Abstract
Fructans from agave have received specific attention because of their highly branched fructan content. We have previously reported that the degree of polymerization (dp) influences their biological activity. Therefore, the aim of this study was to investigate the effect of unfractionated and fractionated fructans (higher and lower dps) from Agave tequilana in high-fat diet-induced (HFD) obese mice. Fructans with a lower dp (HFD+ScF) decreased weight gain by 30 %, body fat mass by 51 %, hyperglycemia by 25 % and liver steatosis by 40 %. Interestingly, unfractionated fructans (HFD+F) decreased glucose and triglycerides (TG), whereas fractionated fructans with a higher dp (HFD+LcF) decreased TG but not glucose; in contrast, HFD+ScF decreased glucose but not TG. Our findings suggest that both higher and lower dp agave fructans have complementary effects in metabolic disorders related to obesity. These findings may contribute to the development of improved food supplements with a specific ratio combination of fructans with different dps.
Electronic supplementary material
The online version of this article (doi:10.1007/s11130-016-0578-x) contains supplementary material, which is available to authorized users.
Keywords: Dietary fiber, Fructans, Agave, Liver steatosis
Introduction
Dietary fibers, such as inulin-type fructans, are selectively used and fermented by the gut, and several studies have demonstrated their health benefits [1–4]. For example, these non-digestible carbohydrates have been demonstrated to reduce weight gain and related metabolic disorders via specific actions on food intake [5–7]. Most of these findings have been obtained following supplementation with inulin-type fructans from Jerusalem artichoke (Helianthus tuberosus) and chicory (Cichorium intybus) [8]. The inulin from these sources comprises linear chains of β (2–1) fructans (unbranched). Interestingly, Agave tequilana Weber var. azul contains complex fructans with demonstrated bioactivity. These fructans are highly branched with both beta (2–1) and beta (2–6) linkages [9, 10]. Moreover, they are resistant to hydrolysis by human digestive enzymes and may be fermented by colonic microbiota; however, their mode of action is not completely elucidated. Fructans from Agave tequilana and Agave angustifolia have been primarily investigated as a complex mix of different chain lengths (total fructans). C57Bl/6J mice fed a standard diet supplemented with fructans from Agave tequilana Gto., exhibited reduced food intake, body weight and plasma glucose and lipids [11]. In diabetic rats with normal weight, fructans from Agave angustifolia diminished hyperglycemia and liver steatosis [12]. A previous study conducted in our laboratory demonstrated that both the degree of polymerization (dp) and the demineralization process influence the biological activity of agave fructans. Our studies indicate that the treatment of obese mice with fructans with a lower dp (dp < 10) from A. tequilana did not increase the count of fecal Bifidobacteria; however, it reduced body weight. In contrast, obese mice that received total fructans exhibited an increased fecal Bifidobacteria count; however, they did not exhibit changes in the lipid profile or body weight [13]. It was subsequently reported that agavins (agave fructans) with a lower dp from A. angustifolia and A. potatorum promoted the release of peptides involved in appetite regulation and may thus be involved in the control of obesity and its associated metabolic disorders [14]. On this basis, the present study aimed to investigate the effect of unfractionated and fractionated fructans (higher and lower dp) from Agave tequilana in high-fat diet-induced obese mice.
Materials and Methods
Agave Fructan Extraction, Purification and Carbohydrate Profile Characterization
Agave tequilana Weber var. azul plants were collected from an endemic growing area in Tequila, Jalisco, Mex. The collection, purification and characterization were performed as previously described [13]. The total contents of the fructans were labeled F, whereas two fractions of fructans with different dp profiles were obtained via ultrafiltration procedures. Agave fructans with a higher dp >10 (labeled LcF) were obtained from the retentate of a 3 kDa membrane, whereas fructans with a lower dp < 10 (labeled ScF) were recovered from the retentate of a 1 kDa membrane.
