Abstract
Parkinson’s Disease (PD) involves the degeneration of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNc) that is thought to cause the classical motor symptoms of this disease. However, motivational and affective impairments are also often observed in PD patients. These are usually attributed to a psychological reaction to the general motor impairment and to a loss of some of the neurons within the ventral tegmental area (VTA). We induced selective lesions of the VTA and SNc DA neurons that did not provoke motor deficits, and showed that bilateral dopamine loss within the SNc, but not within the VTA, induces motivational deficits and affective impairments that mimicked the symptoms of PD patients. Thus, motivational and affective deficits are a core impairment of PD, as they stem from the loss of the major group of neurons that degenerates in this disease (DA SNc neurons) and are independent of motor deficits.
Parkinson’s disease (PD) is mainly characterized by a progressive degeneration of midbrain dopaminergic (DA) neurons along a caudorostral and lateromedial gradient, with a marked loss of neurons in the substantia nigra pars compacta (SNc)1, projecting to the dorsal striatum along the nigrostriatal pathway2, and a more modest loss in the ventral tegmental area (VTA)1, projecting to limbic and cortical areas along the mesolimbic and mesocortical pathways2, respectively.
In addition to the classical motor symptoms, several neuropsychiatric symptoms, such as depression, anxiety and motivational deficits (apathy), are frequently observed in PD patients3, 4. The underlying pathological mechanisms have not yet been elucidated, but these motivational and affective impairments are generally attributed to the patient’s psychological reaction to the profound motor deficit and to the associated loss of dopaminergic neurons in the VTA4, 5. This pathophysiological concept stems from a dichotomous vision of the functional role of mesencephalic DA neurons, in which motor function is attributed to the nigrostriatal system, originating from the SNc, and motivational and affective functions are attributed to the mesocorticolimbic system, originating from the VTA2, 6, 7.
Most of the data that have been used to ground this dichotomous vision of VTA-SNc neurons implication in PD symptoms have been generated using lesions of these neurons, involving various degrees of destruction of bordering zone of the VTA and SNc. Therefore, large SNc lesions inducing profound motor deficits also involve the lateral part of the VTA. Conversely, the VTA lesions used to attribute motivational properties to this structure always involve destruction of the medial part of the SNc6, 8. Consequently, the origins of the motivational symptoms in PD remain unresolved, not least because disentangling the potential motivational and mood-related deficit alterations from motor impairments remains a challenging issue in animal models of PD9.
We have developed a lesional model, based on the stereotaxic injection of the catecholaminergic neurotoxin 6-hydroxydopamine (6-OHDA) into precise areas of the rat brain, in which degenerations of the DA mesocorticolimbic and nigrostriatal systems can be clearly separated and in which the motor skills of the animals are preserved. We evaluated several aspects of behavior and found that bilateral dopamine loss within the SNc, but not within the VTA, caused motivational deficits and affective impairments resembling some of the neuropsychiatric symptoms observed in PD patients.
Results
Bilateral partial lesions of the mVTA or SNc result in distinct, non overlapping, complementary patterns of DA denervation
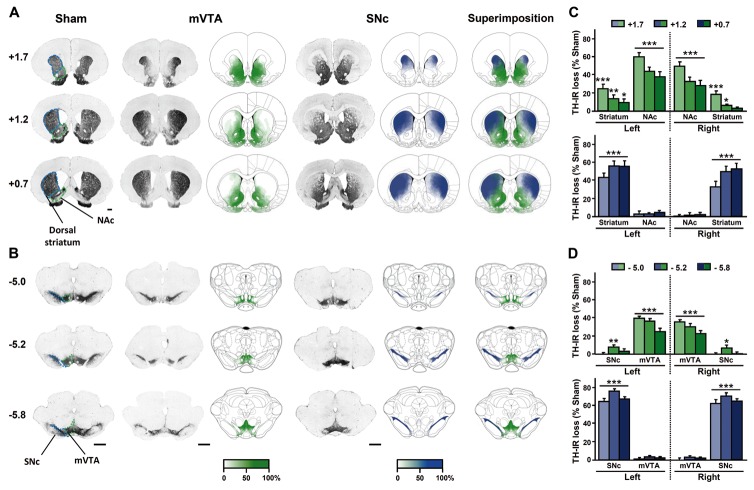
mVTA and SNc lesions produce two distinct, non overlapping, complementary, patterns of bilateral DA denervation throughout the striatum, as revealed by decreases in tyrosine hydroxylase (TH)-immunoreactivity (IR) and striatal DA contents (Fig. 1 and Fig. S1). The mVTA lesions preferentially affected the ventral part of the striatum, resulting in a 40 to 60% decrease in TH-IR density within the nucleus accumbens. The extent of the mVTA lesions therefore mimicked the loss of dopaminergic innervation observed in the ventral head of the caudate nucleus in PD patients10. The SNc lesion was associated with a similar decrease in TH-IR density, along the rostrocaudal extent of the dorsal striatum, predominantly in its lateral portion (~70%).
Fig. 1. Bilateral partial lesions of the mVTA or SNc result in distinct, non overlapping, complementary patterns of DA depletion throughout striatal territories.
(A and B) Representative photomicrographs of coronal sections stained for TH in striatal (+1.7 to 0.7 mm anterior to bregma) (A) and mesencephalic (−5 to −5.8 mm anterior to bregma) (B) regions according to the stereotaxic atlas of Paxinos and Watson36, 37. Bar = 1 mm. The intensity of the gradient of color (white to green or white to blue) in schematic sections corresponds to the measured DA lesioned area in the different brain structures studied for each lesion performed. mVTA lesion are shown in green and SNc lesion in blue. The highest intensity of green or of blue color (100%) indicates that all animals had lesions in the corresponding area, whereas the lowest color intensity (white, 0%) corresponds to a non lesioned or denervated area. (C and D) Quantification of the loss of TH staining in the different mesencephalic (C) and striatal (D) structures, expressed as percentage of the mean value obtained for sham-operated animals. Two-way ANOVAs revealed significant interactions between the lesion and the brain region considered (Fs > 9.49, Ps < 0.001). n = 22–28, *P < 0.05, **P < 0.01, ***P < 0.001. mVTA, medial ventral tegmental area; NAc, nucleus accumbens; SNc, substantia nigra pars compacta.
Bilateral partial DA lesions of the mVTA or SNc do not induce locomotor deficits
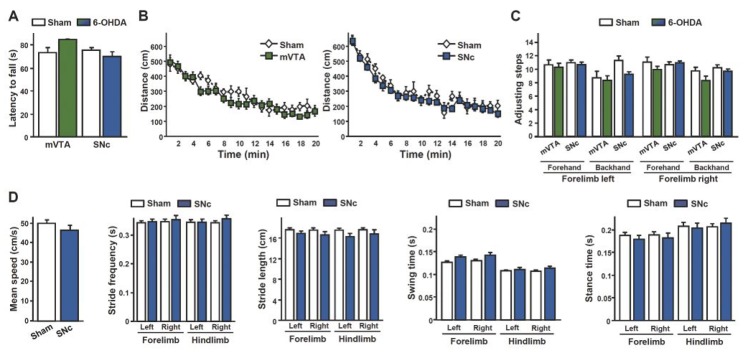
As expected with such partial striatal DA denervation11, neither SNc nor mVTA lesions impaired sensorimotor coordination on an accelerating rotarod or spontaneous locomotor activity (Fig. 2A, B). Furthermore, despite a slight alteration of stepping adjustment in SNc DA-lesioned rats (Fig. 2C), no impairment was observed with regards to fine-motor velocity or ambulatory coordination in an automated laboratory gait analysis system (Fig. 2D).
Fig. 2. Bilateral partial 6-OHDA lesions of either the mVTA or SNc do not impair locomotion.
