Abstract
Objectives
White spot lesions are the most undesired side-effect of fixed orthodontic treatments. The objectives of this study were to combine nanoparticles of silver (NAg) with 2-methacryloyloxyethyl phosphorylcholine (MPC) to develop a modified resin-modified glass ionomer cement (RMGI) as orthodontic cement with double benefits of antibacterial and protein-repellent capabilities for the first time.
Methods
NAg and MPC were incorporated into a commercial RMGI. Another commercial orthodontic adhesive also served as control. Enamel shear bond strengths (SBS) were determined. Protein adsorption was measured via a micro bicinchoninic acid method. A dental plaque microcosm biofilm model with human saliva as inoculum was tested. Biofilms adherent on the cement samples and planktonic bacteria in the culture medium away from the cement surfaces were both evaluated for bacterial metabolic activity, colony-forming units (CFU), and lactic acid production.
Results
Adding 0.1% NAg and 3% MPC to RMGI, and water-aging for 30 days, did not adversely affect the SBS, compared to the unmodified RMGI control (p>0.1). The modified RMGI containing 0.1% NAg and 3% MPC achieved the greatest reduction in protein adsorption, bacterial adhesion, CFU, metabolic activity and lactic acid production. The RMGI containing 0.1% NAg and 3% MPC inhibited not only the bacteria on its surface, but also the bacteria away from the surface in the culture medium.
Conclusions
The incorporation of double agents (antibacterial NAg + protein-repellent MPC) into RMGI achieved much stronger inhibition of biofilms than using each agent alone. The novel antibacterial and protein-repellent RMGI with substantially-reduced biofilm acids is promising as an orthodontic cement to combat white spot lesions in enamel.
Keywords: Orthodontic cement, protein repellent, antibacterial property, shear bond strength, human saliva microcosm biofilm, white spot lesions
1. Introduction
White spot lesions around brackets is a major complication in patients of fixed orthodontic treatments, especially those with poor oral hygiene.1 The lesions are due to demineralization of enamel by acids from biofilms around the brackets.2 Many methods were investigated to decrease the occurrence of white spot lesions: improving oral hygiene, modifying diet (low carbohydrate), and treating with topical fluoride.1,2 However, these methods depend on patient compliance and therefore are unreliable.1,2 Hence, preventive measures that do not rely on patient compliance may be more effective in preventing white spot lesions.
Resin-based materials are increasingly used as dental restorations and bonding agents.3-8 Orthodontic brackets are bonded to teeth via orthodontic cements.9,10 Resin-modified glass ionomer cements (RMGIs) have been used for bracket-bonding to enamel because of their fluoride (F)-releasing capabilities and ability to bond the orthodontic brackets with acceptable bond strengths.9,10 Lower bacterial colonization and less plaque buildup could reduce enamel demineralization and white spot lesions.11 However, previous studies reported that RMGIs could accumulate more bacteria due to their relatively rough surfaces, high surface-free energy and polarity.11-13 Additionally, studies indicated that the duration of F release was short-term.10,14,15 F ions released from RMGIs began with an initial burst at the time of bonding, followed by a rapid decrease over time.10,14,15 Furthermore, proper oral hygiene is difficult to maintain around the brackets, and pH levels lower than 4.5 have been measured in the plaques around the brackets.16,17 Such a low pH hinders the remineralization process, so that more F ions may not yield a better cariostatic effect.16,17 Therefore, it would be desirable to add antimicrobial agents into RMGIs to inhibit biofilms, reduce acid production, maintain a higher local pH, and thereby render the F ions more effective in promoting remineralization and suppressing demineralization in enamel.
In the oral cavity, the acquired salivary pellicle, which is produced by adsorption of salivary proteins on tooth surfaces, acts as the substratum for the attachment of oral bacteria.18,19 Hence, it would be desirable to develop an orthodontic cement that can repel protein adsorption, thereby to reduce bacteria attachment and acid production. There are at least two main types of protein-repellent agents. The first is polyethylene glycol (PEG).20 A novel study treated silicon wafer surfaces with PEG and two pyridinium group-containing methacrylate monomers to investigate the influence of prior protein adsorption on bactericidal activity.20 The second group uses zwitterionic polymers, such as poly(sulfobetaine methacrylate) (pSBMA), carboxybetaine methacrylate (CBMA), and 2-methacryloyloxyethyl phosphorylcholine (MPC).19,21,22 The present study selected MPC because it is the most commonly-used protein-repellent agent, and previous studies showed that surfaces coated with MPC could resist biofilm formation for longer periods of time than PEG-coated surfaces.19,21,22 This is because MPC is a methacrylate with a phospholipid polar group in the side chain, hence it can be co-polymerized with the materials and therefore is not released or lost over time.19,21,22 The biocompatibility and effectiveness of MPC-containing biomaterials have been confirmed by their inertness for biological systems, and their reduction of protein absorption, bacterial adhesion and cellular attachment.21,22 In recent studies, novel protein-repellent dental composites and bonding agents containing MPC were developed.23,24 MPC was also incorporated into a RMGI.25 However, that RMGI-MPC cement was not antibacterial.25 It was reported that dental resin containing nanoparticles of silver (NAg) had a long-distance killing capability due to Ag ion release.26,27 It would be beneficial to combine MPC with NAg to possess protein-repellent and antibacterial abilities, thereby to inhibit white spot lesions beneath orthodontic bracket as well as in its vicinity.
Accordingly, the objectives of this study were to incorporate NAg and MPC into RMGI to develop a bioactive orthodontic cement with a combination of antibacterial and protein-repellent capabilities. A dental plaque microcosm biofilm model with human saliva as inoculum was used.28,29 The following hypotheses were tested: (1) incorporating MPC and NAg into RMGI would not compromise the enamel bond strength; (2) RMGI containing NAg would inhibit not only adherent bacteria on its surface, but also bacteria away from its surface in the culture medium; (3) RMGI containing MPC would have much less protein adsorption than RMGI control; and (4) using dual agents (NAg + MPC) would achieve much greater anti-biofilm potency than a single agent.
2. Materials and methods
2.1. Preparation of RMGI containing NAg and MPC
A resin-modified glass ionomer cement (Vitremer, 3M, St. Paul, MN), referred to as VT, was used as the parent system. VT consisted of fluoroaluminosilicate glass, and a light-sensitive, aqueous polyalkenoic acid. Indications include Class III, V and root-caries restoration, Class I and II in primary teeth, and core-buildup. A powder/liquid mass ratio of 2.5/1 was used according to the manufacturer. VT was selected because RMGIs have been used as orthodontic adhesives due to their fluoride-releasing capability and clinically acceptable bond strengths.9,10 The purpose was to investigate a model system, and then the method of incorporating NAg and MPC could be applied to other orthodontic cements.