Animals, Diets and Experimental Groups
Sixty male mice (C57/BL/6) with body weights of 20–25 g (Harlan/Envigo Inc.) were housed in a controlled environment with free access to food and water. After one week of acclimatization, the mice were randomly divided into two experimentation groups. A) Model validation; with two groups (n = 10/group): 1) was fed a standard diet (SD) with 18 % fat (energy density 3.1 kcal/g), and 2) was fed a high fat diet (HFD) with 60 % fat (energy density 5.1 kcal/g), both for eight weeks (20128S and TD.6414 diets, respectively, from Teklad Harlan/Envigo Inc.). B) Treatments; with four groups (n = 10/group): 3) Orafti sinergy1TM (HFD+OS1), unbranched inulin-type fructans from chicory, 4) unfractionated agave fructans (HFD+F) and fractionated, 5) agave fructans with a higher dp (HFD+LcF) and 6) a lower dp (HFD+ScF). The mice had free access to the HFD or SD in common cages (five per cage); however, they were orally administered with fructans at doses of 125 mg / 25 g of body weight individually three times per week for eight weeks. The study was approved by the local Animal Care and Use Committee and complied with local (NOM-062-ZOO-1999) and International Guidelines (Animal Welfare Assurance A5281-01).
Biochemical Assays
The fasting glucose concentration was determined from tail vein blood using a hand-held glucometer, ONETOUCH® ULTRA®, with reactive strips (Johnson & Johnson). At the end of the experiment, the total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL), low-density lipoproteins (LDL), very low-density lipoproteins (VLDL) and hepatic enzymes AST and ALT were measured via automated enzymatic methods on a Sincron-7 analyzer.
Oral Glucose Tolerance Test
An oral glucose tolerance test was performed on 6 h fasting-mice [15]. In brief, the mice were administered glucose via gavage (1 g/kg glucose, 20 % glucose solution). The blood glucose was determined using reactive strips on a glucose meter from whole blood drawn from the tail-tip capillary region at points 0, 30, 60 and 120 min after gavage.
Fat Pad Collection and Liver Histology
The mice were sacrificed via an i.p. injection with ketamine / xylazine (100/10 mg / kg). White adipose tissues were dissected and weighed. Liver tissues were stained with hematoxylin and eosin and observed under a light microscope (Leica, DMR). Biopsies were classified into three grades [16], in which a sample is classified as grade 1 when fat vacuoles are identified in 5–33 % of hepatocytes, grade 2 when 33–66 % of hepatocytes are affected by fat vacuoles, and grade 3 when fat vacuoles are identified in >66 % of hepatocytes.
Data Analyses
A Kolmogorov-Smirnov test was performed for all data to determine whether the values originate from a Gaussian distribution. In the first experiment “Model validation”, statistical differences between groups were assessed using unpaired t-tests two-tailed, whereas for the second experiment “Treatments”, statistical differences between groups were assessed using one-way ANOVA followed by a Dunnett post-hoc test to compare all treatments vs. HDF with GraphPad Prism 5 Software, Inc., USA. Differences were considered significant at p ≤ 0.05. The obtained data are expressed as the mean ± standard deviation (mean ± SD).
Results and Discussion
A limited number of studies have utilized branched fructans from agave. These studies were primarily designed to analyze the prebiotic effects from agave fructans in in vitro studies [17–21], animal models [13, 22, 23] and clinical trials [24–27], whereas other studies have investigated the effects on food intake, body weight gain and hyperglycemia [11, 14, 22, 28]. Only one study has been conducted to analyze liver steatosis in diabetic rats supplemented with agave fructans [12], and several studies have been designed to investigate the relationship between dp and biological activity [13, 20, 21]. Thus, our study aimed to investigate the effect of unfractionated and fractioned branched fructans (higher and lower dp) from Agave tequilana in HFD induced obese mice and compare them with unbranched –linear- chicory fructans. As expected, the final body weight, weight gain and fat mass in the mice fed a HFD were increased (p < 0.05) compared with the mice fed a SD (supplementary file 1). The weight gain in the HFD was approximately 10 g more than the SD, whereas the fat mass was increased more than 10-fold. The obese mice accumulated more epididymal (1.86 g) and subcutaneous (1.78 g) fat compared with visceral fat (0.68 g). The mice fed a SD attained a total fat accumulation of 0.34 g, whereas the mice fed a HFD accumulated 4.32 g (11.7-fold, compared with the mice fed a SD). The blood glucose, TG, TC and AST concentrations of the HFD mice were also increased (p < 0.05) compared with the SD mice. Finally, the mice fed a HFD exhibited substantially