Abstract
Obliterative bronchiolitis (OB) is a major cause of morbidity and mortality in patients undergoing hematopoietic stem cell transplantation (HSCT). Our objective was to perform a systematic review and meta-analysis of the impact of azithromycin on change in forced expiratory volume in 1 second (FEV1). We searched MEDLINE, EMBASE, Web of Science, Cochrane CENTRAL and Scopus databases. We included studies that compared azithromycin to placebo or no intervention in the treatment of OB or BOS in patients who had undergone allogeneic HSCT. 91 unique publications were identified. 4 studies met inclusion criteria, with a total of 90 patients. Changes in FEV1 were measured between 12 weeks and 24 weeks after initiation of treatment. The meta-analysis demonstrated a mean increase in FEV1 of 30 ml (95% Confidence Interval: -260 to +330 ml, p = 0.82) following initiation of azithromycin. One patient death was reported, but not attributed to azithromycin therapy. In conclusion, current evidence can neither support nor refute the use of azithromycin in the treatment of patients who develop OB/BOS following HSCT. Further studies are needed to determine whether azithromycin is beneficial for the treatment of OB/BOS in this setting.
Keywords: Obliterative bronchiolitis, bronchiolitis obliterans, bone marrow transplant, hematopoietic stem cell transplant, allogeneic
Introduction
Obliterative bronchiolitis (OB) is a disorder characterized by small airway injury and progressive airflow obstruction 1. OB is seen most commonly in patients who have undergone lung transplantation or allogeneic hematopoietic stem cell transplantation (HSCT) 2-4. In the latter group, OB is particularly common in those with chronic graft versus host disease in other organs. OB has also been reported in patients with rheumatoid arthritis 5 and following certain inhalational exposures 6-10. Pathologically, patients with OB have inflammatory and fibrotic narrowing of bronchioles 11. When patients who have undergone lung transplantation or allogeneic HSCT demonstrate clinical, physiologic and imaging findings consistent with OB, without histologic diagnosis, the term bronchiolitis obliterans syndrome (BOS) is used 12, 13.
The optimal treatment for OB in patients with HSCT remains unknown 11. Several potential therapies have been investigated including systemic steroids, inhaled steroids 14, long-acting beta agonists (LABA) 14, 15, leukotriene receptor antagonists (LTRA) 16,27, extracorporeal photophoresis (ECP) 17, 18 and macrolides 27, 16, 19, 20. Macrolides, and azithromycin in particular, have been studied for the treatment of OB in patients who have undergone HSCT and lung transplantation. While the precise mechanism of action of macrolides is not known, it is believed their beneficial effects relate primarily to anti-inflammatory and immunomodulatory effects 11.
In a systematic review of azithromycin for the treatment of OB or BOS following lung transplantation, azithromycin was associated with a small (8.8%) but statistically significant improvement in forced expiratory time in one second (FEV1) at an average of seven months 21. However, the role of azithromycin in the treatment of OB and BOS in patients who have undergone allogeneic HSCT remains unknown. To better characterize the effectiveness of azithromycin in this clinical setting, we performed a systematic review and meta-analysis of azithromycin for the treatment of obliterative bronchiolitis following allogeneic HSCT.
Materials and Methods
This systematic review and meta-analysis was conducted and reported according to Preferred Reporting Items for Systematic review and Meta-Analysis standards of quality 22.
Study eligibility
We included randomized or observational studies that compared azithromycin to placebo or no intervention in the treatment of OB or BOS in patients who had undergone allogeneic HSCT. Observational studies were included if the cohort represented a complete or consecutive center experience. BOS was defined as decline in FEV1 with obstructive lung disease in a patient who had undergone allogeneic HSCT with compatible clinical and/or imaging findings. Eligibility was not restricted by language, publication status, year of publication, outcome or azithromycin dose. For articles that described overlapping cohorts the most recent report was included.