(A, B) DA lesions did not affect the latency to fall from an accelerating rotarod (A) (Ps > 0.42, n = 22–28), or horizontal ambulatory activity (B) during a 20 minutes period in an open area (no effect of lesion: Fs < 0.91, Ps > 0.35 and no lesion x time interaction: FS < 0.64, Ps > 0.88, n = 11–18). (C) A two-way ANOVA showed a marginal effect of the SNc lesions (F1,144 = 4.04, P = 0.05) and lesion x paw interaction (F3,144 = 2.54, P = 0.06) on the number of adjusting steps. No such effects were found for the mVTA lesions (Fs < 1.91, Ps > 0.18), n = 22–28. (D) SNc lesions had no effect on gait parameters analyzed with an automated gait analysis system (no effect of lesion: Fs < 2.37, Ps > 0.14 and no lesion x paw interaction: Fs < 0.92, Ps > 0.43, n = 12–14).
Bilateral partial DA lesions of the SNc, but not of the mVTA, decrease general behavioral activity
The lack of a major motor deficit after partial lesioning of the SNc allowed us to study specifically the role of the DA nigrostriatal system in motivational processes, in the absence of the usual potential bias related to locomotor alterations. Apathy is one of the major non motor symptoms of PD3, 4. Apathy is defined as a lack of motivation, or a reduction in “goal-directed behaviors”12, with a global deficit in self-initiation and maintenance of voluntary and purposeful behavior, resulting in low levels of activity and a loss of interest in sources of reinforcement13–15. Interestingly, the mVTA lesions had no significant effect on general consummatory responses, measured as water and food intake over a 24-hour period, whereas SNc lesions decreased these behaviors (Fig. 3A and B) and were associated with a slower weight gain (Fig. 3C), sometimes associated with a transient starvation state after surgery (see online Methods). Like apathetic patients12, 13, rats with DA lesions of the SNc, but not of the mVTA, displayed a decrease in social interaction (Fig. 3D) that could not be attributed to an olfactory deficit, since neither attraction to an appetitive odor (coconut, Fig. 3E) nor avoidance from an aversive (acetic acid, Fig. 3F) odor was altered by the DA lesions.
Fig. 3. Bilateral partial 6-OHDA lesions of the SNc, but not of the mVTA, decrease consummatory responses, weight gain and social interaction without affecting olfaction.
(A, B) Water (A) and food (B) intake in the homecage were measured over a 24-hour period. (B) Both lesions resulted in a loss of weight after surgery, but mVTA-lesioned animals recovered during the three weeks following surgery, whereas SNc-lesioned animals continued to gain less weight than the controls (Effects of lesion: Fs > 4.48, Ps < 0.05 and significant lesion x day interaction: Fs > 5.16, Ps < 0.001). (D) SNc lesions reduced the time spent in social interaction with a congener. (E, F) 6-OHDA lesions did not affect attraction toward an appetitive (coconut, E) or avoidance from an aversive (acetic acid, F) odor n = 12–18. *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Bilateral partial DA lesions of the SNc, but not of the mVTA, specifically impair motivated behaviors
This aspect of behavior was investigated further, in various non operant and operant tasks, including place preference, instrumental responding and runway tasks for palatable food. The use of this approach made it possible to distinguish between the effects of the lesion on preparatory and consummatory components of motivated behaviors. Specifically, the acquisition of a runway task (progressive reduction in the latency to reach and to start to eat a palatable food at the end of a straight alley) was similar in all conditions, demonstrating an absence of learning deficits (Fig. 4A). However, SNc DA lesions decreased asymptotic performance, essentially due to a greater number of interruptions and route reversals in SNc-lesioned than in sham rats, resulting in a smaller number of direct runs to the goal (Fig. 4A). These observations are consistent with a weaker motivation to reach the goal16 or an approach/avoidance conflict17, that cannot be attributed to deficits in Pavlovian associative processes or in the reinforcing properties of the palatable food, as neither type of lesion affected performance in conditioning place preference (CPP) for the same food (Fig. 4B).
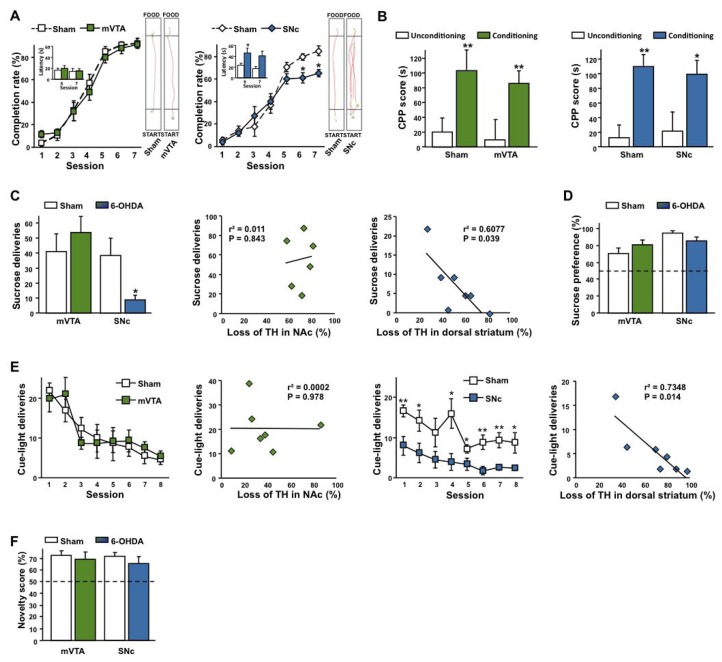
Fig. 4. Bilateral partial 6-OHDA lesions of the SNc, but not of the mVTA, impair motivated behaviors.
(A, B) SNc (lesion x session interaction: F6,108 = 2.65, P < 0.02, n = 19–22) but not mVTA (lesion x session interaction: F6,66 = 0.84, P = 0.55, n = 12–15) lesions increased the latency to reach a palatable food in a runway paradigm at the asymptotic level (A), with no incidence on CPP for the same reward (B, effect of conditioning: FS > 11.61, Ps < 0.001, no effect of lesion: FS < 0.50, Ps > 0.48 and no interaction: FS < 0.16, Ps > 0.69, n = 10–17). (C, D) SNc (*P < 0.05) but not mVTA (P = 0.41) lesions decreased operant sucrose self-administration (C, representation of the mean of the three last FR1 sessions (first graph) and linear regressions between sucrose deliveries and the loss of TH in the NAc (second graph) and the dorsal striatum (third graph) for mVTA and SNc lesions respectively, n = 6–9), while having no significant effect on sucrose preference in a two-bottle choice procedure (D, Ps > 0.08, n = 12–19). (E) SNc but not mVTA lesions (Effect of lesion: F1,98 = 14.1, P < 0.01 and F1,91 = 0.01, P = 0.94 respectively, n = 7–8) reduced self-activation of a cue-light during an operant procedure. Linear regressions between cue-light deliveries and the loss of TH in the NAc and the dorsal striatum for mVTA and SNc lesions respectively. (F) No effects of the 6-OHDA lesions were found on the preference for a novel environment (Ps > 0.73), n = 6–8.*P < 0.05, **P < 0.01, Sham-operated vs. Lesioned.
An effect of SNc lesion on preparatory behavior was further demonstrated by a dramatic impairment of instrumental responding for a sucrose solution in SNc lesioned rats as compared to both sham and mVTA lesioned rats (Fig. 4C and S3A–B), the latter even tending to outperform controls, both during acquisition and when the workload required to obtain the reward increased exponentially under a progressive ratio schedule of reinforcement, an index of motivation18 (Fig. S3C). The reduced behavioral responses of SNc-lesioned rats cannot be attributed to an impairment in instrumental learning, as their capacities to discriminate between the active and inactive (control) lever throughout the task were preserved (Fig. S3B), or to a decrease in sensitivity to the motivational properties of sucrose, as demonstrated by their clear preference for the same sucrose solution in a two-bottle choice procedure (Fig. 4D and S4A). Similar results were obtained with saccharin, a non caloric sweetener (Fig. S4B), excluding an effect of potential metabolic confounding factors. In this test, lesioned animals were also able to differentiate between two saccharin concentrations and to shift their preference toward the concentration supplying the greatest reward (Fig. S4C). Thus, the poorer operant performances of the SNc lesioned animals do not result from a learning deficit or a change in reward or hedonic processing. Instead, they reflect a profound decrease in motivation to work to obtain the reward.