increased blood glucose, 60 mg/dl more than the SD, whereas the TG and TC increases were 100 and 80 mg / dl, respectively. Commercially inulin-type fructans from chicory (unbranched fructans) were used as a reference because they have been extensively investigated as prebiotics and may be used to compare branched fructans from agave. However, we did not identify statistically significant differences in the weight gain, fat mass, lipid profile or transaminases in the obese mice treated with unbranched fructans (HFD+OS1) (Table 1). Fructans from Agave tequilana with a lower dp (HFD+ScF) decreased (p ≤ 0.05) the final body weight (Table 1, Fig. 1a and b), weight gain and fat mass (Table 1). These animals exhibited a weight difference of 7.4 g compared with the HFD, which represents a decrease of 30 % in the body weight gain. HFD+ScF also accumulated 51 % less fat compared with the mice fed a HFD, which resulted in 2.28 g of total fat. We identified a positive linear correlation between the weight increase and fat pad accumulation in all treatments (Fig. 1c) with an R2 of 0.9535; however, the HFD+ScF showed reduced (p ≤ 0.05) epididymal and subcutaneous fat even if they were simultaneously fed a HFD. These findings indicate that ScF from Agave tequilana significantly reduces body weight gain by diminishing fat accumulation in obese mice. In contrast, unfractionated agave fructans (HFD+F) and agave fructans with a higher dp (HFD+LcF) did not exhibit statistical differences in body weight, weight gain or fat mass; however, TG decreased (p ≤ 0.05). Fructan supplementation with inulin-type fructans from chicory (HDF+OS1), unfractionated fructans (HFD+F) and fractionated fructans with a lower dp from agave (HFD+ScF) decreased (p ≤ 0.05) glucose and the area under the curve (AUC) in the oral glucose tolerance test (between 14 and 45 %) compared with the AUC of the obese mice fed a HFD. The obese mice exhibited a concentration of 171 mg / dl, whereas treatment with fructans from chicory (HFD+OS1) decreased hyperglycemia by 19 % (139 mg / dl), the treatment with agave unfractionated fructans (HFD+F) decreased hyperglycemia by 20 % (137 mg/dl) and fractionated fructans with lower dp (HFD+ScF) decreased hyperglycemia by 25 % (129 mg / dl) and the AUC was 24,375 units compared with 27,000 units in the obese mice fed a HFD (Table 1, Fig. 1d and e). In summary, unbranched fructans reduced glycemia; however, there was no change in the serum cholesterol and TG levels. Similar results have been demonstrated in dietary supplementation with inulin in rats fed a HFD [7]. Moreover, the mice fed branched fructans from A. tequilana with a lower dp (HFD+ScF) decreased body weight gain by 30 %, body fat mass by 51 % and glycemia by 25 %. Interestingly, unfractionated branched fructans (HFD+F) decreased blood glucose and TG, whereas fractionated fructans with a higher dp (HFD+LcF) decreased TG but not glucose; moreover, fructans with a lower dp (HFD+ScF) decreased blood glucose but not TG. Linear fructans from chicory exhibited similar effects on glucose to unfractionated branched fructans from agave. These findings may explain the evidence regarding reductions in weight gain and blood glucose concentration in different animal models supplemented with total fructans from Agave spp., including non-obese mice [11, 14], diabetic rats [12] and a hypercholesterolemic model [28]. C57BL/6J male mice fed a HFD share nearly the same human obesity phenotype; visceral adiposity, hyperglycemia, insulin and leptin resistance, as well as hepatic steatosis [29, 30]. In this study, the liver steatosis grades were affected by fructan supplementation (supplementary file 2). Treatment with unbranched fructans from chicory (HFD+OS1) reduced accumulated fat droplets (macrovesicular), which decreased the steatosis percentage (20 %) similar to the animals supplemented with fructans from agave with a higher dp (HFD+LcF). Moreover, treatment with unfractionated fructans (HFD+F) exhibited a less decrease in the degree of steatosis (10 %). The most interesting results were identified in the mice treated with fructans from agave with a lower dp (HFD+ScF), which indicated reduced steatosis (microvesicular) (40 %) with minimal inflammatory invasions and few hypertrophic hepatocytes (grade 1) compared with the untreated mice (HFD) with moderate steatosis (grade 2). These findings are consistent with the reduction in AST levels presented in Table 1 and could explain why liver steatosis decreased in diabetic rats supplemented with fructans from Agave angustifolia as previously reported [12]. Bacteria grew according to the molecular weight of the agave fructans [20], in which fructans with a higher dp exert a more pronounced prebiotic effect [31] in contrast to fructans with a lower dp that exhibit more anti-obesity effects [12, 13].
