (29, 37). Other studies of rodent models of cholestasis suggest that Car and Pxr mediate induction of Cyp2b10 and Cyp3a11, respectively (38). In the present study, hepatic Cyp2b10 but not Cyp3a11 was induced by SRT1720 suggesting a Car mediated upregulation (39). However, SRT1720 administration had no effect on hepatic expression of Car (Fig 3D) or the expression of Sult2a1, another Car activated gene, in agreement with the hepatic BA profiles which demonstrated minimal detection of sulfated BA (data not shown). Whether the SRT1720 mediated changes in BA hydrophilicity observed in mice would translate into human cholestatic liver disease, will need to be studied further.
In contrast, SRT1720 administration almost completely ablated hepatic Cyp7a1 and Cyp27a1 expression (Fig. 3D), thus inhibiting hepatic BA synthesis. Hepatic inhibition of Cyp7a1 in other rodent models of cholestasis is driven either by Fxr mediated Shp activation within the liver (40) and/or is induced via increases in ileal Fgf15 expression that then activates hepatic Fgfr4 signaling and inhibits Cyp7a1 expression by Shp independent mechanisms (41–43). In our current study, SRT1720 significantly induced ileal Fgf15, hepatic Fgfr4, as well as hepatic Shp expression, indicating that both of these pathways could contribute to inhibition of hepatic Cyp7a1 and Cyp27a1 expression (Fig. 3D, 5A and 5C). Interestingly, upon SRT1720 administration hepatic Sirt1 mRNA expression does not change, however, Sirt1 protein expression is significantly induced (Fig. 5A and B). SRT1720 is an agonist of Sirt1 that binds at an allosteric site, positioned at N-terminal with respect to the catalytic domain of the protein and lowers the Michaelis-Menten constant for the acetylated substrates (44, 45). At the same time, SRT1720 also induces SUMOylation of Sirt1 in vivo that is associated with Sirt1 protein stabilization and nuclear translocation (46). Thus, it is possible that even though Sirt1 mRNA is not induced significantly, SRT1720 stabilizes Sirt1 protein resulting in accumulation of protein in the hepatocytes leading to increased deacetylation activity.
In addition, prior studies in cholic acid fed mice have described a significant role for the kidney in eliminating excess circulating BA. This has been attributed to the induction of apical efflux transporters (such as Mrp3, Mrp4 and Oat3) in kidney proximal tubules, thus leading to an increased urinary excretion of bile acids (47, 48). In this study we did not see changes in renal Mrp2 expression in the cholic acid fed mice, however, SRT1720 administration to CA fed mice resulted in significant increases in both renal Mrp2 and Mrp4 expression (Fig. 4). These transporter increases were accompanied by increases in renal mRNA for Sirt1, Fxr, and Car (Fig. 4D and 5B), as well as decreased Fxr acetylation (Fig. 5C). Induction of mouse renal Mrp2 has been shown to be largely independent of Fxr regulation in cholestasis (48), and other studies in rodents suggest that it is Rxr/Rar, and Car, dependent (49). Studies using Car ligand TCPOBOP have also supported Car dependent induction of mouse renal Mrp2 and Mrp4 expression in CBDL in association with increased urinary BA excretion (39). Thus, our data are consistent with a Car dependent induction of renal Mrp2 and Mrp4 after SRT1720 treatment of CA fed mice that may account for the increased urinary BA excretion (Fig. 4D).
Data shown in this study suggests that Car mediated induction of hepatic Cyp2b10, renal Mrp2, and renal Mrp4 is an important target of SRT1720 administration. Previous studies have demonstrated that Sirt1 induction of Car and its downstream target genes occur via Pgc1α, an important downstream target of Sirt1 (28). As seen in Fig. 3D and 4D, SRT1720 administration significantly induced hepatic and renal Pgc1α expression. Thus, SRT1720 mediated change in hepatic BA hydroxylation potentially occurs via induction of hepatic Pgc1α-Car-Cyp2b10 axis. SRT1720 induction of renal Mrp2 and Mrp4 via Car is also potentially via Sirt1-Pgc1α-Car regulatory axis. However, the exact mechanism needs further studies.
