Abstract
Exploiting iron-uptake pathways by conjugating β-lactam antibiotics with iron-chelators such as catechol and hydroxamic acid is a proven strategy to overcome permeability-related resistance in Gram-negative bacteria. Since naturally occurring iron chelating tetramic acids have not been previously examined for this purpose, an exploratory series of novel ampicillin-tetramic acid hybrids that structurally resemble ureidopenicillins was designed and synthesized. The new analogs were evaluated for the ability to chelate iron and their MIC activities determined against a representative panel of clinically significant bacterial pathogens. The tetramic acid β-lactam hybrids demonstrated a high affinity to iron in the order of 10−30 M3. The hybrids were less active against Gram-positive bacteria. However, against Gram-negative bacteria, their activity was species dependent with several hybrids displaying improved activity over ampicillin against wild-type Pseudomonas aeruginosa. The anti-Gram-negative activities of the hybrids improved in the presence of clavulanic acid revealing that the tetramic acid moiety did not provide added protection against β-lactamases. Additionally, the hybrids were found to be efflux pump substrates as their activities markedly improved against pump-inactivated strains. Unlike the catechol and hydroxamic acid siderophore β-lactam conjugates, the activities of the hybrids did not improve under iron-deficient conditions. These results suggest that the tetramic acid hybrids gain permeability via different membrane receptors, or they are out competed by native bacterial siderophores with stronger affinities for iron. This study provides a foundation for the further exploitation of the tetramic acid moiety to achieve novel β-lactam anti-Gram-negative agents, providing that efflux and β-lactamase mediated resistance is addressed.
Introduction
β-lactam antibiotics are the leading marketed drugs for the treatment of bacterial infections, but resistance to this class of agents is now common in both gram-positive and gram-negative pathogens [1, 2]. The underlying mechanisms of resistance include production of β-lactamases that hydrolyze the lactam ring, alterations within the penicillin-binding protein (PBP) binding site that reduce antibiotic affinity, changes in outer membrane permeability that decrease antibiotic uptake, and increased expression of efflux pumps that reduce target access [3]. Studies have shown that permeability-mediated resistance can be overcome by exploiting the iron-uptake pathways as a Trojan horse strategy to enter antibiotics into bacterial cells [4]. Bacteria assimilate iron by synthesizing and utilizing low molecular weight iron-chelating compounds called siderophores and these Fe3+- siderophore complexes are recognized and actively transported into cells by siderophore receptors located in the outer membrane [5]. Iron uptake mechanisms are particularly active in hosts where freely available iron in tissue fluids is scarce. Hence, conjugating antibiotics to siderophores presents an attractive approach to promote the cellular uptake of antibiotics. This strategy has been successfully applied to improve the in vitro activity of ampicillin and amoxicillin against Gram-negatives, including Pseudomonas aeruginosa [6, 7]. Also, novel monobactam antibiotics containing a siderophoric motif were shown to utilize the iron-uptake pathway to enter cells; these compounds also display efficacy in animal models of Gram-negative infections [8–10].
The most common siderophores belong to three main classes: catecholates, hydroxamates, and α-hydroxycarboxylates [11]. Of these, the catecholates and hydroxamates have been widely explored in antibiotic drug discovery [12]. One iron chelator moiety that appears to be underutilized is the class of natural products known as tetramic acids. Tetramic acid motifs are found in a wide variety of pharmacologically active natural products, with many displaying antifungal and antibacterial activities [13–15]. Molecules bearing the tetramic acid core demonstrate diverse modes of action ranging from inhibition of cell wall [16] and RNA synthesis [17] and dissipation of the membrane potential [18]. The tetramic acid motif has the ability to interact with acid and metal binding enzymatic domains in essential bacterial drug targets [19, 20]. However, there are no marketed antibiotics containing the tetramic acid motif. The 