Abstract
Objectives
Calcium phosphate (CaP) composites with Ca and P ion release can remineralize tooth lesions and inhibit caries. But the ion release lasts only a few months. The objectives of this study were to develop rechargeable CaP dental composite for the first time, and investigate the Ca and P recharge and re-release of composites with nanoparticles of amorphous calcium phosphate (NACP) to achieve long-term inhibition of caries.
Methods
Three NACP nanocomposites were fabricated with resin matrix of: (1) bisphenol A glycidyl dimethacrylate (BisGMA) and triethylene glycol dimethacrylate (TEGDMA) at 1:1 mass ratio (referred to as BT group); (2) pyromellitic glycerol dimethacrylate (PMGDM) and ethoxylated bisphenol A dimethacrylate (EBPADMA) at 1:1 ratio (PE group); (3) BisGMA, TEGDMA, and Bis[2-(methacryloyloxy)ethyl] phosphate (BisMEP) at 2:1:1 ratio (BTM group). Each resin was filled with 20% NACP and 50% glass particles, and the composite was photo-cured. Specimens were tested for flexural strength and elastic modulus, Ca and P ion release, and Ca and P ion recharge and re-release.
Results
NACP nanocomposites had strengths 3-fold of, and elastic moduli similar to, commercial resin-modified glass ionomer controls. CaP ion recharge capability was the greatest for PE group, followed by BTM group, with BT group being the lowest (p < 0.05). For each recharge cycle, CaP re-release reached similarly high levels, showing that CaP re-release did not decrease with more recharge cycles. After six recharge/re-release cycles, NACP nanocomposites without further recharge had continuous CaP ion release for 42 d.
Significance
Novel rechargeable CaP composites achieved long-term and sustained Ca and P ion release. Rechargeable NACP nanocomposite is promising for caries-inhibiting restorations, and the Ca and P ion recharge and re-release method has wide applicability to dental composites, adhesives, cements and sealants to achieve long-term caries-inhibition.
Keywords: Dental composite, calcium phosphate nanoparticles, calcium phosphate ion release, recharge, dental caries inhibition, remineralization
1. Introduction
Resin composites and adhesives are increasingly used in dental restorations [1-6]. Tooth cavity restorations cost the United States approximately $46 billion annually [7]. Secondary (recurrent) caries is a frequent reason for the failure of dental restorations [8-10], and replacing the failed restorations accounts for 50-70% of all restorations performed [11,12]. According to the minimally-invasive treatment concept, more dentin tissues are recommended to be preserved, which are accompanied by a certain amount of caries-infected and caries-affected dentin with residual bacteria in the prepared cavity [13-16]. In addition, microleakage along the restoration margins can provide pathways for new invading bacteria from the oral environment [17-20]. The bacteria growth and biofilm formation can produce acids to demineralize the tooth structure and cause caries.
A promising approach to combat caries is the development of calcium phosphate (CaP) composites that can release calcium (Ca) and phosphate (P) ions [21-27]. Traditional CaP composites contained CaP particles with sizes of 1-55 μm and achieved successful remineralization of tooth lesions [21-24]. Re-incorporation of minerals into the demineralized dentin matrix is important since the precipitated mineral may serve as sites for further nucleation, and the remineralized tissues may be more resistant to degradation [28]. Recently, nanocomposites containing nanoparticles of amorphous calcium phosphate (NACP) with a mean particle size of 116 nm were developed [27,29-31]. The nanocomposites released high levels of Ca and P ions while having mechanical properties 2-fold those of traditional CaP composites [25,27]. The NACP nanocomposite was “smart” and can rapidly neutralize lactic acid solutions at a cariogenic pH of 4 and increase the pH to a safe level of above 6 [29]. The NACP nanocomposite successfully remineralized enamel lesions in vitro, achieving a remineralization that was 4-fold that of a commercial fluoride-releasing composite [30]. In a human in situ model, the NACP nanocomposite inhibited secondary caries at the enamel-restoration margins in vivo, reducing the enamel mineral loss at the margins to 1/3 of the mineral loss associated with a control composite without NACP [31].
However, a major drawback of CaP composites is that the Ca and P ion release lasts for only weeks to months, and then the ion release is diminished over time. Previous studies measured Ca and P ion release from composites to at most a couple of months [21,23,27,30,32]. However, clinicians and patients would expect the composite restoration to be effective in vivo for much longer than a few months (e.g., for 10 or 20 years). Therefore, it would be highly desirable for the CaP composite to be able to recharge and re-release Ca and P ions, thereby to release Ca and P ions indefinitely and provide long-term caries-inhibition capability. However, literature and patent searches revealed no report on rechargeable calcium phosphate dental composites and resins.
Therefore, the objectives of this study were to develop rechargeable calcium phosphate dental composite for the first time, and to investigate the effects of different resin matrices on the CaP recharge and re-release efficacy. A previous NACP nanocomposite using bisphenol A diglycidyl methacrylate (BisGMA)-based matrix with high levels of Ca and P ion release and good mechanical properties served as a control [22,27]. Two new NACP nanocomposites were synthesized. One contained pyromellitic glycerol dimethacrylate (PMGDM) and ethoxylated bisphenol A dimethacrylate (EBPADMA) at a mass ratio of 1:1. The other contained Bis[2-(methacryloyloxy)ethyl] phosphate (BisMEP). PMGDM and BisMEP were selected because both are acidic adhesive monomers [33-35], and may chelate with calcium and phosphate ions from a recharge solution to achieve the recharge capability. The following hypotheses were tested: (1) Rechargeable calcium phosphate composites can be developed and the recharge efficacy will depend on matrix resin type; (2) the re-release of Ca and P ions from the NACP nanocomposite will be maintained over time and not decrease with increasing the number of recharge/re-release cycles; (3) the rechargeable NACP nanocomposite will possess mechanical properties matching or exceeding commercial control fluoride-rechargeable restorative materials.
2. Materials and Methods
2.1. NACP nanocomposite fabrication
NACP [Ca3(PO4)2] were synthesized via a spry-drying technique as previously described [27,30]. Briefly, calcium carbonate and dicalcium phosphate anhydrous were dissolved into an acetic acid solution. The concentrations of Ca and P ions were 8 mmol/L and 5.333 mmol/L, respectively. The solution was sprayed into a heated chamber to evaporate the water and volatile acid. The dried particles were collected by an electrostatic precipitator. This yielded NACP with a mean particle size of 116 nm [27,30]. As a co-filler, barium boroaluminosilicate glass particles with a median size of 1.4 μm (Caulk/Dentsply, Milford, DE) were silanized with 4% 3-methacryloxypropyltrimethoxysilane and 2% n-propylamine as previously described [27,30].
