Abstract
Epilepsy has remained a significant social concern and financial burden globally. Current therapeutic strategies are based primarily on neurocentric mechanisms that have not proven successful in at least a third of patients, raising the need for novel alternative and complementary approaches. Recent evidence implicates glial cells and neuroinflammation in pathogenesis of epilepsy with the promise of targeting these cells to complement existing strategies. Specifically, microglial involvement, as a major inflammatory cell in the epileptic brain, has been poorly studied. In this review, we highlight microglial reaction to experimental seizures, discuss microglial control of neuronal activities, and propose the functions of microglia during acute epileptic phenotypes, delayed neurodegeneration and aberrant neurogenesis. Future research that would help fill in the current gaps in our knowledge including epilepsy-induced alterations in basic microglial functions, neuro-microglial interactions during chronic epilepsy, and microglial contributions to developmental seizures. Studying the role of microglia in epilepsy could inform therapies to better alleviate the disease.
Keywords: Microglia, Epilepsy, Seizures, Kainic acid, Pilocarpine
[1] Epilepsy: A Global Neurological Disorder
Epilepsy is a term used to describe a spectrum of neurological disorders in which there is abnormal hypersynchrony of neuronal activity (Fisher et al. 2005). The disorder affects between 50 - 65 million people globally (Thurman et al. 2011), including both children and the elderly with a myriad of etiologies of known (such as genetic risk factors) and unknown factors. Indeed epileptic seizures occur in other neurological conditions being comorbid with stroke and traumatic brain injury (Temkin 2009). Moreover, because of the reorganization of neural circuits and activities in the brain in response to seizures, patients frequently experience cognitive, psychiatric and mood disorders (Jensen 2011). Furthermore, epilepsy has been reported to exhibit increased mortality of 2-10 years earlier than the general population (Gaitatzis et al. 2004). Thus, epilepsy is a significant health concern for the human population. There is, therefore, a critical need for the development of appropriate strategies to ameliorate the progression and / or limit the detrimental consequences of the disease.
Regarding the mechanisms underlying epilepsy, it is generally assumed that because of the resulting hyperactivity in neurons during the condition, there is an imbalance wherein excitatory neurotransmission predominantly through glutamatergic signaling is increased and inhibitory neurotransmission predominantly through GABA-ergic signaling is decreased (Dalby and Mody 2001; Sharma et al. 2007). Despite the widespread acknowledgement of these mechanisms, therapeutic antiepileptic strategies targeting these mechanisms have proved insufficient in a significant proportion of patients (Kwan and Brodie 2006). In recent years, however, a role for inflammation and inflammatory mediators has become increasingly appreciated and is a focus of current research (Choi and Koh 2008; Vezzani et al. 2013).
Our current understanding of epileptic mechanisms have been especially informed by studies from human patients with epilepsy (including examinations of excised tissues) and experimental epilepsy models developed primarily in rodents. Although several such epilepsy models have been developed, the most ubiquitous models include chemically-induced models using kainic acid or pilocarpine, which is sometimes coupled with a lithium chloride pre-treatment (Kandratavicius et al. 2014; Leite et al. 2002). Both chemoconvulsants induce hippocampal sclerosis, a feature that is prominent in clinical epilepsy. These models are now recognized to mimic salient histopathological and clinical features of human mesial temporal lobe epilepsy (MTLE), the most common form of epilepsy in adults. Kainic acid is a glutamate analog that preferentially activates kainate glutamate receptor subtypes (Ben-Ari and Cossart 2000) while pilocarpine is a muscarinic receptor agonist (Vezzani 2009). Various kainic acid model of seizures are employed including intraperitoneal or intracerebral (such as cerebroventricular, striatal, hippocampal and amygdala) injections. Kainic acid in these different brain regions leads to hypersychonized excitatory neuronal activity which may persist, if prolonged, to neuronal death. Although a complex interplay of kainate and non-kainate glutamate receptors have been implicated in the mechanism of kainic acid induced seizures, the CA3 region of the hippocampus is recognized to be extremely susceptible due to a high density of specific kainate receptors in this region (Levesque and Avoli 2013). The pilocarpine model is usually induced by intraperitoneal delivery though it is sometimes coupled with a lithium pre-treatment so lower the threshold for seizure-induction. Because muscarinic acetylcholine receptor receptor 1 (M1) knockout mice are deficient in pilocarpine-induced seizures, this model of seizures must be triggered by the activation of this muscarinic receptor subtype (Hamilton et al. 1997). Downstream of M1 receptor activation, the homeostatic balance of neuronal excitation-inhibition is tipped towards increased excitability presumably at least in part by an increase in glutamate release and sustained NMDA receptor activation (Priel and Albuquerque 2002; Smolders et al. 1997). Although beyond the scope of this review, further details of the mechanics and precise pathological features of these models can be found in several excellent reviews of the kainic acid and pilocarpine models of experimental seizures / epilepsy (Curia et al. 2008; Levesque and Avoli 2013; Levesque et al. 2016; Turski et al. 1989).
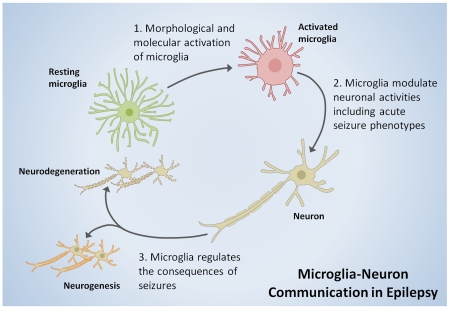
In the following pages, we discuss the literatures highlighting (i) microglial response to epilepsy at both morphological and molecular levels, (ii) microglial regulation of neuronal activities during epilepsy, (iii) microglial contributions to acute seizure phenotypes and seizure-induced neurodegeneration, and (iv) conclude with suggesting areas for future research to understand the role of microglia in epilepsy.
[2] Microglial Morphological and Molecular Activation in Response to Seizures
Microglia are highly adaptable glial cells of the central nervous stem (CNS) that are now recognized to play important roles in the healthy CNS as well as during various CNS pathologies (Morris et al. 2013; Nayak et al. 2014; Ransohoff and Perry 2009; Tremblay et al. 2011; Zhuo et al. 2011). Microglia constitutively scan the brain and spinal cord (Davalos et al. 2005; Dibaj et al. 2010; Nimmerjahn et al. 2005) where they interact physically with neurons (Baalman et al. 2015; Li et al. 2012; Tremblay et al. 2010; Wake et al. 2009) and modulate neurotransmission (Hoshiko et al. 2012; Ji et al. 2013; Li et al. 2012; Paolicelli et al. 2011; Pascual et al. 2012; Zhang et al. 2014a). During development, microglia prune synapses (Paolicelli et al. 2011; Schafer et al. 2012), participate in the clearance of apoptotic neurons (Ahlers et al. 2015; Marin-Teva et al. 2004; Sierra et al. 2010), regulate neurogenesis as well as oligodendrogenesis (Shigemoto-Mogami et al. 2014), promote neural precursor cell development (Arno et al. 2014; Cunningham et al. 2013), and enhance neuronal survival (Ueno et al. 2013). They are thus critical in the early wiring of the central nervous system (Squarzoni et al. 2014; Zhan et al. 2014). In the mature brain, although microglia do not acutely respond to neuronal stimulations (Wu and Zhuo 2008), microglia are important in the processes required for learning and memory (Parkhurst et al. 2013), synaptic plasticity and general cognitive function (Rogers et al. 2011; Sipe et al. 2016). During disease, they undergo activation where their functions are hotly debated as neurotoxic or neuroprotective agents depending on the precise timing and disease context (Block et al. 2007; Hanisch and Kettenmann 2007; Morris et al. 2013; Wu 2014; Wu et al. 2012). Thus, microglia are very relevant to both nervous system physiological function and pathophysiological processes. However, microglial roles in epilepsy have been less studied. Therefore, in this section, we consider some of the evidence for microglial activation in response to seizures or epilepsy at both morphological and molecular levels (Figure 1).
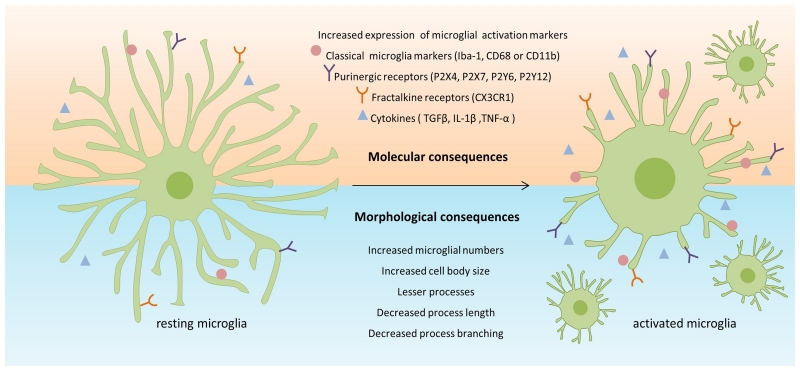
Figure 1. Schematic diagram depicting molecular and morphological changes of microglial activation following seizures.
