Summary
Background
Inorganic polyphosphate (polyP) elicits intracellular signaling responses in endothelial cells through activation of mTOR complexes 1 and 2. Glycogen synthase kinase 3 (GSK-3) is known to be a negative regulator of mTOR and Wnt/β-catenin signaling pathways.
Objective
The objective of this study was to investigate the effect of polyP on the expression, degradation and subcellular localization of the Wnt/β-catenin target gene, cyclin D1, in endothelial cells.
Methods
Regulation of cyclin D1 expression, phosphorylation and subcellular localization by polyP or platelet releasates was monitored in the absence and presence of pharmacological inhibitors and/or siRNA for specific molecules of the upstream mTOR/Wnt/β-catenin signaling network by established methods.
Results
Both purified polyP and boiled-platelet releasates induced the phosphorylation-dependent inactivation of GSK-3, thereby increasing the expression and nuclear localization, but inhibiting the degradation of cyclin D1. Inhibitors of mTORC1 (PI3K, AKT, PLC, PKC), rapamycin and siRNA for raptor (mTORC1-specific component) and β-catenin, all inhibited polyP-mediated regulation of cyclin D1 expression, phosphorylation and subcellular localization in endothelial cells. The signaling effect of polyP was effectively inhibited by the recombinant extracellular domain of the receptor for advanced glycation end products (RAGE) and/or by the RAGE siRNA. Specific pharmacological inhibitors and siRNA knockdown of ERK1/2 and NF-κB pathways indicated that polyP-mediated cyclin D1 expression and nuclear localization are IKKα- and ERK1/2-dependent, whereas its inhibitory effect on phosphorylation-dependent degradation of cyclin D1 is IKKβ-dependent.
Conclusion
We conclude that a RAGE-dependent polyP-mediated crosstalk between mTOR and GSK-3/Wnt/β-catenin signaling network can modulate important physiological processes in endothelial cells.
Keywords: Polyphosphate, GSK-3, Wnt/β-catenin, cyclin D1, mTOR, endothelial cells
Introduction
Inorganic polyphosphates (polyP) are linear polymers of 3 to over 1,000 inorganic phosphate residues, linked together by high-energy phosphoanhydride bonds (1). High levels of medium size polyP polymers (~60–100 phosphate units) are stored in platelets which are known to be released to circulation upon their activation by thrombin or other physiological stimuli during activation of the blood coagulation cascade (2,3). Longer polyP polymers (>1000 phosphate units) may be synthesized by microorganisms under different environmental conditions (1,3). Recent results have established an important role for polyP in regulating numerous physiological processes including coagulation (3–5), tumor metastasis (6,7), proliferation (8), apoptosis (9,10) and inflammation (4,10–12).
PolyP is known to activate mammalian target of rapamycin (mTOR) in breast cancer cells (13). We recently demonstrated polyP, upon interaction with the receptor for advanced glycation end products (RAGE) and P2Y1 purinergic receptor, mediates phosphorylation-dependent inactivation of the upstream regulatory tuberous sclerosis complex 1/2 (TSC1/2), thereby activating both mTOR complexes 1 (mTORC1) and 2 (mTORC2) in HUVECs (11,12). It has been established that the key negative regulator of Wnt/β-catenin signaling, glycogen synthase kinase 3β (GSK-3β), through activation of TSC1/2 can inactivate mTOR signaling, suggesting a crosstalk between the two signaling networks modulates metabolic and proliferative signaling responses in HUVECs (14,15). Upon expression and binding of Wnt proteins to the Frizzled family of receptors, GSK-3β is inactivated by phosphorylation, thus leading to activation of both mTOR and Wnt/β-catenin signaling pathways (15,16). GSK-3β is a kinase involved in degradation of β-catenin, thus as a consequence of GSK-3β inactivation, intact β-catenin can translocate into the nucleus, where it binds to specific transcription factors (i.e., T cell factor, TCF) to induce expression of Wnt/β-catenin target genes (16, 17). Cyclin D1 is a Wnt/β-catenin target gene, mainly involved in regulation of the G1 phase of the cell cycle (18). Cyclin D1 accumulates in the nuclei of cells during the G1 phase and exits into the cytoplasm during the S phase (19). It has been demonstrated that GSK-3β through phosphorylation of the nuclear cyclin D1 regulates cytoplasmic distribution and subsequent degradation of the cell cycle protein (20).
In this study we investigated the role of polyP-70 in modulating Wnt/β-catenin signaling by analysis of polyP-mediated phosphorylation of GSK-3 and regulation of cyclin D1 synthesis in the absence and presence of siRNA for β-catenin and pharmacological inhibitors of specific signaling pathways. Results suggest that polyP-70, through phosphorylation-dependent inactivation of GSK-3, activates Wnt/β-catenin signaling, thereby regulating expression and subcellular localization of cyclin D1 in HUVECs. Possible physiological significance of these results was demonstrated by findings that platelet releasates exerted similar signaling effects in HUVECs by polyP-dependent mechanisms.
Materials and Methods
Reagents
Wnt/β-catenin inhibitors, iCRT3 and PNU-74654, were from Santa Cruz Biotechnology (Santa Cruz, CA). MG-132, was obtained from Sigma-Aldrich Chemical Co., Inc. (St. Louis, MO). Vectashield mounting medium was from Vector Laboratories Inc. (Burlingame, CA). siRNA for β-catenin was from Dharmacon (Lafayette, CO). Rabbit antibodies to cyclin D1, GSK3α, GSK3β, phospho GSK3α, phospho GSK3β, phospho cyclin D1, eukaryotic initiation factor 4E (eIF4E) and Alexa-conjugated secondary antibodies were from Cell Signaling Technology Inc. (Beverly, MA). Anti-eIF4E (phospho S209) antibody was from Abcam Inc. (Cambridge, MA). Extracellular domain of receptor for advanced glycation end products (sRAGE) was prepared as described (11). All other reagents were provided as described (11,12). Scrambled siRNA was used as negative control for all siRNA experiments as described (11,12). PolyP-70 was a generous gift from Dr. James Morrissey (University of Illinois, Urbana). Platelets releasates were prepared by activation of human platelets with TRAP as described (21).
Cell culture
Primary human umbilical vein endothelial cells (HUVECs) and immortalized HUVECs (EA.hy926) were grown as described (11,12).
Western-blot analysis
Cells were treated with stimuli under different conditions (with or without transfection with siRNA and treatment with pharmacological inhibitors) and lysed with Pierce IP lysis buffer as described (12,22).
Immunofluorescence staining
To examine subcellular localization of cyclin D1, cells (2.5×105) were seeded on glass coverslips and fixed with 4% paraformaldehyde for 10min at room temperature. Next, cells were first incubated in blocking solution (2% BSA, 0.1% tritonX-100 in PBS) and then stained with either anti-cyclin D1 or phospho anti-cyclin D1 (Thr-286) monoclonal antibodies, followed by Alexa-conjugated secondary antibodies (Alexa Fluor 488 and 594 conjugates). Coverslips were mounted on glass slides with Vectashield mounting medium containing DAPI to counterstain cell nuclei. Coverslips were then sealed with a mixture of Vaseline and wax. Stained cells were visualized with a Nikon Optiphot-2 microscope fitted with appropriate fluorescence filters.
