Abstract
Background:
In response to food cues, obese vs normal-weight individuals show greater activation in brain regions involved in the regulation of food intake under both fasted and sated conditions. Putative effects of obesity on task-independent low-frequency blood-oxygenation-level-dependent signals—that is, resting-state brain activity—in the context of food intake are, however, less well studied.
Objective:
To compare eyes closed, whole-brain low-frequency BOLD signals between severely obese and normal-weight females, as assessed by functional magnetic resonance imaging (fMRI).
Methods:
Fractional amplitude of low-frequency fluctuations were measured in the morning following an overnight fast in 17 obese (age: 39±11 years, body mass index (BMI): 42.3±4.8 kg m−2) and 12 normal-weight females (age: 36±12 years, BMI: 22.7±1.8 kg m−2), both before and 30 min after consumption of a standardized meal (~260 kcal).
Results:
Compared with normal-weight controls, obese females had increased low-frequency activity in clusters located in the putamen, claustrum and insula (P<0.05). This group difference was not altered by food intake. Self-reported hunger dropped and plasma glucose concentrations increased after food intake (P<0.05); however, these changes did not differ between the BMI groups.
Conclusion:
Reward-related brain regions are more active under resting-state conditions in obese than in normal-weight females. This difference was independent of food intake under the experimental settings applied in the current study. Future studies involving males and females, as well as utilizing repeated post-prandial resting-state fMRI scans and various types of meals are needed to further investigate how food intake alters resting-state brain activity in obese humans.
Introduction
As assessed by functional magnetic resonance imaging (fMRI) in response to food cues, obese (body mass index (BMI)⩾30 kg m−2) vs normal-weight (BMI<25 kg m−2) individuals show increased activation of brain circuits regulating food intake, under both fasted and sated conditions.1, 2, 3, 4 Differences between the obese and normal-weight humans have been further demonstrated in studies using resting-state fMRI (rsfMRI), a measure of task-independent low-frequency blood-oxygenation-level-dependent signals that are assumed to reflect the intrinsic functional interaction of brain regions.5 Compared with normal-weight controls, obese males and females exhibit increased functional connectivity for networks involved in the regulation of food intake, such as the salience network6 and hypothalamic network.7 Moreover, it has been suggested that the intrinsic brain activity patterns of obese and normal-weight females are differently altered by food intake:8 obese vs normal-weight females had greater connectivity in reward-related brain regions (between the right lateral hypothalamus and putamen), and weaker connectivity in homeostasis and gustatory-related brain regions (between the right hypothalamus with posterior insula and the primary interoceptive cortex and parietal cortex), following consumption of a high-sucrose beverage as compared with a low-sucrose drink.8 These results were obtained on two separate experimental days and did not examine pre- and post-prandial differences.8 To our best knowledge, only one study has systematically examined the intrinsic brain activity patterns under both fasting and post-prandial conditions in normal-weight and obese individuals.9 This study, which included male participants only, reported increased resting-state activity in the putamen, and decreased activity in the orbitofrontal cortex and medial prefrontal cortex compared with normal-weight male controls in a fasted state. In contrast, no such differences were observed after consumption of a liquid formula meal.9
Studies have shown that central nervous system mechanisms involved in body weight regulation10, 11, 12 and task-dependent brain activation patterns in regions associated with food intake (such as reward anticipation and food motivation)3 differ between females and males. The effect of food intake on rsfMRI has so far only investigated in males,9 but not in females. The present study therefore sought to measure task-independent (intrinsic) low-frequency blood-oxygenation-level-dependent signals of the brain, both before and after food intake in severely obese and normal-weight females. We hypothesized that obese females would exhibit a stronger intrinsic activation of brain circuits associated with food intake regulation than normal-weight females.
Materials and methods
Participants
Seventeen severely obese (BMI>35 kg m−2) and 12 normal-weight female controls of similar age participated in the study. All participants were right-handed, non-smokers, non-claustrophobic, free from metallic implants, and free from ongoing psychiatric treatment, which was checked during an anamnestic interview with all potential participants preceding the experiment. All obese participants were enrolled from a population of individuals who were seeking bariatric surgery at the University Hospital of Uppsala. Normal-weight volunteers were recruited by advertisements.
