Abstract
Objectives
Bulk fracture is one of the primary reasons for resin-based dental restoration failures. To date, there has been no report on the use of polymerizable dental monomers with acceptable biocompatibility to develop a resin with substantial self-healing capability. The objectives of this study were to: (1) develop a self-healing resin containing microcapsules with triethylene glycol dimethacrylate (TEGDMA)-N,N-dihydroxyethyl-p-toluidine (DHEPT) healing liquid in poly(urea-formaldehyde) (PUF) shells for the first time, and (2) determine the physical and mechanical properties, self-healing efficiency, and fibroblast cytotoxicity.
Methods
Microcapsules of polymerizable TEGDMA-DHEPT in PUF were prepared via an in situ polymerization method. Microcapsules were added into a BisGMA-TEGDMA resin at microcapsule mass fractions of 0%, 5%, 10%, 15% and 20%. A flexural test was used to measure composite strength and elastic modulus. A single edge V-notched beam method was used to measure fracture toughness KIC and self-healing efficiency.
Results
Flexural strength and elastic modulus (mean ± sd; n = 6) of resin containing 5% to 15% microcapsules were similar to control without microcapsules (p > 0.1). Adding microcapsules into the resin increased the virgin KIC, which was about 40% higher at 15% microcapsules than that with 0% microcapsules (p < 0.05). Specimens were fractured and healed, then fractured again to measure the healed KIC. A self-healing efficiency of about 65% in KIC recovery was obtained with 10–20% microcapsules. All specimens with 0–20% microcapsules had fibroblast viability similar to control without resin eluents (p > 0.1).
Significance
Self-healing dental resin containing microcapsules with polymerizable TEGDMA-DHEPT healing liquid in PUF shells were prepared for the first time with excellent self-healing capability. These microcapsules and self-healing resins containing them may be promising for dental restorations to heal cracks/damage and increase durability.
Keywords: Self-healing dental resin, microcapsules, polymerizable healing liquid, mechanical properties, fracture toughness recovery, cytotoxicity
Graphical abstract
1. Introduction
Dental caries is a prevalent problem worldwide with a significant financial burden [1]. Resin composites are popular materials to fill tooth cavities due to their excellent esthetics and direct-filling capabilities [2,3]. However, the durability of these restorations is limited, with half of all restorations failing in less than 10 years, and with fracture as one of the primary reasons for failure [4,5]. It was reported that more than 25% of composite replacements were driven by some forms of fracture [6]. Indeed, fracture of restorations was the main reason for failure in composite restorations in large cavities after 5 years of use [7].
A main reason that composite restorations fail was suggested to be the formation and accumulation of micro-cracks, caused by factors including mastication forces and thermal stresses [8]. Efforts have been made to develop composites with improved resistance to fracture failures [9–13]. These efforts included optimizing the inorganic filler level, reducing filler size to nanoscale, adding different types of fillers such as fibers, whiskers and nanotubes, improving the polymer matrix and optimizing the polymerization reactions. These strategies have yielded composites with better mechanical performance and improved longevity. Nonetheless, composites are still brittle and prone to fracture, especially in large-sized restorations in high load-bearing locations [3]. Therefore, there is a strong need to inhibit crack growth and fracture failures in dental resin-based restorations [14].
Self-healing polymers have the promise of autonomic repair to heal cracks and damage [15]. They have the built-in capability to repair damage and recover the load-bearing capability [16]. A promising method for providing self-healing capability is by embedding microcapsules into the composite [17]. Each microcapsule has an external shell that encapsulates a healing liquid inside. When the polymer experiences damage and cracking, the microcapsules could be ruptured by the damage. They release the healing liquid which flows into the crack planes and the damage site, exposing itself to the catalyst embedded in the polymer matrix. Self-healing then occurs due to the polymerization of the healing agent, which fills and bonds the crack together and inhibits the crack propagation [18]. In a pioneering study, dicyclopentadiene (DCPD) was encapsulated in a poly(urea-formaldehyde) (PUF) shell to form microcapsules [19]. Grubb’s catalyst, a transition metal carbine complex, was used in an epoxy matrix to trigger the polymerization of the released DCPD, which achieved a good self-healing efficiency [19]. Since then, several other studies were reported on microcapsules of various shell and healing liquid compositions, with potential applications ranging from microelectronics to aerospace [20,21], as well as bone cement where effective rebonding of the crack was achieved, with the healed specimens reaching approximately 75% of the original fracture toughness [22].
For dental use, efforts were made to incorporate DCPD-containing microcapsules and Grubb’s catalyst into a composite, achieving a recovery of 57% of the original fracture toughness of composite [23]. Another study tested the mechanical properties of dental resins with DCPD-containing microcapsules, showing that the use of microcapsules did not affect the mechanical performance of resin matrix [24]. However, literature search revealed no further report on the use of DCPD and Grubb’s catalyst in dental materials, likely due to biocompatibility concerns. Toxicity from the self-healing agent inside the microcapsules as well as the catalyst present in the composite needs to be considered [14]. The DCPD toxicity [25], Grubb’s catalyst toxicity, and their high cost [26] remain challenges in using them in dental composite. Another study developed nanocapsules with a polyurethane (PU) shell containing the triethylene glycol dimethacrylate (TEGDMA) liquid, demonstrating that the addition of nanocapsules into a dental adhesive increased the dentin bond strength [27]. However, these nanocapsules contained no catalyst for polymerization, and hence no self-healing effect on adhesive resin was reported [27]. To date, there has been no report on the use of polymerizable dental monomers as healing liquid with proven biocompatibility in dentistry to develop a resin demonstrating a substantial self-healing capability.
The healing liquid should have a relatively low viscosity to flow and fill the cracks in the resin matrix. TEGDMA is flowable and has been used as a dental monomer with acceptable biocompatibility. Furthermore, TEGDMA can form a polymer via free-radical initiation by using a peroxide initiator and a tertiary amine accelerator. A usual dental tertiary amine accelerator, N,N-dihydroxyethyl-p-toluidine (DHEPT), has good solubility and stability in TEGDMA. Therefore, the present study proposed to test DHEPT incorporation in TEGDMA to serve as the self-healing liquid inside microcapsules for the first time. In the present study which was our first study, we focused on the synthesis and characterization of the self-healing microspheres, incorporation into resin, physical and mechanical properties, and fibroblast cytotoxicity. Then in our second study, we further added calcium phosphate and antibacterial monomer to the self-healing resin, and tested oral biofilm response [28].
Accordingly, the objectives of this study were to investigate TEGDMA-DHEPT as a self-healing liquid in PUF microcapsules for dental applications and to examine the mechanical properties and self-healing efficacy. The following hypotheses were tested: (1) The new TEGDMA-DHEPT-in-PUF microcapsules could be incorporated into a dental resin without decreasing the mechanical properties of the resin; (2) The microcapsule-containing resin would have a high self-healing capability after fracture; (3) The microcapsule-containing resin would have a low cytotoxicity against human fibroblasts.
