Abstract
Purpose
Despite the number of publications on orbital decompression surgery for thyroid eye disease (TED), there are few comparative studies and most studies are underpowered. The goal of our study is to use multivariable analysis to identify independent patient and disease-related predictors of response to decompression surgery and of need for secondary decompressions.
Methods
We retrospectively reviewed all patients who underwent transorbital thyroid-related orbital decompression surgery at the Kellogg Eye Center of the University of Michigan between 1999 and 2014. Demographic, medical, and surgical covariates were collected. Decompression techniques included medial, lateral, and balanced decompressions, with or without orbital fat removal. Main outcomes included proptosis reduction and secondary decompressions, both analyzed at the orbital level. Univariate and multivariable analyses (with adjustment for inter-orbit correlation) were conducted to determine predictors of our outcomes of interest.
Results
Mean proptosis reduction was 3.8 ± 2.4 mm (mean ± standard deviation, N = 420 orbits). The secondary decompression rate was 13.8% (82/594). On multivariable mixed linear regression, larger preoperative proptosis (P < 0.0001), balanced decompression (P = 0.0002), TED duration < 4 years (P = 0.0093), and history of orbital radiation (P = 0.0111) were all predictive of greater proptosis reduction. On multivariable survival modeling, factors associated with increased hazard for secondary decompression include younger age (P = 0.0434), larger preoperative proptosis (P = 0.0001), unilateral decompression (P = 0.0272), preoperative steroid treatment (P = 0.0200), and normal thyroid function (P = 0.0148). Factors associated with decreased hazard include adjunctive fat decompression (P = 0.0004), balanced decompression (vs lateral, P = 0.0039), and African-American ethnicity (P = 0.0076).
Conclusions
Despite a diverse study cohort, we have identified factors associated with clinically relevant outcomes of decompression surgery for TED, including proptosis reduction and incidence of secondary decompression. Randomized controlled trials of different treatment algorithms for thyroid eye disease are needed to devise optimized guidelines for individualizing surgical care.
Keywords: thyroid eye disease, ophthalmopathy, decompression surgery, proptosis
Precis
Thyroid-related orbital decompression surgery outcomes of proptosis reduction and secondary decompression can be predicted by select clinical risk factors.
Introduction
With its capacity to cause vision loss and severe facial disfigurement, thyroid eye disease (TED) is the most morbid manifestation of thyroid autoimmunity.1 Unfortunately, TED occurs in up to half of patients with Graves’ disease and even mild TED can substantially impact patients’ quality of life, with residual effects long after the initial disease process has abated.2,3 Twenty percent of patients diagnosed with TED will require one or more surgical procedures,4 and this figure is likely higher now given the increasing usage of decompression surgery for aesthetic rehabilitation. Major indications for decompression surgery include compressive optic neuropathy (CON), aesthetic rehabilitation, corneal exposure, orbital congestion, and severe restrictive strabismus in preparation for strabismus surgery.1 Despite high patient satisfaction with the procedure, the evidence guiding decompression surgery is limited.1,5,6
The therapeutic effect of orbital decompression surgery is achieved by removing one or more walls of the orbit, with or without orbital fat removal, allowing prolapse of diseased and voluminous orbital tissue into the adjoining sinuses or newly created bony defects.1,6 Targeted orbital walls include any combination of the medial, inferior, or lateral walls and choice of technique and approach is guided more by surgeon familiarity and institutional preferences than by evidence-based guidelines.1,6 The amount of proptosis reduction achieved is usually a function of the number of walls removed, with a reported range of −6 to 11 mm and an average of 4.45 millimeters (mm) irrespective of technique in one recent review.6 Strabismus, leading to binocular diplopia, is the most common and most debilitating postoperative complication of decompression surgery, generally with highest rates reported in patients who underwent inferior and/or medial wall decompression and with decreasing rates in balanced and lateral decompressions, respectively.1,6,7
Despite the abundance of publications on orbital decompression surgery for TED, there are few comparative studies and most studies are underpowered. Even for a given decompression technique, there is a wide range of reported proptosis reductions and postoperative strabismus rates.6 The only study we identified in PubMed (using synonyms of thyroid eye disease and decompression surgery as search terms) that constructed a multivariable analysis to predict response to decompression surgery based on preoperative patient characteristics is a 1994 study examining transantral decompressions.8 In that study, the authors found that higher preoperative proptosis and a longer interval to postoperative examination (in the first 6 months after decompression) independently predicted greater proptosis reduction.8 Other studies have examined variations in individual orbital morphology parameters that may affect decompression response.6,9,10 In our search of the literature, we did not identify any studies that have evaluated predictors of repeat or additional decompressions. The goal of our study is to use multivariable analysis to identify independent patient and disease-related predictors of response to decompression surgery and of need for secondary decompressions.