Table 1.
Effects of fructans on weight gain, body fat, lipid profile and liver enzymes in mice fed a HFD for eight weeks
| Obese | Treatments | ||||
|---|---|---|---|---|---|
| Linear chicory fructans | Branched Agave fructans | ||||
| Unfractionated | Fractionated | ||||
| HFD | HFD + OS1 | HFD + F | HFD + LcF | HFD + ScF | |
| Final body weight (g) | 36.71 ± 1.5 | 36.55 ± 3.6 | 35.52 ± 3.7 | 37.49 ± 4.2 | 33.5 ± 2.2* |
| Gain weight (g) | 16.37 ± 2.0 | 13.26 ± 1.0 | 12.54 ± 0.82 | 11.92 ± 1.2 | 8.96 ± 0.42* |
| Fat Mass (g) | 4.32 ± 0.25 | 3.82 ± 0.62 | 4.48 ± 0.37 | 4.20 ± 0.42 | 2.28 ± 0.52* |
| Epididymal (g) | 1.86 ± 0.10 | 1.68 ± 0.30 | 1.85 ± 0.14 | 2.04 ± 0.21 | 1.04 ± 0.15* |
| Visceral (g) | 0.67 ± 0.03 | 0.62 ± 0.09 | 0.51 ± 0.05 | 0.65 ± 0.06 | 0.42 ± 0.11 |
| Subcutaneous (g) | 1.78 ± 0.18 | 1.50 ± 0.25 | 1.19 ± 0.11 | 1.50 ± 0.15 | 0.71 ± 0.19* |
| Triglycerides (mg/dl) | 196.0 ± 60.4 | 136.6 ± 8.8 | 95.0 ± 5.5* | 92.6 ± 5.2* | 129.6 ± 9.8 |
| Total cholesterol (mg/dl) | 147.6 ± 8.5 | 140.0 ± 13.0 | 151.3 ± 2.1 | 145.6 ± 5.2 | 124.3 ± 3.1 |
| HDL (mg/dl) | 71.3 ± 10.9 | 80.0 ± 5.0 | 79.3 ± 4.0 | 70.6 ± 8.7 | 55.3 ± 8.5 |
| LDL (mg/dl) | 37.0 ± 10.6 | 32.67 ± 10.4 | 53.0 ± 2.6 | 56.3 ± 14.1 | 43.3 ± 9.3 |
| VLDL (mg/dl) | 39.3 ± 11.9 | 27.3 ± 1.6 | 19.0 ± 1.1 | 18.6 ± 0.8 | 25.6 ± 2.0 |
| Glucose (mg/dl) | 171.0 ± 17.1 | 139.1 ± 19.5* | 137.1 ± 14.11* | 160.4 ± 20.5 | 129.1 ± 22.4* |
| AST (U/l) | 159.0 ± 9.6 | 134.6 ± 22.5 | 146.6 ± 28.3 | 101.6 ± 21.2 | 66.3 ± 8.5* |
| ALT (U/l) | 64.6 ± 9.6 | 51.3 ± 11.6 | 46.3 ± 4.4 | 52.3 ± 1.4 | 47.0 ± 2.3 |
Mean ± SD, growth parameters n = 10, biochemical parameters n = 6. * p < 0.05
Abbreviations: ALT alanine aminotransferase, AST aspartate aminotransferase, F total fructans, HFD high-fat diet, HDL high-density lipoproteins, LcF fructans with higher dp, LDL low-density lipoproteins, OS1 Orafti sinergy1, ScF fructans with lower dp, SD standard-diet, VLDL very low-density lipoproteins
Fig. 1.
