Abstract
In response to stress, defined as a real or perceived threat to homeostasis or well-being, brain systems initiate divergent physiological and behavioral processes that mobilize energy and promote adaptation. The brainstem contains multiple nuclei that engage in autonomic control and reflexive responses to systemic stressors. However, brainstem nuclei also play an important role in neuroendocrine responses to psychogenic stressors mediated by the hypothalamic-pituitary-adrenocortical axis. Further, these nuclei integrate neuroendocrine responses with stress-related behaviors, significantly impacting mood and anxiety. The current review focuses on the prominent brainstem monosynaptic inputs to the endocrine paraventricular hypothalamic nucleus (PVN), including the periaqueductal gray, raphe nuclei, parabrachial nuclei, locus coeruleus, and nucleus of the solitary tract (NTS). The NTS is a particularly intriguing area, as the region contains multiple cell groups that provide neurochemically-distinct inputs to the PVN. Furthermore, the NTS, under regulatory control by glucocorticoid-mediated feedback, integrates affective processes with physiological status to regulate stress responding. Collectively, these brainstem circuits represent an important avenue for delineating interactions between stress and health.
Keywords: hypothalamic-pituitary-adrenocortical axis, paraventricular hypothalamic nucleus, nucleus of the solitary tract, glucocorticoid
1. Introduction
In response to real or perceived threats to homeostasis or well-being, an organism generates multiple integrated stress responses that provide resources for physiological and behavioral adaptation (de Kloet et al., 2005; Myers et al., 2014b). Physiological stress responses promote energy mobilization and redistribution through two primary systems, the autonomic nervous system and the hypothalamic-pituitary-adrenocortical (HPA) axis (for review see Ulrich-Lai and Herman, 2009). Autonomic responses to stress increase heart rate, blood pressure, and glucose availability, providing energetic substrates for stress adaptation (Ulrich-Lai and Herman, 2009). The neuroendocrine HPA axis causes the secretion of glucocorticoids (principally corticosterone in rodents and cortisol in humans) from the adrenal cortex (Keller-Wood and Dallman, 1984). Activity of the HPA axis is initiated by parvocellular corticotropin-releasing hormone (CRH) neurons of the paraventricular nucleus of the hypothalamus (PVN). At the level of the anterior pituitary, CRH leads to the systemic release of adrenocorticotropic hormone (ACTH), the primary stimulus for glucocorticoid secretion (Ulrich-Lai and Herman, 2009). Glucocorticoids then signal throughout the body to regulate many systemic and neural functions, including hepatic glycogenolysis and neuronal metabolism (Herman, 2013; Herman et al., 2003). Behavioral stress responses depend on a large network of brain systems involved in appraisal, emotion, and memory (Joëls and Baram, 2009; Sousa and Almeida, 2012). Behavioral-regulatory circuits include forebrain sites such as the amygdala, prefrontal cortex, and hippocampus; importantly, these networks also interact with the hypothalamus and brainstem to integrate behavioral responses with HPA axis and autonomic activity (Joëls and Baram, 2009; McKlveen et al., 2015; Ulrich-Lai and Herman, 2009).
Aberrant activation of the HPA axis plays a role in many psychiatric illnesses including depression, anxiety, and post-traumatic stress disorder (for review see Jacobson, 2014; Ströhle and Holsboer, 2003). Glucocorticoids also exert profound effects on somatic health, particularly cardio-metabolic processes (Vogelzangs et al., 2010). In fact, cardiomyocyte glucocorticoid signaling is essential for maintaining cardiac function and survival (Oakley et al., 2013). Glucocorticoids also act in the hindbrain to increase arterial pressure and modulate baroreflex control (Bechtold and Scheuer, 2006; Scheuer et al., 2007). Therefore, the ascending brainstem circuits that regulate HPA axis activity and stress-related behaviors represent an important avenue for delineating the complex relationships between stress, behavior, and health.
The current review considers the key brainstem nuclei providing direct input to the PVN, circuits that have predominantly been mapped in rodents. We discuss their connectivity, chemistry, and role in HPA axis and behavioral stress responses, principally anxiety-, fear-, and depression-related behaviors. Although the autonomic nervous system mediates many important aspects of stress responsiveness, the role of the brainstem in autonomic regulation has been described elsewhere (see Ally, 1998; Bandler et al., 2000; Scislo and O’Leary, 2005 for review). Therefore, the current review will focus on the ascending pathways to the endocrine hypothalamus and their effects on behavior. The brainstem also gives rise to neuromodulatory projections that collateralize throughout the brain. These primarily aminergic systems regulate neural and behavioral function throughout the limbic system and cortex. Cataloging the diversity of these functions is beyond the scope of a single review; thus, we will provide references, where applicable, to reviews that focus on specific messenger systems and their effects on behavior mediated through interactions with the forebrain. Brainstem projections to the forebrain also have indirect effects on HPA axis stress responses via neuromodulation within limbic structures (Radley et al., 2008). These multisynaptic network-level mechanisms have yet to be unraveled; consequently, we will focus on the monosynaptic brainstem inputs to the PVN. Our review outlines brainstem nuclei that directly regulate the HPA axis, from rostral to caudal these are the periaqueductal gray (PAG), raphe nuclei, parabrachial nuclei (PBN), and locus coeruleus (LC). Additionally, we will explore the nucleus of the solitary tract (NTS) in detail and its rich collection of messengers that interact with glucocorticoids, including norepinephrine, glucagon-like peptide-1 (GLP-1), and glutamate. Finally, we will discuss how recent advances have expanded our understanding of the role of ascending brainstem projections, suggesting that these circuits form a critical hub for integrating interoceptive input with descending limbic information to coordinate endocrine and behavioral stress responses.
2. Brainstem inputs to the paraventricular hypothalamus (PVN)
The PVN is composed of a relatively small number of neuropeptide-containing cells that regulate many aspects of endocrine and homeostatic function (for reviews see Herman et al., 2002a; Herman et al., 2002b). Although some PVN neurons project within the central nervous system and act in a pre-autonomic fashion, other PVN cell groups are neuroendocrine (Biag et al., 2012; Hallbeck and Blomqvist, 1999). Thus, multiple subregions of the PVN are defined based on neurochemistry and connectivity (for reviews see Herman et al., 2008; Swanson and Sawchenko, 1983). Generally, the PVN is divided into magnocellular and parvocellular regions. For instance, the anterior, medial, and posterior magnocellular portions of the PVN contain neurons that synthesize oxytocin and vasopressin for release from neurosecretory terminals in the posterior pituitary (van Leeuwen et al., 1979). Within parvocellular regions, the lateral, dorsal, and ventromedial areas give rise to central projections that target the basal forebrain, brainstem, and spinal cord (Swanson and Sawchenko, 1983). These central circuits regulate numerous autonomic and behavioral processes through the release of oxytocin, vasopressin, and other transmitters (Knobloch and Grinevich, 2014). Importantly, the dorsomedial and anterior divisions of parvocellular neurosecretory cells project to the median eminence where they secrete CRH, the primary ACTH secretagogue, as well as vasopressin, which acts synergistically with CRH to promote HPA axis activity (Sawchenko et al., 1984; Vale et al., 1981). Thus, the HPA axis response to stress originates in a circumscribed collection of neurosecretory cells in the PVN (Ulrich-Lai and Herman, 2009). These cells express a multitude of neurotransmitter and peptide receptors that integrate signals from PVN-projecting circuits. Importantly, the PVN only receives direct synaptic input from a restricted number of brain regions (for review see Herman et al., 2003). These PVN-projecting afferents arise from other hypothalamic nuclei, the bed nucleus of the stria terminalis (BST), and importantly, the brainstem (Fig. 1). The intra-hypothalamic interactions have been described in detail (Cullinan et al., 1996; Myers et al., 2014a; Ulrich-Lai et al., 2011), as have projections from the BST (Choi et al., 2007; Cullinan et al., 1996; Dong and Swanson, 2006; Dong et al., 2001; Radley and Sawchenko, 2011). Briefly, these circuits have been proposed to form a hierarchy for mediating limbic stress integration and regulating HPA axis responses to psychogenic stressors (Herman et al., 2005; Myers et al., 2012). In contrast, brainstem projections have been postulated to mediate reflexive responses to systemic stressors. However, the multiple brainstem nuclei innervating the PVN represent a substantial proportion of stress-regulatory input to CRH neurons (Larsen and Mikkelsen, 1995; Ziegler et al., 2012). Accordingly, these structures are well-positioned to regulate endocrine responses to both systemic and psychogenic stress, as well as behavior (for review see Morgane and Mokler, 2006; Morgane et al., 2005).