Methods
Study Cohort
The University of Michigan Institutional Review Board approved this study (No. 00040783) and all data were obtained retrospectively through review of the digitized and electronic medical records (EMR). EMR was available for data collection for operations performed after 9/1/2012, while digitized paper records were used for operations performed earlier. Our Institutional Review Board approved this study with no requirement for patient consent. All consecutive patients who underwent thyroid-related transorbital decompression surgery at the Kellogg Eye Center of the University of Michigan from 4/21/1999 to 12/31/2014 were reviewed; records were not available earlier than the above starting date. The patient list was obtained using procedural billing codes. Decompressions performed for CON were excluded from the study, as the goal of these decompressions is to relieve compression of the optic nerve, and hence, should be examined using visual outcomes, not proptosis reduction. Patients whose medical records lacked either preoperative or postoperative documentation were also excluded. All decompressions were performed through a transorbital approach using small, and usually hidden, incisions.
Outcomes and Covariates
Our outcomes of interest were proptosis reduction attributable to decompression surgery and incidence of secondary decompressions. Both outcomes were analyzed at the orbit level. For proptosis reduction, we collected preoperative proptosis measurements (by Hertel, Mourits, or Naugle exophthalmometers) as close to decompression date as possible and the earliest postoperative proptosis measurement between the third and twelfth postoperative months (using the same exophthalmometer and base as the preoperative measurement whenever available). The difference between the preoperative and postoperative measurement defines the amount of change in proptosis. Attending ophthalmologist documentation was used for all measurements when available. We relied on measurements as close as possible to the third postoperative month for assessing the surgical effect, as this was consistent with several studies we encountered on our literature review.11–13 This time interval is also supported by a prior study conducted at our institution in 2011 which examined the effect of decompression surgery on eyelid retraction; in that study, the authors reported only a 0.2 mm difference in Hertel measurements between the average 3-month postoperative measurement and final postoperative measurement taken at 30 months of average follow-up (20.7 mm and 20.5 mm, respectively), showing that proptosis reduction is largely stable by the third postoperative month.14 Additionally, some patients underwent strabismus surgery as early as 4 months after decompression, which may affect proptosis measurements at a later date.15 Therefore, all of our postoperative measurements were taken before extraocular muscle recession or secondary decompressions, if performed.
We defined secondary decompression as any decompression surgery (fat and/or wall) performed on the operated-on orbit during follow-up. To be considered a secondary decompression (instead of a planned staged decompression), there must have been one postoperative visit three months or more after initial decompression with a proptosis measurement or strabismus surgery in between. In patients who had a postoperative visit with proptosis measurement, subsequent decompressions were counted as secondary decompressions, whereas those who underwent multiple decompressions without a clinic visit and proptosis measurement in between surgeries were considered to have undergone a single staged decompression. In patients who underwent multiple secondary decompressions, the first one was recorded and used for analysis of time to secondary decompression. Indications for performing secondary decompression were also recorded from preoperative clinic visit documentation and operative report.
Baseline/preoperative patient characteristics collected from the medical record included patient gender, age at initial decompression surgery, ethnicity (non-Hispanic Caucasian, African-American, Asian-American, or other), thyroid disease diagnosis at the time of surgery (hyperthyroid disease, hypothyroid disease, or no diagnosed thyroid disease), presence of thyroid dermopathy (on ophthalmologist, endocrinologist, or dermatologist documentation), smoking status (‘current’ if primary exposure or significant second-hand exposure in the year before operation; ex-smoker if no exposure in the year before operation but positive in the past; and ‘never’ if no history of primary exposure or significant second-hand exposure), history of strabismus surgery, history of radioiodine treatment, history of thyroidectomy, oral or intravenous steroid treatments in the 3 months before decompression surgery, primary or reading gaze binocular diplopia (both continuous or incontinuous by history or by ophthalmic exam). Baseline/preoperative orbital characteristics collected from the medical record included ocular symptom duration (time elapsed since onset of symptoms characteristic of TED to date of operation measured in years), history of orbital radiation, history of decompression surgery, orbital ache/pressure, disease activity status (active or inactive), and preoperative proptosis measurement in millimeters (mm). Since disease activity is a clinical diagnosis, activity was based on documentation in surgeon clinical assessment and plan; for instances in which the surgeon did not explicitly document whether disease was “active” or “inactive”, a computed clinical activity score less than 3 and six months of < 2 mm proptosis increase (if available) was used to define inactive, and documented exams that did not satisfy this criteria were considered active.16 Similar to a previous study, TED duration was converted into a categorical variable with an arbitrary cut-off of 4 years to compare patients in the early inflammatory phase with those in the chronic fibrotic phase.17
Intraoperative orbital characteristics we collected included laterality of operation, unilateral operation or part of bilateral operation, decompression technique (medial, lateral, or balanced), and whether intraconal fat was excised. Balanced decompressions included both simultaneous and staged procedures. Faculty surgeons of the Eye Plastic, Orbital and Facial Cosmetic Surgery Service performed all surgeries. All covariates and outcome data extracted from the medical record were routinely assessed throughout the study period; proportion of missing data for each covariate is also reported.