All participants gave their written informed consent before any experimental testing. The present study was approved by the Regional Ethical Review Board in Uppsala, and the procedures followed were in accordance with the Helsinki Declaration. This trial was registered at clinicaltrials.gov as NCT 01815216.
Experimental procedure
rsfMRI measurements were conducted in the morning (between 8.00 and 10.00) after an overnight fast. Upon arrival at the research center, participants reported the number of hours they had slept the night before and were requested to rate their feelings of hunger, fullness, tiredness and stress on a 100-mm visual analog scale. Whole blood was collected via an intravenous cannula to measure fasting concentrations of plasma glucose and serum insulin, and height and weight for the calculation of BMI were assessed. Following these baseline measurements, participants' resting-state brain activity under the fasting conditions was measured by rsfMRI (description of the imaging procedure, see below). Directly after completion of the scan, participants consumed 250 ml of a milk-based, vanilla-flavored drink fortified with protein and carbohydrates (carbohydrates/protein/fat=64/32/4 energy% total energy amount 260 kcal; Gainomax Recovery Vanille; Norrmejerier Umeå). Twenty-five minutes after this caloric load, subjective appetite ratings were repeated and a second resting-state activity scanning session was performed. Finally, another blood sample for repeated measures of glucose and insulin was obtained. Blood samples were centrifuged directly after sampling for the analysis of blood glucose and insulin. Plasma glucose was measured using routine assays (hexokinase method, Aeroset; Abbott Diagnostics, North Chicago, IL, USA), and serum concentrations of insulin were determined using noncompetitive immunometric assays (12017547 122; Roche Diagnostics, North Chicago, IL, USA).
Image acquisition
Structural and functional brain images were acquired with a 3-Tesla Philips scanner (Achieva, Philips Healthcare, Best, The Netherlands) using a 32-channel headcoil. Resting-state data were collected with an echoplanar imaging sequence collecting 180 volumes with 32 slices, using a field of view of 192 × 192 mm and voxel size of 3 × 3 × 3 mm. Slice thickness was set at 3 mm, and 0.9-mm gap between the interleaved scans (repetition time (TR)=2 s, echo time (TE)=30 ms; flip angle=90°).
During both rsfMRI scans (that is, fasting vs post prandial), participants were instructed not to focus their thoughts on anything and to keep their eyes closed without falling asleep. The resting-state imaging sequence lasted for 6 min. Resting-state activity of the brain was investigated by exploring low-frequency fluctuations of blood-oxygenation-level dependent based on a voxel-wise, frequency-based measure. Amplitude of the low-frequency fluctuations (ALFF) reflects regional intensity of spontaneous neural activity,13 and the total power spectrum in the low-frequency band. However, the ALFF may be sensitive to physiological noise from adjacent blood vessels and cisterns.14, 15 We therefore used the fractional amplitude of low-frequency fluctuations (fALFF), a more robust measure—as it incorporates the ratio of the total power spectrum of the low-frequency range to the power spectrum of the full-frequency range measured in the scanner.14
Preprocessing
Statistical parametric mapping 8 (http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB (version R2010a (8.3.0.532), MathWorks, Inc., Sherborn, MA, USA) was used as a platform for preprocessing rsfMRI data before subsequent analyses. To control for bias in signal intensity, the first 10 time points were discarded. Images were realigned to correct for head motion during scanning. None of the participants had moved >3 mm, which was the cutoff for exclusion. Participants' functional images were co-registered to the corresponding structural images. The structural image was segmented to remove non-brain tissue (such as bone, fat and muscle), and together with the functional images normalized to a default brain template from the Monteal Neurological Institute (MNI) space. Finally, functional images were smoothed with an 8-mm full width half-maximum Gaussian kernel.