Figure 1. Anatomical diagram of PVN-projecting brainstem nuclei.
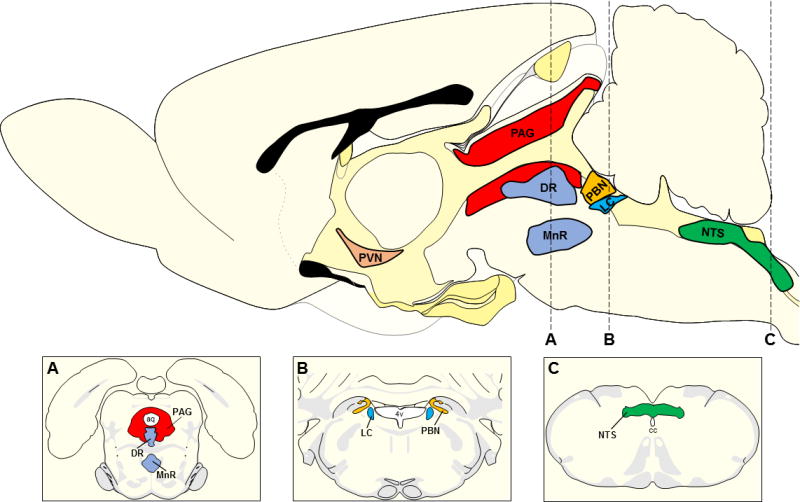
Sagittal rat brain schematic adapted from Swanson 2004 indicating the relative location of major PVN-projecting brainstem regions. The top panel illustrates PVN (light brown), PAG (red), raphe nuclei (DR and MnR; light blue), PBN (orange), LC (cerulean blue), and NTS (green). Panel A depicts a coronal section through the midbrain as indicated by dashed line A. This coronal map adapted from Swanson 2004 indicates the location of the PAG, DR, and MnR relative to the aq. Panel B illustrates a coronal section corresponding to dashed line B and outlines the PBN and LC in relation to the 4v. Panel C is a schematic coronal section through the caudal medulla (dashed line C) depicting the location of the NTS relative to the cc. List of abbreviations aq: cerebral aqueduct, cc: central canal, DR: dorsal raphe, LC: locus coeruleus, MnR: median raphe, NTS: nucleus of the solitary tract, PAG: periaqueductal gray, PBN: parabrachial nuclei, PVN: paraventricular nucleus, 4v: fourth ventricle.
3. Periaqueductal gray (PAG)
The PAG is a midbrain region surrounding the cerebral aqueduct that regulates many vital functions, including analgesia, autonomic responses, and behaviors related to defense, fear, and anxiety (see Bandler and Shipley, 1994; Behbehani, 1995; Carvalho-Netto et al., 2007). The columnar organization within the PAG gives rise to diverse projections with individualized functions (Bandler and Shipley, 1994). Multiple tract-tracing studies have identified specific PAG projections to the PVN arising from the pre-commissural and commissural subdivisions, dorsolateral column, and ventrolateral column (Cameron et al., 1995; Canteras and Goto, 1999; Floyd et al., 1996; Ziegler et al., 2012). Importantly, fibers from the ventrolateral PAG robustly target the medial, dorsal, and anterior parvocellular PVN (Floyd et al., 1996), providing an anatomical substrate for HPA axis regulation. Projections from the lateral PAG to the PVN co-localize with the glutamatergic marker vesicular glutamate transporter 2 (vGluT2) (Ziegler et al., 2012), suggesting the PAG may have an excitatory influence on the HPA axis. Multiple modalities of stress, including predator exposure, loud noise, forced swim, and restraint, increase neuronal activation in the dorsolateral PAG (Campeau and Watson, 1997; Canteras and Goto, 1999; Cullinan et al., 1995). Furthermore, electrical stimulation of the PAG in the open field increases corticosterone, accompanied by flight behaviors (Lim et al., 2011). Interestingly, intra-PAG administration of a cannabinoid receptor agonist also increases plasma corticosterone, yet decreases stress-induced hyper-locomotion and increases freezing (Finn et al., 2004). Thus, there appears to be functional topography of the PAG for regulating fight-or-flight behaviors, with different PAG columns implicated in divergent behavioral strategies. For instance, the ventrolateral PAG may be involved in passive defense strategies (e.g. freezing), while the dorsal PAG may mediate active defense behaviors (e.g. flight) (Monassi et al., 1997).
4. Raphe nuclei
The raphe nuclei are a collection of functionally and anatomically diverse cell groups that span the brainstem and contain the majority of the serotonin-producing neurons in the central nervous system. The organization and physiology of the raphe nuclei are comprehensively reviewed elsewhere (see Jacobs and Azmitia, 1992; Lowry, 2002). In general, the raphe nuclei can be divided into primarily ascending and primarily descending groups. The descending nuclei, including the raphe magnus and raphe pallidus, are spinal-projecting and regulate autonomic functions, among others (Jacobs and Azmitia, 1992). The ascending raphe nuclei, primarily the dorsal raphe (DR) and the median raphe (MnR), send projections throughout the brain and regulate a variety of behaviors related to mood, anxiety, and stress (Jacobs and Azmitia, 1992). The effects of serotonin signaling are dependent not only on the topography of the raphe, but also on a vast array of receptor subtypes. Consequently, serotonin can have divergent and opposing effects on many processes. For detailed reviews of serotonin interactions with the limbic system in behavioral control see Deakin and Graeff, 1991; Hale et al., 2012; Paul et al., Briefly, the MnR is proposed to inhibit stress responses, while the DR appears to facilitate arousal and anxiety-like behaviors (Lowry, 2002). However, both the MnR and DR send serotonergic fibers to the PVN, although, the majority of serotonergic fibers terminate in the region immediately surrounding the PVN (Sawchenko et al., 1983). Models of altered serotonin signaling, including serotonin transporter knockout mice and serotonin receptor overexpression in the medial hypothalamus, suggest that raphe projections, whether to PVN proper or surrounding areas, likely stimulate stress responses (Hanley and Van de Kar, 2003; Li et al., 2004). Importantly, injections of a serotonin-specific neurotoxin directly into the PVN inhibit stress-induced ACTH release (Jorgensen et al., 1998), indicating that serotonin input to the PVN predominantly excites the HPA axis. Whether PVN-projecting raphe neurons directly regulate behavioral responses to stress is somewhat unclear. The largest numbers of serotonin neurons arise from the DR and ascend through two major fiber tracts, the forebrain bundle tract and the peri-ventricular tract (Hale and Lowry, 2011; Hale et al., 2012). The former targets major limbic nuclei, while the later innervates the hypothalamus; thus, it is possible that behavioral and endocrine regulation may be partially segregated within the raphe nuclei. In support of this, decreased serotonin signaling in the medial hypothalamus attenuates ACTH secretion but not anxiety-like behaviors (Li et al., 2004).
5. Parabrachial nuclei (PBN)
The PBN are located in the reticular formation at the midbrain-pontine junction and are primarily composed of lateral and medial nuclei (Gauriau and Bernard, 2002). The PBN are involved in many homeostatic functions including chemoreception, nociception, and autonomic control (Chamberlin, 2004; Gauriau and Bernard, 2002; Spector, 1995). Although the role of the PBN in stress responding has been understudied, the nuclei project to the hypothalamus and may play a role in neuroendocrine-autonomic integration. Specifically, the lateral nuclei, including central lateral, superior lateral, and the external lateral, densely innervate the PVN (Bester et al., 1997). Neurochemically, the lateral PBN projections to the PVN are glutamatergic and predominantly innervate parvocellular neurons (Krukoff et al., 1992; Ziegler et al., 2012), suggesting a possible excitatory influence on HPA axis reactivity. Neurons of the PBN are activated by both systemic (e.g. visceral illness) and psychogenic (e.g. restraint) stressors, including cells that express peptides such as calcitonin gene-related peptide, neurotensin, and CRH (Kainu et al., 1993). While few rodent studies have examined neuroendocrine regulation by the PBN, CRH injections into the PBN increase circulating ACTH in anesthetized cats (Carlson et al., 1994). Behaviorally, the PBN are largely implicated in aversive behaviors related to interoceptive information, such as conditioned taste aversion (Bester et al., 1997; Reilly and Trifunovic, 2001).
