Abstract
Background
Patients with chronic kidney disease (CKD) have a high symptoms burden that is related to a poor health-related quality of life (HRQoL) and high costs of care. Validated instruments may be useful for assessing the symptoms and monitoring outcomes in these patients. The Palliative care Outcome Scale-Symptoms Renal (POS-S Renal) is a patient-reported outcome measure for assessing symptoms in CKD stage 4–5. This study is the first cross-cultural adaptation and psychometric analysis of this clinical tool. The purpose of this study is to carry out a cross-cultural adaptation of the POS-S Renal for Spanish-speaking patients, and to perform an analysis of the psychometric properties of this questionnaire.
Methods
The English version of the POS-S Renal was culturally adapted and translated into Spanish using a double forward and backward method. An expert panel evaluated the content validity. The questionnaire was pilot-tested in 30 patients. A total of 200 patients with CKD stage 4–5 filled in a modified Spanish version of the POS-S Renal and the MSAS-SF. Statistical analysis to evaluate the psychometric properties of the questionnaire was carried out.
Results
The content validity index (CVI) was 0.97, which indicated that the content of the instrument is an adequate reflection of the symptoms in advanced CKD (ACKD). The factor analysis indicated a two-factor solution explaining 35.05% of total variance. The confirmatory factor analysis (CFA) demonstrated that the two factor model was well supported (comparative fit index = 0.98, root mean square error of approximation = 0.068). This assessment tool demonstrated a satisfactory test–retest reliability (r = 0.909 to factor 1, r = 0.695 to factor 2, r = 0.887 to total score), good internal consistency to factor 1 (α = 0.78) and moderate internal consistency to factor 2 (α = 0.56). Concurrent criterion-related validity with MSAS-SF was also demonstrated, with r = 0.860, which indicated a high degree of correlation with a validated instrument that has been used in patients with ACKD.
Conclusions
The Spanish modified version of the POS-S Renal is a reliable and valid instrument that can be used to assess symptoms in Spanish patients with CKD stage 4–5.
Electronic supplementary material
The online version of this article (doi:10.1186/s12882-016-0402-8) contains supplementary material, which is available to authorized users.
Keywords: Outcome measure, Symptoms, ACKD, Psychometrics
Background
A patient with Advanced Chronic Kidney Disease (ACKD)(defined, according to the international guidelines, as chronic kidney disease at stage 4 or 5 with a glomerular filtration rate < 30 ml/min) has an increased burden of physical and psychological symptoms that are associated with a poor health-related quality of life (HRQoL) [1–3]. Symptoms such as tiredness, pruritus, constipation, pain, sleep alterations, anxiety, dyspnoea, nausea, restless legs and depression have a high prevalence in these patients [4, 5]. As uncontrolled symptoms at the end of life lead to greater suffering, their treatment in the advanced phases of the disease is a priority [6].
Patient reported outcome (PRO) instruments are generally used to assess patients’ functional status, quality of life and symptoms [7]. This is especially important in patients with chronic diseases such as chronic kidney disease (CKD), in which PRO measures can be used to evaluate, monitor, and facilitate the introduction of interventions [8].
The use of validated scales may therefore be useful for standardising the evaluation of symptoms and monitoring the healthcare outcomes of these patients [9]. Because of the variety of symptoms that might be suffered by a kidney patient, tools that can evaluate a wide range of symptoms are recommended. In addition, it is useful to have instruments that can be administered pre- and post-dialysis start, in order to better monitor patients as they progress through CKD stages. Among the tools most used for the study of symptoms in kidney patients are questionnaires that are not specific to renal patients, such as the Memorial Symptom Assessment Scale-Short Form(MSAS-SF) [10], questionnaires modified for use with patients on dialysis, such as the Edmonton Symptom Assessment System (ESAS) [11], and other specific questionnaires that have been developed to measure symptoms in ACKD, such as the Dialysis Symptom Index (DSI) [12] and the Palliative care Outcome Scale-Symptoms Renal (POS-S Renal) [13].
There are no specific symptom assessment questionnaires for CKD patients that have been adapted to the Spanish culture, Spanish being one of the most commonly spoken languages in the world and that enable us to evaluate the intensity of the various symptoms in this population [14]. The POS-S Renal is a questionnaire that has been designed to evaluate symptoms in ACKD, and it has demonstrated its usefulness in clinical practice and in research [13, 15]. Thus it is a tool recommended for the evaluation of symptoms in this population [9, 16, 17]. There are at present no studies that have assessed the psychometric properties of this questionnaire.
The aims of this study were to carry out a cross-cultural adaptation of the POS-S Renal into Spanish and to perform an analysis of test-retest reliability, internal consistency, internal structure, and concurrent criterion-related validity with the MSAS-SF, a previously validated PRO measure which has been used in patients with ACKD [18–20].
