Abstract
Community water fluoridation is considered a significant public health achievement of the 20th century. In this paper, the hypothesis that added water fluoridation has contributed to diabetes incidence and prevalence in the United States was investigated. Panel data from publicly available sources were used with population-averaged models to test the associations of added and natural fluoride on the outcomes at the county level in 22 states for the years 2005 and 2010. The findings suggest that a 1 mg increase in the county mean added fluoride significantly positively predicts a 0.23 per 1,000 person increase in age-adjusted diabetes incidence (P < 0.001), and a 0.17% increase in age-adjusted diabetes prevalence percent (P < 0.001), while natural fluoride concentration is significantly protective. For counties using fluorosilicic acid as the chemical additive, both outcomes were lower: by 0.45 per 1,000 persons (P < 0.001) and 0.33% (P < 0.001), respectively. These findings are adjusted for county-level and time-varying changes in per capita tap water consumption, poverty, year, population density, age-adjusted obesity and physical inactivity, and mean number of years since water fluoridation started. Sensitivity analyses revealed robust effects for both types of fluoride. Community water fluoridation is associated with epidemiological outcomes for diabetes.
Keywords: diabetes, fluoride, incidence, prevalence, United States
Introduction
Water fluoridation has reportedly produced great benefit to society, often by reducing dental caries and hence the cost that comes with untreated or advanced periodontal disease (Griffin et al. 2001a, 2001b; Jones et al. 2005), which may itself often be a trigger of other chronic conditions (Cullinan & Seymour 2013). The side effects of water fluoridation have generally appeared to be either inconclusive or minimal (Leone et al. 1954; Morgan et al. 1998; McDonagh et al. 2000; Broadbent et al. 2015), with some notable exceptions for hypothyroidism prevalence (Pearce 2015; Peckham et al. 2015). Taken together, these observations suggest that water fluoridation has indeed been one of the great public health accomplishments of the twentieth century (Centers for Disease Control and Prevention (CDC) 1999).
Research conducted before and after the widespread implementation of water fluoridation in the 1940s in the United States has suggested that fluoride is a potent preservative of blood glucose (Roe et al. 1927; Chan et al. 1989), thereby inhibiting glycolysis (Halpern 1936). Specifically, such glycolytic inhibition from fluoride is thought to mitigate oral bacteria enolase activity (Hüther et al. 1990). Enolase is an enzyme acting late in the glycolytic pathway (Pancholi 2001). It is this mechanism which is considered to prevent dental caries, although there has been no general agreement that the anti-microbial effects of fluoride contribute to the anti-caries effect of the chemical (Hamilton 1990).
However, one issue that remains is the distinction between fluoride's uses as (1) a topical agent in preventing dental caries (as described above) versus (2) its utility as an ingested additive. The topical effect of fluoride, demonstrated by showing caries reduction, was the result of fluoride acting on the external surface of the teeth, not through ingestion of fluoride itself (Bibby et al. 1955). Given the known glycolytic inhibition of sodium fluoride in bacteria, it is plausible that a similar phenomenon could occur in humans. Sodium fluoride that is ingested produced significant decrements in plasma insulin (Rigalli et al. 1990), which is known to regulate glycolysis (Wu et al. 2005). Furthermore, hypothyroidism, whose deleterious association with water fluoridation was documented above, may be the body's attempt to ameliorate the effects of a prolonged hyperglycemic state or uncontrolled diabetes (Mouradian & Abourizk 1983; Celani et al. 1993): type 2 diabetes mellitus patients are more likely to have subclinical hypothyroidism (Han et al. 2015).
Over the past 32 years, from 1980 to 2012, the number of adults with diagnosed diabetes in the United States nearly quadrupled, from 5.5 million to 21.3 million (CDC 2014). Among adults, about 1.7 million new cases of diabetes are diagnosed each year. If this trend continues, as many as one out of every three adults in the United States could have diabetes by 2050. Moreover, previous research has indicated as many as 30–40% of diabetics went undiagnosed in the United States in recent decades (Gregg et al. 2004; Cowie et al. 2009). Persistently elevated blood sugar represents one of the most costly and potentially fatal complications associated with diabetes, including a hyperosmolar hyperglycemic state (Kitabchi et al. 2009). Furthermore, the risk of hypoglycemia remains a serious and costly adverse effect of metabolic disease (Erdogan et al. 2011; Meher et al. 2013) and diabetic pharmacotherapy (Quilliam et al. 2011). Given the profound and growing impacts of diabetes on the American health care system (Seuring et al. 2015) and the potential for continued adverse side effects from diabetes medications such as dipeptidyl peptidase-4 inhibitors (Food and Drug Administration 2016), it is worth re-examining the potential influences of fluoride on its prevalence to ensure that all avenues are investigated and, if appropriate, exonerated from further consideration.
After all these years, the question remains unanswered: to what extent does fluoride predict changes in diabetes outcomes in the United States? The objective of the present study is to robustly examine the associations between added and naturally present fluoride and epidemiological outcomes of diabetes, including prevalence and incidence.
Methods
Data
To analyze the association between diabetes outcomes and community water fluoridation, data were collected from state fluoridation reports, available in the My Water's Fluoride portal through the Centers for Disease Control and Prevention (CDC 2015a). Although the CDC lists 25 states as having available operational reports, only 22 were accessible via their website. Hence, this investigation focuses on these 22 states, which are identified in Figure 1.
Figure 1.
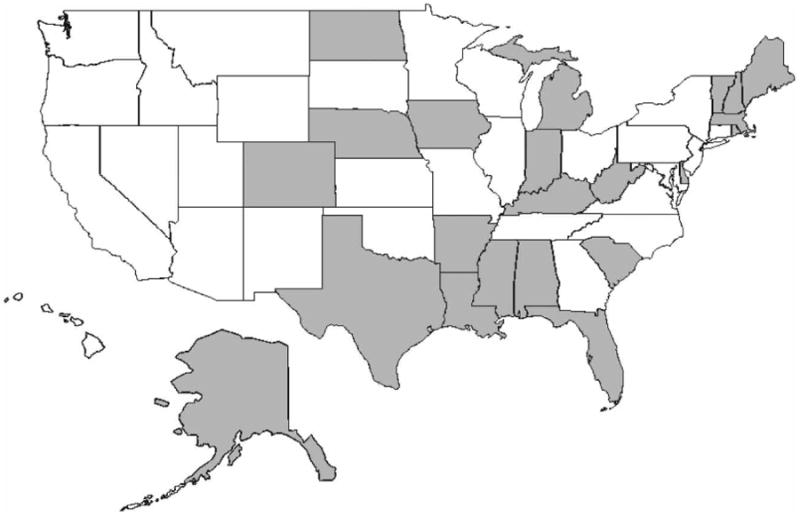
States with operational reports on water fluoridation, available by water system.
