Abstract
Energy balance has in the hypothalamus a central component of integration of food intake and energy expenditure. An accumulating body of evidence indicates that energy homeostasis is largely affected by inflammatory challenges. Severe undernutrition caused by exacerbated inflammatory response may lead to cachexia. On the other hand, prolonged low-grade inflammation such as that observed in obesity and metabolic syndrome, raises the risk for the development of diabetes and heart diseases. Changes in circulating insulin and cytokines such as leptin, interleukins and tumor necrosis factor, as well as changes in their action in the hypothalamus drive the inhibition of food consumption during inflammation. The molecular pathways associated with these responses have only started to be unraveled. One potential candidate is the PI3K signaling, an important player in distinct hypothalamic neurons that control food intake. This study presents an overview of the current knowledge about PI3K role on cytokines and insulin signaling in the hypothalamic regulation of feeding during inflammation.
Keywords: Inflammation, Hypothalamus, Metabolism
1- Introduction
Organism survival basically rests on the ability to store energy and to fight infection. For that reason, metabolic and immunological systems are the most elementary necessities common to a variety of species. Several hormones, signaling molecules, cytokines and transcription factors act in both metabolic and immune systems. Acute inflammatory states are often associated with a significant loss of appetite and body weight as a result of increased cytokine production [Socher et al., 1988; Grunfeld et al., 1989; Langhans 1996; Johnson, 1998; Borges et al., 2007; 2011]. Interleukin (IL) 1β, IL-6, and tumor necrosis factor-α (TNF-α) classically secreted by activated mononuclear myeloid cells, are found in the endocrine system and brain [Johnson, 1998]. Central or peripheral injection of these molecules inhibits food consumption whereas their antagonism abolishes the hypophagia induced by inflammatory stimuli [Socher et al., 1988; Grunfeld et al., 1989; Johnson, 1998]. Compelling evidence has demonstrated that these pro-inflammatory cytokines act on adipocytes and induce secretion of leptin [Grunfeld et al., 1996; Kurosawa et al., 2015]. Leptin is a cytokine-like protein whose activity to reduce food intake and increase energy expenditure is exerted primarily through the hypothalamus and potentially other brain sites [Scott et al., 2009].
The mechanisms regulating energy intake and expenditure during inflammation have being unveiled in studies using the lipopolysaccharide (LPS) component of the Gram-negative bacteria cell wall. LPS activates innate immune system and induces cytokine expression and secretion [Langhans 1996; Johnson, 1998]. Among the cytokines, leptin secretion is stimulated by LPS making it reasonable to hypothesize that leptin is an additional player in the inflammation-induced hypophagia [Sarraf et al., 1997; Berkowitz et al., 1998; Borges et al., 2011]. By utilizing a neutralizing antiserum against rat leptin, Sachot and coworkers [2004] found a reversal of LPS-induced appetite suppression and weight loss, in parallel with an inhibition of the LPS-induced IL-1β and IL-1 receptor (IL-1ra) mRNA expression in the hypothalamus. These findings pointed out to leptin as an adjuvant factor by which inflammatory peripheral anorexigenic signal is conveyed to appetite-regulating centers in the hypothalamus. Inflammatory cascades have also been shown to be linked to insulin secretion and the development of insulin resistance [Wellen and Hotamisligil, 2005; Konner and Bruning, 2010; Jia et al., 2014]. Increased insulin secretion by LPS and attenuation of LPS-induced hypophagia in insulin-deficient streptozotocin treated rats suggest a role for insulin in the endotoxemic suppression of food consumption [Kim et al., 2007].
Although independent, metabolic and immune pathways are closely linked, suggesting the existence of an integrator signal capable of ensuring the maintenance of energy homeostasis during metabolic or immunological challenges. In this regard, the phosphoinositide 3- kinase (PI3K) pathway, largely activated by the above mentioned players, emerges as a potential molecular integrator of metabolic and inflammatory signals in energy balance. The new findings supporting this model are highlighted in this review.
2 - Hypothalamic control of food intake during inflammation
The precise brain circuitry underlying innate immunity-central nervous system (CNS) interactions in the context of suppression of food intake during inflammation remains unsettled. Two key neuronal populations in the arcuate nucleus (ARC), comprised by the orexigenic neuropeptide Y (NPY)/Agouti-related peptide (AgRP) neurons and the anorexigenic proopiomelanocortin (POMC) neurons are targets of peripheral leptin and insulin [Elmquist et al., 1999; Schwatz, 2006; Flak and Myers, 2015]. Their actions result in different acute cellular reponses: leptin depolarizes POMC while hyperpolarizes NPY/AgRP neurons, and insulin hyperpolarizes both POMC and NPY/AgRP neurons [van den Top et al., 2004; Konner et al., 2007; Hill et al., 2008; Williams et al., 2010; Gavello et al., 2016]. Although the metabolic control of energy balance by the ARC is well stablished, how the ARC neurons behave in response to immune challenge is still unclear. Upon binding to the Toll like receptor 4 (TLR4), LPS from invading pathogens promotes IL-1β and TNF-α secretion [Langhans, 1996]. However, the lack of evidence for TLR4 expression in ARC neurons in addition to the scarcity of conclusive studies on the direct effects of the endotoxin over hypothalamic neurons, precludes meaningful assumptions that LPS would directly act on neuronal populations to inhibit feeding. Microglial cells, known for a long time as central sentinels of brain injury, express TLR4 and produce cytokines under LPS stimulation [Milanski et al., 2009; Saijo and Glass, 2011; Borges et al., 2012; Fan et al., 2012]. These findings support the idea of microglia acting to inhibit food intake under endotoxin challenges. LPS would centrally stimulate cytokines secretion by microglia which in turn would act in hypothalamic neurons to suppress feeding. In line with this, a study defining the transcriptome of the hypothalamic leptin responsive neurons [Allison et al., 2015] describes the expression of Il and Tnf receptors genes in LepR neuronal population, suggesting that leptin responsive cells are also reactive to other proinflammatory cytokines and are likely to share intracellular signaling pathways.