6. Locus coeruleus (LC)
The LC, a discrete pontine nucleus contiguous with the fourth ventricle, houses the majority of the norepinephrine-expressing neurons in the brain and innervates the entire neuraxis (Dahlström and Fuxe, 1964; Schwarz and Luo, 2015; Swanson, 1976). The LC modulates behaviors related to arousal, attention, cognitive flexibility, and stress responses (Cassens et al., 1981; Francis et al., 1999; Valentino and Van Bockstaele, 2008). The LC is also the primary source of norepinephrine for the cortex and hippocampus, representing a major component of the central arousal network (Valentino and Van Bockstaele, 2008). Thus, LC-corticolimbic interactions are key for behavioral state regulation (see Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003 for review). The LC provides sparse direct input to the PVN that is limited to the medial parvocellular division and predominantly, but not exclusively, noradrenergic (Cunningham and Sawchenko, 1988; Sawchenko and Swanson, 1982). The LC is activated by numerous modalities of stress, including psychosocial, physiological, and nociceptive stimuli (Cassens et al., 1981; Francis et al., 1999; McCall et al., 2015; Reyes et al., 2008). Accordingly, the role of the LC in modulating the HPA axis differs depending on the duration and intensity of stressors (Armario et al., 2012). Generally, LC function appears more important for neuroendocrine responses to acute stressors than for repeated challenges following chronic stress. For instance, norepinephrine depletion of the LC by neurotoxic lesions blunts HPA axis responses to acute restraint, but do not prevent the induction of HPA axis hyperactivity following chronic variable stress (Ziegler et al., 1999). Although, repeated or prolonged activation of the LC has been implicated in the development of stress-related behaviors (George et al., 2013; Reyes et al., 2015; Valentino et al., 2012). Specifically, recent studies employing chemogenetic and optogenetic modulation of LC norepinephrine neurons determined the necessity and sufficiency of these cells for stress-induced anxiety-like behavior (McCall et al., 2015). Interestingly, the effects of stress on behavior may be mediated, in part, by CRH signaling in the LC. Although CRH input to the LC has been shown to arise from amygdalar and brainstem afferents (McCall et al., 2015; McFadden et al., 2012), the sensitivity of LC neurons to CRH is not static and is influenced by factors such as prior stress exposure (Curtis et al., 1999, 1995).
7. Nucleus of the solitary tract (NTS)
The NTS is an expansive and neurochemically diverse cell group in the dorsal medulla. The NTS is a critical hub for integrating interoceptive and viscero-sensory input with descending affective and cognitive information from the limbic forebrain (for excellent reviews see (Rinaman, 2011, 2007). The best-described functions of the NTS relate to autonomic control, yet emerging evidence points to NTS involvement in many behavioral and neuroendocrine processes (Rinaman, 2011). This diversity of functions is mediated by an array of neurotransmitters and peptides with partially overlapping expression. For instance, the intermediate NTS contains the A2 group of norepinephrine-producing neurons (Dahlström and Fuxe, 1964). This cell group provides a substantially greater proportion of noradrenergic projections to the PVN than the aforementioned locus coeruleus (i.e. A6), and the overwhelming majority of norepinephrine input to medial parvocellular neurons relative to other noradrenergic populations (e.g. A1) (Sawchenko and Swanson, 1982). In addition to norepinephrine, these neurons also co-release glutamate (Stornetta et al., 2002). Furthermore, NTS noradrenergic neurons contain peptides such as prolactin-releasing peptide and neuropeptide Y, which may act synergistically with norepinephrine on PVN CRH neurons (Chen et al., 1999; Sawchenko et al., 1985; Uchida et al., 2010). A separate cell group located in the caudal NTS that expresses glucagon-like peptide-1 (GLP-1), as well as glutamate (Zheng et al., 2015), also gives rise to PVN projections; importantly, these cells do not produce norepinephrine (Ghosal et al., 2013; Larsen et al., 1997). Neurons throughout the NTS are activated by a variety of acute and chronic stressors (Flak et al., 2012; Pezzone et al., 1993; Rinaman, 1999). Moreover, a portion of NTS chronic stress-activated neurons are immunoreactive for the norepinephrine-synthesizing enzyme dopamine-β-hydroxylase (DBH) (Flak et al., 2012). Direct stimulation of the NTS, as well as pharmacological activation of NTS-derived norepinephrine and GLP-1 signaling in the PVN, indicate that the nucleus promotes HPA axis activation (Day et al., 1985; Kinzig et al., 2003). In addition to sending projections that target subcortical limbic regions critical for regulating behavioral responses to stress, the NTS also receives direct input from the amygdala, BST, and prefrontal cortex (van der Kooy et al., 1984). Consequently, the NTS has been implicated in behaviors related to anxiety, depression, and fear memory (Ghosal et al., 2014; Miyashita and Williams, 2002).
7.1. Norepinephrine
Housed within the NTS, A2 noradrenergic neurons form a bidirectional interface linking physiological processes with emotional and cognitive events, particularly in response to stressors (Rinaman, 2011; Sawchenko and Swanson, 1982, 1981). These neurons integrate feedback from physiological systems with affective state and relay this information to the PVN, forming a major HPA axis excitatory pathway (Morilak et al., 2005; Plotsky et al., 1989; Rinaman, 2011; Sawchenko and Swanson, 1981; Sawchenko et al., 1996). Specifically, catecholaminergic lesions of NTS neurons attenuate HPA axis responses (Bundzikova-Osacka et al., 2015); further, electrical stimulation of ascending catecholaminergic afferents to the PVN and intracerebroventricular injection of norepinephrine induce CRH secretion in a α1-adrenergic receptor-dependent manner (Plotsky, 1987; Plotsky et al., 1989). Collectively, these data indicate that norepinephrine is both necessary and sufficient for HPA axis activation. Noradrenergic projections to the PVN are particularly important for challenges to physiological homeostasis. In fact, neurochemically specific interventions have illuminated a role for these circuits in facilitating HPA responses to hypotension (Plotsky, 1987), glucoprivation (Ritter et al., 2003), osmotic challenge (Khan et al., 2011), and inflammation (Bienkowski and Rinaman, 2008). However, noradrenergic neurons also activate HPA axis responses to psychogenic stressors, effects that appear to be weighted towards acute reactivity, as opposed to chronic stress (Bundzikova-Osacka et al., 2015). In fact, PVN injections of saporin toxin conjugated to a DBH antibody (a method to remove epinephrine/norepinephrine inputs to PVN) blunt ACTH and corticosterone responses to acute restraint. However, these inputs do not appear to be necessary for glucocorticoid hyper-responsiveness after chronic variable stress (Flak et al., 2014). In contrast, repeated cold stress increases HPA axis responses to a novel stressor through enhanced α1-adrenergic receptor responsiveness in the PVN (Ma and Morilak, 2005), suggesting that the modality of chronic stress (i.e. heterotypic vs. homotypic) may be an important determinant for the involvement of PVN norepinephrine signaling.
7.2. Glucagon-like peptide-1 (GLP-1)
GLP-1 is an incretin hormone most prominently expressed in epithelial L-cells of the intestine (Holst, 2007). The primary systemic role of GLP-1 is post-prandial stimulation of pancreatic insulin production (Holst, 2007). In addition, there are a restricted collection of hindbrain neurons that produce and transmit GLP-1 to homeostatic regulatory areas, including the PVN. The neuronal form of GLP-1 is derived from differential post-transcriptional processing of preproglucagon (PPG), whereas glucagon is the major product of PPG in the pancreas (Larsen et al., 1997). In the central nervous system, GLP-1 signaling regulates a variety of homeostatic processes, including body weight, blood glucose, heart rate, and blood pressure (reviewed in Ghosal et al., 2013). The vast majority of GLP-1 immunoreactive cells in the hindbrain reside in the visceral NTS, with sparse expression in the ventral and medial reticular nuclei (Han et al., 1986; Jin et al., 1988). This location in the caudal NTS allows GLP-1 neurons to monitor visceral signals and respond to physiological stressors (Larsen et al., 1997; Rinaman, 1999). In addition to activation by systemic/visceral stressors, GLP-1 neurons are also activated by psychogenic stress and have been implicated in anxiety-like behavior (Kinzig et al., 2003). GLP-1 neurons project from the NTS to the hypothalamus where they appose CRH neurons in the medial parvocellular PVN (Sarkar et al., 2003; Tauchi et al., 2008). Furthermore, GLP-1 and vGluT2 co-localize in synaptic terminals targeting the PVN (Zheng et al., 2015). Thus, projections from GLP-1-producing neurons facilitate the HPA axis response to stress. Intracerebroventricular injections of GLP-1 activate the medial parvocellular PVN, an effect prevented by pre-administration of a GLP-1 receptor antagonist (Larsen et al., 1997; Turton et al., 1996). Additionally, GLP-1 injected into the PVN increases plasma ACTH and corticosterone (Kinzig et al., 2003). The HPA axis excitatory effects of GLP-1 in the PVN appear to be divergent from GLP-1 effects on anxiety-like behavior, the later depending on GLP-1 signaling in the amygdala (Kinzig et al., 2003). Interestingly, pharmacological administration of GLP-1 interacts with chronic variable stress to increase sensitization of glucocorticoid responses to a novel stressor (Tauchi et al., 2008), suggesting that GLP-1 may be particularly important for tuning reactivity to chronic stress.
