Abstract
Objectives
The objectives of this study were to develop a self-healing dental composite containing poly(urea-formaldehyde) (PUF) shells with triethylene glycol dimethacrylate (TEGDMA) and N,N-dihydroxyethyl-p-toluidine (DHEPT) as healing liquid, and to investigate the mechanical properties of the composite and its self-healing efficacy after water-aging for 6 months.
Methods
PUF microspheres were synthesized encapsulating a TEGDMA-DHEPT healing liquid. Composite containing 30% of a resin matrix and 70% of glass fillers by mass was incorporated with 0%, 2.5%, 5%, 7.5% and 10% of microcapsules. A flexural test was used to measure flexural strength and elastic modulus. A single edge V-notched beam method was used to measure fracture toughness (KIC) and self-healing efficacy. Specimens were water-aged at 37 °C for 1 d to 6 months and then tested for self-healing. Fractured specimens were healed while being immersed in water to examine self-healing efficacy, in comparison with that in air.
Results
Incorporation of up to 7.5% of microcapsules into the resin composite achieved effective self-healing, without adverse effects on the virgin mechanical properties of the composite (p > 0.1). An excellent self-healing efficacy of 64%–77% recovery was obtained (mean ± sd; n = 6). Six months of water-aging did not decrease the self-healing efficacy compared to 1 d (p > 0.1). Exposure to water did not decrease the healing efficacy, compared to that healed in air (p > 0.1).
Conclusions
A composite was developed with excellent self-healing efficacy even while being immersed in water. The self-healing efficacy did not decrease with increasing water-aging time for 6 months.
Clinical significance
The novel self-healing composite may be promising for dental applications to heal cracks, resist fracture, and increase the durability and longevity.
Keywords: Self-healing, dental composite, microcapsules, polymerizable healing liquid, water-aging, mechanical property recovery
1. Introduction
Tooth-colored composites are increasingly used in dentistry as an alternative to amalgam to fill cavities.1–4 Of the 166 million tooth cavity restorations placed in 2005 in the United States, 52.5 million (31.6%) were amalgams, 77.3 million (46.6%) were composites, and 36.2 million (21.8%) were crowns.5 The increasing popularity of composites is a result of the superior esthetics, direct-filling capability, and improved physical and mechanical properties.6–15 However, studies showed that the durability of composite restorations needs to be improved.16,17 Half of all the restorations fail in less than 10 years, with fracture as one of the primary reasons.16 In a review on the longevity of composite restorations, it was observed that within 0 to 5 years after placement, the failure was primarily caused by restoration fracture, followed by secondary caries.17 The predominant reason for failure of composite restorations in larger cavities was also found to be fracture.18 Deterioration in restorations in vivo can be induced by fatigue from mastication, causing micro-flaws which can coalesce into cracks, eventually resulting in failure.19,20 Therefore, improving the fracture resistance of dental composites is of great significance. Extensive efforts have been undertaken in resin chemistry and fillers to improve the mechanical properties and performance of dental composite.3,21–24 However, inhibiting the crack propagation and fracture in dental composite restorations remains a challenge.3,24
A novel approach is to develop self-healing materials that have an autonomous crack-healing ability, to repair damage and recover the load-bearing capability.25 An important method employs a liquid healing agent encapsulated in a polymeric shell to form microcapsules, which are then incorporated into a matrix material.26 When cracking occurs, the propagating crack would rupture the microcapsules, releasing the healing liquid which flows into the cracked planes via capillary action. Upon contacting the embedded catalyst in the matrix, the polymerization of the healing liquid is triggered, and the material is healed.25 In a previous study, dicyclopentadiene (DCPD) was encapsulated in a poly(urea-formaldehyde) (PUF) shell to form microcapsules.27 Grubb’s catalyst, a transition metal carbine complex, was used in an epoxy matrix to trigger the polymerization of the released DCPD, resulting in self-healing.27 Other researchers synthesized self-healing materials in fields varying from microelectronics to aerospace.28 In the dental field, a self-healing composite was developed with a healing ability to recover 57% of the virgin fracture toughness (KIC).29 Attempts were also made to incorporate DCPD-containing microcapsules into dental resins.30 However, to date, there has been no further report on the use of DCPD and Grubb’s catalyst in Dental Materials, likely because the DCPD toxicity,31 Grubb’s catalyst toxicity, availability and high cost32 remain challenges. In a recent study,33 polyurethane (PU) shell-based triethylene glycol dimethacrylate (TEGDMA)-containing capsules were synthesized and added into a dental adhesive, showing that the dentin bond strength was not compromised. However, that study did not mention the use of a catalyst in the capsules, and self-healing was not reported.33
More recently, novel self-healing poly(urea-formaldehyde) (PUF) microcapsules containing polymerizable TEGDMA and N,N-dihydroxyethyl-p-toluidine (DHEPT) were synthesized and incorporated into dental resin.34 The self-healing resin containing microcapsules had human fibroblast viability similar to control cells in culture medium without resin, indicating little cytotoxicity in vitro.34 The composite containing these microcapsules achieved good self-healing efficacy.35 However, in these pilot studies, the cured specimens were immersed in water for only 1 day before testing. Dental composites in the oral environment are required to survive being wet for long periods of time. It is not clear how water-aging would affect the self-healing resin matrix, the microcapsules and the encapsulated healing liquid, thereby affecting the healing liquid polymerization with the initiator in the matrix. A literature search revealed no report on the effect of water-aging on the self-healing properties of composites.