Statistical Analyses
For proptosis reduction and secondary decompression, we used mixed linear regression and Cox proportional hazards analysis with sandwich estimators, respectively, to investigate associations in univariate and multivariable models. These modeling techniques account for inter-orbit correlation, which otherwise would have resulted in falsely precise confidence intervals due to underestimation of standard errors.18 Survival analysis was used for secondary decompression as this outcome is time-dependent. The proportional hazards assumption was tested with covariate by time interactions and, when violated, the interaction was retained. Kaplan-Meier analysis was used to estimate the time-related probability of secondary decompression.
Multivariable model selection was performed using the best subset selection method with R-squared as the selection criteria for linear models and the score statistic as the selection criteria for survival models. This approach identifies the overall best model as well as closely competing models. Final models which resulted in the largest number of statistically significant independent predictors were chosen. All statistical tests were two-sided and P values < 0.05 were considered statistically significant. To reduce the possibility of Type I statistical errors, the Holm adjustment for multiple comparisons was applied to all univariate analyses. All statistical analyses were performed with commercially available software: SAS 9.4 (SAS Institute, Cary, NC).
Results
Descriptive statistics are presented in Table 1. Descriptive statistics stratified by outcome are presented in the supplemental table. The median patient follow-up interval after decompression surgery was 21 months (IQR: 9–43).
Table 1.
Descriptive statistics of study population (total of 594 orbits of 356 patients).
| Continuous Covariates |
Sample Size N (% of total) |
Average mean ± SD |
Range (lower, upper) |
| Age (years)* | 356 (100.0) | 52.2 ± 13.3 | 15.7, 87.5 |
| Preoperative proptosis (mm)† | 594 (100.0) | 24.8 ± 3.4 | 11, 35 |
| Postoperative proptosis (mm)† | 420 (70.7) | 21.0 ± 3.1 | 13, 32 |
| Proptosis reduction (mm)† | 420 (70.7) | 3.8 ± 2.4 | −1.5, 12.5 |
| Cumulative smoking exposure (pack-years)* | 284 (79.8) | 12.7 ± 17.3 | 0, 108 |
| Categorical Covariates |
Sample Size N (% of total) |
Frequency N (% of sample) |
|
| Gender | 356 (100.0) | ||
| Male | 81 (22.8) | ||
| Female | 275 (77.2) | ||
| Ethnicity | 342 (96.1) | ||
| Caucasian | 287 (83.9) | ||
| African-American | 41 (12.0) | ||
| Asian-American | 9 (2.6) | ||
| Other | 5 (1.5) | ||
| Thyroid disease | 349 (98.0) | ||
| Hyperthyroid disease | 313 (89.7) | ||
| None | 19 (5.4) | ||
| Hypothyroid disease | 17 (4.9) | ||
| Thyroid eye disease duration*† | 568 (95.6) | ||
| < 4 years | 303 (53.3) | ||
| ≥ 4 years | 265 (46.7) | ||
| Smoking status* | 343 (96.3) | ||
| Current smoker | 123 (35.9) | ||
| Ex-smoker | 93 (27.1) | ||
| Never smoker | 127 (37.0) | ||
| Thyroid dermopathy | 286 (80.3) | 18 (6.3) | |
| Orbital radiation | 350 (98.3) | 13 (3.7) | |
| Past decompression surgery† | 591 (99.5) | 41 (6.9) | |
| Past strabismus surgery | 354 (99.4) | 34 (9.6) | |
| Thyroidectomy* | 349 (98.0) | 50 (14.3) | |
| Radioactive iodine | 347 (97.5) | 231 (66.6) | |
| Steroids* | 356 (100.0) | 21 (5.9) | |
| Unilateral operation | 356 (100.0) | 85 (23.9) | |
| Walls decompressed† | 594 (100.0) | ||
| Medial | 34 (5.7) | ||
| Lateral | 299 (50.3) | ||
| Balanced | 261 (43.9) | ||
| Fat decompression† | 594 (100.0) | 388 (65.3) | |
| Activity*† | 590 (99.3) | ||
| Active | 150 (25.4) | ||
| Inactive | 440 (74.6) | ||
| Preoperative primary gaze diplopia | 348 (97.8) | 124 (35.6) | |
| Orbital ache/pressure† | 572 (96.3) | 171 (29.9) | |
| Secondary decompression† | 594 (100.0) | 82 (13.8) | |
When patient-level data differed between orbits of a patient (n ≤ 3), data from the orbit with the first decompression was used.
All numbers and percentages reported for these covariates are at the orbit level.
Proptosis reduction
Pre- and postoperative proptosis measurements were available on 420 orbits of 263 patients. Descriptive statistics of this cohort is comparable to that of the entire cohort of 594 orbits (see supplemental table). Postoperative measurements were taken at a median of 3.8 months with a range of 3 to 12 months after decompression surgery. Preoperative proptosis, irrespective of technique, was on average 24.8 ± 3.2 mm and postoperative proptosis was on average 21.0 ± 3.1 mm. The mean proptosis reduction was 3.8 ± 2.4 mm with a range of −1.5 to 12.5 mm. Medial wall decompressions were performed on 26 (6.2%) orbits and resulted in 2.1 ± 1.6 mm of proptosis reduction. Lateral wall decompressions were performed on 219 (52.1%) orbits and resulted in 3.3 ± 2.1 mm of proptosis reduction. And finally, balanced decompressions were performed on 175 (41.7%) orbits and resulted in 4.7 ± 2.5 mm of proptosis reduction. Table 2 shows univariate and multivariable results of mixed linear regression analysis of proptosis reduction.