7.3. Glucocorticoid interactions
The glucocorticoid receptor (GR) is expressed in NTS GLP-1 neurons (Härfstrand et al., 1986; Rinaman, 2011) and binds to glucocorticoid response elements upstream of the PPG promoter to regulate PPG transcription (Zhang et al., 2009). Acute psychogenic (e.g. restraint) or systemic (e.g. hypoxia or visceral illness) stressors deplete PPG mRNA. Notably, PPG hnRNA is increased in the NTS, suggesting that stress increases PPG transcription (Zhang et al., 2009). Thus, the decrease in PPG mRNA is likely due to RNA degradation mediated by glucocorticoids acting in the NTS. Evidence for this hypothesis comes from studies finding that the effects of stress on PPG mRNA are attenuated in adrenalectomized rats and reproduced by exogenous corticosterone administration (Zhang et al., 2009). While acute stress briefly decreases PPG mRNA, both chronic variable stress and repeated restraint produce sustained down regulation (Ghosal et al., 2013; Zhang et al., 2010), an effect dependent on chronic stress-induced corticosterone secretion (Zhang et al., 2010). Collectively, these data suggest that glucocorticoids are crucial for limiting excess excitatory drive to the PVN through long-term down regulation of PPG mRNA. Additional evidence for glucocorticoid inhibition of HPA axis drive via the NTS come from pharmacological studies demonstrating the necessity and sufficiency of NTS GR signaling for decreasing HPA axis activity (Bechtold et al., 2009; Ghosal et al., 2014). In addition to these neuroendocrine effects, NTS GR is also required to diminish the impact of stress on anxiety- and depression-like behaviors (Ghosal et al., 2014). In aggregate, these studies indicate that glucocorticoid-mediated mechanisms in the NTS are critical for moderating endocrine and behavioral stress responses and provide a basis for promoting organismal adaptation to chronic stress.
7.4. Glutamate
As previously mentioned, the excitatory neurotransmitter glutamate is expressed throughout the NTS, including noradrenergic and GLP-1 neurons (Niciu et al., 2012; Storm-Mathisen et al., 1983; Stornetta et al., 2002; Zheng et al., 2015). Thus, glutamate may serve as a major NTS synaptic input to PVN, with norepinephrine and GLP-1 acting as potent modulators (Fig. 2). Although NTS glutamatergic circuits are not well-characterized, within the PVN, glutamatergic synapses interact with diverse neuromodulatory systems to regulate HPA axis output (Bains et al., 2015). In fact, glutamatergic synapses often combine fast ionotropic signaling with slower-acting metabotropic signaling mediated by co-transmitters, including norepinephrine (Atasoy et al., 2012; Bains et al., 2015; Betley et al., 2013; Daftary et al., 2000). Importantly, HPA axis activity induced by electrical or pharmacological stimulation of the noradrenergic system is blocked by intra-PVN microinjection of glutamate receptor antagonists (Feldman and Weidenfeld, 2004). Furthermore, catecholaminergic inputs from the hindbrain promote glutamatergic excitation of parvocellular PVN neurons in response to glycemic challenge (Johnson and Watts, 2014). Postsynaptic glutamatergic currents in the PVN are also reduced by an adrenergic antagonist, suggesting that norepinephrine tonically enhances glutamate co-release in the PVN (Inoue et al., 2014). Taken together, these data indicate that glutamatergic innervation constitutes an important excitatory input to the PVN and substantially interacts with neuromodulatory systems such as norepinephrine and, perhaps, GLP-1 to regulate HPA axis dynamics.
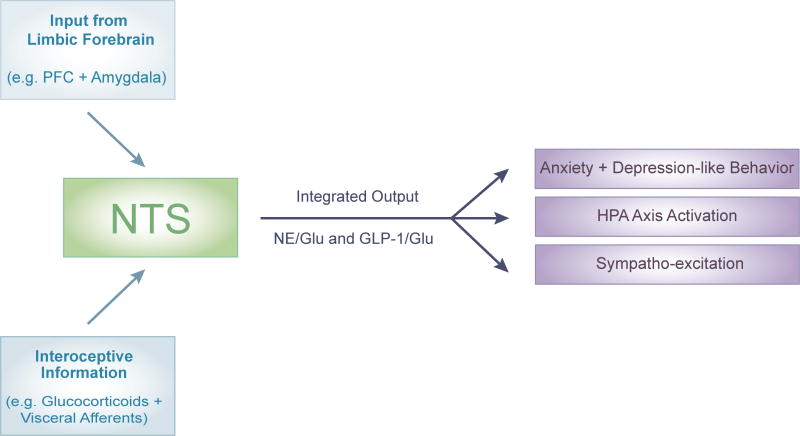
Figure 2. Summary of NTS as nexus for integrating descending limbic input with ascending interoceptive information to generate behavioral, neuroendocrine, and autonomic stress responses.
Regions of the limbic forebrain including the PFC and amygdala, among others, project to the NTS. The NTS also expresses a high density of glucocorticoid receptors and receives ascending visceral signals related to physiological status. At the level of the NTS, this information is integrated and multiple output circuits including NE/Glu and GLP-1/Glu coordinate organismal adaptation to adversity. List of abbreviations GLP-1: glucagon-like peptide-1, Glu: glutamate, NE: norepinephrine, NTS: nucleus of the solitary tract, PFC: prefrontal cortex,
8. Paraventricular hypothalamic integration of inputs
8.1. Synaptic plasticity
Brainstem input to the PVN is dynamically regulated by local circuit mechanisms within neuroendocrine cells. For example, CRH neurons exhibit stress-induced synaptic plasticity and also provide feedback to afferent synapses via retrograde signaling (for reviews see Bains et al., 2015; Herman et al., 2008; Hill and Tasker, 2012; Wamsteeker Cusulin and Bains, 2015). Briefly, chronic stress increases the expression of CRH (Herman et al., 1995) and ionotropic glutamate receptors (Herman et al., 2008), while reducing expression of GR (Herman et al., 1995) and GABA receptor subunits (Cullinan, 2000). Chronic stress also increases glutamatergic and noradrenergic appositions onto the somato-dendritic compartment of PVN CRH neurons (Flak et al., 2009). Interestingly, the chronic stress-induced increases in pre-synaptic input are abolished by PVN noradrenergic lesions (Flak et al., 2014), raising the possibility that plastic glutamate and norepinephrine afferents arise from the same cell population or that noradrenergic-glutamatergic interactions within the PVN are necessary for chronic stress-induced synaptic plasticity. In support of the later, norepinephrine promotes glutamate receptor-dependent synaptic potentiation following stress, allowing the PVN to undergo experience-dependent plasticity (Inoue et al., 2013). Collectively, the structural and functional consequences of chronic stress include up-regulation of neuronal processes promoting excitability, concomitant with decreased capacity for negative feedback. Overall, this plasticity could account for facilitation of PVN output and enhanced HPA axis activity after chronic stress. However, CRH neurons have the capacity to release retrograde inhibitory signals that tune activity of afferent synapses (Di et al., 2003).