The objectives of this study were to develop a self-healing dental composite containing PUF microcapsules with TEGDMA and DHEPT healing liquid, and to investigate the mechanical properties of the composite and its self-healing efficacy after the composite was water-aged for up to 6 months. The following hypotheses were tested: (1) Incorporation of the new microcapsules into the dental composite would not compromise the original mechanical properties of composite without microcapsules; (2) The self-healing efficacy would be directly proportional to the microcapsule filler level in the composite; (3) Water-aging for 6 months would not reduce the composite’s healing efficacy, compared to that at 1 day.
2. Materials and methods
2.1 Synthesis of self-healing microcapsules
Microcapsules were synthesized via in situ polymerization of formaldehyde and urea as described previously.34,35 Briefly, DHEPT (Sigma-Aldrich, St. Louis, MO) at 1% (all mass fractions) was added to TEGDMA monomer (Esstech, Essington, PA). At room temperature, 50 mL of distilled water and 13 mL of a 2.5% aqueous solution of ethylene-maleic anhydride (EMA) copolymer (Sigma-Aldrich) were mixed in a 250 mL round-bottom glass flask.36 The flask was suspended in a water bath on a hotplate (Isotemp, Fisher Scientific, Pittsburg, PA). The EMA solution was used as a surfactant to form an “oil-in-water” emulsion (“oil” being the TEGDMA-DHEPT monomer). Under agitation by a magnetic stir bar (diameter = 7.8 mm, length = 50 mm, Fisher Scientific) at 300 rpm, the shell-forming material urea (1.25 g), ammonium chloride (0.125 g) and resorcinol (0.125 g) (Sigma-Aldrich) were added into the solution. Resorcinol was added in the reaction of shell formation to enhance the rigidity of the shell.36 The pH was adjusted to 3.5 via drop-wise addition of 1 M sodium hydroxide solution. Then, the agitation rate was increased to 400 rpm, and 30 mL of the TEGDMA-DHEPT liquid was added into the flask. A stabilized emulsion of fine TEGDMA-DHEPT droplets was formed after 10 min of agitation. Then, 3.15 g of a 37% aqueous solution of formaldehyde (Sigma-Aldrich) was added, and the flask was sealed with aluminum foil to prevent evaporation. The temperature of the water bath was raised to 55 °C and the shell material was isothermally polymerized for 4 hours (h) under continuous agitation.36 In this process, ammonium chloride catalyzed the reaction of urea with formaldehyde to form PUF at the oil-water interface to develop the shell.36 The microcapsules thus obtained were rinsed with water and acetone, vacuum-filtered, and air-dried for 24 h. The microcapsules were examined by optical microscope (TE2000-S, Nikon, Japan), and their sizes were measured with an image analysis software (Nis-Elements BR2.30, Nikon). The microcapsules were also examined with scanning electronic microscopy (SEM, Quanta 200, FEI, Hillsboro, OR).
2.2 Development of self-healing composite
Bisphenol A glycidyl dimethacrylate (BisGMA) and TEGDMA (Esstech) were mixed at a mass ratio of 1:1, and rendered light-curable by adding 1% by mass of a photo-initiator phenyl-bis (2,4,6-trimethylbenzoyl) phosphine oxide (BAPO, Sigma-Aldrich).37,38 Then 0.5% by mass of benzoyl peroxide (BPO) (Sigma-Aldrich) was dissolved in the mixture. BPO serves as an initiator to react with the DHEPT in the healing liquid to trigger the self-healing polymerization. The glass fillers for the composite were barium boroaluminosilicate with a median particle size of 1.45 μm (Caulk/Dentsply, Milford, DE), which were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine.37,38 These glass particles were mixed into the resin at a filler level of 70% by mass to form a cohesive paste. Self-healing microcapsules were then mixed into this composite paste. The following microcapsule mass fractions in composite were used: 0%, 2.5%, 5%, 7.5% or 10%, resulting in five composites, respectively. Each group had six specimens for each experiment under each tested condition (n = 6). The composite paste was placed into a mold of 2 × 2 × 25 mm and covered with Mylar strips. Each specimen was photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side of the mold.
2.3 Flexural strength and elastic modulus measurement
The cured specimens were stored in distilled water at 37 °C for 24 h. A Universal Testing Machine (5500R, MTS, Cary, NC) was used to fracture the specimens in three-point flexure with a span of 10 mm at a crosshead speed of 1 mm/min. Flexural strength was S = 3PmaxL/(2bh2), where P max is load-at-failure, L is span, b is specimen width and h is thickness. Elastic modulus was E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope in the linear elastic region of the load-displacement curve. The specimens were tested within a few minutes after being taken out of water and were fractured while being wet.