2. Materials and methods
2.1. Synthesis of microcapsules
Microcapsules were prepared by in situ polymerization of formaldehyde and urea, following a previous study [29]. DHEPT (Sigma-Aldrich, St. Louis, MO) at 1% mass fraction was added to TEGDMA monomer (Esstech, Essington, PA). At room temperature, 50 mL of distilled water and 13 mL of a 2.5% aqueous solution of ethylene-maleic anhydride (EMA) copolymer (Sigma-Aldrich) were mixed in a 250 mL round bottom glass flask. The flask was suspended in a water bath on a hotplate (Isotemp, Fisher Scientific, Pittsburg, PA). The EMA solution was used as a surfactant to form an “oil-in-water” emulsion (“oil” being TEGDMA-DHEPT). Under agitation by a magnetic stir bar (diameter = 7.8 mm, length = 50 mm, Fisher Scientific) at 300 rpm, the shell-forming material urea (1.25 g), ammonium chloride (0.125 g) and resorcinol (0.125 g) (Sigma-Aldrich) were added into the solution. The resorcinol was added in the reaction of shell formation to enhance the rigidity of the shells [30]. The pH was adjusted to 3.5 via drop-wise addition of 1 M sodium hydroxide solution. Then, the agitation rate was increased to 400 rpm, and 30 mL of the TEGDMA-DHEPT liquid was added into the flask. A stabilized emulsion of fine TEGDMA-DHEPT droplets was formed after 10 min of agitation. Then, 3.15 g of a 37% aqueous solution of formaldehyde (Sigma-Aldrich) was added, and the flask was sealed with aluminum foil to prevent evaporation. The temperature of the water bath was raised to 55 °C and the shell material was isothermally polymerized for 4 hours (h) under continuous agitation [30]. In this process, ammonium chloride catalyzed the reaction of urea with formaldehyde to form PUF at the oil-water interface to develop the shell [30]. The microcapsules thus obtained were rinsed with water and acetone repeatedly, vacuum-filtered, and air-dried for 24 h in a hood.
2.2. Characterization of microcapsules
An optical microscope (TE2000-S, Nikon, Japan) was used to examine the microcapsules. The microcapsule diameters were measured using the optical microscope together with an image analysis software (Nis-Elements BR2.30, Nikon, Japan). In addition, the surface morphology of TEGDMA-filled microcapsules was examined with scanning electronic microscopy (SEM, Quanta 200, FEI, Hillsboro, OR). Microcapsules were spread onto an adhesive tape and sputter-coated with gold. Some microcapsules were placed on an adhesive tape and cut open with a razor blade to facilitate the examination of the shell structure, following a previous study [29].
The ability of the encapsulated TEGDMA to polymerize was quantified by near-infrared (NIR) spectroscopy (Nicolet 6700, Thermo Scientific, Waltham, MA). A mixture of microcapsules with 0.5% by mass of benzoyl peroxide (BPO) initiator powder (the catalyst) (Sigma-Aldrich) were broken by manual spatulation and placed in a 6 × 3 × 2 mm Teflon mold. NIR spectra were acquired immediately after resin paste mixing and at 24 h post-cure in an FTIR spectrometer (Nicolet 6700, Thermo Scientific). The degree of vinyl conversion (DC) was calculated from the % change in the integrated peak area of methacrylate =CH2 absorption band, normalized by thickness of the specimen before and after curing, following a previous study [31].
The yield of microcapsules was calculated as the ratio of the dry weight of total microcapsules of each batch divided by the weight of the initial substances [29], i.e., urea, resorcinol, ammonium chloride, formaldehyde, TEGDMA and DHEPT. Microcapsules loaded with TEGDMA were dispersed in acetone and sonicated at 40Hz for 5 min (Branson, Fisher Scientific). This broke the microcapsules, and the TEGDMA and DHEPT were dissolved in the acetone solvent. After vacuum filtration and drying for 1 d, the undissolved microcapsule shell fragments were collected and weighed. The TEGDMA-DHEPT mass was calculated as the initial weight of the microcapsules minus the shell weight. The TEGDMA-DHEPT mass fraction in the microcapsules was calculated as the TEGDMA-DHEPT mass divided by the total mass of the microcapsules [27].
To assess the stability of the microcapsules, additional microcapsules were exposed to air at ambient temperature for 30 d. The TEGDMA-DHEPT mass fraction in the aged microcapsules was again calculated following the aforementioned procedures.
The thermal stability of the microcapsules was tested using differential scanning calorimetry (DSC, 2920MDSC, TA, New Castle, DE) to measure the heat flow. Five mg of microcapsules was placed in an air-tight aluminum pan and heated at a heating rate of 10 °C/min from 0 °C to 350 °C in a nitrogen environment. The DSC diagram was recorded by the DSC analysis instrument. With the increase in temperature, any exothermic and endothermic events were recorded on the DSC diagram, thus providing information on how thermally stable the microcapsules were, and at what temperature they became unstable. All assessments were conducted using 6 batches of samples (n = 6).
2.3. Fabrication of self-healing dental resin
Bisphenol A glycidyl dimethacrylate (BisGMA) (Esstech) and TEGDMA monomers were mixed at a 1:1 mass ratio. The mixture was rendered light-curable by adding 1% by mass of a photo-initiator, phenyl bis(2,4,6-trimethylbenzoyl) phosphine oxide (BAPO, Sigma-Aldrich). Then, 0.5% benzoyl peroxide (BPO) was also added to the monomer mixture as the self-healing initiator. BPO could react with DHEPT from the microcapsules in the case of microcapsule rupture to cause self-healing. The 0.5% of BPO was selected because it did not decrease the mechanical properties of the resin and it was sufficient to trigger the free-radical polymerization in a preliminary test.
Microcapsules were mixed into the photo-activated BisGMA-TEGDMA monomers at the following mass fractions: 0%, 5%, 10%, 15% and 20%. Microcapsule mass fractions greater than 20% were not used because the mechanical properties of the resin were degraded in preliminary studies. For mechanical testing, each paste was placed in a stainless steel mold of 2 × 2 × 25 mm and covered with a Mylar strip. Each specimen was photo-cured (Triad 2000, Dentsply, York, PA) for 1 min on each open side of the mold. The specimens were demolded and stored in distilled water at 37 °C for 24 h prior to testing.
2.4. Flexural testing
A computer-controlled Universal Testing Machine (5500R, MTS, Cary, NC) was used to fracture the specimens in three-point flexure using a span of 10 mm and a crosshead speed of 1 mm/min. Flexural strength S was measured as: S = 3PmaxL/(2bh2), where P max is the load-at-failure, L is span, b is specimen width and h is thickness. Elastic modulus E was measured as: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope in the linear elastic region of the load-displacement curve. The specimens were taken out of the water and fracture while being wet. Six specimens of each group were tested [32].
2.5. Fracture toughness testing
Fracture toughness KIC was measured via a single edge V-notched beam (SEVNB) method under mode I condition [33]. A notch with a depth of approximately 500 µm was machined into a resin bar of 2 × 2 × 25 mm using a diamond blade with a 150 µm thickness (Buehler, Lake Bluff, IL). Then, a 3-µm diamond paste (Buehler) was placed into the notch tip, and a new sharp razor blade was used to cut the notch further to a total depth of about 700–800 µm. According to previous studies using the same method, this produced a relatively sharp notch with a tip radius of approximately 20 µm [33,34]. For each specimen, the notch length was measured using an optical microscope (TE2000-S, Nikon) on both sides of the specimen and then the notch length was averaged. The notched specimens were tested in the same three-point fixture with the notch on the tensile side, and the loading pin aligned with the notch under a 3x magnifier. KIC was calculated following an established SEVNB method [34]. The photo-cured BisGMA-TEGDMA resins with 0% to 20% of microcapsules were tested. This yielded the original virgin KIC of the specimens, KIC-virgin.
