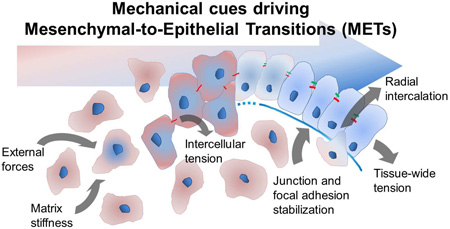
Abstract
The mesenchymal-to-epithelial transition (MET) is an intrinsically mechanical process describing a multi-step progression where autonomous mesenchymal cells gradually become tightly linked, polarized epithelial cells. METs are fundamental to a wide range of biological processes, including the evolution of multicellular organisms, generation of primary and secondary epithelia during development and organogenesis, and the progression of diseases including cancer. In these cases, there is an interplay between the establishment of cell polarity and the mechanics of neighboring cells and microenvironment. In this review, we highlight a spectrum of METs found in normal development as well as in pathological lesions, and provide insight into the critical role mechanics play at each step. We define MET as an independent process, distinct from a reverse-EMT, and propose questions to further explore the cellular and physical mechanisms of MET.
Keywords: MET, EMT, cell mechanics, phenotypic plasticity, cell and tissue polarity, epithelial-to-mesenchymal transition, re-epithelialization, reverse-EMT, epithelialization, polarization
Graphical Abstract
1. Introduction
Mesenchymal-to-epithelial transition (MET) refers to the progressive phenotypic change from loosely associated motile cells to tightly bound cells with a distinct apical-basal polarity. MET is a fundamental cellular process that underlies a range of developmental, regenerative and pathological events relevant to human health. The emergence of MET in eukaryotes likely played a key role in the evolution of multicellular organisms from loose associations of single cells [1]. The invention of specialized intercellular junctions, including tight and adherens junctions, enabled cell-cell communication and allowed the creation of microenvironments isolated from the outside world in the form of lumens. As organisms became increasingly multifaceted in composition and physiological function, so did the programs of morphogenesis, which would require multiple sequences of EMT and MET. Cellular programs of MET from development eventually would be recapitulated during regeneration and under pathological conditions, driving formations of tumors and fibrotic lesions.
In this review, we will highlight extant cases of MET during development, examining the initiation and progression of METs in various contexts of self-assembling tissues, from early embryogenesis to metastatic tumor formations. We direct the interested reader to the excellent reviews that have focused on the molecular signaling pathways that establish cell-cell junctions and apical-basal polarity [2, 3]. Here, we will focus on the specific steps of MET and the contribution of mechanical processes in driving MET, e.g. cell contractility, tensile forces and the mechanical properties of surrounding microenvironment. By relating the mechanical processes that drive mesenchymal cells to more polarized phenotypes we note many parallels with role of mechanics in regulating cellular processes including EMTs [4, 5], cell survival and proliferation, and stem cell differentiation [6, 7]. In our conclusion we pose several questions to guide future research to elucidate how mechanical cues from the dynamically changing microenvironment influence the molecular signaling pathways driving polarity establishment.
2. Defining the Role of Mechanics in the Mesenchymal-to-Epithelial Transition (MET)
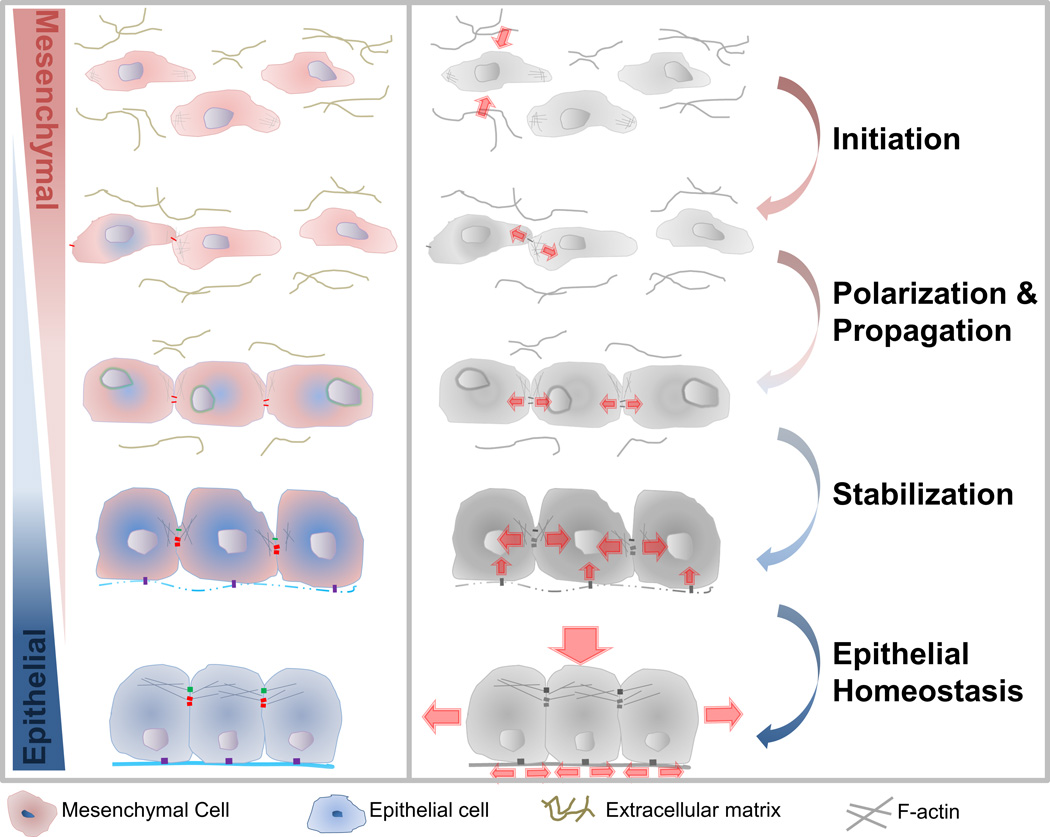
We broadly define a MET as any transition that increases the "epithelial-ness" of a mesenchymal cell, whereby the transitioning cell acquires a more polarized morphology or establishes a more asymmetric distribution of apical junctions. We include in our definitions events described as "epithelialization", "reversion to epithelia," and "re-epithelialization" but note these terms only span segments of a full multi-step MET (Fig. 1). Based on findings from a range of model systems that cover segments of the transitional process we propose multiple steps of MET that cover the entire cellular process (Fig. 1) from (1) triggering the specification of epithelial cells via developmental programs or micro-environmental cues, (2) establishment of cell polarity, (3) propagation of MET through tissue, and (4) stabilization of new tissue architecture. Specific cases of MET and their mechanical regulation will be discussed in later sections.
Figure 1. The role of mechanics in the step-wise progression of mesenchymal-to-epithelial transition (MET).
Scattered or loosely adhered mesenchymal cells (red) undergo multi-step progression to epithelial cells (blue): Initiation: External cues such as forces from surroundings, matrix remodeling, and modulated stiffness induce MET. Polarization and Propagation: Intercellular forces on nascent adherens junctions increase the number and density of adherens junctions, induces actomyosin remodeling, and increases tight junction protein synthesis. Stabilization: The formation of functional tight junctions and focal adhesions increase intercellular tension and extracellular matrix assembly. Epithelial Homeostasis: Contractile actomyosin cortex within cells and collective traction by groups of cells maintain tissue-wide tension and enable the epithelium to withstand loads along apical and basal surfaces.