2.4 Fracture toughness (KIC) and self-healing efficacy measurements
The cured composites were stored in water at 37 °C for 24 h. A single edge V-notched beam (SEVNB) method was used to measure the KIC.39 A notch depth of 500 μm was machined into each specimen using a diamond blade with a thickness of 150 μm (Buehler, Lake Bluff, IL).34,38 Then, a 3-μm diamond paste (Buehler) was placed into the notch tip, and a new razor blade was used to cut the notch further to a total depth of about 700–800 μm.34,38 This resulted in a relatively sharp notch with a tip radius of about 20 μm.38 The notch length was measured using an optical microscope (TE2000-S) on both sides of the specimen and averaged. Specimens were tested in three-point flexure with the notch on the tensile side, and the loading pin aligned with the notch under a 3× magnifier. KIC was calculated following an established method.39 This yielded the virgin fracture toughness of the specimen, KIC-virgin.34
To test self-healing efficacy, immediately following fracture, the two halves of the specimen were placed back into the mold to ensure a good contact of the two fractured planes, following a previous study.34 This would be similar to the case of a composite restoration in a tooth cavity, where the cracked restoration would stay in the tooth cavity to allow the released healing liquid to heal the crack. The healing of a completely cracked specimen would be a more rigorous test than the healing of a partial or small crack in the specimen. Because the fracture ruptured the microcapsules in the composite, the released TEGDMA-DHEPT liquid from the microcapsules would react with the BPO in the resin matrix. This would cause the polymerization of the released liquid to heal and bond the two cracked planes into a cohesive specimen.34 The healing specimens were incubated in a humidor at 37 °C in air for 24 h. The healed specimen was tested, which yielded the healed toughness, KIC-healed.34. The self-healing efficacy (η) was assessed following previous studies:34,40 η = KIC-healed/KIC-virgin. Selected fracture surfaces were sputter-coated with gold and examined in SEM (Quanta 200).
2.5 Water-aging of self-healing composite specimens
The composite with 7.5% microcapsules had a flexural strength similar to that with 0% microcapsules, while having a high self-healing efficacy; the composite with 10% microcapsules had a lower flexural strength. Therefore, the composite containing 7.5% microcapsules was selected for water-aging test for 6 months. The cured specimens were immersed in distilled water at 37 °C for 1 d and 1, 2, 3 and 6 months. For each time period, six specimens were immersed in 200 mL of water in a sealed polyethylene container. The water was changed once every week. After each water-aging time period, the KIC-virgin was measured. Then the two broken halves of each specimen were allowed to heal as described above, and the KIC-healed was measured. This yielded the self-healing efficacy η vs. water-aging time for the composite.
2.6 Self-healing while being immersed in water
The composite containing 7.5% microcapsules was tested. In the previous sections, the healing specimens were placed in a humidor in air with 100% relative humidity for the two broken halves to heal, not immersed in water. It is unclear how water immersion would affect the self-healing process. Therefore, in this section, after the specimen was fractured and the two halves were placed back into the mold, the construct was immediately immersed in water at 37 °C for 24 h, while the specimen healed. KIC-healed was then measured as described above.
2.7 Statistical analysis
One-way and two-way analyses-of-variance (ANOVA) were performed to detect the significant effects. Tukey’s multiple comparison was used to compare the data at p of 0.05.
3. Results
All data in this study were verified to have a normal distribution. The microcapsule diameters were measured (mean ± sd; n = 200) to be 70 ± 24 μm. An SEM image of microcapsules is shown in Fig. 1 (A). These microcapsules were incorporated into the composite, and the mechanical properties of the composite after water-immersion for 1 d are plotted in (B) for flexural strength, and (C) for elastic modulus (mean ± sd; n = 6). There was no significant difference in strength and elastic modulus with microcapsule mass fractions from 0% to 7.5% (p > 0.1). However, the mechanical properties at 10% microcapsules were significantly lower than the other groups (F = 7.45, p = 0.0004 for flexural strength; F = 23.85, p < 0.0001 for elastic modulus; one-way ANOVA).
Figure 1.
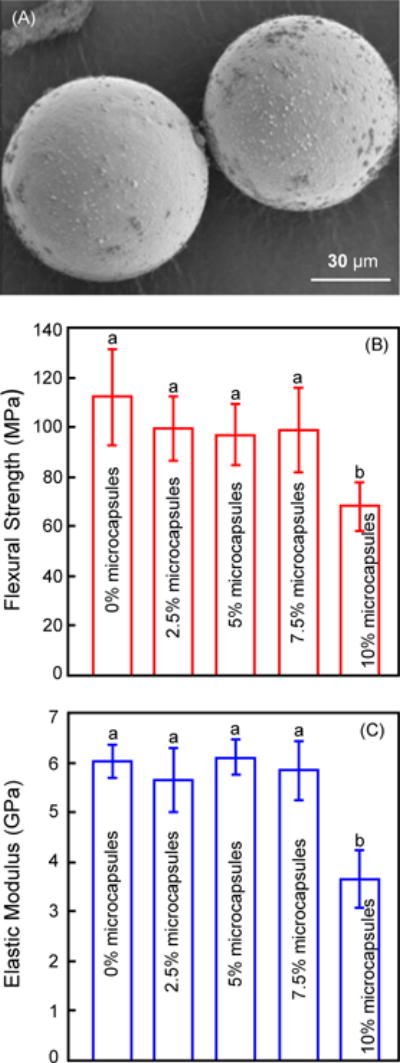
Self-healing microcapsule incorporation into dental composite and flexural testing. (A) Representative SEM image of microcapsules, (B) flexural strength and (C) elastic modulus of composite containing microcapsules at different mass fractions (mean ± sd; n = 6). The cured specimens were immersed in water for 1 d and then tested. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
The cured composite specimens were immersed in water for 1 d and then tested for self-healing. Fig. 2 plots (A) the virgin and healed KIC, and (B) the self-healing efficacy as a function of microcapsule content (mean ± sd; n = 6). Two-way ANOVA was tested in Fig. 2A. Factor 1 was the virgin and healed treatment levels (F = 1139.78, p < 0.0001), factor 2 was the microcapsule content levels (F = 18.32, p < 0.0001), and they had a significant interaction (F = 78.29, p < 0.0001). There is no significant difference in KIC-virgin from 0% to 7.5% microcapsules (p > 0.1). However, further increasing the microcapsule mass fraction to 10% reduced the KIC-virgin, from 1.26 MPa•m1/2 at 0% to 1.01 MPa•m1/2 at 10% microcapsules (p < 0.05). KIC-healed was significantly increased, from no healing at 0% microcapsules, to a maximum healing at 10% microcapsules (p < 0.05). In (B), a KIC recovery of 64% to 77% was achieved for composites with 7.5% and 10% microcapsules (F = 392.50, p < 0.0001; one-way ANOVA).