3-carbonyl tetramic acids are strongly acidic with pKa ~ 2.3–3.5, and they chelate a variety of metal ions such as Fe3+, Zn2+, Ca2+, Mg2+, and Cu2+ [21, 22]. Recently, the tetramic acid harzianic acid was described as a novel siderophore in Trichoderma harzianum [23]. Similarly, based on studies by Kauffman et al. it is thought that physiological degradation of the quorum sensing molecule N-acylhomoserine lactones (AHL) to a tetramic acid may play a role in iron acquisition by P. aeruginosa [24]. However, tetramic acid molecules are not generally well described as siderophores in pathogenic bacteria. While several known siderophore moieties have been applied in discovering siderophore-fused β-lactam antibiotics, there is a lack of literature examining the use of the tetramic acid motif for cellular drug uptake. Considering these factors, we sought to examine the prospect of applying the tetramic acids motif to improve cellular uptake of β-lactam antibiotics. We hypothesized that attaching a 3-acyltetramic acid moiety to β-lactam antibiotics could improve their activity by increasing permeability by exploiting the metal ion-uptake pathways. Since ampicillin has been successfully linked to other siderophores, we tested our hypothesis herein by synthesizing an exploratory series of novel ampicillin-tetramic acid hybrids, similar in molecular shape to ureidopenicillins, and examined their antimicrobial activities.
Results and discussion
Design of ampicillin– tetramic acid hybrids
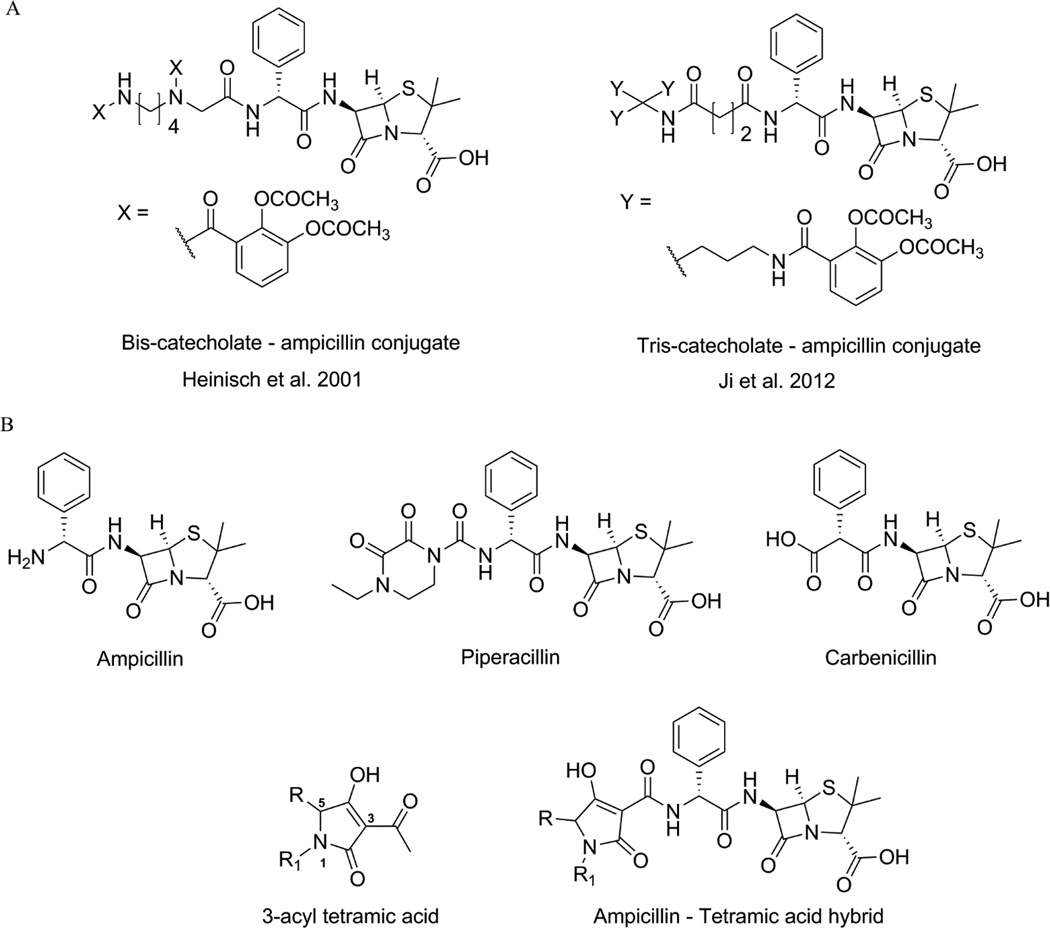
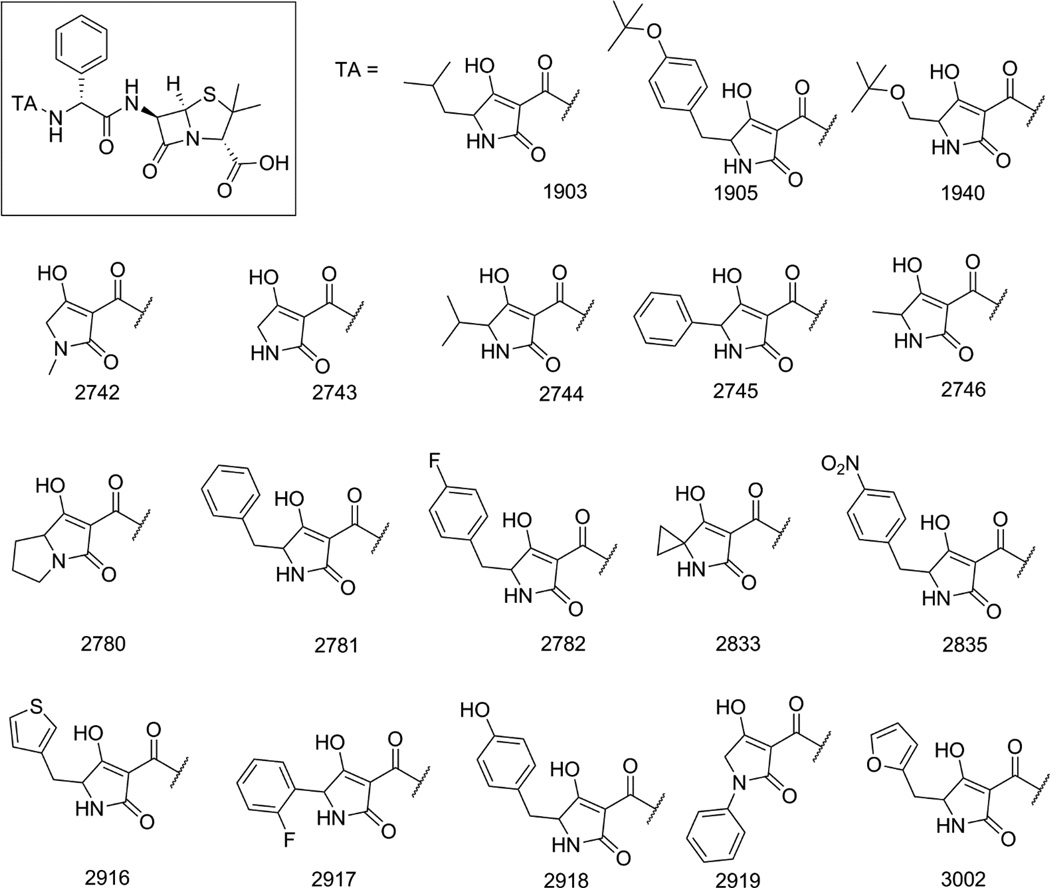
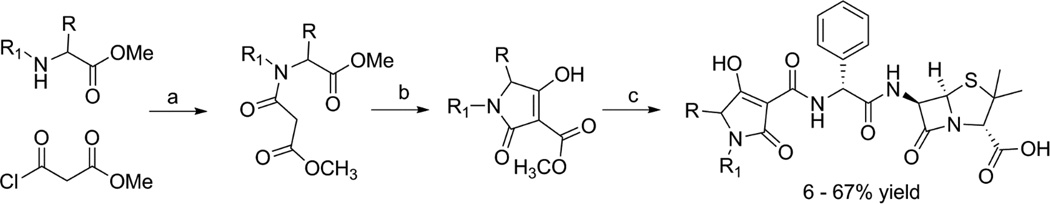
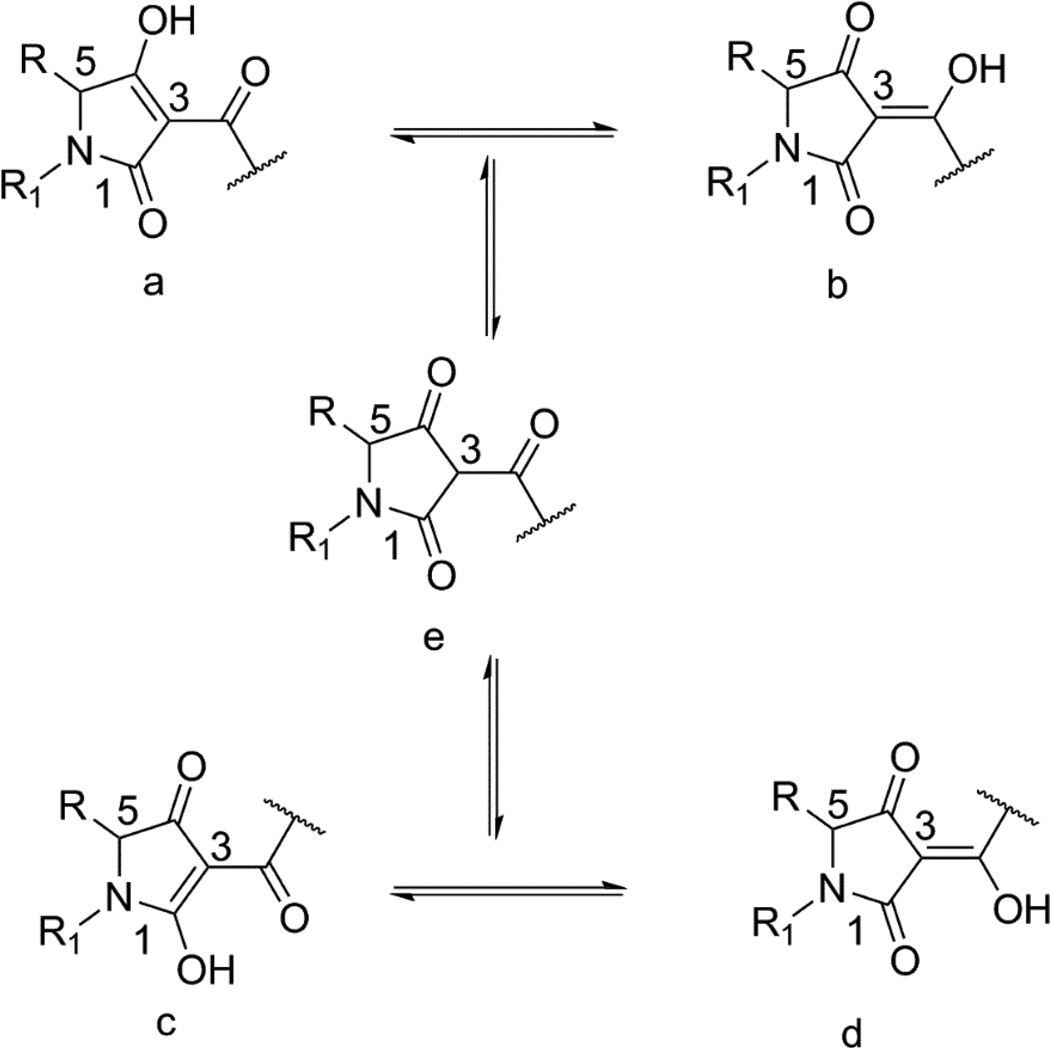
The amine terminal of ampicillin was selected for the attachment of the tetramic acid motif based on the structures of previously reported ampicillin-siderophore conjugates where catecholate siderophores were attached to this position through acylation [6, 7]. These conjugates contained more than one catechol moieties attached to ampicillin via linkers of varying lengths (Figure 1A). For our study, we decided to attach the tetramic acid directly to the amine terminal of ampicillin, which was inspired by the observation that this amine is acylated to form a urea in the ureidopenicillins, such as piperacillin. Ureidopenicillins show broad-spectrum activity against Gram-positive and Gram-negative bacteria including most notably P. aeruginosa [25–27]. It was also noted that an acidic center was also tolerated at this position, as replacement of the ampicillin amine by a carboxylate group produces carbenicillin. Carbenicillin displays significant Gram-negative coverage including strain dependent antipseudomonal activity [28, 29]. The tetramic acid core offers several potential positions for attachment to the ampicillin amine. The 3-position was selected for attachment of ampicillin for synthetic ease and overall pharmacophoric match [30, 31]. Accordingly, we designed ampicillin-tetramic acid hybrids in which ampicillin was linked to the 3-position of tetramic acid via a carboxamide (Figure 1B). This design combined the structural features of both ureidopenicillins and carbenicillin while introducing a chelating tetramic acid. Based on this design a small library of analogs was synthesized to study the structure-activity relationship (SAR) (Figure 2). The intermediate 3-methoxycarbonyl tetramic acids were synthesized from various amino acids creating diversity at the C5 and the N1-positions of the tetramic acid core. These structural modifications allowed us to explore the influence of different physiochemical parameters such as of size, shape, hydrophobicity, and polarity on the activity of the hybrids. The hybrids were synthesized as shown in Scheme 1. The NMR characterization of 3-carbonyl tetramic acids can be complicated due to their ability to undergo keto-enol tautomerism. A total of nine tautomers are possible, but typically only four enolic tautomers are observed in solution (Figure 3) [15]. These tautomers form two pairs of internal tautomers (a ⇌ b and c ⇌ d) which are in rapid equilibrium and a pair of external tautomers (ab ⇌ cd) which is in slow equilibrium. The interconversion of the external tautomers is postulated to proceed via the β-triketo tautomer e [32]. The ratio of the tautomers observed during NMR analysis can be influenced by the solvent [33]. In our study, the NMR for all hybrid molecules were performed in MeOH-d4 and they all displayed only one major tautomer, which is tautomer a.