fALFF calculations
The fALFF calculations were carried out using the Rest toolbox (Resting-State fMRI Data Analysis Toolkit V1.8, REST-Group, http://www.restfmri.net).16 First, the signal was linearly detrended to correct for signal drift. A fast Fourier transformation converted data from the time domain to the frequency domain to obtain the power spectrum, which was square rooted in each voxel and normalized by dividing the ALFF from each voxel with the global mean of the ALFF from all voxels in the brain (confined by the default REST brain mask).13, 16 fALFF, however, considers the power in the low-frequency range (frequency band width 0.01–0.08 Hz), as a fraction of the total power in the entire frequency range (0–0.25 Hz), band-pass filtering was performed after fALFF calculation.14, 15 The fALFF maps were then normalized by dividing fALFF from each voxel with the global mean of fALFF from all voxels in the brain (confined by the default REST brain mask).13, 16
Statistical analyses
A repeated measures analysis of variance with weight status as a between-subject factor was carried out to test for the main and interaction effects of weight status (obese vs normal-weight) and time (before vs after food intake) on hunger, fullness, tiredness and stress. Behavioral data were analyzed using SPSS software (version 22, SPSS Inc, Chicago, IL, USA). Analysis of variance was also used to test for these effects on fALFF. As the change in stress (Δ stress) was different between obese and lean participants, these delta scores were included in the model as covariate. The normalized fALFF maps were imported to statistical parametric mapping for second level analyses. Monte Carlo simulations were performed to identify cluster extent thresholds for a more reliable interpretation of the results. The Monte Carlo simulations were performed using AlphaSim within the Rest toolbox17 with 1000 iterations, including the functional mask of the analysis, with an independent voxel threshold of P<0.001. For our results, the Monte Carlo simulation indicated a cluster size threshold of 23 voxels for a cluster alpha of 0.05.
Results
Descriptives
Baseline characteristics are shown in Table 1. Obese participants had higher fasting plasma glucose and serum insulin levels than normal-weight individuals. Age and self-reported sleep duration did not differ between groups. Moreover, no baseline differences in rated hunger, fullness, tiredness and stress were found between groups.
Table 1. Subject's characteristics and subjective ratingsa on appetite and stress on arrival, and 25 min after food intake (mean±s.d.) of the severely obese and normal-weight females.
|
Severely obese (n=17) |
Normal-weight (n=12) |
Test statistic (t) | P-value | |||
|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | |||
| Body weight (kg) | 119b | 17.5 | 64b | 8.8 | −10.0 | <0.0001 |
| Body mass index (kg m−2) | 43.2b | 4.8 | 22.7b | 1.8 | −14.1 | <0.0001 |
| Age | 39 | 11.0 | 36 | 12.3 | −0.65 | 0.52 |
| Hours of sleep night preceding tests | 7.0 | 1.1 | 7.1 | 0.67 | 0.23 | 0.82 |
| Fasting plasma glucose (mmol l−1)c | 5.42b | 0.54 | 4.85b | 0.53 | −2.66 | 0.01 |
| Fasting insulin (μU ml−1)c | 20.8b | 12.9 | 5.38b | 1.69 | −4.41 | 0.001 |
| Increase in plasma glucose (mmol l−1) | 1.35 | 0.49 | 1.01 | 1.14 | −0.95 | 0.35 |
| Increase in insulin (μU ml−1) | 156.1b | 85.7 | 55.7b | 33.6 | −3.63 | 0.02 |
| Subjective ratings on arrival | ||||||
| Hunger (mm) | 58 | 18 | 54 | 20 | 1.17 | 0.60 |
| Fullness (mm) | 20 | 18 | 17 | 18 | −2.05 | 0.68 |
| Stress (mm) | 18 | 17 | 18 | 21 | 2.04 | 0.91 |
| Tiredness (mm) | 54 | 25 | 39 | 20 | −1.75 | 0.10 |
| Change in subjective ratings | ||||||
| Hunger (mm) | −21 | 24 | −8 | 18 | −1.63 | 0.12 |
| Fullness (mm) | 27 | 24 | 14 | 24 | 1.49 | 0.15 |
| Stress (mm) | −10b | 12 | +3b (increase) | 17 | −2.33 | 0.03 |
| Tiredness (mm) | −20 | 24 | −17 | 23 | −0.037 | 0.72 |
Ratings were obtained using a 100-mm visual analog scale.
Difference between obese and normal-weight participants (P<0.05).