In order to examine whether activated microglia alters ARC neuronal function, Reis et al. [2015] dissected the functional consequences of TLR4-mediated microglia activation on the firing activity of POMC and NPY/AgRP neurons. Interestingly, acute exposure to LPS evoked a predominantly excitatory response in POMC and an inhibitory response in NPY/AgRP neurons. The LPS effects in NPY/AgRP, but not in POMC neurons, were blocked when microglia function was inhibited. The inhibition of the orexigenic NPY/AgRP neurons evoked by the activation of ARC microglial TLR4 advocates for a likely role of microglia in the hypothalamic modulation of food intake in inflammation.
Recent chemo- and opto-genetic studies have emphasized the protagonism of NPY/AgRP neurons in the regulation of food intake [Atasoy et al., 2008; Krashes et al., 2011]. Activation of AgRP neurons rapidly stimulates vigorous feeding whereas its inhibition or ablation suppresses food consumption [Krashes et al., 2011, Liu et al., 2016]. Considering that central AgRP injection blunted the hypophagic effect of LPS [Sartin et al., 2008] it has been postulated that inflammatory hypophagia might be caused by lack of AgRP action. Using pharmacogenetics and the DREADD (designer receptors exclusively activated by designer drug) technique Liu and colleagues [2016] attempted to delineate the exact neural circuits linking LPS-induced hypophagia to the AgRP neuronal pathway. They assessed if activation of AgRP neurons attenuates LPS-induced hypophagia. Data from pharmacogenetically activated AgRP neurons in LPS-treated mice indicate that the absence of AgRP action is unlikely to play a role in inflammation-induced hypophagia, given that LPS similarly reduced food intake in both AgRP-DREADD negative or positive mice. Therefore, AgRP-DREADD neuronal activation is not sufficient to prevent LPS hypophagia in mice [Liu et al., 2016].
In this regard, stimulation of the ARC POMC neurons could be considered a contributing factor to LPS-caused hypophagia. Nevertheless, studies found no detectable changes in LPS-induced c-Fos expression in POMC neurons [Gautron et al., 2005; Liu et al., 2016]. Previous reports by our group and others have described no changes in POMC mRNA expression in the ARC of rats treated with LPS [Gautron et al., 2005; Borges et al., 2007]. Nonetheless, a higher number of cells expressing phosphorylated signal transduced and activator of transcription 3 (STAT3) in the ARC has been observed after LPS treatment [Borges et al., 2011]. STAT3 is an intracellular mediator linking inflammation and metabolism. In ARC cells, leptin and other inflammatory cytokines activate the Janus kinase (JAK)2/STAT3 signaling pathway, that induces the expression of POMC mRNA [Borges et al., 2011; Flak and Myers, 2015].
In a recent study, we have found LPS-induced pSTAT3 in ARC LepR neurons [Borges et al., 2016]. However, LPS failed to acutely (after 2 h) reduce food intake in leptin-deficient mice, although it did reduce food intake in later periods of endotoxemia [Borges et al., 2016]. As previously reported, body weight was similarly decreased in wild type and ob/ob mice [Faggioni et al., 1997]. Interestingly however, ob/ob mice presented a delayed LPS-induced pSTAT3 expression in the ARC, leading us to postulate that leptin-induced pSTAT3 expression in the ARC is not required for the initial LPS hypophagia. On the other hand, pSTAT3 likely induced by other inflammatory cytokines in the ARC may be important for the hypophagia over the course of the endotoxemia. Of note, IL-1β activates and depolarizes POMC neurons in the ARC, indicating that this cytokine participates in the hypophagia during inflammation by acting in ARC neurons [Scarlett et al., 2007]. The precise role of POMC neurons in LPS-induced hypophagia remains inconclusive and further studies are warranted.