Figure 1.
A. Structures of catecholate-based siderophore-ampicillin conjugates. B. Design of ampicillin - tetramic acid hybrids inspired by piperacillin and carbenicillin.
Figure 2.
Structures of ampicillin – tetramic acid hybrids
Scheme 1.
Synthesis of ampicillin – tetramic acid hybrids. Reagents and conditions: a)TEA/DCM b)NaOMe/MeOH c)ampicillin/DMF/MW/100°C/3mins
Figure 3.
Tautomerism of 3-carbonyl tetramic acids
Antimicrobial activity against gram-positive bacteria in Mueller-Hinton broth
In order to determine the antimicrobial potential of these hybrids, they were initially tested against a panel of gram-positive and gram-negative bacteria in cation-adjusted Mueller-Hinton broth, with below MICs being mean values of at least two replicates. In general, against gram-positive bacteria (Table 1), the fusion of ampicillin with tetramic acid led to a moderate loss in potency. Nevertheless, several hybrids displayed good activity with low MICs (<0.2 – 6.25 µg/ml) against methicillin-sensitive S. aureus (MSSA), Streptococcus pyogenes, S. pneumoniae and Bacillus anthracis sterne and B. subtilis. Against Enterococcus faecalis and methicillin-resistant S. aureus (MRSA), most hybrids had MICs >25 µg/ml. Previously, we reported that the antimicrobial activity of tetramic acids belonging to the membrane active reutericyclin class was influenced by hydrophobic substituents at the N1, C5, and 3-positions of the tetramic core and that the simple hydrophilic tetramic acid core alone did not possess measurable antibacterial activity (MICs > 200 µg/ml) against Gram-positive bacteria [18, 34]. In comparison, for tetramic acid-ampicillin hybrids, different substituents at the C5 and N1-positions on the tetramic core, irrespective of their size, shape, hydrophobicity, and polarity produced similar activity, and no clear SAR was observed. Additionally, the position of the substituent (C5 or N1) did not affect the activity, for e.g. 2746 and 2742 which have a methyl group at the C5 and N1-position respectively have comparable activity; similarly, 2745 and 2919 which have a phenyl group at the C5 and N1-positions respectively have comparable activity. The lack of SAR and loss of potency compared to ampicillin might suggest suboptimal binding of these hybrids with target PBPs; however, due to the chelating nature of tetramic acids, the possibility that these hybrids may be functioning via mechanism(s) unrelated to PBP binding cannot be ruled out. Since cytotoxicity is a concern for tetramic acid bearing molecules, such as some reutericyclins, we investigated this property in the ampicillin-tetramic acid hybrids [14, 34]. When tested against HeLa cells, the hybrids were non-toxic producing cytotoxicity values similar to other penicillin antibiotic controls (Supporting information, Table S1).
Table 1.
MIC (µg/ml) of ampicillin-tetramic acid hybrids against gram-positive bacteria.
| Compound | EF | SPy | SP | BA | BS | MSSA | MRSA |
|---|---|---|---|---|---|---|---|
| ampicillin | 3.13 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | 50 |
| piperacillin | 3.13 | <0.2 | <0.2 | 0.39 | 0.39 | <0.2 | 25 |
| carbenicillin | 3.13 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | 6.25 |
| 1903 | 25 | 0.39 | 0.39 | 3.13 | 1.56 | 0.78 | 25 |
| 1905 | 50 | 0.78 | <0.2 | 3.13 | 3.13 | 0.78 | 50 |
| 1940 | 25 | 0.78 | 0.78 | 3.13 | 3.13 | 1.56 | 25 |
| 2742 | 25 | 1.56 | 1.56 | 6.25 | 3.13 | 1.56 | 100 |
| 2743 | 12.5 | <0.2 | <0.2 | 0.78 | 0.78 | 0.78 | 25 |
| 2744 | 12.5 | 0.78 | 0.39 | 6.25 | 3.13 | 1.56 | 25 |
| 2745 | 12.5 | 0.78 | 0.39 | 6.25 | 3.13 | 0.78 | 25 |
| 2746 | 25 | 0.39 | 0.39 | 1.56 | 3.13 | 1.56 | 50 |
| 2780 | 25 | 0.78 | 0.39 | 1.56 | 0.78 | 0.39 | 50 |
| 2781 | 25 | 0.39 | <0.2 | 3.13 | 0.78 | <0.2 | 25 |
| 2782 | 6.25 | 0.78 | 0.39 | 3.13 | 1.56 | 0.39 | 12.5 |
| 2833 | 12.5 | 0.78 | <0.2 | 1.56 | 0.39 | <0.2 | 25 |
| 2835 | 25 | 0.78 | <0.2 | 3.13 | <0.2 | <0.2 | 25 |
| 2916 | 25 | 0.39 | <0.2 | 1.56 | 1.56 | <0.2 | 50 |
| 2917 | 25 | 0.39 | <0.2 | 3.13 | 1.56 | 0.78 | 50 |
| 2918 | 25 | 1.56 | 0.39 | 6.25 | 3.13 | 0.78 | 50 |
| 2919 | 25 | 0.78 | <0.2 | 3.13 | 1.56 | 0.78 | 50 |
| 3002 | 25 | 0.39 | 0.39 | 3.13 | 1.56 | 0.78 | 50 |
Key: EF= E. faecalis ATCC33186; SPy= S. pyogenes ATCC700294; SP= S. pneumoniae R6; BA= B. anthracis sterne; BS= B. subtilis ATCC23857; MSSA= S. aureus -ATCC29213; MRSA= S. aureus NRS 70.