Methods
This study was conducted in two principal phases: translation and cross-cultural adaptation, and then validation.
Phase 1: Translation and cross-cultural adaptation
Translation
Translation of the original English version of the POS-S Renal was performed using a double forward and backward method following recommended guidelines [21]. This method includes: 1) two forward translations from the source language into the target language; 2) reconciliation of the two versions; 3) two backward translations from the target language version to the source language; and 4) reconciliation of the two versions and comparison with the original to create a consensus document.
There were two independent translations into Spanish. The two versions were compared and, after discussion, merged into a single document: the preliminary Spanish version of POS-S Renal. Two native English translators performed a backward translation of this version to the source language to make, after discussion, a single document. This document was compared with the original to check the conceptual and semantic equivalence between the two texts (Fig. 1).
Fig. 1.
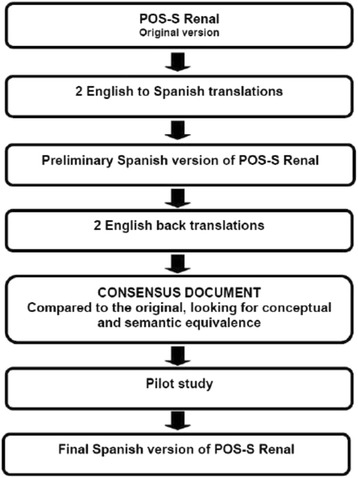
Flowchart of the translation of the POS-S Renal from English to Spanish. Two forward translations were performed by two translators. Two native English translators performed a backward translation. The back-translated version was compared with the original version to create a consensus document, which was pilot tested to provide a final version
Content validity
Content validity occurred during this phase to evaluate the degree to which the content of the instrument is an adequate reflection of the construct to be measured. An expert panel review was conducted to assess content validity. A total of 14 healthcare professionals (experts in palliative care and nephrology), researchers, patients with ACKD and carers of ACKD patients were invited to join the expert panel, but only eight of them (six healthcare professionals, one patient and one carer) agreed to participate. The other six declined because of lack of time (n = 4) or lack of knowledge about the subject (n = 2). Content validity was determined using the COSMIN checklist [22]. Individually, the expert participants were asked to rate their level of agreement with the relevance of each item. One symptom (cramps) was added after discussion. The response choices for this item were set to be identical to the response choices for the other 17items. This symptom was prevalent (29%), but had not previously been included in this symptom assessment tool. Content validity was determined using the Lawshe content validity index (CVI);employing the formula suggested by Lawshe, the CVI was 0.97, with items ranging from 0.75 to 1.00, which is acceptable, according to Lawshe and Davis [23, 24].
Pilot study
A pilot study was conducted with 30 patients to identify problematic items and to ensure that the adapted questionnaire was understandable and acceptable to the patients.
Phase 2: Validation study
Sample, setting and data collection
Two hundred (200) patients (aged 66.45 ± 14.52, 65% male) participated in the present study. Their socio-demographic and clinical characteristics are shown in Table 1. All participants were recruited at the Nephrology Service at Carlos Haya University Hospital in Málaga (Spain), and, after receiving information about the project, signed to give their informed consent. The inclusion criteria were: adult Spanish-speaking patients with ACKD receiving dialysis or renal conservative management (without dialysis).
Table 1.
Socio-demographic and Clinical Characteristics of the Sample (N = 200)
| Characteristics | Tota (N = 200) | Conservative management group (n = 139) | Dialysis group (n = 61) |
|---|---|---|---|
| Age (mean, SD) | 66.45 (±14.5) | 69.65 (±12.8) | 59.15 (±15.4) |
| Gender | |||
| Male | 130 (65%) | 95 (68%) | 35 (57.4%) |
| Female | 70 (35%) | 44 (32%) | 26 (42.6%) |
| Ethnicity | |||
| Caucasian | 199 (99.5%) | 138 (99.3%) | 61 (100%) |
| Spanish descent | 193 (96.5%) | 132 (94.9%) | 61 (100%) |
| British descent | 3 (1.5%) | 3(2.2%) | 0 (0%) |
| German descent | 3 (1.5%) | 3(2.2%) | 0 (0%) |
| Indian | 1 (0.5%) | 1(0.7%) | 0 (0%) |
| Marital status | |||
| Married | 134 (67%) | 94 (68%) | 40 (65.6%) |
| Not married | 66 (33%) | 45 (32%) | 21 (34.4%) |
| Causes of CKD | |||
| Renal vascular disease | 66 (33%) | 56 (40.5%) | 10 (16.4%) |
| Diabetic nephropathy | 32 (16%) | 22 (16%) | 10 (16.4%) |
| Primary glomerular disease | 17 (8.5%) | 9 (6.5%) | 8 (13.1%) |
| Polycystic kidneys | 13 (6.5%) | 8 (6%) | 5 (8.2%) |
| Unknown aetiology | 27 (13.5%) | 19 (14%) | 7 (11.5%) |
| Others | 45 (22.5%) | 24 (17%) | 21 (34.4%) |
| Barthel index (mean, SD) | 94.8 (±9.8) | 94.93 (±9.5) | 94.59 (10.3%) |
| Charlson comorbidity index | |||
| 2–3 | 83 (41.5%) | 56 (40.3%) | 27 (44.3%) |
| 4–5 | 85 (42.5%) | 58 (41.7%) | 27 (44.3%) |
| ≤ 6 | 32 (16%) | 25 (18%) | 7 (11.4%) |
By contrast, there were three exclusion criteria: the patient’s refusal to participate in the study, mild cognitive impairment and the patient being under the age of 18 years. The data were collected between April 2015 and September 2015.