The regulation of energy homeostasis by non-neuronal cell types has been described in several studies. Astrocytes, the most abundant cell type in the CNS, are activated in response to states of positive and negative energy balance, such as obesity and fasting, regulating neuronal activity by altering synaptic function [Buckman and Ellacott, 2014]. Under endotoxemia or high fat diet, astrocytes have essential role in the central innate immune response, where they undergo morphological and proliferative changes, in a process referred to as astrogliosis [Borges et al., 2012; Thaler et al., 2012; Buckman and Ellacott, 2014]. Studies have demonstrated that astrocytes express leptin receptors [Hsushou et al., 2009; Kim et al., 2014] and that leptin signaling in astrocytes plays a role in energy homeostasis [Kim et al., 2014; Wang et al., 2015]. Kim and coworkers [2014] utilizing a mouse model with tamoxifen- inducible deletion of leptin receptors from astrocytes demonstrated that time-specific ablation of leptin receptor led to altered astrocyte morphology and synaptic inputs onto hypothalamic neurons involved in feeding control. The ability of leptin to reduce food intake was partially inhibited following the loss of astrocytic leptin-receptor signaling, suggesting that these cells play an active role in hypothalamic synaptic remodeling and control of feeding by leptin. By combining chemo-genetic approach with cell-type-specific electrophysiology, pharmacology, and feeding assays, Yang et al., [2015] found that astrocyte activation depressed ghrelin-evoked hyperphagia, whereas it facilitated leptin-induced anorexia. On the other hand, astrocyte inactivation potentiated ghrelin-evoked feeding but blunted leptin- induced anorexia. Because of the inhibition of feeding by astrocytes activation, the authors focused on the effects of astrocytes on the firing rate of AgRP neurons. The main findings demonstrated that astrocyte activation negatively regulates ghrelin-evoked feeding by inactivating AgRP neurons. Studies on the specific role of astrocytes in the suppression of food intake during endotoxemia has yet to be developed.
3 - Cytokines activate the PI3K signaling pathway
Upon binding to TLR4, LPS recruits a diverse array of intracellular signaling pathways. One of these pathways engages in the activation of the PI3K enzyme [Guha and Mackman, 2002; Schabbauer et al., 2004; Borges et al., 2016]. The heterodimeric enzyme PI3K consists of a regulatory (p85) and a catalytic (p110) subunit [Cantley, 2002] and its activation results in the conversion of phosphatidylinositol 4, 5 bisphosphate (PIP2) into phosphatidylinositol 3, 4, 5 triphosphate (PIP3). PIP3 allows for the recruitment and activation of downstream molecules such as AKT, that in turn participates in several cellular responses including cell survival, proliferation and metabolism, regulation of gene expression and cytoskeletal rearrangements [Cantley, 2002]. PI3K/AKT pathway negatively regulates LPS actions in monocytes and macrophages due to inhibition of the expression of inflammatory mediators [Schabbauer et al., 2004; Luyendyk et al., 2007; Weichhart and Saemann, 2008]. Both pharmacological and genetic approaches have shown that blockade of PI3K promotes loss of downstream targets activated by LPS, such as phosphorylation of AKT [Guha and Mackman, 2002; Schabbauer et al., 2004]. In human monocytic cells and macrophages, PI3K inhibition enhances LPS-induced TNF-α expression [Guha and Mackman, 2002; Luyendyk et al., 2007].
One of the main downstream targets of the PI3K/AKT pathway is the mammalian target of rapamycin (mTOR). Studies have identified that mTOR complex 1 (mTORC1) is involved in the regulation of the levels of pro- and anti-inflammatory cytokines produced by innate immune cells. These effects result in inhibition of the pro-inflammatory and stimulation of the anti-inflammatory pathways [Weichhart and Saemann, 2008].
Another crucial PI3K/AKT downstream substrate is the transcription factor forkhead box class O1 (FoxO1), known to inhibit the transcription of targeted genes. PI3K/AKT inhibits FoxO1 via phosphorylation and subsequent nuclear exclusion that in turn releases the expression of targeted genes [Matsumoto and Accili, 2005]. LPS-induced IL-6 and TNF-α production are blunted by the FoxO1-specific siRNA in microglial cell culture, indicating that LPS-triggered inflammatory signaling depends in part on FoxO1 [Dong et al., 2014].
4 - PI3K pathway in the hypothalamus participates in Leptin, Insulin and LPS inhibition of food intake
PI3K signaling has emerged as one of the main pathways through which leptin and insulin alter neuronal excitability in the hypothalamus [Xu et al., 2005; Donato et al., 2010; Gavello et al., 2016; Kwon et al., 2016]. Targeted deletion of PI3K from POMC neurons blunted leptin-induced neuronal activation as well as acute suppression of food intake [Hill et al., 2008; Hill et al., 2009]. Interestingly, a study by Williams et al. [2010] showed both histological and electrophysiological evidence that leptin and insulin act differentially in segregated groups of ARC POMC cells. In agreement, leptin and insulin action to reduce food intake is prevented by PI3K inhibitors, suggesting that the crosstalk between leptin and insulin in modulating food intake lies in part on PI3K signaling. Whether this crosstalk occurs within a network of cells or within individual neurons still needs to be directly demonstrated [Niswender et al., 2001; Niswender et al., 2003; Schwartz, 2006; Williams et al., 2010].
