Abstract
Objective
To assess a large national sample of bladder cancer pathology reports to determine if they contained the components necessary for clinical decision making.
Methods
We examined a random sample of 507 bladder cancer pathology reports from the national Veterans Administration (VA) Corporate Data Warehouse to assess whether each included information on the four report components explicitly recommended by the College of American Pathologists’ protocol for the examination of such specimens: histology, grade, presence vs. absence of muscularis propria in the specimen, and microscopic extent. We then assessed variation in the proportion of reports lacking at least one component across VA facilities.
Results
108 of 507 reports (21%) lacked at least one of the four components, with microscopic extent and presence vs. absence of muscularis propria in the specimen most commonly missing (each in 11% of reports). There was wide variation across facilities in the proportion of reports lacking at least one component, ranging from 0% to 80%.
Conclusion
One fifth of bladder cancer pathology reports lack information needed for clinical decision making. The wide variation in incomplete report rates across facilities implies that some facilities already have implemented best practices assuring complete reporting while others have room for improvement. Future work to better understand barriers and facilitators of complete reporting may lead to interventions that improve bladder cancer care.
Keywords: bladder cancer, pathology, pathology reporting, quality
Introduction
Bladder cancer is the third most prevalent cancer in the United States.1 The majority of patients with bladder cancer (approximately 75%) are diagnosed with early stage disease.2 While most patients with early stage disease harbor low-risk cancer which is rarely lethal,3 about a third of patients have high-risk cancer which can progress to life-threatening disease.4,5 Therefore, current guidelines call for more intensive treatment and surveillance for patients with high-risk cancer, while those with low-risk cancer often do not need additional treatment and can undergo tumor surveillance less frequently.6–10
A patient's individual bladder cancer risk depends on the histology, grade, and Tumor (T)-stage of the cancer as well as on the patient's cancer history.11 Specifically, T-staging requires knowledge about absence or presence of invasion, depth of invasion, and absence or presence of muscularis propria in the bladder biopsy specimen. As such, since 1996 the College of American Pathologists’ protocol for the examination of specimens from patients with carcinoma of the bladder recommends explicit reporting of the following four components: histology, grade, microscopic extent (absence or presence and depth of invasion), and absence or presence of muscularis propria in the specimen.12 If information on the four components is explicitly stated in the pathology report, clinicians can easily translate these reports into evidence-based treatment and surveillance recommendations for individual patients. However, if information on the four components is missing or unclear, the patient's care may be at best inefficient (requiring follow up communications between urologist and pathologist for clarification) or worse, poorly aligned with his risk of recurrence and progression. Specifically, the clinician may err on the side of caution and proceed with more intensive treatment or surveillance care, which frequently may not be necessary and subjects the patient to additional procedures with associated procedural risks, discomfort, anxiety, and opportunity costs.
For these reasons, we sought to better understand how often the clinically most pertinent information is readily available in a large national sample of bladder cancer pathology reports from the Department of Veterans Affairs (VA). In addition, we hypothesized that there would be facility-level variation in the availability of this information, which could help with identification of best practices and opportunities for improvement.
Methods
Study population
Our goal was to identify a nationally representative sample of bladder cancer pathology reports from the VA Corporate Data Warehouse. We began by identifying a cohort of patients who were newly diagnosed with bladder cancer between January 2005 and December 2011 based on VA oncology data abstracted by tumor registrars and stored in the Corporate Data Warehouse (12,874 patients). For these patients, we identified all pathology documents from a time window starting 30 days prior to the date of diagnosis to 120 days after the date of diagnosis (8,190 patients, 20,505 documents). We limited pathology documents to those containing the key words “bladder”, “ureteral”, or “urethral” (14,990 documents). After excluding urine cytology specimens, our sample included 11,033 documents. Finally, we selected a random sample of 600 documents (representing 582 unique patients from 81 facilities) from these 11,033 documents for review.