Data were collected during face-to-face interviews. When possible, the questionnaires (POS-S Renal and MSAS-SF) were self-completed. Otherwise, the interviewer read out and filled in the instruments according to the patient’s responses. In the case of patients managed conservatively (without dialysis), the interviews were conducted in the routine medical consultation room, whereas patients in dialysis were interviewed in the dialysis room. The interviewers first recorded the patient’s clinical socio-demographic data, and afterwards asked the patients to complete the POS-S Renal and the MSAS-SF.
Questionnaires
POS-S renal
This is a patient-completed questionnaire that has been developed to assess symptoms in ACKD. The questionnaire identifies the presence and severity of 17 symptoms during the week prior to the completion of the questionnaire [13]. If the symptom is present, the intensity of each symptom is rated on a 5-point Likert scale (0 to 4) ranging from ‘none’ to ‘overwhelming’.
In addition, the questionnaire provides open fields to list the main problems experienced. This open-ended question gives the patient the opportunity to emphasise the importance of a symptom, or to indicate other symptoms not included in the questionnaire. In addition, a symptom severity score can be calculated from the questionnaire as a whole. If one apportions a number to the range of severity of each symptom (from 0 to 4), the maximum global symptom severity score is 68. This simple tool is used widely and is recommended as the tool of choice for ACKD [9]. The original questionnaire is available from: http://pos-pal.org/maix/pos-s-in-english.php.
MSAS-SF
The MSAS-SF is a validated self-reporting questionnaire that includes 32 highly common physical and psychological symptoms [10]. It assesses the presence and severity of 28 physical symptoms on a 5-point Likert scale (from 0 = ‘not bothersome’ to 4 = ‘very bothersome’) and the presence and frequency of four psychological symptoms on a 4-point Likert scale (from 1 = ‘rarely present’ to 4 = ‘almost constantly present’).
The MSAS-SF subscales include: 1) the Global Distress Index (GDI), measuring four psychological symptoms and six physical symptoms; 2) the Physical Symptom Distress Score(PHYS) comprising 12 common physical symptoms; and 3) the Psychological Symptom Distress Score (PSYCH), including six common psychological symptoms. The total number of symptoms (TNS) is the sum of symptoms experienced. The scoring procedure follows the instructions of Portenoy et al. for the short form [25]. The MSAS-SF has been cross-culturally adapted to Spanish speakers and has shown adequate psychometric properties [26]. It has been widely used in patients with ACKD [18–20].
Psychometric evaluation of the questionnaire
Reliability
Reliability was considered as test-retest reliability. To evaluate this, 73 patients completed the Spanish POS-S Renal twice, separated by 7 days. Pearson’s r correlation coefficient was used for assessment of the test-retest reliability.
Internal consistency
Internal consistency concerns whether there is a satisfactory degree of interrelatedness among the items. To evaluate this psychometric property, Cronbach’s α coefficients were calculated using the intraclass correlation coefficient type 2.1two-way mixed effects model, where people effects are random and measures effects are fixed (ICC2.1) [27].
Internal structure
Internal structure refers to the degree to which the scores for a questionnaire are an adequate reflection of the dimensionality of the construct to be measured. Exploratory factor analysis (EFA) with maximum likelihood extraction (MLE) and varimax rotation was used to evaluate this psychometric property. To confirm the factor model, we performed confirmatory factor analysis (CFA).
Concurrent criterion-related validity
Concurrent criterion-related validity refers to the degree to which the scores for an instrument are associated with scores for other instruments that are intended to evaluate similar constructs. This psychometric property was measured using the MSAS-SF as a reference, and Pearson’s r correlation coefficient was used to examine correlations between factor 1 of POS-S Renal and MSAS-SF total score, between factor 2 of POS-S Renal and MSAS-SF total score, and between the total score of POS-S Renal and MSAS-SF.