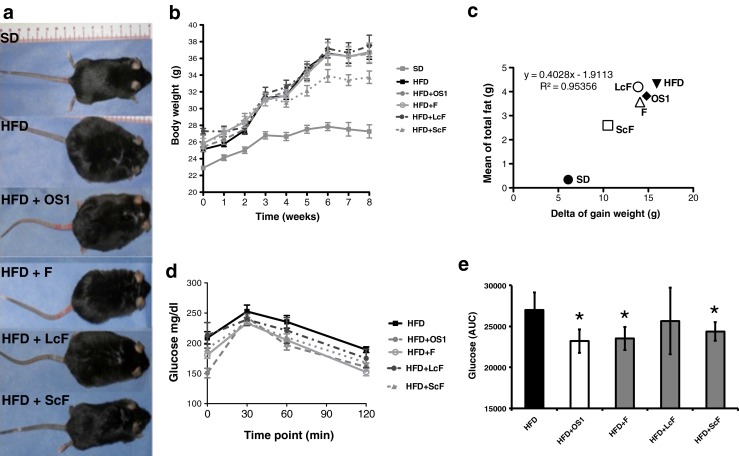
Growth performance and glycemia. Final weight (a), body weight kinetics (b), correlation of gain weight and body fat (c), glucose tolerance test and AUC (d and e) in mice fed a HFD and supplemented with fructans: chicory fructans (OS1), agave fructans unfractionated (F) and fractionated in a higher dp (LcF) and lower dp (ScF). Data are presented as the mean ± SD (n = 10). The asterisk (*) denotes a significant difference compared with a HFD (p < 0.05)
Conclusions
Our findings suggest that branched fructans from agave with a lower dp are responsible for most of the beneficial effects exerted by unfractionated fructans and represent a powerful tool to prevent body weight gain, fat accumulation, liver steatosis and hyperglycemia, despite high fat diet consumption. However, both higher and lower dp agave fructans have complementary effects in metabolic disorders related to obesity. A high concentration of lower dp fructans is required to achieve a reduction in weight gain and liver steatosis. Therefore, it would be desirable to have agave branched fructans in a specific ratio of higher and lower dp to achieve positive effects in all metabolic disorders related to obesity, and studies on this effective ratio should be performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 22 kb)
(PDF 8420 kb)
Acknowledgments
The authors would like to thank the AGARED (Mexican network of agave and derivatives) for the support received.
Abbreviations
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- dp
Degree of polymerization
- F
Total fructans
- HFD
High-fat diet
- LcF
Fructans with a higher dp
- OS1
Orafti sinergy1
- ScF
Fructans with a lower dp
- SD
Standard-diet
- TG
Triglycerides
- TC
Total cholesterol
Compliance with Ethical Standards
Statement on the Welfare of Animals
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human.
Conflict of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
All the authors reviewed the paper and approved the final version.
Contributor Information
A. L. Márquez-Aguirre, Phone: +52(33) 33455200, Email: amarquez@ciatej.mx
R. M. Camacho-Ruíz, Email: rcamacho@ciatej.mx
Y. K. Gutiérrez-Mercado, Email: ygutierrez@ciatej.mx
E. Padilla-Camberos, Email: epadilla@ciatej.mx
M. González-Ávila, Email: mgavila@ciatej.net.mx
F. J. Gálvez-Gastélum, Email: galvez_gastelum@hotmail.com
N. E. Díaz-Martínez, Email: ediaz@ciatej.mx
D. Ortuño-Sahagún, Email: dortuno@cucs.udg.mx
References
- 1.Meyer D. Health benefits of prebiotic fibers. Adv Food Nutr Res. 2015;74:47–91. doi: 10.1016/bs.afnr.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Leotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(Suppl 2):S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 3.Di Bartolomeo F, Van den Ende W. Fructose and fructans: opposite effects on health? Plant Foods Hum Nutr. 2015;70(3):227–237. doi: 10.1007/s11130-015-0485-6. [DOI] [PubMed] [Google Scholar]
- 4.Fuller S, Beck E, Salman H, Tapsell L. New horizons for the study of dietary fiber and health: a review. Plant Foods Hum Nutr. 2016;71(1):1–12. doi: 10.1007/s11130-016-0529-6. [DOI] [PubMed] [Google Scholar]
- 5.Cani PD, Delzenne NM. Interplay between obesity and associated metabolic disorders: new insights into the gut microbiota. Curr Opin Pharmacol. 2009;9(6):737–743. doi: 10.1016/j.coph.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol. 2014;20(43):16079–16094. doi: 10.3748/wjg.v20.i43.16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han KH, Tsuchihira H, Nakamura Y, Shimada K, Ohba K, Aritsuka T, Uchino H, Kikuchi H, Fukushima M. Inulin-type fructans with different degrees of polymerization improve lipid metabolism but not glucose metabolism in rats fed a high-fat diet under energy restriction. Dig Dis Sci. 2013;58(8):2177–2186. doi: 10.1007/s10620-013-2631-z. [DOI] [PubMed] [Google Scholar]