Figure 2.
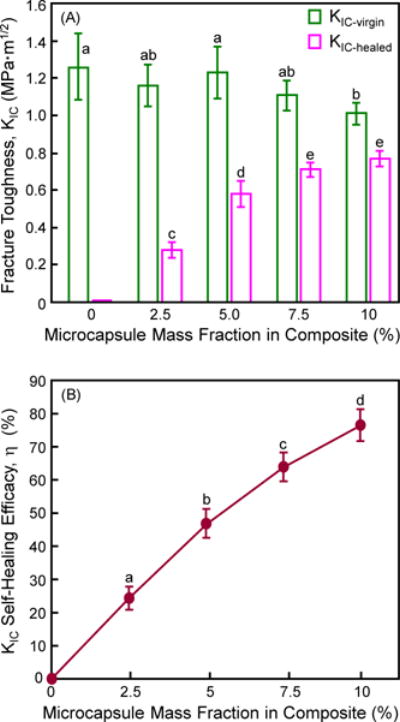
Self-healing of composite as a function of microcapsule mass fraction: (A) Virgin and healed fracture toughness, and (B) self-healing efficacy (mean ± sd; n = 6). The cured specimens were immersed in water for 1 d and then fractured to measure KIC-virgin. They were healed in a humidor in air for 1 d and then re-fractured to measure KIC-healed. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
SEM micrographs of representative fracture surfaces of the composite containing 7.5% microcapsules are shown in Fig. 3: (A) virgin fracture surface, and (B) fractured, healed and re-fractured surface. The virgin fracture surface indicates fractured microcapsules in the resin matrix. The healed and re-fracture surface contained polymer films (arrows), indicating that the healing liquid was released on the fracture surface and polymerized. Such observations of healing liquid polymerization are consistent with the recovery of the load-bearing capability.
Figure 3.
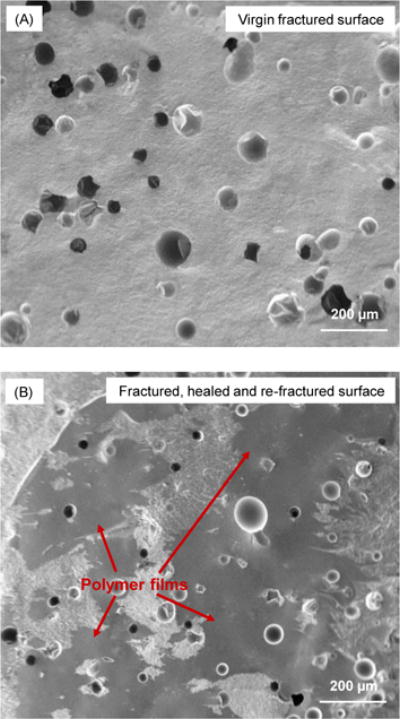
Typical SEM images of fractured surfaces of composite containing 7.5% microcapsules: (A) Virgin fracture surface, and (B) fractured, healed, and re-fractured surface. Arrows in (B) indicate polymer films formed by the released healing liquid that was polymerized at the cracked plane via reaction with the initiator in the resin matrix.
The cured composite specimens containing 7.5% microcapsules were water-aged for up to 6 months and then tested for self-healing. Fig. 4 plots (A) the KIC-virgin and KIC-healed, (B) the self-healing efficacy vs. water-aging time (mean ± sd; n = 6). Two-way ANOVA was used in Fig. 4A. Factor 1 was the virgin and healed treatment levels (F = 323.28, p < 0.0001), factor 2 was the different water-aging time levels (F = 25.77, p < 0.0001), and they had no significant interaction (F = 2.44, p = 0.073). Water-aging significantly decreased the KIC-virgin from 1 d to 30 d. Further immersion from 30 d to 6 months showed little further decrease in KIC-virgin. KIC-healed showed a similar trend to KIC-virgin. In (B), the healing efficacy η varied between 60% to 70%, and there was no significant loss in η from 1 d to 6 months (F = 0.13, p = 0.97; one-way ANOVA). η was 64% after water-aging for 6 months, indicating a good self-healing durability.
Figure 4.
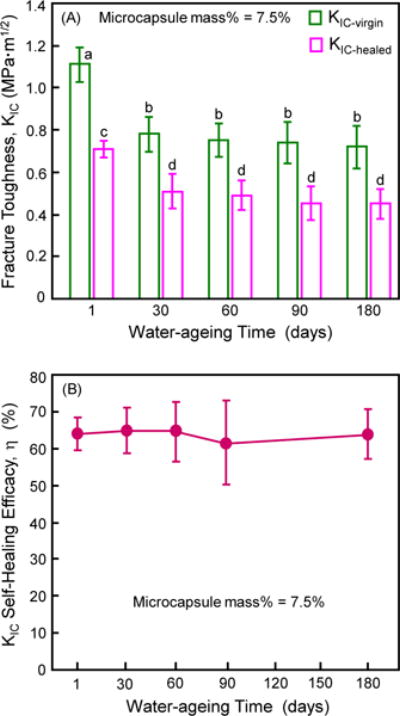
Effect of water-aging time on self-healing of composite containing 7.5% microcapsules. (A) Virgin and healed fracture toughness, and (B) self-healing efficacy (mean ± sd; n = 6). The cured composite specimens were water-aged for 1 d to 6 months (with 6 specimens for each time period), and then fractured to measure KIC-virgin. They were then healed in a humidor in air for 1 d and then re-fractured to measure KIC-healed. In (A), values with dissimilar letters are significantly different from each other (p < 0.05). In (B), all values are statistically similar (p > 0.1).
