Abstract
We sought to establish clinical practice recommendations to redefine the role of allogeneic hematopoietic cell transplantation (allo-HCT) for patients with chronic lymphocytic leukemia (CLL) in an era of highly active targeted therapies. We performed a systematic review to identify prospective randomized controlled trials comparing allo-HCT against novel therapies for treatment of CLL at various disease stages. In the absence of such data, we invited physicians with expertise in allo-HCT and/or CLL to participate in developing these recommendations. We followed the Grading of Recommendations Assessment, Development and Evaluation methodology. For standard-risk CLL we recommend allo-HCT in the absence of response or if there is evidence of disease progression after B cell receptor (BCR) inhibitors. For high-risk CLL an allo-HCT is recommended after failing 2 lines of therapy and showing an objective response to BCR inhibitors or to a clinical trial. It is also recommended for patients who fail to show an objective response or progress after BCR inhibitors and receive BCL-2 inhibitors, regardless of whether an objective response is achieved. For Richter transformation, we recommend allo-HCT upon demonstration of an objective response to anthracycline-based chemotherapy. A reduced-intensity conditioning regimen is recommended whenever indicated. These recommendations highlight the rapidly changing treatment landscape of CLL. Newer therapies have disrupted prior paradigms, and allo-HCT is now relegated to later stages of relapsed or refractory CLL.
Keywords: Chronic lymphocytic leukemia, Allogeneic hematopoietic cell, transplantation, BCR inhibitors, BCL-2 inhibitors
INTRODUCTION
Chronic lymphocytic leukemia (CLL) represents the most common leukemia in the Western hemisphere. In 2016, it is anticipated that 18,960 cases of CLL will be diagnosed in the United States [1]. A better understanding of the biologic, molecular, and genetic aspects of CLL have resulted in better prognostic risk stratification of this disease [2-7] and have brought novel and highly active therapies targeting various kinases downstream of the B cell receptor (BCR) pathway along with a new generation of monoclonal antibodies, among others [8-13]. Although emergence of these therapies has certainly altered the therapeutic landscape of CLL, mostly because of improved efficacy and better tolerability, the disease remains incurable unless patients are offered allogeneic hematopoietic cell transplantation (allo-HCT), especially those with high-risk disease [14-17].
In 2007 Dreger et al. [18] published on behalf of the European Society for Blood and Marrow Transplantation (EBMT) a consensus paper with indications for allo-HCT in patients with CLL. Allo-HCT candidates were considered those with previously treated poor-risk CLL defined by the following: not achieving response, relapsing after 3 scenarios (ie, within 12 months after purine analogue–containing therapy, or within 24 months after purine analogue combination therapy, or autologous HCT), or presence of TP53 mutation or 17p deletion (del17p13) [18]. Emergence of ibrutinib, a BCR inhibitor, and other targeted therapies that have proved to be effective treatment options for CLL even in high-risk disease has undoubtedly challenged the appropriateness of the 2007 EBMT consensus recommendations [19,20]. Several randomized controlled trials (RCT) and a meta-analysis have shown that high-dose chemotherapy and autologous HCT do not offer an overall survival (OS) advantage compared with conventional chemotherapy or chemoimmunotherapy; accordingly, relapsed CLL after an autologous HCT is not considered, today, as a prerequisite for an allo-HCT [21-25]. Moreover, autologous HCT has been abandoned from current treatment algorithms for CLL [21-25]. Recognizing the pressing need to incorporate the new realities of treating CLL in this modern treatment era [19], the American Society for Blood and Marrow Transplantation convened a group of experts to develop clinical practice recommendations related to the role of allo-HCT for CLL.
METHODS
Twenty-six physicians recognized for their expertise in allo-HCT and/or treatment of CLL were invited to contribute to the development of these recommendations. The composition of the panel was both national and international and purposely designed to include both transplant and nontransplant physicians to embrace diversity of opinion with the goal of enhancing applicability of the final recommendations.
Search and Study Selection
We searched the literature using Medline via PubMed from inception until May 28, 2015 using a MeSH and broadly general text terms (“Leukemia, Lymphocytic, Chronic, B-Cell”[Mesh]) AND “Transplantation, Homologous”[Mesh]). In addition, references of relevant nonsystematic review articles were scanned to identify additional relevant studies. No search limits were applied, but we excluded studies that were only presented in abstract form but had not yet been published as a peer-reviewed article.
Panel of Experts
A transplant physician was an individual who spent > 75% of his or her time in the care and management of patients undergoing HCT, whereas a nontransplant physician spent > 75% of his or her time in the care and management of patients outside the transplant setting. A mixed practice was defined as spending approximately 50% of the physician’s time in each of the aforementioned modalities of therapy (ie, HCT and nontransplant-related CLL clinical care). We also included a methodologist (A.K.) with expertise in systematic reviews/meta-analysis and Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology who did not vote in the question prioritization or recommendations process.
Survey Methodology and Survey Questions
GRADE methodology was used to assist in moving from evidence to decision-making and generating recommendations. To generate evidence before making recommendations, we performed the aforementioned systematic review (not meta-analysis) because data were very scarce. Our approach intentionally included a diverse group of panel participants (transplant and nontransplant physicians) because of the rapidly changing therapeutic landscape where new and more effective drugs to treat CLL, even for those with high-risk disease, are becoming available. Toward this goal, we aimed at developing recommendations by a majority vote (defined as >50% of voting participants).
Panelists were surveyed using www.Qualtrics.com (Qualtrics LLC, Provo, UT). Questions included panelists’ demographics (age, gender, years of experience, practice focus), volume of CLL patients seen in a routine week, information relevant to their respective transplant program (number of allo-HCT performed per year, preferred preparative regimen(s), cell source and donor source, criteria for selection of patients and donors), and questions pertaining to risk definition, timeliness, and appropriateness of allo-HCT for CLL.
After the identification of key clinical questions, a second survey was conducted wherein panelists were asked to vote on the direction of recommendations (in favor of versus against) for each key question along with strength (strong versus weak) of rendered recommendations. As previously noted, recommendations were issued based on the majority vote. Questions that were specifically related to the procedural aspects of allo-HCT (eg, donor selection, preferred cell source, and choice of the intensity of the preparative regimen, among others) were addressed to all panel members, but for the purpose of issuance of final recommendations, responses from the panelists who identified themselves as predominantly transplant physicians and those in the mix practice category were taken into account considering the potential lack of familiarity of the nontransplant physicians in this particular area. Finally, a supplemental survey was circulated to all panel members after venetoclax received approval by the US Food and Drug Administration on April 11, 2016 for patients with CLL harboring Del17p who had received at least 1 prior line of therapy.