Silver 2-ethylhexanoate (Strem, Newburyport, MA) of 0.1 g was dissolved into 0.9 g of 2-(tert-butylamino)ethyl meth-acrylate (TBAEMA, Sigma, St. Louis, MO).28,29 TBAEMA improved the solubility by forming Ag-N bonds with Ag ions to facilitate Ag salt to dissolve in resin solution.28,29 TBAEMA contains reactive methacrylate groups which can be chemically bonded in the resin upon photo-polymerization. In a previous study, silver 2-ethylhexanoate was incorporated into an adhesive at silver 2-ethylhexanoate mass fractions of 0.05%, 0.1%, and 0.15%.30 While the antibacterial potency increased with increasing NAg mass fraction from 0.05% to 0.1%, the bond strength was decreased when using 0.15% of NAg.30 Therefore, the present study used a silver 2-ethylhexanoate/(VT + silver 2-ethylhexanoate) mass fraction of 0.1%.
MPC was obtained commercially (Sigma-Aldrich, St. Louis, MO) which was synthesized via a method reported by Ishihara et al.21 The MPC powder was mixed with VT at MPC/(VT + MPC) mass fraction of 3%. Previous study showed that 3% MPC yielded a strong protein-repellent property without compromising the bond strength.25
Another orthodontic cement (Transbond XT, 3M, Monrovia, CA) served as the second control (referred to as TB). According to the manufacturer, TB consisted of silane treated quartz (70-80 % by weight), bisphenol-A-diglycidyl ether dimethacrylate (10-20%), bisphenol-A-bis (2-hydroxyethyl) dimethacrylate (5-10%), silane-treated silica (< 2%) and diphenyliodonium hexafluorophosphate (< 0.2%). TB provides a higher end of the enamel bond strength range for orthodontic cements,31 while VT provides a medium enamel bond strength.9,10 NAg and MPC were incorporated into VT, but not into TB, because TB had no fluoride release, and the purpose here was to formulate an orthodontic cement with fluoride release plus antibacterial and protein-repellent capabilities.
Therefore, five groups were tested:
Transbond XT control (referred to as TB control);
Vitremer control (referred to as VT control);
99.9% Vitremer + 0.1% NAg (referred to as VT+NAg);
97% Vitremer + 3% MPC (referred to as VT+MPC);
96.9% Vitremer + 0.1% NAg + 3% MPC (referred to as VT+NAg+MPC).
2.2. Enamel shear bond strength (SBS) and adhesive remnant index (ARI)
One hundred extracted human maxillary first premolars were randomly divided into 5 groups of 20 teeth for each group. The criteria for tooth selection included intact buccal enamel that had not been pretreated with chemical agents, no visible cracks, and no enamel irregularities.31 The teeth were cleaned and polished with a fluoride-free pumice slurry and rubber cups for 10 s, and thoroughly washed and dried with an oil-free air stream.32 Each tooth was embedded vertically in a self-curing acrylic resin (Lang Dental Manufacturing, Wheeling, IL) taking into account the buccal axis of the clinical crown, so that their labial surface would be parallel to the force during the shear bond testing. Premolar metal orthodontic brackets (Ormco 2000, Sybron Dental, Orange, CA) were used. The average base surface areas of the brackets were calculated with measurements made by a digital caliper (Mitutoyo, Miyazaki, Japan). The bonding procedures were performed as follows.
For group 1, the bonding procedures were performed following the manufacturers' recommendations. Enamel was etched for 30 s with 37% phosphoric acid (Scotchbond, 3M ESPE, St. Paul, MN) and then rinsed for 10 s. The tooth was dried with a stream of air, and TB primer was applied. Then, TB light-cured adhesive paste was applied to the bracket base and pushed against the enamel surface. A bracket placement plier was used to hold and keep the bracket in position on the center of the enamel surface. A 300-g force was applied vertically on the bracket for 5 s using a force gauge (Correx, Bern, Switzerland) to ensure a uniform bonding pressure and adhesive thickness.33 Excess adhesive around the bracket base was removed with a clinical probe and then the specimens were photo-cured (Demetron VCL 401, Demetron, CA) for a total of 40 s. The curing light was held against the bracket and tooth on the mesial aspect for 20 s followed by 20 s against the distal aspect at a constant distance of 3 mm and a 45° angle to the enamel surface.34,35
For groups 2-5, according to the manufacturer and literatures,9,10 VT was used for bonding brackets without acid etching. Hence, the bonding procedure consisted of pumicing the enamel surface for 10 s with flour pumice, followed by rinsing for 10 s with water. Each tooth was then wiped with a moist cotton roll to ensure that the bonding surface was not desiccated, and excess water was removed.34,36 Then, VT paste was applied to the bracket base and the bracket was positioned and bonded to the enamel. The bracket was then light cured for a total of 40 s as described above.34,35
Each bonded group was randomly divided into two sub-groups of ten samples each. Ten samples were stored in distilled water at 37 °C for 1 day (d), and the other ten samples were stored for 30 d. SBS was measured as previously described.33-36 Briefly, a chisel was connected with a computer-controlled Universal Testing Machine (MTS, Eden Prairie, MN) and the chisel tip was positioned on the upper part of the bracket base. An occlusal-gingival load (speed = 0.5 mm/min) was applied to the bracket, producing a shear load at the bracket-tooth interface until the bond failed. A 0.019 × 0.025 inch stainless steel wire was ligated into each bracket slot to reduce deformation of the bracket during debonding. SBS was calculated as the debonding force divided by the bracket contact surface area.33-36
After debonding, each enamel surface was examined by a stereomicroscope (Leica Zoom 2000, Leica, Wetzlar, Germany) at 10× magnification, and the ARI score was assessed following a previous study.37 The ARI score quantifies the remnant resin material on the enamel surface to assess where fracture occurred during the shear bond testing.37 The following scores were used: 0 = no adhesive remained on enamel; 1 = less than half of the enamel bonding area was covered with adhesive; 2 = more than half of the enamel bonding area was covered with adhesive; 3 = all the enamel bonding area was covered with adhesive.
2.3. Measurement of protein adsorption
For protein adsorption and biofilm experiments, each adhesive paste was placed into a disk mold of 9 mm diameter and 2 mm thickness.25,29 The sample was light-cured for 40 s on each open side. The disks were immersed in 200 mL of distilled water and magnetically-stirred with a bar at a speed of 100 rpm for 1 h to remove any uncured monomers, following previous studies.38,39 The disks were then sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC) and de-gassed for 3 d. 40,41
The amount of protein adsorbed on the disks was determined by the micro bicinchoninic acid (BCA) method.25,42 Each disk was immersed in phosphate buffered saline (PBS) for 2 h. The disk then was immersed in bovine serum albumin (BSA) (Sigma-Aldrich) solutions at 37 °C for 2 h. The protein solution contained BSA at a concentration of 4.5 g/L following previous studies.25,42 The disk then was rinsed with fresh PBS by stirring at a speed of 300 rpm for 5 min (Bellco Glass, Vineland, NJ), immersed in sodium dodecyl sulfate (SDS) at 1 wt% in PBS, and sonicated at room temperature for 20 minutes to completely detach the BSA from the disk surfaces. A protein analysis kit (micro BCA protein assay kit, Fisher Scientific, Pittsburgh, PA) was used to determine the BSA concentration in the SDS solution. From the concentration of protein, the amount of protein adsorbed on the resin disk was calculated.25,42 Six disks were evaluated for each group (n = 6).