In order to test the self-healing efficacy, immediately following specimen fracture, the two halves of the specimen were placed back into the same mold which was labeled and was originally used to make the same specimen. This is similar to the case of a resin-based restoration in a tooth cavity, where the cracked restoration would stay in the tooth cavity to allow the released healing liquid to heal the crack. Because the fracture ruptured the microcapsules, the released TEGDMA-DHEPT from the microcapsules would react with the BPO in the resin matrix. This would cause the polymerization of the released liquid to heal and bond the two cracked planes into one cohesive specimen. This healed sample was placed in a humidor at 37 °C for 24 h. The notch length was again measured and made sure that it was the same as the virgin notch length of the same specimen. Then the healed bar was tested again using the same flexural method to measure the KIC. This yielded the healed value, KIC-healed.
The self-healing efficiency (η) was assessed following a previous study as [35]: η = KIC-healed/KIC-virgin
The fractured surfaces of selected self-healed specimens were examined via SEM after sputter-coating with gold. In addition, the fractured and unhealed surfaces, which were rinsed with ethyl alcohol immediately after fracture to remove the released healing agent, were also examined for investigation of fracture mechanisms.
2.6. Cytotoxicity testing
The cytotoxicity of the five groups of resins containing 0%, 5%, 10%, 15% and 20% of microcapsules was examined by measuring cell survival and proliferation in vitro via a MTT assay [36]. To prepare resin specimens for cytotoxicity testing, the cover of a sterile 96-well plate was used as molds, following a previous study [36]. Fifty µL of resin paste of each group was placed into the bottom of the dent and covered with a Mylar strip, then light-cured for 30 s (Optilux VCL 401, Demetron Kerr, Danbury, CT). This formed a disk of about 8 mm in diameter and 0.5 mm in thickness. The disks were sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC). Then, six disks of each group were immersed in 10 mL of a commercial fibroblast culture medium (ScienCell, San Diego, CA), referred to as FM. The medium was agitated for 24 h at 37 °C to obtain resin eluents, following a previous study [36]. The original extract solution was then serially diluted for the cytotoxicity test [36]. Three solutions were prepared at dilutions of 32-fold (1 part of original extract + 31 parts of fresh FM), 64-fold, and 128-fold, respectively, following a previous study [36]. The reason for choosing 128-fold dilution was that the amount of saliva flow for an average person is approximately 1000 to 1500 mL per day [37]. The 128-fold dilution yielded to a total solution volume of 1280 mL for the six resin disks. The 32-fold and 64-fold corresponded to a total solution of 320 mL and 640 mL respectively, which would be equivalent to 1/4 and 1/2 of the normal saliva amount per day, to take into account of reduced saliva production for some patients. The total resin volume of the six disks was 151 mm3. Hence, the resin volume divided by the total solution volume for the three solutions was 0.12, 0.24, and 0.47 mm3/mL, respectively. Fresh FM without any resin eluent (resin/solution volume ratio = 0 mm3/mL) served as the control in cell culture.
Human gingival fibroblasts (HGF, ScienCell) were cultured in a growth medium consisting of FM supplemented with 2% fetal bovine serum, 100 IU/mL penicillin and 100 IU/mL streptomycin. They were seeded into the wells of 96-well micro-plates at a density of 4,000 cells/well [38]. After 24 h of incubation at 37 °C in a humid atmosphere with 5% CO2 in air, the culture medium in the 96-well microplates was removed and replaced with 100 µL of the aforementioned resin extract solutions. Then the cells were cultured for another 48 h. To each well was added 20 µL of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT, Sigma-Aldrich) solution at a concentration of 5 mg/mL [38]. After incubating in a dark room for 4 h, the unreacted dye was replaced by adding150 µL/well of dimethylsulfoxide (DMSO, Sigma-Aldrich). The plate was gently shaken until the crystals were completely dissolved. Then the absorbance of the solutions was measured spectrophotometrically using a microplate reader (Spectra Max M5, Molecular Devices, Sunnyvale, CA) at 492 nm. Pure FM without any resin eluent was used as control to culture fibroblasts, and its absorbance was set as 100%. Cell viability was assessed as Aresin/Acontrol, where Aresin was the absorbance of cells cultured with resin eluents, and Acontrol was the absorbance of cells cultured in FM without resin eluents [38].
2.7. Statistical analysis
One-way analysis of variance (ANOVA) was performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used at a p value of 0.05.
3. Results
Fig. 1A shows a pile of the synthesized microcapsules which had the appearance of a white powder. Fig. 1B shows that the microcapsules had a black ring outside and a relatively bright area inside. This phenomenon was attributed to the two different refractive indices of the PUF shell and the healing liquid inside. This indicates that the TEGDMA was successfully encapsulated by the PUF shell. The SEM image in Fig. 1C showed the external surface of microcapsules. The diameters in two perpendicular directions were measured and averaged for each microcapsule. The microcapsules were measured to have an average diameter of 70 ± 24 µm (n = 200). Higher magnification in Fig. 1D revealed details of the shell, showing numerous nanoparticles attaching to a smooth shell surface to form a rough shell. The smooth shell surface appeared to be dense without voids. Fig. 1E displayed the shell wall of a microcapsule at a high magnification. The thickness of the shell was estimated to be 230 ± 43 nm (mean ± sd; n = 6). Crushed microcapsules with shell fragments and the released healing agent between two glass slides were displayed in Fig. 1F, further demonstrating the liquid filled structure of microcapsules.
Figure 1.
Microcapsules were prepared with polymerizable TEGDMA-DHEPT healing liquid inside PUF shells. (A) Photo showing a pile of the synthesized microcapsules. (B) Transmitting optical image showing the shell structure as a dark ring. (C) SEM image of typical microcapsules. (D) High magnification SEM image of the shell surface, showing nanoparticle deposits on an otherwise smooth shell surface. (E) High magnification SEM image indicating the shell thickness. (F) Optical image of crushed microcapsules showing the released healing liquid films.