Three types of matrix resins were prepared to fabricate the NACP nanocomposite. For type 1, a resin of BisGMA and triethylene glycol dimethacrylate (TEGDMA) (Esstech, Essington, PA) at 1:1 mass ratio was rendered light-curable with 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate, following previous studies [27,30]. BisGMA and TEGDMA are common dental resins used frequently in non-rechargeable commercial dental restoratives and, as such, provide a control to compare with the novel, rechargeable NACP nanocomposites. This resin using BisGMA and TEGDMA is referred to as BT resin.
For type 2, acidic monomer PMGDM and dimethacrylate EBPADMA (Sigma-Aldrich, St, Louis, MO) were mixed at a mass ratio of 1:1 to form the matrix resin [21,24]. This is referred to as PE resin. The combination of PMGDM and EBPADMA were used experimentally in resin-based calcium phosphate cements [21,33]. Experiments showed that PMGDM and EBPADMA had a cytotoxicity similar to that reported for other dental dimethacrylates, but significantly less than the cytotoxicity of BisGMA [33].
For type 3, BisGMA, TEGDMA, and acidic monomer BisMEP (Sigma-Aldrich) were mixed at a mass ratio of 2:1:1 to form the matrix resin [36]. This is referred as BTM resin. BisMEP has been used as a component in commercial self-etch primer systems (Tyrian SPE/One-Step Plus, Bisco, Schaumburg, IL) and in experimental amorphous calcium phosphate (ACP)-based composites [23]. The 0.2% camphorquinone and 0.8% ethyl 4-N,N-dimethylaminobenzoate were the same in all three groups.
Each aforementioned resin was filled with mass fractions of 20% NACP and 50% glass particles to form a readily-mixed and cohesive paste. Each composite paste was placed into a stainless steel mold of 2 × 2 × 25 mm, and light-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side. The three types of experimental composites are denoted BT-NACP, PE-NACP, and BTM-NACP nanocomposites, respectively.
For mechanical testing, four commercial materials were included as comparative controls to provide a range of commercial mechanical properties. A resin-modified glass ionomer (RMGI) cement (Vitremer, 3M, St. Paul, MN) consisted of fluoroaluminosilicate glass, and a light-sensitive, aqueous polyalkenoic acid. According to the manufacturer, indications include Class III, V and root-caries restoration, Class I and II in primary teeth, and core-buildup. A powder/liquid ratio of 2.5/1 was used yielding a filler mass fraction of 71.4%, according to the manufacturer. Another RMGI (Ketac Nano, 3M) consisted of polycarboxilic acid modified with methacrylate groups and fluoroaluminosilicate glass, with a filler level of 69%. It is a two-part, paste/paste system and dispensed using the Clicker Dispensing System. It is recommended for small Class I restorations, and Class III and V restorations. In addition, two composites also served as controls. A nanocomposite Renamel (Cosmedent, Chicago, IL) consisted of nanofillers of 20-40 nm a 60% filler level in a multifunctional methacrylate ester resin. According to the manufacturer, Renamel is indicated for Class III, IV, and V restorations. Another nanocomposite Heliomolar (Ivoclar, Amherst, NY) contained 66.7% of nanofillers of 40-200 nm of silica and ytterbium-trifluoride with fluoride-release (Heliomolar, Ivoclar, Amherst, NY). According to the manufacturer, Heliomolar is indicated for Class I, II, III, IV and V restorations. All specimens were light-cured as described above and treated in the same manner.
2.2. Mechanical testing
All specimens were stored at 37 °C for 24 h. Flexural strength and elastic modulus of specimens were measured using a three-point flexural test with a 10 mm span at a crosshead-speed of 1 mm/min on a computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC) [27,32]. Flexural strength was calculated by: S = 3Pmax/L(2bh2), where Pmax is the fracture load, L is span, b is specimen width and h is thickness. Elastic modulus was calculated by: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope in the linear elastic region.
2.3. Ca and P ion release from NACP nanocomposites
Three groups were tested for Ca and P ion release: BT group, PE group, and BTM group. The commercial controls were used for mechanical testing, but not in Ca and P ion release experiments because they did not release Ca and P ions. A sodium chloride (NaCl) solution (133 mmol/L) was buffered to pH 4 with 50 mmol/L lactic acid to measure ion release, simulating a cariogenic condition [27,31]. As in previous studies [22,27,31,32], three specimens of approximately 2 × 2 × 12 mm were immersed in 50 mL of solution to yield a specimen volume/solution of 2.9 mm3/mL. This was similar to a specimen volume per solution of about 3.0 mm3/mL in a previous study [23]. The concentrations of Ca and P ions released from the specimens were measured at 1, 3, 5, 7, 14, 21, 28, 35, 42, 49, 56, 63, and 70 days (d). At each time, aliquots of 0.5 mL were removed and replaced by fresh solution. The aliquots were analyzed for Ca and P ion concentrations via a spectrophotometric method (DMS-80 UV-visible, Varian, Palo Alto, CA) using known standards and calibration curves [21,23]. Six batches of specimens were tested and averaged for ion release for each group. The released ions were reported in cumulative concentrations [22,27,31,32]. This ion release from NACP nanocomposite was termed “virgin release”, to differentiate from the subsequent recharge and re-release.