Statistical analysis
Descriptive statistical analysis was used to determine the means and standard deviation of the sociodemographic and clinical variables and the POS-S Renal. We tested the differences in the responses from patients in dialysis and patients in conservative management using one-way analysis of variance (ANOVA).
Statistical analysis was conducted to assess the test-retest reliability, internal consistency, internal structure and concurrent criterion validity. The sample distribution was determined by Kolmogorov–Smirnov (KS) test. The Pearson’s r correlation coefficient used the criteria of: poor (an r < 0.49), fair (0.50 ≤ r ≤ 0.74), and strong (an r > 0.75) [28]. We assessed the measurement model of the POS-S Renal using CFA. The model fit indices included chi-square (χ 2), root mean square error of approximation (RMSEA), and the comparative fit index (CFI). For RMSEA, values of 0.08 or below indicate a close fit [29]. This analysis was conducted using SPSS version 20 and LISREL 8.80 [30].
Results
Translation and cultural adaptation
The POS-S Renal was translated and back-translated without language difficulties to provide the Spanish modified version of the instrument (Additional file 1). The mean ± SD time to complete the questionnaire at the first administration was 7 ± 2.5 min. The Spanish modified version of the POS-S Renal showed a low proportion of missing responses (this did not exceed 2%). The characteristics of the study sample are shown in Table 1.
Response from patients in dialysis and patients having conservative management showed no significant differences (p = 0.130 to factor 1 and p = 0.455 to factor 2).
Pilot study
The translated and modified version of the POS-S Renal proved to be comprehensible and easy to complete during the pilot testing, and changes in format were not needed. The questionnaire was readable and acceptable by the target population (Additional file 2).
Validation study
Test-retest reliability
The test–retest reliability was satisfactory. Pearson’s correlation coefficient was: r = 0.909 to factor 1, r = 0.695 to factor 2, r = 0.887 to total score and P < 0.001.
Internal consistency
The adapted questionnaire presented good internal consistency to factor 1 (α = 0.78, item range from 0.74 to 0.83) and moderate internal consistency to factor 2 (α = 0.56, item range from 0.45 to 0.65).
Internal structure: factor analysis
The Kaiser-Meyer-Olkin values (0.773) and Bartlett’s Test of Sphericity (P < 0.001) indicated that the correlation matrix for the POS-S Renal was suitable for MLE. The factor analysis revealed that 35.05% of the variance could be explained with two factors with an eigenvalue higher than 1 and 10% of variance explained, and 61.01% with six factors with an eigenvalue higher than 1. The results are shown in Table 2. These six factors were used for the rotated component matrix. The scree plot indicated a two-factor solution (see Fig. 2). The item loading
Table 2.
Total Variance Explained
| Factor | Eigenvalues | ||
|---|---|---|---|
| Total | % of Variance | Cumulative % | |
| 1 | 4.472 | 24.843 | 24.843 |
| 2 | 1.838 | 10.210 | 35.053 |
| 3 | 1.332 | 7.398 | 42.450 |
| 4 | 1.267 | 7.037 | 49.488 |
| 5 | 1.070 | 5.943 | 55.431 |
| 6 | 1.005 | 5.582 | 61.013 |
Extraction Method: Maximum Likelihood
a. When factors are correlated, sums of squared loadings cannot be added to obtain a total variance
Fig. 2.
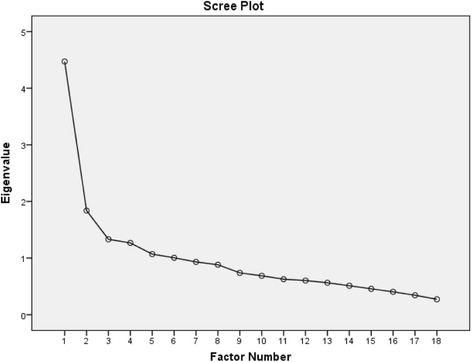
Scree plot of the exploratory two factor solution
for the two-factor solution is shown in Table 3. The fit indices of the two factor model indicated an excellent fit: χ 2 = 222.82, RMESA = 0.68 and CFI = 0.98 (Fig. 3) [31].
Table 3.
Factor Structure of Rotated Component Matrix
| Item | Component | |
|---|---|---|
| 1 | 2 | |
| Pain | 0.555 | −0.162 |
| Shortness of breath | 0.497 | −0.318 |
| Weakness or lack of energy | 0.547 | −0.379 |
| Nausea | 0.251 | −0.779 |
| Vomiting | 0.196 | −0.694 |
| Poor appetite | 0.398 | −0.236 |
| Constipation | 0.305 | −0.001 |
| Mouth problems | 0.558 | −0.305 |
| Drowsiness | 0.433 | −0.320 |
| Poor mobility | 0.672 | −0.050 |
| Itching | 0.361 | −0.317 |
| Difficulty sleeping | 0.506 | −0.386 |
| Restless legs | 0.150 | −0.223 |
| Feeling anxious | 0.537 | −0.303 |
| Feeling depressed | 0.586 | −0.218 |
| Changes in skin | 0.329 | −0.345 |
| Diarrhoea | 0.187 | −0.486 |
| Muscle cramps | 0.229 | −0.358 |
Extraction Method: Maximum Likelihood. Rotation Method: Oblimin with Kaiser Normalisation. Suppression at 0.35
Fig. 3.