For clinical recommendations specifically pertaining to the allo-HCT procedure, for example, determination of donor eligibility, choice of dose intensity of the preparative regimen, preferred cell source, implementation of pre- and post-transplant minimal residual disease (MRD) assessment, and others, these recommendations are issued solely on votes from respondents who identified themselves as predominantly transplant physicians or those who have a mixed practice. The 2008 International Workshop on CLL developed current recommendations pertinent to the assessment of response after treatment. We refer readers to reference the criteria set forth in the 2008 International Workshop on CLL when assessing treatment responses or lack thereof in patients with CLL [26]. Because novel therapies such as ibrutinib, and others, can mobilize CLL cells into the peripheral blood by interfering with their homing [27,28], it is important not to confuse this phenomenon of lymphocytosis with progressive disease unless the patient develops other disease-related signs or symptoms of progressive disease [29].
Summary of Evidence from Systematic Review of the Literature
The initial literature search identified a total of 248 articles, from which 1 additional study was identified by a secondary manual search of references cited within these articles (total = 249). We did not find any RCT that compared allo-HCT with conventional chemotherapy, chemoimmunotherapy, or nonchemotherapy-containing combinations. This remained true regardless of disease risk stratification or the stage at which allo-HCT was performed. In addition, no RCTs compared dose intensity of the preparative regimen used for allografting, specifically, myeloablative versus reduced-intensity (RIC) or nonmyeloablative regimens. Similarly, there were no controlled comparisons of outcomes based on stem cell source (bone marrow [BM] versus filgrastim mobilized peripheral blood stem cells [PBSC] versus umbilical cord blood) or donor type (HLA-matched related versus matched unrelated versus mismatched unrelated versus cord blood versus haploidentical donor) specifically for CLL.
Pertinent to the post-transplant management of CLL, we did not find any published RCTs that addressed the role of donor lymphocyte infusion (DLI) for mixed chimerism, relapsed CLL, or persistent CLL either clinically or by presence of MRD. Similarly, when DLI was deemed indicated, no RCTs compared DLI dose or a prescribed number of DLI infusions. In the end, the overall quality of evidence informing these recommendations was considered to be low/very low as per the GRADE method [30].
Three nonrandomized studies comparing allo-HCT versus nontransplant strategies provide evidence favoring the option of allo-HCT for relapsed or refractory CLL [31-33]. A Markov decision analysis published by Kharfan-Dabaja et al. [31] demonstrated a better overall life expectancy favoring allo-HCT (35 versus 25 months). Similarly, a retrospective comparative analysis of donor versus no-donor, once again in the RIC context, showed a 2-year OS advantage that favored allo-HCT (88% versus 38%, P < .0001) [32]. A third retrospective comparative analysis by Poon et al. [33] using a consult transplant versus a do not consult transplant design, limited to patients with Del17p, also showed a 2-year OS advantage that favored allo-HCT (64% versus 25%, P = .001). These studies are not generalizable beyond the use of RIC regimens for allografting and preceded approval of novel therapies such as ibrutinib, idelalisib, or venetoclax [31-33].
RESULTS OF SURVEYING OF PANEL EXPERTS
Panelist Demographics
The demographic characteristics of participating panel members are summarized in Table 1. The composition of the panel consisted of 13 (50%) nontransplant, 8 (31%) primarily transplant, and 5 (19%) with a mixed practice. The large majority (21 [81%]) had more than 10 years of clinical experience.
Table 1.
Composition of Panel Members (N = 26)
| Demographics | No. of Cases |
|---|---|
| Age | |
| <40 yr | 2 (8%) |
| 40-49 yr | 13 (50%) |
| 50-59 yr | 7 (27%) |
| >59 yr | 4 (15%) |
| Gender | |
| Male | 19 (73%) |
| Female | 7 (27%) |
| Practice domain | |
| Primarily nontransplant | 13 (50%) |
| Primarily transplant | 8 (31%) |
| Mixed (transplant and nontransplant) | 5 (19%) |
| Years in practice | |
| ≤5 | 1 (4%) |
| 6-10 | 4 (15%) |
| 11-15 | 9 (35%) |
| 16-20 | 5 (19%) |
| >20 | 7 (27%) |
| Patients with CLL evaluated (per week) | |
| <5 | 6 (23%) |
| 5-10 | 9 (35%) |
| 11-20 | 5 (19%) |
| >20 | 6 (23%) |
| No. of allo-HCTs performed annually* | |
| 10-50 | 2 (8%) |
| 51-100 | 8 (31%) |
| 101-150 | 7 (27%) |
| >150 | 9 (35%) |
| Total | 26 (100%) |
Represents allo-HCTs for all diseases and not exclusively for CLL.
Transplant Center Demographics and Preferred Regimens for Allo-HCT
Sixteen panelists (62%) described allo-HCT activity at their respective institutions as performing over 100 allo-HCT (not exclusively for CLL) per year, and 25 (97%) reported using RIC (n = 22 [85%]) or nonmyeloablative conditioning (n = 3 [12%]) as the preferred preparative regimen. Only 4 (15%) reported that their centers commonly offer T cell–depleted (in vivo or ex vivo) allografts.
Disease Risk Characterization by Expert Panel
When defining CLL disease risk, we recognize that incidence of high-risk genetic aberrations is higher in the setting of relapsed or refractory disease. For instance, Dohner et al. [4] demonstrated an incidence of Del17p of 7% in treatmentnaive patients. Additionally, studies evaluating novel therapies for relapsed or refractory disease have reported an incidence of Del17p of up to 33% [8].
When defining standard risk, we assume that absence of high-risk features had been confirmed at the time of evaluation for eligibility for allo-HCT. On the other hand, if high-risk abnormalities were present at time of initial diagnosis or they developed over the course of the disease, this would be considered high-risk CLL.
Standard risk
Among 21 panelist survey respondents, the following criteria were used in decreasing percent of frequency to define standard-risk CLL at initial diagnosis and at time of disease relapse/progression: absence of Del17p and/or TP53 mutations (100%), absence of complex karyotype (71%), and absence of Del11q (62%).
High risk
When asked what criteria are commonly used to define high-risk CLL, the responses of the 21 panelists were as follow in decreasing percent of frequency: presence of Del17p and/or TP53 mutations (100%) and presence of complex karyotype (67%).