This deficit in the motivational preparatory responding also affects novelty-seeking, operationalized by the acquisition of instrumental conditioning reinforced only by contingent presentations of a “novel” cue-light19. As shown on Fig. 4E, this cue-light acted as a robust positive reinforcer in both sham groups and mVTA-lesioned rats, but not in SNc-lesioned animals. This result is of particular interest because neither mVTA nor SNc lesions impaired preference for a novel environment in a non instrumental novelty preference procedure (Fig. 4F), thereby suggesting that interest for novelty was unaffected. Again, a marked motivational deficit was observed specifically in animals with SNc DA lesions, when an instrumental preparatory action was required.
These between-subject differences specific to the SNc lesioned rats were further supported by dimensional analyses. Thus, whereas linear regression analyses yielded no significant correlations in the runway (data not shown), robust negative correlations were found between operant performances in the sucrose (Figure 4C) and cue-light self-administration procedure (Figure 4E) and the loss of TH immunoreactivity within the dorsal striatum for the SNc group. Interestingly, these correlations were not found within the NAc for the VTA group. These data therefore strengthen the implication of nigrostriatal DA in motivated behaviors.
Bilateral partial DA lesions of the SNc, but not of the mVTA, induce depressive and anxiety-related behaviors
This generalized lack of motivation seems to present face validity with regards to the pathophysiology of PD, which is frequently accompanied by mood disorders related to DA denervation, including depression and anxiety3–5. Consistent with observations in humans, SNc-lesioned rats also displayed a depressive-like behavior, as revealed by an increase in the time they spent immobile in the forced-swim test, whereas no such effect was observed in mVTA-lesioned rats (Fig. 5A and S5A). Similarly, anxiety-related behaviors, reflected by a reduced latency to enter in the dark side of a light/dark apparatus (Fig. 5B and S5B) and a decreased time spent in the open arms of an elevated plus-maze (Fig. 5C and S5C), were seen in SNc-, but not mVTA-lesioned animals. Therefore, only SNc DA lesions affected mood-related behaviors.
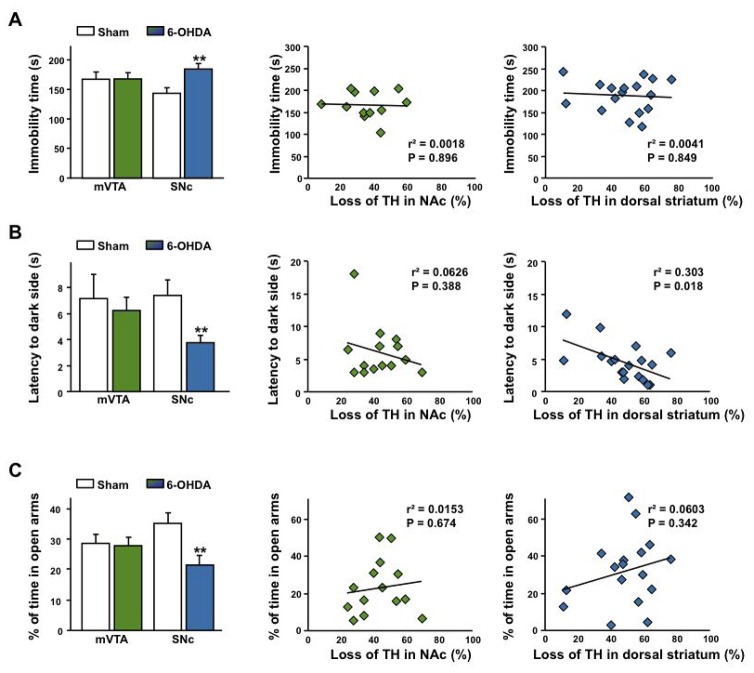
Fig. 5. Bilateral partial 6-OHDA lesions of the SNc, but not of the mVTA, cause a broad spectrum of affective impairments.
(A–C) SNc-lesioned rats displayed increased immobility in the forced-swim test (A, effect of lesion: (F1,47 = 4.71, p < 0.05), no effect of site: (F1,47 < 1) and a significant interaction between both factors: (F1,47 = 4.15, p < 0.05, n = 12–19), and anxiety-like behaviors, as reflected by a reduction in the latency to enter into the black compartment in a light/dark avoidance test (B, effect of lesion: (F1,59 = 4.73, p < 0.05), no effect of site: (F1,59 < 1) and no significant interaction: (F1,59 = 2.07, p = 0.15, n = 12–19) and in the time spent in the open arms of an elevated plus-maze (C, marginal effect of the lesion: (F1,71 = 3.79, p = 0.06), no effect of site: (F1,71 = 0.06, p = 0.80), but a significant interaction between both factors: (F1,71 = 4.37, p < 0.05), n = 15–22). No significant linear regressions were found in the forced-swim test (A) and in the elevated plus-maze (C) while a significant negative correlation was found between the latency to enter into the dark chamber in the light/dark avoidance test and loss of TH within the dorsal striatum (B). **P < 0.01, Sham-operated vs. Lesioned.
For these affective-related behaviors, a significant negative correlation was found between the latency to enter into the dark chamber in the light/dark avoidance test and loss of TH immunoreactivity within the dorsal striatum (Figure 5B) suggesting that the observed increase in anxiety is related to the degree of striatal dopamine depletion. However, no similar correlations were found in the elevated plus-maze and the forced-swim test (Figure 5A,C).
Reversion of the behavioral deficits resulting from nigrostriatal DA denervation by pharmacological DA agents
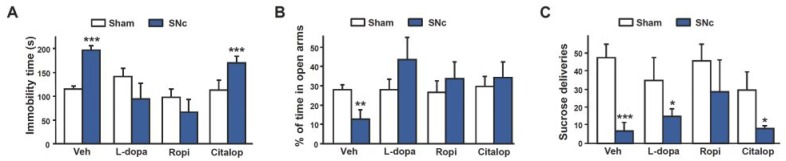
To further investigate the role of DA and validate our experimental approach, we tested whether subchronic administration of pharmacological DA agents classically used in PD could reverse some of the behavioral impairments induced by SNc lesions. The depressive- and anxiety-like behaviors displayed by SNc-lesioned rats were reversed by the D2/D3 agonist ropinirole and by L-dopa (Fig. 6A–B and S6A–B). Both pharmacological DA agents have been shown to have beneficial effects on apathetic symptoms and mood in PD3, 15. These findings thereby provide an interesting predictive validity for this model, in terms of the causal implication of DA. In addition, ropinirole clearly improved motivated behaviors of SNc-lesioned rats, in an operant sucrose self-administration procedure (Fig. 6C and S6C–D). The selective serotonin reuptake inhibitor (SSRI) citalopram had no effect on motivated and depressive-like behaviors in lesioned animals (Fig. 6A and C, and S6), consistent with a selective role for DA, although the effect of this drug on anxiety-like behaviors (Fig. 6B) suggests a possible interaction with the serotoninergic system.
Fig. 6. Effects of L-dopa, ropinirole and citalopram on the behavioral changes induced by the SNc DA lesion.
Effects of chronic intraperitoneal administration of L-dopa (12.5 mg/kg), ropinirole (1 mg/kg) or citalopram (10 mg/kg) evaluated in the forced-swim test, (A) the elevated plus-maze and (B) in an operant sucrose self-administration procedure (C). Significant lesion x treatment interactions were found for (A) and (B) (Fs > 2.94, Ps < 0.05) but not for (C) (significant effect of lesion: F1,63 = 16.53, P < 0.001, but no interaction: F3,63 = 0.78, P = 0.51). n = 6–11. *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Discussion
This study provides new insights into the pathophysiological mechanisms underlying the neuropsychiatric symptoms of PD. Indeed, our data clearly demonstrate that selective bilateral and partial lesions of the SNc, but not of the mVTA, induce a profound deficit in motivated behaviors and related affective changes. These behavioral deficits were selectively reversed by L-dopa and by the direct activation of the D2/D3 receptors with ropinirole, confirming the critical role played by DA.