The molecular consequences of seizures on microglial activation (above) include changes in the expression pattern of an array of microglial molecules such as classical microglial markers, purinergic receptors, fractalkine receptor and cytokines. The morphological consequences of seizures on microglial activation (below) include changes in microglial cell body size, process length, process numbers and complexity of branching. Please refer to “[2] Microglial Morphological and Molecular Activation in Response to Seizures” for references.
[2.1] Microglial Activation: Morphological Considerations
As with other neurological diseases, there are paramount observations with microglia morphological activation in epilepsy. Autopsy analysis from control individuals and human patients with intractable seizures revealed a 3- and 11-fold increase in microglial reactivity to major histocompatibility antigen in the CA3 and CA1 regions of the epileptic hippocampus, respectively (Beach et al. 1995). Similarly, increased microglial immunoreactivity was observed in patients with focal cortical dysplasia known to trigger epilepsy. Moreover, a correlation was evident between the duration and frequency of epilepsy and the degree of microglial activation as shown by the immunohistochemical expression HLA-DR, an MHC class II cell surface receptor often used to identify activated microglia (Boer et al. 2006). These observations indicate that persistent microglial reactivity is a clinical feature of epilepsy.
Consistent with human studies, experimentally-induced seizures in rodents has revealed marked morphological changes of microglia. For example, during kainic acid-induced seizures elicited by intracerebral drug delivery, there is a delayed (up to 48 hours) increase in hippocampal microglial numbers following drug delivery which was presumed to result from ensuing kainic acid-induced neuronal excitotoxic injury in mice (Andersson et al. 1991). A more recent study conducted in transgenic mice that express GFP selectively in microglia corroborated these findings and microglial morphological activation (including shortening and thickening of their processes concomitant with an enlargement of their somata) was confirmed at 24 - 48 hours following intraperitoneal delivery of kainic acid, a time point when neuronal injury was detectable (Avignone et al. 2008). Together, these studies indicated that microglial activation is delayed and may result as a secondary reaction to neuronal injury following the initial seizures.
Despite these observations, there are studies suggested that microglial reactivity following seizures occurs much earlier than 24-48 hours following kainic acid treatment. By 8 hours (but not 4 hours), a time point at which overt neuronal injury is absent, microglial activation as detected by lectin staining was reported and suggested to proceed along propagation pathways of the hippocampal seizures (Taniwaki et al. 1996). These results indicate that microglia may be activated, not merely by delayed neuronal degeneration, but by neuronal hyperactivity that precedes neuronal demise. Another study in rats showed that cortical microglia exhibited a “bushy” appearance with seemingly increased ramifications within 3 hours of intraperitoneal drug delivery of kainic acid introducing the concept of hyper-ramified microglia during acute seizures (Rappold et al. 2006). In all these studies, microglial reactivity was always reported only after the initiating seizures were completed.
In our recent study, we investigated the acute effects of kainic acid-induced seizures on microglial morphologies in the CA1 region of the hippocampus (Eyo et al. 2014). Interestingly, unlike the typical sequelae of microglial activation consisting of a reduction in microglial process ramifications, the acute microglial response to seizures caused an increase in microglial process numbers within 40 minutes of kainic acid treatment by both intraperitoneal and intracerebroventricular delivery at a time when seizure manifestations are elaborate. Furthermore, we showed that this early microglial response is dependent on neuronal NMDA receptor activation. In addition, these observations were recapitulated in real time by bath application of glutamate / NMDA to acute brain slices in our study (Eyo et al. 2014) and independently reported by MacVicar’s group (Dissing-Olesen et al. 2014). These results therefore suggest that microglia respond rapidly (within 5-10 minutes) to acute neuronal hyperactivity triggered by activation of NMDA glutamate receptors during seizures in vivo. The mechanism involves the coupling of neuronal NMDA receptor activation to neuronal ATP release via a pannexin 1-independent pathway that remains to be determined. Released ATP stimulates microglial P2Y12 receptors leading to process extension / outgrowth (Dissing-Olesen et al. 2014; Eyo et al. 2014).
Similar to these observations with seizures induced by kainic acid, pilocarpine-induced seizures have been reported to induce microglial morphological activation within between 3 hours to 3 days in several brain regions following intraperitoneal drug treatment (Borges et al. 2003; Kang et al. 2006; Rosell et al. 2003). These studies also indicate that microglia are hyper-ramified (thickened processes but increased branch points) as early as 3 hours after pilocarpine treatment. At later time points following pilocarpine treatment, microglia exhibited hypertrophic morphology denoted as typical activation (Jung et al. 2009; Shapiro et al. 2008). As with the kainic acid model, microglial reactivity increased following pilocarpine exposure by 24 hours. Taken together, both widely used experimental models of epilepsy indicate that microglia respond rapidly to experimental seizures in a manner that does not merely result from neuronal demise or injury since their activation represented by early morphological hyper-ramification precedes such a demise. In addition, microglial reaction to seizures persists for days to weeks, but with different morphological characteristics.
Although microglial surveillance is known to be critical for microglial functions, the effect of seizures on microglial surveillance have only been addressed by a few studies and both were performed in excised brain slices. In the first study, microglial chemotactic abilities were increased following kainic acid seizures presumably as a result of upregulation of P2Y12 receptor expression (Avignone et al. 2008). A follow up study confirmed this initial study but also showed that kainic acid-induced seizures did not alter their basal surveillance ability (Avignone et al. 2015). In a recent study, we described a novel phenomenon by which microglia and neurons interact during epileptiform activity induced by depletion of extracellular calcium. We have referred to this phenomenon as “microglial process convergence” and is characterized by spontaneous focal targeting of microglial processes towards neuronal dendrites that seem to be independent of metabolic astrocytic activity (Eyo et al. 2015). Although, we confirmed that the phenomena occurs both in excised and intact brain tissue, whether the interaction occurs in the epileptic brain, as well as, the functional consequence of these interactions will need to be determined by future studies.
Resident microglia and peripheral monocytes/macrophages share most common molecular markers (Hickman et al. 2013). Therefore, the increase in microglial-macrophage numbers following both kainic acid and pilocarpine treatment may result from one or a combination of (i) migration / infiltration from other sites such as the blood [a phenomena that does not occur in healthy brain (Ajami et al. 2011; Ajami et al. 2007)], (ii) proliferation of the resident pool, or (iii) de novo generation of microglia from neural progenitor cells. The de novo generation of microglia from nestin-positive progenitors is a relatively new observation that has so far only been described following widespread microglia depletion (Elmore et al. 2014). Whether such a mechanism is relevant during seizures has yet to be evidenced.
Regarding the possibility of infiltration, most studies have failed to distinguish resident microglia and infiltrating cells after seizures. Recent studies, however, suggest that peripheral cells do enter the brain following both pilocarpine and kainic acid-induced seizures in mice as well as in human epileptic brain tissues without permanently taking up residence therein (Bellavance et al. 2015; Longo et al. 2010; Ravizza et al. 2008; Zattoni et al. 2011). These results indicate that monocyte infiltration may at least in part account for the increased microglia-macrophage numbers following epilepsy. Indeed, seizure studies using chimeric PU.1gfp donor marrow cells transplanted into wildtype mice revealed an increase in engraftment of donor cells as early as 6 hours after kainic acid-induced seizures suggesting infiltration of blood-derived monocytes (Bellavance et al. 2015). A similar chimeric strategy has been employed with bone marrow transplants from generic GFP mice and peripheral cells were identified as early as 2 hours after pilocarpine-induced seizures (Longo et al. 2010). However, we have to caution that experimental artifacts of BBB breakage from irradiation/bone-marrow transplantation may complicate the observation of monocyte infiltration after epilepsy (Ajami et al. 2007). In addition to monocyte infiltration, CD45+ CD3+ T-cells, but not CD45+ B220+ B-cells, were detected in both the human and kainic acid-induced mouse epileptic hippocampus that were not found in control tissues. These cells were cytotoxic in nature being CD8+. Similarly, Gr-1+ neutrophils, absent in the naïve brain, were found in the epileptic brain (Zattoni et al. 2011). Together, these data suggest that resident microglia undergo robust morphological activation following seizures as well as proliferation accompanied by infiltration of circulating cells including monocytes, T cells and neutrophils.
[2.2] Microglial Activation: Molecular Considerations
Although it is widely accepted that microglial activation occurs following seizures, less is known about the specific molecules of microglial activation other than classical activation markers like Iba-1, CD68 or CD11b. Currently, data is available regarding the fractalkine receptor, purinergic receptors, certain proteases and cytokine expression on microglia in this experimental context. Investigation into the molecular signatures for microglia in epilepsy will improve our understanding of not only microglial activation but also their potential effector mechanisms in the pathogenesis of epilepsy.