Assessment of MRD
Fourteen of 20 respondents (70%) reported commonly obtaining MRD assessments in CLL patients who were being considered for allo-HCT. However, no respondents considered the presence of MRD positivity (ie, persistent disease) as a contraindication for proceeding to allo-HCT. Pertaining to post-transplant disease monitoring, 14 (70%) acknowledge using MRD for this purpose.
Summary of Recommendations
For standard-risk CLL
As illustrated in Table 2, the panel recommends offering an allo-HCT when there is lack of response or evidence of disease progression after BCR inhibitors.
Table 2.
Summary of Indications for Allo-HCT in Standard-Risk CLL at Time of Transplant Evaluation
| Clinical Scenarios | Strength of Recommendation |
|---|---|
| The panel does not recommend allogeneic HCT for patients who relapse after front-line therapy and demonstrate sensitive disease after second line therapy (not BCR inhibitors) |
Strong |
| The panel does not recommend allogeneic HCT for patients who relapse after front-line therapy, demonstrate refractory disease after second-line (not BCR inhibitors), but show an objective response to BCR inhibitors or to a clinical trial |
Strong |
| The panel recommends offering allogeneic HCT when there is lack of response or evidence of disease progression after BCR inhibitors |
Strong |
Standard-risk is defined as absence of Del17p and/or TP53 mutations and/or absence of complex karyotype, and/or absence of Del 11q.
For high-risk CLL
The panel recommends allo-HCT for patients showing an objective response to BCR inhibitors or to a clinical trial (Table 3). The panel also recommends allo-HCT for patients showing an objective response to BCL-2 inhibitors, namely venetoclax, or to a clinical trial after demonstrating refractory disease to prior therapies including BCR inhibitors (Table 3). Moreover, patients who failed to respond or progressed after BCL-2 inhibitors, namely venetoclax, should also be offered allo-HCT (Table 3). Patients with documented Richter transformation who demonstrate an objective response to treatment should be offered an allo-HCT. Finally, patients with purine analogue relapsed or refractory disease are still considered in the category of high-risk disease; however, in contrast to previous 2007 EBMT consensus recommendations, immediate allo-HCT is not recommended any longer.
Table 3.
Summary of Indications for Allo-HCT in High-Risk CLL at Time of Transplant Evaluation
| Clinical Scenarios | Strength of Recommendation | |
|---|---|---|
| High-risk CLL at time of transplant evaluation |
The panel does not recommend offering an allogeneic HCT in the front-line consolidation setting |
Strong |
| The panel does not recommend offering an allogeneic HCT for patients who relapse after front-line therapy and demonstrate sensitive disease after second line therapy (not BCR inhibitors) |
Weak | |
| The panel recommends allogeneic HCT for patients who relapse after front-line therapy, demonstrate refractory disease after second-line (not BCR inhibitors), but show an objective response to BCR inhibitors or to a clinical trial |
Strong | |
| The panel recommends allogeneic HCT for patients who relapse after front-line therapy, demonstrate refractory disease after second-line therapy including BCR inhibitors (not BCL-2 inhibitors), but show an objective response to BCL-2 inhibitors, namely venetoclax, or to a clinical trial |
Strong | |
| The panel recommends allogeneic HCT when there is lack of response or there is progression after BCL-2 inhibitors, namely venetoclax |
Strong | |
| Richter transformation | The panel recommends allogeneic HCT for patients with Richter transformation after achieving an objective response to anthracycline-based chemotherapy |
Strong |
| Purine analogue relapsed and/or refractory disease |
The panel considers purine analogue relapsed and/or refractory disease high-risk disease but not an indication for immediate allogeneic HCT |
Strong |
High-risk is defined as the presence of Del17p and/or TP53 mutations and/or complex karyotype.
For allo-HCT–specific management
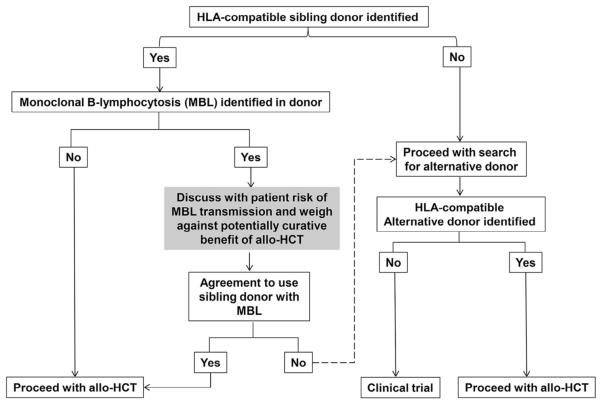
The panel recommends that siblings who are identified as suitable donors should be tested to rule out CLL or monoclonal B cell lymphocytosis (MBL) (Figure 1). Whenever indicated, the panel recommends that RIC regimens should be considered when performing an allo-HCT and that PBSC is the preferred cell source. Other recommendations pertaining to MRD assessment are summarized in Table 4. The panel understands that the prognostic value of MRD assessment is mostly relevant to patients without radiologic and/or BM morphologic evidence of disease.
Figure 1.
Donor selection in the presence of MBL in HLA-compatible sibling donors.
Table 4.
Recommendations for Allo-HCT–Specific Management (Based on Voting Limited to Predominantly Transplant Physicians and Physicians with Mixed Transplant/ Nontransplant Practice)
| Recommendations | Strength of Recommendation | |
|---|---|---|
| Donor eligibility and selection (also refer to Figure 1) |
The panel recommends that siblings who are identified as suitable donors should be tested to rule out CLL or monoclonal B cell lymphocytosis* |
Strong |
| The panel does not recommend initiation of an unrelated donor search as first priority before testing siblings for suitability |
Strong | |
| Dose-intensity of the preparative regimen |
The panel recommends RIC for allo-HCT whenever indicated | Strong |
| Preferred cell source | The panel recommends filgrastim mobilized PBSCs as a preferred cell source for allo-HCT for CLL |
Weak |
| MRD assessment† | The panel recommends performing MRD assessment in patients planned for an allo-HCT | Strong |
| The panel does not recommend considering the presence of MRD positivity (ie, persistent disease) a contraindication for proceeding with an allo-HCT |
Strong | |
| The panel recommends to use MRD for monitoring disease after allo-HCT | Strong | |
| The panel recommends using MRD for disease monitoring after allo-HCT starting no earlier than 30 days and no later than 90 days |
Weak |
According to published literature, the morbidity and mortality risks related to donor MBL appear to be exceedingly rare when compared with the usually known risks of allo-HCT, namely graft-versus-host disease and its associated complications as well as disease relapse or progression. This should be kept in mind when explaining the risks associated with MBL transmission to the patient.