8.2. Retrograde signaling
Excitatory glutamatergic drive of CRH neurons is dampened by retrograde signaling, most prominently endocannabinoids and opioids (Evanson et al., 2010; Patel et al., 2004; Tasker et al., 2006; Wamsteeker Cusulin and Bains, 2015). Release of inhibitory messengers in the PVN is an activity-dependent process that activates pre-synaptic receptors and decreases the firing of afferents terminals (Di et al., 2009), including those from the brainstem. Specifically, CRH neuronal endocannabinoid release at glutamatergic synapses suppresses glutamate release and subsequent excitation of post-synaptic cells (Di et al., 2009). This process is dependent on cannabinoid receptor 1 (CB1) activation (Di et al., 2003), as well as GR (Nahar et al., 2015). Furthermore, CB1 antagonism reverses glucocorticoid feedback inhibition of the HPA axis (Evanson et al., 2010), indicating the necessity of CB1-GR interactions for terminating HPA axis response to stress. Importantly, repeated stress progressively decreases CB1 signaling, as well as GR feedback, resulting in the loss of endocannabinoid-mediated inhibition of PVN afferent neurotransmitter release (Wamsteeker et al., 2010). Thus, exposure to chronic or repeated stress disinhibits glutamatergic drive to parvocellular neurons, providing an additional avenue for sensitization of the HPA axis. More recently, an additional mechanism of retrograde signaling from neuroendocrine cells was described that relies on somato-dendritic release of endogenous opioids acting on pre-synaptic μ-opioid receptors (Wamsteeker Cusulin et al., 2013). In this case, prolonged activation of parvocellular neurons drives the release of opioids that lead to long-term synaptic depression in afferent terminals. Interestingly, this process is enhanced by GR and occurs at both glutamatergic and GABAergic synapses (Bains et al., 2015). This would seem to indicate that protracted HPA axis activity may result in a generalized μ-opioid-dependent inhibition of neurotransmitter release onto CRH neurons, thus reducing responsiveness to inputs from the brainstem.
9. Summary and conclusions
Monosynaptic inputs to PVN parvocellular neurosecretory cells arise from key brainstem nuclei, including the PAG, raphe nuclei, PBN, LC, and NTS. This closely associated group of structures provides diverse neurochemical input to the PVN, including glutamate, serotonin, norepinephrine, and a variety of neuropeptides. Neurons in these regions are activated by multiple modalities of stress and form a major HPA axis-regulatory network. This circuitry also underlies behavioral responses to stress, including, fear-, anxiety-, and depression-like behavior. The NTS appears to be particularly prominent for the integration of affective processes with physiological status, regulating many aspects of stress responding. Neurons of the NTS are also under regulatory control by glucocorticoid-mediated feedback, acting to constrain endocrine and behavioral responses to prolonged stress. At the level of the PVN, brainstem afferents are summated in an experience-dependent manner and modulated by post-synaptic retrograde messengers, providing a highly-plastic environment. The responsiveness of PVN neurons to brainstem afferents is likely increased by chronic stress. In fact, chronic stress enhances glutamatergic signaling within the PVN, a process that requires norepinephrine and that may be facilitated by reduced endocannabinoid inhibition. In totality, the literature reviewed here suggests that the activity of specific brainstem neural circuits could account for many of the long-term deleterious effects of stress.
It is important to note that the current review focuses almost exclusively on literature generated from rodents. This is due to the vast quantity of data on pathways and neurochemical mechanisms that have emerged from rodent studies. Ultimately, advances in clinical outcomes will need to be driven by a better understanding of the parallel circuitry in humans. Currently, functional imaging modalities are limited in their spatial and temporal resolution, hampering the ability to discern the activity and interactions of individual brainstem nuclei. Nonetheless, there is evidence from human studies that brainstem nuclei are significant contributors to behavioral pathology. For instance, electrical stimulation of the PAG in humans produces fear and avoidance (Behbehani, 1995). Additionally, increased cerebrospinal fluid norepinephrine levels are observed in patients with melancholic depression (Wong et al., 2000), suggesting that brainstem noradrenergic neurons may contribute to the pathophysiology of depression.
The basic neurobiology of brainstem circuit integration also has several outstanding questions. There are a number of brainstem structures that have received little attention in relation to HPA axis regulation. For instance, the lateral dorsal tegmental nucleus and peduculopontine nucleus both give rise to cholinergic afferents that, although sparse, do innervate the PVN (Ruggiero et al., 1990; Ziegler and Herman, 2002). Additionally, the A1/C1 regions of the ventrolateral medulla provide norepinephrine/epinephrine input, respectively, to the PVN (Sawchenko and Swanson, 1982). These neurons mainly target magnocellular vasopressin neurons, although most techniques historically used to investigate noradrenergic signaling in the PVN have not clearly differentiated between the cell populations of origin. Furthermore, the complex physiology and functional consequences of co-transmitters and transmitter-peptide co-release have only recently begun to be unraveled. This interface is of paramount importance for a comprehensive understanding of circuit integration, as well as the mechanisms of stress-induced plasticity. These abundant interactions likely represent the endogenous signals guiding synaptic function, as well as endocrine and behavioral stress reactivity. Particularly, in-depth investigations of specific glutamatergic circuits and their co-transmitters could yield major advances in this realm. For instance, the role of PBN glutamate output for stress reactivity is poorly characterized, let alone the role of the numerous peptides likely co-released by these nuclei. Additionally, raphe serotonergic neurons have a mixed pattern of glutamate co-expression (Hioki et al., 2010), a phenomenon that has not been functionally investigated. Finally, the interface of GLP-1 and glutamate signaling within the PVN is unknown. Whether these mixed circuits and messengers interact synergistically or oppositionally is critical for delineating both basic neural physiology, as well as the complexity of neuropsychiatric disorders.
In conclusion, the growing consensus emerging from multiple lines of research on brainstem circuitry supports the idea that this region is vital for mediating many aspects of the relationship between psychogenic stress and behavioral dysregulation. Furthermore, the previous theories that viewed the brainstem simply as a component of the ‘reptilian’ brain, only acting to stimulate reflexive responses to systemic disturbance, is no longer adequate for a thorough and coherent understanding of stress-regulatory neuronal networks. Moving forward, the interconnectedness and microcircuitry of brainstem nuclei, as well as the heterogeneity of synaptic chemistry, represent major frontiers for advancing stress neurobiology.
Highlights.