Studying the non motor functions of the DA nigrostriatal system has remained a critical issue, due to the potential influence of motors deficits on the behavioral performances of the animals and the difficulty of targeting this pathway specifically. For example, large lesions of the DA nigrostriatal system and genetically-induced complete DA depletion lead to the so-called “lateral hypothalamic syndrome”, characterized by a dramatic aphagic and adipsic state6, 20, 21, a phenotype in some ways similar to the decrease in consummatory responses we observed in SNc-lesioned animals. However, it was not clear whether these impairments resulted from a pure motivational deficit or from the strong akinesia induced by the lesion, and whether they were specific to the nigrostriatal system. Similarly, previous studies attempting to model cognitive dysfunctions and neuropsychiatric symptoms in PD with unilateral or bilateral 6-OHDA lesions in rats or MPTP-lesioned monkeys, have rarely been able to circumvent possible motor confounding factors and did not systematically aim to target the DA nigrostriatal system selectively9, 22, 23. In the present study, with the use of partial and bilateral DA denervation, we were able to disentangle the non motor from the motor function, an approach that was further confirmed by the lack of specific relationship between individuals presenting no or mild motor deficits and their behavioral performances (data not shown). Thus, the lack of effect of the 6-OHDA lesions on several aspects of sensorimotor and ambulatory behavior suggests that motor alterations cannot account for the strong phenotype associated with partial DA denervation of the nigrostriatal system evidenced in this study.
These results therefore clearly demonstrate that selective bilateral lesions of the SNc, but not of the mVTA, induce a profound deficit in motivated and affective-related behaviors. While the lack of a robust correlation between affective-related deficits and dorsal striatum DA depletion suggests a potential role of extra-striatal regions receiving DA inputs from the SNc such as the amygdala or the orbitofrontal cortex2 in such behaviors, the clear-cut correlation between deficits in operant performances and the degree of TH loss in the dorsal striatum univocally emphasizes the predominant role of the DA nigrostriatal system in specific motivational processes. This result is particularly striking as, by contrast, a partial DA lesion of the mVTA, an area known to be involved in reinforcement and motivational processes6, 24–26, did not modify any of the non motor behaviors evaluated here. Interestingly, sensitivity to reward and Pavlovian processes, which are dependent on the DA mesocorticolimbic system24, 26, were unaffected by the lesion, which seems to alter the preparatory aspect of instrumental responses in a specific manner. Consistent with these findings, the SNc is located at the interface between the neurobiological systems underlying goal-directed and habitual control of behavior24, 27, 28, where DA neurons encode crucial motivational and reward-related signals30. The lesion generated is therefore likely to strongly interfere with the chain of processes that increase motivation and energize actions for a specific goal.
The behavioral phenotype induced by the SNc DA lesion is also reminiscent of apathy and related neuropsychiatric symptoms observed in PD patients4, 5, suggesting that the DA nigrostriatal system plays a primary role in non motor deficits in PD. It has been suggested that apathy in PD may result from a corticostriatal dysfunction linked to the loss of nigrostriatal DA tone13. Furthermore, a recent study using an implicit incentive task14 reported an association of apathy in PD and non PD patients with changes in the motivational processes normally responsible for translating expected reward into effort and action, with no change in the perception of reward value. These findings are therefore strikingly similar to the motivational deficits observed in our experimental model and its underlying neurobiological substrate.
The adverse phenotype induced by the SNc lesion is reversed, at least partly, by pharmacological DA agents known to have positive effects on apathetic symptoms and mood in PD3, 15. While both L-dopa and ropinirole completely reversed depressive- and anxiety-related behaviors, only ropinirole significantly improved motivated behaviors in lesioned animals. This may reflect the differences in the pharmacological properties of the two DA drugs, with L-dopa acting presynaptically on a denervated system and ropinirole having a stronger effect through the direct activation of postsynaptic receptors, including, in particular, the D3 receptor, a potent regulator of mood and motivated behaviors29. Indeed, it has been suggested that D2/D3 agonists such as ropinirole, may be more effective than other DA agents for the treatment of apathy, due to their high affinity for D2 and D3 receptors3, 15. By contrast, the SSRI citalopram had no beneficial effect on motivated and depressive-like behaviors, consistent with several clinical observations suggesting that SSRIs are much less effective than DA agonists for reducing depressive symptoms in PD30, 31 and may even have a deleterious effect on apathy32. These data strengthen the validity of our experimental approach in regard to PD-related neuropsychiatric symptoms and the critical role of DA.
The effect of citalopram on anxiety-related behaviors indicates however a possible implication of the serotoninergic system. Electrophysiological and neurochemical studies have reported a strong interaction between the dopaminergic and serotoninergic systems, with a prominent modulation of midbrain DA neuronal activity by the raphe nuclei33. Moreover, complex interactions between the serotoninergic and dopaminergic system has been reported in PD34 and related animal models35. Such interactions may account for the complex influence of serotonin on the DA dysfunction highlighted by the present data.
In conclusion, this study highlights a critical role in motivation for the SNc that had been previously largely neglected and attributed to the DA mesoaccumbal pathway2, 27. These data also demonstrate that motivational and affective deficits are a core impairment of PD, independent of the motor deficits and resulting from the loss of the major neuronal group known to degenerate in this disease (DA SNc neurons). This new insight into the pathophysiological mechanisms of mood and motivational dysfunctions in PD will facilitate the design of new treatments through a more balanced approach taking into account the entire spectrum of deficits observed in this brain disease.
Online Methods
Animals
Experiments were performed on male Sprague Dawley rats (Janvier, Le Genest-Saint-Isle, France) weighing 180 g (6 weeks old) at the time of surgery. Animals were housed four per cage until the second week after surgery and then transferred to individual cages until the end of the study, under standard laboratory conditions (12 h light/dark cycle, with lights on at 7:00 am) with food and water available ad libitum, unless otherwise stated. Protocols used complied with the European Community Council Directive of 24 November 1986 (86/609/EEC) for the care of laboratory animals, French Ministry of Agriculture regulations (authorization no. 38-R1001) and French guidelines for the use of living animals in scientific investigations. They were approved by the Grenoble-Institut des Neurosciences ethics committee, under agreement number 004.
Bilateral 6-OHDA lesions
All animals were anesthetized with a mixture of xylazine (15 mg/kg i.p.) and ketamine (100 mg/kg, i.p.) and treated with desipramine hydrochloride (25 mg/kg s.c.; Sigma, St Quentin-Fallavier, France) to protect noradrenergic neurons38, 30 min before 6-OHDA injection. Rats were secured in a Kopf stereotaxic apparatus (Phymep, Paris, France) and 6 μg of 6-OHDA dissolved in 2.3 μl of sterile 0.9% NaCl with 0.2% ascorbic acid (Sigma, St Quentin-Fallavier, France) were injected bilaterally, at a flow rate of 0.5 μl/min. The solution was delivered to the medial plane, to target the medial ventral tegmental area (mVTA group), or into the medial part of the substantia nigra pars compacta (SNc group). The stereotaxic coordinates of the injection site relative to bregma were as follows, according to the stereotaxic atlas of Paxinos and Watson (1998)36: (1) mVTA lesion: anteroposterior (AP), −5.6 mm; lateral (L), +1.0 mm, with a 10° angle toward the midline, and dorsoventral (DV), −8.1 mm; (2) SNc lesion: AP, −5.4 mm; L, ±1.8 mm and DV, −8.1 mm, with the incisor bar at +3.2 mm below the interaural plane. After each injection, the cannula was left in position for 5 min to allow the injected solution to be absorbed and to minimize the spread of the toxin along the needle tract. An identical procedure was used for sham-operated controls, but with 2.3 μl of vehicle (0.9% NaCl, 0.02% ascorbic acid). After recovery from anesthesia, animals returned to the facility for three weeks, to allow the 6-OHDA lesion to develop and stabilize38, before the beginning of the behavioral experiments.