Problems with the blood sampling procedures in the severe obese participants resulted in missing values for fasting glucose and insulin for three obese individuals (data fasting blood parameters presented for n=14), and we were not able to calculate increases in glucose and insulin concentrations for five obese individuals (data increase in blood parameters presented for n=12).
Behavioral measurements, and glucose and insulin levels after food intake
Consumption of the caloric load promoted satiety: following food intake, participants felt less hungry (main effect of time F=12.15, P<0.01), fuller (F=58.78, P<0.001) and less tired (F=16.34, P<0.001) than they did at baseline (Table 1). As expected, circulating levels of glucose (F=43.22, P<0.001) and insulin levels (F=19.91, P<0.01) were also higher after food intake compared with baseline (Table 1). There was no weight status × time interaction effect on hunger (F=2.65, P=0.12), fullness (F=2.21 P=0.15), tiredness (F=0.13, P=0.72) or plasma glucose concentrations (F=0.90, P=0.35). In contrast, we observed a weight status × time interaction effect on insulin concentrations (F=13.21, P<0.01), with a greater increase in obese vs normal-weight individuals after food intake (Table 1). We also observed a significant interaction effect on perceived stress (F=5.44, P=0.03): normal-weight participants reported slightly higher feelings of stress 25 min after food intake (which was directly before the second scanning session) than at baseline, whereas perceived stress levels maintained stable across the session for obese participants (Table 1).
Resting-state fMRI measurements: fALFF
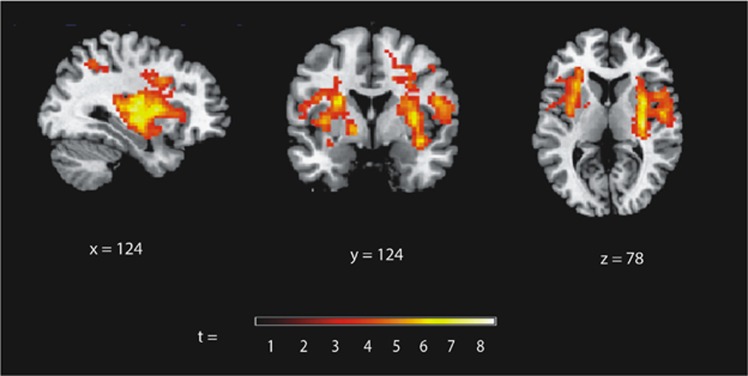
The analyses of the fALFF data revealed a main effect of weight status on activity bilaterally in the putamen, claustrum and insula (Table 2), with increased low-frequency activity in the obese compared with the normal-weight individuals in individual clusters incorporating the right putamen, the left inferior parietal lobe, as well as in the left putamen (reaching the insula) independent of food intake (Table 2; Figure 1). No brain regions showed increased activity in the normal-weight compared with the obese participants and no weight status × time interaction was observed.
Table 2. Detailed information on clusters that show differences in the fractional amplitude of low-frequency fluctuations (fALFF) between severely obese and normal-weight individuals.
| Brain regions included in cluster (BA; nearest GM within 3 mm) | Hemisphere | Cluster sizea |
MNI |
Test statistic | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Interaction weight status × time: cluster with highest f | ||||||
| NS | ||||||
| Main effect BMI | ||||||
| Putamen/claustrum | R | 1641 | 30 | −19 | 13 | f=71.28 |
| Claustrum | R | 33 | 2 | 7 | f=52.21 | |
| Putamen | R | 33 | −13 | 1 | f=50.64 | |
| Putamen/claustrum | L | 513 | −24 | 2 | 19 | f=44.92 |
| Claustrum/insula | L | −30 | 17 | 4 | f=39.98 | |
| Claustrum | L | −36 | −4 | 4 | f=37.94 | |
| Postcentral gyrus/ inferior parietal lobule (BA40) | L | 49 | −54 | −28 | 22 | f=38.19 |
| Obese>normal weight after food intake | ||||||
| Putamen/claustrum | R | 2062 | 30 | −19 | 13 | t=8.44 |
| Claustrum | R | 33 | 2 | 7 | t=7.23 | |
| Putamen | R | 33 | −13 | 1 | t=7.12 | |
| Putamen/ claustrum | L | 771 | −24 | 2 | 19 | t=6.70 |
| Claustrum/insula | L | −30 | 17 | 4 | t=6.32 | |
| Claustrum | L | −36 | −4 | 4 | t=6.16 | |
| Postcentral gyrus/ inferior parietal lobule (BA40) | L | 62 | −54 | −28 | 22 | t=6.18 |
Abbreviations: BA, brodmann area; BMI, body mass index; MNI, Monteal Neurological Institute; NS, not significant; R, right; L, left.