2.4. Saliva collection for biofilm inoculum
The biofilm viability was investigated using a dental plaque microcosm model following previous studies.28,29 Saliva is ideal for growing dental plaque microcosm biofilms in vitro, with the advantage of maintaining much of the complexity and heterogeneity of the dental plaque in vivo.43 Saliva was collected from ten healthy adult donors having natural dentition without active caries or periopathology, and without the use of antibiotics within the last 3 months, following previous studies.28,29 The donors did not brush teeth for 24 hours and abstained from food and drink intake for 2 hours prior to donating saliva. Stimulated saliva was collected during parafilm chewing and was kept on ice. An equal volume of saliva from each of the ten donors was combined to form the saliva sample. The saliva was diluted in sterile glycerol to a concentration of 70% and stored at -80 °C.44
2.5. Dental plaque microcosm biofilm formation and live/dead assay
The saliva-glycerol stock was added, with 1:50 final dilution, to a growth medium as inoculum.28,29 The growth medium contained mucin (type II, porcine, gastric) at a concentration of 2.5 g/L; bacteriological peptone, 2.0 g/L; tryptone, 2.0 g/L; yeast extract, 1.0 g/L; NaCl, 0.35 g/L, KCl, 0.2 g/L; CaCl2, 0.2 g/L; cysteine hydrochloride, 0.1 g/L; hemin, 0.001 g/L; vitamin K1, 0.0002 g/L, at pH 7.45 1.5 mL of inoculum was added to each well of 24-well plates containing a cement disk, and incubated for 8 h. Then, the disks were transferred to new 24-well plates filled with fresh medium and incubated. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. This totaled 2 days of culture, which formed microcosm biofilms as shown previously.28,29 The 2-day biofilms on the disks were used in the following experiments, and the 8-h planktonic bacteria in the growth medium were also collected separately.27 The planktonic bacteria in the growth medium were not collected at 24 h or 48 h, because the disks were transferred to new 24-well plates filled with fresh medium, and the different amount of bacteria adhering on the disks would result in different number of planktonic bacteria in the growth medium.27
Disks with 2-day biofilms were washed with PBS and stained using the BacLight live/dead kit (Molecular Probes, Eugene, OR).28,29 Live bacteria were stained with Syto 9 to produce a green fluorescence, and bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. The stained disks were examined using an inverted epifluorescence microscope (Eclipse TE2000-S, Nikon, Melville, NY). The area of green staining (live bacteria) was computed with NIS Elements imaging software (Nikon). The area fraction of live bacteria = green staining area/total area of the image.29 Six specimens were evaluated for each group. Three randomly chosen fields of view were photographed for each disk, yielding a total of 18 images for each group.
2.6. MTT assay of metabolic activity
The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used to examine the metabolic activity of the 2-day biofilms on disks and planktonic bacteria in the growth medium.27,40 MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. Disks with 2-day biofilms were transferred to new 24-well plate, with 1 mL of MTT dye in each well.40 Separately, the collected medium with planktonic bacteria from each well was transferred to a tube containing 100μL of MTT dye.27 All specimens were incubated at 37°C in 5% CO2 for 1 h. During this process, metabolically active bacteria reduced the MTT to purple formazan. After 1 h, the biofilm specimens were transferred to a new 24-well plate. The planktonic bacteria were collected by centrifugation at 5 kg for 4 min.27 An aliquot of 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals.27,40 After incubation for 20 min in the dark, 200 μL of the DMSO solution was transferred to a 96-well plate, and the absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm on the disk.27,40
2.7. Lactic acid production and colony-forming unit (CFU) counts
Disks with 2-day biofilms were rinsed with cysteine peptone water (CPW) to remove loose bacteria and placed in a new 24-well plate.40 Separately, the planktonic bacteria from each well was transferred to a tube and collected by centrifugation at 5 kg for 4 min.27 An aliquot of 1.5 mL of buffered peptone water (BPW) supplemented with 0.2% sucrose was added to each well or tube.27,40 Samples were incubated at 37 °C in 5% CO2 for 3 h to allow the bacteria to produce acid. The BPW solutions were then stored for lactate analysis. Lactate concentrations in the BPW solutions were determined using an enzymatic (lactate dehydrogenase) method.27,40 The microplate reader was used to measure the absorbance at 340 nm for the collected BPW solutions. Standard curves were prepared using a lactic acid standard (Supelco, Bellefonte, PA) following previous studies.27,40
Disks with 2-day biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA).40,41 Separately, the CFU counts of the planktonic bacteria from the medium were also measured.27 Three types of agar plates were prepared. First, tryptic soy blood agar culture plates were used to determine total microorganisms.45 Second, mitis salivarius agar (MSA) culture plates containing 15% sucrose were used to determine total streptococci.46 Third, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci.45 The bacterial suspensions were serially diluted and spread onto agar plates for CFU analysis.27,40
2.8. Statistical analysis
All data collected from this research were first checked for normal distribution with the Kolmogorov–Smirnov test, and tested for homogeneity with the Levene's test. For MTT metabolic assay and acid production experiments, inter-group differences were estimated by a statistical analysis of variance (ANOVA) for factorial models; individual groups were compared with Fisher's protected least-significant difference test. Statistical analyses were performed by SPSS 13.0 software (SPSS Inc., Chicago, IL) at a significance level of p < 0.05. The chi-square test was used to evaluate the ARI scores.
3. Results
Figure 1 shows: (A-D) the color of the modified VT with different mass fractions of NAg, and (E) the enamel shear bond strengths (SBS) (mean ± sd; n = 10). Incorporating 0.05% and 0.1% NAg into VT caused no noticeable change in paste color, compared to VT control. In contrast, at 0.15% NAg, the paste became visibly darker than the control. Therefore, the NAg mass fraction in VT was limited to 0.1%. Preliminary study determined that adding 3% MPC into VT caused no noticeable color change. In (E), TB had the highest SBS. Adding NAg and MPC into VT did not adversely affect the SBS, compared to VT control (p > 0.1). Water-aging for 30 d had no significant effect on SBS, compared to those at 1 d (p > 0.1).
Figure 1.