The prognostic value of MRD is mostly relevant to patients without radiologic and/or BM morphologic evidence of disease.
DISCUSSION
These clinical practice recommendations are reflective of the changes in CLL treatment algorithms resulting from emergence of novel therapies including BCR, PI3Kδ, and BCL-2 inhibitors. In addition, questions pertaining to donor selection and transplant-related clinical questions are explored.
Despite emergence of new prognostic biomarkers in CLL, panelists considered, unanimously, absence of Del17p and/or TP53 mutations as a criterion to define standard-risk CLL. Absence of complex karyotype or Del11q were considered criteria to define standard-risk CLL by 71% and 62% of respondents, respectively. Alternatively, voting panelists considered, unanimously, the presence of Del17p and/or TP53 mutations as a defining criterion for high-risk CLL. Presence of complex karyotype was considered a criterion to define high-risk CLL by 67% of respondents, but Del11q was considered high risk by only 29%. This has created an undefined gap between standard- and high-risk CLL for patients harboring Del11q, limiting our ability to issue specific recommendations for this particular genetic abnormality. However, we believe that the comparative efficacy of therapies such as ibrutinib [8] or chemoimmunotherapy [34] in patients with Del11q vis-à-vis non-Del11q, suggests that Del11q may be more appropriately incorporated within standard-risk treatment algorithms unless they show evidence of clonal progression with Del17p or complex karyotype or Richter transformation.
Pertaining to standard-risk CLL at time of transplant evaluation, panelists recommended offering an allo-HCT only when there is lack of response or evidence of progression after BCR inhibitors (Table 2). No randomized data exist, to date, that compares allo-HCT versus nontransplant strategies in patients who do not respond or progress on BCR or other novel agents such as PI3Kδ or BCL-2 inhibitors. Several mechanisms of resistance to ibrutinib have been reported predominantly in subjects with high-risk features who develop a cysteine-to-serine mutation in Bruton tyrosine kinase (BTK) at the binding site of ibrutinib and/or activating mutations in PLCγ2, which is directly downstream of BTK [35]. A large study of 308 patients showed that mutations in BTK and PLCγ2 are associated with CLL progression on ibrutinib [36]. In this study most patients had complex cytogenetics (n = 169 [58%]), including in most cases Del17p [36]. Relapse during ibrutinib therapy was described even in patients with Del13q when associated with other adverse genetic aberrations such as Del17p, Del11q, or complex karyotype [36]. Other investigators have shown a median OS of only 3.1 months after discontinuation of ibrutinib for various reasons, emphasizing the urgency in identifying these cases and initiating the process of searching for a HLA suitable donor as soon as possible [37].
In the case of high-risk CLL, our recommendations (Table 3) also highlight a practice shift when compared with 2007 EBMT consensus recommendations where allo-HCT was offered in the front-line setting [18]. This is also explained by encouraging data with novel therapies. In the front-line setting, a phase II study of 51 high-risk CLL patients (Del17p = 47, TP53 [without Del17p] = 4) of whom 35 (69%) were treatmentnaive, received ibrutinib [38]. The overall response rate (ORR) in the treatment-naive patients was 97% and the estimated 2-year OS rate was 84% [38]. In patients with relapsed or refractory CLL, the reported ORR was 80% and 2-year OS rate was 74% [38]. Another phase II study of ibrutinib plus rituximab in high-risk CLL including Del17p or TP53 mutations, Del11q, or patients who had short progression-free survival (PFS) after prior therapies yielded an 18-month PFS rate of 78% (72.4% in Del17p or TP53 mutation) [39]. Recent data demonstrated efficacy of venetoclax in high-risk relapsed/refractory CLL [13]. An open-label, phase II, international study of 107 patients with Del17p relapsed or refractory CLL demonstrated an ORR of 79% [13]. In this study, patients had received a median of 2 prior lines of therapy, including 8% being refractory to bendamustine or fludarabine and 5 (5%) failing prior BCR (3%) or PI3Kδ inhibitors (1%) [13]. This explains the paradigm shift of delaying allo-HCT to later stages of relapsed or refractory CLL, although the concern remains about the difficulty of reinducing remission after relapse and the poor survival of patients relapsing or progressing on ibrutinib. Future research should focus on identifying better methods of stratifying CLL with Del17p to identify those who should still be considered for earlier allo-HCT even in the era of BCR, including perhaps those with Del17p and complex karyotype [40]. A cross-study analysis evaluating efficacy of ibrutinib in Del17p CLL showed inferior PFS and OS in the presence of complex karyotype [40].
In the case of Richter transformation, the panel recommended offering an allo-HCT after patients achieve an objective response to anthracycline-based chemotherapy (Table 3). Therapies combining cyclophosphamide, adriamycin, vincristine, prednisone, and rituximab have shown ORRs of 67% (CR in only 7%), but responses are generally short-lived with a median PFS of only 10 months and treatment appears to be poorly tolerated [41]. Moreover, Maddocks et al. [36] reported a cumulative incidence of Richter transformation of 4.5% at 12 months after ibrutinib treatment for CLL. Although the incidence of Richter transformation appears to be relatively low, early identification of these cases, and prompt referral to transplant centers, is extremely important considering the short-lived responses and the aggressive biology of this disease. Presence of Del17p and NOTCH 1 mutations, among others, are known risk factors for Richter transformation, which carries a serious prognosis after BCR inhibitors [42-45]. There are significant differences in outcomes between the clonally related diffuse large B cell lymphoma and the nonclonal de novo diffuse large B cell lymphoma, and this has to be considered in the allo-HCT decision. Clonally related Richter transformation is reported to occur more frequently (80% versus 20%) [42,46,47] and is generally associated with a worse prognosis (median survival of approximately 14 months versus 63 months) [42].
In the current recommendations, the panel members still consider CLL that is refractory or relapsing within 12 to 24 months after initial purine analogue–based therapy a determinant of high-risk disease but not an indication for immediate allo-HCT. The avoidance of immediate allo-HCT is a major shift from the previous 2007 EBMT consensus recommendations that results from the emergence of promising novel therapies for high-risk CLL [18]. Initial ORRs of 71% are attained with ibrutinib even in heavily pretreated CLL patients (who had received prior nucleoside analogue therapy) and rise over time to 90% with an estimated 30-month PFS rate of 69% [8,48]. Moreover, responses are also attainable in high-risk cytogenetics, namely Del17p [8], hence explaining the shift in offering an allo-HCT until after novel therapies such as ibrutinib and venetoclax are prescribed. Similar encouraging findings were also reported using the PI3Kδ inhibitor, namely idelalisib, when combined with rituximab in previously treated CLL who had failed a purine analogue in over 50% of cases and 45% of whom had Del17p [9]. The high efficacy of these agents would explain how they have disrupted prior treatment paradigms and why allo-HCT is now relegated to later stages of CLL (Figure 2).