Results
PolyP-70 inactivates GSK-3 and promotes cyclin D1 expression
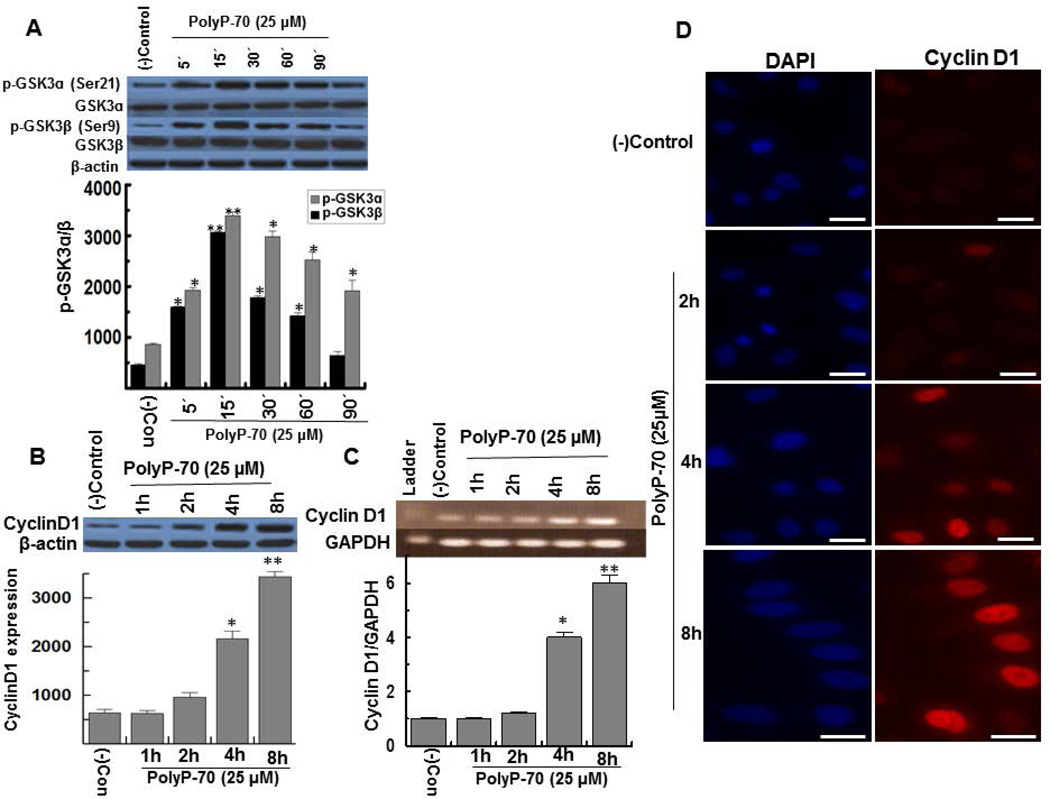
PolyP-70 promoted GSK-3 phosphorylation in HUVECs by a time-dependent mechanism with optimal effect occurring after 15min of treatment (Fig. 1A). GSK-3 is encoded by two genes, GSK-3α and GSK-3β (23). GSK-3α and GSK3-β are inactivated by phosphorylation at Ser-21 and Ser-9, respectively, when Wnt proteins bind to Frizzled receptors to mediate activation of Wnt signaling (24,25). PolyP effectively phosphorylated both isoforms of GSK-3 (Fig. 1A). The Wnt signaling-dependent phosphorylation of GSK-3β inhibits its kinase activity, thereby preventing degradation of β-catenin by GSK-3β (15–17,23). The translocation of intact β-catenin to the nucleus, promotes transcription of Wnt/β-catenin target gene, cyclin D1 (18–20). In quiescent cells, in addition to degradation of β-catenin, GSK-3β is also involved in phosphorylation-dependent degradation of cyclin D1 (18). Results suggested that polyP-mediated inactivation of GSK-3β promotes overexpression of cyclin D1 protein (Fig. 1B), transcript (Fig. 1C) and its nuclear localization (Fig. 1D). PolyP-70 stimulated expression of cyclin D1 in both primary and transformed HUVECs.
Figure 1.
PolyP-70 induces GSK-3 phosphorylation and increases expression and nuclear localization of cyclin D1, in EA.hy926 endothelial cells. (A) Time course of polyP-mediated phosphorylation and inactivation of GSK-3α/β in EA.hy926 cells. (B) Cells were treated with polyP-70 (25 µM) at different time points followed by measuring the expression of cyclin D1. (C) Semiquantitative RT-PCR was performed as described before (22). Results were normalized to expression levels of GAPDH and presented as the fold difference relative to the control group at each time point. The results are shown as mean ± standard deviation of 3 different experiments as determined Student t test. *P<0.05; **P<0.01. (D) The same as B except that the effect of polyP-70 on nuclear localization of cyclin D1 was measured. Cells were stained with DAPI to visualize the nucleus (Blue) and anti-cyclin D1 antibody (Red) and then imaged by confocal microscopy. The scale for the microscopic figure is 20µm.
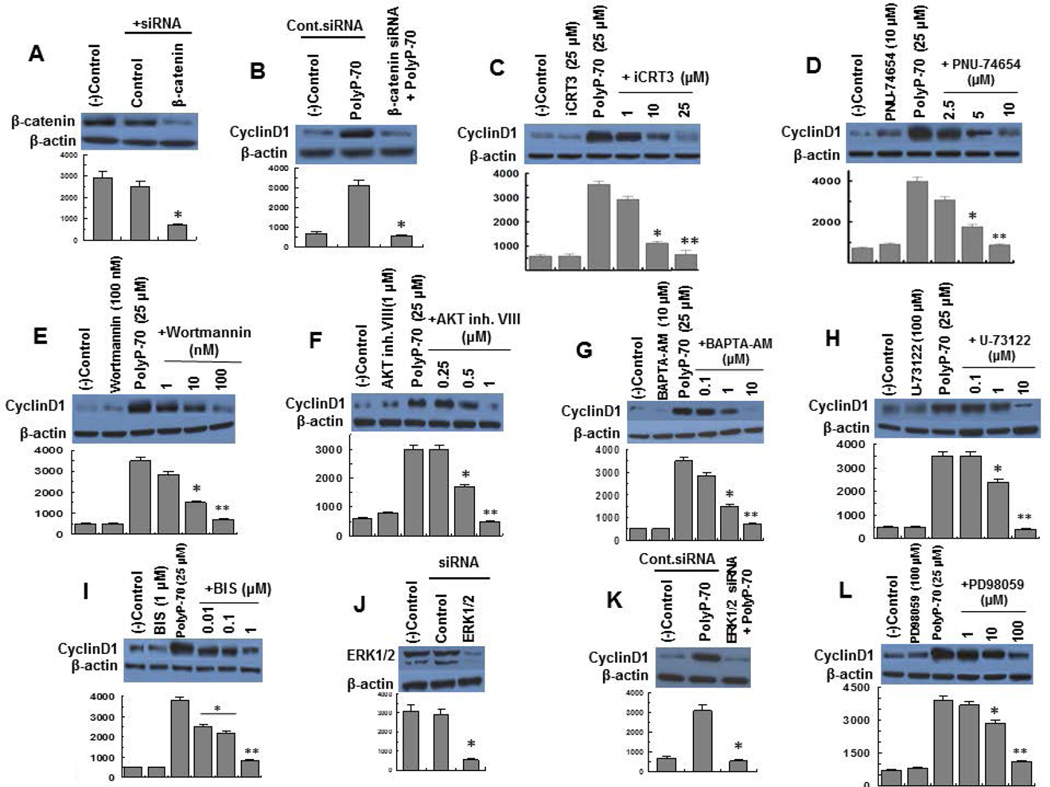
To provide further support for the hypothesis that polyP-70 activates Wnt/β-catenin signaling, expression of cyclin D1 in polyP-70-stimulated cells was studied with and without transfection of cells with β-catenin siRNA. Efficiency of siRNA knockdown of β-catenin is presented in Fig. 2A. siRNA for β-catenin effectively inhibited polyP-70-mediated up-regulation of cyclin D1 expression (Fig. 2B). Up-regulation of cyclin D1 in polyP-stimulated cells was also investigated in the presence of two different Wnt/β-catenin signaling inhibitors, iCRT3 and PNU-74654 which both bind β-catenin, disrupting the interaction of β-catenin with TCF transcription factor, thereby inhibiting expression of Wnt target genes (26). Consistent with β-catenin siRNA results, both inhibitors inhibited polyP-induced cyclin D1 expression (Fig. 2C,D). These results suggest polyP-70-induced cyclin D1 overexpression in is mediated through activation of Wnt/β-catenin signaling.
Figure 2.
PolyP-70-mediated expression of cyclin D1 in the absence and presence of siRNA or specific inhibitors for signaling molecules. (A) The efficiency of gene knockdown of β-catenin (>75%) was determined 48h post transfection by Western-blotting using a specific antibody. The (B) PolyP-mediated up-regulation of cyclin D1 was monitored after siRNA knockdown of β-catenin in EA.hy926 endothelial cells. (C–D) PolyP-mediated overexpression of cyclin D1 in the absence and presence of increasing concentrations of two different Wnt signaling inhibitors (iCRT3 and PNU-74654). (E) PolyP-mediated up-regulation of cyclin D1 in the absence and presence of increasing concentrations of PI3K inhibitor (wortmannin). (F) PolyP-mediated overexpression of cyclin D1 in the absence and presence of increasing concentrations of AKT inhibitor (AKT inhibitor VIII). (G) PolyP-mediated overexpression of cyclin D1 in the absence and presence of increasing concentrations of intracellular calcium chelator (BAPTA-AM). (H) PolyP-mediated up-regulation of cyclin D1 in the absence and presence of increasing concentrations of PLC inhibitor (U-73122). (I) PolyP-mediated overexpression of cyclin D1 in the absence and presence of increasing concentrations of PKC inhibitor (BIS). (J) EA.hy926 cells were transiently transfected with control siRNA or siRNA for ERK1/2 and the efficiency of gene knockdown (>75%) was determined 48h post transfection by Western-blotting using a specific antibody. (K) The same as J except that polyP-mediated overexpression of cyclin D1 was monitored after siRNA knockdown of ERK1/2. (L) PolyP-mediated overexpression of cyclin D1 in the absence and presence of increasing concentrations of ERK inhibitor (PD-98059). The results are shown as mean ± standard deviation of 3 different experiments. *P<0.05; **P<0.01.