Fractalkine is a chemotactic cytokine predominantly expressed by neurons, whose receptor is selectively expressed on microglia in the CNS (Cardona et al. 2006). Fractalkine is upregulated in the serum and cerebrospinal fluid of epileptic patients as well as in a lithium-pilocarpine rat model (Ali et al. 2015). Furthermore, a corresponding increase in fractalkine receptor expression is detected between 1-6 hours and begins to decline by 3 days following seizures (Ali et al. 2015; Yeo et al. 2011). However, following intrastriatal kainic acid treatment, fractalkine receptor expression remained unchanged in microglia despite evident neuronal loss (Hughes et al. 2002). Thus, the regulation and expression of the microglial fractalkine receptor needs to be further clarified in these experimental conditions.
In addition to fractalkine signaling, purinergic signaling is now known to be critical in epileptogenesis. An upregulation of purinergic receptors have been confirmed on microglia following experimental seizures. Rapold et. al., 2006 first reported an increased immunohistochemical expression of the P2X7 receptor predominantly in microglia in rats following kainic acid treatment. Similar results have been confirmed in mice by quantitative PCR and functional electrophysiology (Avignone et al. 2008). Like the P2X7 receptor, upregulated microglial P2X4 expression has been confirmed in the mouse hippocampus following seizures (Avignone et al. 2008; Ulmann et al. 2013). In a similar manner, both P2Y6 and P2Y12 mediated responses and mRNA are increased in hippocampal microglia following kainic-acid induced seizures (Avignone et al. 2008).
In addition to modulation of specific microglial cell surface receptors, seizures also upregulate microglial cytokine expression including TGFβ (Morgan et al. 1993), IL-1β (Eriksson et al. 2000; Vezzani et al. 1999) and TNF-α (Turrin and Rivest 2004). Finally, regarding microglial proteases, cathepsin B, D and S are increased following seizures (Akahoshi et al. 2007; Banerjee et al. 2015). Together, these studies highlight the dramatic upregulation of microglial receptors, cytokines and proteases following seizures.
Taking a cue from macrophage studies, microglial researchers have identified microglial polarization during disease: the M1 (classical) and M2 (alternative) activation states (Boche et al. 2013; Colton 2009; Perry et al. 2010). Although this M1/M2 microglia has been studied in several brain diseases such as Alzheimer’s Disease (Tang and Le 2016; Varnum and Ikezu 2012), ischemia (Frieler et al. 2011; Hu et al. 2012), multiple sclerosis (Mikita et al. 2011; Peferoen et al. 2015; Vogel et al. 2013) and amyotrophic lateral sclerosis (Henkel et al. 2009; Liao et al. 2012), less has been done to investigate this microglial polarization during seizures / epilepsy. One recent study reported that M1 markers (IL-1β, TNFα, CD16, CD86,IL-6, IL-12, Fc receptors 16 and CD86) were upregulated in both the pilocarpine and kainic acid models (Benson et al. 2015). Interestingly, there was a transition from M1 to M2 markers (Arg1, Ym1, FIZZ-1, CD206, IL-4, and IL-10) after pilocarpine treatment by 3 days of seizures (Benson et al. 2015). Here, the authors suggested that the underlying mechanisms for this transition may result from peripheral inflammation since pilocarpine was administered systemically by the intraperitoneal route while kainic acid was only administered directly to the hippocampus. This peripheral inflammation in the pilocarpine model may in turn alter the brain environment and thus microglial cells. Further work is required to ascertain the precise contributions and roles of the differently polarized microglia to the progression of epilepsy. However, although this M1/M2 polarization has been suggested for microglial phenotypes, caution has to be employed with this type of simplistic nomenclature as the validity characterizing microglia and macrophages in this polarized manner has recently been questioned (Murray et al. 2014; Prinz et al. 2014).
[3] Microglial Regulation of Neuronal Activities in Epilepsy
Since seizures and epileptic phenotypes are rooted in aberrations in neuronal function and microglia are homeostatic regulators of the CNS (Hanisch and Kettenmann 2007; Tremblay 2011), microglial contributions to seizure phenotypes and consequences can be expected. Indeed, several lines of evidence have shown that microglia might be able to regulate neuronal activities in healthy and epileptic brain (Figure 2).
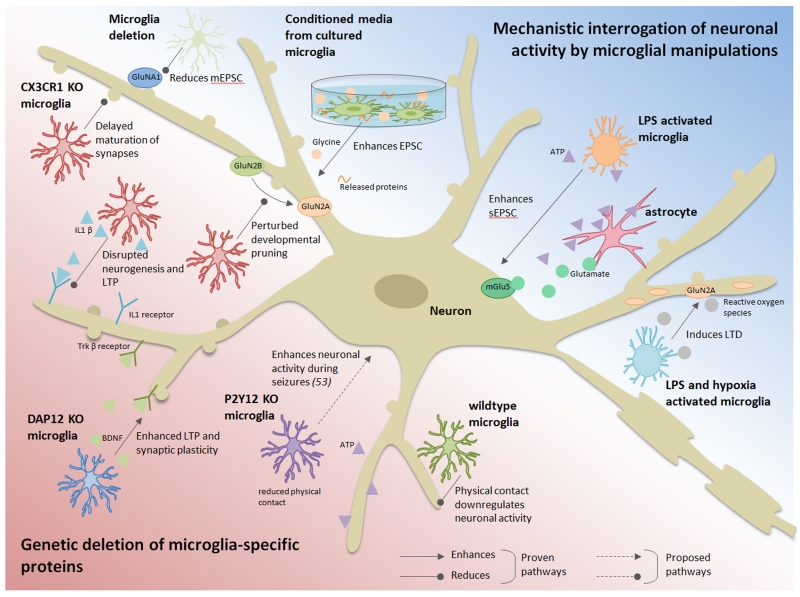
Figure 2. Microglial influence on neuronal activity.
This schematic summarizes the current literature on the effects of microglia on neuronal activity. The studies were broadly classified into two categories based on the experimental approach: (1) genetic deletion of microglial-specific proteins (lower left, pink) and (2) mechanistic interrogation of neuronal activity by microglial manipulations (upper right, blue). Arrowheads indicate an enhancing effect, while rounded head indicate an inhibitory effect. Proven pathways and proposed pathways are represented by solid and dashed lines, respectively. Please refer to “[3] Microglial Regulation of Neuronal Activities in Epilepsy” for references.
[3.1] Evidence for Microglial Regulation of Neuronal Activities
Mounting evidences suggest delicate interactions between microglia and neurons by which microglia can modulate neuronal activities (Bechade et al. 2013; Eyo and Wu 2013). Evidence for this comes primarily from two approaches: (i) genetic deletion of microglial-specific proteins and (ii) mechanistic interrogation of neuronal activity by microglial manipulations.
Evidence from the genetic approach has mainly been provided for by fractalkine signaling. The fractalkine receptor is exclusively expressed on microglial cells in the CNS parenchyma (Cardona et al. 2006). Since neurons express fractalkine, it provides an interesting signaling axis for communication between microglia and neurons that has been extensively investigated (Lauro et al. 2015; Limatola and Ransohoff 2014; Paolicelli et al. 2014). Interestingly, in mice lacking this microglial receptor, there is a delayed functional maturation of neuronal synapses and consequently synaptic plasticity is perturbed. Deficiency of fractalkine signaling was mechanistically linked to impairment in microglial colonization of the CNS, the subsequent lack of developmental pruning, as well as, perturbation of the developmental switch from GluN2B to GluN2A NMDA subunits at the synapse (Hoshiko et al. 2012; Paolicelli et al. 2011). Furthermore, fractalkine receptor deficiency has also been linked to disruptions in neurogenesis, neural connectivity, and long term potentiation (LTP, the cellular basis for learning and memory), with corresponding defects in fear and motor learning (Rogers et al. 2011) as well as social behaviors (Zhan et al. 2014). Similar to the fractalkine receptor, DAP12 is a microglia-specific protein whose genetic disruption results in enhanced LTP and thus synaptic plasticity. The basis for these changes were suggested to occur as a result of alterations in glutamate receptor content at synapses as well as through microglial brain-derived neurotrophic factor (BDNF) (Roumier et al. 2004). Indeed, microglial BDNF has also been otherwise shown to be relevant for learning behaviors (Parkhurst et al. 2013) and development of pathological pain (Coull et al. 2005; Trang et al. 2009).
A second more widely used approach to revealing microglial control of neuronal activities has involved probing neurotransmission with microglial-specific manipulations. This has especially been shown in both dissociated cultures as well as in in tissue contexts. Microglial conditioned media enhanced excitatory postsynaptic potentials and currents in cultured neurons and in acute hippocampal slices through released proteins and / or glycine from microglia (Hayashi et al. 2006; Moriguchi et al. 2003). In contrast, by combining microglial depletion strategies in organotypic cultures with exogenous microglial addition to neuronal cultures, a recent study showed that microglia regulate synaptic transmission by decreasing miniature excitatory postsynaptic currents which are due to microglial pruning synapses containing GluA1 receptors (Ji et al. 2013).