Transient starvation states occurred two to three days after surgery in a subset of SNc-lesioned animals (around 20%). These animals received supplementation with a high-caloric liquid diet and palatable food for 1 to 2 weeks. Animals that did not recover (< 5%) were discarded from the experimental procedure. This phenotype was never observed in the Sham and mVTA lesions groups.
Histological analysis
Immunohistochemistry
Immunohistochemical analysis was carried out as previously described39. Briefly, rats were killed under chloral hydrate anesthesia at the end of the behavioral experiments, perfused intracardially with paraformaldehyde and brains removed. Free-floating 30 μm-thick coronal sections from the mesencephalon and the striatum were incubated with an anti-TH antibody (mouse monoclonal MAB5280, Chemicon, Temecula, USA; 1:2500), and then with a biotinylated goat anti-mouse IgG antibody (BA-9200, Vector Laboratories, Burlingame, CA, USA; 1:500). Immunoreactivity was visualized with avidin-peroxidase conjugate (Vectastain ABC Elite, Vector Laboratories, Burlingame, CA, USA).
Quantification of the extent of the mesencephalic DA lesion and of striatal DA denervation
TH-immunolabelling detection of DA neurons and terminals were evaluated under a light microscope (Nikon, Eclipse 80i) coupled to the ICS FrameWork computerized image analysis system (TRIBVN, 2.9.2 version, Châtillon, France).
For quantification, six selected TH-labeled coronal sections for each experimental animal, corresponding to three antero-posterior levels of the striatum (+0,7 to 1.7 mm anterior to bregma) and of the mesencephalon (−5 to −5.8 mm anterior to bregma), were digitized with a camera (Pike F-421C, ALLIED Vision Technologies, Stadtroda, Germany). For each section, six subregions within the striatum and three subregions within the mesencephalon were chosen, taking into account the topography of DA innervation2, 40, as indicated in Fig. S1A and B.
For all quantitative measurements, masks from these different striatal and mesencephalic subregions were drawn with the computer analysis system to ensure that appropriate comparisons were made between homologous anatomical regions. Optical densities (OD) were measured for each striatal and mesencephalic subregion, and the mean OD was calculated with ICS FrameWork software (TRIBVN, 2.9.2 version, Châtillon, France). OD values were measured for the denervated and non denervated territories of the lesioned animals for each section analyzed and were compared with those for the homologous regions in sham-operated animals. The OD value obtained for an unlabeled area (the corpus callosum) was used as the background and was subtracted from each of the OD values measured.
A simplified and classic subdivision of mesencephalic and striatal areas is shown in Fig. 1, for conciseness and to take the nigrostriatal and mesolimbic projections into account.
Brain tissue dopamine determination
Three weeks after surgery, dorsal striatum and NAc were dissected out and processed as previously described41, for the determination of DA concentration with a liquid chromatography system (Shimadzu, France) coupled to an electrochemical detector (Decade, Antec Leiden, the Netherlands) and a C18 reverse-phase microcolumn (Aquasil, RP-18, 150×1 mm, 3 μm particle size, ThermoHypersil, maintained at 30°C). The mobile phase (50 mM NaH2PO4, 0.1 mM EDTA-Na2, 1.7 mM sodium octyl sulfate, 4.5 mM KCl and 5% acetonitrile (vol/vol), adjusted to pH 3.10) was run at a flow rate of 0.06 ml/min. DA contents were determined by comparing DA peaks with external standards and were expressed as the amount of DA, in ng per mg of brain tissue.
Behavioral procedures
All rats were subjected to a sequence of behavioral tests, as summarized in Fig. S2. Different groups of animals were exposed to different sequences, as some combinations of tests, such as operant sucrose self-administration and cue-light self-administration or conditioned place preference (CPP) and novelty-preference, could not be performed on the same animal. However, all animals were tested for potential locomotor deficits on the rotarod and in the stepping test. When animals were subjected to a long sequence of behavioral tests or pharmacological treatment, two subgroups of animals were constituted, and the order of the tests was reversed in the second subgroup (see group A and B in Fig. S3A and B), to ensure that the effects of the lesion or of a pharmacological treatment did not change over the course of the study. No effect of order was found, in any of the conditions tested (data not shown). In each experiment, all conditions (lesions and/or pharmacological treatments) were counterbalanced among the different test chambers according to a Latin-square design. Each apparatus was cleaned with 10% ethanol and 2% H2O2, and dried with a paper towel after each trial or session.
Accelerating rotarod
Animals were first trained to remain on a rotarod (Harvard Apparatus, Holliston, MA, USA) turning at 4 rpm for more than 30 s. The rotation speed of the rod was then gradually increased, at a rate of 1 rpm every 8 s. Latency to fall from the rod was recorded three times for each rat.
Stepping test
Animals were moved sideways along a smooth-surfaced table number of forelimb adjusting steps measured, as described by Olsson et al., 199542. The test was carried out three times for each paw, by two experimenters blind to the experimental conditions.
Gait analysis
Gait was analyzed with an automated gait analysis system (GaitLab, Viewpoint S.A., Champagne au Mont d'Or, France). Rats were imaged from below, with a high-speed camera (~150 frames per second), while they ran on a narrow glass corridor (7 × 90 cm), to identify paw step positions and moving speed. Different metrics were calculated, including speed, stride length, stance time, swing time and number of strides per second. After a period of training of one week, gait and ambulatory behaviors were recorded three times for each rat, on a final test day.
Locomotor activity
Rats were placed in a dimly lit white Perspex™ open arena (50×50×40 cm) and horizontal distances traveled were recorded with a video-tracking system (Viewpoint S.A., Champagne au Mont d'Or, France), over a 20-minute period.
Olfactory tests
Tests were carried out in a dimly lit white open arena, with a video-tracking system. Olfactory avoidance and preference behaviors were evaluated by comparing the time the rats spent near two filter papers soaked in 40% acetic acid or in 50% coconut milk with the time spent near two filter papers soaked in distilled water. Acetic acid and coconut milk were used as they have been shown to be potent olfactory aversive43 and attractive44 cues, respectively.
Runway task for food
Food-restricted rats (90% of their free-feeding weight) were trained to run from a start box (20x15x40 cm) to the end of a Perspex™ alley (100x15x40 cm) to obtained a palatable food (salted, cheese-flavor cookies, Belin, France) presented in a plastic bowl. Ambulatory pattern was recorded, together with the latency to reach the food at the end of the runway and to start to eat it, with a video-tracking system. Animals were allowed to eat for less than 30 s, to prevent early satiation16. A 120 s cut off was used when animals did not complete the task. This procedure was repeated three times per day over a period of seven days. Performances were represented as a completion score16: (120 – latency to reach food)/120 x 100.
Conditioned place preference for food
CPP chambers consisted of two compartments (40x33x35 cm) differing in wall colors and floor texture, separated by a small (10 cm length) compartment45. A video-tracking system was used to measure the time spent in each compartment. During a preconditioning session, food-restricted rats (90% of their free-feeding weight) were placed in the CPP chamber and allowed to freely explore the three compartments for 15 minutes. Conditioning took place over eight consecutive days. During these sessions, animals were confined alternatively to one compartment with palatable food (Belin, France) for the paired condition, or without food for the unpaired condition, and to the other without food for both the paired and unpaired conditions. For testing, rats were allowed to explore the entire chamber for 15 minutes, as during the preconditioning session. Preference scores were expressed as the difference between the time spent in the food-paired compartment during the CPP test and the preconditioning test.
Evaluation of sucrose preference
Rats were given 24-h concurrent access in their home cage to two graduated 250 ml plastic bottles (Techniplast, France), for three days. One of these bottles contained tap water, whereas the other contained 2% sucrose (Sigma, St Quentin-Fallavier, France) in tap water. Rats and bottles were weighed daily, with the position of the bottles (left or right) alternated, to control for side preference. The first day was used as an acclimation period. The volumes of sucrose solution and water consumed on the second and third days were averaged to determine sucrose, water and total fluid intake (ml/kg), and preference for sucrose over water (sucrose intake/total intake, expressed as a percentage).