Step 1: Initiation - the Decision to Change
The initial decision to transition from a mesenchymal to an epithelial phenotype can be categorized by the input signals, i.e., autonomous vs. non-autonomous. Of the many developmental METs found in development it is not clear how many occur autonomously; by contrast, numerous chemical or mechanical cues from microenvironment are known to drive MET, for instance as secondary metastatic tumors arise, or as iPSCs are generated from adult cells, or as wounds close.
A number of cellular processes are known to be responsive to mechanical cues including stem cell fate decisions [8, 9] and durotaxis in migratory cells [10]. A number of findings suggest mechanical cues contribute to MET; for instance, inner cell mass cells of the early mouse blastocyst undergo MET as they localize the polarity protein, aPKC, upon reaching the fluid-filled surface of the blastocyst cavity [11]. Such environmental cues may influence cancer cells, for instance mechanical properties of the secondary site where circulating mesenchymal tumor cells reside is an important factor in activating their metastatic growth as epithelial tumor [12–15].
Step 2: Polarization - Establishing a New Axis
After making the decision to adopt a more epithelial phenotype, mesenchymal cells need to establish apical-basal polarity. Cycles of actomyosin contractility drive the formation and maturation of cell-cell adhesion (e.g., E-cadherin; [16]) between neighbors. Nascent cell-cell contacts formed via cadherin complexes may require tension before the contacts are reinforced or recruit additional types of complexes. Cells increase their adhesion to the ECM substrate by increasing numbers or increasing the strength of focal adhesions (e.g., integrin engagement through ECM and basement membrane;[17]). Spatial patterns of junctional compliance, e.g. the "deformability" of cell-cell or cell-ECM attachments, localize assembly and activity of polarity proteins (e.g. Par3, Par6/aPKC, and crumbs; [17]) that partition apical and basolateral membranes.
Throughout this process a thin meshwork of F-actin and myosin II under the cell cortex provides both mechanical stability and energy to remodel the cytoarchitecture. For example, soon after fertilization, the one cell embryo of C. elegans quickly clears the pulsatile actomyosin contraction from one side of embryo, stabilizing factors that establish anterior posterior polarity [18, 19]. This mechanically defined polarity translates into precise distribution of polarity-regulating factors (e.g., Par2 and Par6;[18]). The adhesion between E-cadherin expressing, MET undergoing cells, may nucleate actin polymerization and cortical contractility in neighboring cells. Cellular tension transmitted through the adherens junction can provide polarization cues to the rest of the cell cortex and enhance the mechanical stability of apical membranes. [16, 20]
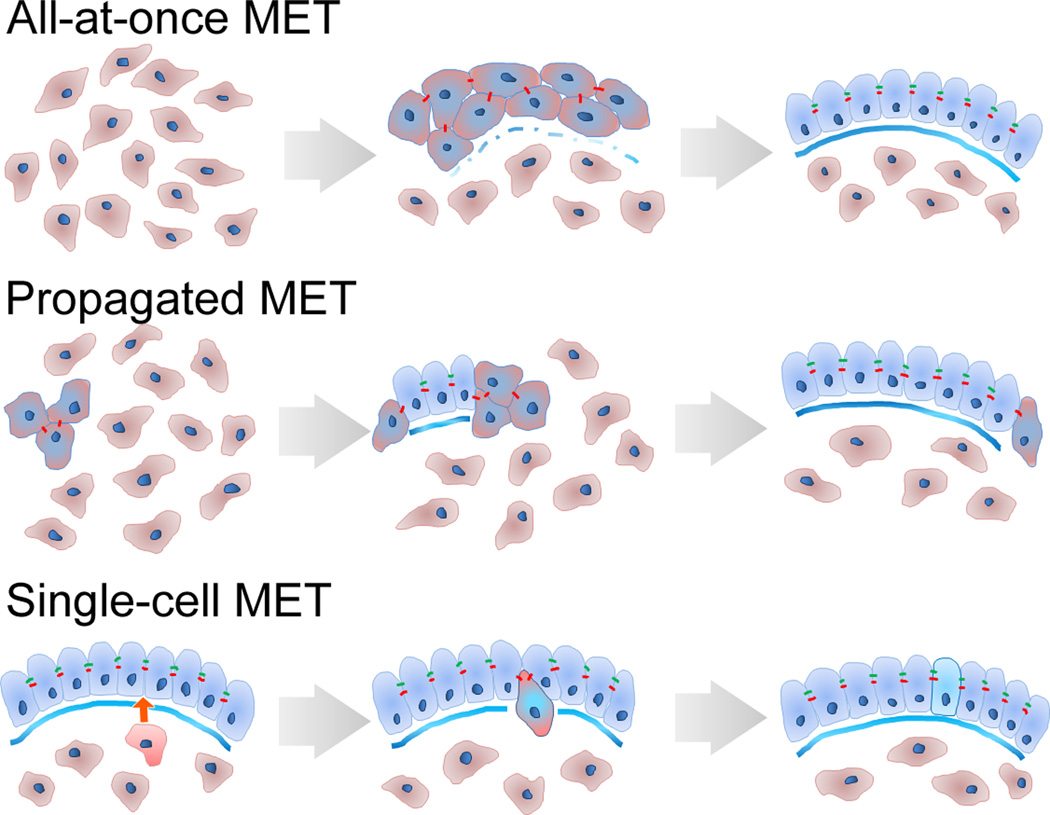
Step 3: Propagation - Spreading Polarity
There are many unanswered questions regarding the propagation of MET due to limited access to the real-time progression of MET in vivo. Several possible scenarios of MET propagation include (Fig. 2): 1) a single cell or a group of cells autonomously becomes epithelial until sufficient numbers form a functional epithelium, 2) a single cell or group of polarized epithelial cells recruit neighboring epithelial cells, through transmission of intercellular forces or signals, thus spreading a nascent epithelial sheet over the tissue, 3) apical insertion of cells from a basal layer of tissue to transition to epithelial. Cellular processes during propagation may be homologous to the processes that induce cell-cell junction formation in cultured epithelial cells after calcium depletion or wounding.
Figure 2. Cellular mechanisms of MET propagation.
Three strategies of MET that use mesenchymal cell sources (red) to expand and diversify an epithelial sheet (blue).
An intriguing possibility is that only a few cells undergoing MET may polarize their contractility and activate mechanoreceptors on neighboring cells that would lead to sequential MET induction along the axis of the tension. Alternatively, development of homogeneous tension over the surface of a compact group of mesenchymal cells or along the surface of a fluid-filled cavity might coordinate the onset MET at that surface.
Step 4: Stabilization - Building New Architecture
Dense circumapical and apico-medial F-actin provide cells in the epithelium mechanical integrity and but also allow cell rearrangement and renewal throughout for the life of the organism. Tension within the epithelium, strength and cohesion of junctional complexes, and composition of the basal ECM may provide cues that guide the insertion of specialized cells recruited from adjacent mesenchymal populations via single-cell MET [21, 22]. Cycles of EMT and MET are common during regeneration of epithelial organs, e.g. after acute kidney injury, yet the role of mechanics in these events remains to be determined.
