Abstract
Children vary greatly in their vocabulary development during preschool years. Importantly, the pace of this early vocabulary growth predicts vocabulary size at school entrance. Despite its importance for later academic success, not much is known about the relation between individual differences in early vocabulary development and later brain structure and function. Here we examined the association between vocabulary growth in children, as estimated from longitudinal measurements from 14 to 58 months, and individual differences in brain structure measured in 3rd and 4th grade (8–10 years old). Our results show that the pace of vocabulary growth uniquely predicts cortical thickness in the left supramarginal gyrus. Probabilistic tractography revealed that this region is directly connected to the inferior frontal gyrus (pars opercularis) and the ventral premotor cortex, via what is most probably the superior longitudinal fasciculus III. Our findings demonstrate, for the first time, the relation between the pace of vocabulary learning in children and a specific change in the structure of the cerebral cortex, specifically, cortical thickness in the left supramarginal gyrus. They also highlight the fact that differences in the pace of vocabulary growth are associated with the dorsal language stream, which is thought to support speech perception and articulation.
Keywords: vocabulary growth, first language acquisition, longitudinal, cortical thickness, white matter connectivity, probabilistic tractography
Babies are born without speech; the word infant comes from Latin infant- ‘unable to speak’2. Nevertheless, they acquire language with remarkable speed and sophistication, starting with phoneme discrimination and babbling during the first months, and continuing to produce their first words by the end of the first year (Kuhl, 2004). Unlike a second language learner who typically receives explicit instruction, babies acquire their vocabulary implicitly, using statistical learning (computing the probability of a syllable being preceded or followed by another syllable) to extract words from continuous speech (Saffran et al., 1996). The acquisition of a word form, in turn, facilitates mapping it to meaning (Estes et al., 2007).
Although most children learn words according to a common trajectory, rate and timing of vocabulary development show striking variability across children (Fenson et al., 1994). Variation in vocabulary development during the first years of life is important because it is associated with later academic success. The size of oral vocabulary at 24 months of age predicts academic achievement (reading and math), as well as behavioral functioning (self-regulation and social behavior), at kindergarten, even after controlling for covariates such as socioeconomic status (SES), gender, birth weight, parenting quality, and maternal health (Morgan et al., 2015). Moreover, the rate of vocabulary growth at 30 months of age can uniquely predict vocabulary skill before entering kindergarten (Rowe et al., 2012).
Despite the ample evidence demonstrating that variations in vocabulary early in development predict subsequent skills, little is known about the biological underpinnings of these effects. In the current study, for the first time, we examined the relation between individual differences in early vocabulary growth at preschool and structural differences in the brain at school age. More specifically, we examined the association between early vocabulary growth from 14 months to 58 months of age and individual variation in grey and white matter in brain regions previously implicated in vocabulary processing at school age (3rd and 4th grade) in 20 typically developing children.
Studying the underlying brain mechanisms of first language vocabulary acquisition can be methodologically challenging because of the nature of the subject population (excessive motion, anxiety, lack of sustained attention, to name but a few) (Dehaene-Lambertz and Spelke, 2015). Nonetheless, there is an increasing number of studies investigating speech processing in infant brain using magnetic resonance imaging (MRI), functional near-infrared spectroscopy, magnetoencephalography, and electroencephalography (Benavides-Varela et al., 2012; Dehaene-Lambertz et al., 2006; 2010; Junge et al., 2012; Mahmoudzadeh et al., 2013; Travis et al., 2011). These studies reveal a continuity between the patterns of brain activation to speech in infants and those observed in adults. Dehaene-Lambertz and Spelke, (2015) thus suggest that developmental changes in behavior may be mediated by maturational changes in cortical regions and their connections. The maturation of frontal, temporal, and parietal cortical areas, and their white matter connectivity, provide children with increasingly efficient networks for language (Dubois et al., 2016; Leroy et al., 2011; Pujol et al., 2006). But in addition to maturational processes, the child’s interaction with her environment has been found to contribute significantly to individual differences in language development (Hackman and Farah, 2009; Johnson, 2001).
Although there is a paucity of neuroimaging studies of young children during the preschool years, a time when vocabulary shows the largest growth (Kuhl, 2010; Nazzi and Bertoncini, 2003), several studies have been done on vocabulary learning in older children (Richardson and Price, 2009). These studies have shown that grey matter density in the posterior supramarginal gyrus (SMG) is positively associated with vocabulary knowledge in young teenagers (Lee et al., 2007; Richardson et al., 2010), as are the posterior superior temporal sulcus (pSTS) and posterior temporo-parietal junction across the lifespan (ages 7–73). Sowell et al. (2004) found that cortical thinning in the left lateral dorsal frontal and the left lateral parietal regions correlates with improvement in vocabulary competence in children between ages 5 and 10 (Sowell et al., 2004).
Second language learning studies in adults also offer some insights into the neural underpinnings of word acquisition. Rodríguez-Fornells et al. (2009) proposed three “interfaces” in the brain that are crucial for second language learning: (1) an interface between auditory and motor processes that enables speech perception and articulation (auditory-motor interface), thought to be mediated by the dorsal language stream; (2) an interface between meaning representations and response selection (meaning integration interface) that enables mapping speech to meaning, mediated by the ventral language stream; and (3) a memory interface enabling consolidation of newly learned words into new lexical representations linked to meaning (memory interface), mediated by the hippocampus and medial temporal lobe (Davis & Gaskell, 2009).
Adult vocabulary learning studies frequently use tasks that tap into a specific interface, be it the meaning integration interface (i.e. the ability to map novel sounds/words to meaning), or the auditory-motor interface (i.e. the ability to map novel sounds/words to articulation). In a study testing the meaning-integration interface, (Wong et al., 2011) trained individuals to match non-native spoken words to pictures of items. They found that white matter fractional anisotropy (FA) in a left parieto-temporal cluster in the extreme capsule fiber system (a ventral language pathway) is associated with learning performance. They suggested that this result was consistent with the word-to-meaning mapping required in their task (Wong et al., 2011). Another set of studies focusing on the auditory-motor interface used continuous speech learning paradigms with non-native (Veroude et al., 2010) or artificial languages (Lopez-Barroso et al., 2013; 2015). Veroude et al. (2010) exposed adult learners to a short video clip in Chinese and later asked them to recognize the phonological form of words they had heard in the clip. They found that successful learners showed increased connectivity between the left and right SMG after exposure to the new language López-Barroso et al. (2015) measured resting-state connectivity before and after word learning training from continuous speech in an artificial language. They found increased connectivity in dorsal fronto-parietal, dorsal auditory-premotor, and ventral frontotemporal networks after training, and a correlation between learning performance and connectivity in a dorsal auditory-premotor network (Lopez-Barroso et al., 2015) The ability to learn words from continuous speech was also related to white matter radial diffusivity (RD) along the left arcuate fasciculus, connecting posterior temporal and inferior frontal area (Lopez-Barroso et al., 2013). These results stress the importance of the dorsal pathway for learning words from exposure to non-native continuous speech. However, this literature is based on adolescent and adult learners who have already acquired a native language. The focus of our study is to determine which of the three interfaces outlined above—auditory-motor interface, meaning integration interface, memory interface—is more closely related to first language vocabulary acquisition.
The measure of vocabulary competence that we used in our study was based on observations of children’s spontaneous interactions with their caregivers. We tallied the number of different word types children uttered at each observation session (taken every 4 months between 14 to 58 months of age). Our measure was thus an expressive rather than receptive vocabulary measure. As a consequence, we would expect contributions from all three interfaces, with an emphasis on the auditory-motor interface. Rather than focus on children’s vocabulary at a single point in time, we modeled the growth of children’s vocabulary between 14 and 58 months to get a picture of the trajectory of vocabulary development.