Study selection and data extraction
A professional librarian (PJE) designed our search strategy using Ovid MEDLINE, Ovid EMBASE, Web of Science, Ovid Cochrane CENTRAL and Scopus databases (the full search is available as an online supplement). Reference lists of included studies and recent comprehensive narrative reviews were reviewed 11, 23. The search was conducted from database inception and last updated on June 28, 2016. Study selection was completed independently and in duplicate by two trained reviewers (HY and CCK). Disagreement was resolved by consensus. Study selection is outlined in Figure 1.
Figure 1.
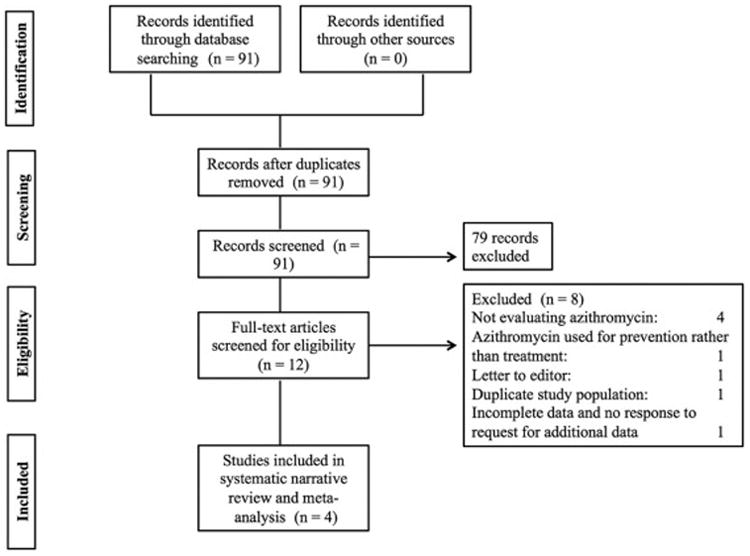
Study Flow Diagram.
We abstracted information on author, year of publication, azithromycin dose, azithromycin duration, patient survival, number of patients included and change in lung function measured by FEV1. To facilitate statistical comparisons between studies that reported FEV1 as percentage predicted instead of absolute change in liters, FEV1 percentage predicted was converted to liters using previously published predicted values corresponding to the age and gender distribution of the cohort 24. The methodological quality of the included studies was assessed using the Cochrane risk of bias tool for randomized control trials and the Newcastle-Ottawa scale for observational studies. Authors were contacted by email for clarification or additional data when necessary.
Statistical analysis
We performed quantitative synthesis of data on absolute change in FEV1 at the endpoint of the study (between 12 and 24 weeks depending on the study) compared to baseline FEV1. Pre-specified subgroup analyses were planned to compare studies with low risk of bias versus high risk of bias, and on studies that compared FAM versus azithromycin therapy alone. We used the generic inverse variance method with random effects models to pool the mean difference using RevMan 5.1 (Copenhagen, Denmark, 2011). We used the I2 statistic to quantify heterogeneity. Statistical significance was set at p < 0.05.
Results
Overview of included studies
Our search yielded 91 citations that were independently reviewed by two abstractors. Twelve studies were retrieved for full text review (HY and CCK, inter-observer agreement κ = 0.95, 95% CI: 0.86 – 1.00). Of these, 4 were excluded for not evaluating azithromycin for treatment of OB/BOS, one was excluded since it evaluated the role of azithromycin in OB/BOS prevention and one was a letter to the editor. One citation was retrieved in abstract form but had insufficient data for abstraction into the systematic review or meta-analysis 25. Attempts at contacting the authors for further data were unsuccessful. Ultimately, four studies were included in the final analysis (Figure 1).
Characteristics and quality of included studies
Four studies were included in the final analysis – one randomized controlled trial 26 and three observational cohort studies 16, 19, 27. The characteristics of the included studies are summarized in Table 1. Enrollment size ranged from 8 to 36. Publication dates ranged from 2005 to 2015. Study quality was assessed independently by two reviewers (HY and CCK) and is presented in Table 2 for the observational studies and in Table 3 for the randomized controlled trial.