3. In vitro Insights to METs
In vitro culture models have provided a valuable context to access and analyze the fine points of cellular mechanisms. Models of junction formation in stable epithelial cell lines and of junction re-establishment in cultured epithelial cells have been essential to identifying mechanisms that control junction formation and maturation, which offers partial insight into the steps of MET. In brief, currently available details of epithelialization (e.g., formation and establishment of adherens and tight junctions) are mostly explored using calcium switch protocols on cultured epithelial cells. Modulating simple factors including cell confluency and the period of calcium depletion have provided insight into various elements and magnitudes of re-epithelialization, including the temporal dynamics of localizing adherens junctions (E-cadherin) and tight junctions (ZO-1)[23, 24], identifying the physical role of actin polymerization in sealing adherens ‘zippers’ [25] and Rho-mediated contractile actin to strengthen epithelial junctions [16]. In addition to switching states of epithelial cells, mesenchymal cells (e.g., mouse fibroblast) expressing E-cadherin have been used to understand how cell polarity is established during MET and showed the role of cadherins in inducing epithelial-like polarization by restricting NaK-ATPase to the basolateral domains of the cell [26].
4. METs and Early Development
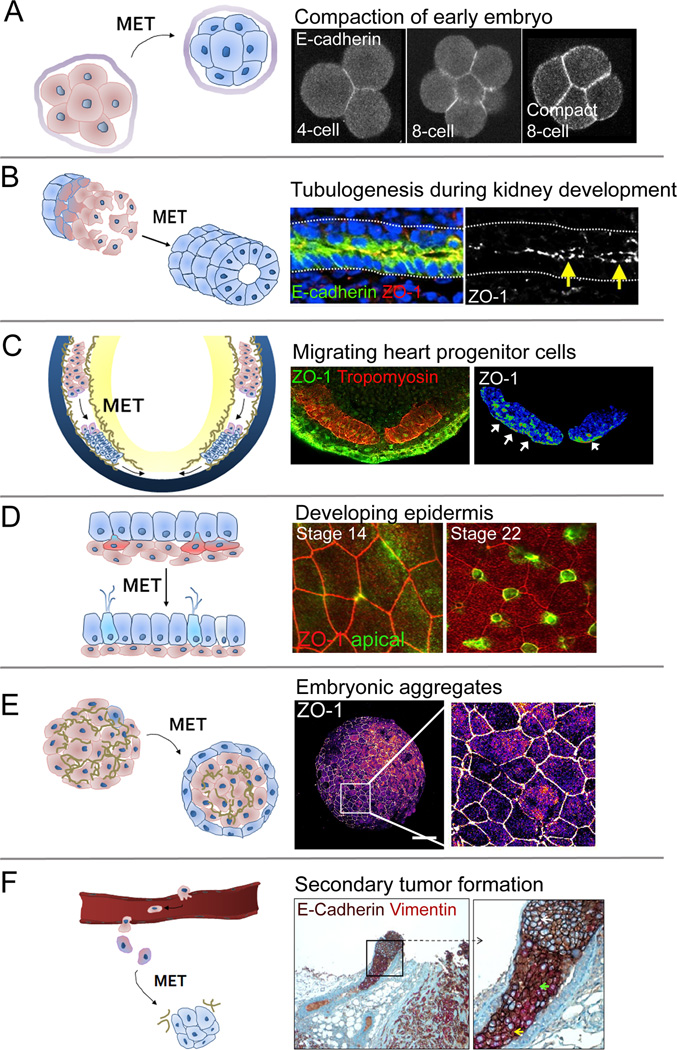
Definitive stages of early development are synonymous with MET. Blastomeres localize their membrane traffic [27] and exhibit polarized membrane domains as early as the two-cell stage in the aquatic frog Xenopus 28]. Early mammalian morphogenesis begins with a MET when the 8-cell embryo undergoes compaction; undifferentiated cells become adherent and establish apical-basally polarized membrane domains (Fig. 3A) after activation of the apical-basal polarity pathways. The resulting polarity allows cells to apply contractile forces on neighbors via their adhesion sites [29–33]. It is also thought that membranes surrounding early embryos, such as the vitelline membrane in C. elegans or the zona pellucida in mammals, exert a passive mechanical force on blastomeres that also contributes to compaction and constrain blastomere movements [31, 34]. Upon division into the 16- and 32-cell stages, only surface cells retain apical membrane polarity and the blastocyst cavity begins to form. In mammals, the surface cells and inner cells give rise to two different cell lineages, the trophoectoderm and the inner cell mass, respectively. Further development of the trophoectoderm requires tight junctions, actomyosin contractility, and cadherin-based adhesions [35], suggesting a continuing role for intercellular tension. Once inner cell mass forms apical tight junctions polarized distribution of NaK-ATPase pumps ions into the blastocoel which inflates from osmotic pressure [27]. In zebrafish, cells forming the embryo are fully derived from an inner cell mass of mesenchymal cells and must undergo MET to establish all embryonic epithelia. The sorting and agglomeration of these cells appear regulated by differential contraction as cell-cortex tension regulates germ-layer specific epithelial aggregation [36]. Gastrulation proceeds with polarized cell orientations, intercalation and migration driving tissue collective migration and convergent extension [37–40] requiring an interplay between cellular mechanics, canonical Wnt signaling and other planar cell polarity signaling pathways.
Figure 3. Six examples of MET during development, regeneration, and cancer.
A) MET during compaction of the mouse embryo. Non-polarized egg initiates MET with E-cadherin mediated compaction of early embryo. Modified with permission from [105]. B) MET during lumen formation of Wolffian duct (WD) in developing chicken embryo. Apical localization of E-cadherin and ZO-1 indicate formation of epithelia along the newly assembled lumen (yellow arrows). Modified with permission from [42]. C) MET during heart development in Xenopus embryo (mid-tailbud; stage 28). Ventrally migrating heart progenitor cells (HPCs; red, tropomyosin) establish apical-basal polarity and form tight junctions (ZO-1, green on middle panel, pseudo-color in right panel - white arrows indicate apical surface; personal communication, T. R. Jackson). D) Single-cell MET during skin development of Xenopus embryo. Basal mesenchymal cells (green) intercalate and integrate with the pre-existing epithelial sheet (red ZO-1) [85]. E) MET in embryonic aggregates. Surface cells on pluripotent mesenchymal aggregate adopt apical-basal polarity and transition to epithelia cells (ZO-1). Pseudo-colored ZO-1 expression shows scattered clusters of epithelial cells across the surface of the mesenchymal-cell aggregate (personal communication, H. Y. Kim), and F) MET in metastatic tumor. Heterogeneously mixed population of mesenchymal (vimentin) and epithelial (E-cadherin) cells found in tumor emboli within local lymphovascular space. Modified with permission from [114]. Schematic diagrams in left panels (A-F) indicate involvement of mesenchymal cells (red) transitioning to epithelial cell type (blue).
5. METs Are Fundamental to Vertebrate Organogenesis
Mesenchymal cells contribute to the formation of many epithelia in the developing embryo through both multicellular and single-cell METs. In the following section we will discuss several examples that highlight specific mechanisms and different stages of MET during multicellular and single cell MET contributions to vertebrate organogenesis.
Kidney Development
MET in kidney development is one of the best-studied METs in organogenesis and has provided detailed insights on the cellular basis and the molecular signaling pathways involved in kidney development, which have been well reviewed [41]. Kidney progenitor cells (KPCs) derive from bilateral fields of mesoderm cells that lie in between the somitic and lateral plate mesoderm. The caudally extending pronephric duct forms as mesenchymal leader and follower KPCs exhibit collective migration [42, 43]. Follower cells undergo MET to form a single-cell thick epithelial tube that extends caudally (Fig. 3B). Studies of collective cell migration models suggest polarized traction generation from the epithelial follower cells drive this process [44], using intercellular tensile forces transmitted through cadherins to establish migration polarity [45]. Such intercellular tensions may be promoting MET in the follower cells [46] yet remain inhibited by FGF signaling in leader cells.