PolyP-70 activates Wnt/β-catenin signaling by PI3K/AKT- and PLC/PKC/ERK-dependent mechanisms
Next, we investigated the role of PI3K/AKT in polyP-mediated Wnt/β-catenin signaling by monitoring expression of cyclin D1 using pharmacological inhibitors of these signaling pathways. Pretreatment of cells with either wortmannin (PI3K inhibitor) or AKT VIII (AKT inhibitor) suppressed up-regulation of cyclin D1 in polyP-70-stimulated cells (Fig. 2E,F). PolyP mediates calcium release from intracellular stores through interaction with P2Y1 (11,12,27). It was found that calcium signaling is required for polyP-mediated Wnt/β-catenin signaling since the Ca2+ chelator, BAPTA-AM, inhibited the effect of polyP-70 on cyclin D1 overexpression (Fig. 2G). Inhibitors of PLC (U-73122) and PKC, bisindolylmaleimide I hydrochloride (BIS), also inhibited polyP-induced overexpression of cyclin D1 (Fig. 2H,I). These results suggest polyP-70 up-regulates cyclin D1 expression through activation of PI3K/AKT and PLC/PKC/Ca2+ signaling cascades.
Next, polyP-70-mediated cyclin D1 overexpression was monitored in the absence and presence of siRNA for ERK1/2. Efficiency of gene knockdown was determined 48h post transfection (Fig. 2J). Results demonstrated ERK1/2 siRNA inhibits polyP-mediated up-regulation of cyclin D1 (Fig. 2K). Consistent with these results, pretreatment of cells with ERK1/2 inhibitor, PD-98059, also abrogated polyP-induced cyclin D1 expression (Fig. 2L). Our previous results indicated polyP exerts its modulatory effect through PI3K/AKT- and PLC/PKC/Ca2+-dependent activation of mTOR independent of ERK1/2 (12). Thus in contrast to mTOR activation, polyP-mediated up-regulation of Wnt/β-catenin signaling required ERK1/2 activation. We have demonstrated that none of the inhibitors used in this study has a toxic effect on EA.hy926 cells (12).
PolyP-70 regulates phosphorylation and degradation of cyclin D1
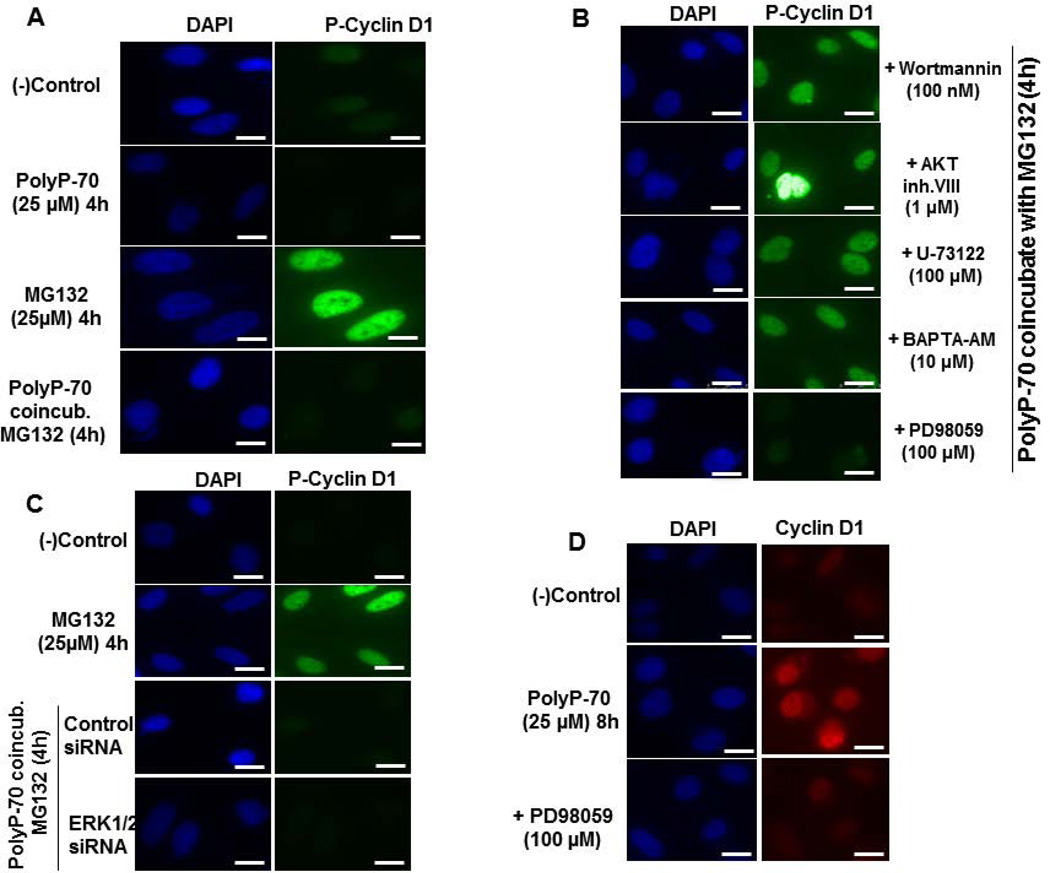
GSK-3β phosphorylates cyclin D1 on Thr-286, thereby mediating its cytoplasmic degradation (20). Since polyP-70 inhibits GSK-3α/β, we investigated effect of polyP-70 on phosphorylation and degradation of cyclin D1 in the absence and presence of the proteasome inhibitor, MG-132. In the presence of MG-132, nuclear-localized phospho-cyclin D1 could be visualized by a specific anti-phospho-cyclin D1 antibody (Fig. 3A). However, co-treatment of cells with MG-132 and polyP-70 significantly decreased the phosphorylated form of cyclin D1 (Fig. 3A), suggesting polyP-70 inhibits phosphorylation-dependent degradation of cyclin D1. To further investigate this question, cells were treated with inhibitors of mTOR upstream signaling and phosphorylation of cyclin D1 was analyzed. Results suggest inhibition of PI3K/AKT and Ca2+-signaling abrogates the inhibitory effect of polyP-70 on cyclin D1 phosphorylation (Fig. 3B). In contrast to the suppressive effect of ERK1/2 inhibitor on cyclin D1, the inhibitory effect of polyP-70 on cyclin D1 phosphorylation was independent of ERK1/2 (Fig. 3B, bottom panel). In agreement with results obtained with the ERK1/2 inhibitor, siRNA for ERK1/2 had no effect on polyP-induced inhibition of cyclin D1 phosphorylation (Fig. 3C). Moreover, consistent with the inhibitory effect of PD-98059 (ERK1/2 inhibitor) on polyP-70-induced cyclin D1 overexpression, PD-98059 significantly suppressed nuclear localization of cyclin D1 in polyP-70-stimulated cells (Fig 3D). These results suggest that effects of polyP-70 on cyclin D1 expression and nuclear localization are both dependent on ERK1/2, whereas PolyP-mediated inhibition of cyclin D1 phosphorylation/degradation is independent of ERK1/2.
Figure 3.
PolyP-70 inhibits phosphorylation and degradation of cyclin D1 in EA.hy926 endothelial cells. (A) Cells were treated with MG132 (25 µM) for 4h in the absence or presence of polyP-70 (25 µM) followed by measuring the phosphorylation of cyclin D1 using anti-phospho cyclin D1 antibody. (B) PolyP-mediated inhibition of cyclin D1 phosphorylation was monitored in the presence of specific inhibitors of mTOR upstream signaling pathway. In the presence of each inhibitor, cells were co-incubated with MG132 (25µM) and PolyP-70 (25µM) for 4h. (C) PolyP-mediated inhibition of cyclin D1 phosphorylation was monitored after siRNA knockdown of ERK1/2. (D) PolyP-mediated nuclear localization of cyclin D1 was measured in the absence and presence of ERK1/2 inhibitor (PD-98059). The scale for the microscopic figure is 20µm.