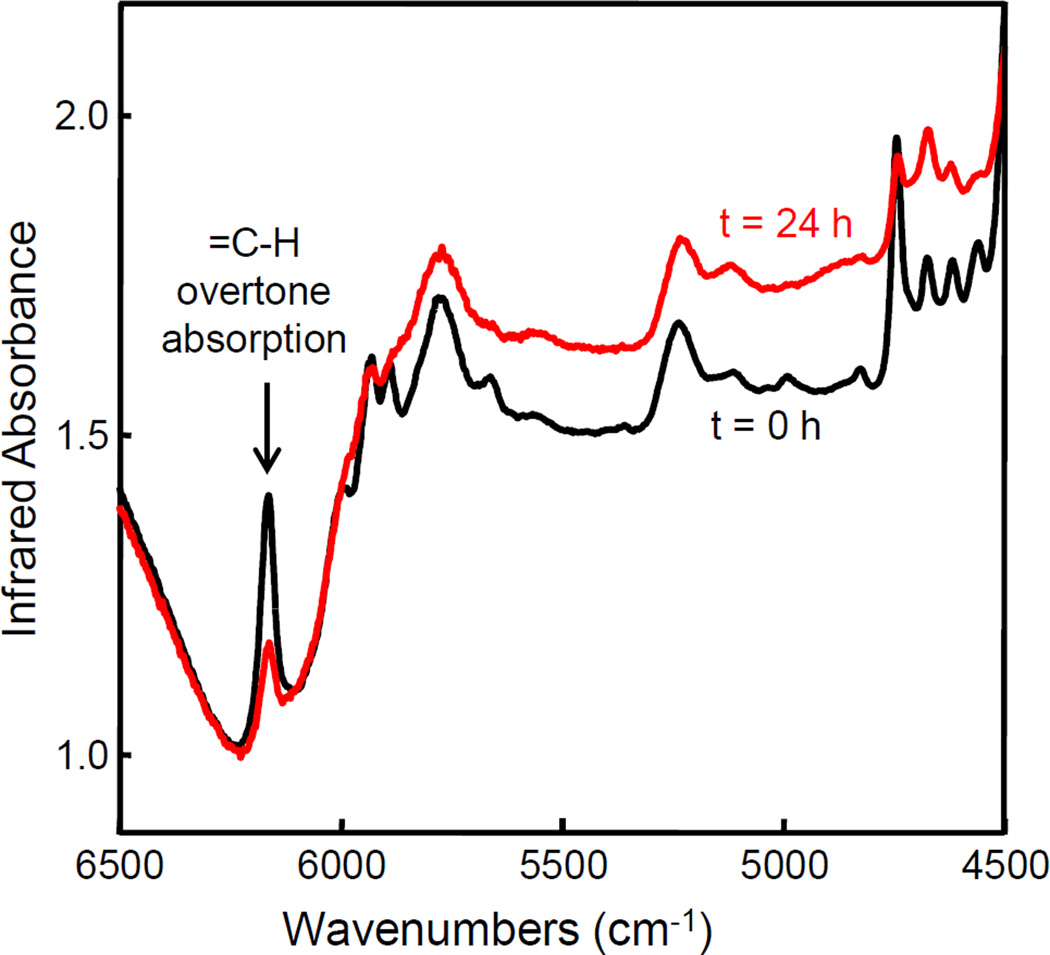
Fig. 2 shows the NIR results of the microcapsules after they were fractured and the released TEGDMA-DHEPT was polymerized. The presence of the =CH overtone band at 6165 cm−1 indicated that TEGDMA was successfully encapsulated. Furthermore, after polymerization for 24 h, reduction in absorbance of this band signified that polymerization of TEGDMA occurred. The degree of conversion for the released TEGDMA-DHEPT polymerization was (67.2 ± 0.6)% (mean ± sd; n = 3). The analysis of the polymer products spectra after the microcapsules were crushed confirmed the reactivity of the microcapsules.
Figure 2.
NIR spectra of microcapsules consisting of TEGDMA-DHEPT healing liquid inside PUF shells. Appearance and absorption of vinyl peak at 6165 cm−1 verified the successful process of encapsulation and excellent polymerizable ability of these microcapsules.
The synthesis yield of the microcapsules was calculated as the weight of the microcapsules of each batch divided by the weight of the initial substances used. This yield was measured (mean ± sd; n = 6) to be (76.8 ± 3.7)%.
The TEGDMA-DHEPT mass fraction in the microcapsules was calculated as the TEGDMA and DHEPT mass divided by the total mass of the microcapsules. This was measured (mean ± sd; n = 6) to be (70.8 ± 5.4)% at 1 d, and (66.9 ± 4.6)% after aging in air for 30 d. These two values are not significantly different from each other (p > 0.1).
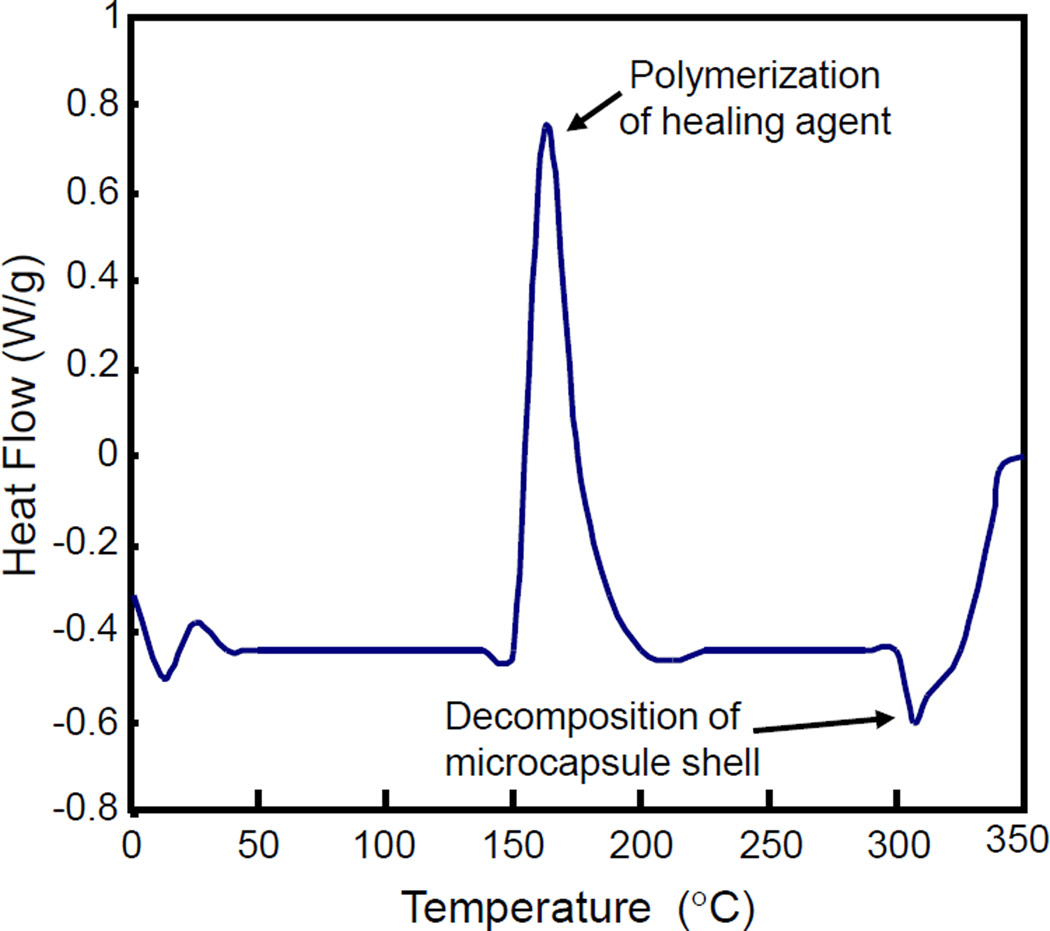
Fig. 3 displays a representative DSC diagram of the microcapsules. A typical exothermic peak and an endothermic valley appeared in the DSC curve at high temperatures. The exothermic peak at 162 °C was due to the polymerization reaction of TEGDMA in the microcapsules, which was triggered by the high temperature. The endothermic valley at about 307 °C was because of the decomposition of the PUF shell [39]. These results demonstrate that the PUF shell-based microcapsules are chemically stable at temperatures of up to about 150 °C, which showed an excellent thermal stability.
Figure 3.
DSC diagram of microcapsules containing TEGDMA-DHEPT healing liquid inside PUF shells. The exothermic peak at 162 °C was due to polymerization of TEGDMA by the high temperature. The endothermic valley at 307 °C was related to PUF shell decomposition. These microcapsules were stable up to 150 °C, indicating suitability for use at temperatures in vivo in dentistry.
