Abstract
Purpose
Quantitative magnetic resonance imaging (QMRI) of the hip with sequences such as T1ρ and T2 mapping has been utilized to detect early changes in cartilage matrix composition. However, QMRI has not been performed in the presence of intra-articular contrast. Thus the purpose of this study was to evaluate the feasibility and use of QMRI during MR-arthrography (MRA) in femoracetabular impingement (FAI) patients.
Materials and Methods
Using a 3T MR-scanner, ten FAI patients underwent a unilateral MRA and standard MRI of the hip joint. Global and sub-regional T1ρ and T2 relaxation times of the acetabular and femoral articular cartilage were computed in the MRA and MRI assessments and agreement of these values were assessed using the Krippendorff’s alpha (α) coefficient and linear regression (μ). T1ρ and T2 relaxation times between the MRA and MRI were compared using a repeated measures analysis of variance.
Results
Both global and sub-regional T1ρ and T2 relaxation times demonstrated strong agreement (α>0.83; μ>0.85) independent of intra-articular contrast. Also, global and sub-regional acetabular T1ρ (p=0.72) and T2 (p=0.94), as well as femoral T1ρ, relaxation times were similar between MRA and MRI (p=0.73) yet femoral T2 relaxation times decreased when using intra-articular contrast (p=0.04).
Conclusion
This study demonstrated the feasibility of T1ρ and T2 mapping for use in hip MRA with FAI patients. The inclusion of QMRI in MRA provides a quantitative assessment of the effects of FAI on hip joint articular cartilage while allowing for detailed assessment of labral pathology with the use of intra-articular contrast.
Keywords: Femoroacetabular Impingement (FAI), MR-Arthrography (MRA), MR-imaging (MRI), T1ρ, T2
Introduction
Femoracetabular impingement (FAI) is a disorder in which morphological variations of the hip result in abnormal contact between the femoral head-neck junction and the acetabular rim during hip joint motion (1). The pathomechanics of this condition can lead to labral tears, articular cartilage damage and may contribute to the early onset of hip joint osteoarthritis (OA) (1). Therefore, the identification of imaging strategies that improve the assessment of chondrolabaral damage, especially in the early stages, is essential to improving intervention strategies and long-term patient outcomes (2).
MR Arthrography (MRA) is an imaging modality that has been proven useful for evaluation of FAI, particularly in the detection of labral pathologies (3,4), through use of a contrast agent to improve visualization of labral tears and cartilage surface anatomy (4). Further, clinicians frequently use MRA as a method to localize the source of hip pain in patients as injection of local anesthetic or steroid along with the contrast agent can temporarily be therapeutic for intra-articular sources of pain (5). However, similar to MR imaging (MRI), MRA lacks the sensitivity to diagnose degeneration of the cartilage matrix in the early stages of FAI (6). For this reason, quantitative MRI (QMRI) techniques such as T1ρ and T2 mapping (7–9) are appealing for use in FAI.
Previous studies have used QMRI to assess global and regional differences between subjects with FAI (7,8). These previous studies have only focused on non-contrast MRI, and it is not well understood whether or not T1ρ and T2 values are equivalent in the presence of a contrast agent. As MRA is used to detect labral pathology, the use of QMRI in the presence of contrast may provide a more comprehensive assessment of joint health than MRA alone, reduce the need for multiple radiological examinations (10) and improve clinical evaluation of FAI patients. Therefore, the purpose of our study was to assess the feasibility of T1ρ and T2 mapping, in the presence of intra-articular contrast, in the FAI population.