PolyP-mediated cyclin D1 overexpression, localization and degradation are mTORC1- and NF-κB-dependent
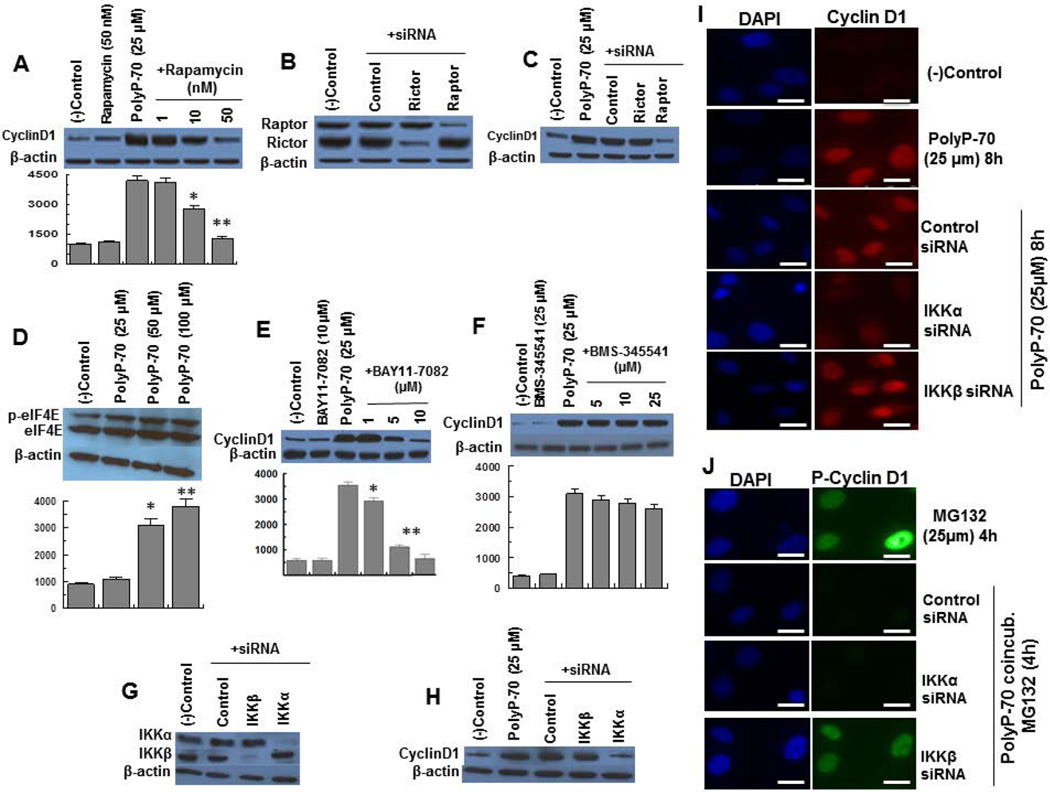
We recently demonstrated that polyP, through interaction with RAGE and P2Y1, activates both mTORC1 and mTORC2 in endothelial cells (12). GSK-3β through phosphorylation of β-catenin and TSC1/2 inhibits Wnt/β-catenin and mTOR signaling, respectively. Since polyP inactivated GSK-3β, we investigated the role of mTORC1 and mTORC2 in Wnt/βcatenin-dependent regulation of cyclin D1. Results indicated the mTORC1 inhibitor, rapamycin, abrogates polyP-induced up-regulation of cyclin D1 expression (Fig. 4A). Since rapamycin may also inhibit mTORC2 (28,29), this question was analyzed in cells transfected with siRNAs specific for components of either mTORC1 (raptor) or mTORC2 (rictor). Both raptor and rictor siRNAs effectively inhibited their expression (Fig. 4B), however, only siRNA for raptor, inhibited polyP-mediated overexpression of cyclin D1 (Fig. 4C), suggesting polyP-mediated Wnt/β-catenin and mTORC1 signaling are linked pathways.
Figure 4.
PolyP-70-mediated regulation of expression, localization and degradation of cyclin D1 are dependent on mTORC1 and NF-κB signaling pathways. (A) PolyP-mediated overexpression of cyclin D1 was monitored in the presence of different concentrations of mTORC1 inhibitor (rapamycin). (B) EA.hy926 endothelial cells were transiently transfected with control siRNA or siRNA specific for either raptor or rictor and the efficiency of gene knockdown (>75%) was determined 48h post transfection. (C) The same as B except that polyP-mediated up-regulation of cyclin D1 was monitored after siRNA knockdown of either raptor or rictor. (D) The expression and phosphorylation of eIF-4E was monitored in EA.hy926 cells treated with different concentrations of polyP-70. (E) PolyP-mediated overexpression of cyclin D1 was monitored in the presence of different concentrations of IKKα inhibitor (BAY11-7082). (F) The same as D except that polyP-mediated up-regulation of cyclin D1 was monitored in the presence of different concentrations of IKKβ inhibitor (BMS-345541). (G) The same as B except that cells were transiently transfected with control siRNA or siRNA specific for either IKKα or IKKβ. (H) The same as C except that polyP-mediated up-regulation of cyclin D1 was monitored after siRNA knockdown of either IKKα or IKKβ. (I) PolyP-mediated nuclear localization of cyclin D1 was measured in EA.hy926 cells transiently transfected with control siRNA or siRNA specific for either IKKα or IKKβ. (J) The same as H except that polyP-mediated inhibition of cyclin D1 phosphorylation was monitored after siRNA knockdown of IKKα or IKKβ. The scale for the microscopic figure is 20µm. The results are shown as mean ± standard deviation of 3 different experiments. *P<0.05; **P<0.01.
mTORC1 promotes protein synthesis through stimulation of phosphorylation of p70S6K and enhancing expression and phosphorylation-dependent release of eukaryotic initiation factor 4E (eIF-4E) from its inhibitory proteins (30). We previously demonstrated polyP effectively up-regulates phosphorylation of p70S6K through activation of mTORC1 in the absence of serum in endothelial cells (12). Analysis of polyP-70-mediated regulation of eIF-4E indicated polyP has no effect on expression of eIF-4E but promotes its phosphorylation (Fig. 4D).
In light of findings that polyP activates NF-κB (10,11) and that there is interplay between mTORC1 and NF-κB pathways in polyP-stimulated cells (12), we investigated the role of NF-κB in Wnt/β-catenin-dependent regulation of cyclin D1 expression in polyP-70-stimulated cells. The IKKα-inhibitor, BAY11-7082, markedly decreased polyP-mediated cyclin D1 overexpression (Fig. 4E) whereas the IKKβ-inhibitor, BMS-345541, had no effect (Fig. 4F). In agreement with inhibitor results, while both IKKα and IKKβ siRNAs effectively inhibited their expression (Fig. 4G), only IKKα siRNA suppressed overexpression of cyclin D1 in the presence of polyP-70 (Fig. 4H). To determine whether polyP-70-mediated cyclin D1 nuclear localization and phosphorylation are NF-κB-dependent, cells were transfected with siRNAs for IKKα and IKKβ and localization and phosphorylation of cyclin D1 were analyzed. Results demonstrated that polyP-mediated nuclear localization of cyclin D1 is IKKα-dependent but the inhibitory effect of polyP on cyclin D1 phosphorylation is specifically dependent on IKKβ (Fig. 4 I,J).
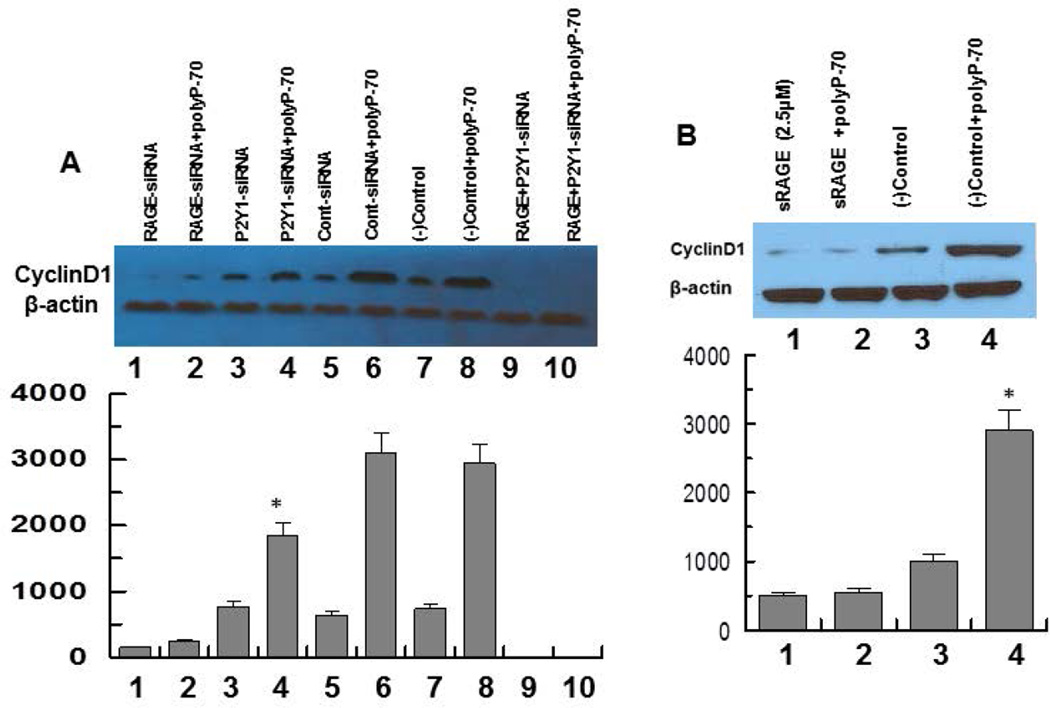
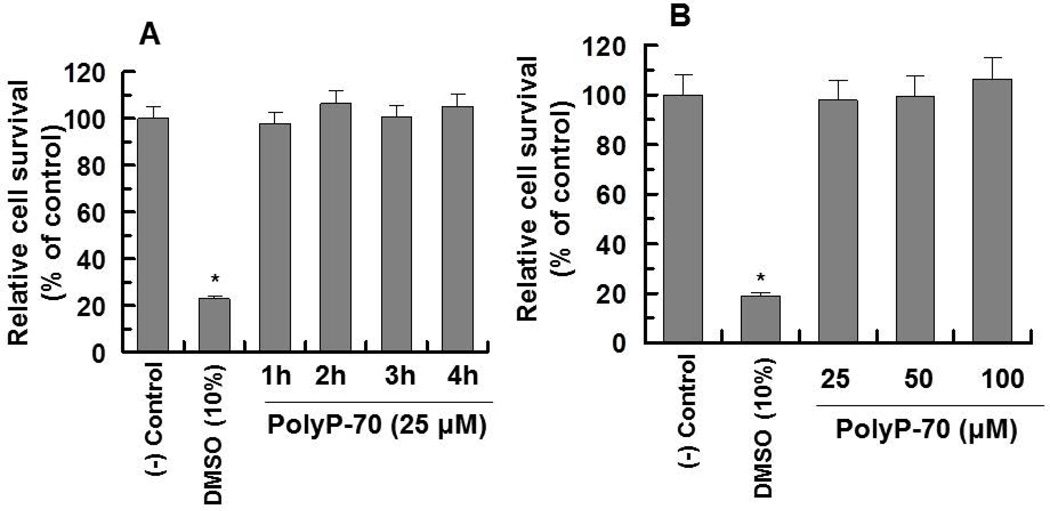
Next, involvements of RAGE and P2Y1 receptors in polyP-mediated cyclin D1 synthesis were investigated by both siRNA approaches and competitive binding studies using the extracellular domain of RAGE (sRAGE) (11). Results suggested while siRNA for either RAGE or P2Y1 significantly decreases the ability of polyP to induce cyclin D1, the combination of two siRNAs completely abrogates polyP-mediated cyclin D1 synthesis (Fig. 5A, lane 10), suggesting that similar to polyP-mediated mTOR activation, up-regulation of cyclin D1 synthesis is also mediated through polyP interacting with RAGE and P2Y1. Interestingly, RAGE siRNA by itself resulted in a dramatic decrease in cyclin D1 synthesis (Fig. 5A, lane 1) and in combination with P2Y1 siRNA, no cyclin D1 could be detected in transfected cells (Fig. 5A, lane 9), suggesting a key role for RAGE-signaling in cyclin D1 synthesis. In support of this hypothesis, sRAGE effectively inhibited cyclin D synthesis and polyP-70 did not up-regulate its expression in the presence of sRAGE (Fig. 5B), confirming siRNA results that RAGE is required for cell growth and that polyP induces cyclin D1 synthesis through stimulation of RAGE-signaling. Treatments of cells with polyP for up to 4h did not have any effect on the viability of endothelial cells as determined by the MTT assay as described (12) (Fig. 6A,B).