2.4. Recharge of CaP composite and re-release of ions
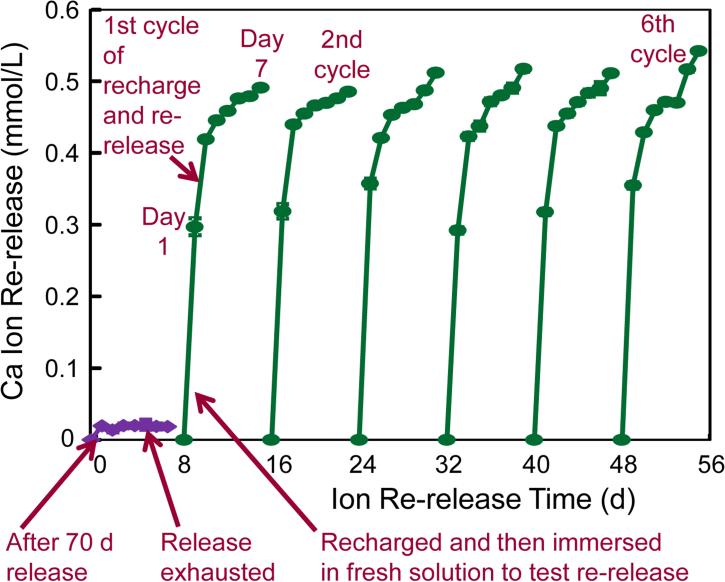
The procedures of recharge and re-release measurement are illustrated in Fig. 1. First, NACP nanocomposite specimens were immersed in pH 4 solution to measure ion release as described in Section 2.3. At 70 d, the ion measurement showed that the ion concentration had plateaued and there was no further release. The composite specimens were removed from the 70-d solution and rinsed with water for 5 min. The specimens were then immersed in a flesh 50 mL solution at pH 4. Then Ca and P ion release was further measured for 7 d, which confirmed that the ion release was indeed exhausted and there was no further release, as indicated by the two arrows at the lower left corner in Fig. 1.
Figure 1.
Illustration of Ca and P recharge and re-release. NACP nanocomposite was first immersed in a pH 4 solution for 70 d to exhaust the ion release, as indicated by the lower left arrow. Then the specimens were immersed in a new pH 4 solution to confirm that the ion release was exhausted, as indicated by the lower middle arrow. The exhausted specimens were recharged in a recharge solution. The specimens were then tested for Ca and P ion re-release for 7 d, as indicated by the third arrow at the bottom of Fig. 1. This constituted the first recharge/re-release cycle. This process was repeated for 6 cycles.
The exhausted specimens were then used for recharge. The recharging solutions for Ca and P ions were prepared respectively. The calcium ion recharging solution consisted of 20 mmol/L CaCl2 and 50 mmol/L HEPES buffer [24,37]. The phosphate ion recharging solution consisted of 12 mmol/L KHPO4 and 50 mmol/L HEPES buffer. Each solution was adjusted to a pH of 7.0 using 1 mol/L KOH [24,37]. Three specimens of approximately 2 × 2 × 12 mm were immersed in 5 mL of the calcium or phosphate solution and gently shaken on a mixing machine (Analog Vortex Mixer, Fisher Scientific, Waltham, MA) at a power level of 3 for 1 min. This immersion and shaking treatment simulated the movement in the mouth rinsing process when a calcium or phosphate mouth-rinse could be used. Then the specimens were rinsed with running distilled water for 1 min to remove any loosely attached deposits on specimen surfaces (hence only the ions recharged into the interior of the composite will be measured in the subsequent re-release test). This recharge process was repeated three times daily at 9:00 am, 12:00 noon and 5:00 pm for 3 d. All specimens for the BT group, PE group and BTM group were treated in the same manner for comparison of the recharge and re-release efficacy.
The recharged specimens were then immersed in 50 mL of the pH 4 solution as described in Section 2.3 to measure Ca and P ion re-release, as indicated by the third arrow in the bottom of Fig. 1. In order to test the recharge/re-release cycle repeatedly for many time to investigate the durability, each cycle of re-release measurement lasted for 7 d (The short arrow in Fig. 1 indicates the measurement from 1 d to 7 d in the first cycle). After 7 d of re-release, the specimens were recharged again and tested for re-release, as cycle 2. This was repeated for 6 cycles in the present study as illustrated in Fig. 1.
After 6 cycles, in order to investigate how long the specimens could further release Ca and P ions, the specimens after the 6th cycle (without further recharge) were immersed in 50 mL of the pH 4 solution as described in Section 2.3. The measurements of Ca and P ion release from these specimens were continued for an additional 42 d. At 1, 2, 3, 4, 5, 6, 7, 14, 21, 28, 35 and 42 d, the Ca and P measurements were performed as described in Section 2.3. For each of the three NACP nanocomposite groups, three batches of specimens were tested and averaged for ion release following previous studies [22, 27, 31,32].
2.5. Statistical analysis
One-way and two-way analyses of variance (ANOVA) were performed to detect the significant effects of the variables. Tukey's multiple comparison tests were used to compare the data at a p value of 0.05.
3. Results
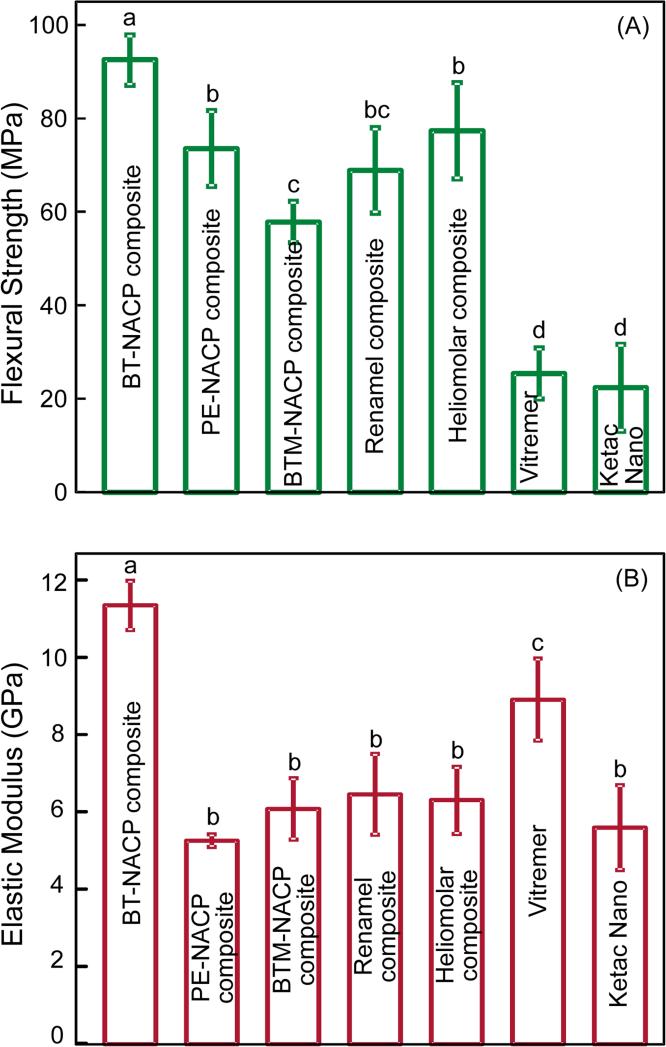
Fig. 2 plots the flexural strength and elastic modulus of the materials (mean ± sd; n = 6). The BT-NACP nanocomposite showed the highest strength (p < 0.05). PE-NACP nanocomposite had strength similar to commercial composite controls (p > 0.1). All three NACP nanocomposites had strengths significantly higher than RMGI controls (p < 0.05). BT-NACP nanocomposite had the greatest elastic modulus. PE-NACP and PEM-NACP nanocomposites had elastic moduli similar to commercial control composites (p > 0.1). NACP nanocomposites had strengths approximately 3 fold of, and elastic moduli generally similar to, those of commercial RMGI controls.