Studies have shown that the PI3K/AKT downstream molecules FoxO1 and mTOR are cellular fuel sensors whose hypothalamic activity are tied to the regulation of energy homeostasis [Kim et al., 2006; Weichhart and Saemann, 2008]. Under negative energy balance condition, FoxO1 inhibits POMC expression, while stimulates AgRP in the ARC. In response to leptin, FoxO1 is phosphorylated and translocate from the nucleus to the cytoplasm, stimulating POMC expression and inhibiting AgRP, leading to a reduction of the food intake [Kitamura et al., 2006; Kwon et al., 2016]. In turn, activation of mTOR signaling suppresses food intake whereas hypothalamic mTOR inhibition markedly increases food intake [Weichhart and Saemann, 2008]. Of note, mTOR inhibitor attenuates leptin-induced hypophagia and weight loss [Cota et al., 2006]. Investigating how these hypothalamic pathways respond to LPS, Yue et al. [2016] recently found that LPS phosphorylates both mTOR and FoxO1 in the ARC. Central blockade of mTOR pathway with rapamycin attenuated both LPS-induced hypophagia and LPS-induced phosphorylation of FoxO1. These data reinforce the likely role of PI3K pathway in the hypophagia during inflammatory states.
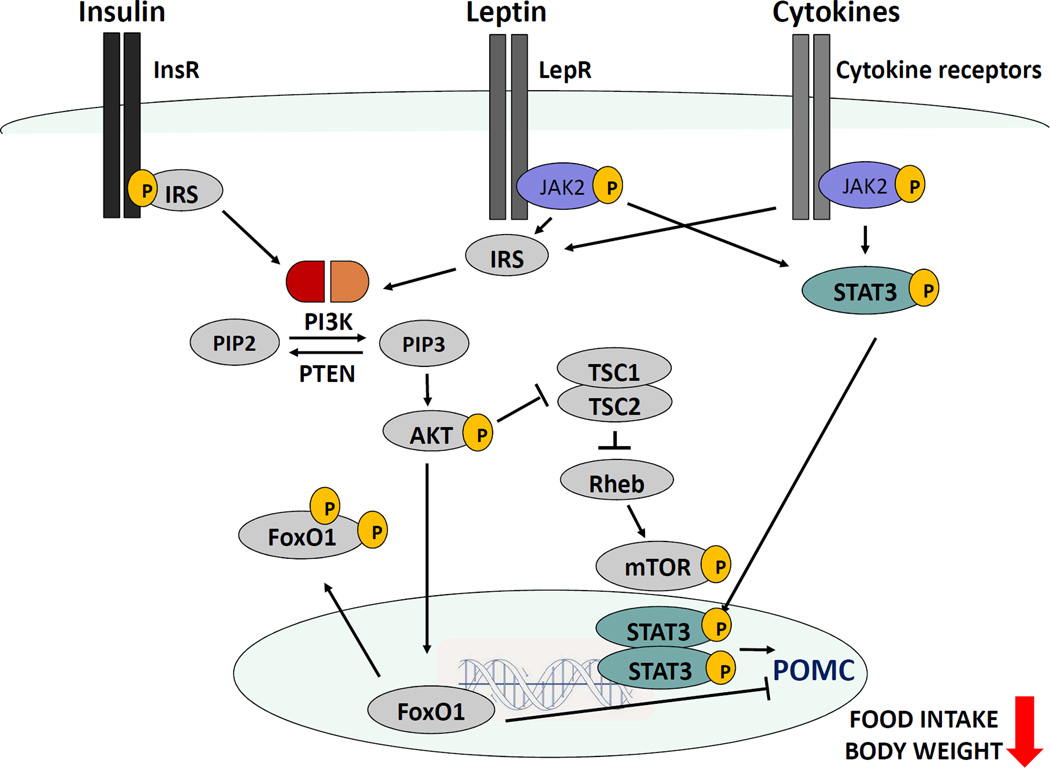
We recently assessed if leptin-mediated PI3K signaling plays a role in LPS-induced hypophagia. PI3K inhibitor prevented hypophagia in mice exposed to endotoxin [Borges et al., 2016]. We further investigated whether genetic deletion of PI3K catalytic subunits in LepR cells has the same effect as the global inhibition. Mice lacking both PI3K p110α and p110β subunits, but not only p110α, selectively in LepR cells showed blunted suppression of food intake by endotoxin [Borges et al., 2016]. Our findings indicate that PI3K via p110β catalytic subunit in LepR expressing cells is required for the acute hypothagic response induced by endotoxemia. LPS-induced AKT phosphorylation in LepR cells in the ARC was attenuated by targeted deletion of PI3K catalytic subunits. Additionally, LPS failed to phosphorylate AKT in the ARC of leptin-deficient mice [Borges et al., 2016]. Taken together, these data demonstrate that the suppression of food intake during an inflammatory challenge depends on PI3K/AKT pathway activated by cytokines and leptin in hypothalamic neurons (Figure 1).
Figure 1. Proposed model for PI3K regulation of food intake and body weight in hypothalamic cells during endotoxemia.