At the time of access, the state fluoridation reports contained the following variables used in the analysis: (1) water system ID, (2) primary county of the water system, (3) fluoridation chemical (sodium fluoride, fluorosilicic acid, or sodium fluorosilicate), (4) fluoridation start date, (5) natural fluoride level and (6) optimal fluoride level (both in parts per million, ppm). Optimal fluoride level was defined by a range of 0.7–1.2 mg/L (ppm = mg/L) issued in 1962 by the US Department of Health and Human Services (HHS). Every water system independently sets their own optimal level. Each water system was designated a status regarding water fluoridation; those with a classification of ‘adjusted’ meant that the level of fluoride in the tap water was manipulated using one of the above listed chemicals to achieve an optimal level. Other reported statuses included ‘non’, ‘nat’, or ‘cons’. ‘Non’ status referred to water systems with an insufficient natural fluoride level (<0.7 mg/L) but no fluoridation chemical identified to achieve optimal status; ‘nat’ status generally denoted water systems with a sufficient natural fluoride level (≥0.7 mg/L); ‘cons’ status identified water systems with missing information for both the natural fluoride concentration and chemical used.
To these list, several additional variables were generated. The five-digit Federal Information Processing Standard (FIPS) code was inserted, which uniquely identifies counties and county equivalents, to facilitate (1) the averaging of natural and optimal levels of fluoride concentrations by county and (2) the merging with other CDC data. The natural and optimal fluoride levels, defined by the water system, were averaged by county. All data available on natural fluoride concentrations (i.e. using water systems with ‘adj’, ‘non’ or ‘nat’ status) were used to produce a county average for natural fluoride. To calculate the average added levels for each county, only water systems with an adjusted status where fluoridation chemicals could be identified were used. To calculate the mean added fluoride concentration, the mean natural level was subtracted from the mean optimal level for the county. Similarly, the criterion for defining the fluoridation chemical was the type of additive used by a water system in a county with an adjusted status. Thus a county could be identified as using all three chemicals if at least three different water systems in the county with an adjusted status used each chemical. A mean ‘years fluoridated’ variable was also defined, which was computed as the number of years before December 31, 2004 and December 31, 2009 that a water system was fluoridated, averaged over the county. A negative outcome here suggested that water fluoridation started after these dates. The ‘years fluoridated’ variable also only reflects the average among water systems in a county with an adjusted status.
The most recent fluoridation start date provided among all water systems in the 22 states identified was January 2011. This was interpreted as the last date of update for these data. Hence, the scope of our analyses was limited to the complete calendar year 2010 and the years prior.
To these fluoride data, other covariates were added that reflected our outcomes and other relevant predictors. These data came from the County Data Indicators profile of the Diabetes Data and Statistics portal through the CDC (CDC 2015b). In particular, county-level data for the years 2005 and 2010 were collected for the following indicators: (1) diabetes incidence and prevalence (the outcomes), (2) obesity prevalence and (3) leisure-time physical inactivity prevalence. The estimates for diagnosed diabetes, obesity and physical inactivity were derived using data from the Census and Behavioral Risk Factor Surveillance System, an ongoing, state-based, random-digit-dialed telephone survey of the US civilian, non-institutionalized population aged 18 years and older. The physical inactivity rates reflected adults who reported no physical activity or exercise other than at their regular job. County-level and year-specific poverty (number of people in poverty) and population per square mile statistics from the Area Health and Resource File for the years 2005 and 2010 (AHRF 2014) were also sourced. Poverty was normalized as a percent (number of persons in poverty divided by the total population for a specific year). The population density variable was logged and its squared term was also included as a covariate.
To compute the primary exposures to fluoride, water consumption data from the US Geological Survey (USGS) (USGS 2015) were collected. The USGS gave two variables of interest: ‘Domestic, deliveries from Public Supply, in Mgal/d’ (labeled DO-PSDel) and ‘Public Supply, total population served, in thousands’ (labeled PS-TOPop). These data were available for the years 2005 and 2010 for all counties. For each FIPS code, a ratio was created, dividing domestic deliveries per county in millions of liters per day by total people supplied to get a per capita water delivery. The USGS estimates that an individual uses 302.8–378.5 L of water a day. It was estimated that each individual drinks 1.9 L of water a day. Dividing 1.9 L by 302.8 (≈0.625%) and 378.5 (=0.5%) liters yields an approximate range of the proportion of the per capita supply that is actually ingested. Multiplying per capita water consumption in liters by 0.625% provides the upper limit (UL) on the water supply that is ingested, whereas multiplying by 0.5% gives the lower limit (LL). The resulting products are estimates in liters of water. Converting these per capita outcomes for water intake to liters and multiplying by the (added or natural) fluoride exposure in milligrams per liter averaged by county gives the unit of measurement for the fluoride covariates in terms of milligrams. For comparison, exposure where the fluoride concentration is unadjusted for per capita tap water consumption was also considered. Thus, there were two sets of regressions for the diabetes outcomes: the aforementioned analysis with the primary exposure in milligrams (mg) and an alternative, unadjusted analysis with the primary exposure in ppm.
Regression analyses
In accordance with the research inquiries, two sets of regressions were composed using generalized estimating equations (GEE) population-averaged models using autoregressive (AR1) correlation structures, common to quantitative longitudinal data (Liang & Zeger 1986; Zeger & Liang 1986; Diggle et al. 1994). A GEE is used to estimate the parameters of a generalized linear model with a possible unknown correlation between outcomes of interest. The unit of analysis is the county. The regression sets focus separately on the analysis of naturally occurring and added fluoride and are specified as the following:
where refers to the outcomes (i.e. incidence rate [E = 1] and prevalence percent [E = 2] of diabetes) for S fluoride exposure in M units in county j of state k at year t (t = 2005 or 2010), gives the fluoride concentration for county j of state k, where S = 1 references natural fluoride level, S = 2 describes the added fluoride concentration, M = 1 denotes the exposure assessed in milligrams (adjusted by county-level per capita tap water consumption) and M = 2 indicates the exposure in ppm (unadjusted for county-level tap water consumption); CHEMi,jk refers to one of three chemicals, i = 1, 2, 3, used to adjust the fluoride concentration (i.e. sodium fluoride, fluorosilicic acid, or sodium fluorosilicate), Xp,jkt refers to the regressor set of seven covariates that might modify the primary relationship of interest, all of which vary by time: (1) the age-adjusted proportion of individuals reporting physical inactivity, (2) the age-adjusted proportion of obese individuals, (3) the average number of years prior to December 31, 2004 (for the 2005 outcome) or December 31, 2009 (for the 2010 outcome) in which county j began fluoridating its water supply, (4) the proportion of individuals in poverty, (5) the logged population per square mile value, (6) a year variable and (7) the logged population per square mile value squared.
Eight (=23 given E = 1, 2; M = 1, 2; S = 1, 2) separate models were analyzed. For each unit model (M = 1, 2), there were eleven estimated parameters in the S = 2 model and seven estimated parameters in the S = 1 model (three fluoridation chemicals and years of fluoridation are excluded in the natural fluoride models). Sensitivity analyses were conducted by combining the natural and added fluoride concentrations in one model for each E, M combination.
All analyses were completed with Stata 11.1 (StataCorp 2009). The significance of variables was indicated with P < 0.05, but also reported for lower significance thresholds (P < 0.01, P < 0.001) where appropriate. No ethics approval was required for the study, given its exclusive reliance on publicly accessible data.