Evaluation of saccharin preference
Rats were given 24-h concurrent access to a bottle containing tap water and another containing 0.002% saccharin (Sigma, St Quentin-Fallavier, France) in tap water, using a procedure similar to that for sucrose. Rats were then given access to two bottles, containing 0.002% and 0.02% saccharin, respectively, for the next three days.
Operant sucrose self-administration
Rats were first habituated to voluntary consume 2% sucrose solution in a two-bottle choice procedure, as described above. They were then trained to self-administer a 2% sucrose solution in operant chambers (Med Associates, St. Albans, VT, USA) as previously described46, under a fixed ratio 1 reinforcement schedule (FR1), with an active, reinforced, lever, for which presses resulted in the delivery of 0.2 ml of the sucrose solution, and an inactive, non reinforced, lever. Once performances had stabilized (less than 20% performance variation over three consecutive sessions), rats were subjected to a progressive ratio schedule session, in which the number of active lever presses required for a reward increased exponentially after each reward, according to Roberts’ equation47. The session ended when the rat failed to complete a response requirement (i.e., a ratio) within one hour. The breakpoint was defined as the final ratio completed by the animal47.
Cue-light self-administration
A naive set of rats (i.e., that had not been subjected to the operant sucrose self-administration procedure) was tested daily for 1 h, during 8 days, for lever responses to the contingent presentation of a 6 s cue-light (FR1), located 4 cm above the active lever, in the self-administration chambers described above. The sides on which the inactive and active levers were located (right or left), were distributed evenly between the different conditions.
Novelty preference
This procedure was adapted from a method described elsewhere48. We used the same place preference chambers as described above for the CPP for food, with the same video-tracking system set-up. Rats were pseudorandomly exposed for 20 min to one compartment (‘familiar’). At the end of this habituation phase, animals were allowed to explore the whole chamber (familiar and new compartments) for 15 min. A novelty preference index was calculated as follows: time spent in the new compartment/(time spent in the new compartment + time spent in the familiar compartment) x 100.
Social interaction
Rats were placed in a dimly lit white Perspex™ arena (50x50x45 cm), for 10 min, for acclimatization to their surroundings. An unfamiliar male congener was then introduced into the arena and social interaction was video-recorded for 10 min. The total time that the test rats spent engaging in social interaction behaviors (listed and defined in49) was scored by two observers blind to the experimental conditions.
Elevated plus-maze
The elevated plus-maze (Viewpoint S.A., Champagne au Mont d'Or, France) consists of two opposing open arms and two opposing arms enclosed by 40 cm high walls, and was placed in a dimly lit room. Each arm was 50 cm long and 10 cm wide and made of black Perspex™, suspended 55 cm above the floor. The rats were placed in the center of the maze and their behavior was recorded for 5 minutes with a video-tracking system. Number of entries into and total time spent in the open and closed arms were quantified by the video-tracking system.
Light/dark avoidance test
The apparatus was made of Plexiglas and consisted of a light and a dark chamber (38x33x35 cm each), separated by an opaque Plexiglas wall with a 7 x 7 cm aperture, to allow the animals to move freely between the chambers. The light chamber was made of white walls, opened at the top, and was lit with a white incandescent light (100 watts) located 70 cm above the floor of the chamber. By contrast, the dark chamber was made of black walls, closed at the top, and was not lit. The animals were placed in the center of the light chamber, facing away from the opening toward the dark chamber, and were video-recorded for 5 min. Latency to the first entry into the dark chamber and the total amount of time spent in the light chamber were determined by two observers blind to the experimental conditions.
Forced swim test
Rats were placed in a cylinder of 40 cm high and 20 cm in diameter, filled with water (24±1°C) to a depth of 30 cm, for 15 min. Twenty four hours later, they were placed in the same cylinder for 5 min. Animal activity was detected and recorded with a video-tracking system.
Pharmacological procedures
Three weeks after 6-OHDA infusion, we initiated a sequence of behavioral tests on mSNc-lesioned rats, as described in Fig. S2B. Intraperitoneal administration of 12.5 mg/kg of L-dopa (together with 15 mg/kg of benserazide), 1 mg/kg of ropinirole, 10 mg/kg of citalopram, or vehicle (0.9% NaCl), at a volume of 1 ml/kg, began two days before the start of the behavioral tests sequence. Injections were carried out 30 min before the beginning of each behavioral session.
Data and statistical analysis
Data were analyzed by t-tests or two-way ANOVAs, depending on the experimental design. When indicated, post-hoc analyses were carried out with the Bonferroni’s correction procedure or the method of contrasts. Dimensional analyses were performed by parametric simple linear regressions.
Supplementary Material
Fig. S1. Extent of DA lesions following the infusion of 6-OHDA into the mVTA or SNc: two distinct patterns. (A) Representation of the mesencephalic and striatal subregions quantified in the selected coronal sections of the mesencephalon and striatum. (B) Mesencephalic and striatal TH-immunoreactivity quantification for the mVTA (green) and SNc (blue) 6-OHDA lesions. lSNc: lateral substantia nigra pars compacta, mSNc: medial substantia nigra pars compacta, mVTA: medial ventral tegmental area, NAc: nucleus accumbens, mTolf: medial olfactory tubercles, lTolf: lateral olfactory tubercles, DMS: dorsomedial striatum, DLS: dorsolateral striatum, n = 22–28. Note the segregation of the denervated striatal areas between the two lesions along a medioventral to dorsolateral gradient, as described by Voorn et al.40, with a preferential loss of TH staining in the shell of the NAc and other parts of the ventral striatum (core of the NAc and olfactory tubercules) for mVTA lesions, and a selective loss of TH staining in the dorsal striatum, predominantly in its lateral part, for SNc lesions. (C) DA contents of the striatum and NAc, n = 8–12. Note that infusion of 6-OHDA into the mVTA lead to a preferential loss of DA in the NAc (69±1.2% DA loss in the NAc with respect to sham-operated animals vs. 24.7±1.8% in the striatum), whereas the opposite pattern was found for SNc 6-OHDA lesions (18.2±3.2% DA loss in the NAc with respect to sham-operated vs. 77.7±1.7% in the striatum). Two-way ANOVAs found significant effects of the lesions (Fs > 34.86, Ps < 0.001) and significant interactions between the lesion and the brain region considered (Fs > 12.42, Ps < 0.01). *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Fig. S2. Behavioral experiment schedules. (A) Evaluation of the behavioral effects of the mVTA and SNc DA lesions. (B) Evaluation of the effects of different pharmacological treatments on the behavioral deficits induced by SNc DA lesions. The behavioral studies began three weeks after surgery and tests were carried out in opposite orders for groups A and B. CPP: conditioned place preference, FST: forced swim test, TBC: two-bottle choice procedure.
Fig. S3. SNc but not mVTA lesions reduced operant sucrose self-administration. (A) The number of sucrose deliveries along the 10 one-hour sucrose self-administration sessions was reduced in SNc-lesioned animals (effect of lesion: F1,135 = 6.68, P < 0.05 and significant lesion x session interaction: F9,135 = 2.39, P < 0.02), whereas similar performances were obtained for animals with mVTA lesions and control animals (no significant effect of lesion: F1,108 = 1.66, P = 0.22 and no lesion x session interaction: F1,108 = 0.54, P = 0.84). (B) Despite the small number of presses on the active, reinforced, lever for the SNc-lesioned animals, a two-way ANOVA nonetheless detected a significant effect of the lever (F1,72 = 6.39, P < 0.05), indicating that the animals pressed the active level significantly more frequently than the inactive level. (C) In a progressive ratio session, SNc (**P < 0.01), but no mVTA (P = 0.12) lesions significantly affected the breakpoint, corresponding to the last ratio completed (i.e., the last sequence of lever presses required to obtain a reward). n = 6–9. *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Fig. S4. 6-OHDA lesions do not alter sensitivity to rewarding sucrose and saccharin solutions. (A) The lesions had no significant effect on water (Ps > 0.05), 2% sucrose (Ps > 0.26) or total fluid intake (Ps > 0.27), in a two-bottle choice procedure, n = 12–19. (B) They also had no significant effect on the preference for a 0.002% saccharin solution (Ps > 0.32), or on water (Ps > 0.15) and saccharin (Ps > 0.20) intake, in a two-bottle choice procedure, n = 4–6. (C) The lesions had no significant effect on preference for a 0.02% over a 0.002% saccharin solution (Ps > 0.47), or on 0.002% saccharin (Ps > 0.15) and 0.02% saccharin (Ps > 0.51) intake, in a two-bottle choice procedure, n =4–6.