As the pronephric duct extends, adjacent mesenchymal cells form arrays of epithelial tubules that branch from the pronephric duct to lengthen medioventrally toward the aorta. The caudal end of the pronephric duct begins to swell to form the ureteric bud, which invades the metanephric mesenchyme and begins branching morphogenesis. At the tips of these branches, the metanephric mesenchyme cells undergo MET to contribute to branch elongation. Branching morphogenesis appears to be highly dependent on extracellular matrix [47], PCP signaling and convergent extension [48] and on Rho/ROCK and MAPK/ERK signaling, as inhibition of ROCK with Y27632 increases branching of epithelial buds and migration of metanephric mesenchyme [49]. Polarization of tubule epithelia appears crucial for kidney development, as perturbations give rise to defects, including polycystic kidney disease [50]. Fully-differentiated kidney epithelia include a diverse population of cell types; patterning of these intercalated cells depend on Notch signaling [51] but how exactly they become incorporated into these epithelia, whether they present in the epithelium de novo or they arrive through single cell MET and intercalation, is not clear.
Early Heart Development
Vertebrate heart organogenesis is an inherently mechanical process whereby planar, bisymmetric fields of heart precursor cells (HPCs) move ventrally to merge on the midline, fold to form a lumen and tube, which loops and septates to form the adults heart. In avian and mammalian development, presumptive HPCs undergo an EMT during gastrulation and establish identities within the definitive mesoderm [4, 52–54] (Fig. 3C). Prior to reaching the midline, HPCs form epithelia that are characterized by basolateral membrane expression of β-catenin and apical aPKC [55, 56] and a belt of N-cadherin expression [57]. Fibronectin is also deposited on the basal surface of HPC epithelia and plays a crucial role in establishing polarity and adherens junctions [55]. MET is required for proper heart function [57] and shortly after epithelialization is when the first cardiac electrical activity is recorded [58, 59]. During their movement to the ventral midline, HPCs move through a dynamically changing mechanical micro-environment, which includes extensive fibronectin remodeling [60, 61] increases in tissue stiffness [62] and tension originating from the convergent extension of the endoderm [63–65]. While knocking down actomyosin based cell contractility prior to heart tube formation and epithelialization consistently causes cardiac defects [63, 66], perturbing cell contractility near the onset of looping, post-MET does not, which implicates a crucial role for cell mechanics in providing cues to guide the early organization of HPCs.
After the myocardial epithelium is established, endocardial cells undergo an EMT to migrate out of the trunk mesoderm and form a loose mesenchymal network located between the myocardium and the endoderm, which deposits and remodels fibrillin to enhance endocardial migration [67]. These cells aggregate to line the innermost lumen of the heart, and polarize their actin cytoskeleton, N-cadherin and integrin expression and NaK-ATPase [58, 68]. Additional trunk mesoderm cells contribute to the outflow tract and the right ventricle of the growing heart; disruption of this MET may have a role in congenital heart defects associated with DiGeorge Syndrome and basal filopodia activity appears crucial to outflow tract development [69]. Both of these processes involve restructuring ECM, directed migration, and polarized actomyosin, suggesting that microenvironment mechanics or intercellular forces play a role in these METs.
Somitogenesis
After gastrulation the internalized presomitic mesoderm (PSM) is further subdivided and organized through a series of transitions that increase order within mesenchymal tissues [70, 71]. New ECM interfaces provide cues to mesenchymal cells allowing them to polarize in shape and organize dynamic behaviors along the anterior-posterior and medial-lateral axes of the embryo [72]. Segmentation of the dorsal paraxial mesoderm into somites involves a further transition toward an epithelial phenotype. Avian exhibit the most polarized example of epithelial somites with clear apical-basal assembly of N-cadherin and ZO-1 demonstrating the somites in these animals generate an inward directed apical polarity [73, 74]. The PSM in teleosts and amphibians, which undergo more rapid larval development, also develop highly ordered somites but these do not fully epithelialize with apical junctions [75, 76]. Interestingly, somitogenesis in these species appear sensitive to same factors that destabilize epithelial somites suggesting that their constituent mesenchyme undergo early phases of MET but do not complete the process.
We can consider the role of mechanics in somitogenesis MET in several stages. First, mechanical cues triggered by the somitogenesis clock may initiate phenotypic changes in cell behaviors and gene expression. Next, mechanical processes likely drive sorting as polarized mesoderm cells separate and engulf non-polarized cells. Lastly, forces produced at new surfaces of the PSM and nascent somite may stabilize junctions by feedback through intracellular trafficking pathways, using cues from surrounding ECM to orient assembly of apical-basal junctions. Patterns of cell-cell tension may enable cells to adjust their phenotype or even gene expression to their position within each new segment. Recent efforts to consider the impact of global patterns of tissue strain [77] and the connection between cell behaviors and tissue mechanics during somitogeneis [78] suggest mechanics may play an important role in the METs driving segmentation.
6. Single-cell MET - Expanding the Function of Existing Epithelia
Once an epithelial tissue or organ has been established, additional cases of single cell MET can generate specialized cells (Fig. 2). During single cell MET, individual cells within the surrounding mesenchyme are induced to become a specialized cell precursor which subsequently migrates from the mesenchyme and insert from the basal surface of the epithelium. The exact origins and the environmental cues generating most specialized cells remain unclear but we will review efforts to identify mechanisms underlying the contribution of single cell MET to the Xenopus embryonic skin and mammalian kidney tubules.
Single cell METs contribute distinct epidermal cell types responsible for ciliary transport and electrophysiology in the larval epidermis of the aquatic frog Xenopus laevis 79] (Fig. 3D). Cells on the embryo surface differ from deeper mesenchymal cells by exhibiting strong apical-basal polarity both morphologically and molecularly [28, 80–82]. Mesenchymal cells associate strongly with nascent fibronectin extracellular matrix, assembling a delineated basement membrane before gastrulation begins [83]. Global movements of epiboly and gastrulation spread epidermal precursors within the embryonic ectoderm over the entire surface of the embryo where local signals mediated by Notch generate a set of precursor cells [84]. Single cell METs in the Xenopus epidermis occur in stages as induced cells disperse along the basal surface of the existing epithelium and extend protrusions toward the apical surface of the epidermis [22, 85]. The cell mechanics of intercalation are poorly understood but appear to be regulated by many of the same pathways that regulate cell motility and establishment of junctions between same-type epithelial cells [22, 86, 87].
7. METs During Drosophila Development
METs play key roles in shaping the Drosophila embryo forming secondary epithelia such as the midgut and the dorsal vessel, adding diverse cell types to the renal rudiment, and later establishing the follicular epithelium of the developing oocyte. In contrast to vertebrates, junctions in epithelia descended from the blastoderm epithelia (e.g. primary epithelia) differ from junctions in midgut and heart tubes that form by MET from mesoderm cells produced by gastrulation (e.g. secondary epithelia; [88, 89]). Drosophila offers many lessons in morphogenesis and we summarize the role of MET in three events: formation of the heart tube, transformation of the mesoderm into the midgut epithelium, and intercalation of mesenchymal cells into the renal epithelium.