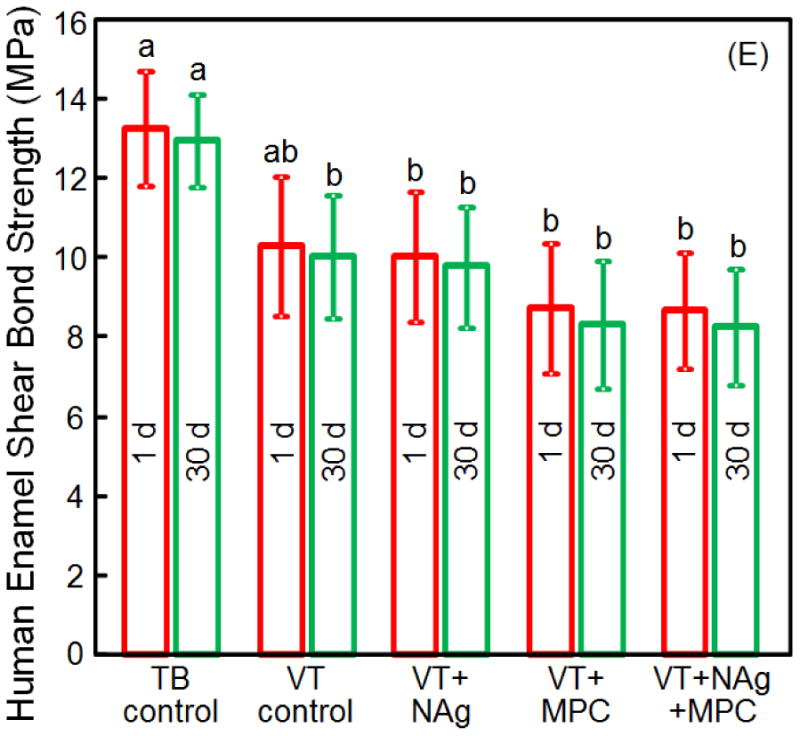
(A-D) The color of the modified RMGI with different mass fractions of NAg. (E) Enamel shear bond strengths (SBS) (mean ± sd; n = 10). There was no noticeable difference in color between RMGI with 0%, 0.05% and 0.1% NAg. However, at 0.15% NAg, the color of the paste became noticeably darker. In (E), adding 0.1% NAg and 3% MPC into VT, and water-aging for 30 d, did not adversely affect the SBS, compared to the commercial VT control (p > 0.1). Bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
The ARI scores are shown in Table 1 (n = 10). TB control had the highest ARI scores. Groups 2-5 had similar ARI scores, which were greatly lower than that of TB control (P < 0.05). The bracket-adhesive interface was the most common site of failure for TB control. Most of the specimens of groups 2-5 failed at the adhesive-enamel interface. There was no noticeable difference between 1 d and 30 d samples (p > 0.1).
Table 1. ARI Scores of Orthodontic Cements (n = 10)*.
| ARI scores | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Group | Water-aging | 0 | 1 | 2 | 3 | ARI scores Sig. |
| TB control | 1 d | 0 | 0 | 6 | 4 | a |
| VT control | 1 d | 3 | 7 | 0 | 0 | b |
| VT+NAg | 1 d | 3 | 7 | 0 | 0 | b |
| VT+MPC | 1 d | 4 | 6 | 0 | 0 | b |
| VT+NAg+MPC | 1 d | 4 | 6 | 0 | 0 | b |
| TB control | 30 d | 0 | 1 | 7 | 2 | a |
| VT control | 30 d | 4 | 6 | 0 | 0 | b |
| VT+NAg | 30 d | 4 | 6 | 0 | 0 | b |
| VT+MPC | 30 d | 4 | 6 | 0 | 0 | b |
| VT+NAg+MPC | 30 d | 4 | 6 | 0 | 0 | b |
Sig. refers to statistical significance, with different letters (a, b) indicating significant differences in ARI scores (p < 0.05).
The amounts of protein adsorption on disks are plotted in Fig. 2 (mean ± sd; n = 6). Adding MPC into VT significantly decreased the protein adsorption (p < 0.05). Adding NAg into VT had no effect on protein adsorption, compared to commercial controls. VT+NAg+MPC had similar protein adsorption as VT+MPC, which was about 10-fold less than those of commercial controls (p < 0.05).
Figure 2.
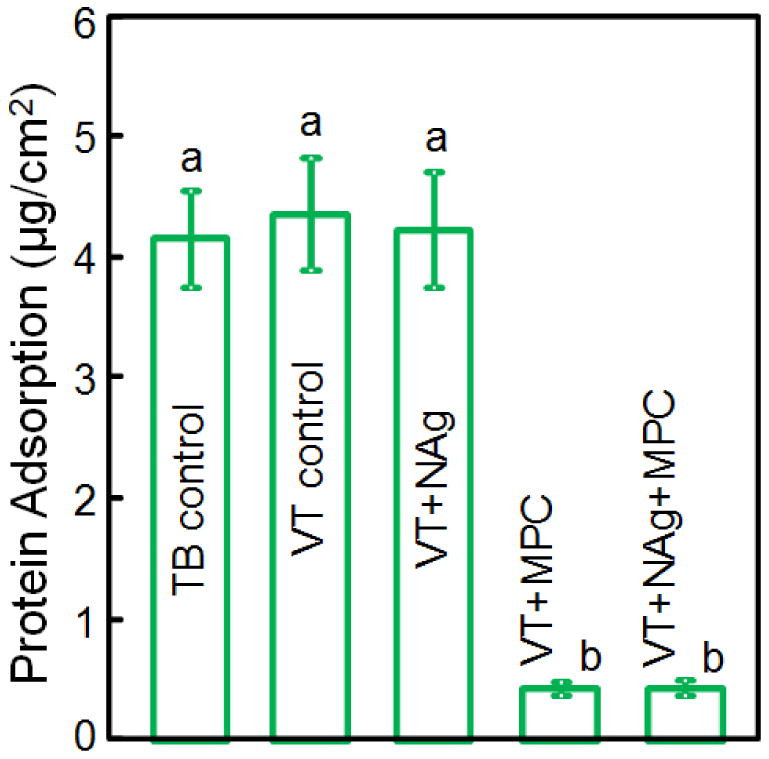
Protein adsorption onto sample surfaces (mean ± sd; n = 6). VT with 3% MPC and VT with 0.1% NAg + 3% MPC both had much less protein adsorption, which was about 1/10 that of commercial controls (p < 0.05). Bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
Representative live/dead images of 2-day biofilms on disks are shown in Fig. 3. In (A), TB was fully covered by a layer of live biofilm. In (B), VT had primarily live bacteria, with slightly more dead bacteria. In (C), VT+NAg had many dead bacteria with red staining. In (D), VT+MPC had much less bacterial adhesion. In (E), VT+NAg+MPC had less bacterial adhesion, and the biofilms consisted of primarily dead bacteria. In (F), VT+NAg+MPC had much less (p < 0.05) biofilm coverage than other groups (mean ± sd; n = 6).
Figure 3.