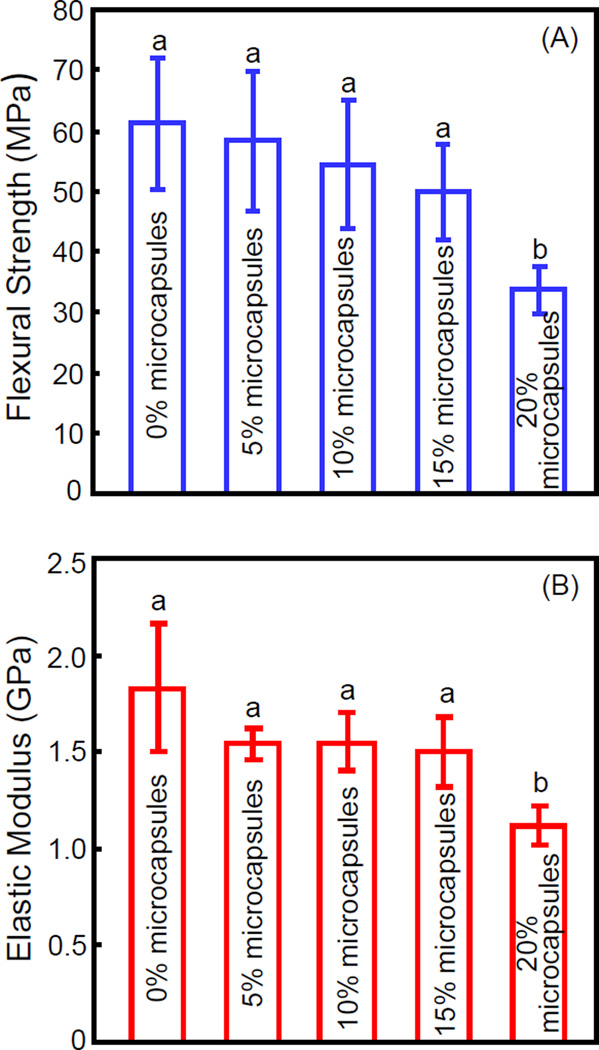
Flexural strength and elastic modulus of resin with different microcapsule mass fractions are plotted in Fig. 4 (mean ± sd; n = 6). The flexural strength and elastic modulus of resins containing 0% to 15% microcapsules were not significantly different from each other (p > 0.1). However, the mechanical properties of the resin with 20% microcapsules were significantly lower than the other groups (p < 0.05).
Figure 4.
Mechanical properties of resin containing microcapsules of various concentrations: (A) flexural strength, and (B) elastic modulus (mean ± sd; n = 6). 0% refers to resin containing 0% microcapsules, 5% refers to resin containing 5% microcapsules, and so on. Addition up to 15% of microcapsules resulted in no significant decrease in strength and elastic modulus of the resin. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
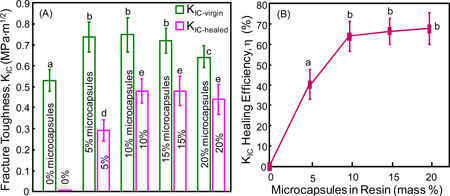
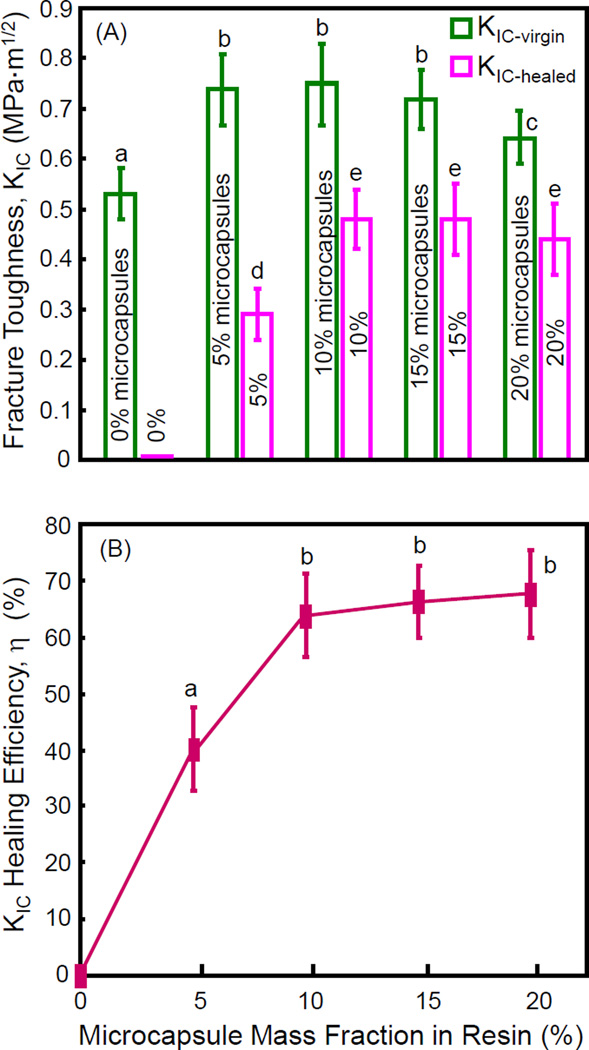
The fracture toughness KIC results are plotted in Fig. 5 (mean ± sd; n = 6). In (A), the virgin and healed KIC of the resins are plotted vs. microcapsule mass fraction. Adding microcapsules into the resin increased the virgin KIC, which was about 40% higher at 15% microcapsules than that at 0% microcapsules (p < 0.05). For the healed KIC, the specimen was completely fractured and then the two halves were placed back into the mold to allow self-healing to occur. The healed KIC significantly increased, from no healing at 0% microcapsules to maximum healing at 10% and 15% microcapsules (p < 0.05). The self-healing efficiency is plotted in (B). A self-healing efficiency of 64%-68% in KIC recovery was obtained when the microcapsule concentration in the resin was ≥ 10%.
Figure 5.
Self-healing of resin containing microcapsules: (A) fracture toughness, and (B) self-healing efficiency (mean ± sd; n = 6). 0% refers to resin containing 0% microcapsules, 5% refers to resin containing 5% microcapsules, and so on. In each plot, values with dissimilar letters are significantly different from each other (p < 0.05).
Representative fracture surfaces of self-healing resin are shown in Fig. 6. The example shown is for the resin containing 15% microcapsules. The initial virgin fractured surface of a specimen is shown in (A). Typical step tail patterns (arrows) following the crack propagation direction could be seen, with an example enlarged in the insert in (A). In (B), the specimen was fractured into two halves, and the two halves were placed into the mold for the released healing liquid to polymerize and heal the specimen. The healed specimen was re-fractured and examined in SEM. Many polymer films and layers (arrows) were visible on the healed surface in (B). These numerous irregularly-shaped films in many different locations on the fracture surface indicate the successful release of TEGDMA from microcapsules and polymerization to bond the cracked planes together. This is consistent with the measured relatively high recovery of the fracture toughness in Fig. 5.
Figure 6.
Representative SEM images of the fractured planes from the specimens containing 15% microcapsules. (A) The initial virgin fracture surface of the resin specimen revealing the step tail structure. The inset in (A) shows a higher magnification of a step tail structure. (B) The healed and re-fractured surface of the resin specimen displaying the presence of released and polymerized healing liquid films.