Since the main resource available to word-learners is the speech they are immersed in, the variation in children’s vocabulary development can be traced back to variations in environmental factors, in particular, to family SES (Hoff, 2006) and to the quantity (Huttenlocher et al., 1991) and quality (Cartmill et al., 2013) of parental linguistic input (Hoff, 2003; Hoff and Naigles, 2002; Montag et al., 2015; Rowe, 2012; Weisleder and Fernald, 2013). Thus, in examining relations between vocabulary development and brain, we included parental SES and input as covariates.
We examined relations between vocabulary development and two brain structure measures: cortical thickness, and white matter connectivity. Cortical thickness measures were extracted from the left inferior frontal gyrus (IFG; pars opercularis and pars triangularis), the middle frontal gyrus (MFG), the posterior middle temporal gyrus (pMTG), the posterior superior temporal gyrus (pSTG), the posterior superior temporal sulcus (pSTS), and the SMG. The areas were selected based on the literature on vocabulary processing, which implicates these regions for processing single words (Davis and Gaskell, 2009; Li et al., 2014; Price, 2010; Rodríguez-Fornells et al., 2009). We focused on cortical thickness because it is a measurable manifestation of important underlying cellular changes: Cortical thickness is tied to the number of neurons in a cortical column, the amount of glial and capillary support, and dendritic branching(Rakic, 2009; 1988), all of which are amenable to change as a result of postnatal experience and learning (Anderson et al., 1994; Black et al., 1990; Kleim et al., 1996). To explore the connectivity between cortical areas involved in word learning, we used probabilistic tractography to map white matter connectivity, our second brain structure measure.
Based on existing data on teenagers learning words in their first language, adults learning words in a second language, as well as computer simulations (Ueno et al., 2011), we hypothesized that variations in the rate of early vocabulary acquisition would be primarily associated with variations in grey and white matter structure in brain regions along the dorsal language pathway. To our knowledge, this is the first study to examine the relation between the developmental trajectory of vocabulary acquisition in preschool years and subsequent brain structure in later years.
MATERIAL AND METHODS
2.1 Participants
Twenty children (age 8–10 years, M = 9.21 years, SD = 0.63 years; right-handed; 10 female), all of whom were native speakers of American English, participated in the study, which is part of a larger longitudinal study on language development (Goldin-Meadow et al., 2014). Each parent gave written informed consent following the guidelines of the Institutional Review Boards for the Division of Biological Sciences at The University of Chicago, and the Office of Research at the University of California, Irvine, which approved the study. Children gave verbal assent. All participants reported normal hearing and normal or corrected-to-normal vision. No parents reported any history of neurological or developmental disorders in their children. Two participants were excluded due to excessive motion during the MRI scan, resulting in a final study sample of 18 children.
Families were recruited through a free monthly magazine and mailings sent directly to them. The families were chosen to be representative of the demographics of the Chicago area, with the caveat that English was the primary language spoken at home. Caregivers provided race and ethnicity information. They reported that 15 children were White, 2 were African-American, and 1 was of mixed race; 1 child was reported to be Hispanic. Parent education (in years) was coded on a categorical scale (10 = less than high school degree, 12 = high school degree, 14 = some college or associate degree, 16 = college degree, 18 = more than college). In this sample, average parent education was 15.1 years (SD = 2.59, Range = 10 – 18) and average family income was $50,278 (SD = $28,309, Range = $7500 – $100,000). We combined the two measures into a composite score of socioeconomic status (SES) using Principal Components Analysis (PCA). The first principal component weighted education and income positively and equally. This component accounted for approximately 66 percent of the original variance in income and education.
2.2 Behavioral procedure
The data coded for this study are part of a larger longitudinal study examining children’s language development. We coded videotapes of parents interacting with their children for approximately 90-minutes during home visits that occurred every 4 months between child ages 14 to 58 months. No toys or directions were provided to the parents, other than asking them to engage in their normal activities. These activities frequently included toy play, book reading, and eating meals and snacks.
2.3 Behavioral measures
2.3.1 Child word growth
All parent and child speech in the videotaped sessions was transcribed. The unit of transcription was the utterance, defined as any sequence of words that was preceded and followed by a pause, a change in conversational turn, or a change in intonational pattern. Transcription reliability was established by having a second individual transcribe 20% of the videotapes with a reliability criterion of 95% agreement on utterance transcription.
We calculated total number of different word types from the transcripts for each child during each session. Our vocabulary growth measure consisted of cumulative word types over time across the 12 sessions from 14 to 58 months. We defined the number of different word types as the number of different intelligible word roots. Morphologically inflected variants of words (e.g., run, running) were considered a single type. Words produced in imitation of the mother and words produced while reading books were included in word type counts. Following previous studies on vocabulary growth (Huttenlocher et al., 1991), cumulative word types served as our measure of vocabulary growth. In other words, if a child produced a word at age 14 months (e.g., “ball”), that word would be considered part of the child’s vocabulary from that point on, whether or not the child produced the word in subsequent sessions. In order to calculate cumulative vocabulary, we first calculated the number of new word types children produced at each visit. Not all sessions were exactly 90 minutes; we therefore pro-rated new word types according to the actual length of the session. The new word types for each session was added to the child’s previous cumulative word types to generate the cumulative word type value for that child at that session. Cumulative word types have been shown to provide less noisy estimates of vocabulary growth than non-cumulative types (Huttenlocher et al., 1991).
Three of the 18 families missed at least one visit (2 families missed one visit each, and 1 family missed 3 visits). We imputed new word types for these visits using a regression equation based on the full sample at the missing age, with age and age-squared as predictors. The imputed value for the missing session was then added to the child’s previous cumulative word types to generate the new cumulative word type value for that child at the missing visit. Overall, 2.3% of the vocabulary growth data were imputed in this way.
2.3.2 Parent word types
We calculated, for each parent, the total number of different word types the parent produced at child age 14 months. We used this input measure to predict child language growth. Previous work has shown that parent word types in first year is a strong predictor of child vocabulary development in later preschool years (Rowe et al., 2012) and variation in parental input has been found to be stable over time (Huttenlocher et al., 2010).
2.3.3 Socioeconomic Status (SES)
As described above, the SES of each family was indexed by a composite factor score that combines parental education and income information.
2.4 MRI Acquisition
Imaging data were acquired on a 3T Siemens Trio scanner with a 32-channel head-coil on the medical campus of Northwestern University. A T1-weighted structural scan was acquired for each participant (1 mm × 1 mm × 1 mm resolution; sagittal acquisition). T1-weighted 3D spoiled gradient echo sequences were obtained with TR = 2300 ms, TE = 2.91 ms, flip angle = 9°, inversion time = 900 ms, and 256 contiguous slices (slice thickness = 1 mm, voxel size = 1 × 1 × 1 mm3, matrix size = 256 × 256).
In addition, we acquired diffusion weighted imaging (DWI) data using a single-shot pulsed gradient spin-echo sequence (TR = 9449 ms, TE = 88 ms, flip angle = 90°). The diffusion weighting orientations were isotropically distributed along 64 directions with a b-value of 1,000 s/mm2. Eight volumes without diffusion weighting (b-value of zero) were acquired, interspersed into the sequence. A total of 68 slices covering the whole brain were acquired (slice thickness = 2mm, voxel size = 2 × 2 × 2 mm3, matrix size= 128 × 128).
2.4.1 Quality assurance
T1-weighted images were inspected visually for quality assurance. Two independent observers (SSA, EODL) performed this inspection and excluded the same two participants. For the DWI data, the mean relative displacement (motion in consecutive time-points) was estimated and participants with displacement larger than 2mm were excluded from further analyses. The two children did not fulfill this criterion were the same participants who were excluded from the cortical thickness analysis.