Materials and Methods
Patient Demographics
Ten patients (3 females) that were diagnosed with FAI were recruited for this study and the group demographics are displayed in Table 1. Patients were included in this study if they were diagnosed with symptomatic FAI as defined using MR-imaging and clinical signs of impingement. MR-based measurements of the lateral center edge (LCE) and alpha angles were determined for each participant by two musculoskeletal radiologists (B.J.S. with 4 years of experience; T.M.L with 25 years of experience.) using anterior-posterior radiographs and oblique axial MR-images, respectively. The diagnosis of FAI was defined as either an LCE angle of greater than 25° (11) and/or an alpha angle of greater than 55° (12) as well as other morphological abnormalities including cartilage and labral abnormalities, osseous bump formation or acetabular over-coverage. In addition, all participants demonstrated positive impingement signs upon examination by an orthopaedic surgeon (A.L.Z.) through use of the flexion, adduction and internal rotation (FADIR) test (13). Participants were excluded from this study if they had: a body mass index (BMI) of greater than 35kg·m−2, previous hip surgery on the affected side, radiographic indication of OA (KL grade > 1 and less than 2mm of joint space) or demonstrated MRI contraindications such as being pregnant, coronary stent, etc. This study was approved by the University Committee on Human Research. Each patient provided informed written consent.
Table 1.
Group average demographic data
| Age(years) | Height(m) | Mass(kg) | BMI(kg/m2) | LCE | Alpha Angle | |
|---|---|---|---|---|---|---|
| Subject01 | 44 | 1.72 | 70.5 | 23.8 | 28 | 59 |
| Subject02 | 31 | 1.55 | 47.7 | 19.9 | 33 | 43 |
| Subject03 | 36 | 1.83 | 66.8 | 19.9 | 36 | 49 |
| Subject04 | 37 | 1.70 | 65.8 | 22.8 | 46 | 63 |
| Subject05 | 42 | 1.85 | 81.6 | 23.8 | 29 | 75 |
| Subject06 | 37 | 1.65 | 69.5 | 25.5 | 49 | 54 |
| Subject07 | 33 | 1.88 | 74.8 | 21.2 | 30 | 51 |
| Subject08 | 49 | 1.85 | 81.6 | 23.8 | 29 | 49 |
| Subject09 | 47 | 1.70 | 68.0 | 23.5 | 27 | 70 |
| Subject10 | 46 | 1.85 | 74.8 | 21.9 | 46 | 53 |
| Mean±St. Dev. | 40.2±6.2 | 1.76±0.11 | 70.1±9.7 | 22.6±1.9 | 35.3±8.5 | 56.6±10.1 |
LCE: Lateral Center Edge
St. Dev.: Standard Deviation
MRI and MRA
Each participant underwent unilateral MRA and MRI within a one month time span. The MR-arthrogram examination was a part of the patient’s clinical examination. A combination of gadolinium and lidocaine (0.5%) was used as an intra-articular contrast and injected under fluoroscopic guidance. Each participant was scanned using a 3Tesla MR scanner (GE MR750, GE Healthcare, Waukesha, WI) and an eight channel cardiac coil (GE Healthcare, Waukesha, WI). For all scans, each participant’s feet were internally rotated and taped together in order to reduce hip movement.
The MRA and MRI protocols included a combined T1ρ and T2 sequence (9,14), in which the T2 echos are acquired immediately after the T1ρ echoes. The scan was applied in the sagittal plane with the slab in the left/right direction. The combined T1ρ and T2 sequence was obtained using a field of view of 14cm, matrix size of 256 × 128, slice thickness of 4mm, recovery time of 1.2 seconds, views per segment is 64, bandwidth of 62.5 kHz, no gap, in-plane resolution of 0.5mm and acquisition time of 13:47. T1ρ images were acquired using a time of spin lock of 0/15/30/45ms, spin lock frequency of 300 Hz. T2 images were acquired using a preparation echo time of 0/10.4/20.8/41.7ms. In addition, sagittal, oblique coronal and axial images were obtained for each patient using intermediate-weighted, fat-suppressed, fast spin-echo (FSE) sequences. These images were obtained with a repetition time of 2400 – 3700ms, echo time of 60ms, field of view of 14 – 20cm, matrix size of 288×224, slice thickness of 3 – 4mm. These FSE images were used to assess morphological abnormalities including cartilage and labrum pathologies and osseous bump formation and acetabular over-coverage in each of the participants in this study.