Table 1.
Characteristics of included studies.
| Study | Year of publication | Country of origin | Center | Study design | Azithromycin dosing and schedule | Comparator group | Number of patients | Duration of follow-up |
|---|---|---|---|---|---|---|---|---|
| Khalid et al. (19) | 2005 | Saudi Arabia | Single-center | Retrospective cohort | 250 mg three times a week. | None | 8 | 12 weeks |
| Lam et al. (20) | 2011 | Hong-Kong | Single-center | Prospective randomized controlled trial | 250 mg daily. | Placebo | 24 | 16 weeks |
| Norman et al. (27) | 2011 | USA | Single-center | Retrospective matched cohort | 250 mg three times a week. | Oral steroids/‘usual care’ | 22 | 24 weeks |
| Williams et al. (16) | 2015 | USA | Multi-center | Prospective cohort | 250 mg three times a week. | None | 36 | 24 weeks |
Table 2.
Risk of bias assessment in included observational studies (based on Newcastle-Ottawa scale). Filled stars indicate where the methodology is considered adequate by these criteria, whereas unfilled stars indicate that the methodology may not be adequate by these criteria.
| Study | Study design | Adequacy of case definition | Is cohort representative | Selection of non-exposed cohort | Ascertainment of exposure | Subjects on azithromycin at start of study | Comparability of cohorts | Assessment of outcome | Adequacy of follow-up | Blinding during analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Khalid et al. (19) | Cohort | ★ | ★ | ☆ | ★ | ★ | ☆ | ★ | ★ | ☆ |
| Independent review of medical record | Yes | No unexposed cohort | Medical record review | No | No unexposed cohort. | Independent review of medical record | Complete (8/8) | No | ||
| Norman et al. (27) | Matched cohort | ★ | ★ | ★ | ★ | ★ | ☆ | ★ | ☆ | ☆ |
| Independent review of medical record | Yes | Unexposed subjects with BOS | Medical record review | No | Matching variables not specified. | Independent review of medical record | Incomplete (1/9 lost to follow-up) | No | ||
| Williams et al. (16) | Cohort | ★ | ★ | ☆ | ★ | ★ | ☆ | ★ | ☆ | ☆ |
| Independent review of medical record | Yes | No unexposed cohort | Medical record review | No | No unexposed cohort. | Independent review of medical record | Incomplete (3 / 36 lost to follow-up) | No |
Table 3.
Assessment of bias in included randomized control trials (Cochrane Risk of Bias Tool).
| Study | Selection Bias | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | |
|---|---|---|---|---|---|---|
| Random Sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | |
| Lam et al. (20) | Unclear risk Random sequence generation not clearly described. |
Unclear risk Allocation concealment not clearly described. |
Unclear risk Methods of blinding not clearly described. |
Unclear risk Methods of blinding not clearly described. |
Medium risk 2/12 dropped out in treatment arm. 0/12 dropped out in control arm. |
Low risk. |
Data synthesis
A total of 64 patients received azithromycin for the treatment of OB/BOS, and 26 received either placebo or no-treatment depending on the study design. Two studies treated patients with fluticasone, azithromycin and montelukast (FAM) 16, 27, whereas two studies treated with azithromycin alone 19, 26. The primary outcome was change in FEV1 measured at between 12 and 24 weeks, depending on study design (Figure 2, Table 1). Mean and median FEV1 data for individual studies are reported in an online data supplement (Electronic Table 1).
Figure 2.
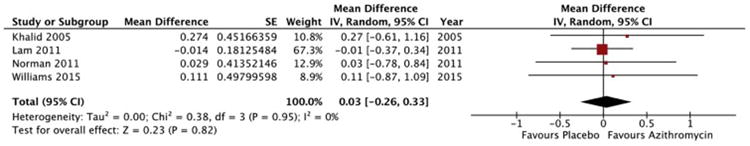
Forest plot of change in FEV1 in liters with azithromycin therapy. The meta-analysis was performed using a generic inverse variance method with a random effects model to pool the mean difference across studies. Studies are listed in order of year of publication.