LPS stimulates insulin and leptin secretion in peripheral tissues as well as secretion of other pro-inflammatory cytokines in microglial cells and periphery. Insulin, leptin and cytokines bind to their receptors in hypothalamic neurons in the arcuate nucleus (ARC) and acutely activate the PI3K/AKT/mTOR/FoxO1 pathway. Simultaneously, leptin and other cytokines trigger the activation of JAK-STAT3 pathway. The final step in both pathways is the stimulation of transcription of POMC, which in turn inhibits food intake and promotes a reduction of the body weight. Abbreviations: InsR: insulin receptor, Lepr: leptin receptor, IRS: insulin receptor substrate, JAK2: Janus kinase 2, STAT3: signal transducer and activator of transcription 3, POMC: proopiomelanocortin, PI3K: Phosphatidylinositol-4,5-bisphosphate 3-kinase, PIP3: Phosphatidylinositol 3,4,5-trisphosphate, PIP2: Phosphatidylinositol 4,5-bisphosphate, PTEN: phosphatase and tensin homolog deleted on chromosome 10, AKT: protein kinase B, FoxO1: transcription factor forkhead box class O1, TSC1/2: Tuberous sclerosis proteins 1 and 2, Rheb: Ras homolog enriched in brain, mTOR: mammalian target of rapamycin.
Obesity is considered a state of chronic low-grade inflammation, featured by increased expression of TNF-α and IL-6 from adipocytes in the periphery [Konner and Bruning, 2010], as well as an increase in these cytokines in the CNS [Milanski et al., 2009; Thaler et al., 2012]. TNF-α and IL-6 inhibit serine phosphorylation in the insulin receptor substrate-1 (IRS-1), disrupting insulin signaling transduction and causing insulin resistance [Wellen and Hotamisligil, 2005]. Animal models of obesity induced by high-fat diet show elevated plasma levels of LPS originated from the gut microbiome. These increased levels are sufficient to induce an inflammatory response [Cani et al., 2007]. Given that central and peripheral inflammation are key components in the development of leptin and insulin resistance in obese subjects, studies focusing on the role of PI3K in low-grade inflammation during obesity will potentially constitute an important advance in human’s health. At present, the role of insulin in PI3K-mediated LPS hypophagia has not been investigated and future studies designed to address this topic are invaluable.
5 -PIP3 phosphatase PTEN and downregulation of PI3K pathway in inflammation
Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) is a tyrosine phosphatase engaged in dephosphorylation of PIP3 into PIP2 [Tsou and Bence, 2013]. PTEN acts as a downstream negative regulator of both leptin and insulin signaling by directly counteracting PI3K function. Taking advantage of the genetic mouse models of conditional gene deletion, studies have shown that LepR-specific deletion of PTEN results in decreased body weight, adiposity and adipocyte size in mice, in spite of unchanged food intake and energy expenditure [Plum et al., 2007]. However, LepR-specific overexpression of PTEN does not result in obese phenotype in mice. POMC neuron-specific PTEN deletion results in a diet-sensitive increase in body weight and hyperphagia, in a sexual dimorphic dependent mechanism [Plum et al., 2006]. Intriguingly, POMC neuron-specific PI3K deletion results in hyperphagia as well [Hill et al., 2009]. These data suggest that PTEN regulates the activity of the PI3K pathway by triggering multiple effects on energy homeostasis. To determine the effect of genetically increased activation of the PI3K/AKT pathway on LPS-induced cytokine expression, Luyendyk et al. [2008] used endotoxemic mice with PTEN deletion in macrophages. LPS induction of TNF-α and IL-6 expression was reduced in mice with PTEN-deficient macrophages, confirming that activation of the PI3K/AKT pathway inhibits LPS effects on cytokine production. Despite a clearly defined intracellular role for PTEN opposing leptin- or insulin-stimulated PI3K signaling, its ability to regulate metabolism during inflammatory challenges remains to be addressed.
6 – Estrogen and PI3K pathway in the hypothalamus: a potential crosstalk between leptin and inflammatory signals
Among the numerous signals that affect the glia-neuron crosstalk, gonadal hormones have received singular attention. Gonadal steroids, known to regulate reproduction, are found to play a role in neuroprotection, energy homeostasis and regulation of diverse cellular, molecular and functional parameters in both neurons and astrocytes [Zhu et al, 2015; Acaz-Fonseca et al., 2014; 2016]. Central inflammatory responses are influenced by sex steroids. For example, microglial cells isolated from male, but not female rats, exhibit an enhanced mRNA expression of IL-1βb after exposure to LPS [Loram et al., 2012]. Sexual dimorphisms regarding the number and morphology of astrocytes have also been reported [Kuo et al., 2010]. Estrogen-induced synaptic remodeling is characterized by morphological changes in ARC astrocytes [Acaz-Fonseca et al., 2016], which may modulate the activity of ARC neurons.
In a recent report, Zhu et al. [2015] demonstrated that estrogen acts upon estrogen receptor α (ERα) to inhibit feeding in female mice, and the anorexigenic effects of estrogens are blunted in mice with selective deletion of ERα in POMC neurons. Treatment with ERα agonist stimulated PI3K pathway specifically in POMC neurons. In turn, mice with deletion of PI3K pathway in POMC neurons show attenuated ERα agonist-induced activation of POMC neurons, as well as attenuated estrogen-induced inhibition of food intake. Taken together, these data indicate that an ERα -PI3K cascade in POMC neurons mediates the hypothalamic suppression of food intake.