Figure 5.
PolyP-mediated expression of cyclin D1 in EA.hy926 endothelial cells transfected with siRNAs for RAGE and/or P2Y1 or treated with recombinant soluble RAGE (sRAGE). (A) Cells were transiently transfected with control siRNA or siRNA specific for either RAGE or P2Y1 followed by stimulating cells with polyP-70 (25 µM) and monitoring the expression of cyclin D1. Lane 1, RAGE siRNA alone; lane 2, RAGE siRNA + polyP-70; lane 3, P2Y1 siRNA alone; lane 4, P2Y1 siRNA + polyP-70; lane 5, control siRNA alone; lane 6, control siRNA + polyp-70; lane 7, control (no siRNA, buffer only); lane 8, control + polyP-70; lane 9, RAGE and P2Y1 siRNAs alone combined; lane 10, RAGE and P2Y1 siRNAs combined + polyP-70. (B) Analysis of expression of cyclin D1 treated with sRAGE (2.5 µM). Lane 1, sRAGE alone; lane 2, sRAGE + polyP-70; lane 3, control (buffer only); lane 4, control + polyP-70. The efficiency of gene knockdown was >75% in all cases. The results are shown as mean ± standard deviation of 3 different experiments. *P<0.05 for lane 4 (P2Y1 siRNA + polyP), compared to control siRNA + polyP-70 in panel A.
Figure 6.
Viability of EA.hy926 cells treated with polyP-70. (A) The time course of cell survival was monitored by treating cells with polyP-70 (25 µM) followed by evaluating the viability by MTT assay as described (22). (B) The same as A except that the polyP-70 concentration-dependence of cell survival was monitored. The percentage of cell viability was calculated by the ratio of the OD540 of wells containing polyP-70 to the OD of the wells lacking polyP-70 × 100. The results are shown as mean ± standard deviation of 3 different experiments. *P<0.05.
Platelet releasates regulate GSK-3/Wnt/β-catenin signaling
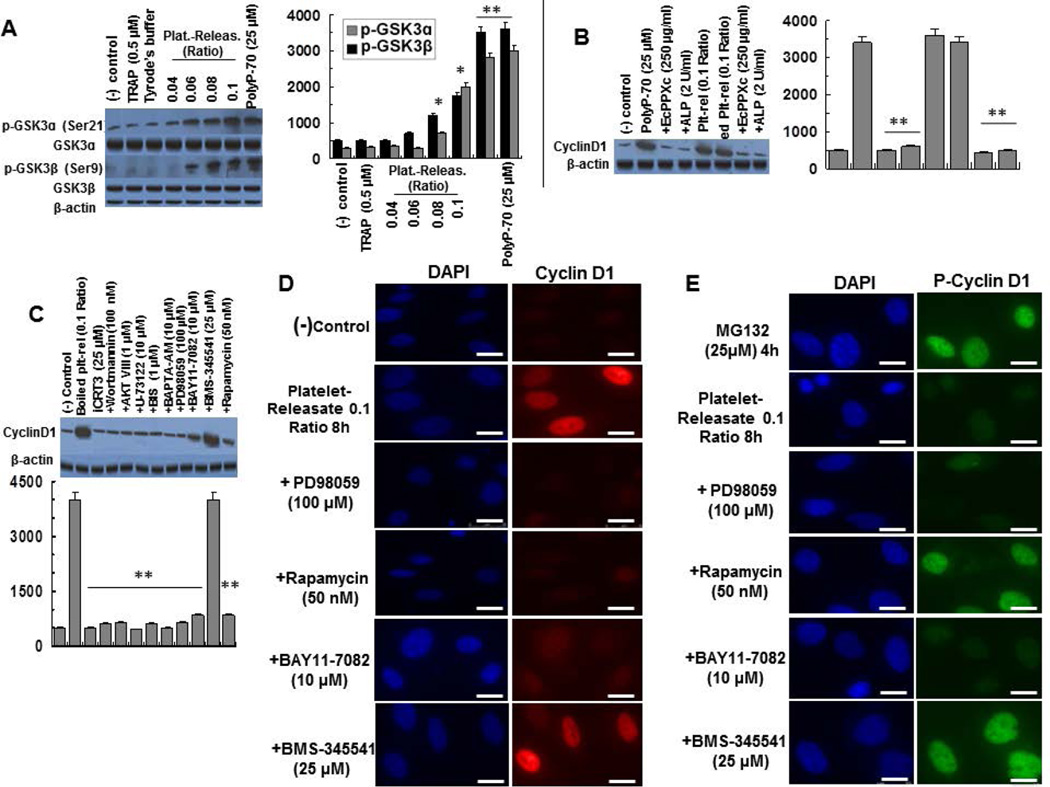
Activated platelets secrete polyP with polymer lengths of ~60–100 phosphate units (2,3). Expression, nuclear localization and phosphorylation of cyclin D1 by activated platelet- releasates were monitored employing the same assays described above. It was found that platelet releasates ratio of 0.04 to 0.1 (platelet releasates/total volume, estimated to be ~10 to 25µM, if the polyP concentration was expressed in terms of phosphate monomer) (12), phosphorylates GSK-3α/β by a dose-dependent manner (Fig. 7A). Since platelets were activated with TRAP, the possible stimulatory effect of a small amount of TRAP present in platelet releasates was analyzed. A TRAP concentration of 0.5µM, which exceeds the concentration of TRAP present in the highest platelet releasates ratio of 0.1 (~25µM) (12), did not have any effect on GSK-3 phosphorylation (Fig. 7A), excluding any contribution from trace amounts of TRAP present in platelet releasates. To establish the hypothesis that polyP derived from platelet releasates is responsible for GSK-3 phosphorylation, incubation of platelet releasates with specific polyP inhibitor EcPPXc (polyP-binding domain of Escherichia coli exopolyphosphatase) (31) or alkaline phosphatase (ALP) abrogated the signaling activity of both polyP-70 and platelet releasates (Fig. 7B). Furthermore, boiling platelet releasates for 30min prior to cell treatment did not impact the platelet releasates-mediated expression of cyclin D1, however, boiling releasates followed by treatment with either recombinant EcPPXc or ALP abrogated the signaling effect (Fig. 7B). It has been previously demonstrated that boiling platelet releasates for 30min denatures all proteins without negatively affecting the cofactor function of polyP (21). These results strongly suggest that platelet polyP is responsible for GSK-mediated cyclin D1 overexpression in endothelial cells.
Figure 7.