Fig. S5. SNc but not mVTA lesions induce depressive and anxiety-related behaviors. (A) SNc (effect of lesion: F1,132 = 8.66, P < 0.01 and no lesion x time interaction: F4,132 = 1.32, P = 0.27), but not mVTA (no effect of lesion: F1,92 = 0.01, P = 0.98 and no interaction: F4,92 = 0.77, P = 0.55) lesions decreased the time spent in activity along a 5 min forced-swim test, n = 11–19. (B) SNc lesions increased the time spent in the dark compartment during a 5-min light/dark avoidance test, whereas mVTA lesions did not, n = 12–19. (C) 6-OHDA lesions did not affect total arm entries (Ps > 0.14), indicating that general ambulatory activity in this test was not affected by the lesions, n = 15–22. *P < 0.05, **P < 0.01, Sham-operated vs. Lesioned.
Fig. S6. Effects of chronic intraperitoneal administration of L-dopa (12.5 mg/kg), ropinirole (1 mg/kg) and citalopram (10 mg/kg), on the depressive and anxiety-related behaviors and motivational deficits induced by SNc lesions. (A) L-Dopa and ropinirole, but not citalopram, reversed the decrease in the time spent in activity induced by the SNc lesions in a five-minute forced-swim test: an effect of the treatment was found for the lesion (F3,120 = 25.88, P < 0.001), but not for the sham-operated (F3,190 = 1.19, P = 0.33) condition. (B) No significant effect of lesion (F1,60 = 0.76, P = 0.38) or of treatment (F3,60 = 0.55, P = 0.65) and no interaction (F3,60 = 0.12, P = 0.95) were found on the total number of arm entries in an elevated plus-maze test, indicating that general ambulatory activity during the test was affected by neither the lesion nor the treatment, n = 6–11. (C) A significant effect of lesions was found for all treatments (Fs > 5.49, Ps < 0.05), except for ropinirole (no effect of lesion: F1,144 = 2.23, P = 0.16 and no lesion x session interaction: F9,144 = 0.62, P = 0.78), on the number of sucrose deliveries over the 10 one-hour sucrose self-administration sessions. (D) A significant effect of lesion (F1,65 = 5.15, P < 0.05) and treatment (F3,65 = 3.22, P< 0.05), but no interaction (F3,65 = 1.31, P = 0.28), were found on the breakpoint, which correspond to the last ratio completed (i.e., the last sequence of lever presses required to obtain a reward), during a progressive ratio session. n = 6–9. *P < 0.05, **P < 0.01, Sham-operated vs. Lesioned.
Acknowledgments
This work was supported by the Institut National de la Santé et de la Recherche Médicale, Fondation NeuroDis, Association France Parkinson, Ministère de la Recherche et de la technologie (MRT), Région Rhône-Alpes (ARC n°2) and Université Joseph Fourier. We would also like to thank Maurice Demattéis and Paul Krack for helpful discussions.
Footnotes
Contributions: G.D., S.C. and M.S. were responsible for overall study design. G.D., S.C., M.F. and S.B. carried out the stereotaxic surgeries and the post-surgery monitoring of the animals. C.C., G.D. and S.C. carried out the neuroanatomical analysis and characterization of the lesions. S.C. and G.D. performed the experiment for the neurochemical characterization of the lesion and A.B. carried out the HPLC process and analysis. S.C. and G.D. carried out the behavioral experiments and analysis. S.C., M.S. and G.D. wrote the paper with the help of the other authors.
References
- 1.Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122( Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 2.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- 4.Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA. Deep brain stimulation: from neurology to psychiatry? Trends Neurosci. 2010;33:474–484. doi: 10.1016/j.tins.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 5.Thobois S, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson's disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 6.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 7.Nieoullon A, Coquerel A. Dopamine: a key regulator to adapt action, emotion, motivation and cognition. Curr Opin Neurol. 2003;16(Suppl 2):S3–9. [PubMed] [Google Scholar]
- 8.Koob GF, Simon H, Herman JP, Le Moal M. Neuroleptic-like disruption of the conditioned avoidance response requires destruction of both the mesolimbic and nigrostriatal dopamine systems. Brain Res. 1984;303:319–329. doi: 10.1016/0006-8993(84)91218-6. [DOI] [PubMed] [Google Scholar]
- 9.Lindgren HS, Dunnett SB. Cognitive dysfunction and depression in Parkinson's disease: what can be learned from rodent models? Eur J Neurosci. 2012;35:1894–1907. doi: 10.1111/j.1460-9568.2012.08162.x. [DOI] [PubMed] [Google Scholar]
- 10.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 11.Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- 12.Marin RS. Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- 13.Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt L, et al. Disconnecting force from money: effects of basal ganglia damage on incentive motivation. Brain. 2008;131:1303–1310. doi: 10.1093/brain/awn045. [DOI] [PubMed] [Google Scholar]
- 15.Czernecki V, et al. Apathy following subthalamic stimulation in Parkinson disease: a dopamine responsive symptom. Mov Disord. 2008;23:964–969. doi: 10.1002/mds.21949. [DOI] [PubMed] [Google Scholar]
- 16.Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher "wanting" but not "liking" for sweet rewards. J Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 19.Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- 20.Olds J. Drives and reinforcements: Behavioral studies of hypothalamic functions. Raven Press; New York: 1977. [Google Scholar]
- 21.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tadaiesky MT, et al. Emotional, cognitive and neurochemical alterations in a premotor stage model of Parkinson's disease. Neuroscience. 2008;156:830–840. doi: 10.1016/j.neuroscience.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 23.Brown CA, et al. Dopamine pathway loss in nucleus accumbens and ventral tegmental area predicts apathetic behavior in MPTP-lesioned monkeys. Exp Neurol. 2012;236:190–197. doi: 10.1016/j.expneurol.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wise RA. Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci. 2009;32:517–524. doi: 10.1016/j.tins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 29.Sokoloff P, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 30.Aarsland D, Pahlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in Parkinson disease--epidemiology, mechanisms and management. Nat Rev Neurol. 2011;8:35–47. doi: 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 31.Barone P. Treatment of depressive symptoms in Parkinson's disease. Eur J Neurol. 2011;18(Suppl 1):11–15. doi: 10.1111/j.1468-1331.2010.03325.x. [DOI] [PubMed] [Google Scholar]
- 32.Barnhart WJ, Makela EH, Latocha MJ. SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract. 2004;10:196–199. doi: 10.1097/00131746-200405000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Adell A, Artigas F. The somatodendritic release of dopamine in the ventral tegmental area and its regulation by afferent transmitter systems. Neurosci Biobehav Rev. 2004;28:415–431. doi: 10.1016/j.neubiorev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Barone P. Neurotransmission in Parkinson's disease: beyond dopamine. Eur J Neurol. 2010;17:364–376. doi: 10.1111/j.1468-1331.2009.02900.x. [DOI] [PubMed] [Google Scholar]
- 35.Navailles S, Benazzouz A, Bioulac B, Gross C, De Deurwaerdere P. High-frequency stimulation of the subthalamic nucleus and L-3,4-dihydroxyphenylalanine inhibit in vivo serotonin release in the prefrontal cortex and hippocampus in a rat model of Parkinson's disease. J Neurosci. 2010;30:2356–2364. doi: 10.1523/JNEUROSCI.5031-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elesvier Academic Press; San Diego: 1998. [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elesvier Academic Press; San Diego: 2005. [Google Scholar]