Figure 2.
Mechanical properties: (A) Flexural strength and (B) elastic modulus. Each value is mean ± sd (n = 6). NACP nanocomposites had strengths approximately 3-fold of, and elastic moduli generally similar to, those of RMGIs. In each plot, values with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
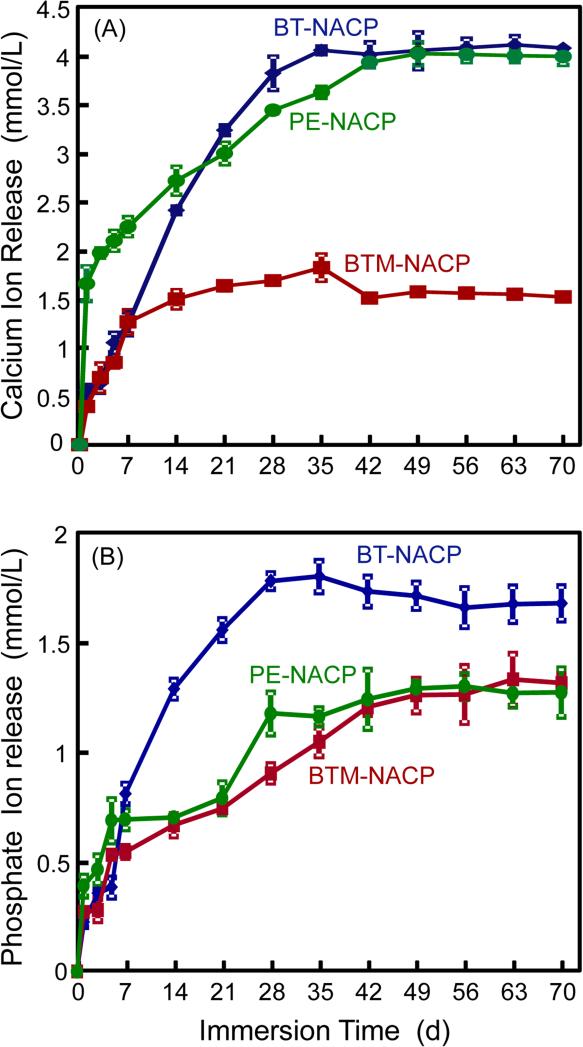
NACP nanocomposites were tested for virgin release of Ca and P ions, and the results are plotted in Fig. 3 (mean ± sd; n = 6). Among the three groups, the Ca ion release was relatively higher for the BT and PE groups, and lower for the BTM group. For P ion release, the PE and BTM groups were similarly lower than the BT group. For all three groups, the released ion concentrations increased with time, reaching a plateau at about 35 d to 42 d, indicating little further release from 42 d to 70 d.
Figure 3.
Calcium and phosphate ion release from virgin NACP nanocomposites. (A) Cumulative calcium, and (B) phosphate ion concentrations. Each value is mean ± sd (n = 6).
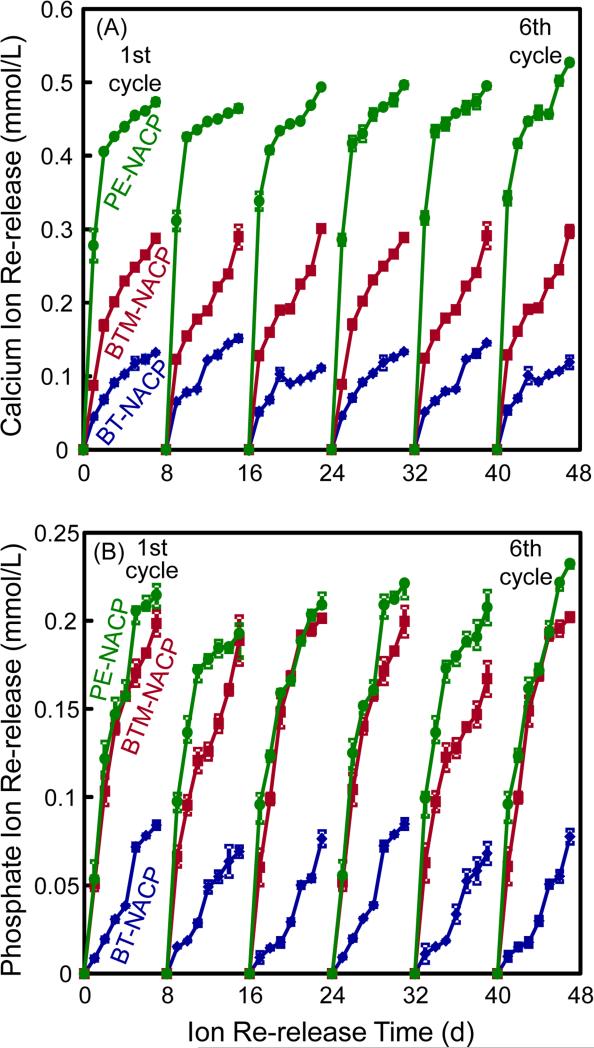
The NACP nanocomposites without further ion release (after 70 d) were recharged and their ion re-release was measured; the results are plotted in Fig. 4. Each value is mean ± sd, with n = 3. Specimens were immersed in flesh solution at pH 4 and the re-release was measured for 7 d, as one cycle. Six recharge/re-release cycles were included in Fig. 4. For Ca ion re-release, the PE-NACP nanocomposite had the greatest re-release, followed by BTM (p < 0.05). BT had the least re-release (p < 0.05). For P ion re-release, PE and BTM groups had similarly high releases, while BT had the lowest re-release (p < 0.05). Their re-release reached similar ion concentration levels for each cycle, showing that the ion re-release from these NACP nanocomposites was maintained with no decrease from cycle 1 to cycle 6.
Figure 4.
NACP nanocomposites first had ion release for 70 d to exhaust the ion release, then were recharged and their ion re-release was measured (mean ± sd; n = 3). Six cycles of recharge/re-release were tested for the three NACP nanocomposites.