Antimicrobial activity against gram-negative bacteria in Mueller-Hinton broth
Compounds within the tetramic acid class (e.g. reutericyclins) are typically inactive against the Gram-negative test panel in Table 2 [15, 18, 34]. However, against the panel of type strains of gram-negative bacteria, fusion of ampicillin with tetramic acid produced variable effects against the different species (Table 2), with a general observed trend that some hybrids showed increased activity over ampicillin and carbenicillin, but were inferior to piperacillin for some key species. Best results were observed against P. aeruginosa, where the activities of the hybrids were 4–16 fold better than ampicillin (>200 µg/ml) and 2–8 fold over carbenicillin. The most active compounds 1903, 2781, and 3002 showed MIC of 12.5 µg/ml, which is 2-fold less active than piperacillin (6.25 µg/ml). Against Acinetobacter baumannii, the MICs ranged from 25–100 µg/ml, with the exception of 1905, 2746, and 2780 (≥200 µg/ml); the compounds were generally better than ampicillin and carbenicillin (100 µg/ml) but were at least 4-fold less active than piperacillin (6.25 µg/ml). Against Klebsiella pneumoniaethe hybrids displayed weaker activities (≥ 200 µg/ml) compared to ampicillin (100 µg/ml), carbenicillin (50 µg/ml), and piperacillin (6.25 µg/ml). Several hybrids displayed low MICs against Proteus mirabilis (1.56–25 µg/ml) and were comparable to ampicillin (3.13 µg/ml) and carbenicillin (1.56 µg/ml) but inferior to piperacillin (0.19 µg/ml). Against the ampicillin-resistant P. vulgaris, 2744 - 45, 2780 - 82, and 2916 - 18 (6.25–12.5 µg/ml) displayed improved activities over ampicillin and carbenicillin (100 µg/ml) but these were not better than piperacillin (1.56 µg/ml). Against Escherichia coli BW25113, the most active hybrids were 2917, 2918, 2919, and 3002 which had MICs of 6.25–12.5 µg/ml that was either comparable to piperacillin (6.25 µg/ml) or slightly inferior to ampicillin (3.13 µg/ml). Other compounds in the series displayed MICs of 25–200 µg/ml, which was comparable to carbenicillin (100 µg/ml). In order to determine whether the introduction of tetramic acid rendered ampicillin more susceptible to exclusion via efflux pumps, we tested the compounds against an E. coli BW25113 strain that lacked tolcan important part of the AcrAB–TolC multidrug efflux system. The hybrids displayed dissimilar MIC activities against both E. coli BW25113 and the TolC-deficient strain, suggesting that they are substrates for the TolC based efflux systems, such as the AcrAB–TolC pump. For instance, 2916 which has MIC of 50 µg/ml against E. coli BW25113 showed a significantly reduced MIC of 1.56 µg/ml in the TolC-deficient strain (Table 2). These shifts are also observed for piperacillin and carbenicillin. These results suggest that efflux is a key factor dictating the relative activities of compounds in the series.
Table 2.
MIC (µg/ml) of ampicillin-tetramic acid hybrids against gram-negative bacteria.
| Compound | AB | EC | ECΔtolC | PV | PM | KP | PA |
|---|---|---|---|---|---|---|---|
| ampicillin | 100 | 3.13 | 3.13 | 100 | 3.13 | 100 | >200 |
| piperacillin | 6.25 | 6.25 | 1.56 | 1.56 | 0.19 | 6.25 | 6.25 |
| carbenicillin | 100 | 100 | 0.09 | 100 | 1.56 | 50 | 100 |
| 1903 | 50 | 50 | 6.25 | 25 | 12.5 | 200 | 12.5 |
| 1905 | 200 | 25 | 6.25 | 50 | 25 | 200 | 25 |
| 1940 | 100 | 50 | 12.5 | 50 | 6.25 | >200 | 25 |
| 2742 | 100 | 100 | 12.5 | 100 | 6.25 | >200 | 25 |
| 2743 | 50 | 200 | 12.5 | 100 | 3.13 | >200 | 25 |
| 2744 | 100 | 100 | 3.13 | 12.5 | 12.5 | >200 | 25 |
| 2745 | 50 | 100 | 3.13 | 12.5 | 3.13 | >200 | 25 |
| 2746 | 200 | 200 | 12.5 | 100 | 3.13 | >200 | 50 |
| 2780 | >200 | 200 | 6.25 | 12.5 | 1.56 | >200 | 25 |
| 2781 | 50 | 100 | 3.13 | 6.25 | 3.13 | >200 | 12.5 |
| 2782 | 25 | 25 | 1.56 | 12.5 | 12.5 | >200 | 25 |
| 2833 | 25 | 100 | 3.13 | 100 | 6.25 | 200 | 25 |
| 2835 | 50 | 100 | 3.13 | 25 | 25 | 200 | 25 |
| 2916 | 50 | 50 | 1.56 | 6.25 | 6.25 | >200 | 25 |
| 2917 | 50 | 12.5 | 6.25 | 6.25 | 3.1 | >200 | 25 |
| 2918 | 100 | 6.25 | 6.25 | 6.25 | 6.25 | >200 | 50 |
| 2919 | 25 | 12.5 | 3.13 | 25 | 50 | 200 | 50 |
| 3002 | 50 | 6.25 | 3.13 | 25 | 1.56 | 200 | 12.5 |
Key: AB= A. baumannii ATCC 19606; EC= E. coli BW25113; ECΔtolC= E. coli BW25113 ΔtolC derivative JW5503-1; PV= P. vulgaris ATCC 33420; PM= P. mirabilis ATCC 25933; KP= K. pneumoniae ATCC 13883; PA= P. aeruginosa PA01.
Activity against gram-negative bacteria in the presence of clavulanic acid
In order to determine whether the activity of these hybrids is affected by β-lactamases, their activity was determined in the presence of sub-inhibitory levels of clavulanic acid (6 µg/ml; Table 3 and Supporting information, Table S2). In the presence of clavulanic acid, hybrids displayed improved activities against wild type E. coli BW25113 with MIC activities comparable to the isogenic strain lacking chromosomally encoded β-lactamase AmpC. Improvement in MIC activity was also observed against P. aeruginosa in the presence of clavulanic acid with many hybrid compounds showing activity in the 3.1–6.3 µg/ml range. Against E. coli ATCC 35218, expressing the TEM-1 β-lactamase, clavulanic acid improved the activity of piperacillin, ampicillin, and compounds in the series with the exception of 2742, 2743 and 2746 (Supporting information, Table S2). For example, in the presence of clavulanic acid activities of 2781, 2782, and 2916 were 25 µg/ml compared to >200 µg/ml in its absence. However, both the hybrids and control drugs did not show any activity against a panel of organisms expressing multiple β-lactamases (e.g. CTX-M-14, KPC-3, and VIM-1) suggesting that they are substrates for these and likely other β-lactamases (Supporting information, Table S2).
Table 3.