Results
Figure 2 describes the changes in diagnosed diabetes from 2005 to 2010 at the county level. The areas identified in the dark color in the figure isolate counties and states with a particularly heavy burden of diabetes: the South and Appalachia. Cross-matched with the states highlighted in Figure 1 this suggests that investigation of the impact of water fluoridation on diabetes outcomes using these data is sufficient to determine the association.
Figure 2.
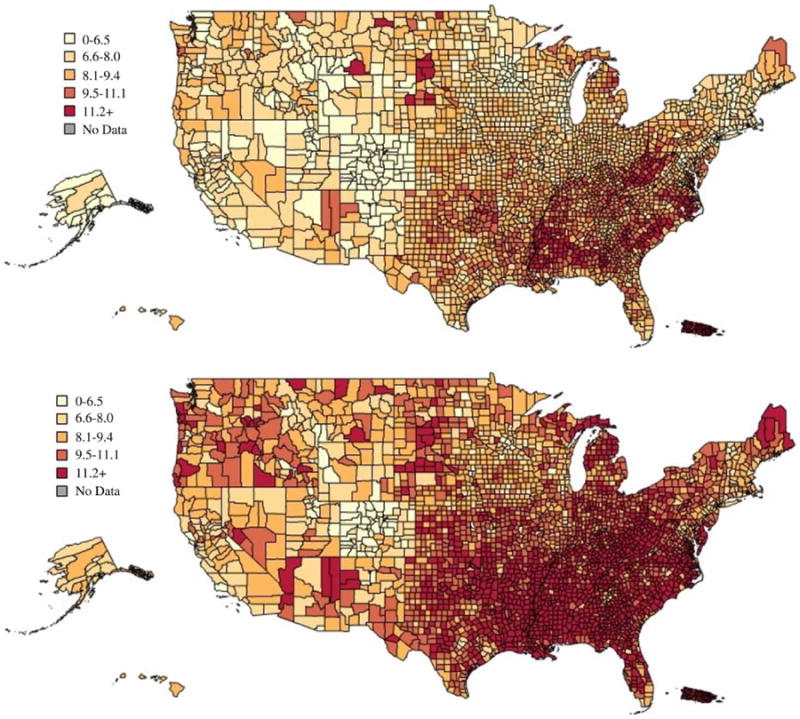
Diagnosed diabetes percentage in 2005 (top) and 2010 (bottom), by county (CDC's Division of Diabetes Translation). The full color version of this figure is available in the online version of this paper, at http://dx.doi.org/10.2166/wh.2016.012.
Figure 3 graphically shows the number and percentage of the US population with diagnosed diabetes from 1958 through 2013 (CDC 2014). An ordinary least squares regression check revealed a significant non-linear relationship between the year and diabetes outcomes: the subset of data from 1993 onwards demonstrated no linearity violation, whereas the period up to 1993 showed significant violations. Thus, in the last two decades, the growth in diabetes has been on a mostly linear trajectory, which has been confirmed in a recent study on comorbidities including diabetes among older US adults (Fluegge 2016), making exhaustive inquiries into the potential causes especially relevant.
Figure 3.
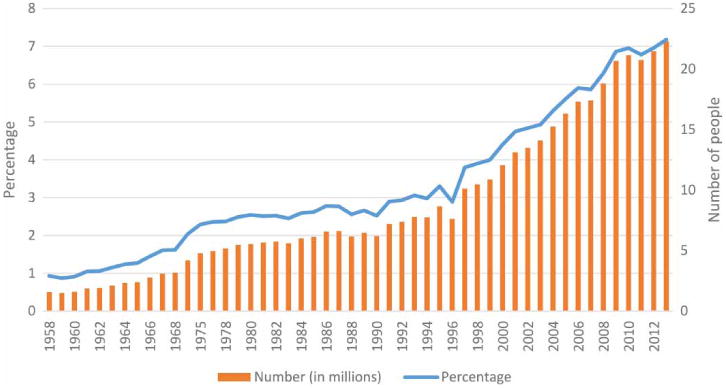
Number and percentage of US population with diagnosed diabetes 1958–2013.
Figure 4 displays the histograms of the outcome variables: age-adjusted incidence (top panel) and age-adjusted prevalence of diabetes (bottom panel) by year. The mean incidence rate declined from 2005 to 2010: mean in 2005 was 10.65 per 1,000 individuals (SD = 2.52) and mean in 2010 was 10.45 per 1,000 individuals (SD = 2.73). A two-sample paired t-test with equal variances revealed an insignificant decline in the incidence (P = 0.10). The mean prevalence percent significantly increased from 2005 to 2010: the mean in 2005 was 8.2% (SD = 1.74) and the mean in 2010 was 9.8% (SD = 2.22). A two-sample paired t-test with equal variances demonstrated a highly significant increase in the prevalence of diabetes (P < 0.001).
Figure 4.
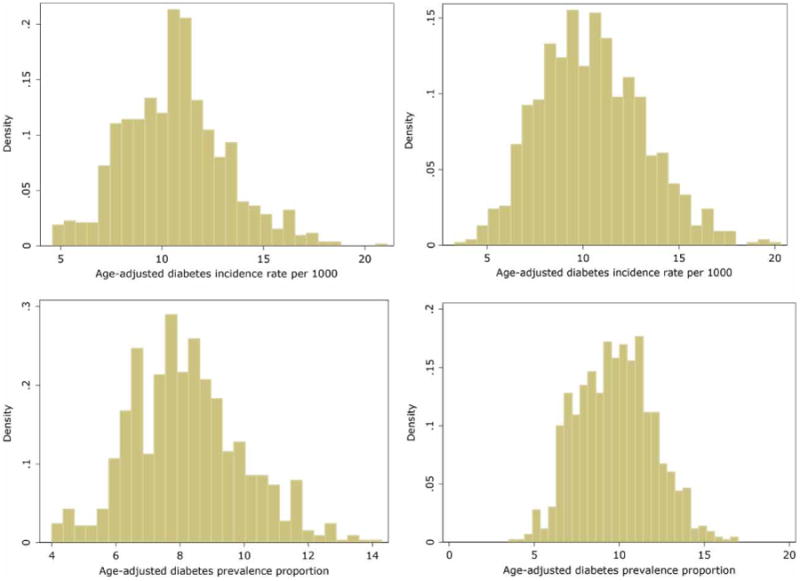
Histograms of outcome variables: age-adjusted incidence (top panel) and age-adjusted prevalence of diabetes (bottom panel), by year (2005 on left; 2010 on right).
Figure 5 includes histograms of the adjusted fluoride exposure variables: added (top panel) and natural fluoride (bottom panel) in milligrams. Recall the added fluoride covariate was calculated as the difference in mean values between the county-defined optimal and natural levels. Thus, it is possible to have negative values if there exists a very high natural level. There were 64 county-year observations with negative mean differences for added fluoride concentration. The LL mean exposure level for added fluoride declined from 2005 to 2010: the mean in 2005 was 1.22 mg (SD = 0.75) and the mean in 2010 was 1.08 mg (SD = 0.51). A two-sample paired t-test with equal variances indicated a significant decline in the exposure level (P < 0.001). The LL mean exposure level for natural fluoride also declined from 2005 to 2010: the mean in 2005 was 0.46 mg (SD = 0.61) and the mean in 2010 was 0.38 (SD = 0.49). A Wilcoxon signed-rank test also produced a significant decline in the exposure level (P < 0.001). Importantly, both of these decreases were attributed to the decline in per capita tap water consumption (reference Table 2).