Quantitative Cartilage Analysis
To avoid artifacts due to possible patient movement during the exam, the T1ρ and T2-weighted images were rigidly registered to the first echo of the T1ρ image using the VTK CISG Registration Toolkit (Kitware Inc., Clifton Park, NY). T1ρ and T2 relaxation maps were created using a two parameter exponential fit (8,9). Femoral and acetabular cartilage were segmented on the first echo of the T1ρ images using a custom in-house software program which incorporates a spline-based semi-automated algorithm (automated edge detection and manual correction) developed in MATLAB (The Mathworks, Natick, MA, USA). The cartilage layers were segmented on approximately 4 consecutive slices near the center of the hip, and the closest matching slices were used for cartilage segmentation in both the MRA and MRI. Segmentations were performed by one trained operator (M.C.G.) and separate regions of interest (ROI) were created for the acetabular and femoral cartilage layers. Intra-rater reliability was assessed in a previous pilot study in order to determine the operator’s ability to perform consistent hip joint cartilage segmentations. The acetabular and femoral ROIs were overlaid on the T1ρ and T2 maps and were used for quantification of global relaxation times. In addition, femoral and acetabular cartilage ROIs were subdivided for localized analysis (8,9). These sub-regions were created by drawing a reference line parallel to the femoral neck on one slice of the echo one image and 8 equal sub-regions were established based upon the reference line. Only sub-regions that consisted of at least 50 pixels for all subjects were analyzed. As a result, sub-regions 1, 7 and 8 were excluded from the femoral analysis while sub-regions 1, 6, 7 and 8 were excluded from the acetabular analysis (Figure 1).
Figure 1.
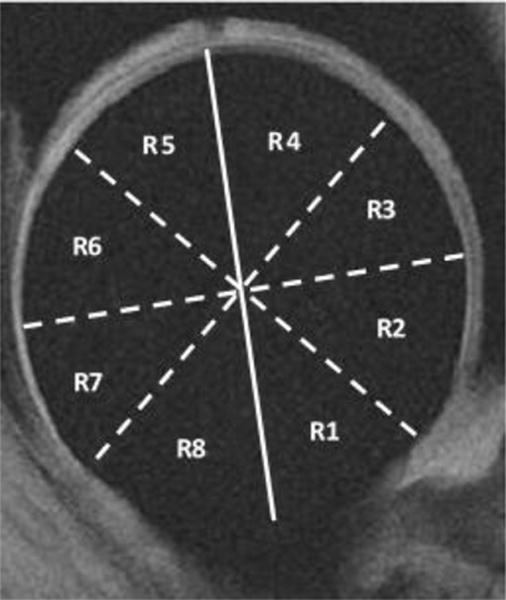
An example of the eight sub-regions of the hip that were created for localized analysis of the femoral and acetabular cartilage layers. In addition to the global femoral and acetabular cartilage assessment, five sub-regions (R2 – R6) of the femoral cartilage layer and four sub-regions (R2 – R5) of the acetabular cartilage region were analyzed. The solid line represents the line drawn parallel to the femoral neck while the dashed lines represent the angle bisectors drawn to create the eight equal sub-regions.
Statistical Analysis
T1ρ and T2 relaxation times determined from the MRA and MRI examinations were assessed for agreement using the Krippendorff alpha coefficient (15) and the estimated slope from linear regression through the origin. The Krippendorff alpha coefficient is a measure of the agreement of two or more methods used to generate data on the same set of objects (15). A bootstrap procedure was used in order to compute a 95% confidence interval for Krippendorff alpha (α). For both the Krippendorff alpha and estimated slopes (μ) from linear regression, a value of zero and one indicated no agreement and perfect agreement, respectively. In addition, the acetabular and femoral T1ρ and T2 relaxation times between the MRI and MRA were compared using a repeated measures analysis of variance, in order to assess the effects of intra-articular contrast on T1ρ and T2 relaxation times. All statistical analyses were performed using SPSS v22 (IBM, Armonk, NY, USA) and alpha was set a priori at the 0.05 level.