MIC (µg/ml) of ampicillin-tetramic acid hybrids against gram-negative bacteria in presence of clavulanic acid.
| Compounds clavulanic acid (6µg/ml) |
EC | KP | ECΔampC | PA | ||||
|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | |
| ampicillin | 1.56 | 3.13 | 25 | 100 | 1.56 | 3.13 | 100 | >200 |
| piperacillin | 3.13 | 6.25 | 6.25 | 6.25 | 1.56 | 1.56 | 3.13 | 6.25 |
| 1903 | 12.5 | 50 | 25 | 200 | 1.56 | 6.25 | 6.25 | 12.5 |
| 1905 | 12.5 | 25 | 100 | 200 | 12.5 | 25 | 12.5 | 25 |
| 1940 | 12.5 | 50 | 25 | >200 | 6.25 | 12.5 | 12.5 | 25 |
| 2742 | 25 | 100 | 100 | >200 | 12.5 | 25 | 12.5 | 25 |
| 2743 | 12.5 | 200 | 25 | >200 | 6.25 | 12.5 | 12.5 | 25 |
| 2744 | 25 | 100 | 25 | >200 | 12.5 | 25 | 25 | 25 |
| 2745 | 12.5 | 100 | 25 | >200 | 6.25 | 6.25 | 25 | 25 |
| 2746 | 50 | 200 | >200 | >200 | 25 | 50 | 25 | 50 |
| 2780 | 50 | 200 | 25 | >200 | 3.13 | 12.5 | 12.5 | 25 |
| 2781 | 6.25 | 100 | 50 | >200 | 3.13 | 6.25 | 3.13 | 12.5 |
| 2782 | 3.13 | 25 | 50 | >200 | 3.13 | 6.25 | 12.5 | 25 |
| 2833 | 12.5 | 100 | 100 | 200 | 6.25 | 12.5 | 6.25 | 25 |
| 2835 | 6.25 | 100 | 100 | 200 | 12.5 | 50 | 6.25 | 25 |
| 2916 | 12.5 | 50 | 100 | >200 | 3.13 | 6.25 | 6.25 | 25 |
| 2917 | 12.5 | 12.5 | 50 | >200 | 12.5 | 25 | 6.25 | 25 |
| 2918 | 12.5 | 6.25 | 50 | >200 | 6.25 | 25 | 6.25 | 50 |
| 2919 | 50 | 12.5 | 100 | 200 | 6.25 | 12.5 | 12.5 | 50 |
| 3002 | 6.25 | 6.25 | 25 | 200 | 6.25 | 6.25 | 3.13 | 12.5 |
Key: EC= E. coli BW25113; KP= K. pneumoniae ATCC 13883; PA= P. aeruginosa PA01; ECΔampC – E. coli BW25113 ΔampC derivative JW4111-2. Clavulanic acid MICs were 100 µg/ml against EC and KP; 200 µg/ml against PA; and 25 µg/ml against ECΔampC.
Comparison of compound activities under iron-depleted media
Unlike laboratory media, iron is not freely available in the body [35]. Hence, testing of β-lactam-siderophore conjugates under iron-depleted conditions improves the activity of these agents due to their increased cellular uptake via siderophore transporters which are more highly expressed under these conditions [36]. This led us to explore if tetramic acid chelation of metal ions may alter cellular uptake and whether testing in iron-depleted Mueller-Hinton media could enhance their activity. We first confirmed that the compounds chelate ferric ion. The addition of iron (III) chloride to 2916 resulted in the formation of an intense red to brown color consistent with the formation of a tetramic acid – iron complex [37]. The stoichiometry of 3:1 tetramic acid: iron for the complex was determined by the continuous variation method [24, 38] (Supporting information, Figure S1). Next, we tested the activity of these hybrids in iron-deficient conditions with 2, 2'-bipyridyl (30 µg/ml) to increase the expression of siderophore transporters [39–41]. A significant increase in siderophore production was confirmed in the test media for P. aeruginosa and E. coli (Supporting information, Table S3), which suggests an increase in cognate siderophore receptors and other transporters for iron uptake[36]. Under iron-depleted conditions, 2916 and other test compounds either showed no change in activities or exhibited only a 2–4-fold difference in activities (Table 4). The trend in the broth MICs for the compounds against P. aeruginosa and E. coli strains was also evident from agar diffusion tests (Supporting information, Table S4). It was hypothesized that bacterial siderophores with higher affinity than tetramic acids for iron might be limiting the activity of these hybrids. Thus, the apparent binding constant (Kd, app) of 2916 for iron (III) was measured as 0.55 × 10−29 M3 by monitoring the loss of absorbance of 2916-iron (III) complex at 460 nm in presence of increasing concentrations of EDTA (Supporting information, Figure S2), which is similar to the previously reported values for tetramic acids [24]. Since in this experiment iron affinity of bidentate chelating tetramic acids is being compared to hexadentate bacterial siderophores, the parameter pM was also calculated for the binding for comparative purposes [42]. The pM value is expressed as –log [Fe3+], where [Fe3+] is the free iron concentration in the presence of 10−5 M total iron chelator and 10−6 M total Fe3+ at pH 7.4. A larger pM value indicates a more effective ligand under the given conditions. Using this equation, the pM for 2916 was calculated to be 20.3. Thus, although the tetramic acid hybrids (Kd 0.55 × 10−29 M3 and pM 20.3) may compete favorably with weak iron chelating siderophores, such as pyochelin (Kd 10−5 M and pM 6) [43, 44], they will likely be outcompeted by siderophores with larger pM values, such as P. aeruginosa pyoverdin (Kd 10−32 M and pM 27) [45, 46] or E. coli enterobactin (Kd 10−52 M and pM 35.6) [42]. Further, the affinities of tetramic acid for the membrane receptors of siderophores and iron uptake transporters are unknown. To explore these hypotheses, we tested the hybrid compounds against P. aeruginosa and E. coli mutants in which siderophore receptors or siderophores were inactivated by single gene deletions. This data is presented in Tables S5 and S6. In summary, deletion on the TonB protein in both species did not influence the activity of compounds; TonB plays a key role in the periplasmic uptake of siderophores as it interacts with several siderophore receptors to provide energy for transport [47]. The loss of enterobactin biosynthesis protein (ΔentB), the ferrienterobactin permease, and other iron transporters did not affect the activity of the compounds against E. coli. Similarly, against P. aeruginosathe loss of pyoverdin biosynthesis (ΔpvcA) [48] and pyoverdin receptors (e.g. ΔfpvA; ΔfpvB) [49, 50] did not affect the activities of the compounds. Together this suggests that the activities of the compounds are not influenced by siderophore competition and point to an alternate mechanism of uptake. In light of the compounds appearing as substrates for efflux pumps, we determined if the efflux pump inhibitor CCCP could enhance the activity of compounds against P. aeruginosa and E. coli. CCCP dissipates the bacterial proton motive force, leading to the inhibition of several efflux pumps [51]. As shown in Table 4, the addition of CCCP also significantly improved the activities of the compounds by 2–8 fold against P. aeruginosa and 2–16 fold against E. coli.
Table 4.
