Abstract
Background
Muscle wasting is a component of the diagnosis of cancer cachexia and has been associated with poor prognosis. However, recommended tools to measure sarcopenia are limited by poor sensitivity or the need to perform additional scans. We hypothesized that pectoralis muscle area (PMA) measured objectively on chest CT scan may be associated with overall survival in non-small cell lung cancer (NSCLC).
Methods
We evaluated two hundred fifty two cases from a prospectively enrolling lung cancer cohort. Eligible cases had CT scans performed prior to the initiation of surgery, radiation, or chemotherapy. PMA was measured in a semi-automated fashion while blinded to characteristics of the tumor, lung, and patient outcomes.
Results
Men had a significantly greater PMA than women (37.59 vs 26.19 cm2, P<0.0001). In univariate analysis, PMA was associated with age and BMI. A Cox proportional hazards model was constructed to account for confounders associated with survival. Lower pectoralis area (per cm2) at diagnosis was associated with an increased hazard of death of 2% (HRadj 0.98 [0.96, 0.99], P=0.044) while adjusting for age, sex, smoking, chronic bronchitis, emphysema, histology, stage, chemotherapy, radiation, surgery, BMI, and ECOG performance status. .
Conclusions
Lower pectoralis muscle area measured from chest CT scans obtained at the time of diagnosis of NSCLC is associated with a worse overall survival.
Impact
Pectoralis muscle area may be a valuable CT biomarker for sarcopenia associated lung cancer survival.
Keywords: Non-small cell lung cancer, Pectoralis muscle area, Sarcopenia
INTRODUCTION
Cancer cachexia has long been recognized as a consequence of malignancy and in lung cancer is associated with decreased physical performance, inability to tolerate therapy, and an overall poor prognosis.(1-4) International consortia have thus identified early recognition of cancer cachexia as a critical goal, calling for interventions to be targeted early in the process rather than when end-stage wasting occurs.(5)
Cachexia is defined by international consensus as weight loss greater than 5% or weight loss greater than 2% in individuals already showing depletion according to body mass index (BMI) or sarcopenia.(6) Thus, accurately measuring muscle loss associated with sarcopenia may dramatically improve early detection of cachexia. Importantly, there is evidence that sarcopenia is associated with a poorer overall prognosis for a number of cancers. (7-9) Current recommendations for measurement of sarcopenia include anthropometric measurement of body dimensions (e.g. mid-upper arm), dual energy x-ray absorbance (DXA), bioimpedance studies, or abdominal computed tomography (CT) scan. (6,10) However, these measurements all have limitations, particularly when applied to patients with lung cancers. Anthropometric measurements of skin fold thickness and body circumference overestimate muscularity by 15-25% compared to abdominal CT and are associated with high within-subject error.(11) Bioimpedance is only considered valid in patients with “grossly altered body composition” limiting its utility for early detection of cachexia (6), while DXA and abdominal CT scanning are not routinely performed for evaluation and follow up of patients with lung cancer.
Patients with chronic obstructive pulmonary disease (COPD), in the absence of cancer, are also plagued by cachexia.(12) Pectoralis muscle area (PMA), measured from CT scans of the chest, has been demonstrated to correlate with bioimpedance measures of fat-free mass, as well as with lung function, symptoms, and exercise capacity.(13) Importantly, PMA is a better predictor of these outcomes than BMI, implying that PMA may be an independent measure of muscularity. We hypothesized that PMA, a novel marker of sarcopenia, may be associated with overall survival for patients with non-small cell lung cancer (NSCLC).