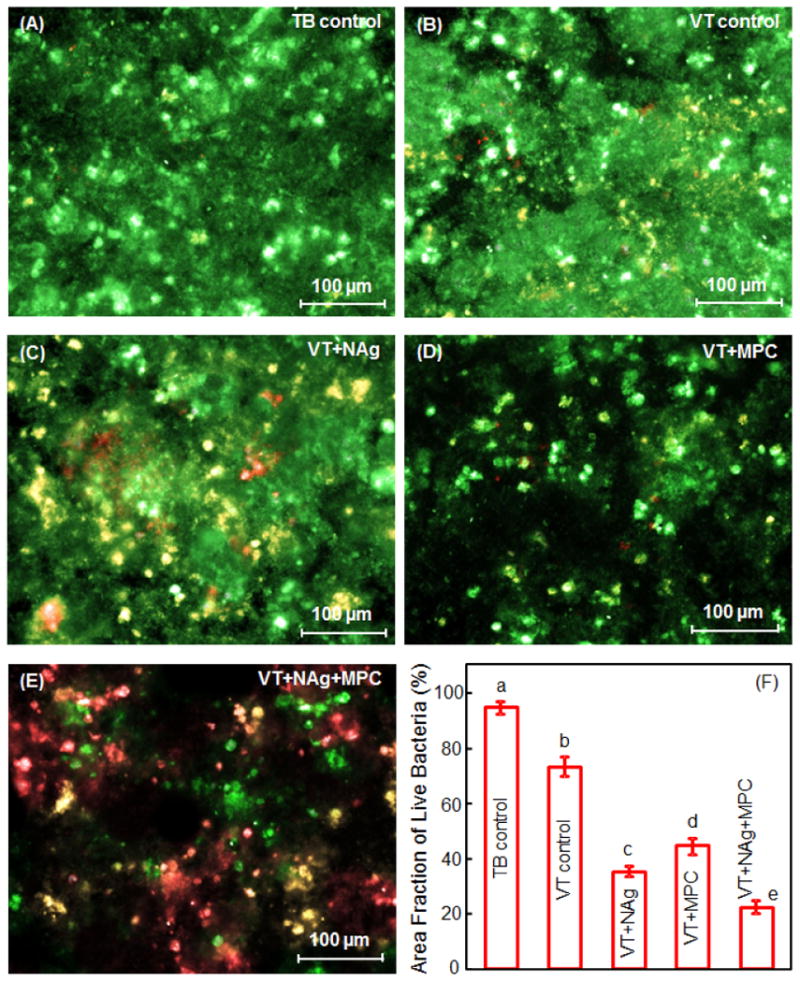
Representative live/dead staining images of 2-day biofilms grown on disks: (A) TB, (B) VT, (C) VT+NAg, (D) VT+MPC, (E) VT+NAg+MPC. (F) Area fraction of live bacteria on disks (mean ± sd; n = 6). The live bacteria were stained green, and the dead bacteria were stained red. When live and dead bacteria were in close proximity or on the top of each other, the staining had yellow or orange colors. Dissimilar letters in (F) indicate values that are significantly different from each other (p < 0.05).
Fig. 4 plots (A) biofilm metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Biofilms on TB had the highest metabolic activity and the most lactic acid production, followed by that on VT. Incorporation of MPC or NAg alone greatly decreased the metabolic activity and lactic acid production, compared to controls (p < 0.05). VT+NAg+MPC had the least metabolic activity and lactic acid production.
Figure 4.
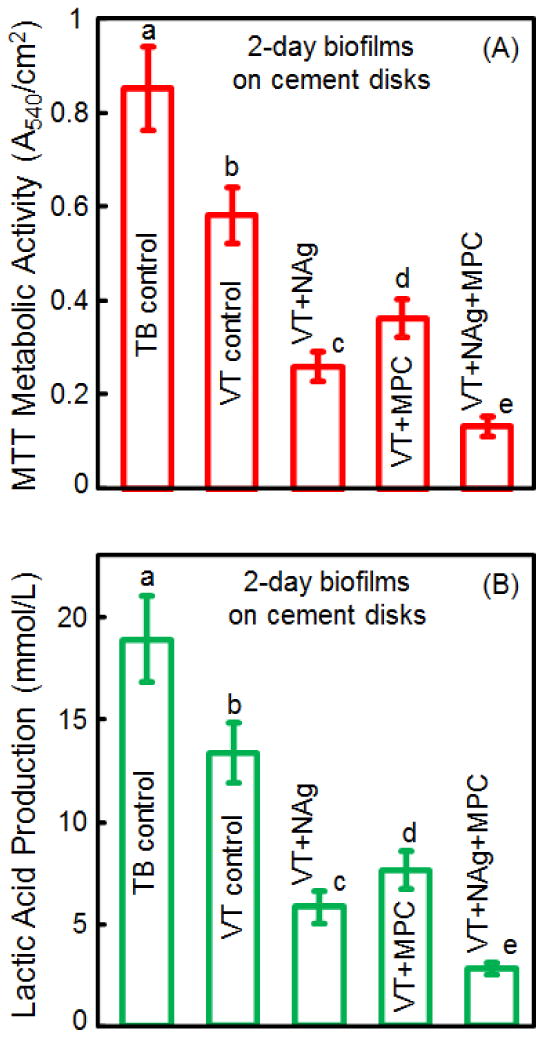
Quantitative viability of 2-day biofilms on disks: (A) metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). VT+NAg+MPC had the least metabolic activity and lactic acid production. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
Figure 5 shows results for planktonic bacteria in growth medium which contained a cement disk and were cultured for 8 hours: (A) metabolic activity, and (B) lactic acid (mean ± sd; n = 6). The planktonic bacteria in growth medium with VT+MPC had the highest metabolic activity and the most acid. Adding NAg into VT greatly decreased the metabolic activity and acid of the planktonic bacteria in growth medium away from cement surface. There was no noticeable difference between VT+NAg and VT+NAg+MPC (p > 0.1).
Figure 5.

Quantitative viability of planktonic bacteria in the growth medium: (A) metabolic activity, and (B) lactic acid production (mean ± sd; n = 6). Adding NAg into VT greatly decreased the metabolic activity and lactic acid production of the planktonic bacteria in the growth medium away from its surface. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
Figure 6 plots 2-day biofilm CFU for: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). Adding MPC or NAg each decreased biofilm CFU, compared to controls (p < 0.05). VT+NAg+MPC had a much stronger antibacterial effect than using MPC or NAg alone (p < 0.05). All three CFU counts for VT+NAg+MPC were two orders of magnitude lower than those on TB control.
Figure 6.
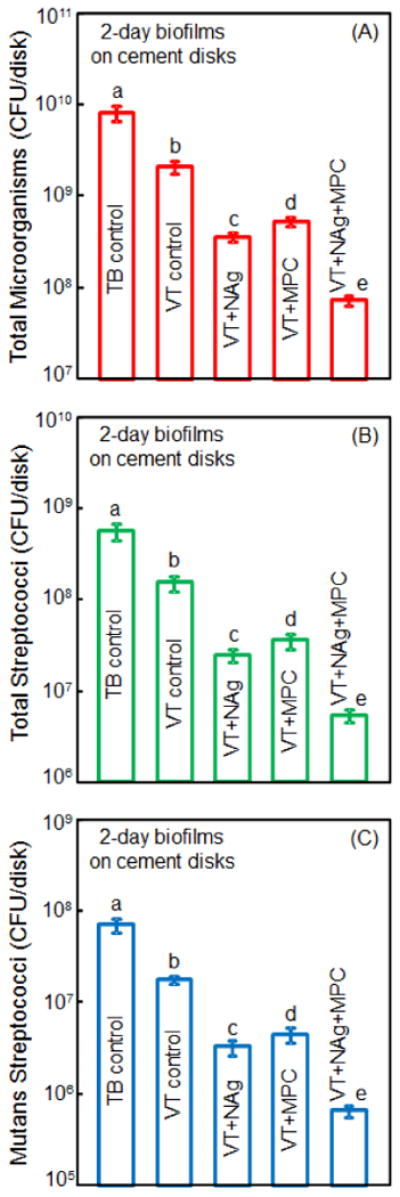
Colony-forming unit (CFU) of 2-day biofilms on disks: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). All three CFU counts on VT+NAg+MPC were two orders of magnitude lower than TB control. In each plot, values with dissimilar letters are significantly different (p < 0.05).