Table 2.
Univariate and multivariate predictors of proptosis reduction. CI = confidence interval. Statistically significant P values are in bold.
| Univariate Analysis* | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Estimate | 95% CI (lower, upper) |
P value | P value† | Estimate | 95% CI (lower, upper) |
P value |
| Preoperative proptosis (per mm) | 0.41 | (0.34, 0.49) | <0.0001 | 0.0024 | 0.40 | (0.33, 0.48) | <0.0001 |
| Walls decompressed | <0.0001 | 0.0024 | |||||
| Medial | −0.72 | (−1.66, 0.22) | 0.1327 | −0.32 | (−1.24, 0.61) | 0.5002 | |
| Lateral | Reference | Reference | Reference | Reference | |||
| Balanced | 1.34 | (0.87, 1.81) | <0.0001 | 0.88 | (0.42, 1.33) | 0.0002 | |
| Thyroid eye disease duration | |||||||
| < 4 years | 0.49 | (−0.07, 1.06) | 0.0861 | 1.0000 | 0.67 | (0.17, 1.18) | 0.0093 |
| ≥ 4 years | Reference | Reference | Reference | Reference | |||
| Orbital Radiation | |||||||
| Yes | 0.63 | (−0.67, 1.93) | 0.3433 | 1.0000 | 1.56 | (0.36, 2.77) | 0.0111 |
| No | Reference | Reference | Reference | Reference | |||
| Activity | |||||||
| Active | 0.91 | (0.28, 1.55) | 0.0050 | 0.1050 | |||
| Inactive | Reference | Reference | |||||
| Preoperative primary gaze diplopia | |||||||
| Yes | 0.93 | (0.35, 1.52) | 0.0019 | 0.0418 | |||
| No | Reference | Reference | |||||
Univariate results: only covariates with either adjusted P values < 0.30 on univariate analysis or that became significant on multivariable analysis are shown.
P values adjusted for multiple comparisons using the Holm method.
Secondary decompressions
Of 594 orbits that underwent orbital decompression surgery, secondary decompressions were necessary in 82 (13.8%). Table 3 shows univariate and multivariable results of cox proportional hazards analysis of secondary decompression. Several covariates had hazard ratios (HR) for secondary decompression that changed over time on univariate modeling. Covariates with P values < 0.3 after adjustment for multiple comparisons (MC) are listed here. African-Americans (vs Caucasians) had a HR of 0.30 one year after decompression that weakened to a HR of 0.37 at 7 years, with P values of < 0.0001 and 0.0025 before and after MC adjustment. ‘Other’ ethnicity (vs Caucasian) had a HR of 12.36 at 1 year, 5.16 at 3 years, 2.16 at 5 years, and 0.90 at 7 years, with P values of 0.0028 and 0.0616 before and after adjustment for MC. And finally, active disease (vs. inactive) had HR of 1.17 at 1 year, 1.23 at 3 years, 1.29 at 5 years, and 1.36 at 7 years with P values of 0.0035 and 0.0735 before and after MC adjustment, respectively.
Table 3.
Univariate and multivariable predictors of time to secondary decompression. Of note, the hazard ratios for preoperative proptosis, ethnicity, and active disease change over time (see results section), and thus only significance of the covariate by time interaction is reported here. Indications for secondary decompression include proptosis (n = 79), exposure keratopathy (n = 74), compressive optic neuropathy (n = 6), and/or other (n = 15). CI = confidence interval. Statistically significant P values are in bold.