Formation of the cardiac heart tube, or dorsal vessel, requires transition of mesenchymal cells to a more epithelial phenotype with bilateral populations of cardiac precursors polarizing lumenal-domains before reaching the dorsal midline. Processes involved in MET and heart tube formation in Drosophila parallel those guiding vertebrate heart formation, including the dependence of MET, but not cell identity on extracellular matrix [90–93], and the role of tension within the embryo to position precursors and enable fusion at the midline [94]. Furthermore, amnioserosa cells, which first appear to engage precursors with E-cadherin and septate junctions must disengage before cardiac cells can fully establish polarized lumenal-domains and fuse [95]. Mechanical cues within the microenvironment during dorsal closure and fusion are likely key to the MET and other phenotypic changes in cardiac precursors in the fly as they form the dorsal vessel.
Shortly after gastrulation mesodermal cells from the anterior and posterior midgut invaginations bridge the length of the embryo to establish the alimentary canal [96]. Mesenchyme progressively adopt apical-basal polarity with junctional E-cadherin and apical arrays of F-actin as they form a columnar epithelium. Just as in the case of the heart, extracellular matrix cues are not required for early patterning but are required for MET [92]. Similar to the dependence of MET and cell migration on laminin, in the forming midgut also depends on instructive cues from netrin expressed by their substrate through the midgut localized receptor frazzled to establish a columnar epithelium.
Once primary and secondary epithelial form in Drosophila they provide a substrate for single cell MET through radial intercalation. Intercalation of mesenchymal precursors into established epithelia, like intercalation of ciliary cells and ionocytes into the Xenopus epidermis, occurs in the midgut (adult midgut precursors and interstitial cell precursors; [97]) and renal epithelia.
8. MET Required for Stem Cell Reprograming
MET is required during reprograming of somatic cells [98, 99]. During the initial phase of reprograming, mouse embryonic fibroblasts transition to an epithelial, unstable intermediate state through a MET[100]. This transition is marked by downregulation of mesenchymal genes (i.e., snail, N-Cadherin, fibronectin) and upregulation of epithelial genes (i.e., E-cadherin and Epcam). Recent studies highlight the role of mechanics in regulating MET during the generation of iPSCs; when cultured on microgrooves, induced fibroblasts align and elongate and show increased efficiency of iPSC formation which depends on MET [101]. Failure to maintain cellular tension generated by the actin myosin network on microgrooves, by inhibiting myosin contractility with blebbistatin, completely abolishes cell reprograming and inhibits MET [101]. Knock-down of these kinases that disrupt the F-actin network, including TESK1 and LIMK2 that phosphorylate the actin-binding protein cofilin [102], induce dramatic cellular phenotypic changes, turns fibroblasts into epithelial cells, and enhances iPSC efficiency. Similarly, MEFs cultured on a soft hydrogels increased MET and iPSC formation [103]. Together these data suggest a key role for cellular mechanics and tension maintained by cytoskeleton in regulating MET during iPSC formation.
9. METs in Secondary Tumor Formation
Metastatic cancers begins with dissemination of mesenchymal tumor cells which undergo MET and form proliferative macro-metastatic colonies. Comparing expression of epithelial junctional proteins including E-cadherin, β-catenin, and connexin, in primary tumor and matched distant metastases in lung, liver, and brain of cancer patients show equal or increased epithelial cells in metastases, indicating that circulating mesenchymal tumor cells undergo MET [42, 104] (Fig. 3F). Furthermore, activation of MET via repression of twist1 or prrx1 promotes cell proliferation and the establishment of metastatic colonies at distant sites [2, 105]. Microenvironmental cues may inhibit or support MET and metastases [8]; for example, a stiff microenvironment created by extensively crosslinked collagen fibrils has been shown to promote MET and secondary site establishment, tumor cell proliferation and metastatic colonization [12, 13]. Dense ECM fibrils at these sites can enhance integrin-mediated tumor cell engagement, activate focal adhesion kinase (FAK), and actomyosin reorganization [14, 15]. Tension generated by the actomyosin network also provides feedback in the form of ECM arrangement and bulk tissue stiffness to drive tumor progression [15]. The critical role of the mechanical microenvironment in cancer metastasis is increasingly appreciated [106] but the role of these cues in driving MET are unclear.
10. Conclusion / Future Directions
The classical definition of MET relies on black and white definitions of what it means to be a mesenchymal cell or an epithelial cell relying on downregulation of mesenchymal markers, such as vimentin, with upregulation of epithelial markers, such as E-cadherin. This rigid, histology-based identification must be broadened in recognition of the diverse spectrum of functional METs observed in development and homeostasis. The number of studies that directly contradict over-rigid definitions of epithelial and mesenchymal are increasing, including Drosophila mesenchymal cells requiring functional E-cadherin to migrate [107], requirement of N-cadherin for the establishment and functional polarity of the outer epithelium in Xenopus embryos [108], and functionally mesenchymal breast cancer cells that neither downregulate E-cadherin nor express vimentin [109–111]. Instead, METs come in various forms and degrees, ranging from a population of autonomous migratory cells that aggregate and form a structured epithelium or endothelium, to a single independent cell intercalating into an epithelium and adopting the polarity of the surrounding cells, to a cell sheet becoming slightly more polarized. In concluding this review we pose a number of key questions whose resolution will expand our understanding of MET and suggest avenues for improved approaches to regeneration and cancer treatment.
How do METs differ and what processes are conserved?
While we have described a broad spectrum of METs that shape different tissues we propose they share common dependence on cell and tissue mechanics. Future studies will need to identify MET instructive cues and demonstrate how these regulators might be used to convert one type of MET to another. This strategy may prove crucial to elucidating key factors that regulate MET in disease progressions, including cancer metastasis, where direct observation is often logistically impractical due to the stochastic nature of MET in animal cancer models.
Is MET simply the reverse of an EMT?
A number of cancer studies have identified what they call reversible EMTs, in response to growth factors [112] and hormones [111]. However, in development, many progenitor cell populations undergo a series of EMTs and METs that accompany dynamic changes to their microenvironment. These transitions do not appear to be the “reverse” of each other, but rather a progression toward terminal differentiation. As studies uncover specific mechanisms driving MET, the contrast between MET and the so-called reverse EMT will become clearer.
What is the role of MET in regeneration?
Mesenchymal-to-epithelial transitions appear to play a central role in regeneration of organs that formed initially via MET including heart and kidney and during regeneration of limb or tail structures where entire germ layers must be reconstructed. In these cases the precise role of MET in regeneration has been difficult to elucidate given the mixed lineage of organs and tissues comprised from multiple germ layers. For instance, kidney tubule regeneration in response to BMP7 has been proposed from renal fibroblasts [113] but tracking the identity of the cells undergoing MET is challenging given the close lineage of the tubule endothelium and renal fibroblast. Still, efficient regeneration strategies may require induction and regulation of MET in order to recreate the diverse populations of cells needed for proper organ function.
Highlights.