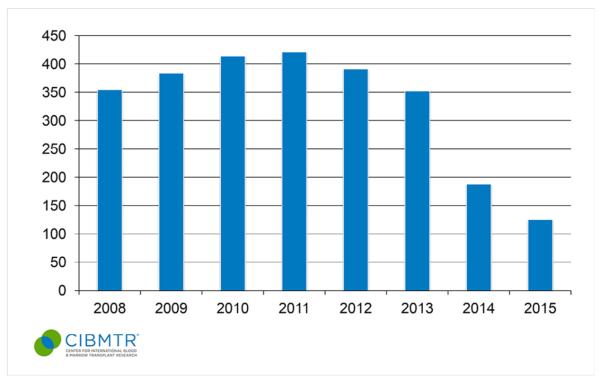
Figure 2.
Number of allogeneic transplants performed in adult CLL patients in the United States during 2008 to 2015. Each bar represents the number of allogeneic transplants for CLL reported per year. (Published with permission from the Center for International Blood and Marrow Transplant Research.)
Although only anecdotal cases of sibling donor to recipient CLL transmission have been reported after allo-HCT [49-52], the panel recommends that siblings who are identified as suitable donors should be tested to rule out asymptomatic CLL or MBL [53] (Figure 1). This recommendation is in line with published guidelines on behalf of the British Committee for Standards in Haematology, which recommended to screen family members for the presence of a circulating clonal B cell population if they are potential allo-HCT donors [54]. Two categories of MBL are currently recognized: high count, which is identified based on relative or absolute lymphocytosis with associated CLL phenotype but below the 5000/μL cut-off needed for diagnosing CLL, and low count, with typically less than 50 cells/μL in peripheral blood [55,56]. Low-count MBL carries a minimal risk of progression to CLL and requires highly sensitive multicolor flow cytometry for detection [55,57]. A population-based registry study from Sweden compared 26,947 first-degree relatives of 9717 patients with CLL to 107,223 first-degree relatives of 38,159 matched control subjects and found that relatives had an increased risk for developing CLL (relative risk = 8.5 (95% CI = 6.1-11.7) [58]. Similarly, Rawstron et al. [53] used flow cytometry to evaluate the incidence of MBL among 1520 individuals aged 62 to 80 years with a normal blood count and 2228 with lymphocytosis (>4000 lymphocytes/mm3). Monoclonal CLL-phenotype B cells were detected in 5.1% of the elderly with normal blood counts and 13.9% of those with lymphocytosis [53]. They also showed that CLL requiring treatment develops in subjects with CLL-phenotype MBL and with lymphocytosis at a rate of 1.1% per year [53]. Del Giudice et al. [59] described a 15.4% incidence of MBL in HLA-matched siblings of CLL patients. These findings highlight the fact that MBL is more common in older sibling donors.
In the case of unrelated donors, several registries generally exclude healthy subjects older than age 60 years from participating in the blood and marrow donor pool. Moreover, allo-HCT using haploidentical donors have been shown to be feasible in patients with CLL [60]. In the event that a HLA histocompatible alternative donor is not found and the only suitable donor is a sibling with diagnosed MBL, it is important to weigh the risk of MBL transmission against the known curative benefit of allo-HCT in CLL (Figure 1). According to published literature, the morbidity and mortality risks related to donor MBL appear to be exceedingly rare when compared with the usually known risks of allo-HCT, namely graft-versus-host disease and its associated complications as well as disease relapse or progression. This should be kept in mind when explaining the risks associated with MBL transmission to the patient according to Figure 1. Moreover, routine donor MBL screening raises complex bioethical dilemmas related to disclosing a neoplastic but most likely harmless condition to otherwise healthy people, which requires broader consideration beyond CLL and HCT experts [61]. Thus, although it has been designated as a strong recommendation according to the rules of this project, the suggestion for routine MBL screening of CLL family donors has to be regarded as preliminary.
The panel recommends using RIC (which also includes nonmyeloablative conditioning within its broad rubric) regimens when patients are deemed eligible for allo-HCT (Table 4). To date, no RCT have been performed comparing RIC versus myeloablative conditioning regimens in patients undergoing an allo-HCT for CLL. It is important to acknowledge that access to allo-HCT has expanded considerably with the introduction of RIC regimens [62]. The Center for International Blood and Marrow Transplant Research reported the outcomes of 1338 patients who received an allo-HCT (RIC = 912, myeloablative = 426) between 2001 and 2011 and showed that the 3-year probability of survival was significantly higher for the RIC allo-HCT group (58% ± 2% versus 50% ± 3%, P < .001) [62]; others have shown post-transplant outcomes favoring RIC regimens in single-center retrospective comparisons [63]. Another reason for favoring RIC regimens is the serious risk of nonrelapse mortality associated with myeloablative doses of chemotherapy or chemoradiotherapy, at times exceeding 40% [14,64-66].
The panel also recommends using filgrastim mobilized PBSCs as a preferred stem cell source for allo-HCT (Table 4). No RCT has been conducted comparing BM versus PBSCs exclusively in patients undergoing allo-HCT for CLL. Eapen et al. [67] did not show any difference in 5-year rates of survival after allografting with PBSCs compared with BM in the setting of RIC allo-HCT using unrelated donors for various hematologic malignancies. On the other hand, Anasetti et al. [68] showed comparable 2-year survival rates when using PBSCs or BM cells for allo-HCT for various hematologic malignancies but at the expense of a higher risk of graft failure when using BM. It is possible that the higher risk of graft failure described in BMT CTN 0201 [68] with use of BM cells with no apparent difference in OS may be among the various reasons for favoring recommending PBSCs as the preferred cell source by our panel.
Finally, our panel recognized the importance of using MRD assays before allo-HCT and to monitor disease after the procedure (Table 4). The prognostic value of MRD is mostly relevant to patients without radiologic and/or BM morphologic evidence of disease. Although more definite studies are needed to address the optimal timing for initiation of MRD monitoring post-allografting, a starting point of no earlier than 30 days and no later than 90 days appears to be reasonable. A standard for MRD assessment exists [26,69], published by the International Workshop on CLL and supported in part by the National Institutes of Health and the European Research Initiative on CLL, among others, and used in several published randomized CLL trials, using a 4-color flow cytometric approach that is able to reach a 10−4 threshold [69]. More recently, a single-tube, 6-color method has been proposed by the European Research Initiative on CLL showing a 10−5 sensitivity and compared with high-throughput Sequencing methods that showed the ability to detect 1 cell out of 1 million (10−6), thus suggesting a possible shift of standard in the future for MRD assessment [70].