Obesity affects both men and women, however premenopausal women are less susceptible to development of obesity-associated metabolic disturbances than males. Moreover, the incidence of obesity increases significantly following menopause [Shi et al, 2009]. Taking advantage of the high fat diet (HFD) experimental model to induce obesity, Morselli et al. [2014] analyzed the sexual dimorphic response in male and female mice. Although males and females showed similar weight gain after HFD, male mice presented higher levels of TNF-α, IL-1β and IL-6 in the hypothalamus than female mice. There was an inverse correlation between ERα and proinflammatory cytokine levels, suggesting a protective role of estrogen against central inflammation during obesity. It is possible that the protective effects of estrogen against central inflammation have an impact on food intake during inflammatory challenges other than obesity, but these responses remain to be addressed
7 - Concluding remarks
Molecules such as IL-1β, TNF-α, leptin and insulin have a role in both immunological and metabolic systems, which are clearly cohesive. Due to their potential crosstalk, the PI3K pathway emerges as an important integrative component for their action in the hypothalamus. PI3K/AKT/mTOR/FoxO1 pathways, widely associated with TLR4 actions in the immune system, are now found to be equally important for the metabolic control during infection/inflammation. The present review aimed to highlight the role of PI3K pathway to better understand the mechanisms underlying energy balance regulation in states of chronic low-grade inflammation such as in obesity or during cachexia, characterized by excessive immune response. Inhibition of severe acute hypophagia (mediated in part by PI3K) may contribute to the prevention of harmful undernutrition during bacterial infections and to warrant organism survival.
Highlights.
-
-
PI3K pathway is commonly activated by leptin, insulin and endotoxin in the hypothalamus
-
-
Inhibition of PI3K reverses the hypophagia induced by leptin, insulin and endotoxin
-
-
PI3K is a potential integrator of the metabolic and immunological responses to suppress food intake
Acknowledgments
We would like to thank Susan J. Allen for her technical support. This work was supported by the National Institutes of Health (R01-HD-069702 to CFE), FAPESP (Sao Paulo Research Foundation, fellowship to BCB and grant to LLKE) and CNPq (Brazilian National Council for Scientific and Technological Development, fellowship to BCB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Beatriz C Borges, Email: beatrizd@med.umich.edu/, borgesbc@rfi.frmp.usp.br.
Carol F Elias, Email: cfelias@med.umich.edu.
Lucila LK Elias, Email: llelias@fmrp.usp.br.
REFERENCES
- Acaz-Fonseca E, Sanchez-Gonzalez R, Azcoitia I, Arevalo MA, Garcia-Segura LM. Role of astrocytes in the neuroprotective actions of 17 beta-estradiol and selective estrogen receptor modulators. Mol Cell Endocrinol. 2014;389:48–57. doi: 10.1016/j.mce.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Acaz-Fonseca E, Avila-Rodriguez M, Garcia-Segura LM, Barreto GE. Regulation of astroglia by gonadal steroid hormones under physiological and pathological conditions. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.06.002. pii: S0301-0082(15)30054-X. [DOI] [PubMed] [Google Scholar]
- Allison MB, Patterson CM, Krashes MJ, Lowell BB, Myers MG, Jr, Olson DP. TRAP-seq defines markers for novel populations of hypothalamic and brainstem LepRb neurons. Mol Metab. 2015;4(4):299–309. doi: 10.1016/j.molmet.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28(28):7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz DE, Brown D, Lee KM, Emala C, Palmer D, An Y, Breslow M. Endotoxin-induced alteration in the expression of leptin and beta3-adrenergic receptor in adipose tissue. Am J Physiol. 1998;274(6 Pt 1):E992–E997. doi: 10.1152/ajpendo.1998.274.6.E992. [DOI] [PubMed] [Google Scholar]
- Borges BC, Antunes-Rodrigues J, Castro M, Bittencourt JC, Elias CF, Elias LL. Expression of hypothalamic neuropeptides and the desensitization of pituitary-adrenal axis and hypophagia in the endotoxin tolerance. Horm Behav. 2007;52:508–519. doi: 10.1016/j.yhbeh.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Borges BC, Rorato R, Avraham Y, da Silva LE, Castro M, Vorobiav L, Berry E, Antunes-Rodrigues J, Elias LL. Leptin resistance and desensitization of hypophagia during prolonged inflammatory challenge. Am J Physiol Endocrinol Metab. 2011;300:858–869. doi: 10.1152/ajpendo.00558.2010. [DOI] [PubMed] [Google Scholar]