MATERIALS AND METHODS
Patients
This study was approved by the Partners Human Research Committee (1999-P-004935/118). Details of enrollment and follow up of this cohort have been described previously.(14-16) Briefly, patients (>18 years old) with pathologically confirmed, newly diagnosed, NSCLC were consecutively recruited and followed at Massachusetts General Hospital between 2002 and 2006. This time period was selected in order to have adequate follow up time for analysis of overall survival. More than 85% of eligible patients were enrolled. Detailed demographic, past medical history, and exposure history were collected via a dedicated questionnaire, as previously described.(17-19) This questionnaire included questions that asked the subject if they had ever been diagnosed, by a physician, with either chronic bronchitis or emphysema (the two components of chronic obstructive pulmonary disease, COPD).
CT Scans
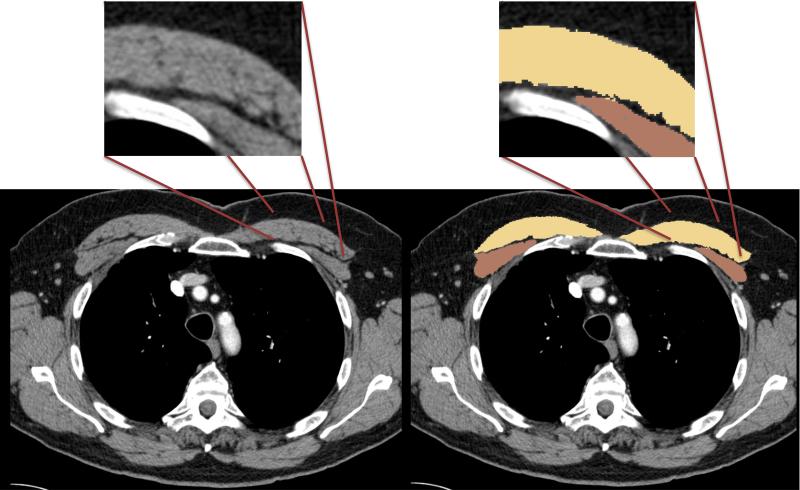
Included cases had to have a chest CT scan with intravenous contrast performed prior to initiation of surgery, radiation, or chemotherapy, and within three months of enrollment in the study. All CT scans were acquired with GE scanners (GE Healthcare, Waukesha WI) at either a 2.5 or 5mm slice thickness and constructed using the “Standard Body” kernel. All CT scans were performed with the arms in the adducted position. Measurement of pectoralis area was performed using 3D Slicer (www.slicer.org).(14,20-23) Quantitative assessment of pectoralis muscle was performed on a single axial slice of the CT scan above the aortic arch by a single reader (CMK). This reader was trained by the senior author (GRW), who has extensive experience with this methodology. (13,24) The reader demonstrated an intrareader variability of 0.97 (concordance correlation coefficient) in this cohort. The slice used for evaluation was selected by scrolling toward the apex of the lungs and identifying the first axial image above the arch. The ability of a reader to select this slice across large cohorts, and among different cohorts, has previously been demonstrated and the subsequent measurement of PMA using this slice shown to have low interreader variability.(13,24) The borders of the pectoralis muscles were then identified objectively using the predefined attenuation thresholds of −50 to 90 Hounsfield Units (Figure 1). Using these predefined thresholds limited potential bias when identifying the borders of the muscles. These measures were performed for each subject and pectoralis muscle area (cm2) presented as the aggregate of right and left pectoralis major and minor muscles.
Figure 1.
Axial CT images demonstrating measurement of pectoralis muscle area. The areas shaded in yellow are the pectoralis major, while the areas in brown are the pectoralis minor. Pectoralis muscle area (PMA) is the sum of the yellow and brown areas. The inset demonstrates the utility of using apriori Hounsfield unit thresholds to define the borders of the pectoralis muscles. This approach allows the border of the muscles to be determined in an unbiased and accurate manner.
Statistical Analysis
All statistical analyses were performed using STATA (Version 12, College Station, TX). P values less than or equal to 0.05 were considered significant and all statistical tests were two-sided. Student's t-test was used to compare normally distributed data between categories. ANOVA was used to evaluate differences in continuous variable among more than two categories. Simple linear regression was used to evaluate the relationship between two continuous variables. Overall survival time was computed as the number of days from study entry until death from any cause. Patients still alive were censored at the most recent follow-up date.