[3.2] Microglial Regulation of Neuronal Activities in Inflammation and Epilepsy
Further evidence of microglial control of neuronal activities has been obtained from studies using lipopolysaccharide (LPS), a component of the outer layer of the cell membrane of gram negative bacteria. Detection of LPS in the brain is predominantly carried out by Toll-like receptor 4 (TLR4) which are mostly expressed by microglia in the parenchyma of the healthy brain (Zhang et al. 2014b). This expression pattern suggests that microglia are the direct target of LPS signaling. Of relevance here, LPS application induced increases in spontaneous excitatory postsynaptic currents (EPSCs) in hippocampal neurons from brain slices in a microglial-dependent manner (Pascual et al. 2012). The mechanism required microglial TLR4 activation inducing ATP release and astrocytes P2Y1 receptor activation, which in turn regulated neuronal EPSC frequency by glutamate release to activate presynaptic neuronal mGluR5. Consistently, in vivo application of LPS also increases neuronal excitability and seizures through TLR4 activation and subsequent IL-1β signaling primarily through microglia (Rodgers et al. 2009). Interestingly, LPS-induced microglial activation was recently shown to dampen synaptic transmission in a hypoxic context where coupling LPS treatment with hypoxic treatment induced long term depression (LTD) (Zhang et al. 2014a). The mechanism required microglial complement receptor function but [unlike the previous study (Pascual et al. 2012)] did not involve TLR4 activation. Downstream of complement activation, superoxide production was induced through activation of NADPH oxidase that subsequently led to AMPA receptor internalization underlying observed LTD (Zhang et al. 2014a).
In the mammalian brain, neuronal NMDA receptor activation elicited robust microglial process outgrowth / extension in a microglial P2Y12-dependent manner which increased microglial contact of neuronal elements (Dissing-Olesen et al. 2014; Eyo et al. 2014). Although the precise function of this contact was not definitively determined (as discussed in more detail below), an abrogation of the microglial response by genetic depletion of the P2Y12 receptor correlated with a worsened seizure phenotype (Eyo et al. 2014). These results suggest a neuroprotective action of P2Y12-dependent microglial contact of neurons that may happen in epilepsy. This neuroprotective hypothesis for microglial contact of neurons is supported by evidence from the developing zebrafish brain where, during physiological activity, microglial processes made increasing contact with hyperactive neurons and such contact served to downregulate neuronal activity (Li et al. 2012). Together, these studies show that in multiple paradigms and in multiple ways, microglia are capable of regulating neuronal activities including during epilepsy (Figure 2).
[4] Microglial Function in Acute Seizures, Neurodegeneration, and Neurogenesis in Epilepsy
Microglia possess both neurotoxic and neuroprotective potential in the context of CNS diseases (Ransohoff and Perry 2009). As discussed above, microglial reactivity during seizures occurs prior to overt neurodegeneration raising questions as to the functional significance of microglial activation in response to the initial events precipitating the seizures. Microglial roles in two aspects of epilepsy can be distinguished: the initial intensity of acute seizures and the subsequent delayed neurodegeneration that occurs. Interestingly, recent studies have also found that microglia also play an important role in epilepsy-induced aberrant neurogenesis (Figure 3).
Figure 3. Microglia at different stages after seizures.
This figure highlights three keynote studies that investigated microglial activation at different time points following seizures. In the acute phase (1-3 hours), microglial P2Y12 receptor-mediated process extension attenuated seizure outcome, playing a neuroprotective role. In the sub-acute phase (48-72 hours), fractalkine signaling is one signaling axis that has been identified that mediates microglial activation resulting in neuronal degeneration. Finally, in the chronic phase (several weeks), microglia was shown to be capable of recognizing DNA from degenerating neurons via TLR9 and TLR9 signaling prevented aberrant neurogenesis following seizures. Please refer to “[4] Microglial Function in Acute Seizures, Neurodegeneration, and Neurogenesis in Epilepsy” for references.
[4.1] Microglial Function in Acute Seizures
One approach recently used to determine general microglial roles in experimental epilepsy has involved the ablation of resident microglia followed by the subsequent exposure of animals to a seizure-inducing stimulus. At present, several microglial ablation strategies have been developed: (1) pharmaco-genetically inducible models using either CD11b-HSVTK or CD11b-DTR mice (Duffield et al. 2005; Heppner et al. 2005); (2) exclusively genetic models such as PU.1 and colony stimulating factor 1 receptor (CSF1R) knockout mice (Erblich et al. 2011; McKercher et al. 1996; Scott et al. 1994); and (3) purely pharmacological models with the use of drugs like clodronate (Cunningham et al. 2013; Faustino et al. 2011; Marin-Teva et al. 2004) or PLX3397, a CSFR1 inhibitor (Elmore et al. 2014), to selectively eliminate brain resident microglia. Despite the availability of these ablation techniques, only one study has attempted to investigate microglial roles in pilocarpine-induced epilepsy using an ablation strategy (Mirrione et al. 2010). Although selective microglial ablation in the dorsal hippocampus using CD11b-HSVTK mice tended towards a slightly worsened acute seizure phenotype, this was not significant and led to the suggestion that resting microglia may not play significant roles during acute seizures (Mirrione et al. 2010). However, using this strategy, microglia were found to be neuroprotective following a 24 hour LPS-preconditioning paradigm in the pilocarpine seizure model (Mirrione et al. 2010).
In our recent study, we approached the question of acute microglial roles during epilepsy using a genetic approach wherein a specific microglial receptor, the P2Y12 receptor, known to be selectively expressed in the brain by microglia (Gu et al. 2015; Haynes et al. 2006) is deleted. This receptor is critical for acute microglial chemotactic responses to ATP in the brain (Haynes et al. 2006; Swiatkowski et al. 2016; Wu et al. 2007). Since ATP is also released during experimental seizures (Santiago et al. 2011) and the P2Y12 receptor mediates microglial process extension in epilepsy (Eyo et al. 2014), we investigated whether microglial chemotactic responses to ATP (released presumably from hyperactive neurons) are functionally significant during acute seizures. Interestingly, using both intraperitoneal and intracerebroventricular models of kainic acid-induced seizures, we found a dramatic exacerbation of seizure behaviors in P2Y12 deficient mice. Consistent with a role for ATP in seizure-induced changes in microglial morphology, P2Y12 deficient microglia exhibited reduced primary process numbers in response to kainic acid treatment as compared with wildtype microglia (Eyo et al. 2014). These results are the first to show (i) such an early (within an hour) morphological response of microglial changes during seizures and (ii) a direct involvement of microglia in the progression of the seizure phenotype, presumably at least in part through their morphological dynamics. This latter point, therefore, suggest neuroprotective roles for microglia through this receptor in acute seizure phenotypes.
Increased seizures in P2Y12 knockout mice may be thought to be in conflict with the other study that failed to observe significant effects of microglial depletion on acute seizure phenotypes (Mirrione et al. 2010). Although the reasons for the seeming discrepancy between both studies are not clear, it is possible that (i) the differences might be a result of the different chemoconvulsants (pilocarpine v. kainic acid) used and can be resolved by investigating differences between wildtype and P2Y12 knockout seizure behaviors in response to pilocarpine treatment; (ii) microglia may possess equally detrimental / beneficial roles during acute seizures that may be masked by whole cell ablation but evident through selective genetic ablation of a single receptor (in this case P2Y12) whose function when present, limits the effect of seizures; (iii) the lack of P2Y12 receptor function in the knockouts through development may cause a developmental deficiency in which the neural environment is compromised and thus more susceptible to seizures. This explanation could be addressed with a temporally controlled depletion or pharmacological inhibition of the receptor’s function; and (iv) it is possible that there are some limitations to the method of microglial elimination which could be addressed by employing other methods of microglial depletion (mentioned above) during epilepsy. Also, local inflammation and astrogliosis are concerns for microglia ablation approaches. Evidently, these results suggest that further studies are required to adequately understand microglial roles during acute seizures.
Although we have identified microglial P2Y12 receptors as modulators of epileptic seizure phenotypes, whether other microglial purinergic receptors are also involved is not known. A recent study failed to detect a significant difference between wildtype and P2X4 deficient animals during acute seizures (Ulmann et al. 2013) suggesting that microglial P2X4 receptors do not modulate acute seizure behaviors. Finally, although P2X7 receptor function is proposed to be neurotoxic during epilepsy (Jimenez-Pacheco et al. 2013), the evidence for the selective contribution of microglial specific P2X7 receptors is currently debated.
Regarding roles for other microglial proteins in acute seizures, at least one study in rats suggest that pharmacological manipulation of the CX3CL1-CX3CR1 signaling axis between neurons and microglia may be neurotoxic in pilocarpine-induced seizures (Yeo et al. 2011), an idea that is consistent with other evidence that indicates reduced seizures in young pentylene tetrazole (PTZ)-treated fractalkine receptor knockout mice (Paolicelli et al. 2011). Nevertheless, future studies would have to appropriately ascertain the role of fractalkine signaling in kainic acid- and or pilocarpine-induced acute seizures.