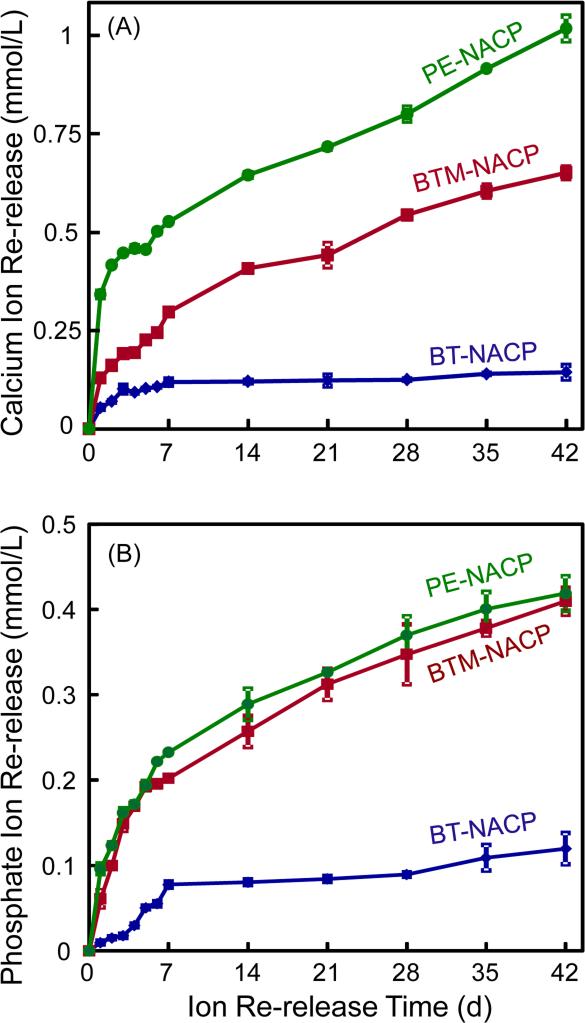
After 6 recharge/re-release cycles, the NACP nanocomposite specimens were used, without further recharge, to measure continuous Ca and P ion release for 42 d, with results in Fig. 5. PE and BTM groups had greater ion release than the BT group (p < 0.05). PE group had the highest level of Ca ion release (p < 0.05), while PE and BTM groups had similarly high levels of P ion release (p > 0.1). These results showed that after the recharge and re-release cycles in Fig. 4, the NACP nanocomposite specimens could continue the re-release for a relatively long period of time.
Figure 5.
NACP nanocomposites after six recharge/re-release cycles were then tested for continuous Ca and P ion release without further recharge (mean ± sd; n = 3). NACP nanocomposites with acidic monomers PMGDM and BisMEP had greater re-release than BisGMA-TEGDMA group (p < 0.05). PE group had the greatest Ca ion release (p < 0.05). PE and BTM groups had similarly high P ion release (p > 0.1).
4. Discussion
In the present study, rechargeable CaP dental composites were developed for the first time, demonstrating successful recharge and prolonged re-release of Ca and P ions. PE-NACP nanocomposite showed the best recharge/re-release capability. The durability of tooth cavity restorations is a main challenge in dentistry [2-4]. Oral bacteria and biofilms produce acids that can cause tooth decay and secondary caries, leading to restoration failure. The net loss or gain in mineral over time ultimately determines whether the tooth decay will advance, stabilize, or regress. Remineralization can be stimulated by the local concentrations of solution calcium and phosphate ions that exceed those existing in ambient oral fluid [38,39]. Therefore, the development of CaP restoratives to increase the local calcium and phosphate ion concentrations is a promising approach to the inhibition of recurrent caries and the prevention of lesion progression [31,40,41]. The novel recharging capability of the NACP nanocomposite to provide sustained Ca and P ion release is promising to impart a highly beneficial long-term inhibition of caries with a continuous remineralization efficacy.
Dental restorative materials with fluoride (F) ion release showed inhibitory effects to caries and were widely used in dentistry [42-43]. The recharging properties of F-releasing materials were investigated using topical fluoride agents or fluoride-containing toothpastes [44-48]. Glass ionomer cements (GIs), RMGIs, and compomers were shown to release, absorb, and re-release fluoride ions. The recharging ability of a restorative material depends on both the chemical composition of the material and the recharging method used. In general, GIs and RMGIs had better capability to act as an effective fluoride reservoir and showed better recharge abilities than composite-based materials [45,49,50]. Furthermore, materials with higher initial F release had a higher F recharge capability [45,47,49,50]. It was suggested that the F ions taken up during the recharge would occupy the sites which had been previously occupied by F ions before the initial release. To improve the F-release and recharge capabilities, organic fluoride salts, F-releasing monomers and F-exchange heavy metal chelates were incorporated into dental composites [45,46].
These F-releasing materials are meritorious in inhibiting caries. In comparison, the new NACP nanocomposite also appeared to be a promising material, which achieved an enamel lesion remineralization that was 4-fold that of a commercial F-releasing composite [30]. In addition, NACP nanocomposite reduced the secondary caries at the enamel-composite margins to 1/3 that of a glass-filled composite under oral biofilms in a human in situ study [31]. Therefore, imparting a recharge and re-release capability to the NACP nanocomposite would be advantageous to provide long-term remineralization and inhibition of caries.
BT-NACP nanocomposite was previously reported to have a high level of Ca and P ion release using a relatively low NACP filler level, thus enabling the use of substantial glass particle fillers in the resin for mechanical reinforcement [22,27]. The small NACP particles had a relatively high specific surface area of 17.76 m2/g [27,30], compared to about 0.5 m2/g of traditional CaP particles in dental resins in previous studies [23,24]. Therefore, high levels of Ca and P ion release can be achieved at lower NACP filler levels [27,31]. The incorporation of glass reinforcement yielded NACP nanocomposites with a unique combination of Ca and P ion release and load-bearing capabilities for dental restorative applications [25,27]. However, the present study showed that the Ca and P ion recharge and re-release was poor for NACP composite using a BisGMA-TEGDMA resin, similar to the resin in previous studies [25,27,31]. Furthermore, the present study also showed that BT-NACP nanocomposite had high virgin CaP ion release. Therefore, unlike the previously-reported F-releasing materials, a high level of initial ion release does not lead to a high capability of recharge and re-release for CaP composites.