2.5 Behavioral analysis
To examine the relation between children’s early vocabulary growth and structural brain measures later in development, we followed two steps. These steps mirror the analysis conducted by Rowe et al. (2012) on the larger dataset; children included in the current study are a subset of this larger set. First, to assess growth of children’s vocabulary skills over time, we built a two-level statistical model for child-specific growth in cumulative word types using hierarchical linear modeling (Raudenbush and Bryk, 2002). The Level 1 model accounted for the variation in repeated measures of vocabulary within each child. The Level 2 model accounted for the variation between children. We used child age in months at each visit as our measure of time and centered age at 3 years (36 months), which is the midpoint of the age range (14 to 58 months). Second, we built prediction models in which we used child specific growth parameters to predict brain structure measures, that is, gray and white matter measures.
2.5.1 The two-level model for child vocabulary growth
Following Rowe et al. (2012), at Level 1 (within children), we represented children’s vocabulary trajectory using a cubic model. So for each child, we had:
In this equation, ti is the age of child i at time t, π0i is child i’s status (average cumulative vocabulary size) at age 3 years, π1i is child i’s velocity at age 3 years, and π2i is child i’s acceleration at age 3 years, and π3i is child i’s cubic change at age 3 years. The residual eti represents that portion of child i’s cumulative vocabulary at age t that is not predicted by his or her age. We presume a heterogeneous level-q variance, meaning that the within-person variation varies over time with child age.
At Level 2 (between children), we tested whether an individual child’s status, velocity, acceleration, and cubic change are predicted by parental socioeconomic status (SES) and parent word types. We therefore have a separate Level 2 equation for each Level 1 coefficient, πpi, where p = 0, 1, 2, 3:
where πpi is the pth growth parameter from the Level 1 model, βp0, βp1, βp2 are linear regression coefficients, and rpi is a random effect.
2.5.2 The prediction model
Next, we examined whether children’s status, velocity, acceleration, and cubic change at age 3 predict later structural differences in gray and white matter at age 9, controlling for background characteristics of parental SES and parent word types. After estimating the two-level model specified in Equations 1 and 2 above, we computed empirical Bayes estimates for each of these predictors, and then produced the empirical Bayes coefficients for each child. These empirical Bayes estimated growth rates were used to predict structural brain differences (see Raudenbush & Bryk, 2002, Chapter 6, and Rowe et al., 2012, for further details on building prediction statistical models).
2.6 MRI Analysis
2.6.1 Cortical parcellation
Cortical reconstruction of white and pial surface models was performed using Freesurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/; for details on the Freesurfer surface-based pipeline, see Dale et al., 1999; Fischl et al., 1999). Once generated, the cortical surface models were manually reviewed and edited for technical accuracy. Sulcal and gyral structures were identified automatically (Fischl et al., 2004) and parcellated using the Destrieux cortical atlas for anatomical labeling (Destrieux et al., 2010). This parcellation scheme results in 148 cortical regions (74 per hemisphere). Cortical thickness was estimated as the average distance between the white and the pial surface reconstructions (Fischl and Dale, 2000). The STG, STS and MTG in the left hemisphere were each sub-parcellated into anterior and posterior sections. This analysis was performed separately for each child, in that child’s native space (i.e. no normalization to a template brain was performed), by drawing a vertical line across these regions, starting from the most inferior part of the transverse sulcus, where it meets the most anterior tip of the planum temporale, thereby separating these areas into two parts. All analyses were performed in native space.
2.6.2 Cortical thickness analysis
We examined the relations between growth parameters and cortical thickness in the IFG (pars opercularis and pars triangularis), the MFG, the pMTG, the pSTG, the pSTS, and the SMG, in the left hemisphere. We selected these areas based on previous findings on word learning (see Introduction).
2.7 DWI Analysis
2.7.1 Preprocessing and tensor fitting of DWI
DWI data were not collected for one child, leaving a sample of 17 children for this analysis. The raw diffusion images were converted from DICOM to Nifti format using dcm2nii. A brain mask was created with FSL’s BET (Smith, 2002), using the first non-diffusion volume. We corrected for the effects of diffusion eddy currents and movements using FSL’s EDDY function and rotated the diffusion sensitizing gradients (bvecs) accordingly. The diffusion tensors were fitted using weighted least squares with the DTIFIT algorithm in FSL. Fractional anisotropy, mean diffusivity ((λ1+λ2+λ3)/3), and radial diffusivity (λ2+λ3/2) images were estimated.
2.7.2 Tract-Based Spatial Statistics
We used FSL’s Tract-Based Spatial Statistics (Smith et al., 2006) to perform voxelwise statistical analyses on the fractional anisotropy (FA), mean diffusivity (MD) and radial diffusivity (RD) data. First, the most representative FA image was aligned to standard MNI space using linear registration; all the images were registered and upsampled into 1×1×1 mm MNI space by using the linear transform from the representative image to standard space and the nonlinear transform of each image to the representative one. The mean FA skeleton was created from the projected individual FA skeletons, thresholded at 0.25, and binarized to create a mask for running statistics. The nonlinear registration was subsequently applied to the MD and RD data. The warped images were then combined and projected onto the mean FA skeleton for voxelwise statistics.
2.7.3 Seed surface region for tractography
The seed region (left supramarginal gyrus) was defined in each participant’s native white matter surface space using Freesurfer’s cortical parcellation (Destrieux atlas). In order to use the Freesurfer parcellation, we computed the following rigid body transformations using FSL’s FLIRT: the Freesurfer volume (norm.mgz) and the diffusion volume were aligned to the skull-stripped anatomical T1 image. The inverse transformation matrices were used to estimate the Freesurfer to DTI alignment. The label file from each subject’s Freesurfer parcellation was then converted into a binary volume mask and used to define the seed space for tractography analysis.
2.7.4 Probabilistic tractography
Tractography was performed using FSL’s FDT toolbox (fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT). We first modeled the distributions of diffusion parameters at each voxel using BEDPOSTX’s ball-and-stick model. Using PROBTRACKX (Behrens et al., 2007), we generated connectivity distributions from our specified seed region (SMG), computing 5,000 trajectories per seed voxel with 2,000 steps per sample with length set to 0.5 mm. Streamline trajectories were terminated when the angle between two steps was ±80° (0.2 curvature threshold). The ventricles and subcortical areas from Freesurfer segmentations were used as exclusion masks, and the white matter served as the waypoint mask. The resulting tracts were thresholded at 1% of the participants’ waytotal (the number of streamlines that are were not rejected) and binarized. They were then used as masks to extract the average fractional anisotropy (FA), mean diffusivity (MD) and radial diffusivity (RD), in the tract for each participant.
RESULTS
In this section, we first present the data on individual growth modeling using HLM. We describe vocabulary growth between 14 and 58 months, integrating parent SES and parent word types as predictors of growth. Second, we use empirical Bayes estimated growth rates from vocabulary growth models to predict gray and white matter structure measures using multiple regression analyses.
3.1 Modeling vocabulary growth
Figure 1 represents the empirical growth curves for children’s cumulative word types, and shows substantial variability in the children’s growth trajectories. To identify the best-fitting model for vocabulary growth, we compared linear, quadratic, and cubic growth models. All models were centered at child age 3 years (36 months), the midpoint of the age range studies. The cubic model revealed the lowest goodness of fit statistic, indicating a better fit (−2 Log Likelihood), and the plot of the cubic model best mirrored the plot of the empirical data (see Figures 1a and 1b). The cubic model was thus considered the best-fitting model. In this model, a fixed slope for the cubic term over children was considered adequate. Model 1 in Table 1 represents this unconditional cubic growth model. This model shows that, on average, children at 3 years of age have an estimated cumulative vocabulary of 470 words, with linear change reflecting cumulative vocabulary increases of 27 words per month. The significant quadratic term shows that the monthly rate of increase itself increases over time, and the significant negative cubic term shows that this increase reduces at later ages. The average fitted growth trajectory is displayed in Figure 1c.
Figure 1.