Initial survival analysis was performed by dichotomizing PMA at the 50th percentile and creating Kaplan-Meier survival curves. Given the known dramatic differences in PMA between men and women, these curves were stratified by gender.(24). For time-to-event analyses, the results of the Wilcoxon (Breslow) test for equality of the survivor function were performed to minimize the effect of the small risk sets in the tails of the survival distributions. The stratified version of this test was used to evaluate for statistical significance among the strata of gender and histology (adenocarcinoma vs other).
Two different Cox proportional hazards models were created to account for confounders associated with survival. We included PMA as a continuous predictor in each model. For categorical predictors, we used Wald tests to evaluate the overall association between the predictor and survival. The “Full” model included all predictors listed in Table 1. The “Reduced” model included only those predictors having a p-value ≤0.1. The proportional hazards assumption was tested via the non-zero slope method of Therneau and Grambsch.(25)
Table 1.
Characteristics of the Cohort
| Age (y):* | 66.2 (±10.9) | 252 |
| Sex: n (%) | ||
| Female | 135 (53.6) | |
| Male | 117 (46.4) | 252 |
| Smoking: n (%) | ||
| Never | 29 (11.5) | |
| Former | 139 (55.2) | |
| Current | 84 (33.3) | 252 |
| Chronic bronchitis: n (%) | 31 (12.6) | 245 |
| Emphysema: n (%) | 41 (16.7) | 245 |
| BMI:* | 26.4 (±4.9) | 237 |
| ECOG Performance: n (%) | ||
| Status 0-2 | 240 (96.5) | |
| Status 3-4 | 9 (3.5) | 249 |
| Histology: n (%) | ||
| Adenocarcinoma | 136 (53.9) | |
| Squamous Cell | 33 (13.1) | |
| Large Cell | 17 (6.8) | |
| NSCLC (NOS/mixed) | 66 (26.2) | 252 |
| Stage: n (%) | ||
| I | 48 (19.1) | |
| II | 10 (4.0) | |
| III | 69 (27.4) | |
| IV | 124 (49.5) | 252 |
| Therapy: | ||
| Surgery: n (%) | 105 (41.7) | 252 |
| Chemotherapy: n (%) | 191 (75.8) | 252 |
| Radiation: n (%) | 76 (30.2) | 252 |
mean ± standard deviation presented
RESULTS
During the time period noted, 304 cases were enrolled in the study. Two hundred sixty cases had CT scans available for evaluation. In eight cases the CT scan was unreadable for PMA. A total of 252 cases were included in the main analysis. The demographics and characteristics of the cohort are shown in Table 1. Data on BMI were available for 237 (94%) cases and on Eastern Cooperative Oncology (ECOG) performance score for 249 (99%) cases. There were no significant differences in demographic data between those with CT scans that were available vs. those without CT data. One-hundered ninety one individuals (75%) received chemotherapy and 105 (41%) underwent surgery. Of the 76 (30%) patients who received radiation therapy only one was Stage I.
PMA was significantly larger for men than for women (37.6 vs 26.2 cm2, P<0.0001). Notably the standard deviation of PMA for men was much larger than for women (10.9 vs. 7.2). Associations of PMA with demographic variables are listed in Table 2. Lower PMA was associated with increased age and lower BMI.
Table 2.