Results
The operator’s intra-rater reliability was found to be greater than 99% for MRI-based hip joint cartilage segmentations. The average global and sub-regional T1ρ and T2 relaxation times in the acetabular and femoral cartilage layers for the group are displayed in Table 2. Both sub-regional and global acetabular T1ρ and T2 relaxation times in both MRA and MRI were similar (P>0.05). The femoral cartilage sub-regional and global T1ρ relaxation times were similar (P>0.05) independent of contrast yet the femoral T2 relaxation times demonstrated a significant overall effect due to the intra-articular contrast (F(4, 6)=6.72, P=0.04). More specifically, T2 relaxation times in regions three (F(1, 9)=7.29, P=0.02), four (F(1, 9)=6.00, P=0.04) and five (F(1, 9)=7.07, P=0.03) were significantly lower in the presence of contrast (Figure 2).
Table 2.
Average (Mean±Standard Deviation) global and sub-regional T1ρ and T2 relaxation times (milliseconds), in both MR-arthrography (MRA) and MR-imaging (MRI), for the group are presented. An X indicates a particular sub-region that was not analyzed. An * indicates a statistically significant difference between MRA and MRI relaxation times.
| Global | Region 2 | Region 3 | Region 4 | Region 5 | Region 6 | |
|---|---|---|---|---|---|---|
| Acetabular | ||||||
| T1ρ (MRA) | 34.4±3.3 | 32.0±5.3 | 38.1±3.70 | 35.1±4.9 | 32.2±3.6 | X |
| T1ρ (MRI) | 34.1±2.6 | 30.5±3.5 | 37.6±2.4 | 33.5±4.7 | 33.1±4.0 | X |
| p-value | 0.72 | 0.38 | 0.74 | 0.30 | 0.44 | X |
| T2 (MRA) | 29.6±2.8 | 25.4±5.6 | 30.2±3.4 | 30.4±5.1 | 30.2±3.8 | X |
| T2 (MRI) | 29.7±2.9 | 24.0±2.8 | 29.6±3.7 | 29.1±5.5 | 31.8±4.5 | X |
| p-value | 0.94 | 0.41 | 0.75 | 0.47 | 0.28 | X |
| Femoral | ||||||
| T1ρ (MRA) | 35.5±3.4 | 35.6±4.4 | 37.8±4.8 | 33.2±4.8 | 36.5±4.5 | 36.0±5.5 |
| T1ρ (MRI) | 36.2±3.4 | 35.2±3.9 | 40.7±5.4 | 33.8±4.8 | 37.7±4.1 | 34.2±4.5 |
| p-value | 0.44 | 0.71 | 0.13 | 0.73 | 0.22 | 0.23 |
| T2 (MRA) | 31.9±3.3 | 31.4±4.4 | 35.9±4.5 | 31.2±4.4 | 32.9±4.7 | 31.3±5.3 |
| T2 (MRI) | 33.6±3.4 | 30.9±3.4 | 39.4±5.0 | 34.1±4.5 | 34.9±3.9 | 30.3±2.9 |
| p-value | 0.09 | 0.60 | 0.02* | 0.04* | 0.03* | 0.56 |
Figure 2.