Review of bladder cancer pathology reports
The 600 pathology documents were reviewed in duplicate by two annotators (FRS and EAP). We used the VA Informatics and Computing Infrastructure ChartReview tool13 to systematically review and annotate these documents. To standardize annotation as much as possible, we developed an a priori variable concept sheet standardizing the information to be abstracted and performed a pilot run on one hundred separate reports that were not included this analysis. Next, we categorized each report by histology (urothelial carcinoma, other pathology, no cancer, or not referring to bladder pathology). Because the focus of this analysis was to understand content of pathology reports referring to urothelial carcinoma, reports indicating no cancer (n=36), other pathology (n=35), or not referring to bladder pathology (n=22) were excluded leaving 507 reports for further analysis.
Within these 507 reports, the annotators then independently classified each report based on inclusion versus exclusion of the four components recommended by the College of American Pathologists:12,14 histology, grade, a statement on presence vs. absence of muscularis propria in the specimen, and a statement on microscopic extent (absence vs. presence and depth of invasion). Because explicit inclusion of the T-stage can avoid misunderstanding,15 we also assessed whether an explicit T-stage was reported. In case of disagreement between the two annotators, we performed adjudication by first correcting any inadvertent errors (e.g. accidental misclassification by one annotator) and then having the report reviewed by a third annotator (JDS) to decide on final classification.
Statistical analyses
We calculated the proportion of reports missing information for each of the four components as well as the proportion missing one or more components. We also assessed the proportion of reports without an explicit T-stage overall and in a subset of reports for which all information was available to provide an explicit T-stage, i.e. those containing all four components and with muscularis propria present in the specimen. We report patient characteristics including age, sex, race, enhanced Elixhauser comorbidity score,16,17 income, education, rural versus urban residence, with the last three obtained from the Health Resources and Services Administration Area Resource File. We used Wilcoxon rank-sum tests and Fisher's exact tests to compare facility characteristics (number of hospital beds, of unique patients per year, of urology outpatient visits per year, of urologist and pathologist FTEs, as well as rural facility location and academic affiliation; obtained from the Veterans Health Administration's Support Service Center and the VA Office of Academic Affiliation) between those with and without any of the four components missing in their pathology reports. Finally, we performed an exploratory analysis to better understand variation in pathology reporting across the facilities from which we had pathology reports. For this, we selected the subset of facilities with at least 5 reports available for evaluation in our sample and then calculated and plotted the proportion of reports with any missing component. In order to better understand potential facilitators of complete pathology reporting, we manually reviewed all pathology reports from facilities with a 0% incomplete report rate and categorized them according to whether they were or were not using a template or synoptic report. All analyses were performed with StataMP version 14. This study was approved by the Dartmouth College Committee for the Protection of Human Subjects and informed consent was waived (Study #28417).
Results
We analyzed 507 pathology reports referring to urothelial carcinoma of the bladder from 494 unique patients seen at 79 facilities. Demographics of the patients are as expected1,18 for a population of Veterans with bladder cancer, with 99% male and mean age of 78 (see Table 1).
Table 1.
Demographics of the patients represented by the 507 bladder cancer pathology reports.
| Age (median, IQR) | 78 (72 - 82) |
| Male Sex (N, %) | 502 (99) |
| Race (N, %) | |
| White | 420 (83) |
| Black | 33 (6.5) |
| Asian | 8 (1.6) |
| Hispanic | 16 (3.2) |
| Native American | 2 (0.4) |
| Unknown | 28 (5.5) |
| Comorbidity (N, %) | |
| 0 | 84 (17) |
| 1 | 114 (22) |
| 2 | 131 (26) |
| ≥ 3 | 178 (35) |
| Grade (N, %) | |
| Low | 155 (31) |
| Intermediate | 37 (7) |
| High | 294 (58) |
| Undifferentiated | 1 (0.2) |
| Missing | 20 (4) |
| Household income (median $, IQR) | 47,724 (42,941-56,090) |
| Lived in County in which 25% or more of adults had a college education (N, %) | |
| No | 230 (45) |
| Yes | 267 (53) |
| Missing | 10 (2.0) |
| Residing in urban area (N, %) | |
| No | 85 (17) |
| Yes | 412 (81) |
| Missing | 10 (2.0) |
108 of the 507 reports (21%, 95% confidence interval 18% - 25%) were missing at least one of the four components, with microscopic extent and presence vs. absence of muscularis propria in the specimen most commonly missing (each in 11% of the reports, Figure 1, supplemental Table 1). Most reports lacked an explicit T-stage (80% overall, 75% among those reporting all four components and with muscularis propria present in the specimen). Facility characteristics did not differ between those with and without any missing components (Table 2). The hospitals were largely approximately 150 bed hospitals with a median of 2.1 urologists and 2.6 pathologists per facility, with approximately 3,000 outpatient visits related to urologic care each year.