Two studies reported FEV1 data for individual patients, and these data were used to derive means and estimates of variance for the meta-analysis 19, 26. One study specified medians and interquartile ranges for change in FEV1 rather than means and standard deviation 27. For the purpose of the meta-analysis, the median was used as the point estimate for the mean, and the estimate of variation of that mean was derived from the interquartile range, in line with methods previously described 28. One study did not provide numeric values for change in FEV1, but represented them in graphical format instead 16. Estimates for the mean and variability were derived from analysis of this graph.
Change in FEV1 with azithromycin
The pooled mean difference in FEV1 for patients receiving azithromycin for the treatment of OB/BOS following allogeneic HSCT was +30 ml (95% CI: -260 ml to +330 ml, p = 0.82, I2 = 0%; Figure 2). Subgroup analyses were performed on studies with low risk of bias versus high risk of bias, and on studies that compared FAM versus azithromycin therapy alone. These subgroup analyses did not show any significant changes in FEV1 compared to placebo or no therapy.
Safety and tolerability of azithromycin
All studies commented on safety and side effects of azithromycin. In total, there was one patient death during the study period across all studies. This death was not attributed to an adverse effect from the treatment. Side effects reported included dyspepsia (n = 1), fever (n = 1), pain (n = 1), pulmonary infection (n = 6, events = 8), cough (n = 1), dyspnea (n = 1), pneumonitis (n = 1), pneumothorax (n = 1), and other respiratory disorders (n = 2). The majority of side effect reporting was from a single study that investigated FAM therapy rather than azithromycin alone 16. Therefore, it is unclear whether the side effects reported are from azithromycin or one of the other components of FAM therapy.
Discussion
Our study is the first meta-analysis evaluating the role of azithromycin for the treatment of patients with OB/BOS following allogeneic HSCT. In this population, pooled data did not show a statistically significant effect of azithromycin on lung function as measured by change in FEV1. Overall mortality across the studies was low, and the studies did not report any survival difference with azithromycin. Azithromycin was potentially associated with some side effects, most notably respiratory infection. However, the majority of side effects were only reported once. Additionally, it is difficult to clearly attribute the side effects to azithromycin since the majority of side effects were reported in a study that used combination fluticasone, azithromycin and montelukast (FAM) for the treatment of OB/BOS.
The risk factors, initial evaluation and therapeutic options for patients who develop OB/BOS following HSCT have been recently reviewed elsewhere 11, 23. Unfortunately, no therapeutic options have been shown to consistently improve symptoms, lung function or survival in patients with OB/BOS. Currently the mainstay of therapy is with inhaled and systemic corticosteroids, oral leukotriene antagonists and macrolide therapy, with an emerging role for extracorporeal photophoresis.
Preclinical studies have suggested potential mechanisms of action for macrolides in OB/BOS. These mechanisms include the reduced secretion of interleukin-8 from alveolar macrophages 29, reduced fibroblast matrix metalloprotease activity 30, reduced pulmonary microbial burden 31 and reduced neutrophil survival 32. Several studies have evaluated the role of azithromycin in the role of OB/BOS. In a recent meta-analysis analyzing the role of azithromycin for the treatment of OB/BOS in lung allograft recipients, pooled estimates suggested a small but statistically significant increase in FEV1 in patients receiving azithromycin at an average of 7 months of therapy (8.8% increase in FEV1, 95% CI: 5.1% - 12.5%).