The observations in the present study present SRT1720 and other small molecule activators of Sirt1 as potential therapeutic targets in cholestatic liver diseases. Further studies with other potent Sirt1 activators in other models of cholestasis will determine the usefulness of these activators in chronic liver diseases such as PBC and PSC.
Supplementary Material
Acknowledgments
This work was supported by grant DK 25636 and P30-34989 (Yale Liver Center) by the National Institute of Diabetes and Digestive and Kidney Diseases. We thank Dr. Shi-Ying Cai, Ms. Kathy Harry, and Dr. Nidhi Vishnoi for their technical support.
List of Abbreviations
- Sirt1
Sirtuin1
- Car
Constitutive androstance receptor
- Fxr
Farnesoid X Receptor
- Pxr
Pregnane X Receptor
- Ppar α
Peroxisome proliferator-activated receptor α
- Hnf4 α
Hepatocyte nuclear factor 4 α
- Lxr α
Liver X receptor α
- ERα
Estrogen receptor alpha
- Rxr α
Retinoid X receptor α
- Pgc1 α
Peroxisome proliferator-activated receptor gamma coactivator 1-α
- TUDCA
Tauroursodeoxycholic acid
- GCA
Glycocholic acid
- Mrp2, Mrp4
Multidrug resistance-associated protein 2 and 4
- Ost α
Organic solute transporter α
- Asbt
Apical Sodium Dependent Bile Acid Transporter
- DCoH2
dimerization co-factor of HNF1α, pterin 4α carbinolamine dehydratase 2/dimerization cofactor of HNF1α 2
REFERENCES
- 1.Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corton JC, Brown-Borg HM. Peroxisome proliferator-activated receptor gamma coactivator 1 in caloric restriction and other models of longevity. J Gerontol A Biol Sci Med Sci. 2005;60:1494–1509. doi: 10.1093/gerona/60.12.1494. [DOI] [PubMed] [Google Scholar]
- 3.Kemper JK, Choi SE, Kim DH. Sirtuin 1 deacetylase: a key regulator of hepatic lipid metabolism. Vitam Horm. 2013;91:385–404. doi: 10.1016/B978-0-12-407766-9.00016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schug TT, Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominy JE, Jr, Lee Y, Gerhart-Hines Z, Puigserver P. Nutrient-dependent regulation of PGC-1alpha's acetylation state and metabolic function through the enzymatic activities of Sirt1/GCN5. Biochim Biophys Acta. 2010;1804:1676–1683. doi: 10.1016/j.bbapap.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa Cdos S, Hammes TO, Rohden F, Margis R, Bortolotto JW, Padoin AV, Mottin CC, et al. SIRT1 transcription is decreased in visceral adipose tissue of morbidly obese patients with severe hepatic steatosis. Obes Surg. 2010;20:633–639. doi: 10.1007/s11695-009-0052-z. [DOI] [PubMed] [Google Scholar]
- 7.Castro RE, Ferreira DM, Afonso MB, Borralho PM, Machado MV, Cortez-Pinto H, Rodrigues CM. miR-34a/SIRT1/p53 is suppressed by ursodeoxycholic acid in the rat liver and activated by disease severity in human non-alcoholic fatty liver disease. J Hepatol. 2013;58:119–125. doi: 10.1016/j.jhep.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell SJ, Martin-Montalvo A, Mercken EM, Palacios HH, Ward TM, Abulwerdi G, Minor RK, et al. The SIRT1 activator SRT1720 extends lifespan and improves health of mice fed a standard diet. Cell Rep. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert RE, Thai K, Advani SL, Cummins CL, Kepecs DM, Schroer SA, Woo M, et al. SIRT1 activation ameliorates hyperglycaemia by inducing a torpor-like state in an obese mouse model of type 2 diabetes. Diabetologia. 2015 doi: 10.1007/s00125-014-3485-4. [DOI] [PubMed] [Google Scholar]
- 10.Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson JK, Hirschfield GM, Adams DH, Beuers U, Mann DA, Lindor KD, Jones DE. Novel therapeutic targets in primary biliary cirrhosis. Nat Rev Gastroenterol Hepatol. 2015;12:147–158. doi: 10.1038/nrgastro.2015.12. [DOI] [PubMed] [Google Scholar]