Specimens were fractured to measure KIC-virgin, the broken halves were placed into a mold and immediately immersed in water, and after 24 h the KIC-healed was measured. Specimens healed in humidor without immersion in water served as control. The results are plotted in Fig. 5. Two-way ANOVA was used for Fig. 5A. Factor 1 was the virgin and healed treatment levels (F = 117.72, p < 0.0001), factor 2 was healing in air in humidor or in water (F = 1.25, p = 0.29), and they had no significant interaction (F = 0.28, p = 0.61). Healing in water achieved the same KIC-healed and the same η compared to specimens without immersion (p > 0.1). In (B), a 60% recovery in fracture toughness was achieved indicating that immersion in water did not significantly compromise the healing process (F = 0.54, p = 0.48; one-way ANOVA).
Figure 5.
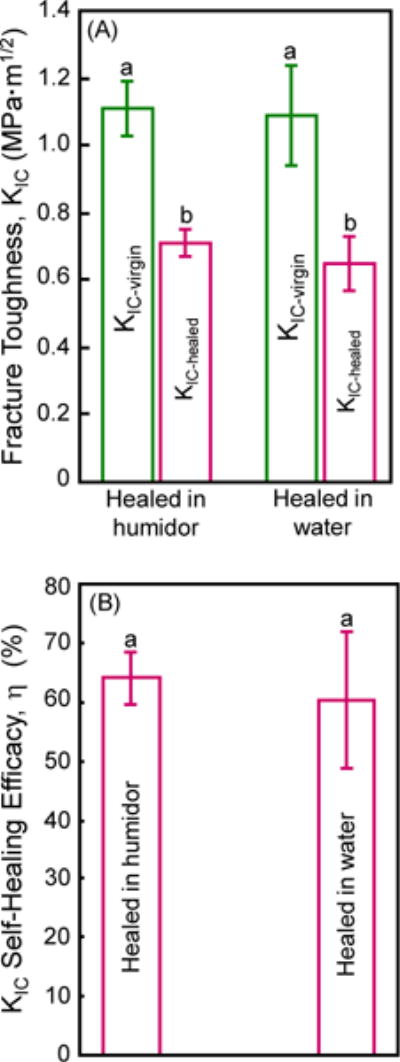
Self-healing while being immersed in water for composite containing 7.5% microcapsules. (A) Virgin and healed fracture toughness, and (B) self-healing efficacy (mean ± sd; n = 6). Healed in humidor: Specimens were water-aged for 1 d and then fractured to measure KIC-virgin. They were then healed in a humidor in air for 1 d and then re-fractured to measure KIC-healed. Healed in water: Specimens were water-aged for 1 d and then fractured to measure KIC-virgin. They were then healed while being immersed in water for 1 d and then re-fractured to measure KIC-healed. Values with the same letter are similar (p > 0.1).
4. Discussion
The present study investigated the effects of immersion in water on the self-healing efficacy of dental composite for the first time. The three hypotheses were accepted that incorporation of the new microcapsules into composite did not compromise the original mechanical properties of the composite, that self-healing efficacy was directly proportional to the microcapsule filler level in composite, and that water-aging for 6 months did not lower the healing efficacy when compared to that at 1 day.
Mechanical properties are important for load-bearing dental composite restorations.41,42 Therefore, incorporation of microcapsules into the composite to achieve self-healing ability must not decrease the original mechanical properties of the composite without microcapsules. In present study, addition up to 7.5% microcapsules into the composite did not decrease the mechanical properties. This was consistent with a previous study showing that the incorporation of up to 6% of microcapsules into a host material did not affect the original flexural strength.30 However, these results are different from another study showing a significant strength increase after inclusion of 5% microcapsules into a polymer.43 In addition, the present study demonstrated that there was a significant reduction in strength and modulus at 10% microcapsules. Furthermore, the majority of previous studies showed that the incorporation of self-healing microcapsules into a polymer matrix increased the KIC-virgin, compared to that without microcapsules.43–46 However, these previous studies used epoxy matrices. The composite in the present study was different from epoxy because the resin was filled with glass fillers. Indeed, a previous study added 5% self-healing microcapsules to a dental composite containing 45% resin and 55% glass fillers, and the KIC-virgin was neither increased nor decreased after the microcapsule addition.29 This is consistent with Fig. 2A of the present study, showing that KIC-virgin was not changed when adding up to 7.5% of microcapsules; but at 10% microcapsules, KIC-virgin was reduced. These studies indicate that whether the addition of microcapsules would increase or decrease the virgin mechanical properties of the composite depends on the different host systems as well as the microcapsule mass fractions used. For the dental composite of the present study, 7.5% of microcapsules appeared to be optimal, achieving the highest KIC-healed, without compromising the KIC-virgin, and without compromising the flexural strength and elastic modulus.