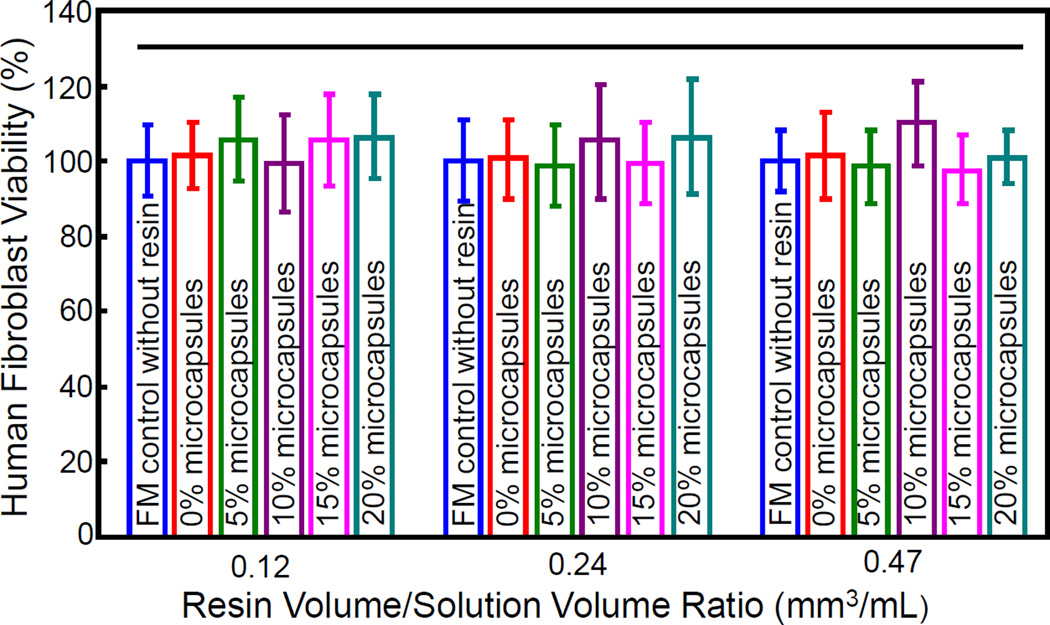
Fig. 7 plots the results on human fibroblast cytotoxicity in vitro. Cell viability in fibroblast medium without any resin eluents was set as 100%. All five groups of resin specimens with 0%, 5%, 10%, 15% and 20% microcapsules had cell viability similar to that of FM control (p > 0.1). Hence the incorporation of microcapsules into resin did not significantly compromise the cell viability.
Figure 7.
Human gingival fibroblast cytotoxicity in vitro (mean ± sd; n = 6). “FM” refers to the cell culture group in fibroblast medium without any resin eluents. 0% refers to resin containing 0% microcapsules, 5% refers to resin containing 5% microcapsules, and so on. All groups had cell viability matching that of FM group, exhibiting a good biocompatibility. The 128-fold dilution (resin/solution volume ratio of 0.12mm3/mL) yielded a total solution volume comparable to the daily saliva volume of 1000 mL −1500mL in vivo. Horizontal line in the plot indicated values that are not significantly different from each other (p > 0.1).
4. Discussion
The present study synthesized TEGDMA-DHEPT containing microcapsules, and the resin with these microcapsules demonstrated successful healing, with substantial recovery of load-bearing capability. Polymeric microcapsules are often prepared via emulsion polymerization techniques [40]. In the present study, microcapsules with a healing liquid of TEGDMA plus 1% DHEPT surrounded by a PUF shell, were manufactured through an in situ polymerization technology in an oil-in-water emulsion. A key requirement of the self-healing process is the rupture of the embedded microcapsules on crack intrusion. The roughness of the microcapsule wall with nanoparticle deposit (Fig. 1D) is of vital importance in obtaining a mechanical interlock between the microcapsule and resin matrix. An interlocking interface would help the microcapsule to be ruptured when a crack propagating in the resin matrix hits the microcapsule. In contrast, with a smooth interface without interlock, the resin matrix crack may go around the microcapsule and not rupture it, without releasing the healing liquid to heal the crack. The microcapsule wall thickness is another key issue. A thinner wall may be easily ruptured, and may be too fragile for handling and mixing of microcapsules into a resin matrix. On the other hand, microcapsule walls that are too thick may make the microcapsule difficult to be broken by the crack, thus failing to release the liquid to cause healing. Therefore, an optimal wall thickness for the microcapsules is vital. A previous study reported average wall thickness values of 160–220 nm [30]. In the present study, the TEGDMA-DHEPT-filled microcapsules with a PUF wall thickness of about 230 nm were shown to successfully heal the dental resin.
The microcapsule stability is another important issue, and the synthesis of stable microcapsules is a necessity to protect the encapsulated healing agent from premature polymerization [41]. The reactive liquid inside microcapsules must not diffuse out of the shell wall during its potentially long shelf-life. In the present study, the microcapsules were exposed to air at ambient temperature for 1 month, and the results suggested that the microcapsules were stable, with negligible permeability and negligible loss of the liquid TEGDMA through the PUF shell. Furthermore, the high temperature experiment of TEGDMA polymerization and PUF shell wall fragmentation with the DSC analysis showed a durable thermal property of PUF shell-based microcapsules up to 150 °C, which will not be experienced in the shelf or in the mouth. These results indicate that these microcapsules likely have a long shelf-life with good thermal stability; however, actual long-term tests need to be performed in further investigations.
Incorporating microcapsules into polymers can inevitably change the properties of the host polymer. The goal would be to obtain self-healing capability without decreasing the original mechanical properties of the resin. Decreasing the mechanical properties of dental polymer including flexural strength and fracture toughness can compromise the durability and reliability of the restoratives [42–44]. A previous study showed different results on the effect of incorporation of microcapsules on the mechanical performance of the host material [24]. Studies showed that the incorporation of microcapsules was accompanied with significant improvements in tensile strength [45] and flexural strength of the polymer matrix [46]. However, another study reported a continuous reduction in the strength of the resin with increasing microcapsule content [47]. In the present study, the flexural strength and elastic modulus of the resin were not significantly reduced or increased by the addition of 0% to 15% microcapsules. However, these properties were significantly decreased at 20% microcapsules. Therefore, whether the addition of microcapsules would affect the mechanical properties of the resin appears to depend on the microcapsule mass fraction.
Previous studies showed that after microcapsule incorporation, the fracture toughness of the polymer matrix generally increased [48–51]. In the present study, a nearly 40% increase in the original virgin KIC was achieved when the microcapsule mass fraction was increased from 0% to 15%. Regarding the toughing mechanism, as shown in the fracture planes (Fig. 6A for virgin fracture surface), numerous step tails originating from the fractured microcapsules were present. This is consistent with the phenomenon reported earlier with self-healing polymers containing microcapsules [47]. It appears that the propagation of the crack tip became discontinuous in the presence of microcapsules, which caused an out-of-surface divergence in crack propagation [47]. After puncturing the microcapsule, the two cracks in different planes met together in order to obtain continuity, thus forming a step tail structure next to the microcapsule [47]. Therefore, the toughening mechanism appeared to be crack pinning and deflection caused by the microcapsules in the resin. In addition, the rupture of the PUF microcapsules, the interfacial debonding, and the liquid burst likely also consumed energy, interfered with the crack, and contributed to an increase in virgin KIC.