MNI coordinates of primary peak locations; x=sagittal plane, y=coronal plane and z=axial plane.
The contrast ‘normal weight>obese after food intake' did not show significant differences in activity.
To identify significant clusters, we applied a cluster size threshold of 23 voxels determined by Monte Carlo simulations.
Figure 1.
Increased fractional amplitude of low-frequency fluctuations (fALFF) in severely obese compared with normal-weight participants (independent of food intake, covaried for subjective stress levels). Significant differences in fALFF are illustrated on a template brain, showing sagittal (x), coronal (y) and axial (z) view, and the slice number in each dimension. The color bar illustrates t-value scores represented by activation clusters on the brain map based on a t-threshold value >t=3.3.
Discussion
Using consecutive measures of rsfMRI before and 30 min after consumption of a standardized meal, we observed greater resting-state low-frequency power in clusters mainly located in the putamen, claustrum and insula in the brain of severely obese females compared with that of normal-weight females. Food intake did not modulate this difference in the resting-state brain activity. These findings suggest that brain regions involved in food processing1, 18, 19 are more active in obese than in normal-weight females, and that this difference is robust to food intake. To which extent this may favor overeating in obese females warrants further investigation.
To the best of our knowledge, to date only one study has examined thec intrinsic brain activity under both fasting and post-prandial conditions in normal-weight and obese individuals.9 In this study, it was demonstrated that obese vs normal-weight males ‘exhibited a higher level of synchronicity of activity in the left putamen prior to food intake' however, in contrast to the main effect of BMI on resting-state activity in the putamen, claustrum and insula in our study, Zhang et al.9 observed differences in resting-state brain activity before, but not after food intake in obese males. There are essential methodological differences between both the studies that could explain these partially discrepant findings. Although our sample consisted of females, the previous study has examined the effects of food intake on brain activity in males, suggesting the existence of gender differences in post-prandial regulation of brain activity. Previous studies demonstrated that the gender influenced task-dependent brain activation patterns in regions associated with reward anticipation3 and brain activation during voluntary inhibition of hunger during food stimulation.20 This could offer an explanation as to why we found differences in post-prandial resting-state brain activity between BMI groups, whereas the previous study9 did not. Another factor that could account for the discrepant post-prandial findings between studies concerns the timing of rsfMRI scans. In the present experiment, rsfMRI scans were scheduled in the morning, whereas resting-state brain activity was measured during evening hours in the previous study. Appetite has been shown to fluctuate across the day,21 and task-dependent activity of brain areas involved in food processing (including the left putamen) are more pronounced in the morning than in the evening.22 Another difference includes the BMI between the obese groups in both studies (present vs previous study: 43 vs 34 kg m−2). In obese individuals, BMI has been found to be correlated negatively with measures of dopamine receptors.23 Dopamine signaling has an important role in reward processing of food.24 Furthermore, it must be kept in mind that the sample size in each of the studies was relatively small and different between the studies (present vs previous study: 17 obese and 12 normal-weight individuals vs 20 individuals in each group9), which may have not only limited power, but could also explain their discrepant rsfMRI results after food intake. Finally, an important difference in study procedures relates to the amount of calories supplied by the test meal (260 kcal vs 40% of resting energy expenditure) and we cannot rule out that a larger caloric preload may have attenuated post-prandial differences in the resting-state activity between BMI groups observed in our study. Our findings suggest that our relatively small caloric load (which could represent a snack or small meal) that promoted subjective satiety, did not seem to fulfill food-reward homeostasis in the morning. As a result, reward-related brain regions remain at higher intrinsic arousal levels in obese females. This could be an explanation for attentional bias for food that has been observed in obese females.25, 26, 27 The obese participants in our study showed increased activity in a cluster that included the right and left putamen, claustrum and insula. Neuroimaging studies have implicated the putamen in aberrant eating processes. For instance, in an fMRI study in obese patients, hypoactivity in the putamen in response to receipt of a palatable food was associated with high BMI, as well as deficiencies in reward prediction.18 It has also been observed that obese individuals exhibit greater resting-state functional connectivity in the putamen nucleus than normal-weight humans.6 Extending these results, we show that the putamen of obese females exhibited increased resting-state low-frequency power independent of food intake. This could suggest that high intrinsic putamen activity may account for variability in eating behavior in obese individuals—as compared with normal weight.