- 38.Schwarting RK, Huston JP. Unilateral 6-hydroxydopamine lesions of meso-striatal dopamine neurons and their physiological sequelae. Prog Neurobiol. 1996;49:215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- 39.Boulet S, et al. Subthalamic stimulation-induced forelimb dyskinesias are linked to an increase in glutamate levels in the substantia nigra pars reticulata. J Neurosci. 2006;26:10768–10776. doi: 10.1523/JNEUROSCI.3065-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Dentresangle C, Le Cavorsin M, Savasta M, Leviel V. Increased extracellular DA and normal evoked DA release in the rat striatum after a partial lesion of the substantia nigra. Brain Res. 2001;893:178–185. doi: 10.1016/s0006-8993(00)03311-4. [DOI] [PubMed] [Google Scholar]
- 42.Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaki E, et al. Maintenance of olfactory neurogenesis requires HSF1, a major heat shock transcription factor in mice. J Biol Chem. 2006;281:4931–4937. doi: 10.1074/jbc.M506911200. [DOI] [PubMed] [Google Scholar]
- 44.Lelan F, et al. Effects of Human Alpha-Synuclein A53T-A30P Mutations on SVZ and Local Olfactory Bulb Cell Proliferation in a Transgenic Rat Model of Parkinson Disease. Parkinsons Dis. 2011;2011:987084. doi: 10.4061/2011/987084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nat Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- 46.Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci U S A. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 48.Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V. High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology. 2011;36:569–579. doi: 10.1038/npp.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.File SE, Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62:19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Extent of DA lesions following the infusion of 6-OHDA into the mVTA or SNc: two distinct patterns. (A) Representation of the mesencephalic and striatal subregions quantified in the selected coronal sections of the mesencephalon and striatum. (B) Mesencephalic and striatal TH-immunoreactivity quantification for the mVTA (green) and SNc (blue) 6-OHDA lesions. lSNc: lateral substantia nigra pars compacta, mSNc: medial substantia nigra pars compacta, mVTA: medial ventral tegmental area, NAc: nucleus accumbens, mTolf: medial olfactory tubercles, lTolf: lateral olfactory tubercles, DMS: dorsomedial striatum, DLS: dorsolateral striatum, n = 22–28. Note the segregation of the denervated striatal areas between the two lesions along a medioventral to dorsolateral gradient, as described by Voorn et al.40, with a preferential loss of TH staining in the shell of the NAc and other parts of the ventral striatum (core of the NAc and olfactory tubercules) for mVTA lesions, and a selective loss of TH staining in the dorsal striatum, predominantly in its lateral part, for SNc lesions. (C) DA contents of the striatum and NAc, n = 8–12. Note that infusion of 6-OHDA into the mVTA lead to a preferential loss of DA in the NAc (69±1.2% DA loss in the NAc with respect to sham-operated animals vs. 24.7±1.8% in the striatum), whereas the opposite pattern was found for SNc 6-OHDA lesions (18.2±3.2% DA loss in the NAc with respect to sham-operated vs. 77.7±1.7% in the striatum). Two-way ANOVAs found significant effects of the lesions (Fs > 34.86, Ps < 0.001) and significant interactions between the lesion and the brain region considered (Fs > 12.42, Ps < 0.01). *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Fig. S2. Behavioral experiment schedules. (A) Evaluation of the behavioral effects of the mVTA and SNc DA lesions. (B) Evaluation of the effects of different pharmacological treatments on the behavioral deficits induced by SNc DA lesions. The behavioral studies began three weeks after surgery and tests were carried out in opposite orders for groups A and B. CPP: conditioned place preference, FST: forced swim test, TBC: two-bottle choice procedure.
Fig. S3. SNc but not mVTA lesions reduced operant sucrose self-administration. (A) The number of sucrose deliveries along the 10 one-hour sucrose self-administration sessions was reduced in SNc-lesioned animals (effect of lesion: F1,135 = 6.68, P < 0.05 and significant lesion x session interaction: F9,135 = 2.39, P < 0.02), whereas similar performances were obtained for animals with mVTA lesions and control animals (no significant effect of lesion: F1,108 = 1.66, P = 0.22 and no lesion x session interaction: F1,108 = 0.54, P = 0.84). (B) Despite the small number of presses on the active, reinforced, lever for the SNc-lesioned animals, a two-way ANOVA nonetheless detected a significant effect of the lever (F1,72 = 6.39, P < 0.05), indicating that the animals pressed the active level significantly more frequently than the inactive level. (C) In a progressive ratio session, SNc (**P < 0.01), but no mVTA (P = 0.12) lesions significantly affected the breakpoint, corresponding to the last ratio completed (i.e., the last sequence of lever presses required to obtain a reward). n = 6–9. *P < 0.05, **P < 0.01, ***P < 0.001, Sham-operated vs. Lesioned.
Fig. S4. 6-OHDA lesions do not alter sensitivity to rewarding sucrose and saccharin solutions. (A) The lesions had no significant effect on water (Ps > 0.05), 2% sucrose (Ps > 0.26) or total fluid intake (Ps > 0.27), in a two-bottle choice procedure, n = 12–19. (B) They also had no significant effect on the preference for a 0.002% saccharin solution (Ps > 0.32), or on water (Ps > 0.15) and saccharin (Ps > 0.20) intake, in a two-bottle choice procedure, n = 4–6. (C) The lesions had no significant effect on preference for a 0.02% over a 0.002% saccharin solution (Ps > 0.47), or on 0.002% saccharin (Ps > 0.15) and 0.02% saccharin (Ps > 0.51) intake, in a two-bottle choice procedure, n =4–6.
Fig. S5. SNc but not mVTA lesions induce depressive and anxiety-related behaviors. (A) SNc (effect of lesion: F1,132 = 8.66, P < 0.01 and no lesion x time interaction: F4,132 = 1.32, P = 0.27), but not mVTA (no effect of lesion: F1,92 = 0.01, P = 0.98 and no interaction: F4,92 = 0.77, P = 0.55) lesions decreased the time spent in activity along a 5 min forced-swim test, n = 11–19. (B) SNc lesions increased the time spent in the dark compartment during a 5-min light/dark avoidance test, whereas mVTA lesions did not, n = 12–19. (C) 6-OHDA lesions did not affect total arm entries (Ps > 0.14), indicating that general ambulatory activity in this test was not affected by the lesions, n = 15–22. *P < 0.05, **P < 0.01, Sham-operated vs. Lesioned.
Fig. S6. Effects of chronic intraperitoneal administration of L-dopa (12.5 mg/kg), ropinirole (1 mg/kg) and citalopram (10 mg/kg), on the depressive and anxiety-related behaviors and motivational deficits induced by SNc lesions. (A) L-Dopa and ropinirole, but not citalopram, reversed the decrease in the time spent in activity induced by the SNc lesions in a five-minute forced-swim test: an effect of the treatment was found for the lesion (F3,120 = 25.88, P < 0.001), but not for the sham-operated (F3,190 = 1.19, P = 0.33) condition. (B) No significant effect of lesion (F1,60 = 0.76, P = 0.38) or of treatment (F3,60 = 0.55, P = 0.65) and no interaction (F3,60 = 0.12, P = 0.95) were found on the total number of arm entries in an elevated plus-maze test, indicating that general ambulatory activity during the test was affected by neither the lesion nor the treatment, n = 6–11. (C) A significant effect of lesions was found for all treatments (Fs > 5.49, Ps < 0.05), except for ropinirole (no effect of lesion: F1,144 = 2.23, P = 0.16 and no lesion x session interaction: F9,144 = 0.62, P = 0.78), on the number of sucrose deliveries over the 10 one-hour sucrose self-administration sessions. (D) A significant effect of lesion (F1,65 = 5.15, P < 0.05) and treatment (F3,65 = 3.22, P< 0.05), but no interaction (F3,65 = 1.31, P = 0.28), were found on the breakpoint, which correspond to the last ratio completed (i.e., the last sequence of lever presses required to obtain a reward), during a progressive ratio session. n = 6–9. *P < 0.05, **P < 0.01, Sham-operated vs. Lesioned.