Platelet releasates regulate expression, localization and stability of cyclin D1 in EA.hy926 cells. (A) Dose response of platelet releasates (30 min) for inducing the phosphorylation of GSK-3α/β in EA.hy926 cells. PolyP-70 is shown as a control in the last lane. (B) Overexpression of cyclin D1 by boiled (30 min) platelet releasates (plt-rel, 0.1 ratio) is inhibited by co-incubation of platelet releasates with EcPPXc (250 µg/ml) and alkaline phosphatase (ALP 2 units/mL). PolyP-70 is shown as a control in the lanes 2–4. (C) Analysis of cyclin D1 overexpression by boiled (30 min) platelet releasates by the inhibitors of mTOR upstream signaling pathway. With all inhibitors, cells were treated with boiled platelet releasates co-incubated with each inhibitor for 8h. (D) Platelet releasates-mediated nuclear localization of cyclin D1 was measured in EA.hy926 cells co-incubated with ERK1/2 inhibitor (PD-98059), mTORC1 inhibitor (rapamycin), IKKα and IKKβ inhibitors (BAY11-7082 and BMS-345541 respectively). (E) The same as D except that platelet releasates-mediated inhibition of cyclin D1 phosphorylation was monitored. The scale for the microscopic figure is 20µm. The results are shown as mean ± standard deviation of 3 different experiments. *P<0.05; **P<0.01.
In agreement with results obtained with polyP-70, iCRT3, wortmannin, AKT VIII, U-73122, BIS, BAPTA-AM, PD-98059, BAY11-7082 (IKKα-inhibitor) and rapamycin all inhibited the platelet releasates-mediated cyclin D1 overexpression (Fig. 7C). As expected based on results presented above, BMS-345541 (IKKβ-inhibitor) had no inhibitory effect on the platelet releasates-mediated regulation of cyclin D1 expression (Fig. 7C).
Similar to results obtained above with 25µM polyP-70, treatment of cells with a 0.1 ratio of boiled platelet releasates (~25µM) enhanced nuclear localization of cyclin D1 and that the effect was eliminated by PD-98059, rapamycin and BAY11-7082 but not by BMS-345541 (Fig. 7D,E). Moreover, consistent with the inhibitory effect of polyP-70 on cyclin D1 phosphorylation, incubation of cells with boiled platelet releasates (0.1 ratio) suppressed cyclin D1 phosphorylation in MG-132-treated cells by mTORC1- and IKKβ-dependent but ERK1/2- and IKKα-independent mechanisms. Taken together, these results suggest that both polyP-70 and platelet-derived polyP regulate GSK-3/Wnt/β-catenin signaling through the same mechanisms in endothelial cells.
Discussion
We have demonstrated here that polyP inhibits the kinase activity of GSK-3 by phosphorylation of both GSK-3α and GSK-3β isoforms in both primary and transformed HUVECs. The phosphorylation-dependent inhibition of GSK-3 by polyP results in stabilization of β-catenin and its subsequent translocation to the nucleus, thus inducing the expression of the β-catenin target gene, cyclin D1 (16–18). Consistent with this hypothesis, Wnt/β-catenin signaling inhibitors, iCRT3 and PNU-74654, both inhibited polyP-mediated up-regulation of cyclin D1 synthesis. Thus, polyP through inhibition of GSK-3 and stabilization of β-catenin/ cyclin D1 appears to play a key role in regulating metabolic and proliferative signaling responses in both primary and transformed HUVECs. The mechanism by which polyP modulates expression and subcellular localization of cyclin D1 is not known. Nevertheless, our recent results, showing that polyP is an effective stimulator of mTOR in endothelial cells (12), suggest that polyP, through activation of mTOR, may also modulate GSK/Wnt/β-catenin signaling. The polyP-induced mTOR signaling in endothelial cells has been found to be primarily mediated through polyP interacting with RAGE (11), a multi-ligand cell surface receptor involved in numerous pathophysiological processes including cell growth, proliferation, inflammation and cancer (32,33). We recently demonstrated that polyP can bind with high affinity to the RAGE ligands, HMGB1 and histones, and present the nuclear proteins to RAGE, thereby dramatically amplifying proinflammatory signaling responses (11). To determine whether polyP-mediated up-regulation of cyclin D1 expression is mediated through the same receptor signaling system, we used RAGE-specific siRNA to effectively down-regulate expression of RAGE in endothelial cells. Results suggested polyP induces cyclin D1 synthesis through the same mechanism since it was ineffective in up-regulating cyclin D1 expression in the presence of RAGE siRNA. In agreement with previous results that polyP interaction with P2Y1 further stimulates mTOR by inducing calcium signaling (11,12), the combination of RAGE and P2Y1 siRNAs completely inhibited cyclin D1 synthesis. Interestingly, RAGE siRNA by itself dramatically down-regulated cyclin D1 expression independent of polyP, suggesting RAGE signaling is required for cyclin D1 synthesis in endothelial cells during nutrient depletion. Stimulation of cells with polyP in the presence of RAGE siRNA did not produce a significant stimulatory effect on expression of cyclin D1. These results suggest that polyP through RAGE signaling regulates expression of cyclin D1. Further support for RAGE/mTOR-dependent regulation of cyclin D1 signaling was provided by the observation that pharmaceutical inhibitors of the upstream mTOR signaling network (PI3K/AKT and PLC/PKC/Ca2+) all abrogated regulatory effects of polyP on cyclin D1 expression, phosphorylation and subcellular localization. PolyP-mediated up-regulation of the cyclin D1 synthesis was mediated specifically through mTORC1 since siRNA for raptor but not rictor effectively inhibited this process.
Results from several studies have indicated that RAGE siRNA arrests a number of human cancer cells in the G1 phase of the cell cycle, thereby inhibiting DNA synthesis, suggesting that RAGE signaling may be involved in promoting cancer growth and metastasis (34–37). Noting that tumor cells can secret significant amounts of HMGB1 and that HMGB1 promotes autophagy (37,38), it has been hypothesized that HMGB1-mediated autophagy through RAGE signaling plays an important role in allowing tumor cells to grow during nutrient depletion (38–41). In this context, we have shown that transformed HUVECs (EA.hy926 endothelial cells) secrete a significant amount of HMGB1 under serum-free growth conditions (42). Thus, it appears that these cells can acquire survival advantage during the serum free culture conditions through HMGB1/RAGE-mediated induction of autophagy, thereby up-regulating cyclin D1 synthesis. In support of this hypothesis, sRAGE, which binds with high affinity to HMGB1, effectively inhibited cyclin D1 synthesis in EA.hy926 cells. We have also demonstrated that polyP can bind to HMGB1 with high affinity and present the nuclear protein to the RAGE, thereby dramatically amplifying proinflammatory signaling responses (11). Several human tumor cell lines are known to synthesize and store a significant amount of polyP in their nuclei and other tissues (1,3,13,43,44). Results presented above raise the possibility that the tumor cell-derived polyP can make a positive contribution to the growth factor-independent proliferation and metastasis of cancer cells by a similar signaling mechanism. Thus, polyP establishes a crosstalk between RAGE, mTOR and Wnt/β-catenin signaling networks that can be critical for survival and regulation of key pathophysiological processes in vascular endothelial cells. The possible physiological significance of these results was demonstrated by findings that boiled human platelet releasates exert similar signaling effects in EA.hy926 endothelial cells by a polyP-dependent mechanism. It is worth noting that the aberrant regulation of both mTOR and Wnt/β-catenin signaling pathways are associated with a number of different tumor developments (16,30,45). A role for platelets in tumor metastasis has also been postulated (46). Thus, it is of interest to further investigate and to determine whether or not polyP, released by activated platelets, in addition to up-regulation of thrombin generation, coagulation and inflammation, can also participate in promoting tumor cell growth and metastasis under certain pathophysiological conditions. It should be emphasized that we monitored HMGB1 expression, RAGE signaling and platelet-releasates experiments only in transformed HUVECs, thus further studies will be required to validate these results in primary endothelial cells.
We previously demonstrated that polyP through activation of mTOR also up-regulates the activation of NF-κB, suggesting that the two pathways are interlinked (12). It was interesting to note in the current study that a polyP-mediated crosstalk between NF-κB and Wnt/β-catenin signaling also modulated expression, phosphorylation and subcellular localization of cyclin D1. This is derived from observations that siRNAs for IKKα and IKKβ and their pharmacological inhibitors down-regulated polyP-mediated overexpression of cyclin D1 and/or abrogated the inhibitory effect of polyP on the phosphorylation-dependent degradation of the cell cycle protein. These two kinases appear to exert different modulatory effects on polyP-mediated up-regulation of cyclin D1. This is based on the observation that inhibition of IKKα, but not IKKβ, inhibited polyP-mediated overexpression and nuclear localization of cyclin D1. By contrast, inhibition of IKKβ, but not IKKα, abrogated inhibitory effect of polyP on the phosphorylation-dependent degradation of cyclin D1. These results provide some insight into the nature of an intricate and complex crosstalk between mTOR, GSK-3/Wnt/β-catenin and NF-κB and envision a key role for polyP in RAGE-dependent modulation of these signaling networks in endothelial cells.