Mechanical cues initiate, propagate, and stabilize polarization MET
Spectrum of METs range from in unison epithelialization to single cell METs
Early development, organogenesis, cancer progression share basic MET framework
Comparative analysis of MET and their relation to EMTs needs to be explored further
Acknowledgments
We would like to thank members of the Davidson lab for their helpful discussions and insights. This review was supported in part by grants to LAD from the NIH (R01 HD044750) and NSF (CBET-1547790). TRJ was supported by the Cardiovascular Bioengineering Training Program (NIH NHLBI T32 HL076124). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Valentine JW. The evolution of multicellular plants and animals. Scientific American. 1978;239:141–158. doi: 10.1038/scientificamerican0978-140. [DOI] [PubMed] [Google Scholar]
- 2.Tsai Jeff H, Donaher Joana L, Murphy Danielle A, Chau S, Yang J. Spatiotemporal Regulation of Epithelial-Mesenchymal Transition Is Essential for Squamous Cell Carcinoma Metastasis. Cancer Cell. 2012;22:725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kowalski PJ, Rubin MA, Kleer CG. E-cadherin expression in primary carcinomas of the breast and its distant metastases. Breast Cancer Res. 2003;5:R217–R222. doi: 10.1186/bcr651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shook D, Keller R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mechanisms of Development. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Gjorevski N, Boghaert E, Nelson CM. Regulation of Epithelial-Mesenchymal Transition by Transmission of Mechanical Stress through Epithelial Tissues. Cancer Microenvironment. 2012;5:29–38. doi: 10.1007/s12307-011-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Developmental Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 7.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 8.Shibue T, Weinberg RA. Metastatic colonization: Settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Seminars in Cancer Biology. 2011;21:99–106. doi: 10.1016/j.semcancer.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–817. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saiz N, Grabarek JB, Sabherwal N, Papalopulu N, Plusa B. Atypical protein kinase C couples cell sorting with primitive endoderm maturation in the mouse blastocyst. Development. 2013;140:4311–4322. doi: 10.1242/dev.093922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cox TR, Bird D, Baker A-M, Barker HE, Ho MW-Y, Lang G, et al. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Research. 2013;73:1721–1732. doi: 10.1158/0008-5472.CAN-12-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, et al. Metastatic Growth from Dormant Cells Induced by a Col-I–Enriched Fibrotic Environment. Cancer Research. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, et al. Inhibition of Metastatic Outgrowth from Single Dormant Tumor Cells by Targeting the Cytoskeleton. Cancer Research. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel Michael S, Lopez Jose I, McGhee Ewan J, Croft Daniel R, Strachan D, Timpson P, et al. Actomyosin-Mediated Cellular Tension Drives Increased Tissue Stiffness and β-Catenin Activation to Induce Epidermal Hyperplasia and Tumor Growth. Cancer Cell. 2011;19:776–791. doi: 10.1016/j.ccr.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. The Journal of Cell Biology. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- 18.Munro E, Nance J, Priess JR. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Developmental cell. 2004;7:413–424. doi: 10.1016/j.devcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Schonegg S, Hyman AA. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development. 2006;133:3507–3516. doi: 10.1242/dev.02527. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, et al. Actin at cell-cell junctions is composed of two dynamic and functional populations. Journal of Cell Science. 2005;118:5549–5562. doi: 10.1242/jcs.02639. [DOI] [PubMed] [Google Scholar]
- 21.Chien Y-H, Keller R, Kintner C, Shook DR. Mechanical Strain Determines the Axis of Planar Polarity in Ciliated Epithelia. Current Biology. 2015 doi: 10.1016/j.cub.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedzinski J, Hannezo E, Tu F, Biro M, Wallingford JB. Emergence of an Apical Epithelial Cell Surface In Vivo. Developmental cell. 2016;36:24–35. doi: 10.1016/j.devcel.2015.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siliciano JD, Goodenough DA. Localization of the tight junction protein, ZO-1, is modulated by extracellular calcium and cell-cell contact in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1988;107:2389–2399. doi: 10.1083/jcb.107.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams CL, Chen Y-T, Smith SJ, James Nelson W. Mechanisms of Epithelial Cell–Cell Adhesion and Cell Compaction Revealed by High-resolution Tracking of E-Cadherin– Green Fluorescent Protein. The Journal of Cell Biology. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Hye Y, Pang M-F, Varner Victor D, Kojima L, Miller E, Radisky Derek C, et al. Localized Smooth Muscle Differentiation Is Essential for Epithelial Bifurcation during Branching Morphogenesis of the Mammalian Lung. Developmental Cell. 34:719–726. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-Epithelial Transition Facilitates Bladder Cancer Metastasis: Role of Fibroblast Growth Factor Receptor-2. Cancer Research. 2006;66:11271–11278. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 27.Roberts SJ, Leaf DS, Moore HP, Gerhart JC. The establishment of polarized membrane traffic in Xenopus laevis embryos. J Cell Biol. 1992;118:1359–1369. doi: 10.1083/jcb.118.6.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chalmers AD, Strauss B, Papalopulu N. Oriented cell divisions asymmetrically segregate aPKC and generate cell fate diversity in the early Xenopus embryo. Development. 2003;130:2657–2668. doi: 10.1242/dev.00490. [DOI] [PubMed] [Google Scholar]
- 29.Winkel GK, Ferguson JE, Takeichi M, Nuccitelli R. Activation of protein kinase C triggers premature compaction in the four-cell stage mouse embryo. Dev Biol. 1990;138:1–15. doi: 10.1016/0012-1606(90)90171-e. [DOI] [PubMed] [Google Scholar]
- 30.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 31.De Vries WN, Evsikov AV, Haac BE, Fancher KS, Holbrook AE, Kemler R, et al. Maternal beta-catenin and E-cadherin in mouse development. Development. 2004;131:4435–4445. doi: 10.1242/dev.01316. [DOI] [PubMed] [Google Scholar]
- 32.Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, et al. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J Cell Sci. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- 33.Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–1433. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 34.Grana TM, Cox EA, Lynch AM, Hardin J. SAX-7/L1CAM and HMR-1/cadherin function redundantly in blastomere compaction and non-muscle myosin accumulation during Caenorhabditis elegans gastrulation. Dev Biol. 2010;344:731–744. doi: 10.1016/j.ydbio.2010.05.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleming TP, McConnell J, Johnson MH, Stevenson BR. Development of tight junctions de novo in the mouse early embryo: control of assembly of the tight junction-specific protein, ZO-1. J Cell Biol. 1989;108:1407–1418. doi: 10.1083/jcb.108.4.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krieg M, Arboleda-Estudillo Y, Puech PH, Kafer J, Graner F, Muller DJ, et al. Tensile forces govern germ-layer organization in zebrafish. Nat Cell Biol. 2008;10:429–436. doi: 10.1038/ncb1705. [DOI] [PubMed] [Google Scholar]
- 37.Jessen JR, Topczewski J, Bingham S, Sepich DS, Marlow F, Chandrasekhar A, et al. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marlow F, Topczewski J, Sepich D, Solnica-Krezel L. Zebrafish Rho kinase 2 acts downstream of Wnt11 to mediate cell polarity and effective convergence and extension movements. Curr Biol. 2002;12:876–884. doi: 10.1016/s0960-9822(02)00864-3. [DOI] [PubMed] [Google Scholar]