Judgments pertaining to evidence and recommendations are generally complex [71]. We acknowledge one limitation in relation to these recommendations. For instance, it was somewhat surprising that, in several occasions, despite the low quality of available evidence (defined by lack of RCTs or well-designed nonrandomized studies) and the relative scarcity of data, panel members issued strong recommendations favoring certain interventions. Although this is important, there is no gold standard for defining scarcity of data [71]. However, to reduce bias and increase transparency, we followed a systematic and explicit approach to make judgments about quality of evidence as per the GRADE method [72]. We believe that the responses of the panel members were based on availability of the most optimal treatment modalities and perhaps an inherent altruism among physicians, exemplified by a sense of obligation to act in patients’ best medical interests [73]. The reasons for making a particular recommendation are complex, and further research is definitely needed to continue to understand the rationale behind such decisions [74].
FUTURE DIRECTIONS FOR RESEARCH AND PRACTICE
These clinical practice recommendations aim at providing therapeutic guidance for the treatment of CLL in the current era where effective targeted therapies are emerging. The complementary role of novel targeted therapies and allo-HCT is an area of active research that may potentially optimize the efficacy of transplantation in CLL. We forecast that future studies would likely incorporate novel therapies either as part of the pre- or periconditioning regimen aiming at a more effective anti-CLL activity or as a post-transplant strategy to target persistent disease or to eradicate MRD. We also anticipate that new therapies including BCR inhibitors or venetoclax, or others, will be incorporated as part of DLI treatment strategies for treatment of post-transplant relapse or progression. Moreover, growing knowledge of T cell engineering and gene transferring techniques is likely to continue to evolve, ultimately aiming at developing more effective anti-CLL specific immune therapies [75,76] .
Although it would be ideal to conduct a large international, multicenter, randomized trial that compares an allogeneic HCT versus novel therapies at various stages of the disease, this may prove to be challenging. Incorporation of MRD assays to help identify patients who might benefit from early post-allografting consolidative strategies with, or without, DLI or other immune therapies should be part of any future studies.
ACKNOWLEDGMENTS
Conflict of interest statement: M.H.: Research funding by Takeda Oncology, Sanofi, and Otsuka; Honoraria from Celgene; Consultancy by Medimmune, Pharmacyclics and Cellerant. S.S.: Honoraria for consultancy, research support, and travel support from AbbVie, Amgen, Boehringer-Ingelheim, Celgene, Genentech, Genzyme, Gilead, GlaxoSmithKline, Janssen, Mundipharma, Novartis, Pharmacyclics, Hoffmann La-Roche, and Sanofi. P.G.: Advisory board for AbbVie, Adaptive Biotechnologies, Gilead, Janssen, Pharmacyclics, and Roche; Research funding from Gilead, GSK, and Roche. P.D.: Research support from Janssen and and Gilead; Honoraria for speaker and advisory board services from Janssen, Gilead, Novartis, and Roche. F.T.A.: Advisory boards for Gilead and Novartis Oncology. J.G.: Honoraria from Janssen, Roche/Genentech, Abbie, Pharmacyclics, Gilead, and Celgene. B.T.H.: Advisory boards for Pharmacyclics and Gilead; Research funding from Abbvie. M.M.: Honoraria for speaking engagements and research support from Janssen. S.O.: Consultant for Amgen, Celgene, and GSK; Advisory board for CLL Global Research Foundation; Research support from Acerta, TG Therapeutics, Regeneron, Gilead, Pharmacyclics, and ProNAi. J.P.-I.: Consultant for Janssen, Gilead, Pharmacyclics, Abbvie, and Celgene; Speaker Bureau for TEVA, Pharmacyclics, and Gilead. N.M.R .: Advisory boards of AbbVie and Gilead.
Footnotes
Financial disclosure: The authors have nothing to disclose.
All other authors report no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Stilgenbauer S. Prognostic markers and standard management of chronic lymphocytic leukemia. Hematol Am Soc Hematol Educ Progr. 2015;2015:368–377. doi: 10.1182/asheducation-2015.1.368. [DOI] [PubMed] [Google Scholar]
- 3.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–1847. [PubMed] [Google Scholar]
- 4.Dohner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343:1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 5.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348:1764–1775. doi: 10.1056/NEJMoa023143. [DOI] [PubMed] [Google Scholar]
- 7.Kharfan-Dabaja MA, Chavez JC, Khorfan KA, Pinilla-Ibarz J. Clinical and therapeutic implications of the mutational status of IgVH in patients with chronic lymphocytic leukemia. Cancer. 2008;113:897–906. doi: 10.1002/cncr.23671. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–1110. doi: 10.1056/NEJMoa1313984. [DOI] [PubMed] [Google Scholar]
- 11.Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–778. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 14.Kharfan-Dabaja MA, Bazarbachi A. Hematopoietic stem cell allografting for chronic lymphocytic leukemia: a focus on reduced-intensity conditioning regimens. Cancer Control. 2012;19:68–75. doi: 10.1177/107327481201900107. [DOI] [PubMed] [Google Scholar]
- 15.Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol. 2008;26:5094–5100. doi: 10.1200/JCO.2008.16.2982. [DOI] [PubMed] [Google Scholar]
- 16.Dreger P, Schnaiter A, Zenz T, et al. TP53, SF3B1, and NOTCH1 mutations and outcome of allotransplantation for chronic lymphocytic leukemia: six-year follow-up of the GCLLSG CLL3X trial. Blood. 2013;121:3284–3288. doi: 10.1182/blood-2012-11-469627. [DOI] [PubMed] [Google Scholar]
- 17.Khouri IF, Saliba RM, Admirand J, et al. Graft-versus-leukaemia effect after non-myeloablative haematopoietic transplantation can overcome the unfavourable expression of ZAP-70 in refractory chronic lymphocytic leukaemia. Br J Haematol. 2007;137:355–363. doi: 10.1111/j.1365-2141.2007.06591.x. [DOI] [PubMed] [Google Scholar]