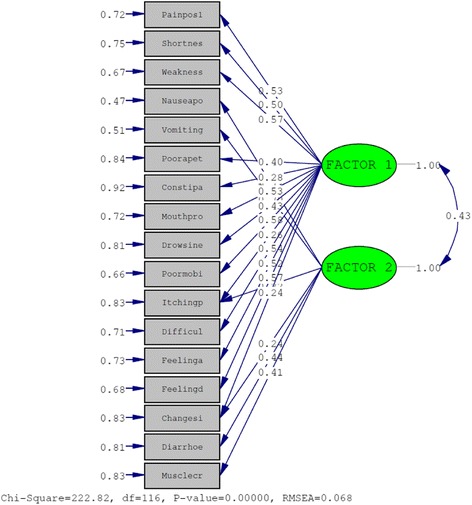
Confirmatory factor analysis of the two factor model of the Spanish version of POS-S Renal
Concurrent criterion-related validity
The correlation between the POS-S Renal and the MSAS-SF was confirmed. The POS-S Renal showed adequate correlations with the MSAS-SF. The values of the correlations were 0.809 (factor 1 – MSAS-SF total score), 0.518 (factor 2 – MSAS-SF total score) and 0.860 (POS-S Renal total score – MSAS-SF total score). The results demonstrated the value of the POS-S Renal as a multidimensional measure.
Discussion
The translation and cross-cultural adaptation of the POS-S Renal into Spanish using recognised international guidelines was achieved satisfactorily. To our knowledge, this study is the first cross-cultural adaptation and psychometric analysis of the POS-S Renal. The study sample included dialysis and conservatively managed patients. Although the POS-S Renal is an instrument to measure symptoms in conservatively managed ACKD patients, in our experience it has been a useful clinical assessment tool for the dialysis population. We tested responses from patients in these two categories, and the results indicated that there were no significant differences between the two groups, although a trend towards a significant difference in the responses between the groups for factor 1 was found.
Excellent content validity of the Spanish modified version was demonstrated, which indicates that all items of this instrument are relevant for the measurement of the symptomatology in ACKD [23, 24]. This instrument showed satisfactory psychometric characteristics in terms of reliability [27], structural validity [31], and concurrent criterion-related validity [32]. The sample size was adequate for all analyses [33].
We added ‘cramps’ to the original 17-item POS-S Renal because of the frequency of cramps among patients with ACKD [4, 5]. Although in our study the prevalence of cramps was lower than has been found in other studies on the Spanish population, this is a common symptom that is often under-evaluated and is an important cause of the early termination of dialysis sessions [34, 35].
The test–retest reliability was high (r = 0.887 to total score), with values beyond those found in the modified ESAS in dialysis patients (r = 0.7 to total score) [11].
Internal consistency analysis indicated a satisfactory degree of interrelatedness among the items of the instrument [27]. Factor 1(α = 0.78) and factor 2 (α = 0.56) showed the highest and lowest results, respectively, with values below those found in the Arabic translation and modification of the DSI (Cronbach’s α = 0.91 overall) [36].
The two-factor solution obtained in the EFA accounted for a significant proportion of variance and showed support for the presence of construct validity, which provides support for evidence of the instrument validity. The factor analysis performed to evaluate the internal structure of the POS-S Renal confirmed the two-factor model, in line with other symptom assessment tools [25]. However, we performed a CFA, and the fit indices of the CFA model were satisfactory [29]. Nonetheless, two POS-S Renal items, namely, restless legs and changes in skin, did not load on either of these two factors. Consequently, it appears that these symptoms function as individual items. A possible explanation for this is that these two symptoms are best regarded as ‘causal indicators’ not ‘indicator variables’, and this suggests inherent problems with the application of the factor analysis [37]. Causal indicators are variables external to the patient that could affect symptoms, and indicator variables are unobservable variables which can be inferred from scores on multiple self-report items. Thus, the inclusion of indicator variables in factor analysis could lead to uninterpretable results [37, 38].