Figure 7 plots the CFU of planktonic bacteria in growth medium which contained a cement disk and were cultured for 8 hours: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). VT+MPC had the highest CFU. Adding NAg substantially decreased the CFU. There was no significant difference between VT+NAg and VT+NAg+MPC for the planktonic bacteria in the growth medium (p > 0.1).
Figure 7.
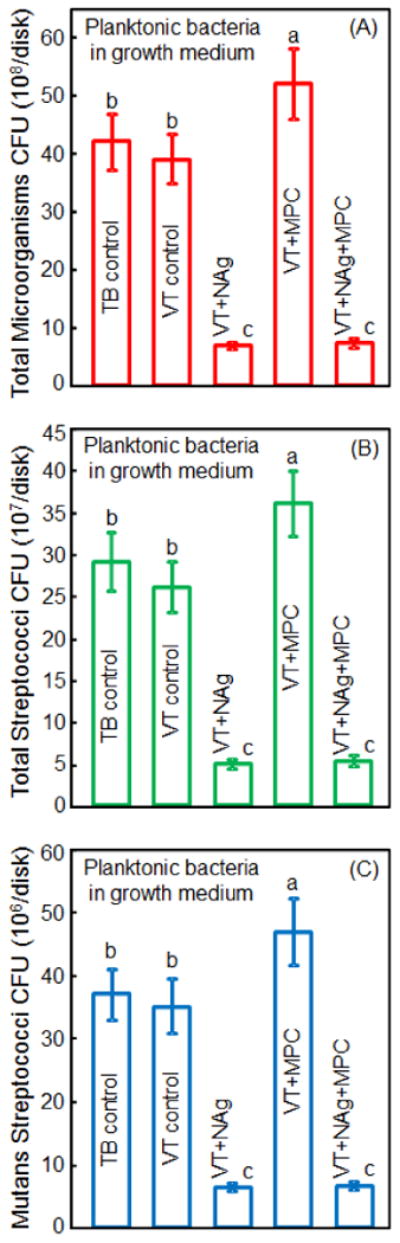
Colony-forming unit (CFU) counts of planktonic bacteria in growth medium: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci (mean ± sd; n = 6). Adding NAg into VT greatly decreased the CFU counts of the planktonic bacteria in the growth medium away from its surface. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
4. Discussion
In this study, a bioactive RMGI was developed to combine NAg for antibacterial activity with MPC for protein-repellent ability as well as with fluoride release for the first time. VT+NAg+MPC greatly reduced protein adsorption, bacterial adhesion, CFU counts, metabolic activity and lactic acid production of dental plaque microcosm biofilms. VT+NAg+MPC inhibited not only the bacteria on the material's surface, but also the bacteria away from the material in the culture medium. This indicates the material's potential ability to combat white spot lesions underneath the orthodontic bracket as well as in the vicinity away from the orthodontic cement. These benefits were achieved without compromising the enamel shear bond strength compared to commercial VT control. Therefore, VT+NAg+MPC is promising for orthodontic adhesives to inhibit biofilm formation and combat white spot lesions, and the approach of using dual agents with protein-repellent and antibacterial capabilities may have applicability to other dental materials.
The combined use of MPC and NAg into RMGI was supported by several benefits. First, MPC could impede bacteria attachment. Salivary proteins adsorbed onto the material surface provide a medium for the attachment of bacteria, thereby initiating the basis for biofilm formation.18-20 MPC has excellent protein-repellent capability to hinder bacterial adhesion.21,22 Regarding the protein-repellent mechanism, it was suggested that MPC is highly hydrophilic and there is an abundance of free water but no bound water in the hydrated MPC polymer.21,22 The presence of bound water would cause protein adsorption.22,47,48 In contrast, the large amount of free water around the phosphorylcholine group is considered to detach proteins effectively, thereby repelling protein adsorption.22,47,48 In the present study, VT+MPC reduced protein adsorption to 1/10 that of commercial controls (Fig. 2).
Second, adding NAg could suppress biofilm growth to a low level unachievable via MPC alone. In addition, Ag has good biocompatibility and low toxicity to human cells, and causes less bacterial resistance than antibiotics.49,50 Regarding the mechanism, it was suggested that the Ag ions could inactivate the vital enzymes of bacteria to cause the bacterial DNA to lose its replication ability, leading to cell death.49,51 NAg were shown to possess potent antibacterial properties due to its small particle size and high surface area.27-29 NAg were recently incorporated into dentin bonding agent and orthodontic cement which greatly reduced oral biofilm growth.28-30,52 Another study tested different concentrations of Ag benzoate (AgBz) in a resin and showed that the resin color turned yellow at 0.002% AgBz, and became a dark brown color at 0.1% AgBz.53 In the present study, silver 2-ethylhexanoate was dissolved in TBAEMA to form the NAg in the resin.28,29 There was no noticeable color change from 0% to 0.1% NAg in the resin, but the color turned darker when the resin contained 0.15% NAg. These results are consistent with a previous study30 in which the incorporation of 0.1% NAg in an adhesive did not affect the dentin bond strength compared to that with 0% NAg, while the bond strength decreased at 0.15% NAg, likely due to the darker color hindering the photo-cure efficacy. Therefore, the effect on color remains the limiting factor in determining how much NAg to be used in a dental resin. While the present study focused on protein-repellent and microcosm biofilm activities with only a preliminary qualitative assessment of the color, further study is needed to quantify the color measurement vs. NAg mass fraction systematically. Further study is also needed to investigate the color differences between the use of silver 2-ethylhexanoate in TBAEMA of the present study vs. the use of AgBz in a previous study.53
Third, the most common sites for demineralization are around the cements and brackets9,10, hence it would be beneficial to inhibit not only the bacteria on the cement, but also the bacteria in the vicinity away from the brackets. This could potentially be achieved via MPC+NAg. The antimicrobial properties of fluoride are limited, its release occurs mainly beneath the orthodontic brackets, and it is often unsuccessful in preventing decalcification away from the brackets.10,16 In contrast, it was reported that NAg-containing resin has a long-distance killing capability and can kill bacteria away from the resin surface, likely due to the release of Ag ions.27,49 Its antibacterial effect appears to be relatively durable. For example, an Ag-containing dental composite was shown to continue to inhibit S. mutans growth when tested for a duration of 6 months.54 Our recent study on bonding agent containing NAg demonstrated that the anti-biofilm activity of the resin specimens after 6 months of water-aging showed no significant decrease when compared to that at 1 day.55 Our on-going study showed a similarly strong antibacterial effect after water-aging for 1 year, compared to that at 1 day, which we plan to report soon. Furthermore, it is beneficial to incorporate NAg into RMGIs, so that the NAg can inhibit biofilms and the fluoride ions can combat demineralization of enamel. These two actions together may be more effective than a single action in inhibiting white spot lesions around the orthodontic brackets. The results of the present study showed that adding 0.1% NAg into VT inhibited not only the bacteria on the surface, but also the bacteria away from the surface in the culture medium. Further studies are needed to measure the release of Ag ions and fluoride ions vs. time to determine their release profiles and if their duration is suitable for orthodontic cement applications, which typically require the effect to last for 1 to 2 years.