Brainstem nuclei provide monosynaptic input to the paraventricular hypothalamus
These distinct cell groups signal through multiple transmitters and peptides
Integration of brainstem inputs is critical for appropriate stress responding
Acknowledgments
The authors are supported by NIH grant K99 HL122454 to B. Myers and NIH grants R01 MH069860, R01 MH049698, and R01 MH101729 to J.P. Herman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ally A. Ventrolateral medullary control of cardiovascular activity during muscle contraction. Neurosci Biobehav Rev. 1998;23:65–86. doi: 10.1016/s0149-7634(97)00069-9. [DOI] [PubMed] [Google Scholar]
- Armario A, Daviu N, Muñoz-Abellán C, Rabasa C, Fuentes S, Belda X, Gagliano H, Nadal R. What can we know from pituitary-adrenal hormones about the nature and consequences of exposure to emotional stressors? Cell Mol Neurobiol. 2012;32:749–58. doi: 10.1007/s10571-012-9814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–50. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–7. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bains JS, Wamsteeker Cusulin JI, Inoue W. Stress-related synaptic plasticity in the hypothalamus. Nat Rev Neurosci. 2015;16:377–88. doi: 10.1038/nrn3881. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–89. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bechtold AG, Patel G, Hochhaus G, Scheuer DA. Chronic blockade of hindbrain glucocorticoid receptors reduces blood pressure responses to novel stress and attenuates adaptation to repeated stress. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1445–54. doi: 10.1152/ajpregu.00095.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold AG, Scheuer DA. Glucocorticoids act in the dorsal hindbrain to modulate baroreflex control of heart rate. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1003–11. doi: 10.1152/ajpregu.00345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bester H, Besson JM, Bernard JF. Organization of efferent projections from the parabrachial area to the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1997;383:245–81. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Betley JN, Cao ZFH, Ritola KD, Sternson SM. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–50. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biag J, Huang Y, Gou L, Hintiryan H, Askarinam A, Hahn JD, Toga AW, Dong H-W. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: a study of immunostaining and multiple fluorescent tract tracing. J Comp Neurol. 2012;520:6–33. doi: 10.1002/cne.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, Rinaman L. Noradrenergic inputs to the paraventricular hypothalamus contribute to hypothalamic-pituitary-adrenal axis and central Fos activation in rats after acute systemic endotoxin exposure. Neuroscience. 2008;156:1093–102. doi: 10.1016/j.neuroscience.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundzikova-Osacka J, Ghosal S, Packard BA, Ulrich-Lai YM, Herman JP. Role of nucleus of the solitary tract noradrenergic neurons in post-stress cardiovascular and hormonal control in male rats. Stress. 2015;18:221–32. doi: 10.3109/10253890.2015.1013531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. II Descending projections. J Comp Neurol. 1995;351:585–601. doi: 10.1002/cne.903510408. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–88. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Goto M. Connections of the precommissural nucleus. J Comp Neurol. 1999;408:23–45. doi: 10.1002/(sici)1096-9861(19990524)408:1<23::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Carlson DE, Nabavian AM, Gann DS. Corticotropin-releasing hormone but not glutamate elicits hormonal responses from the parabrachial region in cats. Am J Physiol. 1994;267:R337–48. doi: 10.1152/ajpregu.1994.267.1.R337. [DOI] [PubMed] [Google Scholar]
- Carvalho-Netto EF, Litvin Y, Nunes-de-Souza RL, Blanchard DC, Blanchard RJ. Effects of intra-PAG infusion of ovine CRF on defensive behaviors in Swiss-Webster mice. Behav Brain Res. 2007;176:222–9. doi: 10.1016/j.bbr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassens G, Kuruc A, Roffman M, Orsulak PJ, Schildkraut JJ. Alterations in brain norepinephrine metabolism and behavior induced by environmental stimuli previously paired with inescapable shock. Behav Brain Res. 1981;2:387–407. doi: 10.1016/0166-4328(81)90020-6. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL. Functional organization of the parabrachial complex and intertrigeminal region in the control of breathing. Respir Physiol Neurobiol. 2004;143:115–25. doi: 10.1016/j.resp.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Chen C, Dun SL, Dun NJ, Chang JK. Prolactin-releasing peptide-immunoreactivity in A1 and A2 noradrenergic neurons of the rat medulla. Brain Res. 1999;822:276–9. doi: 10.1016/s0006-8993(99)01153-1. [DOI] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE. GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J Comp Neurol. 2000;419:344–51. doi: 10.1002/(sici)1096-9861(20000410)419:3<344::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505. doi: 10.1016/0306-4522(94)00355-9. [DOI] [PubMed] [Google Scholar]
- Cunningham ET, Sawchenko PE, Cunningham ET, Jr, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Grigoriadis DE, Valentino RJ. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–50. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- Curtis AL, Pavcovich LA, Valentino RJ. Long-term regulation of locus ceruleus sensitivity to corticotropin-releasing factor by swim stress. J Pharmacol Exp Ther. 1999;289:1211–9. [PubMed] [Google Scholar]
- Daftary SS, Boudaba C, Tasker JG. Noradrenergic regulation of parvocellular neurons in the rat hypothalamic paraventricular nucleus. Neuroscience. 2000;96:743–51. doi: 10.1016/s0306-4522(00)00003-8. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–9. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Day TA, Ferguson AV, Renaud LP. Noradrenergic afferents facilitate the activity of tuberoinfundibular neurons of the hypothalamic paraventricular nucleus. Neuroendocrinology. 1985;41:17–22. doi: 10.1159/000124148. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Graeff FG. 5-HT and mechanisms of defence. J Psychopharmacol. 1991;5:305–15. doi: 10.1177/026988119100500414. [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Maxson MM, Franco A, Tasker JG. Glucocorticoids regulate glutamate and GABA synapse-specific retrograde transmission via divergent nongenomic signaling pathways. J Neurosci. 2009;29:393–401. doi: 10.1523/JNEUROSCI.4546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Watts AG, Swanson LW. Basic organization of projections from the oval and fusiform nuclei of the bed nuclei of the stria terminalis in adult rat brain. J Comp Neurol. 2001;436:430–455. doi: 10.1002/cne.1079. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–4819. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Weidenfeld J. Involvement of endogeneous glutamate in the stimulatory effect of norepinephrine and serotonin on the hypothalamo-pituitary-adrenocortical axis. Neuroendocrinology. 2004;79:43–53. doi: 10.1159/000076044. [DOI] [PubMed] [Google Scholar]
- Finn DP, Jhaveri MD, Beckett SRG, Kendall DA, Marsden CA, Chapman V. Cannabinoids modulate ultrasound-induced aversive responses in rats. Psychopharmacology (Berl) 2004;172:41–51. doi: 10.1007/s00213-003-1629-1. [DOI] [PubMed] [Google Scholar]
- Flak JN, Myers B, Solomon MB, McKlveen JM, Krause EG, Herman JP. Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur J Neurosci. 2014;39:1903–11. doi: 10.1111/ejn.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Ostrander MM, Tasker JG, Herman JP. Chronic stress-induced neurotransmitter plasticity in the PVN. J Comp Neurol. 2009;517:156–65. doi: 10.1002/cne.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Solomon MB, Jankord R, Krause EG, Herman JP. Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur J Neurosci. 2012;36:2547–55. doi: 10.1111/j.1460-9568.2012.08161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd NS, Keay KA, Arias CM, Sawchenko PE, Bandler R. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neurosci Lett. 1996;220:105–8. doi: 10.1016/s0304-3940(96)13240-7. [DOI] [PubMed] [Google Scholar]
- Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46:1153–66. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- Gauriau C, Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–8. doi: 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- George SA, Knox D, Curtis AL, Aldridge JW, Valentino RJ, Liberzon I. Altered locus coeruleus-norepinephrine function following single prolonged stress. Eur J Neurosci. 2013;37:901–9. doi: 10.1111/ejn.12095. [DOI] [PubMed] [Google Scholar]
- Ghosal S, Bundzikova-Osacka J, Dolgas CM, Myers B, Herman JP. Glucocorticoid receptors in the nucleus of the solitary tract (NTS) decrease endocrine and behavioral stress responses. Psychoneuroendocrinology. 2014;45:142–53. doi: 10.1016/j.psyneuen.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S, Myers B, Herman JP. Role of central glucagon-like peptide-1 in stress regulation. Physiol Behav. 2013;122:201–7. doi: 10.1016/j.physbeh.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–64. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hale MW, Shekhar A, Lowry CA. Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol. 2012;32:695–708. doi: 10.1007/s10571-012-9827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallbeck M, Blomqvist A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J Comp Neurol. 1999;411:201–11. [PubMed] [Google Scholar]
- Han VK, Hynes MA, Jin C, Towle AC, Lauder JM, Lund PK. Cellular localization of proglucagon/glucagon-like peptide I messenger RNAs in rat brain. J Neurosci Res. 1986;16:97–107. doi: 10.1002/jnr.490160110. [DOI] [PubMed] [Google Scholar]
- Hanley NR, Van de Kar LD. Serotonin and the neuroendocrine regulation of the hypothalamic--pituitary-adrenal axis in health and disease. Vitam Horm. 2003;66:189–255. doi: 10.1016/s0083-6729(03)01006-9. [DOI] [PubMed] [Google Scholar]
- Härfstrand A, Fuxe K, Cintra A, Agnati LF, Zini I, Wikström AC, Okret S, Yu ZY, Goldstein M, Steinbusch H. Glucocorticoid receptor immunoreactivity in monoaminergic neurons of rat brain. Proc Natl Acad Sci U S A. 1986;83:9779–83. doi: 10.1073/pnas.83.24.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002a;16:381–5. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocr. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–64. doi: 10.1016/S0079-6123(08)00429-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Herman JP, Tasker JG, Ziegler DR, Cullinan WE. Local circuit regulation of paraventricular nucleus stress integration: glutamate-GABA connections. Pharmacol Biochem Behav. 2002b;71:457–68. doi: 10.1016/s0091-3057(01)00681-5. [DOI] [PubMed] [Google Scholar]
- Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. doi: 10.1016/j.neuroscience.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hioki H, Nakamura H, Ma Y-F, Konno M, Hayakawa T, Nakamura KC, Fujiyama F, Kaneko T. Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J Comp Neurol. 2010;518:668–86. doi: 10.1002/cne.22237. [DOI] [PubMed] [Google Scholar]
- Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- Inoue W, Baimoukhametova DV, Füzesi T, Wamsteeker Cusulin JI, Koblinger K, Whelan PJ, Pittman QJ, Bains JS. Noradrenaline is a stress-associated metaplastic signal at GABA synapses. Nat Neurosci. 2013;16:605–12. doi: 10.1038/nn.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue W, Fuzesi T, Pittman Q, Bains JS. Brainstem noradrenergic afferents excite hypothalamic neurons through glutamate co-release [WWW Document] [accessed 2.19.16];Soc Neurosci Abstr. 2014 http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=75b22815-1d9b-47cf-bfda-4fdfc32b7c14&cKey=74fb79a1-f34c-4c59-82fb-748850a6b66e&mKey=54c85d94-6d69-4b09-afaa-502c0e680ca7.
- Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Compr Physiol. 2014;4:715–38. doi: 10.1002/cphy.c130036. [DOI] [PubMed] [Google Scholar]
- Jin SL, Han VK, Simmons JG, Towle AC, Lauder JM, Lund PK. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. J Comp Neurol. 1988;271:519–32. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- Joëls M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–66. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Watts AG. Hindbrain catecholaminergic projections to the paraventricular nucleus are required for activation of glutamatergic terminals by glycemic challenges [WWW Document] [accessed 2.19.16];Soc Neurosci Abstr. 2014 http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=75b22815-1d9b-47cf-bfda-4fdfc32b7c14&cKey=dd75fb6d-af0e-49ad-936b-3b3f334317fa&mKey=54c85d94-6d69-4b09-afaa-502c0e680ca7.
- Jorgensen H, Knigge U, Kjaer A, Vadsholt T, Warberg J. Serotonergic involvement in stress-induced ACTH release. Brain Res. 1998;811:10–20. doi: 10.1016/s0006-8993(98)00901-9. [DOI] [PubMed] [Google Scholar]
- Kainu T, Honkaniemi J, Gustafsson JA, Rechardt L, Pelto-Huikko M. Co-localization of peptide-like immunoreactivities with glucocorticoid receptor- and Fos-like immunoreactivities in the rat parabrachial nucleus. Brain Res. 1993;615:245–51. doi: 10.1016/0006-8993(93)90034-k. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Khan AM, Kaminski KL, Sanchez-Watts G, Ponzio TA, Kuzmiski JB, Bains JS, Watts AG. MAP kinases couple hindbrain-derived catecholamine signals to hypothalamic adrenocortical control mechanisms during glycemia-related challenges. J Neurosci. 2011;31:18479–91. doi: 10.1523/JNEUROSCI.4785-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzig KP, D’Alessio DA, Herman JP, Sakai RR, Vahl TP, Figueiredo HF, Murphy EK, Seeley RJ, Figueredo HF. CNS glucagon-like peptide-1 receptors mediate endocrine and anxiety responses to interoceptive and psychogenic stressors. J Neurosci. 2003;23:6163–70. doi: 10.1523/JNEUROSCI.23-15-06163.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krukoff TL, Morton TL, Harris KH, Jhamandas JH. Expression of c-fos protein in rat brain elicited by electrical stimulation of the pontine parabrachial nucleus. J Neurosci. 1992;12:3582–90. doi: 10.1523/JNEUROSCI.12-09-03582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute “stress” to the hypothalamic paraventricular nucleus. J Neurosci. 1995;15:2609–27. doi: 10.1523/JNEUROSCI.15-04-02609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PJ, Tang-Christensen M, Jessop DS. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138:4445–55. doi: 10.1210/endo.138.10.5270. [DOI] [PubMed] [Google Scholar]
- Li Q, Holmes A, Ma L, Van de Kar LD, Garcia F, Murphy DL. Medial hypothalamic 5-hydroxytryptamine (5-HT)1A receptors regulate neuroendocrine responses to stress and exploratory locomotor activity: application of recombinant adenovirus containing 5-HT1A sequences. J Neurosci. 2004;24:10868–77. doi: 10.1523/JNEUROSCI.3223-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LW, Blokland A, van Duinen M, Visser-Vandewalle V, Tan S, Vlamings R, Janssen M, Jahanshahi A, Aziz-Mohammadi M, Steinbusch HWM, Schruers K, Temel Y. Increased plasma corticosterone levels after periaqueductal gray stimulation-induced escape reaction or panic attacks in rats. Behav Brain Res. 2011;218:301–7. doi: 10.1016/j.bbr.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Lowry CA. Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocr. 2002;14:911–923. doi: 10.1046/j.1365-2826.2002.00861.x. [DOI] [PubMed] [Google Scholar]
- Ma S, Morilak DA. Chronic intermittent cold stress sensitises the hypothalamic-pituitary-adrenal response to a novel acute stress by enhancing noradrenergic influence in the rat paraventricular nucleus. J Neuroendocrinol. 2005;17:761–9. doi: 10.1111/j.1365-2826.2005.01372.x. [DOI] [PubMed] [Google Scholar]
- McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP, Bruchas MR. CRH Engagement of the Locus Coeruleus Noradrenergic System Mediates Stress-Induced Anxiety. Neuron. 2015;87:605–20. doi: 10.1016/j.neuron.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden K, Griffin TA, Levy V, Wolfe JH, Valentino RJ. Overexpression of corticotropin-releasing factor in Barrington’s nucleus neurons by adeno-associated viral transduction: effects on bladder function and behavior. Eur J Neurosci. 2012;36:3356–64. doi: 10.1111/j.1460-9568.2012.08250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27:446–56. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Glutamatergic transmission in the nucleus of the solitary tract modulates memory through influences on amygdala noradrenergic systems. Behav Neurosci. 2002;116:13–21. doi: 10.1037//0735-7044.116.1.13. [DOI] [PubMed] [Google Scholar]
- Monassi CR, Hoffmann A, Menescal-de-Oliveira L. Involvement of the cholinergic system and periaqueductal gray matter in the modulation of tonic immobility in the guinea pig. Physiol Behav. 1997;62:53–9. doi: 10.1016/s0031-9384(97)00134-0. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Galler JR, Mokler DJ. A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol. 2005;75:143–60. doi: 10.1016/j.pneurobio.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Morgane PJ, Mokler DJ. The limbic brain: continuing resolution. Neurosci Biobehav Rev. 2006;30:119–25. doi: 10.1016/j.neubiorev.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–24. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Myers B, Dolgas CM, Kasckow J, Cullinan WE, Herman JP, Dolgas CM, Kasckow J, Cullinan WE, Herman JP. Central stress-integrative circuits: forebrain glutamatergic and GABAergic projections to the dorsomedial hypothalamus, medial preoptic area, and bed nucleus of the stria terminalis. Brain Struct Funct. 2014a;219:1287–303. doi: 10.1007/s00429-013-0566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Front Neuroendocrinol. 2014b;35:180–96. doi: 10.1016/j.yfrne.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B, McKlveen JM, Herman JP. Neural Regulation of the Stress Response: The Many Faces of Feedback. Cell Mol Neurobiol. 2012 doi: 10.1007/s10571-012-9801-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar J, Haam J, Chen C, Jiang Z, Glatzer NR, Muglia LJ, Dohanich GP, Herman JP, Tasker JG. Rapid Nongenomic Glucocorticoid Actions in Male Mouse Hypothalamic Neuroendocrine Cells Are Dependent on the Nuclear Glucocorticoid Receptor. Endocrinology. 2015;156:2831–42. doi: 10.1210/en.2015-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Kelmendi B, Sanacora G. Overview of glutamatergic neurotransmission in the nervous system. Pharmacol Biochem Behav. 2012;100:656–64. doi: 10.1016/j.pbb.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Ren R, Cruz-Topete D, Bird GS, Myers PH, Boyle MC, Schneider MD, Willis MS, Cidlowski JA. Essential role of stress hormone signaling in cardiomyocytes for the prevention of heart disease. Proc Natl Acad Sci U S A. 2013;110:17035–40. doi: 10.1073/pnas.1302546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- Paul ED, Johnson PL, Shekhar A, Lowry CA. The Deakin/Graeff hypothesis: focus on serotonergic inhibition of panic. Neurosci Biobehav Rev 46 Pt. 2014;3:379–96. doi: 10.1016/j.neubiorev.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzone MA, Lee WS, Hoffman GE, Pezzone KM, Rabin BS. Activation of brainstem catecholaminergic neurons by conditioned and unconditioned aversive stimuli as revealed by c-Fos immunoreactivity. Brain Res. 1993;608:310–8. doi: 10.1016/0006-8993(93)91472-5. [DOI] [PubMed] [Google Scholar]
- Plotsky PM. Facilitation of immunoreactive corticotropin-releasing factor secretion into the hypophysial-portal circulation after activation of catecholaminergic pathways or central norepinephrine injection. Endocrinology. 1987;121:924–30. doi: 10.1210/endo-121-3-924. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Cunningham ET, Widmaier EP, Cunningham ET, Jr, Widmaier EP. Catecholaminergic modulation of corticotropin-releasing factor and adrenocorticotropin secretion. Endocr Rev. 1989;10:437–58. doi: 10.1210/edrv-10-4-437. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sawchenko PE. A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. J Neurosci. 2011;31:9683–9695. doi: 10.1523/JNEUROSCI.6040-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Williams B, Sawchenko PE. Noradrenergic innervation of the dorsal medial prefrontal cortex modulates hypothalamo-pituitary-adrenal responses to acute emotional stress. J Neurosci. 2008;28:5806–5816. doi: 10.1523/JNEUROSCI.0552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Trifunovic R. Lateral parabrachial nucleus lesions in the rat: neophobia and conditioned taste aversion. Brain Res Bull. 2001;55:359–66. doi: 10.1016/s0361-9230(01)00517-2. [DOI] [PubMed] [Google Scholar]