- 39.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. J Cell Biol. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, et al. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development. 2003;130:5375–5384. doi: 10.1242/dev.00758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costantini F, Kopan R. Patterning a Complex Organ: Branching Morphogenesis and Nephron Segmentation in Kidney Development. Developmental Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atsuta Y, Takahashi Y. FGF8 coordinates tissue elongation and cell epithelialization during early kidney tubulogenesis. Development. 2015;142:2329–2337. doi: 10.1242/dev.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Attia L, Schneider J, Yelin R, Schultheiss TM. Collective cell migration of the nephric duct requires FGF signaling. Dev Dyn. 2015;244:157–167. doi: 10.1002/dvdy.24241. [DOI] [PubMed] [Google Scholar]
- 44.Trepat X, Wasserman MR, Angelini TE, Millet E, Weitz DA, Butler JP, et al. Physical forces during collective cell migration. Nature Physics. 2009;5:426–430. [Google Scholar]
- 45.Weber GF, Bjerke MA, DeSimone DW. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell. 2012;22:104–115. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasilyev A, Liu Y, Hellman N, Pathak N, Drummond IA. Mechanical stretch and PI3K signaling link cell migration and proliferation to coordinate epithelial tubule morphogenesis in the zebrafish pronephros. PloS one. 2012;7:e39992. doi: 10.1371/journal.pone.0039992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos OF, Nigam SK. HGF-induced tubulogenesis and branching of epithelial cells is modulated by extracellular matrix and TGF-β. Developmental biology. 1993;160:293–302. doi: 10.1006/dbio.1993.1308. [DOI] [PubMed] [Google Scholar]
- 48.Yates LL, Papakrivopoulou J, Long DA, Goggolidou P, Connolly JO, Woolf AS, et al. The planar cell polarity gene Vangl2 is required for mammalian kidney-branching morphogenesis and glomerular maturation. Human molecular genetics. 2010;19:4663–4676. doi: 10.1093/hmg/ddq397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer TN, Schwesinger C, Sampogna RV, Vaughn DA, Stuart RO, Steer DL, et al. Rho kinase acts at separate steps in ureteric bud and metanephric mesenchyme morphogenesis during kidney development. Differentiation. 2006;74:638–647. doi: 10.1111/j.1432-0436.2006.00102.x. [DOI] [PubMed] [Google Scholar]
- 50.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, et al. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- 52.Ohta S, Suzuki K, Tachibana K, Tanaka H, Yamada G. Cessation of gastrulation is mediated by suppression of epithelial-mesenchymal transition at the ventral ectodermal ridge. Development. 2007;134:4315–4324. doi: 10.1242/dev.008151. [DOI] [PubMed] [Google Scholar]
- 53.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Carver EA, Jiang R, Lan Y, Oram KF, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchymal transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–382. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- 56.Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- 57.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 58.Linask KK. N-cadherin localization in early heart development and polar expression of Na+,K(+)-ATPase, and integrin during pericardial coelom formation and epithelialization of the differentiating myocardium. Dev Biol. 1992;151:213–224. doi: 10.1016/0012-1606(92)90228-9. [DOI] [PubMed] [Google Scholar]
- 59.Hirota A, Kamino K, Komuro H, Sakai T. Mapping of early development of electrical activity in the embryonic chick heart using multiple-site optical recording. J Physiol. 1987;383:711–728. doi: 10.1113/jphysiol.1987.sp016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garavito-Aguilar ZV, Riley HE, Yelon D. Hand2 ensures an appropriate environment for cardiac fusion by limiting Fibronectin function. Development. 2010;137:3215–3220. doi: 10.1242/dev.052225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trinh LA, Yelon D, Stainier DY. Hand2 regulates epithelial formation during myocardial diferentiation. Curr Biol. 2005;15:441–446. doi: 10.1016/j.cub.2004.12.083. [DOI] [PubMed] [Google Scholar]
- 62.Zhou J, Kim HY, Wang JH, Davidson LA. Macroscopic stiffening of embryonic tissues via microtubules, RhoGEF and the assembly of contractile bundles of actomyosin. Development. 2010;137:2785–2794. doi: 10.1242/dev.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi Y, Varner VD, Taber LA. Why is cytoskeletal contraction required for cardiac fusion before but not after looping begins? Phys Biol. 2015;12:016012. doi: 10.1088/1478-3975/12/1/016012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Varner VD, Taber LA. Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development. 2012;139:1680–1690. doi: 10.1242/dev.073486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye D, Lin F. S1pr2/Galpha13 signaling controls myocardial migration by regulating endoderm convergence. Development. 2013;140:789–799. doi: 10.1242/dev.085340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li S, Zhou D, Lu MM, Morrisey EE. Advanced cardiac morphogenesis does not require heart tube fusion. Science. 2004;305:1619–1622. doi: 10.1126/science.1098674. [DOI] [PubMed] [Google Scholar]
- 67.Sugi Y, Markwald RR. Formation and early morphogenesis of endocardial endothelial precursor cells and the role of endoderm. Dev Biol. 1996;175:66–83. doi: 10.1006/dbio.1996.0096. [DOI] [PubMed] [Google Scholar]
- 68.Linask KK, Lash JW. Early heart development: dynamics of endocardial cell sorting suggests a common origin with cardiomyocytes. Dev Dyn. 1993;196:62–69. doi: 10.1002/aja.1001960108. [DOI] [PubMed] [Google Scholar]
- 69.Francou A, Saint-Michel E, Mesbah K, Kelly RG. TBX1 regulates epithelial polarity and dynamic basal filopodia in the second heart field. Development. 2014;141:4320–4331. doi: 10.1242/dev.115022. [DOI] [PubMed] [Google Scholar]
- 70.Latimer A, Jessen JR. Extracellular matrix assembly and organization during zebrafish gastrulation. Matrix Biology. 2010;29:89–96. doi: 10.1016/j.matbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Davidson LA, Keller R, Desimone DW. Assembly and remodeling of the fibrillar fibronectin extracellular matrix during gastrulation and neurulation in Xenopus laevis. Dev Dyn. 2004;231:888–895. doi: 10.1002/dvdy.20217. [DOI] [PubMed] [Google Scholar]
- 72.Barrios A, Poole RJ, Durbin L, Brennan C, Holder N, Wilson SW. Eph/Ephrin signaling regulates the mesenchymal-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Current Biology. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 73.Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987;104:1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakaya Y, Kuroda S, Katagiri YT, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Developmental cell. 2004;7:425–438. doi: 10.1016/j.devcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Fagotto F, Gumbiner BM. Beta-catenin localization during Xenopus embryogenesis: accumulation at tissue and somite boundaries. Development. 1994;120(12):3667–3679. doi: 10.1242/dev.120.12.3667. [DOI] [PubMed] [Google Scholar]
- 76.Daggett DF, Domingo CR, Currie PD, Amacher SL. Control of morphogenetic cell movements in the early zebrafish myotome. Dev Biol. 2007;309:169–179. doi: 10.1016/j.ydbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Truskinovsky L, Vitale G, Smit T. A mechanical perspective on vertebral segmentation. International Journal of Engineering Science. 2014;83:124–137. [Google Scholar]
- 78.Benazeraf B, Francois P, Baker RE, Denans N, Little CD, Pourquie O. A random cell motility gradient downstream of FGF controls elongation of an amniote embryo. Nature. 2010;466:248–252. doi: 10.1038/nature09151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dubaissi E, Rousseau K, Lea R, Soto X, Nardeosingh S, Schweickert A, et al. A secretory cell type develops alongside multiciliated cells, ionocytes and goblet cells, and provides a protective, anti-infective function in the frog embryonic mucociliary epidermis. Development. 2014;141:1514–1525. doi: 10.1242/dev.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chalmers AD, Welchman D, Papalopulu N. Intrinsic differences between the superficial and deep layers of the Xenopus ectoderm control primary neuronal differentiation. Dev Cell. 2002;2:171–182. doi: 10.1016/s1534-5807(02)00113-2. [DOI] [PubMed] [Google Scholar]