Figure 5.
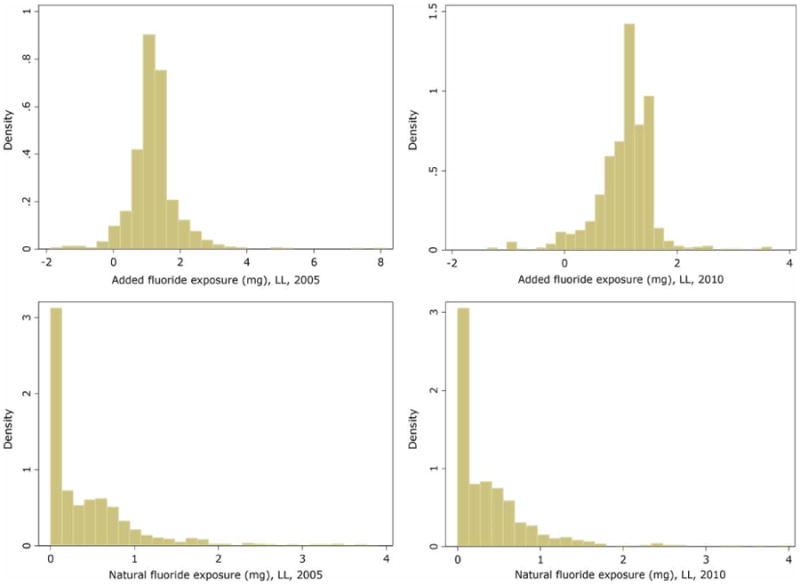
Histograms of primary exposure variables: added fluoride (top panel) and natural fluoride (right) concentrations, by year 2005 (left) and 2010 (right).
Table 2. Descriptive statistics.
| Variable | N | 2005 Mean±SD | 2010 Mean±SD | P-value |
|---|---|---|---|---|
| Population per square mi (log)a | 924 | 4.04 ± 1.42 | 4.06 ± 1.44 | < 0.001 |
| Added fluoride (in ppm) | 925 | 0.71 ± 0.31 | ||
| Natural fluoride (in ppm) | 925 | 0.23 ± 0.27 | ||
| Fluoridation chemical (yes/no) | ||||
| Sodium fluoride | 924 | 0.33 ± 0.47 | 0.33 ± 0.47 | – |
| Fluorosilicic acid | 924 | 0.74 ± 0.44 | 0.74 ± 0.44 | – |
| Sodium fluorosilicate | 924 | 0.14 ± 0.35 | 0.14 ± 0.35 | – |
| Years water system fluoridated | 890 | 28.13 ± 12.95 | 33.13 ± 12.95 | < 0.001 |
| Per capita tap water consumption (LL), in liters | 925 | 1.79 ± 0.86 | 1.56 ± 0.41 | < 0.001 |
| Per capita tap water consumption (UL), in liters | 925 | 2.24 ± 1.08 | 1.94 ± 0.52 | < 0.001 |
| Poverty percent | 924 | 15.16 ± 6.46 | 16.68 ± 6.15 | < 0.001 |
| Age-adjusted obesity prevalence | 924 | 27.01 ± 3.77 | 31.55 ± 4.52 | < 0.001 |
| Age-adjusted physical inactivity prevalence | 924 | 26.83 ± 5.50 | 28.24 ± 5.33 | < 0.001 |
Note: N represents the number of counties.
Two counties in Alaska were missing population per square mile, obesity prevalence, physical inactivity prevalence and poverty (one from 2005 and one from 2010). Hence the total sample size for these variables in each year is 924 instead of the total possible of 925.
Table 1 presents the counties by fluoridation status. Recall the average added fluoride level is generated from water systems in counties with an adjusted status. Average natural fluoride is based on water systems from ‘adj’, ‘non’ or ‘nat’ status.
Table 1. Aggregate fluoridation status reported by the CDC.
| Status | Number of counties | Number of water systems | County average added fluoride (ppm) | County average natural fluoride (ppm) |
|---|---|---|---|---|
| Adj | 925 | 2183 | 0.82 | 0.13 |
| Non | 836 | 8274 | 0.75 | 0.17† |
| Nat | 574 | 3054 | NA | 1.3 |
| Cons | 760 | 3233 | NA | NA |
NA=not available, CDC=Centers for Disease Control and Prevention, ppm=parts per million.
Natural fluoride level is based on 11007 water systems across 1004 counties.
For the ‘non’ status, 2733 water systems did not have data on optimal fluoridation level. The natural fluoride level is based on 11007 water systems in 1562 counties. Average added fluoride for 836 counties is based on data from 8274 water systems in those counties. No data on fluoridation chemical are available for water systems with ‘non’ status.
For the ‘nat’ status water systems, the natural fluoride level is based on 3054 water systems across 574 counties. Optimal levels were reported for 2329 water systems in 484 counties, but no data on fluoridation chemicals were available for water systems with ‘nat’ status. Hence an average added concentration was not calculated. Note, however, that 521 water systems spanning 187 counties with natural fluoride levels under the optimal level indicated were reported under the ‘nat’ status.
For the ‘cons’ status, no data on natural fluoride or fluoridation chemical were available. Only optimal levels were reported. 658 water systems in 116 counties did not have data on optimal fluoridation level.
Table 2 presents the descriptive statistics for the 925 counties in 2005 and 2010 (1,850 total observations). To account for non-normality, Wilcoxon signed-rank tests were used to describe changes in the covariates from the year 2005 to 2010. All variables changed significantly (P < 0.001) in the 5-year lapse between observations. The fluoridation chemical covariates were not time-varying. There were 155 counties where only sodium fluoride was used, 536 where only fluorosilicic acid was added and 54 where sodium fluorosilicate was the only additive. A minority (19%) of counties used more than one additive: 102 where sodium fluoride and fluorosilicic acid were used, 28 where sodium fluoride and sodium fluorosilicate were used, 33 where fluorosilicic acid and sodium fluorosilicate were used and 16 where all three additives were resourced. As a consequence of this minority of multiple additives, all three fluoridation chemicals were retained as binary variables in the regressions. Finally, per capita water consumption (UL/LL) decreased, whereas poverty, obesity and physical inactivity increased significantly (P < 0.001).
Table 3 presents the M = 1 GEE estimates for both diabetes outcomes. The covariates of special interest are the fluoride exposures, using the LL of per capita water consumption. In the incidence models (E = 1), adjusting for changes in physical inactivity, obesity, poverty, log population per square mile, mean number of years fluoridated and year, a 1 mg increase in the amount of added fluoride for an average county significantly increased the diabetes incidence by 0.23 per 1,000 (P < 0.001) as compared to a county without such an increase, whereas for natural fluoride the estimate was of equal magnitude and significance, but negative.