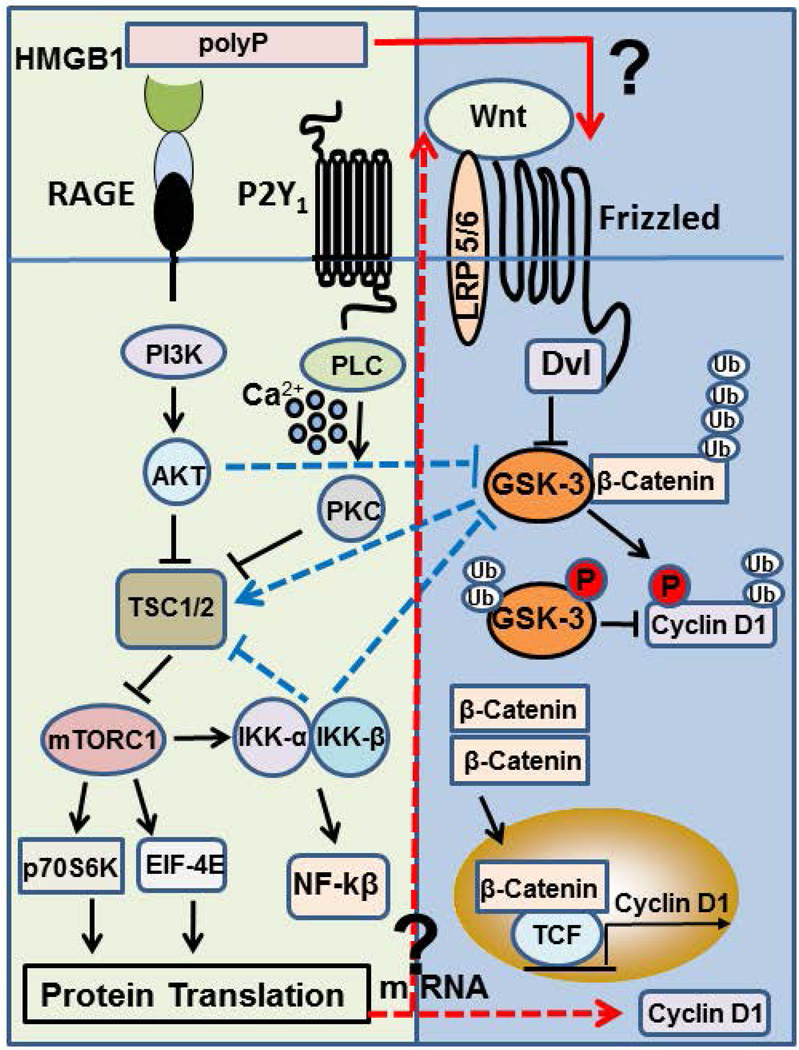
The mechanism through which polyP activates GSK-3/Wnt/β-catenin signaling is not known. It is however known that the pathway is activated when different Wnt ligands bind to Frizzled family of receptors, thereby inactivating GSK-3β by phosphorylation, thus leading to stabilization of β-catenin, its translocation to the nucleus and transcription of the Wnt/β-catenin target gene, cyclin D1 (15–18). Thus, a mechanism through which polyP can activate GSK-3/Wnt/β-catenin signaling is by up-regulation of the synthesis of Wnt proteins by endothelial cells. However, GSK-3 is also known to be phosphorylated/inactivated by AKT/PKB signaling (30,47), which is a pathway effectively activated by polyP (12). Moreover, we previously demonstrated that polyP, through activation of mTORC1, mediates phosphorylation and stimulation of p70S6 kinase (12), thereby increasing mRNA synthesis and translation of ribosomal proteins which promote protein synthesis during cell growth. In support of a role for polyP in promoting protein synthesis through mRNA translation, we discovered in the current study that polyP also promotes phosphorylation of the mRNA cap-binding protein eIF-4E, which has been shown to be involved in the initiation of translation of a subset of mRNAs (including cyclin D1) required for cell growth (48,49). Indeed, the overexpression of elF-4E in NIH 3T3 cells by transfection studies has been found to be associated with increased cyclin D1 mRNA expression and enhanced translation efficiency of cyclin D1 mRNA in serum-deprived cells (50). These results suggest that polyP can influence GSK-3/Wnt/β-catenin signaling through multiple steps, without requirement for Wnt proteins ligating their specific Frizzled receptors. Based on these results we propose the model presented in Fig. 8 depicting different mechanisms through which polyP may modulate the GSK-3/Wnt/β-catenin signaling. The interaction of polyP with RAGE and P2Y1 activates mTORC1 by PI3K/AKT and PLC/PKC dependent inhibition of TSC1/2 complex as we demonstrated previously (12). PolyP, through interaction with the same receptors, can activate GSK-3/Wnt/β-catenin signaling by at least four different mechanisms: 1) polyP-mediated AKT activation can lead to phosphorylation-dependent inhibition of GSK-3, thereby resulting in stabilization of β-catenin independent of Wnt-mediated Frizzled signaling, 2) polyP-mediated improvement of protein translation can result in augmentation of translation of mRNAs for cyclin D1 and/or Wnt proteins, 3) polyP through activation of IKKα/β can down-regulate GSK-3 activity, thereby stabilizing β-catenin and cyclin D1 independent of a direct Wnt/Frizzled signaling, and finally 4) polyP can directly interact with a Frizzled receptor, thereby activating the pathway by a mechanism similar to RAGE signaling. As indicated above, we have demonstrated that polyP can bind HMGB1 with high affinity to dramatically promote RAGE signaling (11). Thus, we hypothesize that low levels of HMGB1 synthesized by cells under stressed conditions can have a profound effect in modulating these signaling pathways in the presence of polyP (11). Further studies will be required to determine the exact mechanisms through which polyP establishes a crosstalk between mTOR and GSK-3/Wnt/β-catenin signaling network that culminates in elevated expression of cyclin D1 in endothelial cells.
Figure 8.
Mechanism of polyP-mediated crosstalk between mTOR and GSK-3/Wnt/β-catenin signaling networks. PolyP, through interaction with RAGE and P2Y1 receptors, activates mTORC1 by PI3K/AKT and PLC/PKC/Ca2+ dependent inhibition of tumor suppressor TSC1/2 complex, thereby augmenting protein translation machinery by phosphorylation of p70S6K and eIF-4E. HMGB1 can dramatically promote these signaling reactions. PolyP by the same mechanism mediates the phosphorylation-dependent activation of IKK-α/β, thereby activating NF-kβ. PolyP can promote the expression of cyclin D1 by several different mechanisms: It can indirectly promote cyclin D1 synthesis by the phosphorylation-dependent inhibition of GSK-3 by either AKT and/or IKK-α/β, thereby preventing the ubiquitin- and proteasome-dependent degradation of β-catenin and cyclin D1 independent of Wnt-Frizzled receptor interaction (dashed blue lines). Alternatively, polyP can promote the translation of cyclin D1 mRNA and/or Wnt mRNAs, thereby leading to up-regulation Wnt proteins and their interaction with and activation of Frizzled receptors (dashed red arrows). Finally, polyP can directly interact with Frizzled (and LRP5/6) receptors to improve the expression of cyclin D1 (solid red arrow). HMGB1, high mobility group box 1; RAGE, receptor for advanced glycation end products; TSC1/2, tuberous sclerosis complex 1/2; GSK-3, glycogen synthase kinase 3; TCF, T cell factor; LRP5/6, Dvl, Dishevelled; LRP5/6, low-density lipoprotein receptor-related protein 5/6, mTORC1, mammalian target of rapamycin complex 1; eIF-4E, eukaryotic initiation factor 4E.
Essentials.
Polyphosphate (polyP) activates mTOR but its role in Wnt/β-catenin signaling is not known.
PolyP-mediated cyclin D1 expression (β-catenin target gene) was monitored in endothelial cells.
PolyP and boiled platelet-releasates induced the expression of cyclin D1 by similar mechanisms.
PolyP establishes crosstalk between mTOR and Wnt/β-catenin signaling in endothelial cells.
Acknowledgments
We thank Stephanie A. Smith from University of Illinois, Urbana for preparing the platelet releasates and Audrey Rezaie for proofreading the manuscript.
Funding Sources
This research was partly supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health HL 101917 and HL 62565 to A.R.R.
Footnotes
Addendum
S. M. Hassanian designed, performed experiments and wrote the manuscript. A. Ardeshirylajimi and P. Dinarvand performed experiments. A. R. Rezaie supervised the project and wrote the manuscript.
Disclosure of Conflict of Interests
The authors declare no competing financial interest.
Supporting Information
The source of reagents can be found in the online version of this article.