- Reyes BAS, Valentino RJ, Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–30. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BAS, Zitnik G, Foster C, Van Bockstaele EJ, Valentino RJ. Social Stress Engages Neurochemically-Distinct Afferents to the Rat Locus Coeruleus Depending on Coping Strategy(1,2,3) eNeuro. 2015;2 doi: 10.1523/ENEURO.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am J Physiol Regul Integr Comp Physiol. 2011;300:R222–35. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Visceral sensory inputs to the endocrine hypothalamus. Front Neuroendocrinol. 2007;28:50–60. doi: 10.1016/j.yfrne.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L. Interoceptive stress activates glucagon-like peptide-1 neurons that project to the hypothalamus. Am J Physiol. 1999;277:R582–90. doi: 10.1152/ajpregu.1999.277.2.R582. [DOI] [PubMed] [Google Scholar]
- Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology. 2003;144:1357–67. doi: 10.1210/en.2002-221076. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ. Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol. 1990;292:1–53. doi: 10.1002/cne.902920102. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Fekete C, Légrádi G, Lechan RM. Glucagon like peptide-1 (7-36) amide (GLP-1) nerve terminals densely innervate corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Brain Res. 2003;985:163–8. doi: 10.1016/s0006-8993(03)03117-2. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Brown ER, Chan RK, Ericsson A, Li HY, Roland BL, Kovács KJ. The paraventricular nucleus of the hypothalamus and the functional neuroanatomy of visceromotor responses to stress. Prog Brain Res. 1996;107:201–22. doi: 10.1016/s0079-6123(08)61866-x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res. 1982;257:275–325. doi: 10.1016/0165-0173(82)90010-8. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–7. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM. Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol. 1985;241:138–153. doi: 10.1002/cne.902410203. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA. The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res. 1983;277:355–60. doi: 10.1016/0006-8993(83)90945-9. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Vale WW. Co-expression of corticotropin-releasing factor and vasopressin immunoreactivity in parvocellular neurosecretory neurons of the adrenalectomized rat. Proc Natl Acad Sci U S A. 1984;81:1883–1887. doi: 10.1073/pnas.81.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Vernon KA. Chronic activation of dorsal hindbrain corticosteroid receptors augments the arterial pressure response to acute stress. Hypertension. 2007;49:127–33. doi: 10.1161/01.HYP.0000250088.15021.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L. Organization of the Locus Coeruleus-Norepinephrine System. Curr Biol. 2015;25:R1051–R1056. doi: 10.1016/j.cub.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O’Leary DS. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurol Res. 2005;27:182–94. doi: 10.1179/016164105X21959. [DOI] [PubMed] [Google Scholar]
- Sousa N, Almeida OFX. Disconnection and reconnection: the morphological basis of (mal)adaptation to stress. Trends Neurosci. 2012;35:742–51. doi: 10.1016/j.tins.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Spector AC. Gustatory function in the parabrachial nuclei: implications from lesion studies in rats. Rev Neurosci. 1995;6:143–75. doi: 10.1515/revneuro.1995.6.2.143. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Leknes AK, Bore AT, Vaaland JL, Edminson P, Haug FM, Ottersen OP. First visualization of glutamate and GABA in neurones by immunocytochemistry. Nature. 1983;301:517–20. doi: 10.1038/301517a0. [DOI] [PubMed] [Google Scholar]
- Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Holsboer F. Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry. 2003;36(Suppl 3):S207–14. doi: 10.1055/s-2003-45132. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R. Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology. 2006;147:5549–56. doi: 10.1210/en.2006-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D’Alessio DA, Stern JE, Herman JP. Distribution of glucagon-like peptide-1 immunoreactivity in the hypothalamic paraventricular and supraoptic nuclei. J Chem Neuroanat. 2008;36:144–149. doi: 10.1016/j.jchemneu.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turton MD, O’Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996;379:69–72. doi: 10.1038/379069a0. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kobayashi D, Das G, Onaka T, Inoue K, Itoi K. Participation of the prolactin-releasing peptide-containing neurones in caudal medulla in conveying haemorrhagic stress-induced signals to the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2010;22:33–42. doi: 10.1111/j.1365-2826.2009.01935.x. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Jones KR, Ziegler DR, Cullinan WE, Herman JP. Forebrain origins of glutamatergic innervation to the rat paraventricular nucleus of the hypothalamus: differential inputs to the anterior versus posterior subregions. J Comp Neurol. 2011;519:1301–1319. doi: 10.1002/cne.22571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science (80- ) 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology. 2012;62:13–20. doi: 10.1016/j.neuropharm.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kooy D, Koda LY, McGinty JF, Gerfen CR, Bloom FE. The organization of projections from the cortex, amygdala, and hypothalamus to the nucleus of the solitary tract in rat. J Comp Neurol. 1984;224:1–24. doi: 10.1002/cne.902240102. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, de Raay C, Swaab DF, Fisser B. The localization of oxytocin, vasopressin, somatostatin and luteinizing hormone releasing hormone in the rat neurohypophysis. Cell Tissue Res. 1979;202:189–201. doi: 10.1007/BF00232234. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman ATF, Milaneschi Y, Bandinelli S, Ferrucci L, Penninx BWJH. Urinary cortisol and six-year risk of all-cause and cardiovascular mortality. J Clin Endocrinol Metab. 2010;95:4959–64. doi: 10.1210/jc.2010-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsteeker Cusulin JI, Bains JS. Embedded synaptic feedback in the neuroendocrine stress axis. J Neuroendocrinol. 2015;27:481–6. doi: 10.1111/jne.12260. [DOI] [PubMed] [Google Scholar]
- Wamsteeker Cusulin JI, Füzesi T, Inoue W, Bains JS. Glucocorticoid feedback uncovers retrograde opioid signaling at hypothalamic synapses. Nat Neurosci. 2013;16:596–604. doi: 10.1038/nn.3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsteeker JI, Kuzmiski JB, Bains JS. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J Neurosci. 2010;30:11188–96. doi: 10.1523/JNEUROSCI.1046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–30. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Jankord R, Flak JN, Solomon MB, D’Alessio DA, Herman JP. Role of glucocorticoids in tuning hindbrain stress integration. J Neurosci. 2010;30:14907–14. doi: 10.1523/JNEUROSCI.0522-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Packard BA, Tauchi M, D’Alessio DA, Herman JP. Glucocorticoid regulation of preproglucagon transcription and RNA stability during stress. Proc Natl Acad Sci U S A. 2009;106:5913–5918. doi: 10.1073/pnas.0808716106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Stornetta RL, Agassandian K, Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Struct Funct. 2015;220:3011–22. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. J Neuroendocr. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Edwards MR, Ulrich-Lai YM, Herman JP, Cullinan WE. Brainstem origins of glutamatergic innervation of the rat hypothalamic paraventricular nucleus. J Comp Neurol. 2012;520:2369–2394. doi: 10.1002/cne.23043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol. 2002;42:541–51. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]