On the other hand, the PE-NACP nanocomposite showed a much higher re-release of Ca and P ions than the BT group. PMGDM is an acidic adhesive monomer that was previously used in dental bonding agent and in a CaP-based cement [33,51]. Due to its active carboxylate group, it can chemically chelate with calcium or phosphate ions of dentin or of the exterior environment such as a recharge solution. There likely exists a dynamic equilibrium between the chelation of calcium ions to the PMGDM monomer and the release of calcium ions from the monomer, which is dependent on the local pH of the immersion solution. The source solution for recharge (simulating a mouth-rinse) had a pH of 7. The PMGDM in the NACP nanocomposite may chelate with the calcium ions diffusing from the recharge solution. After recharging, the bond between PMGDM and calcium ions might be severed in the solution of pH 4 in which the ion re-release was measured, simulating a local cariogenic pH from biofilm acids. This may be a mechanism for the significant recharging capability detected in the PE composite. In previously studies on the recharge of F-releasing materials [45,46,49], a short-term re-release of F ions was observed after recharging, with an initial burst of release for a day after recharge, and then sharply decreased release on the second day. In the present study, the Ca and P ion release after recharge could last at least 7 d. The specimens were rinsed with running distilled water each time after recharging to remove calcium and phosphate ions that freely deposited on the surfaces of the specimen, hence the re-release of ions was from the inside of the NACP nanocomposite, not due to surface deposits.
Four points should be noted for the PE-NACP nanocomposite. (1) After recharge, the re-releasing effect detected in the PE composite lasted for at least 7 d (Fig. 4). (2) After repeated recharge/re-release cycles, the extent of Ca and P ion re-release showed no trend of decrease with increasing the number of cycles, which indicated a long-term caries-inhibition potential and could be highly beneficial clinically. (3) After the 6th cycle of recharge and the specimens were re-released for 7 d, the specimens without further recharge could continue the re-release for at least another 42 d (Fig. 5). (4) The specimens were immersed in a pH 4 solution to measure the ion release in the present study as an accelerated experiment. In the oral environment, acidogenic bacteria ferment carbohydrates and produce organic acids including lactic, formic, acetic and propionic acids [52]. As a result, the oral plaque pH after a sucrose rinse can decrease to 4.5 or even 4 [53]. The Stephan Curve shows that the plaque pH, following a glucose mouthrinse, stays in the cariogenic area for about 30 min, and then increases back to a safe pH of 5.5 or higher, after the bacteria have completed their metabolization of the glucose and the saliva has buffered the acid [53]. Therefore, the low pH environment in vivo would last only about 30 min after a glucose rinse or a meal. This would account for only a couple of hours of accumulated low pH time per day in vivo. This time is a much shorter than the 24 h immersion/day in pH 4 solution in the experiments which could exhaust the ion release much faster from the composite. Therefore, after the recharge, the re-release of Ca and P ions from the PE-NACP nanocomposite could potentially last much longer than 42 d in vivo with only intermittent acid attacks. It is possible that the patients could potentially use the mouthrinse three times per day for three days to recharge the NACP nanocomposite, and then the re-release could last for several months before another recharge would be needed. Further study is needed to investigate and optimize the recharge method and the Ca and P ion re-release process for clinical applications.
The other NACP nanocomposite using an acidic monomer BTM had lower virgin release of Ca and P ions than the BT group. However, its recharge and re-release of Ca and P ions were much higher than those of the BT group. These results suggest that the approach of using an acidic monomer in the NACP nanocomposite to enhance the chelation of ions to facilitate the recharge process was successful. The different results of the PE and BTM groups showed that different acidic monomers exerted a different efficacy in enhancing the recharge/re-release of Ca and P ions. In the recharge/re-release results in Fig. 4, the BTM group had Ca and P ion concentrations with a Ca/P ratio of about 1.5, similar to the starting Ca/P ratio in the spray-drying synthesis of the NACP. However, the PE group had the highest Ca re-release and a lower P ion re-release, yielding a Ca/P ratio of > 2. These results indicate that for the PE group, the Ca ions may likely diffuse and transport through the resin more easily than the phosphate ions. Further study is needed to understand the effects of different acidic monomers on Ca and P ion recharge/re-release for dental composites, and the diffusion of different ions through resin matrix. Nonetheless, the present study showed that the usual BisGMA-TEGDMA dimethacrylates without acidic monomer do not yield a significant Ca and P ion recharge capability, and an acidic monomer needs to be incorporated into the NACP nanocomposite for substantial recharge and sustained Ca and P ion release.
Another important factor for dental restorations is the load-bearing capability. While the BT group had the best mechanical properties, its recharge capability was minimal. Both PE and BTM groups had flexural strengths similar to commercial control composites, and 3-fold those of commercial fluoride-rechargeable RMGIs. The flexural strength for PE and BTM composites were 73.6 ± 8.1 MPa and 57.8 ± 4.4 MPa, respectively. According to the requirements of the ISO standard [54], dental resin-based restorative materials should have a flexural strength no lower than 50 MPa. Both the PE and BTM composites exceeded this minimum requirement. Furthermore, PE and BTM composites had lower elastic modulus than the BT group. According to previous studies, restorative materials with a low elastic modulus could reduce the cervical gap-formation and marginal leakage [55,56]. Indeed, microfilled or nanofilled composites with relatively low elastic moduli have been speculated to reduce stresses at the adhesive interface generated by occlusal forces associated with cervical lesions [57]. In addition, flowable restorative materials with lower elastic modulus were used in Class V cavity restorations [58]. Therefore, the rechargeable PE-NACP nanocomposite with good strength and a relatively lower elastic modulus could be useful for these restorations. Among the two rechargeable NACP nanocomposites, the PE group had a significantly higher flexural strength than the BTM group, while their elastic moduli were statistically similar. It should be noted that this was the first rechargeable NACP composite study focusing on recharge and re-release, and Fig. 2 was measured with specimens after 1 d of water immersion. A previous two-year water-aging study showed that a NACP nanocomposite with BisGMA-TEGDMA resin had mechanical properties matching those of a commercial composite control after long-term water immersion [59]. However, the new rechargeable NACP nanocomposite requires a further study on its mechanical properties after the initial ion release, as well as after repeated recharge and re-release cycles. Furthermore, whether the Ca and P ion recharge and re-release will be affected by the oral microenvironment, such as a coating of salivary proteins on the composite surface in vivo, remains to be investigated. In addition, previous studies examined other load-bearing properties of Ca and P ion-releasing composites including three-body wear and fracture toughness [25,59]. However, further study is needed to evaluate the wear resistance, toughness and other properties of the new CaP rechargeable composites. Considering the generally higher recharge/re-release of the PE group, the present study suggests that NACP nanocomposite using the PMGDA-EBPADMA resin matrix was the best composition for Ca and P ion recharge/re-release to inhibit caries. Furthermore, the novel method of Ca and P ion recharge and re-release is promising to have a wide applicability to other dental composites, adhesives, sealants, coatings, glass ionomer materials, orthodontic cements and crown cements, to achieve long-term remineralization and caries-inhibition capabilities.