Figure 1.

Proportion of pathology reports with missing information recommended for inclusion by the College of American Pathologists. Data are from a national random sample of pathology reports from the Department of Veterans Affairs which were annotated in duplicate.
Table 2.
Facility characteristics (obtained from the Veterans Health Administration's Support Service Center) represented by the 507 bladder cancer pathology reports overall and stratified by whether the pathology report did or did not have missing components.
| Overall (n=507) | No missing component (n=399) | ≥1 missing component (n=108) | p | |
|---|---|---|---|---|
| Number of hospital beds, median (IQR) | 154 (100-242) | 154 (100-242) | 154 (100-234) | 0.855* |
| Number of unique patients per year, median (IQR) | 51,229 (40,270-62,921) | 51,229 (38,197-62,921) | 51,759 (41,363-62,019) | 0.796* |
| Number of urology outpatient visits per year | 3,128 (2,184-4,116) | 3,138 (2,184-4,116) | 2,922 (2,276-4,156) | 0.482* |
| Number of urologist FTEs, median (IQR) | 2.1 (1.4-2.9) | 2.1 (1.4-2.9) | 2.1 (1.4-2.8) | 0.447* |
| Number of pathologist FTEs, median (IQR) | 2.6 (1.9-4.1) | 2.5 (1.9-4.1) | 2.8 (1.9-3.9) | 0.612* |
| Proportion rural facility, n (%) | 44 (8.7) | 37 (9.3) | 7 (6.5) | 0.443 |
| Academic affiliation, n (%) | 492 (97) | 387 (97) | 105 (97) | 1.000 |
Wilcoxon rank-sum test, all other p-values from Fisher's exact test.
51 facilities had at least five reports available for evaluation, representing 436 (86%) of the 507 reports. Among these facilities, the median number of reports was 8 (range 5 – 17). At the facility level, the proportion of reports with any missing component varied widely from 0% to 80% (Figure 2). Fourteen facilities (27%) had a zero percent incomplete report rate.
Figure 2.
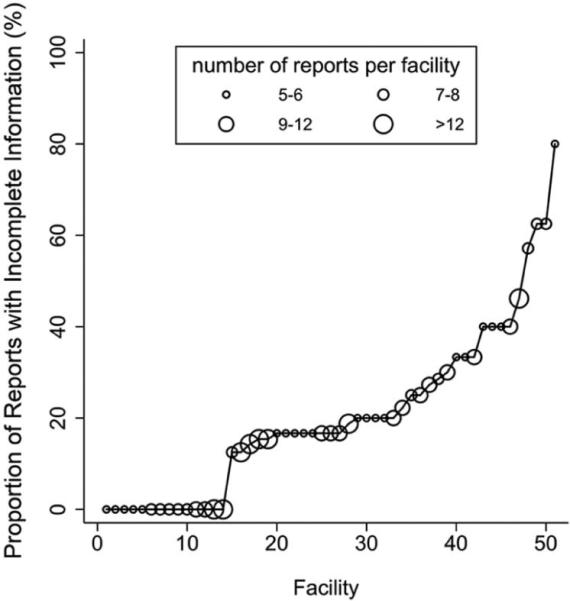
Proportion of reports with incomplete information (defined as one or more missing components) across 51 facilities within the VA which had at least five reports available for review. Fourteen facilities had no reports with incomplete information. Larger size of circle represents larger number of reports per facility.
We then reviewed all 116 reports from these fourteen facilities. This revealed that 20 of the 116 reports (17%) were based on a template. The majority of facilities (9 of 14) did not use templates. Only one facility consistently used a template for reporting, while four facilities used templates in some but not all reports.