- 18.Dreger P, Corradini P, Kimby E, et al. Indications for allogeneic stem cell transplantation in chronic lymphocytic leukemia: the EBMT transplant consensus. Leukemia. 2007;21:12–17. doi: 10.1038/sj.leu.2404441. [DOI] [PubMed] [Google Scholar]
- 19.Dreger P, Schetelig J, Andersen N, et al. Managing high-risk CLL during transition to a new treatment era: stem cell transplantation or novel agents? Blood. 2014;124:3841–3849. doi: 10.1182/blood-2014-07-586826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharfan-Dabaja MA, Wierda WG, Cooper LJ. Immunotherapy for chronic lymphocytic leukemia in the era of BTK inhibitors. Leukemia. 2014;28:507–517. doi: 10.1038/leu.2013.311. [DOI] [PubMed] [Google Scholar]
- 21.Sutton L, Chevret S, Tournilhac O, et al. Autologous stem cell transplantation as a first-line treatment strategy for chronic lymphocytic leukemia: a multicenter, randomized, controlled trial from the SFGM-TC and GFLLC. Blood. 2011;117:6109–6119. doi: 10.1182/blood-2010-11-317073. [DOI] [PubMed] [Google Scholar]
- 22.Michallet M, Dreger P, Sutton L, et al. Autologous hematopoietic stem cell transplantation in chronic lymphocytic leukemia: results of European intergroup randomized trial comparing autografting versus observation. Blood. 2011;117:1516–1521. doi: 10.1182/blood-2010-09-308775. [DOI] [PubMed] [Google Scholar]
- 23.Brion A, Mahe B, Kolb B, et al. Autologous transplantation in CLL patients with B and C Binet stages: final results of the prospective randomized GOELAMS LLC 98 trial. Bone Marrow Transplant. 2012;47:542–548. doi: 10.1038/bmt.2011.117. [DOI] [PubMed] [Google Scholar]
- 24.Magni M, Di Nicola M, Patti C, et al. Results of a randomized trial comparing high-dose chemotherapy plus Auto-SCT and R-FC in CLL at diagnosis. Bone Marrow Transplant. 2014;49:485–491. doi: 10.1038/bmt.2013.214. [DOI] [PubMed] [Google Scholar]
- 25.Reljic T, Kumar A, Djulbegovic B, Kharfan-Dabaja MA. High-dose therapy and autologous hematopoietic cell transplantation as front-line consolidation in chronic lymphocytic leukemia: a systematic review. Bone Marrow Transplant. 2015;50:1069–1074. doi: 10.1038/bmt.2015.69. [DOI] [PubMed] [Google Scholar]
- 26.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo. Blood. 2012;119:1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3‘-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118:3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheson BD, Byrd JC, Rai KR, et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J Clin Oncol. 2012;30:2820–2822. doi: 10.1200/JCO.2012.43.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Kharfan-Dabaja MA, Pidala J, Kumar A, Terasawa T, Djulbegovic B. Comparing efficacy of reduced-toxicity allogeneic hematopoietic cell transplantation with conventional chemo-(immuno) therapy in patients with relapsed or refractory CLL: a Markov decision analysis. Bone Marrow Transplant. 2012;47:1164–1170. doi: 10.1038/bmt.2012.71. [DOI] [PubMed] [Google Scholar]
- 32.Herth I, Dietrich S, Benner A, et al. The impact of allogeneic stem cell transplantation on the natural course of poor-risk chronic lymphocytic leukemia as defined by the EBMT consensus criteria: a retrospective donor versus no donor comparison. Ann Oncol. 2014;25:200–206. doi: 10.1093/annonc/mdt511. [DOI] [PubMed] [Google Scholar]
- 33.Poon ML, Fox PS, Samuels BI, et al. Allogeneic stem cell transplant in patients with chronic lymphocytic leukemia with 17p deletion: consult- transplant versus consult-no-transplant analysis. Leuk Lymph. 2015;56:711–715. doi: 10.3109/10428194.2014.930848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsimberidou AM, Tam C, Abruzzo LV, et al. Chemoimmunotherapy may overcome the adverse prognostic signifficance of 11q deletion in previously untreated patients with chronic lymphocytic leukemia. Cancer. 2009;115:373–380. doi: 10.1002/cncr.23993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of ibrutinib therapy discontinuation and outcomes in patients with chronic lymphocytic leukemia. JAMA Oncol. 2015;1:80–87. doi: 10.1001/jamaoncol.2014.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125:2062–2067. doi: 10.1182/blood-2014-09-603670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16:169–176. doi: 10.1016/S1470-2045(14)71182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2014;15:1090–1099. doi: 10.1016/S1470-2045(14)70335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones J, Coutre S, Byrd JC, et al. Evaluation of 243 patients with deletion 17P chronic lymphocytic leukemia treated with ibrutinib: a cross-study analysis of treatment outcomes. EHA Learning Center. 135185:Abstract: S429. 2016. [Google Scholar]
- 41.Langerbeins P, Busch R, Anheier N, et al. Poor efficacy and tolerability of R-CHOP in relapsed/refractory chronic lymphocytic leukemia and Richter transformation. Am J Hematol. 2014;89:E239–E243. doi: 10.1002/ajh.23841. [DOI] [PubMed] [Google Scholar]
- 42.Rossi D, Spina V, Deambrogi C, et al. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117:3391–3401. doi: 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 43.Parikh SA, Rabe KG, Call TG, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol. 2013;162:774–782. doi: 10.1111/bjh.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi D, Spina V, Forconi F, et al. Molecular history of Richter syndrome: origin from a cell already present at the time of chronic lymphocytic leukemia diagnosis. Int J Cancer. 2012;130:3006–3010. doi: 10.1002/ijc.26322. [DOI] [PubMed] [Google Scholar]
- 45.Rossi D, Cerri M, Capello D, et al. Biological and clinical risk factors of chronic lymphocytic leukaemia transformation to Richter syndrome. Br J Haematol. 2008;142:202–215. doi: 10.1111/j.1365-2141.2008.07166.x. [DOI] [PubMed] [Google Scholar]
- 46.Mao Z, Quintanilla-Martinez L, Raffeld M, et al. IgVH mutational status and clonality analysis of Richter’s transformation: diffuse large B-cell lymphoma and Hodgkin lymphoma in association with B-cell chronic lymphocytic leukemia (B-CLL) represent 2 different pathways of disease evolution. Am J Surg Pathol. 2007;31:1605–1614. doi: 10.1097/PAS.0b013e31804bdaf8. [DOI] [PubMed] [Google Scholar]