Table 3. GEE regression sets, M = 1.
| Outcomes: Diabetes incidence and prevalence, M = 1 (adjusted exposure in mg) | ||||
|---|---|---|---|---|
|
| ||||
| Covariates | Incidence E=1 | Prevalence E=2 | Incidence E=1 | Prevalence E=2 |
| Added fluoride (in mg) | 0.23 (0.06)*** | 0.17 (0.05)*** | – | – |
| Natural fluoride (in mg) | – | – | –0.23 (0.06)*** | −0.15 (0.04)*** |
| AA physical inactivity | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.05 (0.01)*** | 0.06 (0.01)*** |
| AA obesity | 0.31 (0.01)*** | 0.22 (0.01)*** | 0.31 (0.01)*** | 0.22 (0.01)*** |
| Poverty percent | 0.13 (0.01)*** | 0.10 (0.01)*** | 0.14 (0.01)*** | 0.10 (0.01)*** |
| Fluoridation chemical (yes/no) | ||||
| Sodium fluoride | 0.09 (0.09) | 0.11 (0.07) | – | – |
| Fluorosilicic acid | − 0.45 (0.11)*** | − 0.33 (0.08)*** | – | – |
| Sodium fluorosilicate | − 0.13 (0.11) | − 0.17 (0.08)* | – | – |
| Years water system fluoridated | − 0.005 (0.003) | − 0.003 (0.002) | – | – |
| Population per square mile (log) | 0.93 (0.08)*** | 0.64 (0.06)*** | 0.87 (0.09)*** | 0.58 (0.06)*** |
| Year = 2010 | − 1.85 (0.07)*** | 0.33 (0.05)*** | − 1.91 (0.07)*** | 0.28 (0.05)*** |
| Number of counties | 887 | 887 | 923 | 923 |
| Number of observations | 1,774 | 1,774 | 1,846 | 1,846 |
Robust standard errors in parentheses.
P < 0.001,
P < 0.05.
The effect of added fluoride on incidence was slightly greater than half the effect of age-adjusted obesity prevalence (β = 0.31, P < 0.001), which suggests that a 1% increase in the obesity prevalence for an average county drives incidence up by 0.31 per 1,000 persons as compared to a county not experiencing a comparable increase in obesity prevalence. The coefficient for year indicates that the incidence of diabetes declined in the selected states from 2005 to 2010 (β = –1.85, P < 0.001). Finally, the type of fluoridation chemical used may also make a significant difference in diabetes incidence. Among the three used in this data set, fluorosilicic acid was most significantly associated with reduced diabetes incidence (β = –0.45, P < 0.001), inducing the greatest decline in incidence apart from the year variable.
The results for the E = 2 models with age-adjusted diabetes prevalence percent as the outcome largely mirrored the incidence models, with a notable exception for the year variable, which now produced a positive effect (β = 0.33, P < 0.001). Importantly, the results for the added fluoride concentration (β = 0.17, P < 0.01) and fluorosilicic acid (β = –0.33, P < 0.001) were mostly unchanged.
Fluoridation chemical is included as a confounder in the S = 2 models, but it could be an effect modifier rather than a confounder. The county-level correlation between the number of chemicals used and the mean added fluoride (in ppm) was 0.20 (P < 0.01). Furthermore, the parameter estimates in both the E = 1 and E = 2 models for added fluoride (in mg) increased by 15–20% when the fluoridation chemical variables were excluded from the models. Thus in these analyses, it was necessary to model fluoridation chemicals as potential confounders.
To further refine our understanding of the associations between fluoridation chemicals and diabetes outcomes, simple bivariate models were used to analyze each fluoridation chemical's association with the diabetes outcomes using the GEE structure. Sodium fluoride produced significantly positive associations with incidence (β = 0.93, P < 0.001) and prevalence (β = 0.76, P < 0.001), whereas fluorosilicic acid and sodium fluorosilicate produced significantly negative associations respectively (fluorosilicic acid: β = –0.72, P < 0.001 and β = −0.54, P = 0.002; sodium fluorosilicate: β = −0.55, P = 0.05 and β = –0.49, P = 0.02). Thus the comparisons are all relative. The protective effects of fluorosilicic acid and/or sodium fluorosilicate in the multivariable GEE models are, alternatively stated, a deleterious consequence of sodium fluoride use.
Table 4 presents the M = 2 GEE estimates for both regression sets, using ppm as the exposure measure. For both added fluoride and natural fluoride, the estimates aligned with the set of M = 1 results, only greater in magnitude. For added fluoride, a 1 ppm increase produced a 0.35 per 1,000 increase in diabetes incidence (P < 0.001) and a 0.27% increase in prevalence (P < 0.001), whereas the respective estimates for natural fluoride were 0.73 per 1,000 decline (P < 0.001) and a 0.55% decline (P < 0.001).
Table 4. GEE regression sets, M = 2.
| Outcomes: Diabetes incidence and prevalence, M=2 (unadjusted exposure in ppm) | ||||
|---|---|---|---|---|
|
| ||||
| Covariates | Incidence E=1 | Prevalence E=2 | Incidence E=1 | Prevalence E = 2 |
| Added fluoride (in ppm) | 0.35 (0.11)** | 0.27 (0.09)** | – | – |
| Natural fluoride (in ppm) | – | – | −0.73 (0.12)*** | −0.55 (0.09)*** |
| AA physical inactivity | 0.06 (0.01)*** | 0.06 (0.01)*** | 0.05 (0.01)*** | 0.06 (0.01)*** |
| AA obesity | 0.31 (0.01)*** | 0.22 (0.01)*** | 0.31 (0.01)*** | 0.23 (0.01)*** |
| Poverty percent | 0.13 (0.01)*** | 0.10 (0.01)*** | 0.13 (0.01)*** | 0.10 (0.01)*** |
| Fluoridation chemical (yes/no) | ||||
| Sodium fluoride | 0.09 (0.09) | 0.11 (0.07) | – | – |
| Fluorosilicic acid | −0.47 (0.12)*** | −0.36 (0.08)*** | – | – |
| Sodium fluorosilicate | −0.11 (0.11) | −0.15 (0.08) | – | – |
| Years water system fluoridated | −0.006 (0.003)* | −0.004 (0.002)* | – | – |
| Population per square mile (log) | 0.94 (0.08)*** | 0.64 (0.06)*** | 0.86 (0.08)*** | 0.58 (0.06)*** |
| Year=2010 | −1.88 (0.07)*** | 0.31 (0.05)*** | −1.89 (0.07)*** | 0.30 (0.05)*** |
| Number of counties | 887 | 887 | 923 | 923 |
| Number of observations | 1,774 | 1,774 | 1,846 | 1,846 |
Robust standard errors in parentheses.
P < 0.001,
P < 0.01,
P < 0.05.
The counterintuitive findings for significance and sign of the ‘years water system fluoridated’ between the M = 1 and M = 2 models was noted. There was a low (but significant) and negative correlation (rho = −0.18, P < 0.001) between the average years a county's water supply has been fluoridated and per capita consumption of tap water in that county. It appears that the negative parameter estimates may be the result of a selection issue (the longer a water system is fluoridated, the more migration to alternative water sources may be occurring). Given the significance disappears when the per capita tap water consumption component is integrated into the models suggests this may be the case.