The concurrent criterion-related validity analysis with the MSAS-SF was supported by a strong correlation with factor 1 and total score, and a fair correlation with factor 2, which provides support for evidence that the scores of the POS-S Renal are an adequate reflection of a previously validated instrument that has been widely used in patients with ACKD. These results are in line with those found by the authors of the Arabic translation of the DSI and the modified ESAS with the Kidney Dialysis Quality of Life-Short Form (KDQOL-SF) [11, 36].
The extent and severity of the symptom burden in patients with ACKD demonstrates the importance of using an appropriate clinical tool [4, 5, 13, 15]. Likewise, the multidimensional nature of the symptoms should be considered in these patients [39]. The POS-S Renal is a validated instrument that provides information about a large number of physical and psychological symptoms. This also demonstrates the value of POS-S Renal as a useful and clinically appropriate symptom assessment tool to facilitate a multidimensional symptom evaluation in ACKD.
A patient with many mild symptoms can have a score identical to a patient with fewer, but more distressing symptoms, and a high score on even a single symptom could indicate a high level of patient distress for one symptom even if the patient’s total score is low. Thus, clinicians must look at the patient’s score on each of the 18 Spanish modified version of the POS-S Renal symptoms on its own, even though a total score can be obtained.
This study provides access to a PRO instrument to assess symptoms in CKD stage 4–5 for Spanish speaking populations. This single-page 18-item PRO is a self-administered questionnaire that is comprehensible and easy to complete. Hence, this study shows that this instrument will be of value in the symptom assessment of patients with ACKD in clinical practice and in research. Although this study contributes by filling the knowledge gap on the validation and usefulness of POS-S Renal, as well as, on symptoms assessment in ACKD, subsequent studies should be carried out to develop more refined measuring tools in this area [40].
The limitations to consider in this study include the lack of longitudinal data concerning other psychometric characteristics. Although our study shows a preliminary analysis of the psychometric properties of a modified POS-S Renal, sensitivity to change and minimally important differences were not evaluated. Further work using longitudinal data to determine responsiveness is needed to define the use of these measures in cohort studies and clinical trials that evaluate interventions. Finally, given the lower internal consistency for factor 2, a confirmatory factor analysis in a wide sample should be carried out to explore and interpret the behaviour of this factor.
Conclusions
The Spanish modified version of the POS-S Renal demonstrated a two-factor structure and provided a PRO specific to the Spanish population that is valid and reliable. This clinical tool is simple to complete and easily understood. This instrument is comprehensive and can capture the multidimensional aspects of a range of symptoms. Consequently, the POS-S Renal can be recommended for clinical and research purposes in Spanish speaking populations.
Acknowledgements
We would like to thank the Cudeca Foundation and the Nephrology Department at the General University Hospital of Carlos Haya of Málaga for their contribution to the development of this project.
Funding
No funding was obtained for this study.
Availability of data and materials
The datasets generated during the current study are available in the Repositorio Institucional de la Universidad de Málaga (RIUMA) repository, (available from: http://riuma.uma.es/xmlui). All data analysed during this study are included in this published article and in supplementary information files.
Authors’ contributions
DGS participated in the conception and design of the study, and in the data collection, analysis and interpretation of the data, and drafted the manuscript. JPL and RSH participated in the conception of the study and its critical revision, and drafted the manuscript. DHM participated in the data collection and critical revision of the manuscript. AIC participated in the conception and design of the study and the analysis and interpretation of the data, drafted the manuscript and critical revision of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Consent for publication is not applicable in this study.
Ethics approval and consent to participate
The Provincial Ethics Committee of Málaga approved this study.
Abbreviations
- ACKD
Advanced chronic kidney disease
- ANOVA
Analysis of variance
- CFA
Confirmatory factor analysis
- CFI
Comparative fit index
- CKD
Chronic kidney disease
- CVI
Content validity index
- DSI
Dialysis Symptom Index
- EFA
Exploratory factor analysis
- ESAS
Edmonton Symptom Assessment System
- GDI
Global Distress Index
- HRQoL
Health-related quality of life
- ICC2.1
Intraclass correlation coefficient type 2.1
- KDQOL-SF
Kidney Dialysis Quality of Life-Short Form
- KS
Kolmogorov-Smirnov
- MLE
Maximum likelihood extraction
- MSAS-SF
Memorial Symptom Assessment Scale-Short Form
- PHYS
Physical Symptom Distress Score
- POS-S Renal
Palliative care Outcome Scale-Symptoms Renal
- PRO
Patient reported outcome
- PSYCH
Psychological Symptom Distress Score
- RMSEA
Root mean square error of approximation
- TNS
Total number of symptoms
- χ2
Chi-square
Additional files
The questionnaire. It contains the Spanish modified version of the POS-S Renal that has been validated by the authors in this study. (DOCX 19 kb)
Pilot study. It contains the results of the pilot study. (DOCX 46 kb)
Contributor Information
Daniel Gutiérrez-Sánchez, Email: danieltunie@hotmail.com.