At 7.5% microcapsules, a 64% self-healing efficacy was achieved in the present study. This was supported by the existence of polymer films on the fractured, healed and re-fractured surfaces, confirming microcapsule rupture and in situ polymerization of the healing liquid. This demonstrated that the glass fillers in the composite at a filler mass fraction of 70% did not prevent the microcapsules from releasing the liquid containing the catalyst DHEPT to react with the initiator BPO in the resin matrix to cause self-healing.
Our previous study did not test the effect of water-aging on self-healing composite properties.34 Dental composite in vivo function in a wet oral environment which could degrade the mechanical properties.47–51 Previous studies on non-self-healing dental composites showed that the composites were weakened by long-term water-aging, which could degrade the fillers,48 soften the resin matrix due to the plasticizing action of water,47 and cause hydrolytic breakdown of the interfaces between the fillers and the resin matrix.47,50 Two key issues need to be investigated. First, after long-term water-aging, will the composite still be able to undergo self-healing when cracking happens? How does the self-healing efficacy of the dental composite change as a function of increasing water-aging time? Second, when the composite is immersed in water and wet, will the wet environment negatively affect the healing liquid’s reaction and polymerization to cause self-healing?
Regarding the first issue, water-aging did decrease the KIC-virgin of the self-healing composite, which was similar to previous results.47,50 The KIC-virgin of the composite after 6 months of water-aging was 0.72 MPa·m1/2, within the reported KIC range for commercial dental composites which were not water-aged and contained no microcapsules for self-healing.52 The KIC-healed vs. water-aging time showed a similar trend to KIC-virgin, and as a result, the self-healing efficacy η showed no significant decrease vs. water-aging time from 1 d to 6 months (Fig. 4). Therefore, after water-aging for 6 months, the composite was still able to self-healing and recover about 60–70% of the load-bearing capability. This indicates that water-aging for 6 months did not significantly degrade the microcapsules, the healing liquid, and the polymerization reaction efficacy between the catalyst and the initiator in the matrix.
A literature search revealed no report on the effect of water-aging time on self-healing. A previous study tested the aging in air, and reported that solvent-based healing systems showed that the healing efficacy decreased quickly after one month of aging in the air, and decreased to zero after eight months due to the lack of residual functionality groups.53 On the other hand, long-term stability was achieved for self-healing epoxy, showing 68% healing efficacy after aging for six months in ambient air.54 This was because the initiator was maintained to be stable over that period of time.54 The present study investigated the water-aging effect on self-healing of a composite for the first time. Aging the specimens in water could gradually degrade the composite and cause the leach-out of any uncured monomers.50 Indeed, KIC-virgin was decreased after water-aging for 1–6 months, compared to 1 d (Fig. 4A). However, water-aging for six months caused no significant reduction in self-healing efficacy for the dental composite. This may be because that the embedded microcapsules maintained an effective rupture during cracking to release the healing liquid, and the initiator BPO in the resin matrix was still able to trigger the polymerization of the released healing agent. In addition, the extent of decrease in KIC-healed was similar to that in KIC-virgin, thus resulting in a similar η vs. time. This indicates that the water-aging-induced degradation was moderate, and the extent of degradation in the composite matrix was similar to that in the self-healing system. Therefore, the self-healing system did not degrade any more than the dental composite matrix itself in water-aging for 6 months. However, further study is needed to investigate the self-healing of this promising dental composite after longer periods of aging, such as several years.
Regarding the second issue, our previous study tested self-healing of cracked dental composite in air in a humidor, without immersing the composite in water during the healing reaction.34 In the present study, the cracked specimens in water achieved similar self-healing as that in air, indicating that water immersion did not adversely affect the healing polymerization reaction (Fig. 5). Literature search revealed only one report on a resin’s self-healing in a wet environment.55 Our results are consistent with this previous study which reported successful self-healing for a resin while being immersed in a simulated body fluid solution (SBF), achieving a 64% recovery in KIC.55 This was likely because that the healing liquid with catalyst was able to react with the initiator in the resin matrix in the presence of water, and the polymerization was able to proceed. Two factors may have contributed to this result. First, the cured BisGMA-TEGDMA matrix of the composite is relatively hydrophobic and not very wettable,56 hence it would act to moderately repel water. This will likely make it difficult for water to infiltrate the crack in the composite. Second, water would not block the polymerization,56 and the initiator-catalyst reaction occurs in the resin because water is not penetrating into the chemical structure. Even if there is water, it would not affect the polymerization. Indeed, previous studies have shown the presence of water to have little appreciable effect on photo-polymerization rate or the degree of double bond conversion.56,57 Additionally, when methacrylate-based hydrogels were made, it was the same type of free-radical polymerization and that occurred completely in water.58 Hence it is anticipated that this novel composite would be effective in self-healing in the wet oral environment and retain its self-healing ability after water-aging treatments. Therefore, this self-healing composite is promising for crack-inhibiting and fracture-resistant dental restorations. Further studies are needed to test immersion in saliva and investigate other clinically-relevant properties of this self-healing dental composite.
5. Conclusions
This study investigated a self-healing dental composite containing PUF microcapsules with TEGDMA-DHEPT healing liquid and the composite properties vs. water-aging for the first time. Incorporating microcapsules at 7.5% mass fraction into the composite did not compromise the flexural strength, elastic modulus and KIC-virgin, compared to that without microcapsules. Water-aging for 6 months did not decrease the self-healing efficacy compared to that at 1 d, indicating that water-aging did not degrade the microcapsules and the healing reaction any more than the moderate degradation of the composite matrix itself. The composite being immersed in water achieved the same self-healing efficacy as that in air, indicating that the water immersion did not adversely affect the healing liquid’s catalyst reaction with the initiator in the matrix for the polymerization to occur. In light of restoration fracture as one of the primary failure reasons, the novel self-healing dental composite may be promising for crack-resistant restorations to heal fractures and prolong the service life for restorations.