In contrast to the findings of the meta-analysis in the lung allograft population, our study in the post-HSCT population does not find evidence to either support or refute the use of azithromycin in the treatment of OB/BOS. There are several possible reasons for this. Firstly, our search only found four studies with a total of 90 patients. It may be that azithromycin can improve lung function in patients with OB/BOS, but a larger number of patients are needed to see this effect. However, if this is the case, and larger studies are able to determine a statistically significant benefit of azithromycin in OB/BOS, it will be important to also consider whether such a difference is clinically meaningful 33. As the timing of the initiation of azithromycin in the course of OB naturally varied in these studies, it is also unknown whether earlier therapy might prevent progression. Secondly, it may be the studies included in our analysis did not follow patients for sufficiently long to see the full benefits of azithromycin. All studies in our analysis followed patients for between 12 and 24 weeks. In the lung allograft meta-analysis, follow up for included studies was between 12 and 52 weeks with an average of 30 weeks. Limited long-term data exists regarding the use of azithromycin for OB/BOS, and further investigation is needed. Thirdly, it may be that the pathophysiology of OB/BOS is distinct between the lung allograft population and the post-HSCT population. In that setting, what is potentially beneficial in the post-lung allograft population may not be beneficial in the post-HSCT OB/BOS population. The clinical contexts, although related, are clearly different. Lung allograft OB/BOS is hypothesized to be an alloimmune reaction by the host immune system against the graft, whereas post-HSCT OB/BOS is hypothesized to be an alloimmune reaction by the donor-derived immune system against the recipient lung. Allograft HSCT is almost always fully HLA-matched, whereas lung allograft recipients may not have full HLA matching. Conversely, little preclinical or clinical data suggests that OB/BOS have distinct pathophysiological mechanisms in lung allograft and bone marrow transplant recipients, and histologically OB/BOS appears similar between lung allograft and HSCT recipients.
If there truly is no beneficial role for the use of azithromycin in OB/BOS following allogeneic HSCT, then our study has some important implications. Currently many experts routinely recommend the initiation of macrolide therapy as part of combination therapy in patients with persistent moderate or severe airflow obstruction. However, as our analysis shows, there is limited evidence to support the use of azithromycin in this setting. Use of azithromycin in the absence of demonstrated efficacy unnecessarily exposes patients to potential side effects, drug-drug interactions and the possibility of colonization with multidrug resistant organisms.
Importantly, while our analysis does not provide evidence of benefit with azithromycin, it also does not refute the possibility that azithromycin may be beneficial. In general, treatments for OB/BOS may be considered effective even if they stabilize rather than improve pulmonary function. Above all, our study highlights the need for further research in this field. OB/BOS is a devastating condition with limited therapeutic options. Further experimental studies, and in particular randomized control trials evaluating the role of azithromycin and other therapies are urgently needed. The majority of existing studies in the field are observational cohort series, with substantial risk of bias. Future studies should also focus on longer-term follow-up and outcomes, and additionally evaluate quality-of-life and functional outcome measures rather than pulmonary function alone.
Our study has some important limitations. The studies included are all small, and only one is a randomized controlled trial. Consequently, there is a substantial risk of bias in the underlying data. While our test for heterogeneity in results was negative (I2=0), our studies had heterogeneity in trial design, subject selection, duration of follow-up, and whether azithromycin was studied alone or as part of combination therapy. Specifically, two of the four studies evaluated co-interventions (FAM) that could potentially influence the results, and none of the studies explicitly controlled systemic corticosteroid exposure. There was also a lack of a standardized definition of OB used across studies, and one study included some patients who did not have obstructive physiology on PFTs 19. The studies also included patients at different stages in the natural history of OB. The included studies also provided limited data on side effects and mortality. Given the paucity of studies available, publication bias could not be meaningfully assessed in the form of a funnel plot or other statistical tool 34. However, undoubtedly a risk of publication bias exists. Lastly, one citation published in abstract form had potentially relevant data but without sufficient information to include in our systematic review or meta-analysis. We were unsuccessful in our efforts to contact the authors for the original data.