- Borges BC, Rorato R, Antunes-Rodrigues J, Elias LL. Glial cell activity is maintained during prolonged inflammatory challenge in rats. Braz J Med Biol Res. 2012;45(8):784–791. doi: 10.1590/S0100-879X2012007500069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges BC, Garcia-Galiano D, Rorato R, Elias LLK, Elias CF. PI3K p110β subunit in leptin receptor expressing cells is required for the acute hypophagia induced by endotoxemia. Mol Metab. 2016;5(6):379–391. doi: 10.1016/j.molmet.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman LB, Ellacott KL. The contribution of hypothalamic macroglia to the regulation of energy homeostasis. Front Syst Neurosci. 2014;8:212. doi: 10.3389/fnsys.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312(5775):927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, Frazão R, Elias CF. The PI3K signaling pathway mediates the biological effects of leptin. Arq Bras Endocrinol Metabol. 2010;54(7):591–602. doi: 10.1590/s0004-27302010000700002. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang X, Dai X, Lu S, Gui B, Jin W, Zhang S, Zhang S, Qian Y. Lithium ameliorates lipopolysaccharide-induced microglial activation via inhibition of toll-like receptor 4 expression by activating the PI3K/Akt/FoxO1 pathway. J Neuroinflammation. 2014;11(1):140. doi: 10.1186/s12974-014-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: hypothalamic control of food intake and body weight. Neuron. 1999;22(2):221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Faggioni R, Fuller J, Moser A, Feingold KR, Grunfeld C. LPS-induced anorexia in leptin-deficient (ob/ob) and leptin receptor-deficient (db/db) mice. Am J Physiol Regul Integr Comp Physiol. 1997;273:R181–R186. doi: 10.1152/ajpregu.1997.273.1.R181. [DOI] [PubMed] [Google Scholar]
- Fan K, Wu X, Fan B, Li N, Lin Y, Yao Y, Ma J. Up-regulation of microglial cathepsin C expression and activity in lipopolysaccharide-induced neuroinflammation. J Neuroinflammation. 2012;9:96. doi: 10.1186/1742-2094-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flak JN, Myers MG., Jr Minireview: CNS Mechanisms of Leptin Action. Mol Endocrinol. 2016;30(1):3–12. doi: 10.1210/me.2015-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron L, Mingam R, Moranis A, Combe C, Layé S. Influence of feeding status on neuronal activity in the hypothalamus during lipopolysaccharide-induced anorexia in rats. Neuroscience. 2005;134(3):933–946. doi: 10.1016/j.neuroscience.2005.03.063. [DOI] [PubMed] [Google Scholar]
- Gavello D, Carbone E, Carabelli V. Leptin-mediated ion channel regulation: PI3K pathways, physiological role, and therapeutic potential. Channels (Austin) 2016;28:1–15. doi: 10.1080/19336950.2016.1164373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunfeld C, Wilking H, Neese R, Gavin LA, Moser AH, Gulli R, Serio MK, Feingold KR. Persistence of the hypertriglyceridemic effect of tumor necrosis factor despite development of tachyphylaxis to its anorectic/cachectic effects in rats. Cancer Res. 1989;49(10):2554–2560. [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277(35):32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150(11):4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsuchou H, Pan W, Barnes MJ, Kastin AJ. Leptin receptor mRNA in rat brain astrocytes. Peptides. 2009;30:2275–2280. doi: 10.1016/j.peptides.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;12(5):3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RW. Immune and endocrine regulation of food intake in sick animals. Domest Anim Endocrinol. 1998;15:309–319. doi: 10.1016/s0739-7240(98)00031-9. [DOI] [PubMed] [Google Scholar]
- Kahn CR. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes. 1994;43(8):1066–1084. doi: 10.2337/diab.43.8.1066. [DOI] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9(7):901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kim YW, Kim KH, Ahn DK, Kim HS, Kim JY, Lee DC, Park SY. Time-course changes of hormones and cytokines by lipopolysaccharide and its relation with anorexia. J Physiol Sci. 2007;57(3):159–165. doi: 10.2170/physiolsci.RP003407. [DOI] [PubMed] [Google Scholar]
- Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci. 2014;17:908–910. doi: 10.1038/nn.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12(5):534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab. 2007;5(6):438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Könner AC, Brüning JC. Toll-like receptors: linking inflammation to metabolism. Trends Endocrinol Metab. 2011;22(1):16–23. doi: 10.1016/j.tem.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Hamid N, Bondar G, Dewing P, Clarkson J, Micevych P. Sex differences in hypothalamic astrocyte response to estradiol stimulation. Biol Sex Differ. 2010;1:7. doi: 10.1186/2042-6410-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa N, Shimizu K, Seki K. The development of depression-like behavior is consolidated by IL-6-induced activation of locus coeruleus neurons and IL-1β-induced elevated leptin levels in mice. Psychopharmacology (Berl) 2016;233(9):1725–1737. doi: 10.1007/s00213-015-4084-x. [DOI] [PubMed] [Google Scholar]
- Kwon O, Kim KW, Kim MS. Leptin signalling pathways in hypothalamic neurons. Cell Mol Life Sci. 2016;73(7):1457–1477. doi: 10.1007/s00018-016-2133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W. Bacterial products and the control of ingestive behavior: clinical implications. Nutrition. 1996;12(5):303–315. doi: 10.1016/s0899-9007(96)80052-9. [DOI] [PubMed] [Google Scholar]