- 12.Ali AH, Carey EJ, Lindor KD. Current research on the treatment of primary sclerosing cholangitis. Intractable Rare Dis Res. 2015;4:1–6. doi: 10.5582/irdr.2014.01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemper JK, Xiao Z, Ponugoti B, Miao J, Fang S, Kanamaluru D, Tsang S, et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purushotham A, Xu Q, Lu J, Foley JF, Yan X, Kim DH, Kemper JK, et al. Hepatic deletion of SIRT1 decreases hepatocyte nuclear factor 1alpha/farnesoid X receptor signaling and induces formation of cholesterol gallstones in mice. Mol Cell Biol. 2012;32:1226–1236. doi: 10.1128/MCB.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kazgan N, Metukuri MR, Purushotham A, Lu J, Rao A, Lee S, Pratt-Hyatt M, et al. Intestine-specific deletion of SIRT1 in mice impairs DCoH2-HNF-1alpha-FXR signaling and alters systemic bile acid homeostasis. Gastroenterology. 2014;146:1006–1016. doi: 10.1053/j.gastro.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chothe PP, Swaan PW. Resveratrol promotes degradation of the human bile acid transporter ASBT (SLC10A2) Biochem J. 2014;459:301–312. doi: 10.1042/BJ20131428. [DOI] [PubMed] [Google Scholar]
- 17.Chanda D, Xie YB, Choi HS. Transcriptional corepressor SHP recruits SIRT1 histone deacetylase to inhibit LRH-1 transactivation. Nucleic Acids Res. 2010;38:4607–4619. doi: 10.1093/nar/gkq227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Rodriguez JL, Barbier-Torres L, Fernandez-Alvarez S, Gutierrez-de Juan V, Monte MJ, Halilbasic E, Herranz D, et al. SIRT1 controls liver regeneration by regulating bile acid metabolism through farnesoid X receptor and mammalian target of rapamycin signaling. Hepatology. 2014;59:1972–1983. doi: 10.1002/hep.26971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferreira DM, Afonso MB, Rodrigues PM, Simao AL, Pereira DM, Borralho PM, Rodrigues CM, et al. c-Jun N-terminal kinase 1/c-Jun activation of the p53/microRNA 34a/sirtuin 1 pathway contributes to apoptosis induced by deoxycholic acid in rat liver. Mol Cell Biol. 2014;34:1100–1120. doi: 10.1128/MCB.00420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Lin Q, Lin R, Zhang J, Ren F, Zhang J, Ji M, et al. PPARalpha agonist fenofibrate attenuates TNF-alpha-induced CD40 expression in 3T3-L1 adipocytes via the SIRT1-dependent signaling pathway. Exp Cell Res. 2013;319:1523–1533. doi: 10.1016/j.yexcr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Ahn K, Park SH, Kang HJ, Jang BG, Oh SJ, Oh SM, et al. SIRT1 regulates tyrosine hydroxylase expression and differentiation of neuroblastoma cells via FOXO3a. FEBS Lett. 2009;583:1183–1188. doi: 10.1016/j.febslet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Arduini A, Serviddio G, Escobar J, Tormos AM, Bellanti F, Vina J, Monsalve M, et al. Mitochondrial biogenesis fails in secondary biliary cirrhosis in rats leading to mitochondrial DNA depletion and deletions. Am J Physiol Gastrointest Liver Physiol. 2011;301:G119–G127. doi: 10.1152/ajpgi.00253.2010. [DOI] [PubMed] [Google Scholar]
- 23.Frampton G, Ueno Y, Quinn M, McMillin M, Pae HY, Galindo C, Leyva-Illades D, et al. The novel growth factor, progranulin, stimulates mouse cholangiocyte proliferation via sirtuin-1-mediated inactivation of FOXO1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G1202–G1211. doi: 10.1152/ajpgi.00104.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai SY, He H, Nguyen T, Mennone A, Boyer JL. Retinoic acid represses CYP7A1 expression in human hepatocytes and HepG2 cells by FXR/RXR-dependent and independent mechanisms. J Lipid Res. 2010;51:2265–2274. doi: 10.1194/jlr.M005546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soroka CJ, Mennone A, Hagey LR, Ballatori N, Boyer JL. Mouse organic solute transporter alpha deficiency enhances renal excretion of bile acids and attenuates cholestasis. Hepatology. 2010;51:181–190. doi: 10.1002/hep.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta M, Hernandez-Juviel JM, Waring AJ, Walther FJ. Function and inhibition sensitivity of the N-terminal segment of surfactant protein B (SP-B1-25) in preterm rabbits. Thorax. 2001;56:871–876. doi: 10.1136/thorax.56.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta S, Stravitz RT, Dent P, Hylemon PB. Down-regulation of cholesterol 7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway. J Biol Chem. 2001;276:15816–15822. doi: 10.1074/jbc.M010878200. [DOI] [PubMed] [Google Scholar]