| Univariate Analysis* | Multivariable Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Covariate | Hazard Ratio |
95% CI (lower, upper) |
P value | P value† | Hazard Ratio |
95% CI (lower, upper) |
P value |
| Age (per year) | 0.93 | (0.85, 1.02) | 0.3640 | 1.0000 | 0.91 | (0.83, 1.00) | 0.0434 |
| Preoperative proptosis (per mm) | 0.0240 | 0.4560 | 1.20 | (1.10, 1.32) | 0.0001 | ||
| Unilateral operation | |||||||
| Yes | 1.92 | (1.10, 3.45) | 0.0221 | 0.4420 | 2.00 | (1.08, 3.70) | 0.0272 |
| No | Reference | Reference | Reference | Reference | |||
| Steroids | |||||||
| Yes | 1.72 | (0.66, 4.49) | 0.2642 | 1.0000 | 2.48 | (1.15, 5.32) | 0.0200 |
| No | Reference | Reference | Reference | Reference | |||
| Fat decompression | |||||||
| Yes | 0.26 | (0.14, 0.45) | <0.0001 | 0.0025 | 0.32 | (0.17, 0.60) | 0.0004 |
| No | Reference | Reference | Reference | Reference | |||
| Walls decompressed | 0.0021 | 0.0483 | 0.0059 | ||||
| Medial | 1.46 | (0.61, 3.51) | 0.3975 | 1.73 | (0.75, 3.97) | 0.1993 | |
| Lateral | Reference | Reference | Reference | Reference | |||
| Balanced | 0.42 | (0.25, 0.72) | 0.0014 | 0.41 | (0.23, 0.75) | 0.0039 | |
| Ethnicity | 0.0390 | ||||||
| Caucasian | Reference | Reference | Reference | Reference | |||
| African-American | <0.0001 | 0.0025 | 0.33 | (0.15, 0.75) | 0.0076 | ||
| Asian-American | 0.2640 | 1.0000 | 0.72 | (0.09, 5.60) | 0.7536 | ||
| Other | 0.0028 | 0.0616 | 1.81 | (0.31, 10.4) | 0.5084 | ||
| Thyroid disease | 0.0591 | 1.0000 | 0.0347 | ||||
| Hyperthyroid disease | Reference | Reference | Reference | Reference | |||
| None | 2.79 | (1.08, 7.21) | 0.0337 | 2.77 | (1.22, 6.28) | 0.0148 | |
| Hypothyroid disease | 1.94 | (0.67, 5.60) | 0.2206 | 1.62 | (0.65, 4.08) | 0.3033 | |
| Activity | |||||||
| Active | 0.0035 | 0.0735 | |||||
| Inactive | Reference | Reference | |||||
Univariate results: only covariates with either adjusted P values < 0.30 on univariate analysis or that became significant on multivariable analysis are shown.
P values adjusted for multiple comparisons using the Holm method.
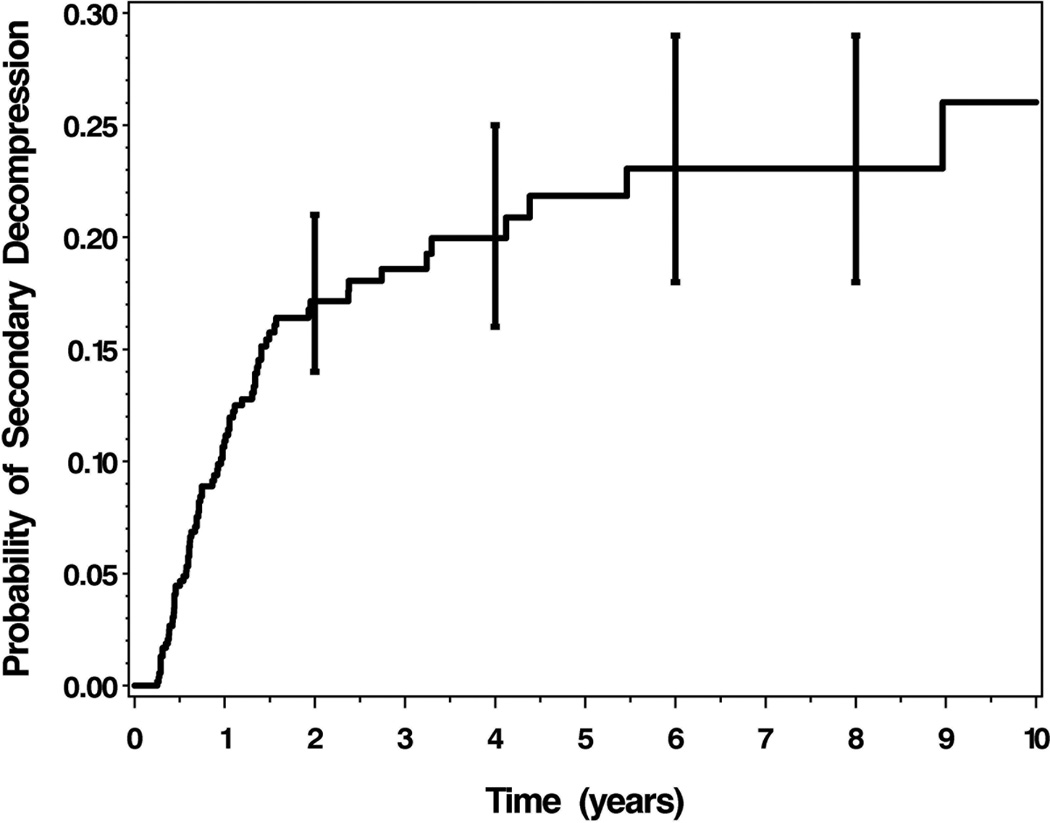
Figure 1 displays the time-related probability of secondary decompression. Most secondary decompressions occurred within the first 2 years after initial decompression (88%, 72 of 82). The probability of secondary decompression 1 year after initial decompression was 0.11, and this probability increased to 0.20, 0.23, and 0.23 at years 4, 6, and 8, respectively.