Acknowledgments
We thank Dr. Joseph M. Antonucci for discussions and Esstech (Essington, PA) for kindly donating materials. This study was supported by NIH R01 DE17974 (HX), ZR2014HM073 (JLW) from Shandong Provincial Natural Science Foundation in China, and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. Journal of the American Dental Association. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 2.Lynch CD. Successful posterior composites. London: Quintessence Publishing Co; 2008. [Google Scholar]
- 3.Ferracane JL. Resin composite-State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Oei JD, Mishriky M, Barghi N, Rawls HR, Cardenas HL, Aguirre R, et al. Development of a low-color, color stable, dual cure dental resin. Dental Materials. 2013;29:405–412. doi: 10.1016/j.dental.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Reports. 2007;122:657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. Journal of Dental Research. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 7.Xu X, Ling L, Wang R, Burgess JO. Formation and characterization of a novel fluoride-releasing dental composite. Dental Materials. 2006;22:1014–1023. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Sadeghipour K, Baran G. Finite element analysis of the effect of an interphase on toughening of a particle reinforced polymer composite. Composites Part A: Applied Science and Manufacturing. 2008;39:956–964. doi: 10.1016/j.compositesa.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei YJ, Silikas N, Zhang ZT, Watts DC. Hygroscopic dimensional changes of self-adhering and new resin-matrix composites during water sorption/desorption cycles. Dental Materials. 2011;27:259–266. doi: 10.1016/j.dental.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Pashley DH, Tay FR, Imazato S. How to increase the durability of resin-dentin bonds. Compendium of Continuing Education in Dentistry. 2011;32:60–64. [PubMed] [Google Scholar]
- 14.Hosoya Y, Shiraishi T, Odatsu T, Nagafuji J, Kotaku M, Miyazaki M, Powers JM. Effects of polishing on surface roughness, gloss, and color of resin composites. Journal of Oral Science. 2011;53:283–291. doi: 10.2334/josnusd.53.283. [DOI] [PubMed] [Google Scholar]
- 15.Lynch CD, McConnell RJ, Wilson NH. Posterior composites: the future for restoring posterior teeth? Primary Dental Journal. 2014;3:49–53. doi: 10.1308/205016814812143923. [DOI] [PubMed] [Google Scholar]
- 16.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. International Dental Journal. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 17.Brunthaler A, Konig F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clinical Oral Investigations. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- 18.van Dijken JW. Direct resin composite inlays /onlays: an 11-year follow-up. Journal of Dentistry. 2000;28:299–306. doi: 10.1016/s0300-5712(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 19.Baran GR, Boberick KG, McCool JI. Fatigue of restorative materials. Critical Reviews in Oral Biology and Medicine. 2001;12:350–360. doi: 10.1177/10454411010120040501. [DOI] [PubMed] [Google Scholar]
- 20.Lohbauer U, Belli R, Ferracane JL. Factors involved in mechanical fatigue degradation of dental resin composites. Journal of Dental Research. 2013;92:584–591. doi: 10.1177/0022034513490734. [DOI] [PubMed] [Google Scholar]
- 21.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dental Materials. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 22.Schneider LF, Pfeifer CS, Consani S, Prahl SA, Ferracane JL. Influence of photoinitiator type on the rate of polymerization, degree of conversion, hardness and yellowing of dental resin composites. Dental Materials. 2008;24:1169–1177. doi: 10.1016/j.dental.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Lee VA, Cardenas HL, Rawls HR. Rubber-toughening of dimethacrylate dental composite resin. Journal of Biomedical Materials Research B. 2010;94:447–454. doi: 10.1002/jbm.b.31674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jandt KD, Sigusch BW. Future perspectives of resin-based Dental Materials. Dental Materials. 2009;25:1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 25.Murphy EB, Wudl F. The world of smart healable materials. Progress in Polymer Science. 2010;35:223–251. [Google Scholar]
- 26.Samadzadeh M, Boura SH, Peikari M, Kasiriha SM, Ashrafi A. A review on self-healing coatings based on micro/nanocapsules. Progress in Organic Coatings. 2010;68:159–164. [Google Scholar]
- 27.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, et al. Autonomic healing of polymer. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Urban MW. Self-healing polymeric materials. Chemical Society Reviews. 2013;42:7446–7467. doi: 10.1039/c3cs60109a. [DOI] [PubMed] [Google Scholar]
- 29.Wertzberger BE, Steere JT, Pfeifer RM, Nensel MA, Latta MA, Gross SM. Physical characterization of a self-healing dental restorative material. Journal of Applied Polymer Science. 2010;118:428–434. [Google Scholar]
- 30.Then S, Neon GS, Kasim NH. Performance of melamine modified urea-formaldehyde microcapsules in a dental host material. Journal of Applied Polymer Science. 2011;122:2557–2562. [Google Scholar]
- 31.Bevan C, Snellings WM, Dodd DE, Egan GF. Subchronic toxicity study of dicyclopentadiene vapor in rats. Toxicology and Industrial Health. 1992;8:353–367. [PubMed] [Google Scholar]