Relationship of PMA and Demographic Variables
| Women | Men | |||
|---|---|---|---|---|
| PMA (cm2) | P | PMA (cm2) | P | |
| Total: | 26.2 (±7.9) | 37 (±10.2) | ||
| Age: (change per year) | −0.235 | 0.047 | −0.321 | 0.001 |
| Smoking | ||||
| Never | 27.3 (±7.3) | 39.1 (±13.1) | ||
| Former | 26.3 (±7.8) | 36.9 (±9.4) | ||
| Current | 25.5 (±8.3) | 0.707 | 38.5 (±10.8) | 0.651 |
| Chronic Bronchitis | ||||
| No | 25.9 (±8.1) | 31.5 (±10.9) | ||
| Yes | 27.8 (±8.0) | 0.334 | 31.9 (±9.8) | 0.731 |
| Emphysema | ||||
| No | 26.7 (±8.2) | 31.8 (±10.9) | ||
| Yes | 22.7 (±5.8) | 0.050 | 30.4 (10.1) | 0.416 |
| Histology: | ||||
| Adenocarcinoma | 26.3 (±7.3) | 39.1 (±11.3) | ||
| Squamous Cell | 24.9 (±6.1) | 36.2 (±7.6) | ||
| Large Cell | 26.6 (±12.7) | 32.1 (±10.2) | ||
| NSCLC (NOS/mixed) | 26.1 (±8.0) | 0.955 | 36.1 (±8.6) | 0.243 |
| Stage | ||||
| I | 26.0 (±6.6) | 34.2 (±8.4) | ||
| II | 26.7 (±8.0) | 39.9 (±10.5) | ||
| III | 27.3 (±7.2) | 39.3 (±7.0) | ||
| IV | 25.6 (±8.9) | 0.788 | 37.7 (±11.8) | 0.341 |
| ECOG Performance | ||||
| Status 0-2 | 26.2 (±7.9) | 37.7 (±10.0) | ||
| Status 3-4 | 27.0 (±9.2) | 0.811 | 38.1 (±15.2) | 0.927 |
| BMI (change per unit) | 0.603 | <0.000 | 0.874 | <0.001 |
All data presented as mean ± standard deviation with the exception of age and BMI
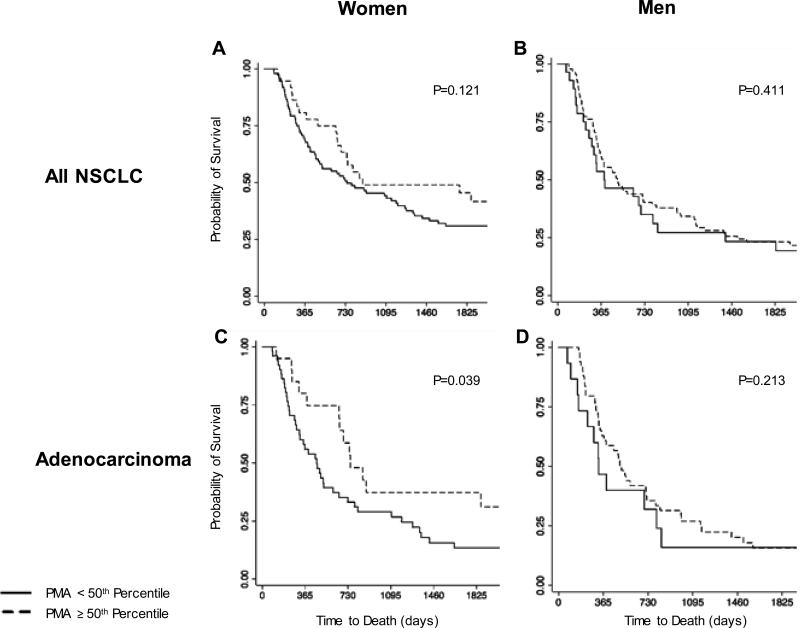
The median follow up time for the cohort was 2412 days (range: 63 to 3373 days). Figure 2 shows the Kaplan-Meier analysis of overall survival according to gender. As demonstrated in the survival curves in Figure 2, PMA < 50th percentile was associated with a possible trend toward worse overall survival for both men and women, although this was not statistically significant (Figure 2, Panels A and B). When limited to the most common histology of adenocarcinoma, the association of PMA <50th percentile with a worse overall survival was stronger in each analysis (Panels C and D), reaching statistical significance independently for the strata of women (P=0.039).