The self-healing efficiency η, was measured to quantify the recovery of the original KIC. The polymerized films on the fracture plane of the healed specimen (Fig. 6B) indicate that the healing agent containing the amine accelerator had the capability to react with the BPO initiator in resin bulk. The polymerization capability of the healing agent was also consistent with the result of NIR analysis. The self-healing efficiency increased to a recovery of about 65% in KIC when the microcapsule mass fraction was increased from 5% to 10% and 15%. A lower recovery at 5% microcapsules was due to the insufficient dosage of the microcapsules, leading to insufficient quantity of the released healing agent to cover the broken planes. The better healing effect deriving from higher microcapsule concentrations may be explained by more healing agent release, resulting in re-bonding of the cracked planes. There was little difference in healing efficacy from a microcapsule concentration of 10% to 20%. This was likely because the healing agent released from the microcapsules was sufficient to completely cover the crack plane [52]. The self-healing efficiency of about 65% is consistent with previous studies reporting a 57% average recovery of the original fracture toughness [23], and a 75% recovery in toughness [19]. It should be noted that on the fractured, healed and refractured surfaces, some microcapsules in the surface did not undergo fracture. It appeared that the crack went through the microsphere-resin interface without rupturing the microsphere in some cases, hence the self-healing liquid was not released, and the self-healing effect of that microsphere was not utilized. It is possible that the healing efficacy could be further increased if more microspheres could be ruptured by the crack. Further study needs to investigate whether silanizing the microspheres to achieve microsphere-resin matrix bonding would lead to more microsphere rupture during fracture.
While previous studies [19,23] used DCPD and Grubb’s catalyst with toxicity concerns [25,26], the present study used dental monomers (TEGDMA, DHEPT, and BPO) for healing liquid polymerization; these components have already been used in commercial dental resins approved by the Food and Drug Administration. Indeed the cytotoxicity test (Fig. 7) showed similar results to culture medium without resin eluents. With good self-healing efficiency of the TEGDMA, DHEPT and BPO system, if the restoration experiences cracks in service, the ruptured microcapsules could deliver the healing agent in time to the damaged region for a recovery. Further study is needed to test the self-healing of dental restorations in vivo.
The effect of possible leakage of unreacted free formaldehyde from PUF shells on cytotoxicity needs to be determined [53]. To date, there has been no report and no data available on the biocompatibility of PUF-based microcapsule inclusion into a dental resin. The present study showed that the addition of microcapsules did not affect cell viability, compared to that without microcapsules, as well as to the control using commercial fibroblast medium without any resin eluents. The results showed that the amount of microcapsules (e.g., 15%) was enough to repair damage; however, it was rather a small amount and could well be below the toxicity threshold. Hence, the present self-healing resin containing microcapsules had minimal toxicity similar to cells in fibroblast medium without any resin eluents. Although there were limitations regarding the correlation between in vitro and in vivo testing, this result did provide insight into the potential promise of this healing system.
5. Conclusions
Novel self-healing dental resin containing microcapsules with polymerizable TEGDMA-DHEPT healing liquid in PUF shells were prepared via in situ polymerization, and exhibited a substantial self-healing capability. Once crack and damage ruptured the microcapsules, the released TEGDMA-DHEPT healing liquid could react with the BPO in the resin matrix and polymerize to bond the cracked planes together. The shell wall of the microcapsules had a proper thickness with a smooth surface roughened by nanoparticle deposits, which promoted micromechanical interlocking of microcapsules in the resin matrix.
The flexural strength and elastic modulus of the resin were not adversely affected when the microcapsule mass fraction was ≤ 15%. The inclusion of microcapsules increased the virgin fracture toughness of resin. The self-healing efficiency showed that about 65% recovery of the virgin fracture toughness could be achieved when using 15% microcapsules. The self-healing resin containing microcapsules appeared to have good cellular cytotoxicity in vitro. Therefore, the novel self-healing dental resin containing microcapsules with polymerizable TEGDMA-DHEPT liquid in PUF shells may be promising for crack-inhibiting and self-healing dental restorations to prolong the lifetime.
Highlights.
Bulk fracture is one of the primary reasons for resin-based dental restoration failures. This study developed a self-healing resin containing microcapsules with triethylene glycol dimethacrylate (TEGDMA)-N,N-dihydroxyethyl-p-toluidine (DHEPT) healing liquid in poly(urea-formaldehyde) (PUF) shells for the first time. Specimens were fractured and healed, then fractured again to measure the healed fracture toughness KIC. A self-healing efficiency of about 65% in KIC recovery was obtained with 10–20% microcapsules. In conclusion, self-healing dental resin containing microcapsules with polymerizable TEGDMA-DHEPT healing liquid in PUF shells were prepared for the first time with excellent self-healing capability. These microcapsules and self-healing resins containing them may be promising for dental restorations to heal cracks/damage and increase durability. The figure below shows: (A) fracture toughness, and (B) self-healing efficiency (mean ± sd; n = 6). 0% refers to resin containing 0% microcapsules, 5% refers to resin containing 5% microcapsules, and so on. Values with dissimilar letters are significantly different from each other (p < 0.05).
Acknowledgments
We thank Esstech (Essington, PA) for kindly donating the BisGMA and TEHDMA monomers. This study was supported by NIH R01 DE17974 (HX) and a seed grant from the University of Maryland Baltimore School of Dentistry (HX).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nash RW. Direct composite resin restorations for today’s practice. Dentistry Today. 2013;32 114,116,118. [PubMed] [Google Scholar]
- 2.Ilie N, Hickel R. Resin composite restorative materials. Australian Dental Journal. 2011;56:59–66. doi: 10.1111/j.1834-7819.2010.01296.x. [DOI] [PubMed] [Google Scholar]
- 3.Ferracane JL. Resin composite-state of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Mjor IA, Moorhead JE, Dahl JE. Reasons for replacement of restorations in permanent teeth in general dental practice. International Dental Journal. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 5.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Van Nieuwenhuysen JP, D’Hoore W, Carvalho J, Qvist V. Long-term evaluation of extensive restorations in permanent teeth. Journal of Dentistry. 2003;31:395–405. doi: 10.1016/s0300-5712(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 7.Brunthaler A, Konig F, Lucas T, Sperr W, Schedle A. Longevity of direct resin composite restorations in posterior teeth. Clinical Oral Investigations. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- 8.Baran GR, Boberick KG, McCool JI. Fatigue of restorative materials. Critical Reviews in Oral Biology and Medicine. 2001;12:350–360. doi: 10.1177/10454411010120040501. [DOI] [PubMed] [Google Scholar]