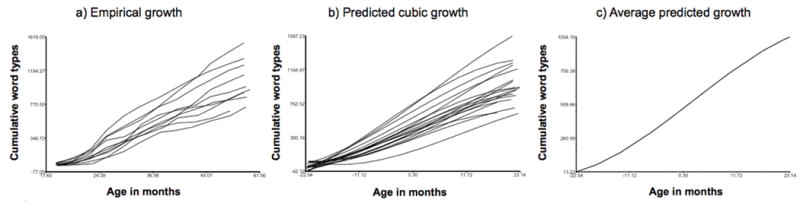
Plots of (a) empirical growth trajectories of child vocabulary types, (b) predicted growth trajectories from the cubic model, and average vocabulary growth from the cubic model.
Table 1.
Estimates of fixed effects, random effects, and goodness of fit for cubic growth models using parent SES, and parent word types to predict intercept and change in children’s cumulative vocabulary.
| Parameter Estimate (SD)
|
|||
|---|---|---|---|
| Model 1 (Cubic) | Model 2 (Adding SES) | Model 3 (Adding Input) | |
| Fixed Effects | |||
| Intercept | 470.29*** (33.44) |
470.28*** (32.25) |
470.28*** (32.91) |
| Linear change | 26.52*** (1.36) | 26.52*** (1.19) | 26.56*** (1.21) |
| Quadratic change | 0.07 (0.04) | 0.06 (0.04) | 0.07 (0.05) |
| Cubic change | −0.01*** (0.01) | −0.01*** (0.01) | −0.01*** (0.01) |
| SES | 50.27 (33.18) | 65.64 (42.40) | |
| SES × Age | 3.06** (1.17) | 3.62** (1.50) | |
| SES × Age2 | 0.06 (0.05) | 0.03 (0.06) | |
| Input | −0.16 (0.27) | ||
| Input × Age | −0.01 (0.01) | ||
| Input × Age2 | 0.01 (0.01) | ||
| Random effects | |||
| Level 2 | |||
| Intercept | 20,059*** (141.63) |
18,635.98*** (136.51) |
19,410.39*** (139.32) |
| Linear change | 30.87*** (5.56) | 22.89*** (4.78) | 23.83 (4.88) |
| Quadratic change | 0.04*** (0.19) | 0.03*** (0.19) | 0.04*** (0.19) |
| Goodness of fit | |||
| Deviance (−2LL) | 2217.41 (7) | 2197.53 (7) | 2227.87 (7) |
p<.05,
p<.01,
p<.001
We next included two background variables that have been found in previous work to strongly predict vocabulary growth—parent SES, and parent word types (Rowe et al., 2012). We first added SES to the model. Model 2 in Table 1 represents the effects of SES on children’s vocabulary growth. In this model, there is a significant relation between parent SES and linear change in cumulative child word types. We next added parent word types to the model. Model 3 in Table 1 shows that parent SES remained a significant predictor of linear change, controlling for parent word types as a predictor. Overall, the developmental trajectory of children’s vocabulary growth varies as a function of parental background factors. Including these background variables enabled us to estimate growth parameters and examine their relation to measures of brain structure, controlling for differences in children’s environmental background.
3.2 Predicting gray matter structure
We used the empirical Bayes intercept and growth estimates (See Equation 4) from Model 3 (Table 1), which included parent SES and parent word types, as predictors of children’s brain structural measures. Importantly, intercept, linear change, and quadratic change estimates were related to each other (Intercept and Linear Change, r = .93, p < .001, Intercept and Quadratic Change, r = −.69, p < .01, Linear and Quadratic Change, r = −.39, p = .11). Because we were specifically interested in differences in rate of vocabulary growth, and because previous behavioral findings on the larger dataset revealed a stronger relation of linear growth to children’s vocabulary outcomes than to intercept or quadratic growth (see Rowe et al., 2012), we only used linear change estimates in the models described below. Cubic growth estimates did not vary between children and were thus not used to predict later structural measures. Given our small sample size, we used slope as a categorical variable with median split.
To examine the relation between linear growth and cortical thickness in brain areas identified on the basis of previous work, we ran multivariate regression analyses, using cortical thickness in each region as the dependent variable, and child-specific linear growth estimates as the independent variable. We included parent SES, parent word types, as well as average cortical thickness, age, gender, and their interaction, as covariates in these models. Results from the MANOVA showed a statistically significant difference in cortical thickness in left SMG, F(1, 16) = 9.68, p=.01, partial η2 = .52, and a marginally significant difference in left MFG, F(1, 16) = 5.08, p = .05, partial η2 = .36, as a function of linear change in cumulative word types. Children with vocabulary growth rates below the median slope (M = 3.22, SD = 0.12) had higher cortical thickness than children with vocabulary growth rates above the median (M = 3.05, SD = 0.16). Cortical thickness in other areas was not significantly related to linear change (all p’s > .10). Of the covariates included in the model, only average cortical thickness emerged as a significant predictor of cortical thickness in the pars opercularis, F(1, 16) = 23.11, p < .001, partial η2 = .62, and pars triangularis, F(1, 16) = 19.94, p=.002, partial η2 = .29, of the left IFG, and the left SMG, F(1, 16) = 26.06, p=001, partial η2 = .78. No significant associations were observed at the whole brain level.
Relations were confirmed at the child level using additional chi-square analyses. Children were divided into two groups (high, low) based on median cortical thickness in left SMG and again on median cortical thickness in left MFG. First, 7 of 9 children with high SMG thickness also had high left MFG thickness χ2= 5.56, p = 0.02. Moreover, 6 of the 7 children with high linear change in vocabulary also had low left SMG cortical thickness, and 8 of the 11 children with low linear change had high cortical thickness in this area, χ2 = 5.84, p =0.02. Linear change and cortical thickness in the left middle frontal gyrus were also associated. Six of the 7 children with high linear change had low cortical thickness in left MFG, and 8 of the 11 children who had low linear change had high cortical thickness in this area as well, χ2 = 5.84, p =0.02.
3.3 Predicting white matter structure
We used the empirical Bayes growth estimate that was used in the cortical thickness analysis to predict children’s white matter structural measures. We used FSL’s randomize command to run voxelwise statistics on the skeletonized FA, MD and RD data. For each diffusion metric, we ran multiple regression analyses, using the child-specific linear growth estimates as the independent variable, along with parent SES, parent word types, age, gender, and their interaction as covariates. Nonparametric permutation methods were used to define statistically significant clusters (Winkler et al., 2014). No significant associations were found between vocabulary slope estimates and FA, MD, and RD data at the whole brain level.
3.4 SMG white matter connectivity
Following the cortical thickness findings, our goal was to examine the structural properties of the white matter fiber tracts connecting the SMG to other brain regions. To do so, we used multivariate regression to predict these structural properties using the same predictors that we used for the cortical thickness measurements. In particular, the dependent measures in this analysis were the average FA, MD, and RD in each child’s traced tract (see Table 2 for descriptive statistics), and the independent variables were the child-specific linear growth estimates, with parent SES, parent word types, age, and gender, and their interaction term included in the model as covariates. The three DTI metrics were strongly correlated; FA was negatively correlated with MD [ r(15) = −.61, p = .008] and RD [ r(15) = −.74, p < .0005], and MD and RD were positively correlated [r(15) = .96, p <.0001]. The MANOVA outcome did not reveal any significant effect of vocabulary linear growth estimates on FA, MD, or RD (all p’s > .10). Of the covariates included in the model, only SES was a marginal predictor of mean FA, b = 0.01851, t(10) = 1.849, p =.09. This result should be interpreted with caution as SES correlated significantly with motion (mean absolute displacement) [r(15)=−.54, p=.0024].
Table 2.