Figure 2.
Kaplan-Meier estimates of overall survival by dichotomized pectoralis muscle area (PMA) among different strata within the cohort. Curves were generated for all NSCLC cases among women (Panel A) and men (Panel B),. Panels C (women), and D (men) demonstrate survival curves in patients with adenocarcinoma histology (representing over half of all cases).
To account for possible confounders of the relationship between PMA and NSCLC survival we performed a regression analysis using the Cox proportional hazards model. The “Full” model included PMA and all variables included in Table 1. Lower PMA (per cm2) at diagnosis was associated with a 2% increase in the hazard ratio for death while adjusting for age, sex, smoking, chronic bronchitis, emphysema, histology, stage, radiation, chemotherapy, surgery, BMI, and ECOG performance score (HRadj 0.98 [0.96, 0.99], P=0.044, Table 3). A test of the proportional hazards assumption found no evidence that the assumption was violated.
Table 3.
Proportional Hazards Models for Risk of Death
| Adjusted Estimates | ||||||
|---|---|---|---|---|---|---|
| Full Model | Reduced Model | |||||
| HRAdj | CI | P | HRAdj | CI | P | |
| Pectoralis Muscle Area* | 0.98 | [0.96, 0.99] | 0.044 | 0.97 | [0.96, 0.99] | 0.002 |
| Age: | 1.01 | [0.99, 1.03] | 0.297 | |||
| Sex: | ||||||
| Male | 1.65 | [1.11, 2.46] | 0.014 | 1.80 | [1.24, 2.63] | 0.002 |
| Female | Ref | Ref | Ref | Ref | ||
| Smoking | ||||||
| Never | Ref | Ref | ||||
| Former | 0.85 | [0.49, 1.43] | ||||
| Current | 0.84 | [0.48, 1.44] | 0.797 | |||
| Chronic Bronchitis | 1.09 | [0.66, 1.80] | 0.745 | |||
| Emphysema | 1.51 | [0.98, 2.33] | 0.064 | 1.51 | [0.98, 2.19] | 0.070 |
| Histology: | ||||||
| Adenocarcinoma | Ref | Ref | Ref | Ref | ||
| Squamous Cell | 0.82 | [0.50, 1.36] | 0.83 | [0.50, 1.36] | 0.451 | |
| Large Cell | 0.66 | [0.36, 1.21] | 0.64 | [0.35, 1.17] | 0.147 | |
| NSCLC (NOS/mixed) | 0.62 | [0.41, 0.94] | 0.121 | 0.61 | [0.41, 0.94] | 0.113 |
| Stage | ||||||
| I | Ref | Ref | Ref | Ref | ||
| II | 1.72 | [0.52, 5.65] | 1.46 | [0.45, 4.73] | ||
| III | 4.05 | [1.56, 10.5] | 3.46 | [1.36, 8.81] | ||
| IV | 7.32 | [3.07, 17.4] | <0.001 | 6.28 | [2.69, 14.7] | <0.001 |
| Radiation | 1.03 | [0.59, 1.79] | 0.915 | |||
| Chemotherapy | 0.73 | [0.37, 1.41] | 0.344 | |||
| Surgery | 0.57 | [0.37, 0.87] | 0.010 | 0.59 | [0.39, 0.90] | 0.015 |
| ECOG Performance | ||||||
| Status 0-2 | Ref | Ref | Ref | Ref | ||
| Status 3-4 | 2.01 | [0.95, 4.25] | 0.070 | 2.15 | [1.03, 4.446] | 0.101 |
| BMI | 0.98 | [0.94, 1.02] | 0.262 | |||
per 1 cm2 increase in pectoralis muscle area (PMA)
ECOG PS: Eastern Cooperative Oncology Group Performance Status, BMI: Body Mass Index
For chronic bronchitis and emphysema references are “no chronic bronchitis and “no emphysema”, respectively
For radiation, chemotherapy, and surgery references are “no radiation”, “no chemotherapy, and “no surgery”, respectively
We also performed a backward selection to build a second “reduced” model. This was achieved via inclusion of predictors that met a significance level of less than or equal to 0.10. This reduced model did not result in a significantly different result with regard to PMA (HRadj 0.97 [0.96, 0.99], P=0.002, Table 3). PMA remained a statistically significant predictor of overall survival while adjusting for sex, histology, stage, surgery, emphysema, and performance status.