5. Conclusion
This study developed the first generation of rechargeable CaP composites with substantial recharge and sustained long-term release of Ca and P ions for remineralization and caries-inhibition. The incorporation of an acidic monomer in the composite was shown to impart Ca and P ion recharge and re-release. Different acidic monomers showed different efficacy of recharge/re-release, with NACP nanocomposite using the PMGDM-EBPAGMA resin achieving the best recharge and re-release. The re-release was sustained without any decrease with increasing the number of recharge/re-release cycles during the six cycles tested. Furthermore, the composites showed continuous re-release of Ca and P ions for 42 d without a need for further recharge. NACP nanocomposite in the PMGDM-EBPAGMA resin had flexural strength matching commercial control composites, and was 3-fold those of commercial rechargeable fluoride-releasing resin-modified glass ionomer materials. The new rechargeable NACP nanocomposite is promising for caries-inhibiting dental restorations, and the novel Ca and P ion recharge and re-release method may have a wide range of applications in various dental composites, adhesives, cements and sealants.
Acknowledgments
We thank Dr. Ning Zhang of the University of Maryland School of Dentistry for discussions and experimental help. This study was supported by NIH R01 DE17974 (HX), National Natural Science Foundation of China 51373198 (LZ), China Scholarship Council (LZ), University of Maryland seed grant (HX), and University of Maryland School of Dentistry bridge fund (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer
Certain commercial materials and equipment are identified to specify the experimental procedures. This does not imply recommendation or endorsement by NIST or the ADA. Official contribution of the National Institute of Standards and Technology (NIST); not subject to copyright in the United States.
References
- 1.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 2.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 3.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, Wei Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Ferracane JL. Resin composite - state of the art. Dent Mater. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 6.Ferracane JL. Resin-based composite performance: Are there some things we can't predict? Dent Mater. 2013;29:51–58. doi: 10.1016/j.dental.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Report. 2007;122:657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 9.Imazato S. Bioactive restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dent Mater J. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 10.Spencer P, Ye Q, Misra A, Goncalves SE, Laurence JS. Proteins, Pathogens, and Failure at the Composite-Tooth Interface. J Dent Res. 2014;93:1243–1249. doi: 10.1177/0022034514550039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 12. National Institute of Dental and Craniofacial Research (NIDCR) announcement # 13-DE-102, Dental Resin Composites and Caries, March 5, 2009.
- 13.Horowitz AM. Introduction to the symposium on minimal intervention techniques for caries. J Public Health Dent. 1996;56:133–4. doi: 10.1111/j.1752-7325.1996.tb02422.x. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee A, Watson TF, Kidd EA. Dentine caries: take it or leave it? Dental Update. 2000;27:272–6. doi: 10.12968/denu.2000.27.6.272. [DOI] [PubMed] [Google Scholar]
- 15.Mount GJ, Ngo H. Minimal intervention: advanced lesions. Quintessence Int. 2000;31:621–9. [PubMed] [Google Scholar]
- 16.Lynch CD, Frazier KB, McConnell RJ, Blum IR, Wilson NH. Minimally invasive management of dental caries: contemporary teaching of posterior resin-based composite placement in U.S. and Canadian dental schools. J Am Dent Assoc. 2011;142:612–20. doi: 10.14219/jada.archive.2011.0243. [DOI] [PubMed] [Google Scholar]
- 17.Fruits TJ, Knapp JA, Khajotia SS. Microleakage in the proximal walls of direct and indirect posterior resin slot restorations. Oper Dent. 2006;31:719–727. doi: 10.2341/05-148. [DOI] [PubMed] [Google Scholar]
- 18.Coelho-De-Souza FH, Camacho GB, Demarco FF, Powers JM. Fracture resistance and gap formation of MOD restorations: influence of restorative technique, bevel preparation and water storage. Oper Dent. 2008;33:37–43. doi: 10.2341/07-27. [DOI] [PubMed] [Google Scholar]
- 19.Totiam P, González-Cabezas C, Fontana MR, Zero DT. A new in vitro model to study the relationship of gap size and secondary caries. Caries Res. 2007;41:467–73. doi: 10.1159/000107934. [DOI] [PubMed] [Google Scholar]
- 20.Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. doi: 10.1016/j.dental.2013.08.201. [DOI] [PubMed] [Google Scholar]
- 21.Dickens SH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–66. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 22.Regnault WF, Icenogle TB, Antonucci JM, Skrtic D. Amorphous calcium phosphate/urethane methacrylate resin composites. I. Physicochemical characterization. J Mater Sci Mater Med. 2008;19:507–15. doi: 10.1007/s10856-007-3178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Donnell JN, Schumacher GE, Antonucci JM, Skrtic D. Structure-composition-property relationships in polymeric amorphous calcium phosphate-based dental composites. Materials. 2009;2:1929–1959. doi: 10.3390/ma2041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langhorst SE, O'Donnell JN, Skrtic D. In vitro remineralization of enamel by polymeric amorphous calcium phosphate composite: quantitative microradiographic study. Dent Mater. 2009;25:884–91. doi: 10.1016/j.dental.2009.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HH, Weir MD, Sun L, Moreau JL, Takagi S, Chow LC, et al. Strong nanocomposites with Ca, PO4, and F release for caries inhibition. J Dent Res. 2010;89:19–28. doi: 10.1177/0022034509351969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh Y, Suzuki M, Kato C, Shinkai K, Ogawa M, Yamauchi J. Observation of calcium phosphate powder mixed with an adhesive monomer experimentally developed for direct pulp capping and as a bonding agent. Dent Mater J. 2010;29:15–24. doi: 10.4012/dmj.2009-023. [DOI] [PubMed] [Google Scholar]