- 9.Venhoven BA, de Gee AJ, Werner A, Davidson CL. Influence of filler parameters on the mechanical coherence of dental restorative resin composites. Biomaterials. 1996;17:735–740. doi: 10.1016/0142-9612(96)86744-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferracane JL. Current trends in dental composites. Critical Reviews in Oral Biology & Medicine. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 11.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dental Materials. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 12.Zhang H, Darvell BW. Mechanical properties of hydroxyapatite whisker -reinforced bis-GMA-based resin composites. Dental Materials. 2012;28:824–830. doi: 10.1016/j.dental.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 13.Feilzer AJ, de Gee AJ, Davidson CL. Setting stresses in composites for two different curing modes. Dental Material. 1993;9:2–5. doi: 10.1016/0109-5641(93)90095-8. [DOI] [PubMed] [Google Scholar]
- 14.Jandt KD, Sigusch BW. Future perspectives of resin-based dental materials. Dental materials. 2009;25:1001–1006. doi: 10.1016/j.dental.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Wu DY, Meure S, Solomon D. Self-healing polymeric materials: A review of recent developments. Progress in Polymer Science. 2008;33:479–4522. [Google Scholar]
- 16.Brochu AB, Craig SL, Reichert WM. Self-healing biomaterials. Journal of Biomedical Materials Research. 2011;96:492–506. doi: 10.1002/jbm.a.32987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samadzadeh M, Boura SH, Peikari M, Kasiriha SM, Ashrafi A. A review on self-healing coatings based on micro/nanocapsules. Progress in Organic Coatings. 2010;68:159–164. [Google Scholar]
- 18.Kessler MR, Sottos NR, White SR. Self-healing structural composite materials. Composites Part A: Applied Science and Manufacturing. 2003;34:743–753. [Google Scholar]
- 19.White SR, Sottos NR, Geubelle PH, Moore JS, Kessler MR, Sriram SR, Brown EN, Viswanathan S. Autonomic healing of polymer composites. Nature. 2001;409:794–797. doi: 10.1038/35057232. [DOI] [PubMed] [Google Scholar]
- 20.Murphy EB, Wudl F. The world of smart healable materials. Progress in Polymer Science. 2010;35:223–251. [Google Scholar]
- 21.Yang Y, Urban MW. Self-healing polymeric materials. Chemical Society Reviews. 2013;42:7446–7467. doi: 10.1039/c3cs60109a. [DOI] [PubMed] [Google Scholar]
- 22.Dailey MM, Silvia AW, McIntire PJ, Wilson GO, Moore JS, White SR. A self-healing biomaterial based on free-radical polymerization. J Biomed Mater Res A. 2014;102(9):3024–3032. doi: 10.1002/jbm.a.34975. [DOI] [PubMed] [Google Scholar]
- 23.Wertzberger BE, Steere JT, Pfeifer RM, Nensel MA, Latta MA, Gross SM. Physical characterization of a self-healing dental restorative material. Journal of Applied Polymer Science. 2010;118:428–434. [Google Scholar]
- 24.Then S, Neon GS, kasim NH. Performance of melamine modified urea-formaldehyde microcapsules in a dental host material. Journal of Applied Polymer Science. 2011;122:2557–2562. [Google Scholar]
- 25.Bevan C, Snellings WM, Dodd DE, Egan GF. Subchronic toxicity study of dicyclopentadiene vapor in rats. Toxicology and Industrial Health. 1992;8:353–367. [PubMed] [Google Scholar]
- 26.Caruso MM, Delafuente DA, Ho V, Sottos NR, Moore JS, White SR. Solvent-promoted self-healing epoxy materials. Macromolecules. 2007;40:8830–8832. [Google Scholar]
- 27.Ouyang X, Huang X, Pan Q, Zuo C, Huang C, Yang X, Zhao Y. Synthesis and characterization of triethylene glycol dimethacrylate nanocapsules used in a self-healing bonding resin. Journal of Dentistry. 2011;39:825–833. doi: 10.1016/j.jdent.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Zhou H, Weir MD, Melo MAS, Levine ED, Xu HHK. Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. Journal of Dentistry (2015, accepted for publication) doi: 10.1016/j.jdent.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blaiszik BJ, Caruso MM, McIlroy DA, Moore JS, White SR, Sottos NR. Microcapsules filled with reactive solutions for self-healing materials. Polymer. 2009;50:990–997. [Google Scholar]
- 30.Brown EN, Kessler MR, Sottos NR, White SR. In situ poly(urea-formaldehyde) microencapsulation of dicyclopentadiene. Journal of microencapsulation. 2003;20:719–730. doi: 10.1080/0265204031000154160. [DOI] [PubMed] [Google Scholar]
- 31.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dental Materials. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 32.Zhou C, Weir MD, Zhang K, Deng D, Cheng L, Xu HH. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into CaP nanocomposite. Dental Materials. 2013;29:859–870. doi: 10.1016/j.dental.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HH, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. Journal of Dental Research. 2004;83:930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- 34.Kuebler J. VAMAS Report No. 37, TWA3, ESIS Report D2–99, EMPA, CH-8600 Duebendorf, Switzerland. 1999. Fracture toughness of ceramics using the SEVNB method; Round robin. [Google Scholar]
- 35.Wool RP, O’Connor KM. A theory of crack healing in polymers. Journal of Applied Physics. 1981;52:5953–5963. [Google Scholar]
- 36.Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, Bai Y, Xu HH. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. Journal of Dentistry. 2013;47:464–474. doi: 10.1016/j.jdent.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. Journal of Prosthetic Dentistry. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 38.Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, Chen JH. Antibacterial activity and cytotoxicity of two novel crosslinking antibacterial monomers on oral pathogens. Archives of Oral Biology. 2011;56:367–373. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Camino G, Operti L, Trossarelli L. Mechanism of thermal degradation of urea-formaldehyde polycondensates. Polymer Degradation and Stability. 1983;5:161–172. [Google Scholar]
- 40.Asua JM. Miniemulsion polymerization. Progress in Polymer Science. 2002;27:1283–1346. [Google Scholar]
- 41.Yuan L, Liang GZ, Xie JQ, Guo J, Li L. Thermal stability of microencapsulated epoxy resins with poly(urea-formaldehyde) Polymer Degradation and Stability. 2006;91:2300–2306. [Google Scholar]
- 42.Takahashi A, Sato Y, Uno S, Pereira PN, Sano H. Effects of mechanical properties of adhesive resins on bond strength to dentin. Dental Materials. 2002;18:263–268. doi: 10.1016/s0109-5641(01)00046-x. [DOI] [PubMed] [Google Scholar]
- 43.Carrilho MR, Tay FR, Pashley DH, Tjäderhane L, Carvalho RM. Mechanical stability of resin-dentin bond components. Dental Materials. 2005;21:232–241. doi: 10.1016/j.dental.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Brosh T, Ganor Y, Belov I, Pilo R. Analysis of strength properties of light-cured resin composites. Dental Materials. 1999;15:174–179. doi: 10.1016/s0109-5641(99)00029-9. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Siddaramaiah Kim NH, Hui D, Lee JH. Effects of dual component microcapsules of resin and curing agent on the self-healing efficiency of epoxy. Composites Part B: Engineering. 2013;55:79–85. [Google Scholar]
- 46.Yuan L, Huang S, Gu A, Liang G, Chen F, Hu Y, Nutt S. A cyanate ester/microcapsule system with low cure temperature and self-healing capacity. Composites Science and Technology. 2013;87:111–117. [Google Scholar]
- 47.Brown EN, White SR, Sottos NR. Microcapsule induced toughening in a self-healing polymer composite. Journal of Materials Science. 2004;39:1703–1710. [Google Scholar]
- 48.Jin H, Miller GM, Sottos NR, White SR. Fracture and fatigue response of a self-healing epoxy adhesive. Polymer. 2011;52:1628–1634. [Google Scholar]
- 49.Wilson GO, Moore JS, White SR, Sottos NR, Andersson HM. Autonomic healing of epoxy vinyl esters via ring opening metathesis polymerization. Advanced Functional Materials. 2008;18:44–52. [Google Scholar]
- 50.Keller MW, White SR, Sottos NR. A self-healing poly(dimethyl siloxane) elastomer. Advanced Functional Materials. 2007;17:2399–2404. [Google Scholar]
- 51.Brown EN, Sottos NR, White SR. Fracture testing of a self-healing polymer-composite. Experimental Mechanics. 2002;42:372–379. [Google Scholar]
- 52.Jin H, Miller GM, Pety SJ, Griffin AS, Stradley DS, Roach D, Sottos NR, White SR. Fracture behavior of a self-healing, toughened epoxy adhesive. International Journal of Adhesion and Adhesives. 2013;44:157–165. [Google Scholar]
- 53.Duong A, Steinmaus C, McHale CM, Vaughan CP, Zhang L. Reproductive and developmental toxicity of formaldehyde: A systematic review. Mutation Research. 2011;728:118–138. doi: 10.1016/j.mrrev.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]