MIC (µg/ml) of ampicillin-tetramic acid hybrids against P. aeruginosa and E. coli in the presence of carbonyl cyanide m-chlorophenyl hydrazone and 2, 2′-bipyridyl.
| PA |
EC |
ECΔtolC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MH | MH+CCCP | MH+BP | MH+BP/CCCP | MH | MH+CCCP | MH+BP | MH+BP/CCCP | MH | MH+CCCP | MH+BP | MH+BP/CCCP | |
| Ampicillin | >200 | >200 | >200 | 12.5 | 3.13 | 1.56 | 3.13 | 0.78 | 3.13 | 1.56 | 3.13 | 0.39 |
| Piperacillin | 6.25 | 3.13 | 6.25 | 3.13 | 12.5 | 6.25 | 1.56 | 0.78 | 0.09 | 0.09 | 0.09 | 0.09 |
| Carbenicillin | 200 | 25 | 50 | 1.56 | 6.25 | 12.5 | 6.25 | 0.78 | 1.56 | 0.78 | 0.56 | 0.78 |
| 1903 | 12.5 | 3.13 | 12.5 | 1.56 | 50 | 25 | 50 | 12.5 | 6.2 | 1.56 | 1.56 | 3.13 |
| 1905 | 25 | 3.13 | 6.25 | 0.78 | 25 | 12.5 | 12.5 | 6.25 | 6.25 | 1.56 | 1.56 | 0.78 |
| 1940 | 25 | 12.5 | 12.5 | 6.25 | 50 | 25 | 25 | 12.5 | 12.5 | 6.25 | 3.13 | 1.56 |
| 2742 | 25 | 12.5 | 12.5 | 6.25 | 100 | 50 | 100 | 12.5 | 12.5 | 6.25 | 6.25 | 1.56 |
| 2743 | 25 | 6.25 | 12.5 | 3.13 | 200 | 25 | 50 | 12.5 | 12.5 | 3.13 | 6.25 | 1.56 |
| 2744 | 25 | 12.5 | 50 | 25 | 100 | 25 | 50 | 25 | 3.13 | 1.56 | 3.13 | 0.78 |
| 2745 | 25 | 12.5 | 50 | 12.5 | 100 | 12.5 | 50 | 12.5 | 3.13 | 1.56 | 1.56 | 0.78 |
| 2746 | 50 | 12.5 | 50 | 12.5 | 200 | 12.5 | 100 | 12.5 | 12.5 | 3.13 | 6.25 | 1.56 |
| 2780 | 25 | 6.25 | 25 | 6.25 | 200 | 25 | 50 | 25 | 6.25 | 3.13 | 6.25 | 3.13 |
| 2781 | 12.5 | 3.13 | 6.25 | 1.56 | 100 | 25 | 25 | 3.13 | 3.13 | 1.56 | 3.13 | 1.56 |
| 2782 | 25 | 12.5 | 25 | 12.5 | 25 | 25 | 12.5 | 3.13 | 1.56 | 1.56 | 3.13 | 1.56 |
| 2833 | 25 | 12.5 | 25 | 12.5 | 100 | 50 | 50 | 6.25 | 3.13 | 1.56 | 1.56 | 0.78 |
| 2835 | 25 | 12.5 | 25 | 12.5 | 100 | 25 | 25 | 12.5 | 3.13 | 1.56 | 1.56 | 0.78 |
| 2916 | 25 | 12.5 | 25 | 6.25 | 50 | 25 | 25 | 12.5 | 1.56 | 0.78 | 1.56 | 0.78 |
| 2917 | 25 | 6.25 | 25 | 12.5 | 50 | 25 | 25 | 12.5 | 3.13 | 1.56 | 3.13 | 1.56 |
| 2918 | 50 | 6.25 | 25 | 6.25 | 200 | 12.5 | 25 | 6.25 | 1.56 | 0.78 | 1.56 | 0.78 |
| 2919 | 50 | 25 | 50 | 12.5 | 100 | 100 | 100 | 25 | 6.25 | 3.13 | 3.13 | 1.56 |
| 3002 | 12.5 | 6.25 | 12.5 | 3.13 | 6.25 | 1.56 | 3.13 | 1.56 | 3.13 | 1.56 | 3.13 | 0.78 |
Key: MH= Mueller-Hinton (MH) broth; MH+CCCP= MH broth containing 50 µg/ml carbonyl cyanide m-chlorophenyl hydrazone (CCCP); MH+BP= MH broth containing 30 µg/ml 2, 2′-bipyridyl; MH+BP/CCCP= MH broth containing 30 µg/ml 2,2’-bipyridyl and 50 µg/ml carbonyl cyanide m-chlorophenyl hydrazine. PA= P. aeruginosa PA01; EC= BW25113; ECΔtolC= E. coli BW25113 ΔtolC derivative JW5503-1.
Conclusions
Our strategy for improving the activity of the β-lactam antibiotic ampicillin by fusing its amine terminal with iron chelating tetramic acids produced compounds with weak to moderate antibacterial activities. Compounds in this series were identified with improved activity against P. aeruginosa. The P. aeruginosa quorum-sensing signal N-3-oxododecanoyl-l-homoserine degrades under physiological conditions to produce a tetramic acid. The role of the rearranged tetramic acid product is not yet clear, but it has been proposed to play a function in iron acquisition [24]. Other β-lactams siderophores conjugates have been reported to utilize cognate siderophore receptors for entry through the outer Gram-negative membrane. However, tetramic acids are in a different chemical class to hydroxamates and catechols that are typically utilized in siderophore drug conjugates. These conjugates must be recognized by specific outer membrane receptors whose expression levels are highest when iron concentrations are low. The activity of the tetramic acids β-lactam hybrids was independent of media iron concentration and hence we speculate that they use a different receptor or porin to enter P. aeruginosa. Future elucidation of this receptor or uptake mechanism could lead to compounds that are optimized to gain entry into P. aeruginosa and other Gram-negative pathogens. In addition, key limitations of the current series include susceptibility to efflux pumps and inactivation by β-lactamases. These liabilities may be addressed through combinations with efflux pump or β-lactamase inhibitors combined with future chemical optimization of the tetramic acid-β-lactam hybrids. Our study provides the first description of tetramic acid-β-lactam hybrids and suggests that further studies are required to determine the mechanism of entry to the periplasmic space to fully explore the utility of tetramic acids as Trojan horses in overcoming outer-membrane permeability mediated resistance to β-lactam antibiotics.
Experimental procedure
Chemistry
Details for the preparation of the amipicillin-tetramic acid hybrids are provided in the Supporting information. Briefly, various amino acid methyl esters were first acylated with methyl malonyl chloride and this intermediate was cyclized under Lacey-Dieckmann condition to afford 3-methoxycarbonyl tetramic acids. Microwave heating of the 3-methoxycarbonyl tetramic acids with ampicillin in DMF at 100°C for three minutes followed by purification by reverse phase column chromatography afforded the ampicillin – tetramic acid hybrids in 6 – 67% yields. The final compounds were characterized by LC-MS, 13CNMR, and 1HNMR. All reported compounds were ≥95% pure.
Biology
Determination of minimum inhibitory concentrations (MICs) in Mueller-Hinton media
The MICs of compounds were determined in Mueller-Hinton (MH) broth according to the Clinical Laboratory Standards Institute (CLSI), using the microbroth dilution method in 96-well round bottom plates. The bacterial inocula were prepared by growing colonies from MH agar in MH broth. The final test concentration of compounds ranged from 200-0.2 µg/ml and bacterial inocula of 105 CFU/ml. After incubation at 37°C for 18–20 hours the MICs were recorded as the lowest concentration of test compound inhibiting visual growth [34]. MICs were also determined in the presence of the β-lactamase inhibitor clavulanic acid (6 µg/ml). MICs were performed in at least duplicate and the mean MIC is reported.
Determination of minimum inhibitory concentrations (MICs) in iron-depleted media
MICs in iron-depleted media were similarly determined with the following exceptions. Firstly, bacteria were grown on MH agar containing 30 µg/ml 2,2'-bipyridyl that chelates ferric iron.[52] Bacterial inocula were then prepared by growing colonies in MH broth containing 2, 2'- bipyridyl (30 µg/ml) to mid-logarithmic phase. MICs were then perform as above, but using MH broth that contained 2, 2'- bipyridyl (30 µg/ml); to permit adequate iron chelation, the 2,2'- bipyridyl was added to broth at least 3 hours before setting up MIC test. The MICs were read after 18–20 hours as above. MICs under iron-replete conditions were also assessed in the presence of the proton pump inhibitor 50 µg/ml carbonyl cyanide m-chlorophenyl (CCCP). MICs were performed in at least duplicate and the mean MIC is reported.