- 32.Caruso MM, Delafuente DA, Ho V, Sottos NR, Moore JS, White SR. Solvent-promoted self-healing epoxy materials. Macromolecules. 2007;40:8830–8832. [Google Scholar]
- 33.Ouyang X, Huang X, Pan Q, Zuo C, Huang C, Yang X, et al. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. Journal of Dentistry. 2011;39:825–833. doi: 10.1016/j.jdent.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Weir MD, Melo MS, Zhang Q, Zhou C, Xu HH. Novel self-healing dental resin with microcapsules of polymerizable triethyleneglycol dimethacrylate and N,N-dihydroxyethyl-p-toluidine. Dental Materials. 2015 doi: 10.1016/j.dental.2015.11.014. http://dx.doi.org/10.1016/j.dental.2015.11.014. [DOI] [PMC free article] [PubMed]
- 35.Wu J, Weir MD, Melo MS, Xu HH. Development of novel self-healing and antibacterial dental composite containing calcium phosphate nanoparticles. Journal of Dentistry. 2015;43:317–326. doi: 10.1016/j.jdent.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaiszik BJ, Caruso MM, McIlroy DA, Moore JS, White SR, Sottos NR. Microcapsules filled with reactive solutions for self-healing materials. Polymer. 2009;50:990–997. [Google Scholar]
- 37.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. Journal of Microencapsulation. 2003;20:719–730. doi: 10.1080/0265204031000154160. [DOI] [PubMed] [Google Scholar]
- 38.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–870. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu HH, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. Journal of Dental Research. 2004;83:930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 40.Kuebler J. Fracture toughness of ceramics using the SEVNB method. EMPA; CH-8600 Duebendorf, Switzerland: 1999. (Round robin VAMAS Report No 37, TWA3, ESIS Report D2-99). [Google Scholar]
- 41.Wool RP, O’Connor KM. A theory of crack healing in polymers. Journal of Applied Physics. 1981;52:5953–5963. [Google Scholar]
- 42.Ilie N, Hickel R. Investigations on mechanical behaviour of dental composites. Clinical Oral Investigations. 2009;13:427–438. doi: 10.1007/s00784-009-0258-4. [DOI] [PubMed] [Google Scholar]
- 43.Bohaty BS, Ye Q, Misra A, Sene F, Spencer P. Posterior composite restoration update: focus on factors influencing form and function. Clinical Cosmetic and Investigational Dentistry. 2013;5:33–42. doi: 10.2147/CCIDE.S42044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan L, Huang S, Gu A, Liang G, Chen F, Hu Y, et al. A cyanate easter/microcapsule system with low cure temperature and self-healing capacity. Composites Science and Technology. 2013;87:111–117. [Google Scholar]
- 45.Brown EN, Sottos NR, White SR. Fracture testing of a self-healing polymer composite. Experimental Mechanics. 2002;42:372–379. [Google Scholar]
- 46.Brown EN, White SR, Sottos NR. Microcapsule induced toughening in a self-healing polymer composite. Journal of Materials Science. 2004;39:1703–1710. [Google Scholar]
- 47.Keller MW, White SR, Sottos NR. A self-healing poly(dimethyl siloxane) elastomer. Advanced Functional Materials. 2007;17:2399–2404. [Google Scholar]
- 48.Ferracane JL, Berge HX, Condon JR. In vitro aging of dental composites in water-effect of degree of conversion, filler volume, and filler/matrix coupling. Journal of Biomedical Materials Research. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 49.Xu HH. Long-term water aging of whisker-reinforced polymer-matrix composites. Journal of Dental Research. 2003;82:48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- 50.Spencer P, Wang Y, Bohaty B. Interfacial chemistry of moisture-aged class II composite restorations. Journal of Biomedical Materials Research B. 2006;77:234–240. doi: 10.1002/jbm.b.30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sideridou ID, Karabela MM, Vouvoudi EC. Physical properties of current dental nanohybrid and nanofill light-cured resin composites. Dental Materials. 2011;27:598–607. doi: 10.1016/j.dental.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 53.Ilie N, Hickel R, Valceanu AS, Huth KC. Fracture toughness of dental restorative materials. Clinical Oral Investigations. 2012;16:489–498. doi: 10.1007/s00784-011-0525-z. [DOI] [PubMed] [Google Scholar]
- 54.Caruso MM, Blaiszik BJ, White SR, Sottos NR, Moore JS. Full recovery of fracture toughness using a nontoxic solvent-based self-healing system. Advanced Functional Materials. 2008;18:1898–1904. [Google Scholar]
- 55.Jin H, Mangun CL, Stradley DS, Moore JS, Sottos NR, White SR. Self-healing thermoset using encapsulated epoxy-amine healing chemistry. Polymer. 2012;53:581–587. [Google Scholar]
- 56.Dailey MM, Silvia AW, McIntire PJ, Wilson GO, Moore JS, White SR. A self-healing biomaterial based on free-radical polymerization. Journal of Biomedical Materials Research A. 2014;102:3024–3032. doi: 10.1002/jbm.a.34975. [DOI] [PubMed] [Google Scholar]
- 57.Park J, Ye Q, Topp EM, Misra A, Kieweg SL, Spencer P. Effect of photoinitiator system and water content on dynamic mechanical properties of a light-cured bisGMA/HEMA dental resin. Journal of Biomedical Materials Research A. 2010;93:1245–1251. doi: 10.1002/jbm.a.32617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fong H. Effects of water contents and postcuring conditions on Bis-GMA/TEGDMA dental restorative composite resins. Journal of Applied Polymer Science. 2004;94:492–502. [Google Scholar]
- 59.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–4314. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]