Discussion
We found that more than one in five bladder cancer pathology reports from the Department of Veterans Affairs did not contain information recommended for inclusion by the College of American Pathologists. Further, there was substantial variation at the facility level in the proportion of reports with incomplete information across 51 facilities which frequently treat patients with bladder cancer in the VA healthcare system. While a quarter of facilities had consistently complete reports, other facilities demonstrated room for improvement and were commonly missing important components necessary for high-quality cancer care.
It has long been known that incomplete pathology reporting is of significant concern in cancer care. For example, a report from more than two decades ago evaluated the quality of 15,940 colon cancer pathology reports from 532 institutions. It demonstrated that important information such as tumor grade, number of lymph nodes with metastases, and margin status was missing in a significant proportion of reports of resected colorectal carcinomas, with some institutions lacking this information in more than 95% of pathology reports.19 Similarly, a more recent study examined the frequency of inclusion of all elements required by the College of American Pathologists in pathology reports for colorectal carcinoma, prostate carcinoma, and breast carcinoma. This study included reports from 86 institutions and found that more than a third of colorectal and breast cancer reports and 6% of prostate carcinoma reports were missing required elements.20
The current study is the first to examine the completeness of bladder cancer pathology reports. Our 21% rate of missing information recommended for inclusion by the College of American Pathologists is consistent with the previous reports focused on other cancers. In addition, our study is the first to examine completeness of pathology reports in the VA. The VA is the largest integrated health care system in the nation with many processes standardized across facilities.21 As such, one could expect a more standardized way of pathology reporting. However, we found significant variation in the completeness of pathology reports across institutions, similar to previous studies performed outside of the VA system.19,20
While 21% of reports had missing information on components recommended for inclusion, a much larger proportion – 75% – did not report an explicit T-stage. Although reporting of an explicit T-stage is not required by the College of American Pathologists for bladder biopsy and transurethral resection specimens, we would posit that explicit T-stage reporting could potentially limit the chance of improper care secondary to poor understanding of bladder cancer stage.15 Bladder cancer pathology reports are not only read by urologists, but also by non-urologist physicians including primary care providers and internists who may not be familiar with the varying language used by pathologists to describe presence and depth of invasion. In addition, electronic health records and programs such as “My HealtheVet” increasingly give patients access to their pathology reports.22 Patients likely have significant difficulty understanding the stage of their bladder cancer if no explicit T-stage is reported. However, pathologists may have valid concerns about reporting an explicit T-stage based on a bladder biopsy, such as the limited amount of tissue or muscularis propria available for review. We believe that addressing these concerns will be a worthwhile endeavor and could lead to improved communication of T-stage to clinicians and patients.
While this study is based on thorough review of a large number of pathology reports, it is not without limitations. First, generalizability is limited as we reviewed bladder pathology reports from within the VA. Given the Veteran population, almost all reports are from male patients, with only 1% from female patients. However, previous studies examining colorectal, breast, and prostate cancer reports outside of the VA system found a similar frequency of missing information.19,20 Therefore, it is likely that bladder cancer pathology reports outside of the VA system have similar issues with missing information. Second, although we report facility-level estimates of report completeness only for facilities with five or more reports, our point estimates likely have limited reliability due to a fairly small denominator of reports per facility. However, our intention was to gain insight into variability across facilities in general, rather than assessing any single facility's performance. Third, it is possible that subjective judgement of the annotators affected our results. To address this as much as possible, we developed an a priori variable concept sheet standardizing the information to be abstracted, performed a pilot run on one hundred separate reports that were not included in this analysis, annotated each report in duplicate, and performed third party adjudication as necessary.
We acknowledge that the content of the reviewed pathology reports not only reflects the pathologist's work, but also the level of communication between the pathologist and the urologist. Better communication between the pathologist and the urologist could improve the pathologist's knowledge of what information is most important for clinical care and the urologist's understanding of how to obtain a better specimen for pathological review. Such communication could also lead to revised or amended pathology reports in cases where the clinician asks the pathologist to provide additional information on microscopic extent, grade, or presence vs. absence of muscularis propria in the specimen. In our study, if pathology reports were amended, we included the information from the amendment in our annotations. However, it is possible that verbal communication took place without documentation in the pathology report, in which case we classified the report as missing information that may have been relayed in verbal form.