- 47.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123:1647–1657. doi: 10.1182/blood-2013-11-516229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125:2497–2506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aikawa V, Porter D, Luskin MR, Bagg A, Morrissette JJ. Transmission of an expanding donor-derived del(20q) clone through allogeneic hematopoietic stem cell transplantation without the development of a hematologic neoplasm. Cancer Genet. 2015;208:625–629. doi: 10.1016/j.cancergen.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Ferrand C, Garnache-Ottou F, Collonge-Rame MA, et al. Systematic donor blood qualiffication by flow cytometry would have been able to avoid CLL-type MBL transmission after unrelated hematopoietic stem cell transplantation. Eur J Haematol. 2012;88:269–272. doi: 10.1111/j.1600-0609.2011.01741.x. [DOI] [PubMed] [Google Scholar]
- 51.Pavletic SZ, Zhou G, Sobocinski K, et al. Genetically identical twin transplantation for chronic lymphocytic leukemia. Leukemia. 2007;21:2452–2455. doi: 10.1038/sj.leu.2404928. [DOI] [PubMed] [Google Scholar]
- 52.Perz JB, Ritgen M, Moos M, Ho AD, Kneba M, Dreger P. Occurrence of donor-derived CLL 8 years after sibling donor SCT for CML. Bone Marrow Transplant. 2008;42:687–688. doi: 10.1038/bmt.2008.230. [DOI] [PubMed] [Google Scholar]
- 53.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359:575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 54.Oscier D, Dearden C, Eren E, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012;159:541–564. doi: 10.1111/bjh.12067. [DOI] [PubMed] [Google Scholar]
- 55.Nieto WG, Almeida J, Romero A, et al. Increased frequency (12%) of circulating chronic lymphocytic leukemia-like B-cell clones in healthy subjects using a highly sensitive multicolor flow cytometry approach. Blood. 2009;114:33–37. doi: 10.1182/blood-2009-01-197368. [DOI] [PubMed] [Google Scholar]
- 56.Brown JR. Inherited susceptibility to chronic lymphocytic leukemia: evidence and prospects for the future. Ther Adv Hematol. 2013;4:298–308. doi: 10.1177/2040620713495639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fazi C, Scarfo L, Pecciarini L, et al. General population low-count CLL-like MBL persists over time without clinical progression, although carrying the same cytogenetic abnormalities of CLL. Blood. 2011;118:6618–6625. doi: 10.1182/blood-2011-05-357251. [DOI] [PubMed] [Google Scholar]
- 58.Goldin LR, Bjorkholm M, Kristinsson SY, Turesson I, Landgren O. Elevated risk of chronic lymphocytic leukemia and other indolent non-Hodgkin’s lymphomas among relatives of patients with chronic lymphocytic leukemia. Haematologica. 2009;94:647–653. doi: 10.3324/haematol.2008.003632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Del Giudice I, Mauro FR, De Propris MS, et al. Identiffication of monoclonal B-cell lymphocytosis among sibling transplant donors for chronic lymphocytic leukemia patients. Blood. 2009;114:2848–2849. doi: 10.1182/blood-2009-06-228395. [DOI] [PubMed] [Google Scholar]
- 60.Brammer JE, Khouri I, Gaballa S, et al. Outcomes of haploidentical stem cell transplantation for lymphoma with melphalan-based conditioning. Biol Blood Marrow Transplant. 2016;22:493–498. doi: 10.1016/j.bbmt.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 61.Hardy NM, Grady C, Pentz R, et al. Bioethical considerations of monoclonal B-cell lymphocytosis: donor transfer after haematopoietic stem cell transplantation. Br J Haematol. 2007;139:824–831. doi: 10.1111/j.1365-2141.2007.06862.x. [DOI] [PubMed] [Google Scholar]
- 62.Pasquini MC, Zhu X. Current uses and outcomes of hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides. Available at: http://www.cibmtr.org. 2014. Accessed May 26, 2016. [Google Scholar]
- 63.Peres E, Braun T, Krijanovski O, et al. Reduced intensity versus full myeloablative stem cell transplant for advanced CLL. Bone Marrow Transplant. 2009;44:579–583. doi: 10.1038/bmt.2009.61. [DOI] [PubMed] [Google Scholar]
- 64.Toze CL, Galal A, Barnett MJ, et al. Myeloablative allografting for chronic lymphocytic leukemia: evidence for a potent graft-versus-leukemia effect associated with graft-versus-host disease. Bone Marrow Transplant. 2005;36:825–830. doi: 10.1038/sj.bmt.1705130. [DOI] [PubMed] [Google Scholar]
- 65.Malhotra P, Hogan WJ, Litzow MR, et al. Long-term outcome of allogeneic stem cell transplantation in chronic lymphocytic leukemia: analysis after a minimum follow-up of 5 years. Leuk Lymphoma. 2008;49:1724–1730. doi: 10.1080/10428190802263535. [DOI] [PubMed] [Google Scholar]
- 66.Doney KC, Chauncey T, Appelbaum FR. Allogeneic related donor hematopoietic stem cell transplantation for treatment of chronic lymphocytic leukemia. Bone Marrow Transplant. 2002;29:817–823. doi: 10.1038/sj.bmt.1703548. [DOI] [PubMed] [Google Scholar]
- 67.Eapen M, Logan BR, Horowitz MM, et al. Bone marrow or peripheral blood for reduced-intensity conditioning unrelated donor transplantation. J Clin Oncol. 2015;33:364–369. doi: 10.1200/JCO.2014.57.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–964. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 70.Rawstron AC, Fazi C, Agathangelidis A, et al. A complementary role of multiparameter flow cytometry and high-throughput sequencing for minimal residual disease detection in chronic lymphocytic leukemia: a European Research Initiative on CLL study. Leukemia. 2016;30:929–936. doi: 10.1038/leu.2015.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64:380–382. doi: 10.1016/j.jclinepi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 73.Glannon W, Ross LF. Are doctors altruistic? J Med Ethics. 2002;28:68–69. doi: 10.1136/jme.28.2.68. discussion 74-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar A, Miladinovic B, Guyatt GH, Schunemann HJ, Djulbegovic B. GRADE guidelines system is reproducible when instructions are clearly operationalized even among the guidelines panel members with limited experience with GRADE. J Clin Epidemiol. 2016;75:115–118. doi: 10.1016/j.jclinepi.2015.11.020. [DOI] [PubMed] [Google Scholar]
- 75.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mato A, Porter DL. A drive through cellular therapy for CLL in 2015: allogeneic cell transplantation and CARs. Blood. 2015;126:478–485. doi: 10.1182/blood-2015-03-585091. [DOI] [PubMed] [Google Scholar]