DISCUSSION
In this report, lower pectoralis muscle area at diagnosis was associated with a worse overall survival for patients with NSCLC, despite adjustment for BMI and performance status. Measurement of PMA for determining sarcopenia has several advantages. First, the measurement is straightforward to perform using open-source software (3D Slicer) and reliable. Prior investigations have demonstrated that the slice used to make the measurement is easily identified on CT scans across multiple cohorts.(13,24) The technique for measurement of pectoralis muscle area has also been shown to be highly reproducible (concordance correlation coefficient: 0.985) between different readers.(21,24) Here, we also used density threshold limits to remove any potential bias when selecting the borders of the muscles.
PMA is readily available from clinically acquired CT scans and does not require specific research protocols. It may be performed from diagnostic or screening studies, and potentially could be followed over time given that chest CT scans are commonly obtained to assess treatment response. In contrast the standard cross-sectional imaging measurement of sarcopenia, lumbar spinal muscle index measured at L3, requires performance of an abdominal CT scan. One of the largest studies that applied this measure to cancers of the respiratory and gastrointestinal tract reported that approximately 25% of patients in the study didn't have CT scans that could be used for lumbar skeletal muscle index analysis.(7) In contrast, only 3% of CT scans in this cohort were not evaluable for PMA. This is strikingly similar to the number of CT scans not evaluable for PMA reported from the ECLIPSE and COPDgene cohorts.(13) Additionally, abdominal CT scans are not necessarily performed for evaluation or follow up of cancers of the respiratory tract limiting their utility in evaluating all patients with lung cancer for changes in muscularity over time. One limitation of the current study is the inability to compare the chest CT measure of PMA to the lumbar spinal muscle index, due to the rarity with which abdominal CT scans are performed.
PMA may be a sensitive measure of cancer-associated sarcopenia. In COPD, fat-free mass as measured by bioimpedance is highly correlated with PMA.(13) Interestingly, bioimpedance is only considered useful in the setting of “grossly altered body composition” in cancer cachexia.(6) Measurement of PMA may be more sensitive. In this cohort, we were able to identify differences in survival above and below the 50th percentile of pectoralis muscle area. This is striking since these data were not adjusted for stage, a predictor associated with a very large magnitude of effect on lung cancer survival, and implies consistency of the effect across stages. In contrast, sarcopenia associated with cancer cachexia has traditionally been defined as muscularity less than the 5th percentile.(6)
In order to insure that PMA was not a measure specifically tied to COPD we also adjusted for physician diagnosed chronic bronchitis and emphysema in the proportional hazards model. Adjusting for these covariates was particularly important in this case since COPD (using the identical measures as reported here) has previously been reported to be associated with a worse overall survival.(26) Additionally, PMA appeared to have a stronger relationship to survival (in unadjusted analysis) in the strata of those with adenocarcinoma potentially implying a cancer specific effect. A known limitation of stratified analysis is the decrease in sample size that limits power to detect differences in the groups. We did not have the statistical power to further assess potential differences by histology in stratified analysis. Although there was a clear effect among those with adenocarcinoma, adjustment for histology in the proportional hazards model demonstrated that the effect was present across categories of histology and not limited to adenocarcinoma.