[4.2] Microglial Function in Delayed Neurodegeneration
In addition to acute seizure phenotypes, microglia play important roles in seizure-induced neurodegeneration. Both pilocarpine and kainic acid models of epilepsy result in delayed neurodegeneration beginning at about 24 hours after drug treatment (Curia et al. 2008; Levesque and Avoli 2013). So far, microglial function in chronic neurodegeneration in seizures have largely employed a pharmacological approach. Several studies have indicated that minocycline, a tetracycline-derivative and microglial inhibitor, is protective in several models of rodent (Abraham et al. 2012; Heo et al. 2006; Wang et al. 2012; Wang et al. 2015) and human epilepsy (Nowak et al. 2012). Minocycline’s neuroprotective effects include a reduction in the degree of neurodegeneration that result from the initial insult, mitigation of pro-inflammatory cytokines presumable released from tissue microglia, as well as the subsequent development of spontaneous recurring seizures (SRS) (Wang et al. 2015). An alternative pharmacological approach that has been employed involved the use of macrophage inhibitory factor (MIF) to reduce microglial activation. This approach revealed that under such conditions, intrahippocampal kainic acid-induced neurodegeneration is dramatically reduced (Rogove and Tsirka 1998). The emerging data, therefore, suggest that subsequent to the initial seizures, microglia may play detrimental pro-convulsive roles in epileptogenesis because minocycline (Yrjanheikki et al. 1999) and MIF (Thanos et al. 1993) inhibit microglial activation. However, caution needs to be taken in interpreting the neuroprotective effects of these drugs (especially minocycline) as the precise mechanisms of their action are not clear and may occur directly on neurons independent of microglial effects due to its lack of specificity of cellular action (Domercq and Matute 2004; Huang et al. 2010). Alternative pharmacological and complementary genetic approaches are thus required to adequately ascertain microglial roles in seizure-induced neurodegeneration.
Microglia have also been implicated in seizure-induced neurodegeneration e.g. through the microglial-specific fractalkine receptor. Inhibiting fractalkine signaling through fractalkine receptor antibodies reduced seizure-induced neurodegeneration in the hippocampus (Ali et al. 2015). Interestingly, a recent report also showed that functional maturity of newborn neurons following kainic acid induced seizures was delayed in fractalkine receptor knockouts (Xiao et al. 2015). Although roles for fractalkine signaling have been suggested in epilepsy, other molecular mechanisms, yet to be identified are sure to be involved and should be a focus of future studies. Together, these studies suggest that microglial roles in delayed neurodegeneration following acute seizures may be complex and more stringent approaches to resolve the current disparities in results.
[4.3] Microglial Function in Aberrant Neurogenesis
Neurogenesis is a phenomenon that occurs throughout life in the adult brain and in most cases is thought to be helpful. For example, promoting adult neurogenesis reduces anxiety- and depression-like behaviors in mice (Hill et al. 2015). Moreover expanding the pool of adult-born neurons has been shown to improve the ability to differentiate between similar contexts in mice (Sahay et al. 2011). Thus, the importance of lifelong neurogenesis has been recognized in both regulating mood and cognitive functions (Christian et al. 2014). Recent studies also highlighted the roles for microglia in adult neurogenesis including providing trophic support for neuronal survival, proliferation and differentiation as well as phagocytic clearance of apoptotic neurons as new cells are birthed (Gemma and Bachstetter 2013; Ribeiro Xavier et al. 2015; Sierra et al. 2014; Sierra et al. 2010).
In addition to this ongoing adult neurogenesis, seizures induce aberrant neurogenesis where it is thought to be dysfunctional (Cho et al. 2015). Whether microglia are involved in this seizure-induced aberrant neurogenesis has only begun to be investigated in recent years. Inhibiting microglial activation with minocycline reduced the pilocarpine-induced aberrant neurogenesis while activating microglia with LPS further exacerbated this neurogenesis (Yang et al. 2010). More recently, mechanisms involved in microglial regulation of seizure-induced aberrant neurogenesis have been determined by microglial TLR9 activation following kainic acid seizures (Matsuda et al. 2015). TLR9 senses nucleic acids including DNA released from damaged cells and was demonstrated to reduce aberrant seizure-induced neurogenesis (Matsuda et al. 2015). Together, these results indicate that microglia are critically involved in the modulation of acute seizure phenotypes as well as the delayed consequences of such seizures on neuronal survival and subsequent proliferation (Figure 3).
[5] Future Direction and Conclusions
Current results as discussed above show that microglia are both activated during / following seizures at both morphological and molecular levels and capable of attenuating or exacerbating seizure intensity during and neuronal dysfunction following seizures. Yet, research into microglial function during seizures / epilepsy has not been extensive. In this section, we highlight three poorly studied areas of microglia roles in seizures / epilepsy that warrant future investigation.
Microglia are widely recognized to be highly morphologically dynamic cells but real time in vivo or ex vivo descriptions of microglial dynamics during seizures and subsequent epilepsy are lacking. More importantly, detailed descriptions and the underlying mechanisms governing their cellular interactions with neurons under these conditions are not known. Despite the evidence of increased P2Y12-dependent chemotactic responses following kainic acid-induced seizures (Avignone et al. 2008), the consequence of this increased dynamics on neuronal function remains elusive. Thus, we still do not actually know (i) the real-time dynamics of microglial activities during acute seizures since no high resolution imaging studies exist on this and (ii) the dynamics of the physical interaction between microglia and neuronal elements and (iii) the functional consequence of such cellular interactions of epileptic phenotypes. Future research should be directed towards understanding these questions.
A second line of research that deserves further attention is with regards to microglial function in spontaneous epilepsy. Although chemical convulsants such as kainic acid and pilocarpine are well known to induce seizures acutely, seizures, in themselves, are technically not considered epilepsy. Rather, epilepsy disorders subsequently develop some time following a latent period in these models from days to weeks after the initial insult in the form of spontaneous recurrent seizures [SRS; (Curia et al. 2008; Dudek et al. 2002)]. Microglial roles in these delayed phenotypes that are more reminiscent of clinical epilepsy and results from the underlying re-organization of neuronal networks have not been extensively studied. Following experimental seizures and during SRS, do (and if so how do) microglia modulate neuronal networks? Could these mechanisms, once understood, be targeted in the clinic to ameliorate the consequences of seizures and epilepsy in general? These questions will need to be addressed.
Thirdly, seizures are a relatively common phenomenon in the developing brain and can occur as typically benign “febrile” seizures or more malignant seizure disorders in which some of the underlying mechanisms including inflammatory mechanisms are known (Glass 2014; Nardou et al. 2013; Patterson et al. 2013; Patterson et al. 2014). However, specific microglial involvement has not been determined. For example, the pro-inflammatory cytokine IL-1β promotes febrile seizures (Heida et al. 2009; Yu et al. 2012) and humans with polymorphisms in the IL-1β gene have increased susceptibility to febrile seizures (Kira et al. 2010; Virta et al. 2002). Although microglia are known to release IL-1β, it is not clear that its release under these conditions require or stem from microglia. Therefore, the effect of developmental seizures on microglial functions and the consequence of microglial activity on seizure behaviors need further investigation. Because emerging data suggest that microglia as the brain resident immune cells play crucial roles in neural development (Eyo and Dailey 2013; Hoshiko et al. 2012; Paolicelli et al. 2011; Paolicelli and Gross 2011; Pont-Lezica et al. 2011; Schafer et al. 2012; Schlegelmilch et al. 2011; Squarzoni et al. 2014), microglial roles in developmental seizures should hold promise in advancing new approaches in the treatment of such disorders.
In summary, we have reviewed some of the relevant literature with regards to the role microglia in human and experimental seizure / epilepsy disorders. Microglia show morphological/molecular alterations in response to seizures and in turn regulate neuronal activities under these conditions. This microglia-neuron communications play a critical role in acute seizures, delayed neurodegeneration, and aberrant neurogenesis in experimental models. Finally we highlight three areas that demand further research in the context of microglial roles in epilepsy including: (i) microglial dynamics and physical interactions with neurons at the cellular level, (ii) microglial roles in the pathogenesis of chronic epilepsy at the circuit level, and (iii) microglial responses and contributions to the pathogenesis of developmental epilepsy. In light of the current established experimental epilepsy models and given the availability of genetic tools (knockout mice), replacement (e.g. cell transplantation) and pharmaco-genetic abilities to eliminate, inhibit or otherwise manipulate microglial functions, these questions can now and should begin to be addressed.
Main points.
Microglia undergo morphological and molecular activation in epilepsy.
Microglia regulate neuronal activities in healthy and epileptic brain.
Microglia contribute to seizure-induced neurodegeneration and neurogenesis.