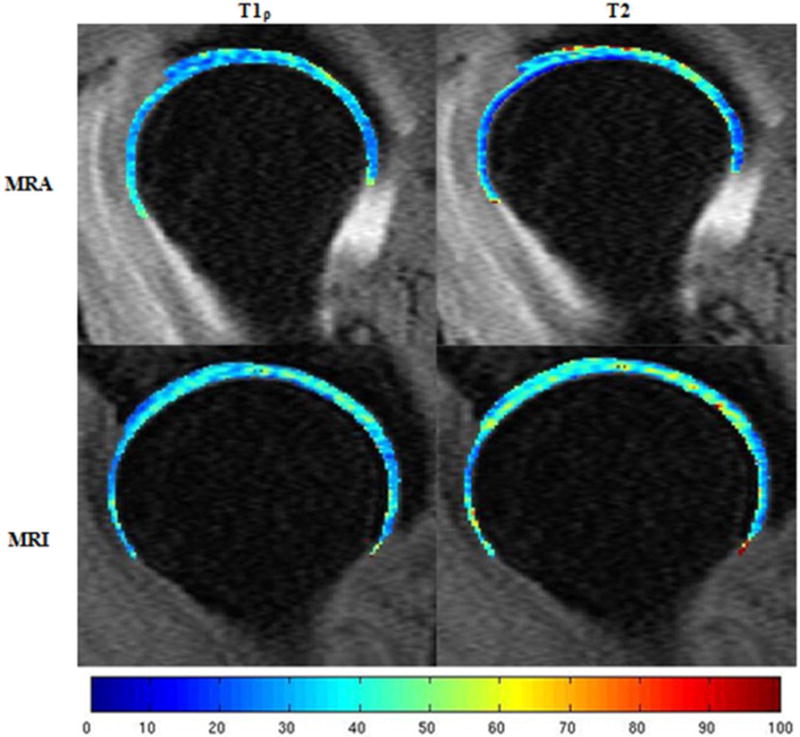
T1ρ and T2 mapping in both MR-arthrography (MRA) and MR-imaging (MRI) of the femoral and acetabular articular cartilage layers of one femoroacetabular impingement patient. The color scale indicates the relaxation time measured in milliseconds. Higher femoral T2 relaxation times can be observed in the MRI compared to the MRA.
Overall, the Krippendorff alpha values for both the acetabular and femoral cartilage layers ranged from 0.83 – 0.97 and demonstrated good agreement between the MRA and MRI values of T1ρ and T2 relaxation times (Table 3). The results of the linear regression produced estimated slopes of 0.85 – 0.97 and provided more evidence of strong agreement in T1ρ and T2 relaxation times between MRA and MRI examinations in both the acetabular and femoral cartilage layers.
Table 3.
Krippendorff alpha (α[95%CI]) values and the slopes (R) of the linear regression analyses obtained from comparison of acetabular and femoral articular cartilage T1ρ and T2 relaxation times between MR-arthrography and MR-imaging. An X indicates a sub-region that was not analyzed.
| Global | Region 2 | Region 3 | Region 4 | Region 5 | Region 6 | ||
|---|---|---|---|---|---|---|---|
| Acetabular | |||||||
| T1ρ α | 0.96[0.92–0.99] | 0.88[0.70–0.98] | 0.95[0.89–0.99] | 0.92[0.82–0.99] | 0.95[0.90–0.98] | X | |
| T1ρ R-values | 0.96 | 0.89 | 0.95 | 0.93 | 0.95 | X | |
| T2 α | 0.96[0.93–0.99] | 0.83[0.71–0.94] | 0.84[0.67–0.96] | 0.88[0.74–0.97] | 0.91[0.83–0.97] | X | |
| T2 R-values | 0.96 | 0.85 | 0.84 | 0.88 | 0.92 | X | |
| Femoral | |||||||
| T1ρ α | 0.97[0.94–0.99] | 0.96[0.93–0.98] | 0.90[0.81–0.96] | 0.90[0.81–0.97] | 0.97[0.95–0.99] | 0.93[0.89–0.96] | |
| T1ρ R-values | 0.97 | 0.96 | 0.92 | 0.90 | 0.97 | 0.94 | |
| T2 α | 0.96[0.92–0.98] | 0.96[0.92–0.99] | 0.91[0.82–0.98] | 0.91[0.81–0.98] | 0.96[0.93–0.99] | 0.88[0.76–0.97] | |
| T2 R-values | 0.97 | 0.96 | 0.95 | 0.95 | 0.98 | 0.88 |
Discussion
The purpose of this study was to evaluate the feasibility of T1ρ and T2 mapping for use in MRA as a quantitative measurement of cartilage degeneration in FAI patients. Our findings suggest that both global and sub-regional values of the acetabular T1ρ and T2 relaxation times, as well as the femoral T1ρ relaxation times, were similar between MRA and MRI in subjects with FAI yet the femoral T2 relaxation times were affected by the presence of intra-articular contrast. Strong agreement in T1ρ and T2 relaxation values, as demonstrated by linear regression and the calculation of Krippendorff’s alpha, between MRA and MRI of the femoral and acetabular cartilage layers support the feasibility of quantitative MRA. The results of this study suggest that T1ρ and T2 measurements are feasible independent of intra-articular contrast.