- 28.Ding X, Lichti K, Kim I, Gonzalez FJ, Staudinger JL. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. J Biol Chem. 2006;281:26540–26551. doi: 10.1074/jbc.M600931200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, et al. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290:G923–G932. doi: 10.1152/ajpgi.00490.2005. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, et al. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. 2015 doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 32.Bowlus CL, Gershwin ME. The diagnosis of primary biliary cirrhosis. Autoimmun Rev. 2014;13:441–444. doi: 10.1016/j.autrev.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghonem NS, Assis DN, Boyer JL. On fibrates and cholestasis: A review. Hepatology. 2015 doi: 10.1002/hep.27744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, Helgason CD, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Li F, Patterson AD, Wang Y, Krausz KW, Neale G, Thomas S, et al. Abcb11 deficiency induces cholestasis coupled to impaired beta-fatty acid oxidation in mice. J Biol Chem. 2012;287:24784–24794. doi: 10.1074/jbc.M111.329318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marschall HU, Wagner M, Bodin K, Zollner G, Fickert P, Gumhold J, Silbert D, et al. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–592. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Fickert P, Wagner M, Marschall HU, Fuchsbichler A, Zollner G, Tsybrovskyy O, Zatloukal K, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–481. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J, Huang W, Qatanani M, Evans RM, Moore DD. The constitutive androstane receptor and pregnane X receptor function coordinately to prevent bile acid-induced hepatotoxicity. J Biol Chem. 2004;279:49517–49522. doi: 10.1074/jbc.M409041200. [DOI] [PubMed] [Google Scholar]
- 39.Wagner M, Halilbasic E, Marschall HU, Zollner G, Fickert P, Langner C, Zatloukal K, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 40.Chen W, Owsley E, Yang Y, Stroup D, Chiang JY. Nuclear receptor-mediated repression of human cholesterol 7alpha-hydroxylase gene transcription by bile acids. J Lipid Res. 2001;42:1402–1412. [PubMed] [Google Scholar]
- 41.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Yu C, Wang F, Jin C, Huang X, McKeehan WL. Independent repression of bile acid synthesis and activation of c-Jun N-terminal kinase (JNK) by activated hepatocyte fibroblast growth factor receptor 4 (FGFR4) and bile acids. J Biol Chem. 2005;280:17707–17714. doi: 10.1074/jbc.M411771200. [DOI] [PubMed] [Google Scholar]
- 43.Kong B, Wang L, Chiang JY, Zhang Y, Klaassen CD, Guo GL. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hubbard BP, Gomes AP, Dai H, Li J, Case AW, Considine T, Riera TV, et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong C, Morrison A, Mattison S, Qian S, Bryniarski M, Rankin B, Wang J, et al. Impaired SIRT1 nucleocytoplasmic shuttling in the senescent heart during ischemic stress. FASEB J. 2013;27:4332–4342. doi: 10.1096/fj.12-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soroka CJ, Velazquez H, Mennone A, Ballatori N, Boyer JL. Ostalpha depletion protects liver from oral bile acid load. Am J Physiol Gastrointest Liver Physiol. 2011;301:G574–G579. doi: 10.1152/ajpgi.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, et al. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39:480–488. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 49.Lee YC, Wang HP, Huang SP, Chang YT, Wu CT, Yang CS, Wu MS, et al. Obstructive jaundice caused by hepatocellular carcinoma: detection by endoscopic sonography. J Clin Ultrasound. 2001;29:363–366. doi: 10.1002/jcu.1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.