This study has several important limitations. Although BMI and performance status correlate with loss of muscle mass, we do not have other direct measures of sarcopenia available from this cohort. Thus we cannot report frequency of sarcopenia in this cohort. Prospective studies in cancer patients simultaneously using PMA and other established metrics for muscularity will be needed to better define the operating characteristics and establish cutoffs for PMA determined sarcopenia. We also don't have longitudinal data on BMI and PMA, and can't comment further on how PMA at diagnosis (or over time) may be associated with changes in BMI over time. These data would prove valuable to determine if change in PMA may be more important than a single measurement at diagnosis and to assess the effect of differing therapies, which may have differential effects on muscle loss. PMA as a predictor of survival may be stronger in women. Men have a larger variance of measured PMA, implying that larger samples sizes may be needed to demonstrate significance within this strata. Regardless, the proportional hazards model suggests that the effect of PMA on survival is present across the strata of both men and women.
Here, we report the first data to demonstrate that lower pectoralis muscle area at diagnosis is associated with a worse overall survival for NSCLC. This association remains after adjustment for predictors associated with NSCLC, COPD, ECOG performance status, and BMI.
ACKNOWLEDGEMENTS
FINANCIAL SUPPORT: Supported by Grants: University of Vermont Cancer Cancer/Lake Champlain Cancer Center (C.M. Kinsey), RO1 CA092824 (D.C. Christiani), RO1 CA074386(D.C. Christiani), RO1 CA090578(D.C Christiani), K25 HL104085(R.S. Estepar), and R01HL116931(R.S. Estepar), R01 HL122464 (G.R. Washko)
ABBREVIATIONS LIST
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CXR
chest x-ray
- ECOG
Eastern Cooperative Oncology Group
- EGFR TKI
epidermal growth factor receptor tyrosine kinase inhibitor
- NSCLC
non-small cell lung cancer
- PMA
pectoralis muscle area
Footnotes
CONFLICTS OF INTEREST
Dr. GR Washko receives consulting fees from GlaxoSmithKline
Per the definition of conflicts of interest set forth by the International Committee of Medical Journal Editors, the other authors of this manuscript report no financial or other conflicts of interest related to the work.
Contributor Information
C. Matthew Kinsey, Division of Pulmonary and Critical Care, University of Vermont Medical Center.
Raul San Jose Estepar, Department of Radiology, Brigham and Women's Hospital.
Jos van der Velden, Department of Pathology, University of Vermont Medical Center.
Bernard F. Cole, College of Engineering and Mathematics, University of Vermont
David C. Christiani, Pulmonary and Critical Care Unit, Massachusetts General Hospital Department of Environmental Health and Epidemiology, Harvard School of Public Health
George R. Washko, Pulmonary and Critical Care Medicine, Brigham and Women's Hospital
REFERENCES
- 1.Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23:1699–708. doi: 10.1007/s00520-014-2534-3. [DOI] [PubMed] [Google Scholar]
- 2.Ross PJ, Ashley S, Norton A, Priest K, Waters JS, Eisen T, et al. Do patients with weight loss have a worse outcome when undergoing chemotherapy for lung cancers? Br J Cancer. 2004;90:1905–11. doi: 10.1038/sj.bjc.6601781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG performance status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A:1135–41. doi: 10.1016/0959-8049(95)00664-8. [DOI] [PubMed] [Google Scholar]
- 4.Langer C, Li S, Schiller J, Tester W, Rapoport BL, Johnson DH, et al. Randomized phase II trial of paclitaxel plus carboplatin or gemcitabine plus cisplatin in Eastern Cooperative Oncology Group performance status 2 non-small-cell lung cancer patients: ECOG 1599. Journal of Clinical Oncology. 2007;25:418–23. doi: 10.1200/JCO.2005.04.9452. [DOI] [PubMed] [Google Scholar]
- 5.Aapro M, Arends J, Bozzetti F, Fearon K, Grunberg SM, Herrstedt J, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Annals of Oncology. 2014;25:1492–9. doi: 10.1093/annonc/mdu085. [DOI] [PubMed] [Google Scholar]