References
- 1.Kornberg A, Rao NN, Ault-Riche D. Inorganic polyphosphate: essential for growth and survival. Annu Rev Biochem. 2009;78:605–647. doi: 10.1146/annurev.biochem.77.083007.093039. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- 3.Morrissey JH, Choi SH, Smith SA. Polyphosphate: an ancient molecule that links platelets, coagulation, and inflammation. Blood. 2012;119:5972–5979. doi: 10.1182/blood-2012-03-306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renné T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SA, Morrissey JH. Polyphosphate as a general procoagulant agent. J Thromb Haemost. 2008;6:1750–1756. doi: 10.1111/j.1538-7836.2008.03104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galasso A, Zollo M. The Nm23-H1-h-Prune complex in cellular physiology: a 'tip of the iceberg' protein network perspective. Mol Cell Biochemistry. 2009;329:149–159. doi: 10.1007/s11010-009-0115-4. [DOI] [PubMed] [Google Scholar]
- 7.Tammenkoski M, Koivula K, Cusanelli E, Zollo M, Steegborn C, Baykov AA. Human metastasis regulator protein H-prune is a short-chain exopolyphosphatase. Biochemistry. 2008;47:9707–9713. doi: 10.1021/bi8010847. [DOI] [PubMed] [Google Scholar]
- 8.Shiba T, Nishimura D, Kawazoe Y, Onodera Y, Tsutsumi K, Nakamura R, Ohshiro M. Modulation of mitogenic activity of fibroblast growth factors by inorganic polyphosphate. J Biol Chem. 2003;278:26788–26792. doi: 10.1074/jbc.M303468200. [DOI] [PubMed] [Google Scholar]
- 9.Hernandez-Ruiz L, Gonzalez-Garcia I, Castro C, Brieva JA, Ruiz FA. Inorganic polyphosphate and specific induction of apoptosis in human plasma cells. Haematologica. 2006;91:1180–1186. [PubMed] [Google Scholar]
- 10.Bae JS, Lee W, Rezaie AR. Polyphosphate elicits pro-inflammatory responses that are counteracted by activated protein C in both cellular and animal models. J Thromb Haemost. 2012;10:1145–1151. doi: 10.1111/j.1538-7836.2012.04671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarvand P, Hassanian SM, Qureshi SH, Manithody C, Eissenberg JC, Yang L, Rezaie AR. Polyphosphate amplifies proinflammatory responses of nuclear proteins through interaction with receptor for advanced glycation end products and P2Y1 purinergic receptor. Blood. 2014;123:935–945. doi: 10.1182/blood-2013-09-529602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassanian SM, Dinarvand P, Smith SA, Rezaie AR. Inorganic polyphosphate elicits proinflammatory responses through activation of mTOR complexes 1 and 2 in vascular endothelial cells. J Thromb Haemost. 2015;5:860–871. doi: 10.1111/jth.12899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Fraley CD, Faridi J, Kornberg A, Roth RA. Inorganic polyphosphate stimulates mammalian TOR, a kinase involved in the proliferation of mammary cancer cells. Proc Natl Acad Sci (USA) 2003;100:11249–11254. doi: 10.1073/pnas.1534805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 15.Vigneron F, Dos Santos P, Lemoine S, Bonnet M, Tariosse L, Couffinhal T, Duplaà C, Jaspard-Vinassa B. GSK-3β at the crossroads in the signalling of heart preconditioning: implication of mTOR and Wnt pathways. Cardiovasc Res. 2011;1:49–56. doi: 10.1093/cvr/cvr002. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;3:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi-Yanaga F, Sasaguri T. GSK-3beta regulates cyclin D1 expression: a new target for chemotherapy. Cell Signal. 2007;4:581–589. doi: 10.1016/j.cellsig.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 19.Baldin VJ, Lukas MJ, Marcote MP, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes & Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 20.Diehl JA, Cheng M, Roussel MF, Sherr CJ. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes & Dev. 1998;15:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Smith SA, Morrissey JH. Polyphosphate is a cofactor for activation of factor XI by thrombin. Blood. 2011;118:6963–6970. doi: 10.1182/blood-2011-07-368811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassanian SM, Dinarvand P, Rezaie AR. Adenosine regulates the proinflammatory signaling function of thrombin in endothelial Cells. J Cell Physiol. 2014;229:1292–1300. doi: 10.1002/jcp.24568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soutar MP, Kim WY, Williamson R, Peggie M, Hastie CJ, McLauchlan H, Snider WD, Gordon-Weeks PR, Sutherland C. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. Neurochem. 2010;114:974–983. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- 24.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Ryder J, Su Y, Zhou Y, Liu F, Ni B. FRAT1 peptide decreases Abeta production in swAPP(751) cells. FEBS Lett. 2003;553:347–350. doi: 10.1016/s0014-5793(03)01042-1. [DOI] [PubMed] [Google Scholar]
- 26.Hahne G, Grossmann TN. Direct targeting of β-catenin: Inhibition of protein-protein interactions for the inactivation of Wnt signaling. Bioorg Med Chem. 2013;21:4020–4026. doi: 10.1016/j.bmc.2013.02.050. [DOI] [PubMed] [Google Scholar]
- 27.Holmström KM, Marina N, Baev AY, Wood NW, Gourine AV, Abramov AY. Signalling properties of inorganic polyphosphate in the mammalian brain. Nat Commun. 2013;4:1362. doi: 10.1038/ncomms2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci (USA) 2008;105:17414–17419. doi: 10.1073/pnas.0809136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner TP, Amrhein N, Freimoser FM. Specific localization of inorganic polyphosphate (polyP) in fungal cell walls by selective extraction and immunohistochemistry. Fungal Genet Biol. 2007;44:845–852. doi: 10.1016/j.fgb.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Fritz G. RAGE: a single receptor fits multiple ligands. Trends Biochem Sci. 2011;36:625–632. doi: 10.1016/j.tibs.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 34.Lata K, Mukherjee TK. Knockdown of receptor for advanced glycation end products attenuate 17α-ethinyl-estradiol dependent proliferation and survival of MCF-7 breast cancer cells. Biochim Biophys Acta. 2014;1840:1083–1091. doi: 10.1016/j.bbagen.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 35.Radia AM, Yaser AM, Ma X, Zhang J, Yang C, Dong Q, Rong P, Ye B, Liu S, Wang W. Specific siRNA targeting receptor for advanced glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci. 2013;14:7959–7978. doi: 10.3390/ijms14047959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik P, Chaudhry N, Mittal R, Mukherjee TK. Role of receptor for advanced glycation end products in the complication and progression of various types of cancers. Biochim Biophys Acta. 2015;1850:1898–1904. doi: 10.1016/j.bbagen.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Kuhla A, Ludwig SC, Kuhla B, Münch G, Vollmar B. Advanced glycation end products are mitogenic signals and trigger cell cycle reentry of neurons in Alzheimer's disease brain. Neurobiol Aging. 2015;36:753–761. doi: 10.1016/j.neurobiolaging.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 38.Kang R, Livesey KM, Zeh HJ, 3rd, Lotze MT, Tang D. Metabolic regulation by HMGB1-mediated autophagy and mitophagy. Autophagy. 2011;7:1256–1258. doi: 10.4161/auto.7.10.16753. [DOI] [PubMed] [Google Scholar]
- 39.Verma N, Manna SK. Advanced glycation end products (AGE) potently induce autophagy through activation of RAF kinase and nuclear factor kB (NF-kB) J Biol Chem. 2016;291:1481–1491. doi: 10.1074/jbc.M115.667576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su Z, Wang T, Zhu H, Zhang P, Han R, Liu Y, Ni P, Shen H, Xu W, Xu H. HMGB1 modulates Lewis cell autophagy and promotes cell survival via RAGE-HMGB1-Erk1/2 positive feedback during nutrient depletion. Immunobiology. 2015;220:539–544. doi: 10.1016/j.imbio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Xie J, Méndez JD, Méndez-Valenzuela V, Aguilar-Hernández MM. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cell Signal. 2013;25:2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Bae JS, Rezaie AR. Activated protein C inhibits high mobility group box 1 signaling in endothelial cells. Blood. 2011;118:3952–3959. doi: 10.1182/blood-2011-06-360701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jimenez-Nuñez MD, Moreno-Sanchez D, Hernandez-Ruiz L, Benítez-Rondán A, Ramos-Amaya A, Rodríguez-Bayona B, Medina F, Brieva JA, Ruiz FA. Myeloma cells contain high levels of inorganic polyphosphate which is associated with nucleolar transcription. Haematologica. 2012;97:1264–1271. doi: 10.3324/haematol.2011.051409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dedkova EN, Blatter LA. Role of β-hydroxybutyrate, its polymer poly-β-hydroxybutyrate and inorganic polyphosphate in mammalian health and disease. Front Physiol. 2014;5:260. doi: 10.3389/fphys.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 46.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiao M, Sheng S, Pardee AB. Metastasis and AKT activation. Cell Cycle. 2008;7:2991–2996. doi: 10.4161/cc.7.19.6784. [DOI] [PubMed] [Google Scholar]
- 48.Rosenwald IB, Kaspar R, Rousseau D, Gehrke L, Leboulch P, Chen JJ, Schmidt EV, Sonenberg N, London IM. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J Biol Chem. 1995;270:21176–21180. doi: 10.1074/jbc.270.36.21176. [DOI] [PubMed] [Google Scholar]
- 49.Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, Schmidt EV. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol. 1993;13:7358–7363. doi: 10.1128/mcb.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature. 1990;345:544–547. doi: 10.1038/345544a0. [DOI] [PubMed] [Google Scholar]