Discussion
The goal of our study was to identify factors that influence outcomes of orbital decompression. We constructed statistical models to identify predictors of proptosis reduction and predictors of secondary decompression. Factors that were directly associated with increased proptosis reduction include larger preoperative proptosis, balanced decompression technique, and active disease. Factors associated with an increased hazard of secondary decompression include younger age, higher preoperative proptosis, unilateral operations, preoperative steroid treatment, and normal thyroid function. Factors associated with decreased hazard for secondary decompression include fat decompression in conjunction with bone removal, balanced decompression technique (compared to lateral), and African-American ethnicity (compared to Caucasian). This study shows that a few select clinical risk factors can influence response to orbital decompression surgery. Knowledge of these factors can help guide orbital surgeons in caring for and counseling thyroid eye disease patients.
In a recent review examining the predictability of exophthalmos reduction after decompression surgery, Borumani et al concluded that there was a wide range of proptosis reduction for a given technique, suggesting that factors other than surgical technique may influence decompression response.6 The authors mentioned individual orbital morphology, size of the globe, globe-orbital volume ratio, and stiffness of orbital tissue as factors that may be contributory. Studies examining orbital morphology, however promising, are still incipient, and have not yet provided an evidence base for its role.6,9,10 The relevance of orbital tissue stiffness, or compliance, results from the natural history of TED. In the early phase of the disease, tissue is inflamed and congested due to autoimmune activity, while in later end-stages, the disease becomes fibrotic, with a theoretical decrease in compliance and decreased potential for herniation into surgically created spaces.6,10 Our results are consistent with this concept as TED duration < 4 years at the time of surgery was an independent predictor of higher proptosis reduction after adjusting for preoperative proptosis level and decompression technique. Additionally, in agreement with prior studies, we found that proptosis reduction was a function of the number of walls removed and that higher preoperative proptosis predicted more proptosis reduction.6,8,12,18,19 We propose two explanations for the latter finding: the difference between absolute versus relative reduction in proptosis, and increased surgeon aggressiveness with higher preoperative proptosis measurements: regardless of surgical technique or other parameters, surgeons may be more aggressive in patients with higher preoperative proptosis.
Interestingly, despite having only 24 orbits of 13 patients with a history of orbital radiation in our study, orbital radiation was an independent predictor of higher proptosis reduction. A prior retrospective comparative case series involving 61 patients found no effect on proptosis reduction attributable to orbital radiation, although the study was limited to aesthetic patients without strabismus, with a radiation cohort of only 29 patients.20 As orbital radiation has been shown to modestly improve extraocular motility impairment, perhaps it can also lessen extraocular muscle swelling after decompression surgery, and as a result, lead to greater proptosis reduction.15,21 Further studies are needed, as a Type I statistical error with this result is possible given the small sample size and the contradictory reports from literature.
Secondary decompression was necessary in 13.8% of orbits, most of which occurred in the first two years after primary decompression, with fewer secondary decompressions noted during longer follow-up. Our incidence rate is similar to the 9% reported in a descriptive study in 2014.22 Proptosis and exposure keratopathy were the most common indications for secondary decompression. As expected, larger preoperative proptosis confers increased hazard for secondary decompression, suggesting that more surgeries are necessary to correct larger preoperative proptosis. Thus, to decrease the number of needed surgeries, it may be advisable to perform balanced decompressions and to use fat decompressions for orbits with larger preoperative proptosis, as these interventions are associated with fewer secondary decompressions.
Our results also present several factors associated with hazard of secondary decompression. Firstly, younger age predisposes to secondary decompressions. It is unclear why younger patients are predisposed to needing multiple decompression surgeries. Based on our clinical experience, we speculate that younger patients are more likely than older patients to undergo additional surgeries for aesthetic concerns. Importantly, facial appearance is a significant quality of life issue for patients and should not be trivialized.2 Patients who underwent unilateral operations for asymmetric disease may be prone to secondary decompressions due to residual asymmetry, as studies otherwise have not suggested that unilateral disease is more severe than the more usual bilateral phenotype.23 Patients without thyroid disease may be prone to secondary decompressions due to the fact that a significant proportion of these patients will eventually present with overt thyroid dysfunction, which may exacerbate their TED.24 In fact, as many as 41% of patients present with eye findings before manifesting thyroid dysfunction.3 Additionally, thyroid hormone imbalance is a known risk factor for severe TED and may possibly lead to further decompression surgeries.25 Alternatively, preoperative treatment with steroids likely increased the hazard of secondary decompressions because steroid usage is a marker for severe disease. Factors associated with decreased hazard of secondary decompression include African-American ethnicity and balanced decompressions as opposed to lateral decompression, as this operation will attain a more robust proptosis reduction, decreasing risk of residual proptosis.26 The decreased hazard of secondary decompressions in African-American patients is interesting because (1) TED in African-Americans has been generally under-studied, and (2) the baseline higher exophthalmometry measurements in African Americans may make it easier to achieve a natural appearance following primary decompression surgery. Additional factors may be involved, and future studies of TED in African American patients are warranted.