Descriptive Statistics for DTI Metrics
| DTI Metric | Mean | SD |
|---|---|---|
| Fractional Anisotropy | .3231 | .020 |
| Mean Diffusivity | .00082 | .000026 |
| Radial Diffusivity | .00068 | .000030 |
Note. N=17
DISCUSSION
We investigated the association between individual variation in vocabulary development during preschool years and cortical thickness and white matter connectivity during later school years in a sample of typically developing children. A large body of literature highlights the importance of examining the trajectory of vocabulary development prior to school entry. The size of a child’s vocabulary in preschool years has been found to predict the child’s vocabulary at school entry (Rowe and Goldin-Meadow, 2009), and the size of a child’s vocabulary at school entry has been found to predict later school success (Snow et al., 1998).
Our study explored the relation between individual differences in vocabulary growth prior to school and individual differences in brain structure during the school years. First, we used hierarchical linear modeling to fit the best longitudinal model to children’s vocabulary growth trajectory from 14 to 58 months of age. We then used estimates of children’s vocabulary growth to predict individual differences in brain structure. More specifically, we used empirical Bayes estimates of growth rate to predict gray and white matter structure. Our results show that individual differences in early vocabulary growth are associated with cortical thickness in the left SMG. Importantly, relations between vocabulary growth and cortical thickness were over and above back round factors which strongly predict children’ vocabulary skill, such as parent socioeconomic status (Hart and Risley, 1992; Rowe et al., 2012). Our findings extend previous behavioral findings (Rowe et al., 2012) by establishing a relation between early vocabulary growth and differences at the neural level measured after school entrance. The findings are in line with literature showing that the left the supramarginal gyrus plays a role in word learning in adolescents (Lee et al., 2007; Richardson et al., 2010). Probabilistic tractography in our sample also replicated previous findings showing that dorsal fiber pathways between the left SMG and the IFG (pars opercularis) and the ventral premotor cortex via what is most likely the third component of the superior longitudinal fasciculus (SLF III) (Dick et al., 2014; Pandya and Schmahmann, 2006). No associations were found between FA, MD and RD, and vocabulary growth.
Seen in terms of the different streams for word acquisition, our findings lend support to the hypothesis that variation in the rate of vocabulary acquisition is more likely to be associated with the dorsal than with the ventral language stream. The left SMG is part of this stream and appears to be connected via a dorsal tract to the frontal lobes, probably providing an interface for speech perception and articulation (Rodríguez-Fornells et al., 2009). The ventral language stream is also important for word acquisition, functioning as an interface for sound-meaning representations. However, the ability to extract words from continuous speech and map their phonological form to articulatory gestures seems to precede the acquisition of semantics and is more important for first language vocabulary development than the ability to map the forms to meaning. This is in alignment with longitudinal evidence showing that early segmentation skills predict infants’ vocabulary development (Singh et al., 2012). It is also consistent with adult vocabulary learning studies in which participants learn new words from continuous speech (Lopez-Barroso et al., 2015; 2013; Veroude et al., 2010). Lastly, the importance of the dorsal stream in first language acquisition is also supported by computational modeling that takes into account the dorsal and ventral neuroanatomical streams. A ventral-stream only model of language lacks the ability to acquire new word forms (Ueno et al., 2011); the dorsal stream appears to play an important role in the acquisition of new forms by encoding sound-articulation statistical regularities and enabling speech repetition and pronunciation (Ueno et al., 2011).
4.1 Vocabulary Growth and Cortical Thickness in the Left SMG
We narrowed down our cortical thickness analysis to brain areas identified in previous studies of spoken word processing (Davis and Gaskell, 2009; Li et al., 2014; Price, 2010; Rodríguez-Fornells et al., 2009). These areas included the IFG (pars opercularis and pars triangularis), the middle frontal gyrus, the posterior middle temporal gyrus, the posterior superior temporal gyrus, the posterior superior temporal sulcus, and the supramarginal gyrus in the left hemisphere. Linear growth in the number of unique words a child produced from 14 – 58 months of age was significantly associated with cortical thickness in the left SMG.
Notably, the association was negative, meaning that the faster the vocabulary growth early in development, the thinner the cortex in the left SMG later on. Cortical thinning in the SMG has previously been found to correlate with verbal working memory in a large sample of children and adolescents, even after controlling for age (Walhovd et al., 2011). The parietal cortex has been shown to have a cubic developmental trajectory in humans, with an initial increase in thickness during childhood (up until nine years of age), a decline during adolescence, followed by stabilization during adulthood (Lerch et al., 2008). Sowell et al. (2004) found evidence of cortical thinning in parieto-occipital regions even earlier than adolescence, in children aged between 5–11yrs. The children in our sample were at the cusp of the two first stages, that is, between the periods of cortical thickening and subsequent thinning. Cortical thinning in this age range is most likely driven by synaptic pruning (Petanjek et al., 2011) and/or increase in myelination (Sowell et al., 2004).
Activation in the left SMG has also been correlated with proficiency in adults learning new words in their first language (Breitenstein et al., 2005; Cornelissen et al., 2004), and gray matter structure in this area has been related to vocabulary proficiency in individuals’ first (Lee et al., 2007; Richardson et al., 2010) or second (Mechelli et al., 2004) language. Our study is the first to show that gray matter structure in the left SMG is also associated with rate of vocabulary growth in children over the course of development.
Previous studies have assigned the left SMG a role in phonological working memory (Li et al., 2014), phonological store and rehearsal (Rodríguez-Fornells et al., 2009), auditory–motor integration (Rogalsky et al., 2016), and as a part of a ‘phonological-articulatory loop’ linking phonological to motor representations (Rauschecker and Scott, 2009). Direct electrical stimulation of the white matter underlying this area induces dysarthria, lending more support to the area’s articulatory role (Maldonado et al., 2011). As such, the left SMG is a component of the auditory-motor interface for language learning and part of the dorsal language stream (Rodríguez-Fornells et al., 2009). However, we need to keep in mind that the left SMG is not only activated during language tasks, but is also activated during perceptual and motor reorienting, episodic memory encoding and retrieval, and number processing. The overarching function of the ventral parietal cortex (SMG and neighboring angular gyrus) may therefore be bottom-up attention (Cabeza et al., 2012). More specifically, Cabeza et al. (2012) have proposed that the SMG is important in “capturing and sustaining activated representations in the service of thought, planning, and action”. The area is strategically located at the crossroads between dorsal and ventral streams for perception and action (Culham and Valyear, 2006); its connectivity to other areas is therefore important for understanding the area’s role in vocabulary acquisition.
4.2 Connectivity of the Left SMG
Using probabilistic tractography with the left SMG as seed region, we traced the white matter connectivity of this inferior parietal area to other areas in the left hemisphere. Tractography allows the precise definition of only the seed region by the researcher. Tractography mainly traces the stem portion for each fiber pathway, which originates from the researcher-defined seed region (Makris et al., 2005). Thus, the real anatomical origins and terminations of the tracts can only be inferred from non-human primate and post-mortem anatomical data. In our study, the left SMG appeared to be connected anteriorly to the inferior frontal cortex (pars opercularis and, less frequently, pars triangularis), the ventral premotor cortex, and, in a few cases, the middle frontal cortex (see Figure 2). This tract, given its origin and terminations, is most likely the SLF III, which has been previously traced in the macaque (Petrides and Pandya, 2009; Schmahmann and Pandya, 2009; Yeterian et al., 2012) and humans (Agosta et al., 2010; Frey et al., 2008; Makris et al., 2005; Ruschel et al., 2014; Rushworth, 2006). It is sometimes referred to as the anterior direct segment of the arcuate fasciculus that connects Broca’s to Geschwind’s territory (Budisavljevic et al., 2015; Catani et al., 2005). Due to methodological limitations prevent us from reliably separating the SLF III from the arcuate fasciculus (Frey et al., 2008; Friederici, 2009).
Figure 2.