Limitations notwithstanding, we believe our findings have important implications. Our data highlight that there is room for improvement in bladder cancer pathology reporting within the VA. It is noteworthy that 14 facilities had not a single report with missing information. Five of these facilities were at least sometimes using templates, a practice that was consistently associated with an increased likelihood of communicating more complete information content in several previous studies.19,20,23 However, nine of the fourteen did not use templates. Thus, they may have implemented other best practices besides the use of templates. To better understand these practices, a qualitative assessment of barriers and facilitators of complete pathology reporting at these facilities will be needed.24,25 Moreover, it will be worthwhile to examine the extent to which incomplete information in bladder cancer pathology reports affects the care patients with bladder cancer receive.
Conclusions
One fifth of bladder cancer pathology reports in the VA lack complete information important for clinical decision making. The rate of reports with incomplete information varied widely across facilities, with more than a quarter of facilities having uniformly complete bladder cancer pathology reporting. This implies that some facilities may already have practices in place that assure complete reporting. Future work could entail better understanding the work processes and priorities that make it easier for pathologists to render complete reports as well as evaluating the extent to which incomplete information in bladder cancer pathology reports represents a target for future quality improvement initiatives.
Supplementary Material
Acknowledgments
Funding: FRS is supported by the Department of Veterans Affairs, Veterans Health Administration, VISN1 Career Development Award, by a pilot grant from the American Cancer Society (IRG-82-003-30), by a Conquer Cancer Foundation Career Development Award, and by the Dow-Crichlow Award of the Department of Surgery at the Dartmouth-Hitchcock Medical Center. PPG is supported by a grant from the Food and Drug Administration FDA (U01FD005478-01, Sedrakyan = PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Conceptualization (FRS, DJR, BS, PPG), Methodology (FRS, DWD, OVP, SLD), Software (DWD, OVP, SLD), Validation (FRS, EAP, DWD, OVP, SLD), Formal Analysis (FRS), Investigation (FRS, EAP, DWD, OVP, JDS), Resources (FRS, SLD, BS, PPG), Data Curation (SLD), Drafting (FRS) and Editing (all authors) of Manuscript, Visualization (FRS), Supervision (FRS, SLD, BS, PPG), Project Administration (FRS, SLD), Funding Acquisition (FRS, PPG)
Conflicts of Interest: none
Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. [June 14, 2016];Bethesda MD Natl. Cancer Inst. 2016 Available at: http://seer.cancer.gov/csr/1975_2013/
- 2.Snyder C, Harlan L, Knopf K, et al. Patterns of care for the treatment of bladder cancer. J. Urol. 2003;169:1697–1701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. NIH; Bethesda, MD: 2007. [August 12, 2014]. Pub. No. 07-6215. Available at: http://seer.cancer.gov/archive/publications/survival/seer_survival_mono_lowres.pdf. [Google Scholar]
- 4.Scosyrev E, Noyes K, Feng C, et al. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 5.Sylvester RJ, van der Meijden A, Witjes JA, et al. High-grade Ta urothelial carcinoma and carcinoma in situ of the bladder. Urology. 2005;66:90–107. doi: 10.1016/j.urology.2005.06.135. [DOI] [PubMed] [Google Scholar]
- 6.Schroeck FR, Montie JE, Hollenbeck BK. Surveillance Strategies for Non-Muscle Invasive Bladder Cancer. AUA Update Ser. 2012;31:313–323. [Google Scholar]
- 7.Babjuk M, Boehle A, Burger M, et al. [March 25, 2016];European Association of Urology (EAU) Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1 and CIS) 2016 Available at: https://uroweb.org/guideline/non-muscle-invasive-bladder-cancer/
- 8.The National Comprehensive Cancer Network [August 13, 2014];NCCN Clinical Practice Guidelines in Oncology: bladder cancer Version 2.2014. 2014 Available at: http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 9.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J. Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Chang SS, Boorjian SA, Chou R, et al. [May 3, 2016];Non-Muscle Invasive Bladder Cancer: American Urological Association / SUO Guideline. 2016 Available at: https://www.auanet.org/education/guidelines/non-muscle-invasive-bladder-cancer.cfm.