The use of quantitative MRI (QMRI) during arthrography has been limited (16) and the implementation of QMRI may add to the importance of arthrography as a clinical tool (5). This study demonstrated that there is strong agreement in T1ρ values in both the acetabular and femoral cartilage layers independent of intra-articular contrast. These results suggest that assessment of T1ρ in MRA, as a measure of proteoglycan content, are similar to those measured in MRI and may add to the benefits of MRA in clinical evaluation of FAI.
T2 relaxation times in both the acetabular and femoral cartilage layers demonstrated strong agreement between MRA and MRI measured values. Despite this strong agreement, femoral T2 relaxation times were affected by the presence of intra-articular contrast. In particular, regions 3 – 5 of the femoral cartilage layer demonstrated significantly lower T2 relaxation times in the MRA compared to the MRI. Similar to the results of this study, previous work demonstrated that T2 relaxation times in the femoral cartilage of those with knee OA were reduced as an effect of contrast (17). In those with knee OA, T2 relaxation times were found to be significantly lower (18), as determined using post-contrast imaging, compared to pre-contrast imaging and may be attributed to the fact that pathologic cartilage may exhibit T2 shortening due to elevated concentrations of contrast (18). It is possible that a similar relationship is present in the FAI patients of this study, where the femoral cartilage within regions 3 – 5 (weight-bearing region of the hip joint) may be more damaged and therefore, exhibit T2 shortening due to the elevated levels of contrast within these regions of the femoral cartilage layer (18). The paramagnetic nature of the gadolinium contrast agent causes an inverse relationship between contrast absorption and proteoglycan content within the cartilage layer (19). More specifically, a non-uniform T2 mapping occurs, throughout the cartilage layer, with shorter and longer T2 values occurring in the superficial and deep layers of the articular cartilage, respectively (20). The regions of interest (ROIs) used in the current study, represent the average signal intensity within a particular ROI and may be affected by this non-uniform T2 mapping within regions 3 – 5. Also, the accumulation of contrast within the femoral cartilage layer, during mechanical unloading of the hip joint during the MRA examination, may be a possible source of change in relaxation time (21) and therefore, there may be a time-dependent effect of contrast on T2 mapping. A pattern of decreased T1ρ relaxation times occur in regions 3 – 5 in the femoral cartilage layer, with contrast, yet these changes are not significantly different and may be due to a lack of statistical power. The observed differences in T2 relaxation times within regions 3–5 of the femoral cartilage layer may not be clinically significant yet the effect of contrast agent on both T1ρ and T2 relaxation times warrant further investigation using a larger cohort.