- Liu Y, Huang Y, Liu T, Wu H, Cui H, Gautron L. Lipopolysacharide rapidly and completely suppresses AgRP neuron-mediated food intake in male mice. Endocrinology. 2016;25:en20152081. doi: 10.1210/en.2015-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2007;180(6):4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyendyk JP, Schabbauer GA, Tencati M, Holscher T, Pawlinski R, Mackman N. Genetic analysis of the role of the PI3K-Akt pathway in lipopolysaccharide-induced cytokine and tissue factor gene expression in monocytes/macrophages. J Immunol. 2008;180(6):4218–4226. doi: 10.4049/jimmunol.180.6.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Accili D. All roads lead to FoxO1. Cell Metab. 2005;1:215–216. doi: 10.1016/j.cmet.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Milanski M, Degasperi G, Coope A, Morari J, Denis R, Cintra DE, Tsukumo DM, Anhe G, Amaral ME, Takahashi HK, Curi R, Oliveira HC, Carvalheira JB, Bordin S, Saad MJ, Velloso LA. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29(2):359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morselli E, Criollo A, Rodriguez-Navas C, Clegg DJ. Chronic High Fat Diet Consumption Impairs Metabolic Health of Male Mice. Inflamm Cell Signal. 2014;1(6):e561. doi: 10.14800/ics.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413(6858):794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Gallis B, Blevins JE, Corson MA, Schwartz MW, Baskin DG. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. J Histochem Cytochem. 2003;51(3):275–283. doi: 10.1177/002215540305100302. [DOI] [PubMed] [Google Scholar]
- Plum L, Schubert M, Brüning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16(2):59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Münzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Brüning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116(7):1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Rother E, Münzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Könner AC, Alber J, Suzuki A, Krone W, Horvath TL, Rahmouni K, Brüning JC. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 2007;6(6):431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Reis WL, Yi CX, Gao Y, Tschöp MH, Stern JE. Brain innate immunity regulates hypothalamic arcuate neuronal activity and feeding behavior. Endocrinology. 2015;156(4):1303–1315. doi: 10.1210/en.2014-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachot C, Poole S, Luheshi GN. Circulating leptin mediates lipopolysaccharide-induced anorexia and fever in rats. J Physiol. 2004;15:263–272. doi: 10.1113/jphysiol.2004.074351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11(11):775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Frederich RC, Turner EM, Ma G, Jaskowiak NT, Rivet DJ, 3rd, Flier JS, Lowell BB, Fraker DL, Alexander HR. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. J Exp Med. 1997;185(1):171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartin JL, Marks DL, McMahon CD, Daniel JA, Levasseur P, Wagner CG, Whitlock BK, Steele BP. Central role of the melanocortin-4 receptors in appetite regulation after endotoxin. J Anim Sci. 2008;86(10):2557–2567. doi: 10.2527/jas.2008-0916. [DOI] [PubMed] [Google Scholar]
- Scarlett JM, Jobst EE, Enriori PJ, Bowe DD, Batra AK, Grant WF, Cowley MA, Marks DL. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology. 2007;148(9):4217–4225. doi: 10.1210/en.2007-0017. [DOI] [PubMed] [Google Scholar]
- Schabbauer G, Tencati M, Pedersen B, Pawlinski R, Mackman N. PI3K-Akt pathway suppresses coagulation and inflammation in endotoxemic mice. Arterioscler Thromb Vasc Biol. 2004;24(10):1963–1969. doi: 10.1161/01.ATV.0000143096.15099.ce. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. Central nervous system regulation of food intake. Obesity (Silver Spring) Suppl. 2006;1:1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. doi: 10.1002/cne.22025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30:396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socher SH, Friedman A, Martinez D. Recombinant human tumor necrosis factor induces acute reductions in food intake and body weight in mice. J Exp Med. 1988;167(6):1957–1962. doi: 10.1084/jem.167.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou RC, Bence KK. Central regulation of metabolism by protein tyrosine phosphatases. Front Neurosci. 2013;6:192. doi: 10.3389/fnins.2012.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hsuchou H, He Y, Kastin AJ, Pan W. Role of Astrocytes in Leptin Signaling. J Mol Neurosci. 2015;56(4):829–839. doi: 10.1007/s12031-015-0518-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichhart T, Säemann MD. The PI3K/Akt/mTOR pathway in innate immune cells: emerging therapeutic applications. Ann Rheum Dis. 2008;67(Suppl 3):iii70–iii74. doi: 10.1136/ard.2008.098459. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30(7):2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302(5651):1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115(4):951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qi Y, Yang Y. Astrocytes control food intake by inhibiting AGRP neuron activity via adenosine A1 receptors. Cell Rep. 2015;11(5):798–807. doi: 10.1016/j.celrep.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Yue Y, Wang Y, Li D, Song Z, Jiao H, Lin H. A central role for the mammalian target of rapamycin in LPS-induced anorexia in mice. J Endocrinol. 2015;224(1):37–47. doi: 10.1530/JOE-14-0523. [DOI] [PubMed] [Google Scholar]
- Zhu L, Xu P, Cao X, Yang Y, Hinton AO, Jr, Xia Y, Saito K, Yan X, Zou F, Ding H, Wang C, Yan C, Saha P, Khan SA, Zhao J, Fukuda M, Tong Q, Clegg DJ, Chan L, Xu Y. The ERα-PI3K Cascade in Proopiomelanocortin Progenitor Neurons Regulates Feeding and Glucose Balance in Female Mice. Endocrinology. 2015;156(12):4474–4491. doi: 10.1210/en.2015-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]