Another brain region that showed greater resting-state activity in obese than in normal-weight females was the insula. As part of the primary taste cortex and primary olfactory cortex, the insula is responsive to different flavors and mediates interoceptive awareness of body state.28 As food intake is normally associated with a decrease in insular regional cerebral blood flow,29 our results suggest an abnormal reward and/or sensory processing of food responses in obesity, as others have also suggested in a meta-analysis of fMRI studies in the obese.30 Several limitations should be kept in mind, when interpreting our results. We measured the resting-state brain activity during morning hours in a small sample of female adults. Thus, no conclusion can be made as to whether similar intrinsic brain patterns would be observed at other daytimes (for example, in the evening) or in males, and extrapolating results to other BMI classes or other age groups should be done with caution. Moreover, we did not have access to a dummy scanner to habituate the participants with the procedure, although stress ratings were low. We included the change in subjective stress ratings over the course of the experimental procedure, but this did not change the results. Participants were instructed not to fall asleep during the rsfMRI scans. Nonetheless, given that we have not recorded sleep polysomnography simultaneously with rsfMRI, we cannot disclaim that some of our subjects may have experienced brief episodes of sleep during the fMRI procedure. Although promoting satiety, our caloric preload was relatively small. We did not observe an interaction of ‘weight status × food intake' on resting-state brain activity or a main effect of food intake. This suggests that our caloric load may have hampered our ability to detect differences in the pre- vs post-prandial contrasts between BMI groups, yet the decrease in hunger and increase in fullness following food intake argues against this possibility. We only ran a single post-prandial rsfMRI scan 30 min after food intake. Additional studies employing repeated post-prandial rsfMRI scans are needed to examine if food intake affects the resting-state brain activity at different post-prandial time points, although studies employing various portion sizes and different macronutrient compositions may help further our understanding of how food intake links to the intrinsic brain activity in obese and normal-weight humans. Finally, we did not take into account individual food preferences, which may have helped reduce noise because of inter-individual variance in brain activity after food intake.
Our findings show that the brain regions involved in reward processing are more active in obese than normal-weight females. This difference was independent of food intake under the experimental settings applied. Obese females in our study exhibited signs of peripheral insulin resistance (for example, they had in average higher fasting serum insulin levels than normal-weight females). As previous studies have shown that peripheral insulin resistance concurs with the altered resting-state brain activity,31, 32 it must be kept in mind that obesity-related health risks, such as insulin resistance, could have contributed to the observed differences in the brain activity between obese and normal-weight females in our study.
Acknowledgments
We thank Dr Owen O'Daly for his expert advice. This work is supported by the Swedish Society for Medical Research (SSMF), A Karlssons and L Erikssons stiftelse, Ingrid Thurings Foundation, Lars Hiertas Minne and Tore Nilsons stiftelse. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare no conflict of interest.