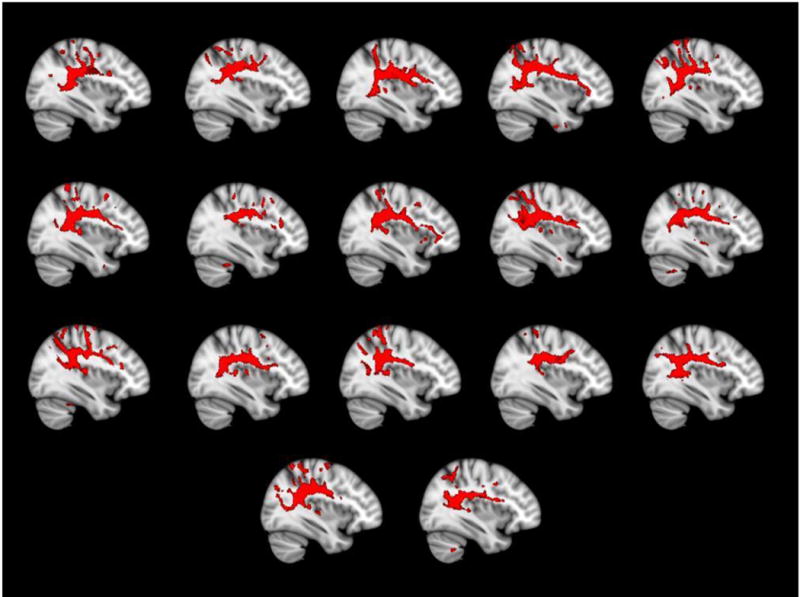
Sagittal view of each child’s tract thresholded at 1% and normalized to MNI space for demonstration purposes.
Our analysis did not reveal any significant associations between the slope of vocabulary growth and the average FA, MD, and RD of the left SMG-seed identified tract. Null results are difficult to interpret, especially since previous studies have shown a negative association between RD and vocabulary learning in left dorsal tracts (Rodríguez-Fornells et al., 2009) and a positive association between FA and word learning in left posterior temporo-parietal areas (Wong et al., 2011). We speculate that the small sample size in our study prevented us from finding significant correlations between early behavioral measures and later acquired diffusion metrics. There was a marginal association between FA and parent SES, which may suggest that environmental factors included in our model (parent education, income), as well other factors not included (parent sensitivity, stress, nutrition), might play a role in white matter microstructure in this tract. Indeed, recent studies highlight relations between parent SES, specifically parent income, and brain structure in areas supporting language and reading skills (Noble et al., 2015). However, children from lower SES backgrounds also moved more during scanning and thus had noisier data. The fact that motion heavily confounds diffusion weighted imaging (Yendiki et al., 2014) warrants caution when interpreting the FA - SES result. In addition, our measure of parent language input was limited to parent word types when the child was 14 months old. Including a wider set of measures to assess parent language input over a longer time span might also reveal significant associations. Future studies using larger sample sizes are needed to explore the relations between parent language input, child early vocabulary growth, and child white matter structure later in development.
Although the microstructural properties of the tract were not significantly associated with the slope of vocabulary growth, its macrostructural properties warrant closer examination. The tract provides a direct connection between the pars opercularis of left IFG and the (ipsilateral) SMG. As such, it may facilitate sensory-motor mapping of sound to articulation (Frey et al., 2008; Makris et al., 2005; Saur et al., 2008). Functional and structural connectivity along the dorsal tracts has been associated with individual differences in the ability to learn non-native words from continuous speech in adults (Lopez-Barroso et al., 2015; 2013). Fractional anisotropy along the SLF III has been correlated with verbal working memory (Peters et al., 2012). MD in the SLF III decreases with age (Budisavljevic et al., 2015), although a meta-analysis of DTI studies in adolescents found that FA increases with age (Peters et al., 2012).
Catani and Bambini (2014) refer to this tract as the “fronto-parietal network for informative actions”, which acts as a filter of incoming sensory information, prioritizing information that is relevant for communication (informative agents and informative actions) (Catani and Bambini, 2014). From a developmental perspective, this basic function may enable the child to engage in interactions with its social environment, as well as the ability to imitate (Saur et al., 2008), an important step in language acquisition (Bates, 1979; Tomasello, 2009).
The frontal terminations to the MFG are of particular interest given the marginal effect found between vocabulary growth and cortical thickness in the left MFG. In a meta-analysis of fMRI studies on language, activation in the left middle frontal gyrus was consistently found for word retrieval (Price, 2010). The areas within the MFG are associated with monitoring and control, and hence may be indirectly involved in novel word acquisition via those processes (Baddeley, 2003). With respect to its connectivity, in the macaque, the SLF III connects the SMG in part to BA46 (within MFG) and it could be important for monitoring the processing of mnemonic information (Petrides and Baddeley, 1996). As noted earlier, the function of the SMG appears to be to sustain activated representations (Cabeza et al., 2012), which is critical for the formation and maintenance of lexical representations, which, in turn, helps the acquisition of novel words in a new language (Baddeley et al., 1998). Connectivity between the SMG and the MFG may enable higher order monitoring and control of this process.
4.3 Conclusions
Cortical thickness in the left SMG is significantly associated with linear growth in vocabulary size, even after controlling for age, gender, SES and parental input. Children with steeper vocabulary growth at 14 to 58 months of age have thinner left SMG cortex at 7 to 9 years of age. Although previous studies have shown an association between the structure of the SMG and word learning in adults, this is the first study to demonstrate that the area is influenced by vocabulary acquisition early in development. Its anatomical position, along with its connectivity to the inferior, middle frontal and precentral gyri via a dorsal tract (most likely the SLF III), highlight the importance of dorsal pathways in first language learning. The temporo-parietal cortical areas have been reported to be the slowest regions to mature among the perisylvian language network (Leroy et al., 2011). Similarly, the dorsal pathways are less mature than the ventral ones at birth, but catch up within the first postnatal weeks (Dubois et al., 2016) and continue to mature until adulthood (Lebel et al., 2008). These developmental changes provide the infant who is not yet able to speak with a dorsal, fronto-parietal neural circuit that captures relevant sensory input in the environment (such as words in continuous speech), sustains relevant information online (such as word representations), and maps it to articulatory actions (through imitation and repetition).
Highlights.
Children differ greatly in the pace at which they acquire their vocabulary.
We estimated the pace of vocabulary growth using longitudinal measurements in 20 children from 14 to 58 months of age.
The pace of vocabulary growth in preschool significantly predicted cortical thickness in the left supramarginal gyrus at school age.
The white matter connectivity of the supramarginal gyrus revealed direct frontal connections via a dorsal white matter tract.