- 8.Chang WC, Jia H, Aw W, Saito K, Hasegawa S, Kato H. Beneficial effects of soluble dietary Jerusalem artichoke (Helianthus tuberosus) in the prevention of the onset of type 2 diabetes and non-alcoholic fatty liver disease in high-fructose diet-fed rats. Br J Nutr. 2014;112(5):709–717. doi: 10.1017/S0007114514001421. [DOI] [PubMed] [Google Scholar]
- 9.Lopez MG, Mancilla-Margalli NA, Mendoza-Diaz G. Molecular structures of fructans from Agave tequilana Weber var. azul. J Agric Food Chem. 2003;51(27):7835–7840. doi: 10.1021/jf030383v. [DOI] [PubMed] [Google Scholar]
- 10.Praznik W, Loppert R, Cruz Rubio JM, Zangger K, Huber A. Structure of fructo-oligosaccharides from leaves and stem of Agave tequilana Weber, var. azul. Carbohydr Res. 2013;381:64–73. doi: 10.1016/j.carres.2013.08.025. [DOI] [PubMed] [Google Scholar]
- 11.Urias-Silvas JE, Cani PD, Delmee E, Neyrinck A, Lopez MG, Delzenne NM. Physiological effects of dietary fructans extracted from Agave tequilana Gto. and Dasylirion spp. Br J Nutr. 2008;99(2):254–261. doi: 10.1017/S0007114507795338. [DOI] [PubMed] [Google Scholar]
- 12.Rendon-Huerta JA, Juarez-Flores B, Pinos-Rodriguez JM, Aguirre-Rivera JR, Delgado-Portales RE. Effects of different sources of fructans on body weight, blood metabolites and fecal bacteria in normal and obese non-diabetic and diabetic rats. Plant Foods Hum Nutr. 2012;67(1):64–70. doi: 10.1007/s11130-011-0266-9. [DOI] [PubMed] [Google Scholar]
- 13.Marquez-Aguirre AL, Camacho-Ruiz RM, Arriaga-Alba M, Padilla-Camberos E, Kirchmayr MR, Blasco JL, Gonzalez-Avila M. Effects of Agave tequilana fructans with different degree of polymerization profiles on the body weight, blood lipids and count of fecal Lactobacilli/Bifidobacteria in obese mice. Food Funct. 2013;4(8):1237–1244. doi: 10.1039/c3fo60083a. [DOI] [PubMed] [Google Scholar]
- 14.Santiago-Garcia PA, Lopez MG. Agavins from Agave angustifolia and Agave potatorum affect food intake, body weight gain and satiety-related hormones (GLP-1 and ghrelin) in mice. Food Funct. 2014;5(12):3311–3319. doi: 10.1039/C4FO00561A. [DOI] [PubMed] [Google Scholar]
- 15.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295(6):E1323–E1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ, Nonalcoholic Steatohepatitis Clinical Research Network (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321. doi:10.1002/hep.20701 [DOI] [PubMed]
- 17.Castro-Zavala A, Juarez-Flores BI, Pinos-Rodriguez JM, Delgado-Portales RE, Aguirre-Rivera JR, Alcocer-Gouyonnet F. Prebiotic effects of Agave salmiana fructans in Lactobacillus acidophilus and Bifidobacterium lactis cultures. Nat Prod Commun. 2015;10(11):1985–1988. [PubMed] [Google Scholar]
- 18.Gomez E, Tuohy KM, Gibson GR, Klinder A, Costabile A. In vitro evaluation of the fermentation properties and potential prebiotic activity of Agave fructans. J Appl Microbiol. 2010;108(6):2114–2121. doi: 10.1111/j.1365-2672.2009.04617.x. [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Vilet L, Garcia-Hernandez MH, Delgado-Portales RE, Corral-Fernandez NE, Cortez-Espinosa N, Ruiz-Cabrera MA, Portales-Perez DP. In vitro assessment of agave fructans (Agave salmiana) as prebiotics and immune system activators. Int J Biol Macromol. 2014;63:181–187. doi: 10.1016/j.ijbiomac.