Acknowledgements
This work is supported by grants from the National Institute of Health (R01NS088627, R21DE025689, and T32ES007148) and New Jersey Commission on Spinal Cord Research (CSCR15ERG015).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Abraham J, Fox PD, Condello C, Bartolini A, Koh S. Minocycline attenuates microglia activation and blocks the long-term epileptogenic effects of early-life seizures. Neurobiol Dis. 2012;46:425–30. doi: 10.1016/j.nbd.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ, Dailey ME. Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia. 2015;63:1694–713. doi: 10.1002/glia.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci. 2011;14:1142–9. doi: 10.1038/nn.2887. [DOI] [PubMed] [Google Scholar]
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–43. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Akahoshi N, Murashima YL, Himi T, Ishizaki Y, Ishii I. Increased expression of the lysosomal protease cathepsin S in hippocampal microglia following kainate-induced seizures. Neurosci Lett. 2007;429:136–41. doi: 10.1016/j.neulet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Ali I, Chugh D, Ekdahl CT. Role of fractalkine-CX3CR1 pathway in seizure-induced microglial activation, neurodegeneration, and neuroblast production in the adult rat brain. Neurobiol Dis. 2015;74:194–203. doi: 10.1016/j.nbd.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Andersson PB, Perry VH, Gordon S. The kinetics and morphological characteristics of the macrophage-microglial response to kainic acid-induced neuronal degeneration. Neuroscience. 1991;42:201–14. doi: 10.1016/0306-4522(91)90159-l. [DOI] [PubMed] [Google Scholar]
- Arno B, Grassivaro F, Rossi C, Bergamaschi A, Castiglioni V, Furlan R, Greter M, Favaro R, Comi G, Becher B. Neural progenitor cells orchestrate microglia migration and positioning into the developing cortex. Nat Commun. 2014;5:5611. doi: 10.1038/ncomms6611. others. [DOI] [PubMed] [Google Scholar]
- Avignone E, Lepleux M, Angibaud J, Nagerl UV. Altered morphological dynamics of activated microglia after induction of status epilepticus. J Neuroinflammation. 2015;12:202. doi: 10.1186/s12974-015-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avignone E, Ulmann L, Levavasseur F, Rassendren F, Audinat E. Status epilepticus induces a particular microglial activation state characterized by enhanced purinergic signaling. J Neurosci. 2008;28:9133–44. doi: 10.1523/JNEUROSCI.1820-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baalman K, Marin MA, Ho TS, Godoy M, Cherian L, Robertson C, Rasband MN. Axon initial segment-associated microglia. J Neurosci. 2015;35:2283–92. doi: 10.1523/JNEUROSCI.3751-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Sasse VA, Wang Y, Maulik M, Kar S. Increased levels and activity of cathepsins B and D in kainate-induced toxicity. Neuroscience. 2015;284:360–73. doi: 10.1016/j.neuroscience.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Beach TG, Woodhurst WB, MacDonald DB, Jones MW. Reactive microglia in hippocampal sclerosis associated with human temporal lobe epilepsy. Neurosci Lett. 1995;191:27–30. doi: 10.1016/0304-3940(94)11548-1. [DOI] [PubMed] [Google Scholar]
- Bechade C, Cantaut-Belarif Y, Bessis A. Microglial control of neuronal activity. Front Cell Neurosci. 2013;7:32. doi: 10.3389/fncel.2013.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellavance MA, Gosselin D, Yong VW, Stys PK, Rivest S. Patrolling monocytes play a critical role in CX3CR1-mediated neuroprotection during excitotoxicity. Brain Struct Funct. 2015;220:1759–76. doi: 10.1007/s00429-014-0759-z. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–7. doi: 10.1016/s0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- Benson MJ, Manzanero S, Borges K. Complex alterations in microglial M1/M2 markers during the development of epilepsy in two mouse models. Epilepsia. 2015;56:895–905. doi: 10.1111/epi.12960. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Boche D, Perry VH, Nicoll JA. Review: activation patterns of microglia and their identification in the human brain. Neuropathol Appl Neurobiol. 2013;39:3–18. doi: 10.1111/nan.12011. [DOI] [PubMed] [Google Scholar]
- Boer K, Spliet WG, van Rijen PC, Redeker S, Troost D, Aronica E. Evidence of activated microglia in focal cortical dysplasia. J Neuroimmunol. 2006;173:188–95. doi: 10.1016/j.jneuroim.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Exp Neurol. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. others. [DOI] [PubMed] [Google Scholar]
- Cho KO, Lybrand ZR, Ito N, Brulet R, Tafacory F, Zhang L, Good L, Ure K, Kernie SG, Birnbaum SG. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Koh S. Role of brain inflammation in epileptogenesis. Yonsei Med J. 2008;49:1–18. doi: 10.3349/ymj.2008.49.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–62. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, Gravel C, Salter MW, De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Martinez-Cerdeno V, Noctor SC. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J Neurosci. 2013;33:4216–33. doi: 10.1523/JNEUROSCI.3441-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–57. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby NO, Mody I. The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol. 2001;14:187–92. doi: 10.1097/00019052-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dibaj P, Nadrigny F, Steffens H, Scheller A, Hirrlinger J, Schomburg ED, Neusch C, Kirchhoff F. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58:1133–44. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- Dissing-Olesen L, LeDue JM, Rungta RL, Hefendehl JK, Choi HB, MacVicar BA. Activation of neuronal NMDA receptors triggers transient ATP-mediated microglial process outgrowth. J Neurosci. 2014;34:10511–27. doi: 10.1523/JNEUROSCI.0405-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domercq M, Matute C. Neuroprotection by tetracyclines. Trends Pharmacol Sci. 2004;25:609–12. doi: 10.1016/j.tips.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res. 2002;135:53–65. doi: 10.1016/S0079-6123(02)35007-6. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Tipping PG, Kipari T, Cailhier JF, Clay S, Lang R, Bonventre JV, Hughes J. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol. 2005;167:1207–19. doi: 10.1016/S0002-9440(10)61209-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–97. doi: 10.1016/j.neuron.2014.02.040. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson C, Zou LP, Ahlenius S, Winblad B, Schultzberg M. Inhibition of kainic acid induced expression of interleukin-1 beta and interleukin-1 receptor antagonist mRNA in the rat brain by NMDA receptor antagonists. Brain Res Mol Brain Res. 2000;85:103–13. doi: 10.1016/s0169-328x(00)00251-5. [DOI] [PubMed] [Google Scholar]
- Eyo UB, Dailey ME. Microglia: key elements in neural development, plasticity, and pathology. J Neuroimmune Pharmacol. 2013;8:494–509. doi: 10.1007/s11481-013-9434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Gu N, De S, Dong H, Richardson JR, Wu LJ. Modulation of microglial process convergence toward neuronal dendrites by extracellular calcium. J Neurosci. 2015;35:2417–22. doi: 10.1523/JNEUROSCI.3279-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Peng J, Swiatkowski P, Mukherjee A, Bispo A, Wu LJ. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J Neurosci. 2014;34:10528–40. doi: 10.1523/JNEUROSCI.0416-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyo UB, Wu LJ. Bidirectional Microglia-Neuron Communication in the Healthy Brain. Neural Plast. 2013;2013:456857. doi: 10.1155/2013/456857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–3001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, Engel J., Jr Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–2. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- Frieler RA, Meng H, Duan SZ, Berger S, Schutz G, He Y, Xi G, Wang MM, Mortensen RM. Myeloid-specific deletion of the mineralocorticoid receptor reduces infarct volume and alters inflammation during cerebral ischemia. Stroke. 2011;42:179–85. doi: 10.1161/STROKEAHA.110.598441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitatzis A, Johnson AL, Chadwick DW, Shorvon SD, Sander JW. Life expectancy in people with newly diagnosed epilepsy. Brain. 2004;127:2427–32. doi: 10.1093/brain/awh267. [DOI] [PubMed] [Google Scholar]
- Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:229. doi: 10.3389/fncel.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass HC. Neonatal seizures: advances in mechanisms and management. Clin Perinatol. 2014;41:177–90. doi: 10.1016/j.clp.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu N, Eyo UB, Murugan M, Peng J, Matta S, Dong H, Wu LJ. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.11.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM. Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci U S A. 1997;94:13311–6. doi: 10.1073/pnas.94.24.13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Ishibashi H, Hashimoto K, Nakanishi H. Potentiation of the NMDA receptor-mediated responses through the activation of the glycine site by microglia secreting soluble factors. Glia. 2006;53:660–8. doi: 10.1002/glia.20322. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Heida JG, Moshe SL, Pittman QJ. The role of interleukin-1beta in febrile seizures. Brain Dev. 2009;31:388–93. doi: 10.1016/j.braindev.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel JS, Beers DR, Zhao W, Appel SH. Microglia in ALS: the good, the bad, and the resting. J Neuroimmune Pharmacol. 2009;4:389–98. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- Heo K, Cho YJ, Cho KJ, Kim HW, Kim HJ, Shin HY, Lee BI, Kim GW. Minocycline inhibits caspase-dependent and -independent cell death pathways and is neuroprotective against hippocampal damage after treatment with kainic acid in mice. Neurosci Lett. 2006;398:195–200. doi: 10.1016/j.neulet.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–52. doi: 10.1038/nm1177. others. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology. 2015;40:2368–78. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci. 2012;32:15106–11. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–70. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- Huang WC, Qiao Y, Xu L, Kacimi R, Sun X, Giffard RG, Yenari MA. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat Cell Biol. 2010;43:325–31. doi: 10.5115/acb.2010.43.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–27. [PubMed] [Google Scholar]
- Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52(Suppl 1):1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Pacheco A, Mesuret G, Sanz-Rodriguez A, Tanaka K, Mooney C, Conroy R, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC, Engel T. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia. 2013;54:1551–61. doi: 10.1111/epi.12257. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim JH, Kang KM, Song EC, Kim SJ, Park HK, Kim M, Lee SK. Region-specific plasticity in the epileptic rat brain: a hippocampal and extrahippocampal analysis. Epilepsia. 2009;50:537–49. doi: 10.1111/j.1528-1167.2008.01718.x. others. [DOI] [PubMed] [Google Scholar]
- Kandratavicius L, Balista PA, Lopes-Aguiar C, Ruggiero RN, Umeoka EH, Garcia-Cairasco N, Bueno-Junior LS, Leite JP. Animal models of epilepsy: use and limitations. Neuropsychiatr Dis Treat. 2014;10:1693–705. doi: 10.2147/NDT.S50371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TC, Kim DS, Kwak SE, Kim JE, Won MH, Kim DW, Choi SY, Kwon OS. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia. 2006;54:258–71. doi: 10.1002/glia.20380. [DOI] [PubMed] [Google Scholar]
- Kira R, Ishizaki Y, Torisu H, Sanefuji M, Takemoto M, Sakamoto K, Matsumoto S, Yamaguchi Y, Yukaya N, Sakai Y. Genetic susceptibility to febrile seizures: case-control association studies. Brain Dev. 2010;32:57–63. doi: 10.1016/j.braindev.2009.09.018. others. [DOI] [PubMed] [Google Scholar]
- Kwan P, Brodie MJ. Refractory epilepsy: mechanisms and solutions. Expert Rev Neurother. 2006;6:397–406. doi: 10.1586/14737175.6.3.397. [DOI] [PubMed] [Google Scholar]
- Lauro C, Catalano M, Trettel F, Limatola C. Fractalkine in the nervous system: neuroprotective or neurotoxic molecule? Ann N Y Acad Sci. 2015;1351:141–8. doi: 10.1111/nyas.12805. [DOI] [PubMed] [Google Scholar]
- Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93–103. doi: 10.1016/s0920-1211(02)00072-4. [DOI] [PubMed] [Google Scholar]
- Levesque M, Avoli M. The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev. 2013;37:2887–99. doi: 10.1016/j.neubiorev.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Avoli M, Bernard C. Animal models of temporal lobe epilepsy following systemic chemoconvulsant administration. J Neurosci Methods. 2016;260:45–52. doi: 10.1016/j.jneumeth.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Liao B, Zhao W, Beers DR, Henkel JS, Appel SH. Transformation from a neuroprotective to a neurotoxic microglial phenotype in a mouse model of ALS. Exp Neurol. 2012;237:147–52. doi: 10.1016/j.expneurol.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limatola C, Ransohoff RM. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front Cell Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo B, Romariz S, Blanco MM, Vasconcelos JF, Bahia L, Soares MB, Mello LE, Ribeiro-dos-Santos R. Distribution and proliferation of bone marrow cells in the brain after pilocarpine-induced status epilepticus in mice. Epilepsia. 2010;51:1628–32. doi: 10.1111/j.1528-1167.2010.02570.x. [DOI] [PubMed] [Google Scholar]
- Marin-Teva JL, Dusart I, Colin C, Gervais A, van Rooijen N, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–47. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Murao N, Katano Y, Juliandi B, Kohyama J, Akira S, Kawai T, Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat Commun. 2015;6:6514. doi: 10.1038/ncomms7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H, Klemsz M, Feeney AJ, Wu GE, Paige CJ. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J. 1996;15:5647–58. others. [PMC free article] [PubMed] [Google Scholar]
- Mikita J, Dubourdieu-Cassagno N, Deloire MS, Vekris A, Biran M, Raffard G, Brochet B, Canron MH, Franconi JM, Boiziau C. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. others. [DOI] [PubMed] [Google Scholar]
- Mirrione MM, Konomos DK, Gravanis I, Dewey SL, Aguzzi A, Heppner FL, Tsirka SE. Microglial ablation and lipopolysaccharide preconditioning affects pilocarpine-induced seizures in mice. Neurobiol Dis. 2010;39:85–97. doi: 10.1016/j.nbd.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Nichols NR, Pasinetti GM, Finch CE. TGF-beta 1 mRNA increases in macrophage/microglial cells of the hippocampus in response to deafferentation and kainic acid-induced neurodegeneration. Exp Neurol. 1993;120:291–301. doi: 10.1006/exnr.1993.1063. [DOI] [PubMed] [Google Scholar]
- Moriguchi S, Mizoguchi Y, Tomimatsu Y, Hayashi Y, Kadowaki T, Kagamiishi Y, Katsube N, Yamamoto K, Inoue K, Watanabe S. Potentiation of NMDA receptor-mediated synaptic responses by microglia. Brain Res Mol Brain Res. 2003;119:160–9. doi: 10.1016/j.molbrainres.2003.09.007. others. [DOI] [PubMed] [Google Scholar]
- Morris GP, Clark IA, Zinn R, Vissel B. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardou R, Ferrari DC, Ben-Ari Y. Mechanisms and effects of seizures in the immature brain. Semin Fetal Neonatal Med. 2013;18:175–84. doi: 10.1016/j.siny.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Nowak M, Strzelczyk A, Reif PS, Schorlemmer K, Bauer S, Norwood BA, Oertel WH, Rosenow F, Strik H, Hamer HM. Minocycline as potent anticonvulsant in a patient with astrocytoma and drug resistant epilepsy. Seizure. 2012;21:227–8. doi: 10.1016/j.seizure.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bisht K, Tremblay ME. Fractalkine regulation of microglial physiology and consequences on the brain and behavior. Front Cell Neurosci. 2014;8:129. doi: 10.3389/fncel.2014.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–8. doi: 10.1126/science.1202529. others. [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Gross CT. Microglia in development: linking brain wiring to brain environment. Neuron Glia Biol. 2011;7:77–83. doi: 10.1017/S1740925X12000105. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Ben Achour S, Rostaing P, Triller A, Bessis A. Microglia activation triggers astrocyte-mediated modulation of excitatory neurotransmission. Proc Natl Acad Sci U S A. 2012;109:E197–205. doi: 10.1073/pnas.1111098109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JL, Carapetian SA, Hageman JR, Kelley KR. Febrile seizures. Pediatr Ann. 2013;42:249–54. doi: 10.3928/00904481-20131122-09. [DOI] [PubMed] [Google Scholar]
- Patterson KP, Baram TZ, Shinnar S. Origins of temporal lobe epilepsy: febrile seizures and febrile status epilepticus. Neurotherapeutics. 2014;11:242–50. doi: 10.1007/s13311-014-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peferoen LA, Vogel DY, Ummenthum K, Breur M, Heijnen PD, Gerritsen WH, Peferoen-Baert RM, van der Valk P, Dijkstra CD, Amor S. Activation status of human microglia is dependent on lesion formation stage and remyelination in multiple sclerosis. J Neuropathol Exp Neurol. 2015;74:48–63. doi: 10.1097/NEN.0000000000000149. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica L, Bechade C, Belarif-Cantaut Y, Pascual O, Bessis A. Physiological roles of microglia during development. J Neurochem. 2011;119:901–8. doi: 10.1111/j.1471-4159.2011.07504.x. [DOI] [PubMed] [Google Scholar]
- Priel MR, Albuquerque EX. Short-term effects of pilocarpine on rat hippocampal neurons in culture. Epilepsia. 2002;43(Suppl 5):40–6. doi: 10.1046/j.1528-1157.43.s.5.18.x. [DOI] [PubMed] [Google Scholar]
- Prinz M, Tay TL, Wolf Y, Jung S. Microglia: unique and common features with other tissue macrophages. Acta Neuropathol. 2014;128:319–31. doi: 10.1007/s00401-014-1267-1. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–8. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–60. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Ribeiro Xavier AL, Kress BT, Goldman SA, Lacerda de Menezes JR, Nedergaard M. A Distinct Population of Microglia Supports Adult Neurogenesis in the Subventricular Zone. J Neurosci. 2015;35:11848–61. doi: 10.1523/JNEUROSCI.1217-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers KM, Hutchinson MR, Northcutt A, Maier SF, Watkins LR, Barth DS. The cortical innate immune response increases local neuronal excitability leading to seizures. Brain. 2009;132:2478–86. doi: 10.1093/brain/awp177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–50. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogove AD, Tsirka SE. Neurotoxic responses by microglia elicited by excitotoxic injury in the mouse hippocampus. Curr Biol. 1998;8:19–25. doi: 10.1016/s0960-9822(98)70016-8. [DOI] [PubMed] [Google Scholar]