Acknowledgments
This research was supported by a program project grant P01HD40605 from the National Institute of Child Health and Human Development (S. Goldin-Meadow, PI; S. Small, Imaging Project PI). We thank the participating families for sharing their child’s language development with us; Christine Bascetta, Karyn Brasky, Megan Broughan, Laura Chang, Elaine Croft, Kristin Duboc, Sam Engel, Jennifer Griffin, Sarah Gripshover, Kelsey Harden, Lauren King, Max Masich, Carrie Meanwell, Erica Mellum, Molly Nikolas, Jana Oberholtzer, Lilia Rissman, Becky Seibel, Meredith Simone, Calla Trofatter, Kevin Uttich, Julie Wallman, and Kristin Walters, Virginia Li for help in collecting and transcribing the data; Peter Huttenlocher and Martin Staudt for assisting one of the authors (SLS) in coding brain scans; Meredith Rowe for assistance with growth-modeling analyses; and Kristi Schonwald, Jodi Khan, and Jason Voigt for administrative and technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Random House Kernerman Webster’s College Dictionary. (2010). Retrieved December 22 2015 from http://www.thefreedictionary.com/infant
References
- Agosta F, Henry RG, Migliaccio R, Neuhaus J, Miller BL, Dronkers NF, Brambati SM, Filippi M, Ogar JM, Wilson SM, Gorno-Tempini ML. Language Networks In Semantic Dementia. Brain. 2010;133:286–299. doi: 10.1093/brain/awp233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baddeley A, Gathercole S, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295X.105.1.158. [DOI] [PubMed] [Google Scholar]
- Bates E. The Emergence of Symbols. Academic Press; New York: 1979. [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides-Varela S, Hochmann JR, Macagno F, Nespor M, Mehler J. Newborn’s brain activity signals the origin of word memories. Proceedings of the National Academy of Sciences. 2012;109:17908–17913. doi: 10.1073/pnas.1205413109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proceedings of the National Academy of Sciences. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Jansen A, Deppe M, Foerster A-F, Sommer J, Wolbers T, Knecht S. Hippocampus activity differentiates good from poor learners of a novel lexicon. NeuroImage. 2005;25:958–968. doi: 10.1016/j.neuroimage.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Budisavljevic S, Dell’Acqua F, Rijsdijk FV, Kane F, Picchioni M, McGuire P, Toulopoulou T, Georgiades A, Kalidindi S, Kravariti E, Murray RM, Murphy DG, Craig MC, Catani M. Age-Related Differences and Heritability of the Perisylvian Language Networks. Journal of Neuroscience. 2015;35:12625–12634. doi: 10.1523/JNEUROSCI.1255-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends in Cognitive Sciences. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartmill EA, Armstrong BF, Gleitman LR, Goldin-Meadow S, Medina TN, Trueswell JC. Quality of early parent input predicts child vocabulary 3 years later. Proceedings of the National Academy of Sciences. 2013;110:11278–11283. doi: 10.1073/pnas.1309518110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Bambini V. A model for Social Communication And Language Evolution and Development (SCALED) Current Opinion in Neurobiology. 2014;28:165–171. doi: 10.1016/j.conb.2014.07.018. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cornelissen K, Salmelin R, Laine M, Renvall K, Saarinen T, Martin N. Learning new names for new objects: Cortical effects as measured by magnetoencephalography✩. Brain and Language. 2004;89:617–622. doi: 10.1016/j.bandl.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Culham JC, Valyear KF. Human parietal cortex in action. Current Opinion in Neurobiology. 2006;16:205–212. doi: 10.1016/j.conb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical Surface-Based Analysis. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends in Neurosciences. 2006;29:367–373. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Montavont A, Jobert A, Allirol L, Dubois J, Hertz-Pannier L, Dehaene S. Language or music, mother or Mozart? Structural and environmental influences on infants’ language networks. Brain and Language. 2010;114:53–65. doi: 10.1016/j.bandl.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Spelke ES. The Infancy of the Human Brain. Neuron. 2015;88:93–109. doi: 10.1016/j.neuron.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The Language Connectome: New Pathways, New Concepts. The Neuroscientist. 2014;20:453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dubois J, Poupon C, Thirion B, Simonnet H, Kulikova S, Leroy F, Hertz-Pannier L, Dehaene-Lambertz G. Exploring the Early Organization and Maturation of Linguistic Pathways in the Human Infant Brain. Cerebral Cortex. 2016;26:2283–2298. doi: 10.1093/cercor/bhv082. [DOI] [PubMed] [Google Scholar]
- Estes KG, Estes KG, Evans JL, Alibali MW, Saffran JR. Can Infants Map Meaning to Newly Segmented Words?: Statistical Segmentation and Word Learning. Psychological Science. 2007;18:254–260. doi: 10.1111/j.1467-9280.2007.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenson L, Reznick JS, Bates E, Dale PS, Thal DJ, Pethick SJ, Tomasello M, Mervis CB, Stiles J. Variability in Early Communicative Development. Monographs of the Society for Research in Child Development. 1994;59:i. doi: 10.2307/1166093. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical Surface-Based Analysis. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically Parcellating the Human Cerebral Cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M, Campbell JSW, Pike GB. Dissociating the Human Language Pathways with High Angular Resolution Diffusion Fiber Tractography. Journal of Neuroscience. 2008;28:11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13:175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Goldin-Meadow S, Levine SC, Hedges LV, Huttenlocher J, Raudenbush SW, Small SL. New evidence about language and cognitive development based on a longitudinal study: Hypotheses for intervention. American Psychologist. 2014;69:588–599. doi: 10.1037/a0036886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. American parenting of language-learning children: Persisting differences in family-child interactions observed in natural home environments. Developmental Psychology. 1992;28:1096–1105. doi: 10.1037/0012-1649.28.6.1096. [DOI] [Google Scholar]
- Hoff E. How social contexts support and shape language development✩. Developmental Review. 2006;26:55–88. doi: 10.1016/j.dr.2005.11.002. [DOI] [Google Scholar]
- Hoff E. The Specificity of Environmental Influence: Socioeconomic Status Affects Early Vocabulary Development Via Maternal Speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Hoff E, Naigles L. How Children Use Input to Acquire a Lexicon. Child Development. 2002;73:418–433. doi: 10.1111/1467-8624.00415. [DOI] [PubMed] [Google Scholar]
- Huttenlocher J, Haight W, Bryk A, Seltzer M, al N E, et al. Early vocabulary growth: Relation to language input and gender. Developmental Psychology. 1991;27:236–248. doi: 10.1037/0012-1649.27.2.236. [DOI] [Google Scholar]
- Huttenlocher J, Waterfall H, Vasilyeva M, Vevea J, Hedges LV. Sources of variability in children’s language growth. Cognitive Psychology. 2010;61:343–365. doi: 10.1016/j.cogpsych.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nat Rev Neurosci. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Junge C, Kooijman V, Hagoort P, Cutler A. Rapid recognition at 10 months as a predictor of language development. Developmental Science. 2012;15:463–473. doi: 10.1111/j.1467-7687.2012.1144.x. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. Journal of Neuroscience. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain Mechanisms in Early Language Acquisition. Neuron. 2010;67:713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nat Rev Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Lee H, Devlin JT, Shakeshaft C, Stewart LH, Brennan A, Glensman J, Pitcher K, Crinion J, Mechelli A, Frackowiak RSJ, Green DW, Price CJ. Anatomical Traces of Vocabulary Acquisition in the Adolescent Brain. Journal of Neuroscience. 2007;27:1184–1189. doi: 10.1523/JNEUROSCI.4442-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerch JP, Shaw P, Kabani NJ, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental Trajectories of the Human Cerebral Cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy F, Glasel H, Dubois J, Hertz-Pannier L, Thirion B, Mangin JF, Dehaene-Lambertz G. Early Maturation of the Linguistic Dorsal Pathway in Human Infants. Journal of Neuroscience. 2011;31:1500–1506. doi: 10.1523/JNEUROSCI.4141-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Legault J, Litcofsky KA. Neuroplasticity as a function of second language learning: Anatomical changes in the human brain. Cortex. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Lopez-Barroso D, Catani M, Ripollés P, Dell’Acqua F, Rodríguez-Fornells A, de Diego-Balaguer R. Word learning is mediated by the left arcuate fasciculus. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1301696110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barroso D, Ripollés P, Marco-Pallarés J, Mohammadi B, Münte TF, Bachoud-Lévi AC, Rodríguez-Fornells A, de Diego-Balaguer R. Multiple brain networks underpinning word learning from fluent speech revealed by independent component analysis. NeuroImage. 2015;110:182–193. doi: 10.1016/j.neuroimage.2014.12.085. [DOI] [PubMed] [Google Scholar]