Detection of siderophore production by CAS liquid assay
The increased production of siderophores in iron-depleted media was determined using the reported chrome azurol S assay (CAS) liquid assay [53]. The following organisms were used to test for siderophore production: P. aeruginosa PAO1 and E. coli strains BW25113. The CAS reagent was prepared by adding 10 mL of 10 mM ferric chloride in hydrochloric acid (100 mM) to 590 mL of a 1 mM of aqueous solution of CAS. The Fe-CAS solution was added to 400 mL of a 2 mM aqueous hexadecyl-trimethyl-ammonium bromide (HDTMA) to yield CAS-Fe-HDTMA. Bacteria were grown for 18–20 hours in MH broth with and without 2, 2'- bipyridyl (30µg/ml) and culture turbidity determined at 600 nm. Subsequently, supernatants were filtered through 0.2 µM PES membrane filters to remove bacteria. Supernatants (700 µl) were mixed with 350 µl of the CAS-Fe-HDTMA reagent, incubated in the dark for an hour and absorbance recorded at 630 nm. The relative percentage of siderophore production was then calculated based on changes in absorbance readings as previously reported [53]. Triplicate cultures were used and all data were normalized based on the turbidity of the corresponding cultures.
Cytotoxicity assay
The HeLa cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) in a humidified incubator (37°C, 5% CO2). Cells were cultured to semi-confluence, trypsinized, and collected by centrifugation. Washed cells were resuspended in fresh DMEM at ~106 cells/mL and dispensed into 96-well microtitre plates (100 µL/well). After overnight incubation at 37°C, compounds were added at two-fold serial dilutions of test compounds (512-0.2 µg/mL) and plates incubated for 48 hours. Cytotoxicity was evaluated using Promega CellTiter Glo® luminescence assay. IC50 data were obtained from dose–response curves plotted using Graphpad prism 5.
Determination of antibiotic susceptibility by agar diffusion
The susceptibilities of bacteria to test compounds were also evaluated by the agar diffusion technique as described,[54] with minor changes. Briefly, bacteria was cultured in low-iron MH broth and 100 µl of a 106 CFU/ml sample was spread onto MH agar that contained 2,2'- bipyridyl (30 µg/ml). The plates were allowed to dry, before antibiotics (50 µl; 0.2 mM) were added to wells that were prepared with a core borer (6.35 mm). The plates were incubated at 37°C overnight and the diameter of zone of inhibition was measured (mm).
Supplementary Material
Acknowledgments
We thank Dr. Karen Bush at Indiana University, Bloomington for providing the β-lactamase producing bacterial strains. E. coli mutants were from the Keio Collection at The Coli Genetic Stock Center, Yale, Connecticut, U.S.A.; P. aeruginosa mutants were from the PA Two-Allele Library, Manoil Lab, University of Washington, Seattle, U.S.A. This work was funded by Grant 5R01AT006732 from the National Center for Complementary and Alternative Medicine at the National Institutes of Health, with additional funds from the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children’s Research Hospital.
References
- 1.WHO. Antimicrobial resistance: global report on surveillance. 2014
- 2.CDC. Antibiotic Resistance Threats in the US. 2013 [Google Scholar]
- 3.Rice LB. Mechanisms of resistance and clinical relevance of resistance to beta-lactams, glycopeptides, and fluoroquinolones. Mayo Clin Proc. 2012;87(2):198–208. doi: 10.1016/j.mayocp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh A, et al. Iron transport-mediated drug delivery using mixed-ligand siderophore-β-lactam conjugates. Chemistry & Biology. 1996;3(12):1011–1019. doi: 10.1016/s1074-5521(96)90167-2. [DOI] [PubMed] [Google Scholar]
- 5.Guerinot ML. Microbial Iron Transport. Annual Review of Microbiology. 1994;48(1):743–772. doi: 10.1146/annurev.mi.48.100194.003523. [DOI] [PubMed] [Google Scholar]
- 6.Heinisch L, et al. Highly Antibacterial Active Aminoacyl Penicillin Conjugates with Acylated Bis-Catecholate Siderophores Based on Secondary Diamino Acids and Related Compounds. Journal of Medicinal Chemistry. 2002;45(14):3032–3040. doi: 10.1021/jm010546b. [DOI] [PubMed] [Google Scholar]
- 7.Ji C, Miller PA, PA MJ. Iron Transport-Mediated Drug Delivery: Practical Syntheses and In Vitro Antibacterial Studies of Tris-Catecholate Siderophore–Aminopenicillin Conjugates Reveals Selectively Potent Antipseudomonal Activity. Journal of the American Chemical Society. 2012;134(24):9898–9901. doi: 10.1021/ja303446w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MF, et al. Pyridone-Conjugated Monobactam Antibiotics with Gram-Negative Activity. Journal of Medicinal Chemistry. 2013;56(13):5541–5552. doi: 10.1021/jm400560z. [DOI] [PubMed] [Google Scholar]
- 9.van Delden C, Page MG, Kohler T. Involvement of Fe uptake systems and AmpC beta-lactamase in susceptibility to the siderophore monosulfactam BAL30072 in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57(5):2095–2102. doi: 10.1128/AAC.02474-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russo TA, et al. In vivo and in vitro activity of the siderophore monosulfactam BAL30072 against Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy. 2011;66(4):867–873. doi: 10.1093/jac/dkr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hider RC, Kong X. Chemistry and biology of siderophores. Natural Product Reports. 2010;27(5):637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 12.Page MGP. Siderophore conjugates. Annals of the New York Academy of Sciences. 2013;1277(1):115–126. doi: 10.1111/nyas.12024. [DOI] [PubMed] [Google Scholar]
- 13.Mo X, Li Q, Ju J. Naturally occurring tetramic acid products: isolation, structure elucidation and biological activity. RSC Advances. 2014;4(92):50566–50593. [Google Scholar]
- 14.Royles BJL. Naturally Occurring Tetramic Acids: Structure, Isolation, and Synthesis. Chemical Reviews. 1995;95(6):1981–2001. [Google Scholar]
- 15.Schobert R, Schlenk A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorganic & Medicinal Chemistry. 2008;16(8):4203–4221. doi: 10.1016/j.bmc.2008.02.069. [DOI] [PubMed] [Google Scholar]
- 16.Zhu W, et al. Antibacterial drug leads targeting isoprenoid biosynthesis. Proceedings of the National Academy of Sciences. 2013;110(1):123–128. doi: 10.1073/pnas.1219899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pronin SV, et al. Chemical Synthesis Enables Biochemical and Antibacterial Evaluation of Streptolydigin Antibiotics. Journal of the American Chemical Society. 2011;133(31):12172–12184. doi: 10.1021/ja2041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherian PT, et al. Chemical Modulation of the Biological Activity of Reutericyclin: a Membrane-Active Antibiotic from Lactobacillusreuteri. Sci. Rep. 2014;4 doi: 10.1038/srep04721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee LV, et al. Biophysical Investigation of the Mode of Inhibition of Tetramic Acids, the Allosteric Inhibitors of Undecaprenyl Pyrophosphate Synthase. Biochemistry. 2010;49(25):5366–5376. doi: 10.1021/bi100523c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, et al. Structures of Kibdelomycin Bound to Staphylococcus aureus GyrB and ParE Showed a Novel U-Shaped Binding Mode. ACS Chemical Biology. 2014;9(9):2023–2031. doi: 10.1021/cb5001197. [DOI] [PubMed] [Google Scholar]