Forth, MPC makes NAg more effective. A previous study found that protein adsorption on NAg-containing resin decreased its antibacterial activity.56 This may be due to the antibacterial mode of NAg-containing material which involves Ag ion release.27,49 Ag ions could target the thiol groups (-SH) of proteins exposed to the extracellular portion of the bacterial membrane and inactivate the vital enzymes of bacteria, thus causing DNA in the bacteria to lose its replication ability, which leads to cell death.49,51 However, when covered with salivary proteins, the negatively-charged N-terminal domain of the acidic proline-RICH proteins (PRPs), which comprise 37% of the salivary proteins adhering to NAg-containing material,57 could capture the positively-charged Ag ions and work as a barrier to hinder Ag ion release.56,57 Because MPC can greatly reduce protein adsorption, the combined use of NAg and MPC could likely enhance the antibacterial effect of NAg-containing material in vivo. The present study confirmed that VT+NAg+MPC had much stronger contact-inhibition antibacterial effects than using NAg alone (Figs. 3, 4 and 6).
The recommended bond strength of metal bracket to enamel should be between 8-9 MPa to permit adequate adhesion to enamel, while allowing for debonding when the treatment is finished.58 In the present study, the SBS of groups 2-5 were within this range. RMGIs were used for bonding brackets without acid etching, due to the ionic bond formation between the hydroxyapatite of tooth hard tissues and carboxyl groups of polyalkenoic acid.33-36 Therefore, the adhesives of groups 2-5 were applied without acid etching. There are several advantages of bonding brackets without acid etching, such as reduced loss of enamel, prevention of saliva contamination, and less chair time.30 Additionally, formation of white spot lesions on enamel might be reduced by eliminating the acid-etching procedure.30 TB required acid etching and had a higher SBS. TB had the highest ARI scores, indicating that after debonding, most teeth retained more than half of the adhesive. This would require removing the adhesive on the tooth surfaces. In contrast, groups 2-5 had less adhesive on enamel. This could be advantageous because cleanup after bracket debonding would be simpler.33-36 Further studies are needed to investigate the long-term enamel bond strength, protein-repellent and antibacterial properties of RMGI containing NAg and MPC under in vivo conditions, as well as their effects on enamel while spot lesion reduction.
5. Conclusions
The present study reported a novel RMGI with a combination of antibacterial activity from NAg and protein-repellent capabilities from MPC to combat biofilms and white spot lesions in orthodontic treatments. RMGI with 0.1% NAg exhibited a strong antibacterial activity. RMGI with 3% MPC repelled protein and bacteria attachment. Furthermore, dual agents NAg+MPC in RMGI achieved the greatest reduction in biofilm growth, metabolic activity and lactic acid production. RMGI with NAg+MPC inhibited not only the bacteria on surface, but also the bacteria away from the surface in the culture medium. Adding NAg and MPC into RMGI did not adversely affect the SBS, compared to unmodified RMGI. The novel RMGI with NAg plus MPC is promising to reduce biofilm formation and plaque buildup, thereby reducing white spot lesios. The method of dual agents (NAg + MPC) may have wide applicability to other dental adhesives, composites, sealants and cements to inhibit caries.
Acknowledgments
We thank Dr. Mary Anne S. Melo and Dr. Junling Wu for experimental help, and Dr. Joseph M. Antonucci and Dr. Satoshi Imazato for fruitful discussions. This study was supported by the National Natural Science Foundation of China (NSFC grant No. 81500879) (NZ), NIH R01 DE17974 (HX), and a Seed Grant (HX) from the University of Maryland School of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuxing Bai, Email: byuxing@263.net.
Hockin H. K. Xu, Email: hxu@umaryland.edu.
References
- 1.Enaia M, Bock N, Ruf S. White-spot lesions during multibracket appliance treatment: A challenge for clinical excellence. American Journal of Orthodontics and Dentofacial Orthopedics. 2011;140:e17–e24. doi: 10.1016/j.ajodo.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 2.Guzma'n-Armstrong S, Chalmers J, Warren JJ. Ask us. White spot lesions: prevention and treatment. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;138:690–6. doi: 10.1016/j.ajodo.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Lynch CD. Successful posterior composites. London: Quintessence Publishing Co.; 2008. [Google Scholar]
- 4.Spencer P, Ye Q, Park JG, Topp EM, Misra A, Marangos O, Wang Y, Bohaty BS, Singh V, Sene F, Eslick J, Camarda K, Katz JL. Adhesive/dentin interface: The weak link in the composite restoration. Annals of Biomedical Engineering. 2010;38:1989–2003. doi: 10.1007/s10439-010-9969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dental Materials. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Uysal T, Amasyali M, Ozcan S, Koyuturk AE, Sagdic D. Effect of antibacterial monomer-containing adhesive on enamel demineralization around orthodontic brackets: An in-vivo study. American Journal of Orthodontics and Dentofacial Orthopedics. 2011;139:650–6. doi: 10.1016/j.ajodo.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 10.Rogers S, Chadwick B, Treasure E. Fluoride-containing orthodontic adhesives and decalcification in patients with fixed appliances: a systematic review. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;138:390.e1–8. doi: 10.1016/j.ajodo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 11.Badawi H, Evans RD, Wilson M, Ready D, Noar JH, Pratten J. The effect of orthodontic bonding materials on dental plaque accumulation and composition in vitro. Biomaterials. 2003;24:3345–50. doi: 10.1016/s0142-9612(03)00166-2. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SJ, Lim BS, Lee SJ. Surface characteristics of orthodontic adhesives and effects on streptococcal adhesion. American Journal of Orthodontics and Dentofacial Orthopedics. 2010;137:489–95. doi: 10.1016/j.ajodo.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature Journal of Clinical Periodontology. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Godoy F, Flaitz C, Hicks J. Role of fluoridated dentifrices in root caries formation in vitro. American Journal of Dentistry. 2014;27:23–8. [PubMed] [Google Scholar]
- 15.Lynch CD. Summary of: A retrospective, practice-based, clinical evaluation of Fuji IX restorations aged over five years placed in load-bearing cavities. British Dental Journal. 2013;215:290–1. doi: 10.1038/sj.bdj.2013.909. [DOI] [PubMed] [Google Scholar]
- 16.Buyukyilmaz T, Øgaard B. Caries preventive effects of fluoride releasing materials. Advances in Dental Research. 1995;9:377–83. [Google Scholar]
- 17.Derks A, Katsaros C, Frencken JE, van't Hof MA, Kuijpers-Jagtman AM. Caries-inhibiting effect of preventive measures during orthodontic treatment with fixed appliances. A systematic review Caries Research. 2004;38:413–20. doi: 10.1159/000079621. [DOI] [PubMed] [Google Scholar]
- 18.Pratt-Terpstra IH, Weerkamp AH, Busscher HJ. The effects of pellicle formation on streptococcal adhesion to human enamel and artificial substrata with various surface free-energies. Journal of Dental Research. 1989;68:463–7. doi: 10.1177/00220345890680030501. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Archives of Oral Biology. 2004;49:789–98. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Müller R, Eidt A, Hiller KA, Katzur V, Subat M, Schweikl H, Imazato S, Ruhl S, Schmalz G. Influences of protein films on antibacterial or bacteria-repellent surface coatings in a model system using silicon wafers. Biomaterials. 2009;30(28):4921–9. doi: 10.1016/j.biomaterials.2009.05.079. [DOI] [PubMed] [Google Scholar]