Juan P. Leiva-Santos, Email: jpleiva@icare.es
Rosa Sánchez-Hernández, Email: rsanchezher@gmail.com.
Domingo Hernández-Marrero, Email: domingohernandez@gmail.com.
Antonio I. Cuesta-Vargas, Email: acuesta@uma.es
References
- 1.K/DOQI Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl1):1–266. [PubMed] [Google Scholar]
- 2.KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. KidneyInt (Suppl) 2013;3(1):1–308. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 3.Iyasere O, Brown EA. Determinants of quality of life in advanced kidney disease: time to screen? Postgrad Med J. 2014;90(1064):340–7. doi: 10.1136/postgradmedj-2013-132251. [DOI] [PubMed] [Google Scholar]
- 4.Murtagh FEM, Addington-Hall J, Higginson IJ. The prevalence of symptoms in end-stage renal disease: a systematic review. ACKD. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor NR, Kumar P. Conservative management of end-stage renal disease without dialysis: a systematic review. J Palliat Med. 2012;15(2):228–35. doi: 10.1089/jpm.2011.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan D. Death and the research imperative. N Engl J Med. 2000;342:654–6. doi: 10.1056/NEJM200003023420910. [DOI] [PubMed] [Google Scholar]
- 7.Garratt A. Patient-reported outcome measures in trials, Editorial. BMJ. 2009;338:2597. doi: 10.1136/bmj.a2597. [DOI] [PubMed] [Google Scholar]
- 8.Perrone RD, Coons SJ, Cavanaugh K, Finkelstein F, Meyer KB. Patient-reported outcomes in clinical trials of CKD-related therapies: report of a symposium sponsored by the National Kidney Foundation and the US Food and Drug Administration. In: American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation [Internet]. 2013 [cited 2015 Oct 20].pp. 1046–1057. Available from: https://www.ncbi.nlm.nih.gov/pubmed/23988757 [DOI] [PubMed]
- 9.Brown MA, Crail SM, Masterson R, Foote C, Robins J, Brown MA, Crail SM, Masterson R, Foote C, Robins J, Katz I, et al. ANZSN Renal Supportive Care Guidelines 2013. Nephrology(Carlton, Vic) 2013;18(6):401–54. doi: 10.1111/nep.12065. [DOI] [PubMed] [Google Scholar]
- 10.Chang VT, Hwang SS, Feuerman M, KasimisBS THT. The memorial symptom assessment scale short form (MSAS-SF) Cancer. 2000;89:1162–71. doi: 10.1002/1097-0142(20000901)89:5<1162::AID-CNCR26>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Davison SN, Jhangri GS, Johnson JA. Cross-sectional validity of a modified Edmonton symptom assessment system in dialysis patients: a simple assessment of symptom burden. Kidney Int. 2006;69(9):1621–5. doi: 10.1038/sj.ki.5000184. [DOI] [PubMed] [Google Scholar]
- 12.Weisbord SD, Fried LF, Arnold RM, Rotondi AJ, Fine MJ, Levenson DJ, et al. Development of a symptom assessment instrument for chronic hemodialysis patients: the Dialysis Symptom Index. J Pain Symptom Manage. 2004;27(3):226–40. doi: 10.1016/j.jpainsymman.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Murphy EL, Murtagh FEM, Carey I, Sheerin NS. Understanding symptoms in patients with advanced chronic kidney disease managed without dialysis: use of a short patient-completed assessment tool. Nephron Clin Pract. 2009;111(1):74–80. doi: 10.1159/000183177. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez DG, Leiva-Santos JP, Sánchez-Hernández R, García RG. Prevalencia y evaluación de síntomas en enfermedad renal crónica avanzada [Internet]. Enfermería Nefrológica. 2015. http://www.redalyc.org/articulo.oa?id=359841633010. Accessed 2 Nov 2015.
- 15.Brennan F, Collett G, Josland EA, Brown MA. The symptoms of patients with CKD stage 5 managed without dialysis. Progress in Palliative Care [Internet].2014 Dec 8 http://www.tandfonline.com/doi/abs/10.1179/1743291X14Y.0000000118. Accessed 21 May 2015.
- 16.Guía para el tratamiento conservador en pacientes con Enfermedad Renal Crónica Avanzada, Govern de les Illes Balears, 2015. Disponible en: http://www.caib.es/sacmicrofront/archivopub.do?ctrl=MCRST3145ZI190069&id=190069. Accessed 30 Apr 2014.