Table 5 displays the sensitivity results from the combined exposures. In 32 counties, the mean quantity of natural fluoride was greater than the mean optimal level. In these cases, the added fluoride concentration was negative, which might confound any protective effect of the natural fluoride concentration seen in the analyses thus far. Therefore, these 64 county-year observations were removed and the GEE analyses re-run for both outcomes in the sensitivity analyses. For the M = 1 models, the results coincided with those presented in Tables 3 and 4. That is, added fluoride exerted a positive and significant change on incidence (0.26 per 1,000 increase) and prevalence (0.22% increase), whereas natural fluoride exhibited a protective effect (0.45 per 1,000 decrease and 0.32% decline, respectively). For the M = 2 model set, however, both exposures demonstrated negative relationships, with the effect of natural fluoride (3.12 per 1,000 decline in incidence and 2.3% reduction in prevalence) being about twice that of added fluoride (1.68 per 1,000 decrease in incidence and 1.09% decline in prevalence). Thus, the sensitivity analyses showed that only the adjusted exposures (using milligrams to account for per capita tap water consumption) revealed robustly consistent associations with diabetes outcomes.
Table 5. Sensitivity analyses: GEE regression sets for combined exposures.
| Outcomes: Diabetes incidence and prevalence | ||||
|---|---|---|---|---|
|
| ||||
| M=1 (exposure in mg) | M=2 (exposure in ppm) | |||
|
|
|
|||
| Covariates | Incidence E=1 | Prevalence E=2 | Incidence E=1 | Prevalence E=2 |
| Added fluoride | 0.26 (0.08)** | 0.22 (0.06)** | −1.68 (0.54)** | −1.09 (0.37)** |
| Natural fluoride | −0.45 (0.15)** | −0.32 (0.06)** | −3.12 (0.59)*** | −2.30 (0.42)*** |
| AA physical inactivity | 0.04 (0.01)*** | 0.06 (0.01)*** | 0.03 (0.01)* | 0.05 (0.01)*** |
| AA obesity | 0.32 (0.02)*** | 0.23 (0.01)*** | 0.33 (0.02)*** | 0.23 (0.01)*** |
| Poverty percent | 0.13 (0.01)*** | 0.10 (0.01)*** | 0.12 (0.01)*** | 0.09 (0.01)*** |
| Fluoridation chemical (yes/no) | ||||
| Sodium fluoride | 0.02 (0.09) | 0.06 (0.07) | 0.16 (0.10) | 0.15 (0.07)* |
| Fluorosilicic acid | −0.46 (0.12)*** | −0.35 (0.09)*** | −0.32 (0.12)** | −0.26 (0.09)*** |
| Sodium fluorosilicate | −0.002 (0.12) | −0.08 (0.09) | 0.05 (0.11) | −0.04 (0.09) |
| Years water system fluoridated | −0.007 (0.003)* | −0.004 (0.002) | −0.003 (0.003) | −0.002 (0.002) |
| Population per square mile (log) | 0.91 (0.09)*** | 0.61 (0.06)*** | 0.76 (0.09)*** | 0.51 (0.07)*** |
| Year=2010 | −1.85 (0.08)*** | 0.36 (0.06)*** | −1.88 (0.08)*** | 0.34 (0.06)*** |
| Number of counties | 759 | 759 | 759 | 759 |
| Number of observations | 1,518 | 1,518 | 1,518 | 1,518 |
Robust standard errors in parentheses.
P < 0.001,
P < 0.01,
P < 0.05.
Discussion
In this report, the relationship between added fluoride and diabetes in 22 states using population-averaged models was examined, which revealed the following main findings:
Fluoride added to achieve optimal levels (defined as between 0.7–1.2 ppm) was significantly positively and robustly associated with increases in both the incidence and prevalence of diabetes from 2005 to 2010 when accounting for per capita consumption of tap water.
Among the three fluoridation chemicals used in this data set (sodium fluoride, fluorosilicic acid, or sodium fluorosilicate), only fluorosilicic acid was significantly and robustly associated with decreases in incidence and prevalence of diabetes.
The first main finding is valuable because of the adjustment for per capita tap water consumption, whereas the second is useful in discriminating between similar yet distinct exposures. Ten independent US and Canadian studies published from 1958 to 1987 have shown that dietary fluoride intakes by adults ranged from 1.4 to 3.4 mg/day in areas where the water fluoride concentration was 1.0 mg/L (Institute of Medicine (IOM) 1999). Applying the upper bound of this range to the model results of this analysis (Table 5) suggests that within a 5-year span, the incidence of diabetes could increase by up to 0.88 per 1,000 persons and prevalence may grow by as much as 0.85% as a consequence of water fluoridation alone. However, these are likely liberal estimates, since the IOM makes no distinction between added and natural fluoride as done in the present analysis.
The differences present in the combined estimates for adjusted (M = 1 model) versus unadjusted (M = 2 model) added fluoride exposure were especially remarkable. Per capita consumption of tap water has declined significantly from 2005 to 2010 in the states examined (see Table 1). This result is informative when also considering the larger substitution effect potentially at play: per capita consumption of bottled water has increased in the USA during the same time: 25.4 gallons per capita in 2005 to 28.3 gallons per capita in 2010 (Rodwan 2011). Note that, unfortunately, this consumption statistic is not available at county level, the unit of analysis in the current inquiry. Bottled water has less fluoride compared to community tap water. According to the United States Department of Agriculture, the average level of fluoride in bottled water is 0.14 ppm (Cutrufelli et al. 2005), with less than 15% of bottled waters surveyed (2/14 brands) containing more than 0.3 ppm (0.31 and 0.34 ppm). This is 83% lower than the mean fluoride concentration for tap water across all municipal regions in the United States (0.81 ppm). Hence, the explanation for the sign reversal for the added fluoride concentration in the unadjusted analysis may be explained, in part, by the increased collective consumption of bottled water. Interacting added fluoride concentration with declining per capita consumption of tap water produced the robustly positive results with diabetes outcomes. No interaction produced the negative results, likely attributable to the increased consumption of bottled water with a lower fluoride concentration.
More work is needed to understand the significant association between fluorosilicic acid and diabetes outcomes before a policy recommendation is made for use of one chemical or another. Recent research has demonstrated significant cost saving from using sodium fluoride in place of fluorosilicic acid (Hirzy et al. 2013). However, the prior cost-benefit analysis did not consider costs associated with diabetes, which represents a major and growing disease burden. Fluorosilicic acid is listed in Section 8(b) of the US Toxic Substances Control Act of 1976. However, harm from exposure to fluorosilicic acid via drinking water is expected to be minimal since it hydrolyzes almost completely under these conditions (Haneke & Carson 2001).