- 21.Ishihara K, Ueda T, Nakabayashi N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polymer Journal. 1990;22:355–60. [Google Scholar]
- 22.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? Journal of Biomedical Materials Research. 1998;39:323–30. doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Zhang N, Melo MA, Bai Y, Xu HH. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. Journal of Dentistry. 2014;42:1284–91. doi: 10.1016/j.jdent.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang N, Ma J, Melo MA, Weir MD, Bai Y, Xu HH. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. Journal of Dentistry. 2015;43:225–34. doi: 10.1016/j.jdent.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang N, Zhang K, Melo MA, Chen C, Fouad AF, Bai Y, Xu HH. Novel protein-repellent and biofilm-repellent orthodontic cement containing 2-methacryloyloxyethyl phosphorylcholine. Journal of Biomedical Materials Research Part B. 2015 May 13; doi: 10.1002/jbm.b.33444. [DOI] [PubMed] [Google Scholar]
- 26.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 27.Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dental Materials. 2013;29:450–61. doi: 10.1016/j.dental.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L, Weir MD, Xu HH, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial amorphous calcium phosphate nanocomposite with quaternary ammonium salt and silver nanoparticles. Dental Materials. 2012;28:561–72. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng L, Zhang K, Melo MA, Weir MD, Zhou X, Xu HH. Antibiofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–52. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scougall-Vilchis RJ, Yamamoto S, Kitai N, Hotta M, Yamamoto K. Shear bond strength of a new fluoride-releasing orthodontic adhesive. Dental Materials Journal. 2007;26:45–51. doi: 10.4012/dmj.26.45. [DOI] [PubMed] [Google Scholar]
- 32.Poosti M, Ramazanzadeh B, Zebarjad M, Javadzadeh P, Naderinasab M, Shakeri MT. Shear bond strength and antibacterial effects of orthodontic composite containing TiO2 nanoparticles. European Journal of Orthodontics. 2013;35:676–9. doi: 10.1093/ejo/cjs073. [DOI] [PubMed] [Google Scholar]
- 33.Bishara SE, Soliman M, Laffoon J, Warren JJ. Effect of antimicrobial monomer-containing adhesive on shear bond strength of orthodontic brackets. Angle Orthodontist. 2005;75:397–9. doi: 10.1043/0003-3219(2005)75[397:EOAMAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Cheng HY, Chen CH, Li CL, Tsai HH, Chou TH, Wang WN. Bond strength of orthodontic light-cured resin-modified glass ionomer cement. European Journal of Orthodontics. 2011;33:180–4. doi: 10.1093/ejo/cjq056. [DOI] [PubMed] [Google Scholar]
- 35.Paschos E, Kleinschrodt T, Clementino-Luedemann T, Huth KC, Hickel R, Kunzelmann KH, Rudzki-Janson I. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. American Journal of Orthodontics and Dentofacial Orthopedics. 2009;135:603–12. doi: 10.1016/j.ajodo.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Tuncer C, Tuncer BB, Ulusoy C. Effect of fluoride-releasing light-cured resin on shear bond strength of orthodontic brackets. American Journal of Orthodontics and Dentofacial Orthopedics. 2009;135:14.e1–6. doi: 10.1016/j.ajodo.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. American Journal of Orthodontics and Dentofacial Orthopedics. 1984;85:333–40. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 38.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–6. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–9. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 40.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–70. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H, Li F, Weir MD, Xu HH. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. Journal of Dentistry. 2013;41:1122–31. doi: 10.1016/j.jdent.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moro T, Kawaguchi H, Ishihara K, Kyomoto M, Karita T, Ito H. Wear resistance of artificial hip joints with poly( 2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials. 2009;30:2995–3001. doi: 10.1016/j.biomaterials.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 43.McBain AJ. Chapter 4: In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 44.Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM. Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Research. 2011;45:87–92. doi: 10.1159/000324084. [DOI] [PubMed] [Google Scholar]
- 45.McBain AJ, Sissons C, Ledder RG, Sreenivasan PK, De Vizio W, Gilbert P. Development and characterization of a simple perfused oral microcosm. Journal of Applied Microbiology. 2005;98:624–34. doi: 10.1111/j.1365-2672.2004.02483.x. [DOI] [PubMed] [Google Scholar]
- 46.Lima JP, Sampaio de Melo MA, Borges FM, et al. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–74. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 47.Yamasaki A, Imamura Y, Kurita K, Iwasaki Y, Nakabayashi N, Ishihara K. Surface mobility of polymers having phosphorylcholine groups connected with various bridging units and their protein adsorption-resistance properties. Colloids and Surfaces B: Biointerfaces. 2003;28:53–62. [Google Scholar]
- 48.Goda T, Konno T, Takai M, Ishihara K. Photoinduced phospholipid polymer grafting on Parylene film: Advanced lubrication and antibiofouling properties. Colloids and Surfaces B: Biointerfaces. 2007;54:67–73. doi: 10.1016/j.colsurfb.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 49.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, Yacaman MJ. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 50.Damm C, Munsted H, Rosch A. Long-term antimicrobial polyamide 6/silver nanocomposites. Journal of Materials and Science. 2007;42:6067–73. [Google Scholar]
- 51.Rai M, Yada A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Ahn SJ, Lee SJ, Kook JK, Lim BS. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dental Materials. 2009;25:206–13. doi: 10.1016/j.dental.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K. Development of an antimicrobial resin--a pilot study. Dental Materials. 2011;27:322–8. doi: 10.1016/j.dental.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida K, Tanagawa M, Atsuta M. Characterization and inhibitory effect of antibacterial dental resin composites incorporating silver-supported materials. Journal of Biomedical Materials Research. 1999;47:516–22. doi: 10.1002/(sici)1097-4636(19991215)47:4<516::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 55.Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH. Effect of water-aging on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. Journal of Dentistry. 2013;41:504–13. doi: 10.1016/j.jdent.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li F, Weir MD, Fouad AF, Xu HH. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dental Materials. 2014;30:182–91. doi: 10.1016/j.dental.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennick A, Chau G, Goodlin R, Abrams S, Tustian D, Madapallimattam G. The role of human salivary acidic proline-rich proteins in the formation of acquired dental pellicle in vivo and their fate after adsorption to the human enamel surface. Archives of Oral Biology. 1983;28:19–27. doi: 10.1016/0003-9969(83)90022-5. [DOI] [PubMed] [Google Scholar]
- 58.Reynolds IR. A review of direct orthodontic bonding. British Journal of Orthodontics. 1975;2:171–8. [Google Scholar]