Conclusion
In our systematic review and meta-analysis on the impact of azithromycin on change in forced expiratory volume in 1 second (FEV1), we did not find sufficient evidence to support nor refute the use of azithromycin in the treatment of patients who develop OB/BOS following HSCT. Further studies are needed to determine whether azithromycin is beneficial for the treatment of OB/BOS in this setting.
Supplementary Material
Highlights.
There is a paucity of studies studying therapies for the treatment of OB/BOS.
Pooled change in FEV1 with azithromycin was not statistically significant.
Current evidence cannot support or refute the use of azithromycin in this setting.
Further studies are needed to evaluate therapies for OB/BOS following HSCT.
Acknowledgments
This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The manuscript was also supported by a Mayo Clinic Department of Medicine Write-up and Publish grant.
Funding: This publication was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The manuscript was supported by a Mayo Clinic Department of Medicine Write-up and Publish grant.
Footnotes
Conflicts of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication As a service to our customers we are providing this early version of the manuscript The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168:1277–1292. doi: 10.1164/rccm.200301-053SO. [DOI] [PubMed] [Google Scholar]
- 2.Au BK, Au MA, Chien JW. Bronchiolitis obliterans syndrome epidemiology after allogeneic hematopoietic cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2011;17:1072–1078. doi: 10.1016/j.bbmt.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dudek AZ, Mahaseth H, DeFor TE, Weisdorf DJ. Bronchiolitis obliterans in chronic graft-versus-host disease: analysis of risk factors and treatment outcomes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2003;9:657–666. doi: 10.1016/s1083-8791(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 4.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Perez T, Remy-Jardin M, Cortet B. Airways involvement in rheumatoid arthritis: clinical, functional, and HRCT findings. Am J Respir Crit Care Med. 1998;157:1658–1665. doi: 10.1164/ajrccm.157.5.9710018. [DOI] [PubMed] [Google Scholar]
- 6.van Rooy FG, Rooyackers JM, Prokop M, Houba R, Smit LA, Heederik DJ. Bronchiolitis obliterans syndrome in chemical workers producing diacetyl for food flavorings. Am J Respir Crit Care Med. 2007;176:498–504. doi: 10.1164/rccm.200611-1620OC. [DOI] [PubMed] [Google Scholar]
- 7.Ghanei M, Mokhtari M, Mohammad MM, Aslani J. Bronchiolitis obliterans following exposure to sulfur mustard: chest high resolution computed tomography. Eur J Radiol. 2004;52:164–169. doi: 10.1016/j.ejrad.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmet AJ, Von Essen S. Rapidly progressive, fixed airway obstructive disease in popcorn workers: a new occupational pulmonary illness? J Occup Environ Med. 2002;44:216–218. doi: 10.1097/00043764-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Kreiss K, Gomaa A, Kullman G, Fedan K, Simoes EJ, Enright PL. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. doi: 10.1056/NEJMoa020300. [DOI] [PubMed] [Google Scholar]
- 11.Barker AF, Bergeron A, Rom WN, Hertz MI. Obliterative bronchiolitis. N Engl J Med. 2014;370:1820–1828. doi: 10.1056/NEJMra1204664. [DOI] [PubMed] [Google Scholar]
- 12.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 13.Meyer KC, Raghu G, Verleden GM, et al. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron A, Belle A, Chevret S, et al. Combined inhaled steroids and bronchodilatators in obstructive airway disease after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:547–553. doi: 10.1038/sj.bmt.1705637. [DOI] [PubMed] [Google Scholar]
- 15.Bergeron A, Chevret S, Chagnon K, et al. Budesonide/Formoterol for bronchiolitis obliterans after hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2015;191:1242–1249. doi: 10.1164/rccm.201410-1818OC. [DOI] [PubMed] [Google Scholar]