These population-based results offer greater insight into a future cost-benefit analysis of widespread community water fluoridation. Other policy adjustments may carry forward from this research, including reducing the optimal levels of fluoride to minimize impacts on diabetes outcomes and/or using chemicals that do not exacerbate the burden of diabetes as much (use of fluorosilicic acid, for example). This research partly supports the April 2015 decision by the HHS that the new optimal level of added fluoride be reduced to 0.7 mg of fluoride per liter of water to prevent tooth decay, which is a revision downward from the previous recommended range of 0.7 to 1.2 mg/L issued in 1962.
The significant results for natural fluoride were surprising, albeit not unwelcome. They are explainable by drawing upon the quality of water, specifically its hardness. Naturally hard water may contain greater amounts of inorganic fluoride minerals, such as fluorite (CaF2)), which are not as well absorbed when compared to sodium fluoride (NaF) (Shannon 1977). Hard water is also indicative of the presence of higher levels of magnesium, which may itself offer protection from developing diabetes (Lopez-Ridaura et al. 2004; Hruby et al. 2014). Furthermore, greater consumption of dairy products may reduce bioavailability of consumed fluoride (Ekstrand & Ehrnebo 1979). Such dietary alterations have distinctly shown great promise in reducing the risk of diabetes (Choi et al. 2005; Tong et al. 2011; Chen et al. 2014).
There are several limitations to this work. First, it is difficult to unequivocally state that these results are the specific consequences of water fluoridation. This is for two reasons. Although the hypothesis and evidence here are suggestive, it is inherently difficult to establish such a connection with aggregate data vulnerable to the ecological fallacy. Furthermore, fluoridation is not the only source of exposure to fluoride. Research over the years has also indicated another nontrivial source of fluoride exposure through foods (Rao 1984; Rankin et al. 2012). Second, as the secondary results demonstrate, diabetes most likely has a multifactorial etiology, even including epigenetic processes. Although the presence of added fluoride may play a role, its influence is almost certainly modified by other important and pervasive exposures and physiological processes that should not be marginalized. It has been shown that the association is robust to only a small, albeit notable and well-recognized, set of factors, including obesity, physical inactivity and poverty (Everson et al. 2002; Hamilton et al. 2007; Garber 2012). Finally, the analyses presented here were limited by the availability of data. Access and use of publicly available data that fit with the time period of interest (the years 2005 and 2010) was attempted; however, comparable and more extensive analyses should be completed in other countries to replicate the findings presented here.
Acknowledgments
This research was supported by NIH National Heart Lung and Blood Institute grant T32HL007567.
Footnotes
Disclaimer: The CDC's portal for My Water's Fluoride and the data it contained changed after the original data used in this analysis were downloaded in April 2015. Currently accessible portal data (including data from 2011 and after) are not reflective of the data used in this analysis. The original source data can be freely accessed by contacting the author.
References
- Area Health Resources Files (AHRF) US Department of Health and Human Services, Health Resources and Services Administration, Bureau of Health Workforce. Rockville, MD: 2013–2014. [Google Scholar]
- Bibby BG, Wilkins E, Witol E. A preliminary study of the effects of fluoride lozenges and pills on dental caries. Oral Surg Oral Med Oral Pathol. 1955;8(2):213–216. doi: 10.1016/0030-4220(55)90195-x. [DOI] [PubMed] [Google Scholar]
- Broadbent JM, Thomson WM, Ramrakha S, Moffitt TE, Zeng J, Foster Page LA, Poulton R. Community water fluoridation and intelligence: prospective study in New Zealand. Am J Public Health. 2015;105(1):72–76. doi: 10.2105/AJPH.2013.301857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celani MF, Bonati ME, Stucci N. Prevalence of abnormal thyrotropin concentrations measured by a sensitive assay in patients with type 2 diabetes mellitus. Diabetes Res. 1993;27(1):15–25. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Ten great public health achievements-United States, 1900–1999. MMWR Morb Mortal Wkly Rep. 1999;48(12):241. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Long-term Trends in Diabetes. 2014 Available from: Division of Diabetes Translation. National Diabetes Surveillance System at www.cdc.gov/diabetes/statistics/slides/long_term_trends.pdf (Retrieved 2 May 2015)
- Centers for Disease Control and Prevention. My Water's Fluoride. 2015a Available from: https://nccd.cdc.gov/DOH_MWF/Default/ParticipatingStates.aspx?Mode=Reporting (Retrieved 14 April 2015)
- Centers for Disease Control and Prevention. County Data Indicators. 2015b Available from: www.cdc.gov/diabetes/atlas/countydata/County_ListofIndicators.html (Retrieved 25 March 2015)
- Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989;35(2):315–317. [PubMed] [Google Scholar]
- Chen M, Sun Q, Giovannucci E, Mozaffarian D, Manson JE, Willett WC, Hu FB. Dairy consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. BMC Med. 2014;12(1):215. doi: 10.1186/s12916-014-0215-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Willett WC, Stampfer MJ, Rimm E, Hu FB. Dairy consumption and risk of type 2 diabetes mellitus in men: a prospective study. Arch Intern Med. 2005;165(9):997–1003. doi: 10.1001/archinte.165.9.997. [DOI] [PubMed] [Google Scholar]
- Cowie C, Rust K, Ford E, Eberhardt M, Byrd-Holt D, Li C. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005–2006. Diabetes Care. 2009;32(2):287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontology. 2013;200062(1):271–286. doi: 10.1111/prd.12007. [DOI] [PubMed] [Google Scholar]
- Cutrufelli R, Pehrsson P, Haytowitz D, Patterson K, Holden J. USDA National Fluoride Database of Selected Beverages and Foods, Release 2. 2005 Available from: www.ars.usda.gov/SP2UserFiles/Place/80400525/Data/Fluoride/F02.pdf (Retrieved 4 April 2016)
- Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford University Press; Oxford, UK: 1994. [Google Scholar]
- Ekstrand J, Ehrnebo M. Influence of milk products on fluoride bioavailability in man. Eur J Clin Pharmacol. 1979;16(3):211–215. doi: 10.1007/BF00562063. [DOI] [PubMed] [Google Scholar]
- Erdogan M, Canataroglu A, Ganidagli S, Kulaksizoglu M. Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest. 2011;34(7):488–492. doi: 10.3275/7202. [DOI] [PubMed] [Google Scholar]
- Everson SA, Maty SC, Lynch JW, Kaplan GA. Epidemiologic evidence for the relation between socioeconomic status and depression, obesity, and diabetes. J Psychosom Res. 2002;53(4):891–895. doi: 10.1016/s0022-3999(02)00303-3. [DOI] [PubMed] [Google Scholar]