- 17.Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, et al. Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88(3):447–59. doi: 10.1038/ki.2015.110. [DOI] [PubMed] [Google Scholar]
- 18.Weisbord SD, Carmody SS, Bruns FJ, Rotondi AJ, Cohen LM, Zeidel ML, et al. Symptom burden, quality of life, advance care planning and the potential value of palliative care in severely ill haemodialysis patients. Nephrol Dial Transplant. 2003;18(7):1345–52. doi: 10.1093/ndt/gfg105. [DOI] [PubMed] [Google Scholar]
- 19.Murtagh FE, Addington-Hall J, Edmonds P, Donohoe P, Carey I, Jenkins K, et al. Symptoms in the month beforedeath for stage 5 chronic kidney disease patients managed without dialysis. JPSM. 2010;40(3):342–52. doi: 10.1016/j.jpainsymman.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Murtagh FEM, Addington-Hall JM, Edmonds PM, Donohoe P, Carey I, Jenkins K, et al. Symptoms in advanced renal disease: a cross-sectionalsurvey of symptom prevalence in stage 5 chronic kidney disease managed without dialysis. J PalliatMed. 2007;10(6):1266–76. doi: 10.1089/jpm.2007.0017. [DOI] [PubMed] [Google Scholar]
- 21.Muñiz J, Elosua P, Hambleton RK. International Test Commission Guidelines for test translation and adaptation: 2nd ed. Psicothema. 2013;25:151–7. doi: 10.7334/psicothema2013.24. [DOI] [PubMed] [Google Scholar]
- 22.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res. 2010;19:539–49. doi: 10.1007/s11136-010-9606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawshe CH. A quantitative approach to content validity. Pers Psychol. 1975;28(4):563–75. doi: 10.1111/j.1744-6570.1975.tb01393.x. [DOI] [Google Scholar]
- 24.Davis L. Instrument review: getting the most from your panel of experts. Appl Nurs Res. 1992;5:194–7. doi: 10.1016/S0897-1897(05)80008-4. [DOI] [Google Scholar]
- 25.Portenoy RK, Thaler HT, Kornblith AB, McCarthy Lepore J, Friedlander-Klar H, Kiyasu E, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. EJC. 1994;30(9):1326–36. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 26.Dapueto JJ, del Abreu M C, Francolino C, Levin R. Psychometric assessment of the MSAS-SF and the FACIT-Fatigue Scale in Spanish-speaking patients with cancer in Uruguay. JPSM. 2014;47(5):936–45. doi: 10.1016/j.jpainsymman.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 27.Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334. doi: 10.1007/BF02310555. [DOI] [Google Scholar]
- 28.Portney LG, Watkins MP. Foundations of clinical research: applications to practice. Upper Saddle River: Prentice Hall Health; 2009. [Google Scholar]
- 29.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 30.Jöreskog KG. Sörbom D.LISREL 8.80. Chicago: Scientific Software International; 2007. [Google Scholar]
- 31.Costello AB, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval. 2005;10(7):1–9. [Google Scholar]
- 32.Terwee CB, Bot SD, de Boer MR, van der Windt DA, Knol DL, Dekker J, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Polit DF, Beck CT. Nursing research: principles and methods. 7. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 34.Alvarez-Ude F, Fernández-Reyes MJ, Vázquez A, Mon C, Sánchez R, Rebollo P. Physical symptoms and emotional disorders in patient on a periodic hemodialysis programme. Nefrologia. 2001;21(2):191–9. [PubMed] [Google Scholar]
- 35.Rocco MV. BurkartJM: Prevalence of missed treatments and early sign-offs in hemodialysis patients. J Am SocNephrol. 1993;4:1178–83. doi: 10.1681/ASN.V451178. [DOI] [PubMed] [Google Scholar]
- 36.Almutary H, Bonner A, Douglas C. Arabic translation, adaptation and modification of the Dialysis Symptom Index for chronic kidney disease stages four and five. BMC Nephrol. 2015;16:36. doi: 10.1186/s12882-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fayers PM, Hand DJ. Causal variables, indicator variables and measurement scales: an example from quality of life. J R Stat Soc. 2002;165:233–61. doi: 10.1111/1467-985X.02020. [DOI] [Google Scholar]
- 38.Siegert RJ, Gao W, Walkey FH, Higginson IJ. Psychological well-being and quality of care: a factor-analytic examination of the palliative care outcome scale. J Pain Symptom Manage. 2010;40(1):67–74. doi: 10.1016/j.jpainsymman.2009.11.326. [DOI] [PubMed] [Google Scholar]
- 39.Jablonski A. The multidimensional characteristics of symptoms reported bypatients on hemodialysis. Nephrol Nurs J. 2007;34(1):29–38. [PubMed] [Google Scholar]
- 40.Mc D, Newell C. Measuring health: a guide to rating scales and questionnaires. 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are available in the Repositorio Institucional de la Universidad de Málaga (RIUMA) repository, (available from: http://riuma.uma.es/xmlui). All data analysed during this study are included in this published article and in supplementary information files.