- 27.Xu HH, Moreau JL, Sun L, Chow LC. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent Mater. 2011;27:762–9. doi: 10.1016/j.dental.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res. 2011;90:953–68. doi: 10.1177/0022034510391799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreau JL, Sun L, Chow LC, Xu HH. Mechanical and acid neutralizing properties and bacteria inhibition of amorphous calcium phosphate dental nanocomposite. J Biomed Mater Res B Appl Biomater. 2011;98:80–8. doi: 10.1002/jbm.b.31834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weir MD, Chow LC, Xu HH. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J Dent Res. 2012;91:979–84. doi: 10.1177/0022034512458288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melo MA, Weir MD, Rodrigues LK, Xu HH. Novel calcium phosphate nanocomposite with caries-inhibition in a human in situ model. Dent Mater. 2013;29:231–40. doi: 10.1016/j.dental.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu HH, Weir MD, Sun L. Nanocomposites with Ca and PO4 release: effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–1491. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boland EJ, MacDougall M, Carnes DL, Dickens SH. In vitro cytotoxicity of a remineralizing resin-based calcium phosphate cement. Dent Mater. 2006;22:338–345. doi: 10.1016/j.dental.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Milward PJ, Adusei GO, Lynch CD. Improving some selected properties of dental polyacid-modified composite resins. Dent Mater. 2011;27:997–1002. doi: 10.1016/j.dental.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 35.Feitosa VP, Sauro S, Ogliari FA, Ogliari AO, Yoshihara K, Zanchi CH, et al. Impact of hydrophilicity and length of spacer chains on the bonding of functional monomers. Dent Mater. 2014;30:e317–23. doi: 10.1016/j.dental.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Amirouche-Korichi A, Mouzali M, Watts DC. Effects of monomer ratios and highly radiopaque fillers on degree of conversion and shrinkage-strain of dental resin composites. Dent Mater. 2009;25:1411–8. doi: 10.1016/j.dental.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Meyer-Lueckel H, Hopfenmuller W, von Klinggraff D, Kielbassa AM. Microradiographic study on the effects of mucin-based solutions used as saliva substitutes on demineralised bovine enamel in vitro. Arch Oral Biol. 2006;51:541–7. doi: 10.1016/j.archoralbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds EC, Cai F, Shen P, Walker GD. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J Dent Res. 2003;82:206–11. doi: 10.1177/154405910308200311. [DOI] [PubMed] [Google Scholar]
- 39.Besinis A, van Noort R, Martin N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent Mater. 2014;30:249–62. doi: 10.1016/j.dental.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 40.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Xie X, Wang Y, Yin W, Antoun JS, Farella M, et al. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: a systematic review. J Dent. 2014;42:769–77. doi: 10.1016/j.jdent.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Marinho VC. Cochrane reviews of randomized trials of fluoride therapies for preventing dental caries. Eur Arch Paediatr Dent. 2009;10:183–91. doi: 10.1007/BF03262681. [DOI] [PubMed] [Google Scholar]
- 43.Ijaz S, Croucher RE, Marinho VC. Systematic reviews of topical fluorides for dental caries: a review of reporting practice. Caries Res. 2010;44:579–92. doi: 10.1159/000322132. [DOI] [PubMed] [Google Scholar]
- 44.Miller FY, Campus G, Giuliana G, Piscopo MR, Pizzo G. Topical fluoride for preventing dental caries in children and adolescents. Curr Pharm Des. 2012;18:5532–41. doi: 10.2174/138161212803307464. [DOI] [PubMed] [Google Scholar]
- 45.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–61. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 46.Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006;22:1014–23. doi: 10.1016/j.dental.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 47.Naoum S, Ellakwa A, Martin F, Swain M. Fluoride release, recharge and mechanical property stability of various fluoride-containing resin composites. Oper Dent. 2011;36:422–32. doi: 10.2341/10-414-L. [DOI] [PubMed] [Google Scholar]
- 48.Davis HB, Gwinner F, Mitchell JC, Ferracane JL. Ion release from, and fluoride recharge of a composite with a fluoride-containing bioactive glass. Dent Mater. 2014;30:1187–94. doi: 10.1016/j.dental.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahn SJ, Lee SJ, Lee DY, Lim BS. Effects of different fluoride recharging protocols on fluoride ion release from various orthodontic adhesives. J Dent. 2011;39:196–201. doi: 10.1016/j.jdent.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent Mater J. 2013;32:296–304. doi: 10.4012/dmj.2012-144. [DOI] [PubMed] [Google Scholar]
- 51.Dickens SH, Kelly SR, Flaim GM, Giuseppetti AA. Dentin adhesion and microleakage of a resin-based calcium phosphate pulp capping and basing cement. Eur J Oral Sci. 2004;112:452–7. doi: 10.1111/j.1600-0722.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 52.Featherstone JDB. The continuum of dental caries – Evidence for a dynamic disease process. J Dent Res. 2004;83:C39–C42. doi: 10.1177/154405910408301s08. [DOI] [PubMed] [Google Scholar]
- 53.Thylstrup A, Fejerskov O. Textbook of cariology. Copenhagen; Denmark, Munksgaard: 1986. pp. 145–146. [Google Scholar]
- 54.International Organization for Standardization, Standard 4049-1988 (E) Dentistry: resin-based dental filling materials. Geneva, Switzerland: [Google Scholar]
- 55.Kemp-Scholte CM, Davidson CL. Complete marginal sealing of Class V resin composite restorations effected by increased flexibility. J Dent Res. 1990;69:1240–1243. doi: 10.1177/00220345900690060301. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Li H, Fok AS, Watts DC. Multiple correlations of material parameters of light-cured dental composites. Dent Mater. 2009;25:829–36. doi: 10.1016/j.dental.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 57.Heymann HO, Sturdevant JR, Bayne S, Wilder AD, Sluder TB, Brunson WD. Examining tooth flexure effects on cervical restorations: a two-year clinical study. J Am Dent Assoc. 1991;122:41–7. doi: 10.1016/s0002-8177(91)25015-1. [DOI] [PubMed] [Google Scholar]
- 58.EI-Safty S, Akhtar R, Silikas N, Watts DC. Nanomechanical properties of dental resin-composites. Dent Mater. 2012;28:1292–300. doi: 10.1016/j.dental.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 59.Moreau JL, Weir MD, Giuseppetti AA, Chow LC, Antonucci JM, Xu HHK. Long-term mechanical durability of dental nanocomposites containing amorphous calcium phosphate nanoparticles. J Biomed Mater Res B. 2012;100:1264–1273. doi: 10.1002/jbm.b.32691. [DOI] [PMC free article] [PubMed] [Google Scholar]