- Fluegge KR. Comorbidities among persons with incident psychiatric condition. Gerontol Geriatr Med. 2016;2:1–10. doi: 10.1177/2333721416635001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration (FDA) FDA Drug Safety Communication: FDA adds warnings about heart failure risk to labels of type 2 diabetes medicines containing saxagliptin and alogliptin. 2016 Available from: www.fda.gov/Drugs/DrugSafety/ucm486096.htm (Retrieved 11 April 2016)
- Garber AJ. Obesity and type 2 diabetes: which patients are at risk? Diabetes, Obesity and Metabolism. 2012;14(5):399–408. doi: 10.1111/j.1463-1326.2011.01536.x. [DOI] [PubMed] [Google Scholar]
- Gregg E, Cadwell B, Cheng Y, Cowie C, Williams D, Geiss L, Engelgau MM, Victor F. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27(12):2806–2812. doi: 10.2337/diacare.27.12.2806. [DOI] [PubMed] [Google Scholar]
- Griffin SO, Jones K, Tomar SL. An economic evaluation of community water fluoridation. J Public Health Dentist. 2001a;61(2):78–86. doi: 10.1111/j.1752-7325.2001.tb03370.x. [DOI] [PubMed] [Google Scholar]
- Griffin SO, Gooch BF, Lockwood SA, Tomar SL. Quantifying the diffused benefit from water fluoridation in the United States. Community Dent Oral Epidemiol. 2001b;29(2):120–129. doi: 10.1034/j.1600-0528.2001.290206.x. [DOI] [PubMed] [Google Scholar]
- Halpern L. The transfer of inorganic phosphorus across the red blood cell membrane. J Biol Chem. 1936;114(3):747–770. [Google Scholar]
- Hamilton IR. Biochemical effects of fluoride on oral bacteria. J Dent Res. 1990;69(2 suppl):660–667. doi: 10.1177/00220345900690S128. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes. 2007;56(11):2655–2667. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- Han C, He X, Xia X, Li Y, Shi X, Shan Z, Teng W. Subclinical hypothyroidism and type 2 diabetes: a systematic review and meta-analysis. PloS One. 2015;10(8):e0135233. doi: 10.1371/journal.pone.0135233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneke KE, Carson BL. Toxicological summary for sodium hexafluorosilicate [16893-85-9] and fluorosilicic acid [16961-83-4] Prepared for National Institute of Environmental Health Sciences. 2001 Available from: https://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/fluorosilicates_508.pdf (Retrieved September 2015)
- Hirzy JW, Carton RJ, Bonanni CD, Montanero CM, Nagle MF. Comparison of hydrofluorosilicic acid and pharmaceutical sodium fluoride as fluoridating agents – a cost-benefit analysis. Environ Sci Pol. 2013;29:81–86. [Google Scholar]
- Hruby A, Meigs JB, O'Donnell CJ, Jacques PF, McKeown NM. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. Diabetes Care. 2014;37(2):419–427. doi: 10.2337/dc13-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüther FJ, Psarros N, Duschner H. Isolation, characterization, and inhibition kinetics of enolase from Streptococcus rattus FA-1. Infect Immun. 1990;58(4):1043–1047. doi: 10.1128/iai.58.4.1043-1047.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Burt BA, Petersen PE, Lennon MA. The effective use of fluorides in public health. Bull World Health Organ. 2005;83(9):670–676. [PMC free article] [PubMed] [Google Scholar]
- Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone NC, Shimkin MB, Arnold FA, Jr, Stevenson CA, Zimmermann ER, Geiser PB, Lieberman JE. Medical aspects of excessive fluoride in a water supply. Public Health Rep. 1954;69(10):925–936. [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lopez-Ridaura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB. Magnesium intake and risk of type 2 diabetes in men and women. Diabetes Care. 2004;27(1):134–140. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- McDonagh MS, Whiting PF, Wilson PM, Sutton AJ, Chestnutt I, Cooper J, Kleijnen J. Systematic review of water fluoridation. BMJ. 2000;321(7265):855–859. doi: 10.1136/bmj.321.7265.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher LK, Raveendranathan SK, Kota SK, Sarangi J, Jali SN. Prevalence of hypothyroidism in patients with metabolic syndrome. Thyroid Res Pract. 2013;10(2):60–64. [Google Scholar]
- Morgan L, Allred E, Tavares M, Bellinger D, Needleman H. Investigation of the possible associations between fluorosis, fluoride exposure, and childhood behavior problems. Pediatr Dent. 1998;20:244–252. [PubMed] [Google Scholar]
- Mouradian M, Abourizk N. Diabetes mellitus and thyroid disease. Diabetes Care. 1983;6(5):512–520. doi: 10.2337/diacare.6.5.512. [DOI] [PubMed] [Google Scholar]
- Pancholi V. Multifunctional α-enolase: its role in diseases. Cell Mol Life Sci. 2001;58(7):902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN. Is fluoridated drinking water associated with increased hypothyroidism risk? Clin Thyroidol. 2015;27(4):100–101. [Google Scholar]
- Peckham S, Lowery D, Spencer S. Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J Epidemiol Commun Health. 2015;69:619–624. doi: 10.1136/jech-2014-204971. [DOI] [PubMed] [Google Scholar]
- Quilliam BJ, Simeone JC, Ozbay AB, Kogut SJ. The incidence and costs of hypoglycemia in type 2 diabetes. Am J Managed Care. 2011;17(10):673–680. [PubMed] [Google Scholar]
- Rankin SJ, Levy SM, Warren JJ, Gilmore JE, Broffitt B. Fluoride content of solid foods impacts daily intake. J Public Health Dent. 2012;72(2):128–134. doi: 10.1111/j.1752-7325.2011.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao GS. Dietary intake and bioavailability of fluoride. Ann Rev Nutr. 1984;4(1):115–136. doi: 10.1146/annurev.nu.04.070184.000555. [DOI] [PubMed] [Google Scholar]
- Rigalli A, Ballina JC, Roveri E, Puche RC. Inhibitory effect of fluoride on the secretion of insulin. Calcif Tissue Int. 1990;46(5):333–338. doi: 10.1007/BF02563825. [DOI] [PubMed] [Google Scholar]
- Rodwan JG., Jr . Bottled water 2010: The recovery begins. U.S. and International Developments and Statistics; 2011. Apr-May. pp. 10–17. Available from: www.bottledwater.org/files/2010BWstats.pdf (Retrieved 2 April 2016) [Google Scholar]
- Roe JH, Irish OJ, Boyd JI. The preservation of blood for chemical analysis by the use of sodium fluoride. J Biol Chem. 1927;75(3):685–695. [Google Scholar]
- Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: A global systematic review. PharmacoEconomics. 2015;33(8):1–21. doi: 10.1007/s40273-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon IL. Biochemistry of fluoride in saliva. Caries Res. 1977;11(Suppl. 1):206–225. doi: 10.1159/000260301. [DOI] [PubMed] [Google Scholar]
- Standing Committee on the Scientific Evaluation of Dietary Reference Intakes, Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academy Press; Washington, DC: 1999. Available from: http://books.nap.edu/books/0309063507/html/288.html#pagetop (Retrieved July 2015) [Google Scholar]
- StataCorp. Stata statistical software: Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a metaanalysis of cohort studies. Eur J Clin Nutr. 2011;65(9):1027–1031. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- United States Geological Survey (USGS) Water-use data available from USGS. 2015 Available from: http://water.usgs.gov/watuse/data/index.html (Retrieved 28 April 2015)
- Wu C, Khan SA, Lange AJ. Regulation of glycolysis – role of insulin. Exp Gerontol. 2005;40(11):894–899. doi: 10.1016/j.exger.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. The analysis of discrete and continuous longitudinal data. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]