- 6.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. The Lancet Oncology. 2011;12:489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 7.Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. The Lancet Oncology. 2008;9:629–35. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 8.Tan BHL, Tan BHL, Birdsell LA, Birdsell LA, Martin L, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:6973–9. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 9.Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. American Association for Cancer Research. 2009;15:2920–6. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 10.Yip C, Dinkel C, Mahajan A, Siddique M, Cook GJR, Goh V. Imaging body composition in cancer patients: visceral obesity, sarcopenia and sarcopenic obesity may impact on clinical outcome. Insights Imaging. 2015;6:489–97. doi: 10.1007/s13244-015-0414-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukaski H. Sarcopenia: assessment of muscle mass. J Nutr. 1997;127:994S–997S. doi: 10.1093/jn/127.5.994S. [DOI] [PubMed] [Google Scholar]
- 12.Morley JE, Thomas DR, Wilson M-MG. Cachexia: pathophysiology and clinical relevance. Am J Clin Nutr. 2006;83:735–43. doi: 10.1093/ajcn/83.4.735. [DOI] [PubMed] [Google Scholar]
- 13.McDonald M-LN, Diaz AA, Ross JC, San José Estépar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326–34. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinsey CM, Estépar RSJ, Zhao Y, Yu X, Diao N, Heist RS, et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer. 2014;84:145–50. doi: 10.1016/j.lungcan.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heist RS, Zhai R, Liu G, Zhou W, Lin X, Su L, et al. VEGF polymorphisms and survival in early-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:856–62. doi: 10.1200/JCO.2007.13.5947. [DOI] [PubMed] [Google Scholar]
- 16.Kinsey CM, San José Estépar R, Wei Y, Washko GR, Christiani DC. Regional Emphysema of a Non-Small Cell Tumor Is Associated with Larger Tumors and Decreased Survival Rates. Ann Am Thorac Soc. 2015;12:1197–205. doi: 10.1513/AnnalsATS.201411-539OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ter-Minassian M, Zhai R, Asomaning K, Su L, Zhou W, Liu G, et al. Apoptosis gene polymorphisms, age, smoking and the risk of non-small cell lung cancer. Carcinogenesis. 2008;29:2147–52. doi: 10.1093/carcin/bgn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BW, Wain JC, Kelsey KT, Wiencke JK, Christiani DC. Association of cigarette smoking and asbestos exposure with location and histology of lung cancer. Am J Respir Crit Care Med. 1998;157:748–55. doi: 10.1164/ajrccm.157.3.9707025. [DOI] [PubMed] [Google Scholar]
- 19.Heist RS, Zhou W, Wang Z, Liu G, Neuberg D, Su L, et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5596–602. doi: 10.1200/JCO.2008.18.0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz AA, Valim C, Yamashiro T, Estépar RSJ, Ross JC, Matsuoka S, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138:880–7. doi: 10.1378/chest.10-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diaz AA, Come CE, Ross JC, San José Estépar R, Han MK, Loring SH, et al. Association between airway caliber changes with lung inflation and emphysema assessed by volumetric CT scan in subjects with COPD. Chest. 2012;141:736–44. doi: 10.1378/chest.11-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. Clinical and Radiographic Predictors of GOLD–Unclassified Smokers in the COPDGene Study. Am J Respir Crit Care Med. 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz AA, Zhou L, Young TP, McDonald M-L, Harmouche R, Ross JC, et al. Chest CT measures of muscle and adipose tissue in COPD: gender-based differences in content and in relationships with blood biomarkers. Academic Radiology. 2014;21:1255–61. doi: 10.1016/j.acra.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. Biometrika Trust. 1994;81:515–26. [Google Scholar]
- 26.Zhai R, Yu X, Shafer A, Wain JC, Christiani DC. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest. 2014;145:346–53. doi: 10.1378/chest.13-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]