Finally, while we did not find that fat decompression resulted in more proptosis reduction, we did find that it was associated with a reduced hazard for secondary decompression (commonly, 0.5 to 2 milliliters of fat are removed when combined with bony decompressions). This may be due to the fact that our surgeons have a lower threshold for secondary decompression surgery if fat removal was not done in the primary decompression. Another hypothesis may be that the removal of adipose tissue and its pockets of orbital fibroblasts (a primary player in TED pathophysiology) may improve the natural course of thyroid eye disease, though this hypothesis remains entirely speculative. Irrespective of mechanism, removing fat along with bone during primary decompression may be advisable to reduce the risk of needing additional surgeries.
Our study has important limitations. Our study was retrospective, the study cohort was diverse, and the study length was long to increase sample size. Out of 594 orbits included in our study, only 420 orbits had proptosis reduction calculated and analyzed. The other 174 orbits either did not have postoperative exophthalmos measurements, didn’t have measurements that fell into our defined time points, or underwent strabismus surgery or secondary decompression surgery before a usable proptosis measurement was made. It is possible that many of these 174 orbits had a robust response to decompression and as a result, the surgeons did not feel it was necessary to measure proptosis, in which case our results would underestimate proptosis reductions. A larger proportion of patients who underwent surgery for more minor aesthetic rehabilitation did not have postoperative proptosis measurements, and hence our results may be more applicable to patients who underwent surgery for medical indications. And finally, our patients underwent decompression for a variety of indications and decompressions were performed by multiple surgeons. Different surgeon cohorts or cohorts decompressed for a specific indication will naturally have baseline differences in preoperative proptosis level, disease activity, and epidemiological characteristics. However, our study patients undergo a similar treatment algorithm (unlike patients with CON, who undergo a different and more aggressive treatment algorithm, and in whom proptosis is not an indicator of disease severity) and that our use of multivariable analysis could adjust for measured baseline differences and calculate the contributions of each of these factors to our outcomes of interest.
In conclusion, we have presented several risk factors that were associated with response to orbital decompression surgery for thyroid eye disease, affecting proptosis reduction and incidence of secondary decompressions. Further studies seeking to increase the predictability of decompression surgery are warranted. Most importantly, randomized controlled trials of different surgical intervention algorithms for thyroid eye disease are needed in order to optimize treatment guidelines for individualizing surgical care.
Supplementary Material
Acknowledgments
This work was funded in part by a grant from the Alliance for Vision Research and the Helmut Stern Professorship. AK is the recipient of a Physician-Scientist award from Research to Prevent Blindness, Inc. The authors would like to gratefully acknowledge support from an unrestricted departmental grant from RPB to Ophthalmology and Visual Sciences. This work utilized the Core Center for Vision Research funded by P30 EY007003 from the National Eye Institute.
Supported in part by a departmental grant from Research to Prevent Blindness, New York, NY
The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
No conflicting relationships exist for any author
Presented at the ASOPRS 46th Annual Fall Scientific Symposium, Las Vegas, Nevada, November 12, 2015
References
- 1.Boboridis KG, Bunce C. Surgical orbital decompression for thyroid eye disease. Cochrane Database Syst Rev. 2011;(12):CD007630. doi: 10.1002/14651858.CD007630.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Estcourt S, Quinn AG, Vaidya B. Quality of life in thyroid eye disease: impact of quality of care. Eur J Endocrinol. 2011;164:649–655. doi: 10.1530/EJE-11-0055. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves’ ophthalmopathy. Thyroid. 2002;12:855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 4.Bartley GB, Fatourechi V, Kadrmas EF, et al. The treatment of Graves’ ophthalmopathy in an incidence cohort. Am J Ophthalmol. 1996;121:200–206. doi: 10.1016/s0002-9394(14)70585-9. [DOI] [PubMed] [Google Scholar]
- 5.Tehrani M, Krummenauer F, Mann WJ, et al. Disease-specific assessment of quality of life after decompression surgery for Graves’ ophthalmopathy. Eur J Ophthalmol. 2004;14:193–199. [PubMed] [Google Scholar]