- 11.Sylvester RJ, van der Meijden APM, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006;49:466–475. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 12.Hammond EH, Henson DE. Practice protocol for the examination of specimens removed from patients with carcinoma of the urinary bladder, ureter, renal pelvis, and urethra. Arch. Pathol. Lab. Med. 1996;120:1103–10. [PubMed] [Google Scholar]
- 13.DuVall S, Forbush T, Cornia RC, et al. Reducing the Manual Burden of Medical Record Review through Informatics. Pharmacoepidemiol. Drug Saf. 2014;23:415. [Google Scholar]
- 14.Amin MB, Delahunt B, Bochner BH, et al. Protocol for the Examination of Specimens From Patients With Carcinoma of the Urinary Bladder. Coll. Am. Pathol. 2013 Available at: http://www.cap.org/ShowProperty?nodePath=/UCMCon/Contribution%20Folders/WebContent/pdf/urinary-13protocol-3210.pdf.
- 15.McKenney JK. Genitourinary Pathology: Practical Advances. Edited by C Magi-Galluzzi and CG Przybycin. Springer; New York: 2015. Reporting of Bladder Cancer in Transurethral Resection of Bladder Tumor and Cystectomy Specimens. pp. 201–204. Available at: https://books.google.com/books/about/Genitourinary_Pathology.html?id=97RnBwAAQBAJ. [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med. Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 18.Dobbs RW, Hugar LA, Revenig LM, et al. Incidence and clinical characteristics of lower urinary tract symptoms as a presenting symptom for patients with newly diagnosed bladder cancer. Int. Braz. J. Urol. 2014;40:198–203. doi: 10.1590/S1677-5538.IBJU.2014.02.09. [DOI] [PubMed] [Google Scholar]
- 19.Zarbo RJ. Interinstitutional assessment of colorectal carcinoma surgical pathology report adequacy. A College of American Pathologists Q-Probes study of practice patterns from 532 laboratories and 15,940 reports. Arch. Pathol. Lab. Med. 1992;116:1113–1119. [PubMed] [Google Scholar]
- 20.Idowu MO, Bekeris LG, Raab S, et al. Adequacy of Surgical Pathology Reporting of Cancer: A College of American Pathologists Q-Probes Study of 86 Institutions. Arch. Pathol. Lab. Med. 2010;134:969–974. doi: 10.5858/2009-0412-CP.1. [DOI] [PubMed] [Google Scholar]
- 21.Asch SM, McGlynn EA, Hogan MM, et al. Comparison of Quality of Care for Patients in the Veterans Health Administration and Patients in a National Sample. Ann. Intern. Med. 2004;141:938–945. doi: 10.7326/0003-4819-141-12-200412210-00010. [DOI] [PubMed] [Google Scholar]
- 22.Department of Veterans Affairs [January 12, 2016];My HealtheVet Account Types. 2016 Available at: https://www.myhealth.va.gov/mhv-portal-web/anonymous.portal?_nfpb=true&_nfto=false&_pageLabel=spotlightArchive&contentPage=ipa/mhv_account_status-definitions.html#premium&WT.ac=splash_account_premium.
- 23.Srigley JR, McGowan T, MacLean A, et al. Standardized synoptic cancer pathology reporting: A population-based approach. J. Surg. Oncol. 2009;99:517–524. doi: 10.1002/jso.21282. [DOI] [PubMed] [Google Scholar]
- 24.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 25.Palinkas LA, Horwitz SM, Green CA, et al. Purposeful Sampling for Qualitative Data Collection and Analysis in Mixed Method Implementation Research. Adm. Policy Ment. Health Ment. Health Serv. Res. 2015;42:533–544. doi: 10.1007/s10488-013-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