- 16.Williams KM, Cheng GS, Pusic I, et al. Fluticasone, Azithromycin, and Montelukast Treatment for New-Onset Bronchiolitis Obliterans Syndrome after Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015 doi: 10.1016/j.bbmt.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brownback KR, Simpson SQ, Pitts LR, et al. Effect of extracorporeal photopheresis on lung function decline for severe bronchiolitis obliterans syndrome following allogeneic stem cell transplantation. J Clin Apher. 2015 doi: 10.1002/jca.21404. [DOI] [PubMed] [Google Scholar]
- 18.Sengsayadeth SM, Srivastava S, Jagasia M, Savani BN. Time to explore preventive and novel therapies for bronchiolitis obliterans syndrome after allogeneic hematopoietic stem cell transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2012;18:1479–1487. doi: 10.1016/j.bbmt.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Khalid M, Al Saghir A, Saleemi S, et al. Azithromycin in bronchiolitis obliterans complicating bone marrow transplantation: a preliminary study. Eur Respir J. 2005;25:490–493. doi: 10.1183/09031936.05.00020804. [DOI] [PubMed] [Google Scholar]
- 20.Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46:1551–1556. doi: 10.1038/bmt.2011.1. [DOI] [PubMed] [Google Scholar]
- 21.Kingah PL, Muma G, Soubani A. Azithromycin improves lung function in patients with post-lung transplant bronchiolitis obliterans syndrome: a meta-analysis. Clin Transplant. 2014;28:906–910. doi: 10.1111/ctr.12401. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG Group TP. Preferred Reporting Items for Systematic Reviews and Meta-analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aguilar PR, Michelson AP, Isakow W. Obliterative Bronchiolitis. Transplantation. 2016;100:272–283. doi: 10.1097/TP.0000000000000892. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 25.Pena T, Ansari S, Soubani A. Effect of azithromycin on the course of Fev1 in bronchiolitis obliterans syndrome after stem cell transplantation? American Journal of Respiratory and Critical Care Medicine Conference: American Thoracic Society International Conference, ATS. 2011;183:A4665. [Google Scholar]
- 26.Lam DC, Lam B, Wong MK, et al. Effects of azithromycin in bronchiolitis obliterans syndrome after hematopoietic SCT--a randomized double-blinded placebo-controlled study. Bone Marrow Transplant. 2011;46:1551–1556. doi: 10.1038/bmt.2011.1. [DOI] [PubMed] [Google Scholar]
- 27.Norman BC, Jacobsohn DA, Williams KM, et al. Fluticasone, azithromycin and montelukast therapy in reducing corticosteroid exposure in bronchiolitis obliterans syndrome after allogeneic hematopoietic SCT: a case series of eight patients. Bone Marrow Transplant. 2011;46:1369–1373. doi: 10.1038/bmt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurdowska A, Noble JM, Griffith DE. The effect of azithromycin and clarithromycin on ex vivo interleukin-8 (IL-8) release from whole blood and IL-8 production by human alveolar macrophages. J Antimicrob Chemother. 2001;47:867–870. doi: 10.1093/jac/47.6.867. [DOI] [PubMed] [Google Scholar]
- 30.Kanai K, Asano K, Hisamitsu T, Suzaki H. Suppression of matrix metalloproteinase production from nasal fibroblasts by macrolide antibiotics in vitro. Eur Respir J. 2004;23:671–678. doi: 10.1183/09031936.04.00057104. [DOI] [PubMed] [Google Scholar]
- 31.Tateda K, Comte R, Pechere JC, Kohler T, Yamaguchi K, Van Delden C. Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2001;45:1930–1933. doi: 10.1128/AAC.45.6.1930-1933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasawa H, Oshikawa K, Ohno S, Sugiyama Y. Macrolides inhibit epithelial cell-mediated neutrophil survival by modulating granulocyte macrophage colony-stimulating factor release. Am J Respir Cell Mol Biol. 2004;30:569–575. doi: 10.1165/rcmb.2003-0105OC. [DOI] [PubMed] [Google Scholar]
- 33.Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun-Niggli G, Worthy G. Relationship between FEV1 change and patient-reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respir Res. 2011;12:40. doi: 10.1186/1465-9921-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