References
- Rothemund Y, Preuschhof C, Bohner G, Bauknecht H-C, Klingebiel R, Flor H et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 2007; 37: 410–421. [DOI] [PubMed] [Google Scholar]
- Boutelle KN, Wierenga CE, Bischoff-Grethe A, Melrose AJ, Grenesko-Stevens E, Paulus MP et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes (Lond) 2015; 39: 620–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Callister RJ, Brain K, Collins CE, Burrows TL. Neural responses to visual food cues according to weight status: a systematic review of functional magnetic resonance imaging studies. Front Nutr 2014; 1: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S, Gibson C, Benson L, Ochner CN, Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes Rev 2012; 13: 43–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8: 700–711. [DOI] [PubMed] [Google Scholar]
- García-García I, Jurado MÁ, Garolera M, Segura B, Sala-Llonch R, Marqués-Iturria I et al. Alterations of the salience network in obesity: a resting-state fMRI study. Hum Brain Mapp 2013; 34: 2786–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Linder K, Zipfel S, Häring H-U, Veit R et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp 2014; 35: 6088–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Coveleskie K, Connolly L, Labus JS, Ebrat B, Stains J et al. Influence of sucrose ingestion on brainstem and hypothalamic intrinsic oscillations in lean and obese women. Gastroenterology 2014; 146: 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Tian D, Yu C, Zhang J, Tian X, von Deneen KM et al. Altered baseline brain activities before food intake in obese men: a resting state fMRI study. Neurosci Lett 2015; 584: 156–161. [DOI] [PubMed] [Google Scholar]
- Benedict C, Kern W, Schultes B, Born J, Hallschmid M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J Clin Endocrinol Metab 2008; 93: 1339–1344. [DOI] [PubMed] [Google Scholar]
- Lovejoy JC, Sainsbury A. Sex differences in obesity and the regulation of energy homeostasis. Obes Rev 2009; 10: 154–167. [DOI] [PubMed] [Google Scholar]
- Scherer T, Lehnert H, Hallschmid M. Brain insulin and leptin signaling in metabolic control: from animal research to clinical application. Endocrinol Metab Clin North Am 2013; 42: 109–125. [DOI] [PubMed] [Google Scholar]
- Yu-Feng Z, Yong H, Chao-Zhe Z, Qing-Jiu C, Man-Qiu S, Meng L et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 2007; 29: 83–91. [DOI] [PubMed] [Google Scholar]
- Zou Q-H, Zhu C-Z, Yang Y, Zuo X-N, Long X-Y, Cao Q-J et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 2008; 172: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X-N, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF et al. The oscillating brain: complex and reliable. Neuroimage 2010; 49: 1432–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 2011; 6: e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for FMRI Data 2000. Available from http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf accessed 16 June 2016.
- Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008; 322: 449–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. Neuroimage 2004; 23: 1486–1493. [DOI] [PubMed] [Google Scholar]
- Wang G-J, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K et al. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci USA 2009; 106: 1249–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf C, Jas P, van der Kooy K, Leenen R. Circadian rhythms of appetite at different stages of a weight loss programme. Int J Obes Relat Metab Disord 1993; 17: 521–526. [PubMed] [Google Scholar]
- Masterson TD, Kirwan CB, Davidson LE, LeCheminant JD. Neural reactivity to visual food stimuli is reduced in some areas of the brain during evening hours compared to morning hours: an fMRI study in women. Brain Imaging Behav 2016; 10: 68–78. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W et al. Brain dopamine and obesity. Lancet 2001; 357: 354–357. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med 2016; 374: 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos EH, Charboneau E, Dietrich MS, Park S, Bradley BP, Mogg K et al. Obese adults have visual attention bias for food cue images: evidence for altered reward system function. Int J Obes (Lond) 2009; 33: 1063–1073. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity 2011; 19: 1775–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenkamp PS, Sundbom M, Nilsson VC, Benedict C, Schiöth HB. Patients lacking sustainable long-term weight loss after gastric bypass surgery show signs of decreased inhibitory control of prepotent responses. PLoS One 2015; 10: e0119896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Front Hum Neurosci 2013; 7: 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Parigi A, Gautier J-F, Chen K, Salbe AD, Ravussin E, Reiman E et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci 2002; 967: 389–397. [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One 2013; 8: e60393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann S, Heni M, Veit R, Ketterer C, Schick F, Häring H-U et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum Brain Mapp 2012; 33: 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Jiao Y, Cui Y, Shang S-A, Ding J, Feng Y. Aberrant brain functional connectivity related to insulin resistance in type 2 diabetes: a resting-state fMRI study. Diabetes Care 2014; 37: 1689–1696. [DOI] [PubMed] [Google Scholar]