Various limitations exist in the current study and should be considered when interpreting the results of this study. The ROIs used in the MRA may better represent the femoral and acetabular cartilage layers compared to the ROIs used in the MRI as contrast enhanced MRI using an intra-articular injection was shown to better delineate the hip joint cartilage compared to non-enhanced MRI (22) and may reduce the possibility of including both joint compartments within a single ROI. The increased delineation of the articular cartilage may allow for more accurate segmentations and therefore, may affect T1ρ and T2 quantification of the femoral and acetabular cartilage ROIs. In addition, there is a lack of knowledge on the effects of intra-articular contrast on T1ρ and T2 mapping as no clinical trials or external validations have been performed to determine the effect of contrast on T1ρ and T2 mapping of hip joint articular cartilage. In addition, the small cohort size in the current study does not allow for definitive conclusions as to the reliability of cartilage relaxation times in MR-arthrography. Despite these limitations, this study demonstrated the possible added clinical benefit of T1ρ and T2 mapping in MRA. Future studies should evaluate the effects of FAI on cartilage degeneration using T1ρ and T2 mapping as markers of articular cartilage degeneration within the FAI population. In a future study using a larger cohort size, a conversion factor may be determined in order to properly interpret the signal changes in the femoral cartilage layer in MRA studies.
In conclusion, this study demonstrated the feasibility of T1ρ and T2 mapping in MRA within the FAI population. The strong agreement of T1ρ and T2 relaxation times between MRA and MRI suggests that the inclusion of QMRI in MRA may add value to the use of MRA in this population, by providing clinicians with more quantitative measures in detection of early cartilage damage. In the future, clinicians may consider adding T1ρ and T2 mapping to MR-arthrograms in order to better evaluate the effects of FAI on hip joint articular cartilage degeneration.
Acknowledgments
Grant Support:
This study was supported by NIH-NIAMS P50 AR060752 and the New Orthopaedic Vision Award (Department of Orthopaedic Surgery, University of California – San Francisco). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ganz R, Parvizi J, Beck M, Leunig M, Nötzli H, Siebenrock KA. Femoroacetabular Impingement: A Cause for Osteoarthritis of the Hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 2.Clohisy J, St John L, Schutz A. Surgical treatment of femoracetabular impingement: A systematic review of the literature. Clin Orthop Relat Res. 2010;468(2):555–564. doi: 10.1007/s11999-009-1138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leunig M, Werlen S, Ungersbock A, Ito K, Ganz R. Evaluation of the acetabular labrum by MR Arthrography. J Bone Joint Surg Br. 1997;79-B(2):230–234. doi: 10.1302/0301-620x.79b2.7288. [DOI] [PubMed] [Google Scholar]
- 4.Petersilge CA, Haque MA, Petersilge WJ, Lewin JS, Lieberman JM, Buly R. Acetabular labral tears: evaluation with MR arthrography. Radiology. 1996;200(1):231–235. doi: 10.1148/radiology.200.1.8657917. [DOI] [PubMed] [Google Scholar]
- 5.Botser I, Safran MR. MR imaging of the hip: pathologies and morphologies of the hip joint, what the surgeon wants to know. Magnetic resonance imaging clinics of North America. 2013;21(1):169–182. doi: 10.1016/j.mric.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 6.Schmid MR, Nötzli HP, Zanetti M, Wyss TF, Hodler J. Cartilage lesions in the hip: Diagnostic effectiveness of MR arthrography. Radiology. 2003;226(2):382–386. doi: 10.1148/radiol.2262020019. [DOI] [PubMed] [Google Scholar]
- 7.Rakhra KS, Lattanzio P-J, Cárdenas-Blanco A, Cameron IG, Beaulé PE. Can T1-rho MRI detect acetabular cartilage degeneration in femoroacetabular impingement?: A pilot study. J Bone Joint Surg Br. 2012;94-B(9):1187–1192. doi: 10.1302/0301-620X.94B9.29981. [DOI] [PubMed] [Google Scholar]