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Velazquez-Martinez JR, Gonzalez-Cervantes RM, Hernandez-Gallegos MA, Mendiola RC, Aparicio AR, Ocampo ML. Prebiotic potential of Agave angustifolia Haw fructans with different degrees of polymerization. Molecules. 2014;19(8):12660–12675. doi: 10.3390/molecules190812660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mueller M, Reiner J, Fleischhacker L, Viernstein H, Loeppert R, Praznik W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J Funct Foods. 2016;24:264–275. doi: 10.1016/j.jff.2016.04.010. [DOI] [Google Scholar]
- 22.Garcia-Curbelo Y, Bocourt R, Savon LL, Garcia-Vieyra MI, Lopez MG. Prebiotic effect of Agave fourcroydes fructans: an animal model. Food Funct. 2015;6(9):3177–3182. doi: 10.1039/C5FO00653H. [DOI] [PubMed] [Google Scholar]
- 23.Jasso-Padilla I, Juarez-Flores B, Alvarez-Fuentes G, De la Cruz-Martinez A, Gonzalez-Ramirez J, Moscosa-Santillan M, Gonzalez-Chavez M, Oros-Ovalle C, Prell F, Czermak P, Martinez-Gutierrez F. Effect of prebiotics of Agave salmiana fed to healthy Wistar rats. J Sci Food Agric. 2016 doi: 10.1002/jsfa.7764. [DOI] [PubMed] [Google Scholar]
- 24.Holscher HD, Bauer LL, Gourineni V, Pelkman CL, Fahey GC, Jr, Swanson KS. Agave inulin supplementation affects the fecal microbiota of healthy adults participating in a randomized, double-blind, placebo-controlled, crossover trial. J Nutr. 2015;145(9):2025–2032. doi: 10.3945/jn.115.217331. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Velazquez G, Diaz-Garcia L, Anzo A, Parra-Ortiz M, Llamosas-Gallardo B, Ortiz-Hernandez AA, Mancilla-Ramirez J, Cruz-Rubio JM, Gutierrez-Castrellon P. Safety of a dual potential prebiotic system from Mexican agave “Metlin(R) and Metlos(R)”, incorporated to an infant formula for term newborn babies: a randomized controlled trial. Rev Investig Clin Organo Hosp Enferm Nutr. 2013;65(6):483–490. [PubMed] [Google Scholar]
- 26.Lopez-Velazquez G, Parra-Ortiz M, Mora Ide L, Garcia-Torres I, Enriquez-Flores S, Alcantara-Ortigoza MA, Angel AG, Velazquez-Aragon J, Ortiz-Hernandez R, Cruz-Rubio JM, Villa-Barragan P, Jimenez-Gutierrez C, Gutierrez-Castrellon P. Effects of fructans from Mexican agave in newborns fed with infant formula: a randomized controlled trial. Nutrients. 2015;7(11):8939–8951. doi: 10.3390/nu7115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramnani P, Costabile A, Bustillo AG, Gibson GR. A randomised, double- blind, cross-over study investigating the prebiotic effect of agave fructans in healthy human subjects. J Nutr Sci. 2015;4:e10. doi: 10.1017/jns.2014.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayago-Ayerdi SG, Mateos R, Ortiz-Basurto RI, Largo C, Serrano J, Granado-Serrano AB, Sarria B, Bravo L, Tabernero M. Effects of consuming diets containing Agave tequilana dietary fibre and jamaica calyces on body weight gain and redox status in hypercholesterolemic rats. Food Chem. 2014;148:54–59. doi: 10.1016/j.foodchem.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81(2):243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 30.de Meijer VE, Le HD, Meisel JA, Akhavan Sharif MR, Pan A, Nose V, Puder M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism. 2010;59(8):1092–1105. doi: 10.1016/j.metabol.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Wiele T, Boon N, Possemiers S, Jacobs H, Verstraete W. Inulin-type fructans of longer degree of polymerization exert more pronounced in vitro prebiotic effects. J Appl Microbiol. 2007;102(2):452–460. doi: 10.1111/j.1365-2672.2006.03084.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 22 kb)
(PDF 8420 kb)