- 21.Lebrun M-H, et al. Complexation of the fungal metabolite tenuazonic acid with copper (II), iron (III), nickel (II), and magnesium (II) ions. Journal of Inorganic Biochemistry. 1985;24(3):167–181. doi: 10.1016/0162-0134(85)85001-7. [DOI] [PubMed] [Google Scholar]
- 22.Steyn PS, Rabie CJ. Characterization of magnesium and calcium tenuazonate from Phoma sorghina. Phytochemistry. 1976;15(12):1977–1979. [Google Scholar]
- 23.Vinale F, et al. Harzianic acid: a novel siderophore from Trichoderma harzianum. FEMS Microbiology Letters. 2013;347(2):123–129. doi: 10.1111/1574-6968.12231. [DOI] [PubMed] [Google Scholar]
- 24.Kaufmann GF, et al. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc Natl Acad Sci U S A. 2005;102(2):309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bush LM, Johnson CC. Ureidopenicillins and beta-lactam/beta-lactamase inhibitor combinations. Infectious disease clinics of North America. 2000;14(2):409–433. doi: 10.1016/s0891-5520(05)70255-5. [DOI] [PubMed] [Google Scholar]
- 26.Ferres H, Basker MJ, O'Hanlon PJ. Beta-lactam antibiotics. I Comparative structure-activity relationships of 6-acylaminopenicillanic acid derivatives and their 6-[D-alpha-acylaminophenylacetamido] penicillanic acid analogues. The Journal of Antibiotics. 1974;27(12):922–930. doi: 10.7164/antibiotics.27.922. [DOI] [PubMed] [Google Scholar]
- 27.Ferres H, et al. Beta-lactam antibiotics. II Structure-activity relationships of 6-[alpha-(alpha-ureidoacylamino)acylamino] penicillanic acids. The Journal of Antibiotics. 1978;31(10):1013–1022. doi: 10.7164/antibiotics.31.1013. [DOI] [PubMed] [Google Scholar]
- 28.Knudsen ET, Rolinson GN, Sutherland R. Carbenicillin: a new semisynthetic penicillin active against Pseudomonas pyocyanea. British Medical Journal. 1967;3(5557):75–78. doi: 10.1136/bmj.3.5557.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chadwick P. Effect of Carbenicillin on Pseudomonas aeruginosa. Canadian Medical Association Journal. 1969;101(7):74–80. [PMC free article] [PubMed] [Google Scholar]
- 30.Peukert S, et al. Design and structure-activity relationships of potent and selective inhibitors of undecaprenyl pyrophosphate synthase (UPPS): tetramic, tetronic acids and dihydropyridin-2-ones. Bioorg Med Chem Lett. 2008;18(6):1840–1844. doi: 10.1016/j.bmcl.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Folkes A, et al. Design, synthesis and in vitro evaluation of potent, novel, small molecule inhibitors of plasminogen activator inhibitor-1. Bioorg Med Chem Lett. 2002;12(7):1063–1066. doi: 10.1016/s0960-894x(02)00078-1. [DOI] [PubMed] [Google Scholar]
- 32.Nolte MJ, Steyn PS, Wessels PL. Structural investigations of 3-acylpyrrolidine-2,4-diones by nuclear magnetic resonance spectroscopy and X-ray crystallography. Journal of the Chemical Society, Perkin Transactions. 1980;1:1057–1065. [Google Scholar]
- 33.Jeong Y-C, Moloney MG. Synthesis of and Tautomerism in 3-Acyltetramic Acids. The Journal of Organic Chemistry. 2011;76(5):1342–1354. doi: 10.1021/jo102304y. [DOI] [PubMed] [Google Scholar]
- 34.Yendapally R, et al. N-Substituted 3-Acetyltetramic Acid Derivatives as Antibacterial Agents. Journal of Medicinal Chemistry. 2008;51(5):1487–1491. doi: 10.1021/jm701356q. [DOI] [PubMed] [Google Scholar]
- 35.Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- 36.Zheng T, Nolan EM. Enterobactin-mediated delivery of beta-lactam antibiotics enhances antibacterial activity against pathogenic Escherichia coli. J Am Chem Soc. 2014;136(27):9677–9691. doi: 10.1021/ja503911p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lebrun MH, et al. Relationships between the structure and the phytotoxicity of the fungal toxin tenuazonic acid. Phytochemistry. 1988;27(1):77–84. [Google Scholar]
- 38.Renny JS, et al. Method of continuous variations: applications of job plots to the study of molecular associations in organometallic chemistry. Angew Chem Int Ed Engl. 2013;52(46):11998–12013. doi: 10.1002/anie.201304157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter BM, Whitmire JM, Merrell DS. This is not your mother's repressor: the complex role of fur in pathogenesis. Infect Immun. 2009;77(7):2590–2601. doi: 10.1128/IAI.00116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji C, Miller PA, Miller MJ. Iron transport-mediated drug delivery: practical syntheses and in vitro antibacterial studies of tris-catecholate siderophore-aminopenicillin conjugates reveals selectively potent antipseudomonal activity. J Am Chem Soc. 2012;134(24):9898–9901. doi: 10.1021/ja303446w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown MF, et al. Pyridone-conjugated monobactam antibiotics with gram-negative activity. J Med Chem. 2013;56(13):5541–5552. doi: 10.1021/jm400560z. [DOI] [PubMed] [Google Scholar]
- 42.Carrano CJ, Raymond RN. Ferric Ion Sequestering Agents. 2. Kinetics and Mechanism of Iron Removal from Transferrin by Enterobactin and Synthetic Tricatechols. J Am Chem Soc. 1979;101:5401–5404. [Google Scholar]
- 43.Cox CD, Graham R. Isolation of an iron-binding compound from Pseudomonas aeruginosa. Journal of Bacteriology. 1979;137(1):357–364. doi: 10.1128/jb.137.1.357-364.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng T, Nolan EM. Siderophore-based detection of Fe(iii) and microbial pathogens. Metallomics. 2012;4(9):866–880. doi: 10.1039/c2mt20082a. [DOI] [PubMed] [Google Scholar]
- 45.Meyer JM, Abdallah MA. The Fluorescent Pigment of Pseudomonas fluorescens: Biosynthesis, Purification and Physicochemical Properties. Microbiology. 1978;107(2):319–328. [Google Scholar]
- 46.Albrecht-Gary A-M, et al. Bacterial Iron Transport: Coordination Properties of Pyoverdin PaA, a Peptidic Siderophore of Pseudomonas aeruginosa. Inorganic Chemistry. 1994;33(26):6391–6402. [Google Scholar]
- 47.Adams H, et al. Interaction of TonB with the outer membrane receptor FpvA of Pseudomonas aeruginosa. J Bacteriol. 2006;188(16):5752–5761. doi: 10.1128/JB.00435-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drake EJ, Gulick AM. Three-dimensional structures of Pseudomonas aeruginosa PvcA and PvcB, two proteins involved in the synthesis of 2-isocyano-6,7-dihydroxycoumarin. J Mol Biol. 2008;384(1):193–205. doi: 10.1016/j.jmb.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar RJ, et al. Interactions between an anti-sigma protein and two sigma factors that regulate the pyoverdine signaling pathway in Pseudomonas aeruginosa. BMC Microbiol. 2014;14:287. doi: 10.1186/s12866-014-0287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghysels B, et al. FpvB, an alternative type I ferripyoverdine receptor of Pseudomonas aeruginosa. Microbiology. 2004;150(Pt 6):1671–1680. doi: 10.1099/mic.0.27035-0. [DOI] [PubMed] [Google Scholar]
- 51.Lomovskaya O, et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45(1):105–116. doi: 10.1128/AAC.45.1.105-116.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mochizuki H, et al. Bactericidal activity of M14659 enhanced in low-iron environments. Antimicrob Agents Chemother. 1988;32(11):1648–1654. doi: 10.1128/aac.32.11.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dale SE, et al. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect Immun. 2004;72(1):29–37. doi: 10.1128/IAI.72.1.29-37.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wencewicz TA, et al. Trihydroxamate siderophore-fluoroquinolone conjugates are selective sideromycin antibiotics that target Staphylococcus aureus. Bioconjug Chem. 2013;24(3):473–486. doi: 10.1021/bc300610f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.