- Mahmoudzadeh M, Dehaene-Lambertz G, Fournier M, Kongolo G, Goudjil S, Dubois J, Grebe R, Wallois F. Syllabic discrimination in premature human infants prior to complete formation of cortical layers. Proceedings of the National Academy of Sciences. 2013;110:4846–4851. doi: 10.1073/pnas.1212220110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Pandya DN, Kennedy DN, Makris N, Kennedy DN, McInerney S, McInerney S, Sorensen AG, Sorensen AG, Wang R, Wang R, Caviness VS, Caviness VS, Pandya DN. Segmentation of Subcomponents within the Superior Longitudinal Fascicle in Humans: A Quantitative, In Vivo, DT-MRI Study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Maldonado IL, Moritz-Gasser S, Duffau H. Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct. 2011;216:263–274. doi: 10.1007/s00429-011-0309-x. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O’Doherty J, Ashburner J, Frackowiak RS, Price CJ. Neurolinguistics: Structural plasticity in the bilingual brain. Nature. 2004;431:757–757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Montag JL, Montag JL, Jones MN, Jones MN, Smith LB, Smith LB. The Words Children Hear: Picture Books and the Statistics for Language Learning. Psychological Science. 2015;26:1489–1496. doi: 10.1177/0956797615594361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PL, Farkas G, Hillemeier MM, Hammer CS, Maczuga S. 24-Month-Old Children With Larger Oral Vocabularies Display Greater Academic and Behavioral Functioning at Kindergarten Entry. Child Development. 2015;86:1351–1370. doi: 10.1111/cdev.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazzi T, Bertoncini J. Before and after the vocabulary spurt: two modes of word acquisition? Developmental Science. 2003;6:136–142. doi: 10.1111/1467-7687.00263. [DOI] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, Akshoomoff N, Amaral DG, Bloss CS, Libiger O, Schork NJ, Murray SS, Casey BJ, Chang L, Ernst TM, Frazier JA, Gruen JR, Kennedy DN, Van Zijl P, Mostofsky S, Kaufmann WE, Kenet T, Dale AM, Jernigan TL, Sowell ER. Family income, parental education and brain structure in children and adolescents. Nat Neurosci. 2015;18:773–778. doi: 10.1038/nn.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Schmahmann JD. Fiber Pathways of the Brain. New York: Oxford University Press. Oxford University Press; 2006. [DOI] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK. White Matter Development in Adolescence: Diffusion Tensor Imaging and Meta-Analytic Results. Schizophrenia Bulletin. 2012;38:1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Baddeley A. Specialized Systems for the Processing of Mnemonic Information within the Primate Frontal Cortex [and Discussion] Philosophical Transactions of the Royal Society B: Biological Sciences. 1996;351:1455–1462. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Distinct Parietal and Temporal Pathways to the Homologues of Broca’s Area in the Monkey. PLoS Biol. 2009 doi: 10.1371/journal.pbio.1000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Pujol J, Soriano-Mas C, Ortiz H, Sebastian-Galles N, Losilla JM, Deus J. Myelination of language-related areas in the developing brain. Neurology. 2006;66:339–343. doi: 10.1212/01.wnl.0000201049.66073.8d. [DOI] [PubMed] [Google Scholar]
- Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models. SAGE; 2002. [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson FM, Price CJ. Structural MRI studies of language function in the undamaged brain. Brain Struct Funct. 2009;213:511–523. doi: 10.1007/s00429-009-0211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson FM, Thomas MSC, Filippi R, Harth H, Price CJ. Contrasting Effects of Vocabulary Knowledge on Temporal and Parietal Brain Structure across Lifespan. Journal of Cognitive Neuroscience. 2010;22:943–954. doi: 10.1162/jocn.2009.21238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Fornells A, Cunillera T, Mestres-Missé A, de Diego-Balaguer R. Neurophysiological mechanisms involved in language learning in adults. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364:3711–3735. doi: 10.1098/rstb.2009.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalsky C, Chen KH, Poppa T, Anderson SW. Frontiers | Relative Contributions of the Dorsal vs. Ventral Speech Streams to Speech Perception are Context Dependent: a lesion study. doi: 10.3389/conf.fpsyg.2014.64.00024/event_abstract. n.d. frontiersin.org. [DOI]
- Rowe ML. A Longitudinal Investigation of the Role of Quantity and Quality of Child-Directed Speech in Vocabulary Development. Child Development. 2012;83:1762–1774. doi: 10.1111/j.1467-8624.2012.01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, Goldin-Meadow S. Early gesture selectivelypredicts later language learning. Developmental Science. 2009;12:182–187. doi: 10.1111/j.1467-7687.2008.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, Raudenbush SW, Goldin-Meadow S. The Pace of Vocabulary Growth Helps Predict Later Vocabulary Skill. Child Development. 2012;83 doi: 10.1111/j.1467-8624.2011.01710.x. no–no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruschel M, Knösche TR, Friederici AD, Turner R, Geyer S, Anwander A. Connectivity Architecture and Subdivision of the Human Inferior Parietal Cortex Revealed by Diffusion MRI. Cerebral Cortex. 2014;24:2436–2448. doi: 10.1093/cercor/bht098. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS. Connection Patterns Distinguish 3 Regions of Human Parietal Cortex. Cerebral Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Saffran JR, Aslin RN, Newport EL. Statistical Learning by 8-Month-Old Infants. Science. 1996;274:1926–1928. doi: 10.1126/science.274.5294.1926. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, Huber W, Rijntjes M, Hennig J, Weiller C. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya D. Fiber Pathways of the Brain. OUP USA; 2009. [Google Scholar]
- Singh L, Reznick JS, Xuehua L. Infant word segmentation and childhood vocabulary development: a longitudinal analysis. Developmental Science. 2012;15:482–495. doi: 10.1111/j.1467-7687.2012.01141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-Based Spatial Statistics: Voxelwise Analysis of Multi-Subject Diffusion Data. FMRIB Technical Report TR05SS1. 1:1–26. doi: 10.1016/j.neuroimage.2006.02.024. n.d. [DOI] [PubMed] [Google Scholar]
- Snow CE, Burns MS, Griffin P. Preventing Reading Difficulties in Young Children. National Academies Press; 1998. [Google Scholar]
- Sowell ER, Toga AW, Thomson PM, Leonard CM, Welcome SE, Kan E. Longitudinal Mapping of Cortical Thickness and Brain Growth in Normal Children. Journal of Neuroscience. 2004 doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello M. THE CULTURAL ORIGINS OF HUMAN COGNITION. Harvard University Press; 2009. [Google Scholar]
- Travis KE, Leonard MK, Brown TT, Hagler DJ, Curran M, Dale AM, Elman JL, Halgren E. Spatiotemporal Neural Dynamics of Word Understanding in 12- to 18-Month-Old-Infants. Cerebral Cortex. 2011;21:1832–1839. doi: 10.1093/cercor/bhq259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: Synthesizing Aphasia and the Neural Basis of Language in a Neurocomputational Model of the Dual Dorsal-Ventral Language Pathways. Neuron. 2011;72:385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Veroude K, Norris DG, Shumskaya E, Gullberg M, Indefrey P. Functional connectivity between brain regions involved in learning words of a new language. Brain and Language. 2010;113:21–27. doi: 10.1016/j.bandl.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Østby Y, Tamnes CK, Fjell AM. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49:3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Weisleder A, Fernald A. Talking to Children Matters: Early Language Experience Strengthens Processing and Builds Vocabulary. Psychological Science. 2013;24:2143–2152. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong FCK, Chandrasekaran B, Garibaldi K, Wong PCM. White Matter Anisotropy in the Ventral Language Pathway Predicts Sound-to-Word Learning Success. Journal of Neuroscience. 2011;31:8780–8785. doi: 10.1523/JNEUROSCI.0999-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Fischl B, Koldewyn K, Kakunoori S, Kanwisher N. Spurious group differences due to head motion in a diffusion MRI study. NeuroImage. 2014;88:79–90. doi: 10.1016/j.neuroimage.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012;48:58–81. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