- 81.Jones E, Woodland H. The development of animal cap cells in Xenopus: the effects of environment on the differentiation and the migration of grafted ectodermal cells. Development. 1987;101:23–32. [Google Scholar]
- 82.Chalmers AD, Lachani K, Shin Y, Sherwood V, Cho KW, Papalopulu N. Grainyhead-like 3, a transcription factor identified in a microarray screen, promotes the specification of the superficial layer of the embryonic epidermis. Mech Dev. 2006;123:702–718. doi: 10.1016/j.mod.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 83.Ramos JW, DeSimone DW. Xenopus embryonic cell adhesion to fibronectin: position-specific activation of RGD / Synergy site-dependent migratory behavior at gastrulation. J Cell Biol. 1996;134:1–14. doi: 10.1083/jcb.134.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deblandre GA, Wettstein DA, Koyano-Nakagawa N, Kintner C. A two-step mechanism generates the spacing pattern of the ciliated cells in the skin of Xenopus embryos. Development. 1999;126:4715–4728. doi: 10.1242/dev.126.21.4715. [DOI] [PubMed] [Google Scholar]
- 85.Stubbs JL, Davidson L, Keller R, Kintner C. Radial intercalation of ciliated cells during Xenopus skin development. Development. 2006;133:2507–2515. doi: 10.1242/dev.02417. [DOI] [PubMed] [Google Scholar]
- 86.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 87.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Current Biology. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tepass U. Epithelial differentiation in Drosophila. Bioessays. 1997;19:673–682. doi: 10.1002/bies.950190807. [DOI] [PubMed] [Google Scholar]
- 89.Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux's archives of developmental biology. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- 90.Knox J, Moyer K, Yacoub N, Soldaat C, Komosa M, Vassilieva K, et al. Syndecan contributes to heart cell specification and lumen formation during Drosophila cardiogenesis. Developmental biology. 2011;356:279–290. doi: 10.1016/j.ydbio.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 91.Chartier A, Zaffran S, Astier M, Sémériva M, Gratecos D. Pericardin, a Drosophila type IV collagen-like protein is involved in the morphogenesis and maintenance of the heart epithelium during dorsal ectoderm closure. Development. 2002;129:3241–3253. doi: 10.1242/dev.129.13.3241. [DOI] [PubMed] [Google Scholar]
- 92.Yarnitzky T, Volk T. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Developmental biology. 1995;169:609–618. doi: 10.1006/dbio.1995.1173. [DOI] [PubMed] [Google Scholar]
- 93.Harpaz N, Ordan E, Ocorr K, Bodmer R, Volk T. Multiplexin promotes heart but not aorta morphogenesis by polarized enhancement of slit/robo activity at the heart lumen. Plos Genetics. 2013;9:e1003597. doi: 10.1371/journal.pgen.1003597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Haack T, Schneider M, Schwendele B, Renault AD. Drosophila heart cell movement to the midline occurs through both cell autonomous migration and dorsal closure. Developmental biology. 2014;396:169–182. doi: 10.1016/j.ydbio.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 95.Medioni C, Astier M, Zmojdzian M, Jagla K, Sémériva M. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. The Journal of cell biology. 2008;182:249–261. doi: 10.1083/jcb.200801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reuter R, Grunewald B, Leptin M. A role for the mesoderm in endodermal migration and morphogenesis in Drosophila. Development. 1993;119:1135–1145. doi: 10.1242/dev.119.4.1135. [DOI] [PubMed] [Google Scholar]
- 97.Tepass U, Hartenstein V. Neurogenic and proneural genes control cell fate specification in the Drosophila endoderm. Development. 1995;121:393–405. doi: 10.1242/dev.121.2.393. [DOI] [PubMed] [Google Scholar]
- 98.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, et al. A Mesenchymal-to-Epithelial Transition Initiates and Is Required for the Nuclear Reprogramming of Mouse Fibroblasts. Cell stem cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 99.Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell. 2010;7:64–77. doi: 10.1016/j.stem.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 101.Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, et al. Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater. 2013;12:1154–1162. doi: 10.1038/nmat3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sakurai K, Talukdar I, Patil Veena S, Dang J, Li Z, Chang K-Y, et al. Kinome-wide Functional Analysis Highlights the Role of Cytoskeletal Remodeling in Somatic Cell Reprogramming. Cell stem cell. 2014;14:523–534. doi: 10.1016/j.stem.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi B, Park KS, Kim JH, Ko KW, Kim JS, Han DK, et al. Stiffness of Hydrogels Regulates Cellular Reprogramming Efficiency Through Mesenchymal-to-Epithelial Transition and Stemness Markers. Macromolecular bioscience. 2015 doi: 10.1002/mabi.201500273. [DOI] [PubMed] [Google Scholar]
- 104.Chao Y, Wu Q, Acquafondata M, Dhir R, Wells A. Partial Mesenchymal to Epithelial Reverting Transition in Breast and Prostate Cancer Metastases. Cancer Microenvironment. 2012;5:19–28. doi: 10.1007/s12307-011-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ocaña Oscar H, Córcoles R, Fabra Á, Moreno-Bueno G, Acloque H, Vega S, et al. Metastatic Colonization Requires the Repression of the Epithelial-Mesenchymal Transition Inducer Prrx1. Cancer Cell. 22:709–724. doi: 10.1016/j.ccr.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 106.Nagelkerke A, Bussink J, Rowan AE, Span PN. The mechanical microenvironment in cancer: How physics affects tumours. Semin Cancer Biol. 2015;35:62–70. doi: 10.1016/j.semcancer.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 107.Campbell K, Casanova J. A role for E-cadherin in ensuring cohesive migration of a heterogeneous population of non-epithelial cells. Nat Commun. 2015;6:7998. doi: 10.1038/ncomms8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- 109.Hiscox S, Jiang WG, Obermeier K, Taylor K, Morgan L, Burmi R, et al. Tamoxifen resistance in MCF7 cells promotes EMT-like behaviour and involves modulation of beta-catenin phosphorylation. Int J Cancer. 2006;118:290–301. doi: 10.1002/ijc.21355. [DOI] [PubMed] [Google Scholar]
- 110.Hiscox S, Morgan L, Green TP, Barrow D, Gee J, Nicholson RI. Elevated Src activity promotes cellular invasion and motility in tamoxifen resistant breast cancer cells. Breast Cancer Res Treat. 2006;97:263–274. doi: 10.1007/s10549-005-9120-9. [DOI] [PubMed] [Google Scholar]
- 111.Planas-Silva MD, Waltz PK. Estrogen promotes reversible epithelial-to-mesenchymal-like transition and collective motility in MCF-7 breast cancer cells. The Journal of steroid biochemistry and molecular biology. 2007;104:11–21. doi: 10.1016/j.jsbmb.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 112.Gal A, Sjoblom T, Fedorova L, Imreh S, Beug H, Moustakas A. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene. 2008;27:1218–1230. doi: 10.1038/sj.onc.1210741. [DOI] [PubMed] [Google Scholar]
- 113.Zeisberg M, Shah AA, Kalluri R. Bone morphogenic protein-7 induces mesenchymal to epithelial transition in adult renal fibroblasts and facilitates regeneration of injured kidney. Journal of Biological Chemistry. 2005;280:8094–8100. doi: 10.1074/jbc.M413102200. [DOI] [PubMed] [Google Scholar]
- 114.Gunasinghe NP, Wells A, Thompson EW, Hugo HJ. Mesenchymal-epithelial transition (MET) as a mechanism for metastatic colonisation in breast cancer. Cancer Metastasis Rev. 2012;31:469–478. doi: 10.1007/s10555-012-9377-5. [DOI] [PubMed] [Google Scholar]