- 8.Subburaj K, Valentinitsch A, Dillon AB, et al. Regional variations in MR relaxation of hip joint cartilage in subjects with and without femoralacetabular impingement. Magn Reson Imaging. 2013;31(7):1129–1136. doi: 10.1016/j.mri.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wyatt C, Kumar D, Subburaj K, et al. Cartilage T1ρ and T2 Relaxation Times in Patients with Mild-to-Moderate Radiographic Hip Osteoarthritis. Arthritis Rheumatol. 2015;67(6):1548–1556. doi: 10.1002/art.39074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Czerny C, Hofmann S, Neuhold A, et al. Lesions of the acetabular labrum: accuracy of MR imaging and MR arthrography in detection and staging. Radiology. 1996;200(1):225–230. doi: 10.1148/radiology.200.1.8657916. [DOI] [PubMed] [Google Scholar]
- 11.Kappe T, Kocak T, Bieger R, Reichel H, Fraitzl CR. Radiographic Risk Factors for Labral Lesions in Femoroacetabular Impingement. Clin Orthop Relat Res. 2011;469(11):3241–3247. doi: 10.1007/s11999-011-1978-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domayer SE, Ziebarth K, Chan J, Bixby S, Mamisch TC, Kim YJ. Femoroacetabular cam-type impingement: Diagnostic sensitivity and specificity of radiographic views compared to radial MRI. Eur J Radiol. 2011;80(3):805–810. doi: 10.1016/j.ejrad.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Philippon MJ, Maxwell RB, Johnston TL, Schenker M, Briggs KK. Clinical presentation of femoroacetabular impingement. Knee Surg Sports Traumatol Arthrosc. 2007;15(8):1041–1047. doi: 10.1007/s00167-007-0348-2. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Wyatt C, Rivoire J, et al. Simultaneous acquisition of T1ρ and T2 quantification in knee cartilage: Repeatability and diurnal variation. J Magn Reson Imaging. 2014;39(5):1287–1293. doi: 10.1002/jmri.24253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krippendorff K. Content analysis: An introduction to its methodology. 3rd. Thousand Oaks: Sage; 2013. p. 440. [Google Scholar]
- 16.Bittershohl B, Hosalkar HS, Werlen S, Trattnig S, Siebenrock KA, Mamisch TC. Intravenous Versus Intra-Articular Delayed Gadolinium-Enhance Magnetic Resonance Imaging in the Hip Joint. Invest Radiol. 2010;45(9):538–542. doi: 10.1097/RLI.0b013e3181ea5bb5. [DOI] [PubMed] [Google Scholar]
- 17.Yoon HJ, Yoon YC, Choe B-K. T2 values of femoral cartilage of the knee joint: Comparison between pre-contrast and post-contrast. Korean J Radiol. 2014;15(1):123–129. doi: 10.3348/kjr.2014.15.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurkijärvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, Nieminen MT. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: Topographical variation and relationships to mechanical properties. Magn Reson Med. 2004;52(1):41–46. doi: 10.1002/mrm.20104. [DOI] [PubMed] [Google Scholar]
- 19.Bashir A, Gray M, Burstein D. Gd-DTPA(2-) as a measure of cartilage degradation. Magn Reson Med. 1996;36:665–673. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 20.Maroudas A, Bayliss M, Venn M. Further studies on the composition of human fmeoral head cartilage. Ann Rheum Dis. 1980;39:514–523. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apprich S, Mamisch TC, Welsch GH, et al. Evaluation of articular cartilage in patients with femoracetabular impingement (FAI) using T2* mapping at different time points at 3.0 Tesla MRI: a feasibility study. Skeletal Radiol. 2012;41:987–995. doi: 10.1007/s00256-011-1313-1. [DOI] [PubMed] [Google Scholar]
- 22.Boesen M, Jensen KE, Qvistgaard E, et al. Delayed gadolinium-enhanced magnetic resonance imaging (dGEMRIC) of hip joint cartilage: Better cartilage delineation after intra-articular than intravenous gadolinium injection. Acta Radiol. 2006;47(4):391–396. doi: 10.1080/02841850600596792. [DOI] [PubMed] [Google Scholar]