- 6.Borumandi F, Hammer B, Kamer L, von Arx G. How predictable is exophthalmos reduction in Graves’ orbitopathy? A review of the literature. Br J Ophthalmol. 2011;95:1625–1630. doi: 10.1136/bjo.2010.181313. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg RA, Perry JD, Hortaleza V, Tong JT. Strabismus after balanced medial plus lateral wall versus lateral wall only orbital decompression for dysthyroid orbitopathy. Ophthal Plast Reconstr Surg. 2000;16:271–277. doi: 10.1097/00002341-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fatourechi V, Bergstralh EJ, Garrity JA, et al. Predictors of response to transantral orbital decompression in severe Graves’ ophthalmopathy. Mayo Clin Proc. 1994;69:841–848. doi: 10.1016/s0025-6196(12)61785-6. [DOI] [PubMed] [Google Scholar]
- 9.Kamer L, Noser H, Schramm A, et al. Anatomy-based surgical concepts for individualized orbital decompression surgery in graves orbitopathy. I. Orbital size and geometry. Ophthal Plast Reconstr Surg. 2010;26:348–352. doi: 10.1097/IOP.0b013e3181c9bb52. [DOI] [PubMed] [Google Scholar]
- 10.Wilson WB, Manke WF. Orbital decompression in Graves’ disease. The predictability of reduction of proptosis. Arch Ophthalmol. 1991;109:343–345. doi: 10.1001/archopht.1991.01080030045035. [DOI] [PubMed] [Google Scholar]
- 11.Sasim IV, de Graaf ME, Berendschot TT, et al. Coronal or swinging eyelid decompression for patients with disfiguring proptosis in Graves’ orbitopathy? Comparison of results in one center. Ophthalmology. 2005;112:1310–1315. doi: 10.1016/j.ophtha.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 12.European Group on Graves’ Orbitopathy (EUGOGO) Mourits MP, Bijl H, et al. Outcome of orbital decompression for disfiguring proptosis in patients with Graves’ orbitopathy using various surgical procedures. Br J Ophthalmol. 2009;93:1518–1523. doi: 10.1136/bjo.2008.149302. [DOI] [PubMed] [Google Scholar]
- 13.Kikkawa DO, Pornpanich K, Cruz RC, Jr, et al. Graded orbital decompression based on severity of proptosis. Ophthalmology. 2002;109:1219–1224. doi: 10.1016/s0161-6420(02)01068-0. [DOI] [PubMed] [Google Scholar]
- 14.Cho RI, Elner VM, Nelson CC, Frueh BR. The effect of orbital decompression surgery on lid retraction in thyroid eye disease. Ophthal Plast Reconstr Surg. 2011;27:436–438. doi: 10.1097/IOP.0b013e3182232465. [DOI] [PubMed] [Google Scholar]
- 15.Gomi CF, Yang SW, Granet DB, et al. Change in proptosis following extraocular muscle surgery: effects of muscle recession in thyroid-associated orbitopathy. J AAPOS. 2007;11:377–380. doi: 10.1016/j.jaapos.2007.01.115. [DOI] [PubMed] [Google Scholar]
- 16.Mourits MP, Prummel MF, Wiersinga WM, Koornneef L. Clinical activity score as a guide in management of patients with Graves’ ophthalmopathy. Clin Endocrinol (Oxf) 1997;47:9–14. doi: 10.1046/j.1365-2265.1997.2331047.x. [DOI] [PubMed] [Google Scholar]
- 17.Baldeschi L, Wakelkamp IM, Lindeboom R, et al. Early versus late orbital decompression in Graves’ orbitopathy: a retrospective study in 125 patients. Ophthalmology. 2006;113:874–878. doi: 10.1016/j.ophtha.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 18.Murdoch IE, Morris SS, Cousens SN. People and eyes: statistical approaches in ophthalmology. Br J Ophthalmol. 1998;82:971–973. doi: 10.1136/bjo.82.8.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalmann R, Mourits MP, van der Pol JP, Koornneef L. Coronal approach for rehabilitative orbital decompression in Graves’ ophthalmopathy. Br J Ophthalmol. 1997;81:41–45. doi: 10.1136/bjo.81.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baldeschi L, MacAndie K, Koetsier E, et al. The influence of previous orbital irradiation on the outcome of rehabilitative decompression surgery in graves orbitopathy. Am J Ophthalmol. 2008;145:534–540. doi: 10.1016/j.ajo.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 21.Alsuhaibani AH, Carter KD, Policeni B, Nerad JA. Effect of orbital bony decompression for Graves’ orbitopathy on the volume of extraocular muscles. Br J Ophthalmol. 2011;95:1255–1258. doi: 10.1136/bjo.2010.188946. [DOI] [PubMed] [Google Scholar]
- 22.Zhang-Nunes SX, Dang S, Garneau HC, et al. Characterization and outcomes of repeat orbital decompression for thyroid-associated orbitopathy. Orbit. 2015;34:57–65. doi: 10.3109/01676830.2014.949784. [DOI] [PubMed] [Google Scholar]
- 23.Kashkouli MB, Kaghazkanani R, Heidari I, et al. Bilateral versus unilateral thyroid eye disease. Indian J Ophthalmol. 2011;59:363–366. doi: 10.4103/0301-4738.83612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo DH, Eng PH, Ho SC, et al. Graves’ ophthalmopathy in the absence of elevated free thyroxine and triiodothyronine levels: prevalence, natural history, and thyrotropin receptor antibody levels. Thyroid. 2000;10:1093–1100. doi: 10.1089/thy.2000.10.1093. [DOI] [PubMed] [Google Scholar]
- 25.Prummel MF, Wiersinga WM, Mourits MP, et al. Effect of abnormal thyroid function on the severity of Graves’ ophthalmopathy. Arch Intern Med. 1990;150:1098–1101. [PubMed] [Google Scholar]
- 26.Dunsky IL. Normative data for hertel exophthalmometry in a normal adult black population. Optom Vis Sci. 1992;69:562–564. doi: 10.1097/00006324-199207000-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.