- Rosell DR, Nacher J, Akama KT, McEwen BS. Spatiotemporal distribution of gp130 cytokines and their receptors after status epilepticus: comparison with neuronal degeneration and microglial activation. Neuroscience. 2003;122:329–48. doi: 10.1016/s0306-4522(03)00593-1. [DOI] [PubMed] [Google Scholar]
- Roumier A, Bechade C, Poncer JC, Smalla KH, Tomasello E, Vivier E, Gundelfinger ED, Triller A, Bessis A. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci. 2004;24:11421–8. doi: 10.1523/JNEUROSCI.2251-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O’Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472:466–70. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, Meda P, Scemes E. Targeting pannexin1 improves seizure outcome. PLoS One. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch T, Henke K, Peri F. Microglia in the developing brain: from immunity to behaviour. Curr Opin Neurobiol. 2011;21:5–10. doi: 10.1016/j.conb.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265:1573–7. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Wang L, Ribak CE. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia. 2008;49(Suppl 2):33–41. doi: 10.1111/j.1528-1167.2008.01491.x. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Reams RY, Jordan WH, Miller MA, Thacker HL, Snyder PW. Mesial temporal lobe epilepsy: pathogenesis, induced rodent models and lesions. Toxicol Pathol. 2007;35:984–99. doi: 10.1080/01926230701748305. [DOI] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34:2231–43. doi: 10.1523/JNEUROSCI.1619-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014;2014:610343. doi: 10.1155/2014/610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, Tsirka SE, Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipe GO, Lowery RL, Tremblay ME, Kelly EA, Lamantia CE, Majewska AK. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat Commun. 2016;7:10905. doi: 10.1038/ncomms10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders I, Khan GM, Manil J, Ebinger G, Michotte Y. NMDA receptor-mediated pilocarpine-induced seizures: characterization in freely moving rats by microdialysis. Br J Pharmacol. 1997;121:1171–9. doi: 10.1038/sj.bjp.0701231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8:1271–9. doi: 10.1016/j.celrep.2014.07.042. [DOI] [PubMed] [Google Scholar]
- Swiatkowski P, Murugan M, Eyo UB, Wang Y, Rangaraju S, Oh SB, Wu LJ. Activation of microglial P2Y12 receptor is required for outward potassium currents in response to neuronal injury. Neuroscience. 2016;318:22–33. doi: 10.1016/j.neuroscience.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181–94. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- Taniwaki Y, Kato M, Araki T, Kobayashi T. Microglial activation by epileptic activities through the propagation pathway of kainic acid-induced hippocampal seizures in the rat. Neurosci Lett. 1996;217:29–32. doi: 10.1016/0304-3940(96)13062-7. [DOI] [PubMed] [Google Scholar]
- Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(Suppl 2):10–3. doi: 10.1111/j.1528-1167.2008.02005.x. [DOI] [PubMed] [Google Scholar]
- Thanos S, Mey J, Wild M. Treatment of the adult retina with microglia-suppressing factors retards axotomy-induced neuronal degradation and enhances axonal regeneration in vivo and in vitro. J Neurosci. 1993;13:455–66. doi: 10.1523/JNEUROSCI.13-02-00455.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, Hesdorffer DC, Hauser WA, Kazis L, Kobau R. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. others. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009;29:3518–28. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME. The role of microglia at synapses in the healthy CNS: novel insights from recent imaging studies. Neuron Glia Biol. 2011;7:67–76. doi: 10.1017/S1740925X12000038. [DOI] [PubMed] [Google Scholar]
- Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–9. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004;16:321–34. doi: 10.1016/j.nbd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Turski L, Ikonomidou C, Turski WA, Bortolotto ZA, Cavalheiro EA. Review: cholinergic mechanisms and epileptogenesis. The seizures induced by pilocarpine: a novel experimental model of intractable epilepsy. Synapse. 1989;3:154–71. doi: 10.1002/syn.890030207. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–51. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Levavasseur F, Avignone E, Peyroutou R, Hirbec H, Audinat E, Rassendren F. Involvement of P2X4 receptors in hippocampal microglial activation after status epilepticus. Glia. 2013;61:1306–19. doi: 10.1002/glia.22516. [DOI] [PubMed] [Google Scholar]
- Varnum MM, Ikezu T. The classification of microglial activation phenotypes on neurodegeneration and regeneration in Alzheimer’s disease brain. Arch Immunol Ther Exp (Warsz) 2012;60:251–66. doi: 10.1007/s00005-012-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A. Pilocarpine-induced seizures revisited: what does the model mimic? Epilepsy Curr. 2009;9:146–8. doi: 10.1111/j.1535-7511.2009.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Aronica E, Mazarati A, Pittman QJ. Epilepsy and brain inflammation. Exp Neurol. 2013;244:11–21. doi: 10.1016/j.expneurol.2011.09.033. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, De Simoni MG. Interleukin-1beta immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. 1999;19:5054–65. doi: 10.1523/JNEUROSCI.19-12-05054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virta M, Hurme M, Helminen M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr Neurol. 2002;26:192–5. doi: 10.1016/s0887-8994(01)00380-0. [DOI] [PubMed] [Google Scholar]
- Vogel DY, Vereyken EJ, Glim JE, Heijnen PD, Moeton M, van der Valk P, Amor S, Teunissen CE, van Horssen J, Dijkstra CD. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J Neuroinflammation. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Englot DJ, Garcia PA, Lawton MT, Young WL. Minocycline- and tetracycline-class antibiotics are protective against partial seizures in vivo. Epilepsy Behav. 2012;24:314–8. doi: 10.1016/j.yebeh.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Mi X, Gao B, Gu J, Wang W, Zhang Y, Wang X. Minocycline inhibits brain inflammation and attenuates spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neuroscience. 2015;287:144–56. doi: 10.1016/j.neuroscience.2014.12.021. [DOI] [PubMed] [Google Scholar]
- Wu LJ. Voltage-gated proton channel HV1 in microglia. Neuroscientist. 2014;20:599–609. doi: 10.1177/1073858413519864. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Vadakkan KI, Zhuo M. ATP-induced chemotaxis of microglial processes requires P2Y receptor-activated initiation of outward potassium currents. Glia. 2007;55:810–21. doi: 10.1002/glia.20500. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, Luo HR, Feener EP, Clapham DE. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15:565–73. doi: 10.1038/nn.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–32. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- Xiao F, Xu JM, Jiang XH. CX3 chemokine receptor 1 deficiency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen Res. 2015;10:772–7. doi: 10.4103/1673-5374.156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu ZR, Chen J, Zhang SJ, Quan QY, Huang YG, Jiang W. Roles of astrocytes and microglia in seizure-induced aberrant neurogenesis in the hippocampus of adult rats. J Neurosci Res. 2010;88:519–29. doi: 10.1002/jnr.22224. [DOI] [PubMed] [Google Scholar]
- Yeo SI, Kim JE, Ryu HJ, Seo CH, Lee BC, Choi IG, Kim DS, Kang TC. The roles of fractalkine/CX3CR1 system in neuronal death following pilocarpine-induced status epilepticus. J Neuroimmunol. 2011;234:93–102. doi: 10.1016/j.jneuroim.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Yrjanheikki J, Tikka T, Keinanen R, Goldsteins G, Chan PH, Koistinaho J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc Natl Acad Sci U S A. 1999;96:13496–500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Liu WH, He XH, Peng BW. IL-1beta: an important cytokine associated with febrile seizures? Neurosci Bull. 2012;28:301–8. doi: 10.1007/s12264-012-1240-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zattoni M, Mura ML, Deprez F, Schwendener RA, Engelhardt B, Frei K, Fritschy JM. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31:4037–50. doi: 10.1523/JNEUROSCI.6210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–6. doi: 10.1038/nn.3641. others. [DOI] [PubMed] [Google Scholar]
- Zhang J, Malik A, Choi HB, Ko RW, Dissing-Olesen L, MacVicar BA. Microglial CR3 activation triggers long-term synaptic depression in the hippocampus via NADPH oxidase. Neuron. 2014a;82:195–207. doi: 10.1016/j.neuron.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014b;34:11929–47. doi: 10.1523/JNEUROSCI.1860-14.2014. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Wu G, Wu LJ. Neuronal and microglial mechanisms of neuropathic pain. Mol Brain. 2011;4:31. doi: 10.1186/1756-6606-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]